Abstract
α-Dystroglycan (α-DG) was recently identified as a receptor for lymphocytic choriomeningitis virus (LCMV) and several other arenaviruses, including Lassa fever virus (W. Cao, M. D. Henry, P. Borrow, H. Yamada, J. H. Elder, E. V. Ravkov, S. T. Nichol, R. W. Compans, K. P. Campbell, and M. B. A. Oldstone, Science 282:2079–2081, 1998). Data presented in this paper indicate that the affinity of binding of LCMV to α-DG determines viral tropism and the outcome of infection in mice. To characterize this relationship, we evaluated the interaction between α-DG and several LCMV strains, variants, and reassortants. These viruses could be divided into two groups with respect to affinity of binding to α-DG, dependence on this protein for cell entry, viral tropism, and disease course. Viruses that exhibited high-affinity binding to α-DG displayed a marked dependence on α-DG for cell entry and were blocked from infecting mouse 3T6 fibroblasts by 1 to 4 nM soluble α-DG. In addition, high-affinity binding to α-DG correlated with an ability to infiltrate the white pulp (T-dependent) area of the spleen, cause ablation of the cytotoxic T-lymphocyte (CTL) response by day 7 postinfection, and establish a persistent infection. In contrast, viruses with a lower affinity of binding to α-DG were only partially inhibited from infecting α-DG−/− embryonic stem cells and required a concentration of soluble α-DG higher than 100 nM to prevent infection of mouse 3T6 fibroblasts. These viruses that bound at low affinity were mainly restricted to the splenic red pulp, and the host generated an effective CTL response that rapidly cleared the infection. Reassortants of viruses that bound to α-DG at high and low affinities were used to map genes responsible for the differences described to the S RNA, containing the virus attachment protein glycoprotein 1.
The initial stage of any viral infection involves interaction of the virus with a host cell receptor(s). Identifying the cellular receptor(s) and defining this interaction can provide data on viral tropism and pathogenesis and can have potential therapeutic value by aiding drug design. Recently, α-dystroglycan (α-DG) was identified as a common receptor for lymphocytic choriomeningitis virus (LCMV) and several arenaviruses that are pathogenic to humans, including Lassa fever virus (9).
LCMV, the prototypic arenavirus, is an enveloped, bisegmented, negative-strand RNA virus with an ambisense coding strategy (reviewed in references 30 and 36). The long (L) segment of RNA contains genes encoding the viral polymerase and Z, a zinc finger motif protein thought to play a role in regulation of transcription (22). The short (S) segment of RNA contains genes encoding the viral nucleoprotein (NP) and the glycoprotein (GP) precursor, GP-C, that is posttranslationally cleaved into GP-1 and GP-2. Several lines of evidence show that LCMV interacts with the cellular receptor via the GP-1 subunit that is exposed on the top of the virion surface GP. Epitopes recognized by neutralizing antibodies are contained within GP-1 (29), and these antibodies prevent the binding of LCMV to cells in vitro (3).
α-DG is a peripheral membrane GP that is noncovalently associated with the transmembrane protein β-dystroglycan (β-DG) (20). α-DG interacts with components of the extracellular matrix, whereas β-DG interacts with the cytoskeleton, thereby linking the exterior and the interior of cells (reviewed in references 16, 17, and 19). The DG complex is expressed in most organs, at differing levels (13). The seemingly diverse roles played by DG in a number of essential physiologic processes make it an ideal choice of receptor for the virus.
LCMV has been used extensively in its natural rodent host to study virus-host interactions, including those involving the virus-immune and -autoimmune, virus-nervous, and virus-endocrine systems (5, 8, 27, 28, 41). In a number of comparative studies in vivo, LCMV strains demonstrating a high degree of homology have been observed to display markedly different tissue tropisms and distinct courses of disease, despite having a common receptor (4, 10). Earlier studies defined differences between LCMV Armstrong 53b (Arm) and its variant Clone 13 (Cl 13). Both virus strains cause a persistent infection as a result of congenital, in utero, or neonatal infection. However, when inoculated intravenously (i.v.) into adult mice, only Cl 13, and not Arm, causes a persistent infection (1, 33, 34). Biologically, persistent infections initiated early in life differ from those developing in adulthood; the former are due to thymic deletion of virus-specific T cells, whereas the latter are due to exhaustion of virus-specific T cells and are associated with a generalized immunosuppression (4, 25, 38). Furthermore, Cl 13 injected i.v. into adult mice was noted to replicate preferentially in the white pulp of the spleen and infect interdigitating dendritic cells, while, in contrast, Arm localized primarily to the red pulp of the spleen, with almost a complete absence from the inner white pulp (4). Genetically, Cl 13 differs from Arm by 5 nucleotides, of which only two result in residue changes in open reading frames (33). In our laboratory, the molecular basis of persistence and suppression of the anti-LCMV cytotoxic T-lymphocyte (CTL) response has been mapped to a single amino acid change in the GP (residue 260, Leu [Cl 13] to Phe [Arm]) (11, 34), while other researchers have implicated this mutation and a mutation within L (residue 1079, Q [Cl 13] to K [Arm]) in these phenotypes (23). Recently it was observed that Cl 13, which caused immunosuppression, bound more vigorously to α-DG than did Arm (9).
We designed experiments to answer the following five questions. First, was there a quantitative difference in binding to α-DG between Cl 13, which suppresses the anti-LCMV CTL response (CTL−) and establishes viral persistence (P+), and Arm, which does not suppress the anti-LCMV CTL response (CTL+) and does not establish persistence (P−)? Second, do other CTL− P+ and CTL+ P− strains of LCMV bind to α-DG with different affinities? Third, are all CTL− P+ viruses associated with tropism for the white pulp of the spleen? Fourth, is α-DG an absolute requirement for cell entry by CTL− P+ and CTL+ P− viruses? Lastly, in studies using CTL+ P−-CTL− P+ LCM virus reassortants, do the heightened ability to bind α-DG and selective tropism to the white pulp of the spleen map to a specific viral RNA segment?
MATERIALS AND METHODS
Mice.
Female BALB/cByJ mice were 6 to 8 weeks old at the onset of the experiments. All mice were obtained from the rodent breeding colony at The Scripps Research Institute (La Jolla, Calif.). All mice were bred and maintained under specific-pathogen-free conditions.
Virus strains, virus quantification, and routes of infection.
Origin, passage history, sequences, and quantitation of LCMV strains Cl 13, Traub, E350, WE54, and WE2.2 have been described elsewhere (14, 34, 35, 37). These viruses and the reassortant viruses Cl 13/ARM (long RNA/short RNA), ARM/Cl 13, Traub/ARM, and ARM/Traub were triply plaque purified on baby hamster kidney (BHK) cells and characterized as described previously (23, 32). Seed stocks of all viruses were prepared by growth on BHK-21 cells, and their titers were determined by plaque assay on Vero cells. Plaque assays were also used to quantitate viral titers in mouse sera and spleen homogenates. Mice were infected with LCMV by i.v. inoculation of 2 × 106 PFU of virus in 200 μl of saline.
Cytotoxicity (CTL) assay.
LCMV-specific CTL activity was assessed in erythrocyte-depleted single-splenocyte suspensions by using a standard 5-h 51Cr release assay. Target cells were 51Cr labeled, LCMV infected (multiplicity of infection [MOI] = 1, 20 h) and uninfected, major histocompatibility complex-matched (BALB/c17 [H-2d]) and mismatched (MC57 [H-2b]) cells. Effector:target ratios of 50:1, 25:1, and 12.5:1 were used.
ES cells.
α-DG knockout (DG−/− embryonic stem (ES) cells (B11), generated as described elsewhere (18), and wild-type (DG+/+) (R1) cells were maintained in Dulbecco's modified Eagle medium (Gibco BRL, Grand Island, N.Y.) containing 15% (vol/vol) fetal calf serum (HyClone, Logan, Utah), 1% (vol/vol) penicillin, 1% (vol/vol) streptomycin (both from Gibco), 1% (vol/vol) glutamine (Gibco), 0.001% (vol/vol) β-mercaptoethanol (Sigma; tissue culture grade), and 103 U of leukemia inhibitory factor (Gibco)/ml in tissue culture flasks pretreated with 0.1% (wt/vol) gelatin (Sigma). For infection studies, ES cells (4 × 105/well) were plated in gelatin-pretreated 24-well plates and incubated for 24 h at 37°C. LCMV was added at an MOI of 0.1, 1, or 5. After 1 h of incubation at 37°C, the nonadherent virus particles were removed, the cells were gently washed three times with medium, and the medium was replaced. Cells were then incubated for 24 or 48 h at 37°C, trypsinized, and transferred onto heavy Teflon-coated 10-well 7-mm-diameter microscope slides (Cel-line, Newfield, N.J.). After 15 min, excess medium was removed, and cells were air dried, fixed in acetone for 10 min, and immunostained with monoclonal antibody (MAb) 113, which is specific for LCMV NP (7). Briefly, cells were incubated with primary antibody (1:100 dilution) for 30 min, washed three times for 5 min in phosphate-buffered saline (PBS), and incubated with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G (Cappel, Costa Mesa, Calif.; 1:40 dilution) for 30 min. The percentage of infected cells was determined with an Olympus BH-2 fluorescence microscope by counting at least 350 cells per well. Duplicate samples were counted for each virus and cell line at each MOI and time point. Results are presented as percentages of infected cells per total cells counted.
Immunocytochemistry.
Mice were sacrificed, and their spleens were immediately embedded in Tissue-Tek O.C.T. compound (Miles Diagnostics Division, Elkhart, Ind.) and frozen on dry ice. Six-micrometer-thick sections were cut on a cryostat, fixed for 2 min in acetone, air dried, and stored at −20°C until stained. After being fixed in acetone for an additional 8 min, sections were washed for 20 min in PBS, blocked for 1 h with 1.5% normal goat serum (Vector Laboratories Inc., Burlingame, Calif.), and stained with a 1:1,000 dilution of a guinea pig anti-LCMV serum (6) overnight at 4°C. Sections were washed in PBS twice for 30 min and then incubated with fluorescein isothiocyanate-conjugated goat anti-guinea pig IgG (Cappel; 1:200 dilution) for 3 h at room temperature. Sections were washed in PBS twice for 10 min and mounted under glass coverslips by using Vectashield mounting medium (Vector Laboratories).
Virus overlay protein blot assay (VOPBA).
α-DG was derived from rabbit skeletal muscle or α-DG+/+ cells (9, 18). Purified α-DG from rabbit skeletal muscle was log serially diluted (from 1 μg/lane) and electrophoresed on 6% polyacrylamide gels. Proteins separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were electrophoretically transferred onto nitrocellulose membranes (0.45 μm pore size; Schleicher and Schuell, Keene, N.H.) by using a Bio-Rad (Hercules, Calif.) transfer apparatus. The membranes were blocked in 5% (wt/vol) skim milk powder in PBS for 1 h. The membranes were then placed in heat-sealable bags, and virus (4 × 107 PFU) was added in PBS with 1% bovine serum albumin. After overnight incubation at 4°C with mild agitation, the membranes were rinsed in wash buffer (PBS–0.1% Tween 20) three times for 5 min each at room temperature. Virus binding to α-DG was detected with pooled MAbs to LCMV GP-1- and GP-2 (WE-36.1 and WE-33.1, respectively) as described in reference 9. After a 1-h incubation, the membranes were washed three times with wash buffer, incubated with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (Pierce, Rockford, Ill.; 1:5,000 dilution in wash buffer) for 45 min at room temperature with mild agitation, and developed for peroxidase activity by using Supersignal chemiluminescent substrate (Pierce). Specific signals were recorded on autoradiographic film (Kodak, Rochester, N.Y.).
α-DG was also enriched from ES cells. Briefly, cells were cultured to approximately 80% confluence in T75 flasks. Cells were washed twice with PBS and solubilized in 4 ml of solubilization buffer (50 mM HEPES [pH 7.5], 200 mM NaCl, 1% [wt/vol] NP-40, 1.2 mM EDTA, complete protease inhibitor cocktail [Boehringer Mannheim, Indianapolis, Ind.], 1 mM phenylmethylsulfonyl fluoride [Boehringer]). Cells were removed by using a cell scraper, pipetted up and down several times, placed on a shaker for 15 min at 4°C, and centrifuged at 14,000 rpm for 10 min in an Eppendorf centrifuge (model 5415 C). The cell lysate was transferred to fresh tubes, and 5 mM MgCl2 and 5 mM CaCl2 were added. Jacalin-Sepharose (Vector Laboratories) was added (2 μl/ml of lysate), and the lysate was gently shaken overnight at 4°C. The lectin matrix with bound material was washed three times with wash buffer (50 mM HEPES [pH 7.5], 200 mM NaCl, 0.05% [wt/vol] NP-40, 1.2 mM EDTA, complete protease inhibitor cocktail, 1 mM phenylmethylsulfonyl fluoride, 5 mM CaCl2, 5 mM MgCl2) at 14,000 rpm for 30 s in the same centrifuge. SDS-PAGE sample buffer was added, and samples were boiled for 5 min.
Competitive inhibition assay of virus binding to α-DG.
As reported previously, inhibition of binding of virus to α-DG was measured in a competitive inhibition assay using soluble α-DG (9). Mouse 3T6 fibroblasts (105/well) were plated in 24-well plates and incubated at 37°C overnight. Virus (2 × 105 PFU) was incubated with serially diluted, soluble α-DG (or bovine serum albumin as a control) at concentrations of 0 to 100 nM for 20 min at 4°C. In preliminary experiments, the use of ≥400 nM soluble α-DG was often toxic to the cultured cells. Cells were then incubated with virus and α-DG at 37°C for 30 min. The medium was replaced, and the cells were cultured for a further 16 h at 37°C. Cells were washed with PBS, fixed with acetone for 10 min, air dried, and stained for LCMV NP by using MAb 113.
RESULTS
LCMV strains exhibit two distinct patterns of infection kinetics.
BALB/cByJ mice were infected i.v. with equivalent doses of one of six different LCMV isolates, and viral titers were determined per gram of spleen tissue at various time points postinfection (p.i.) (Fig. 1A). All strains of LCMV increased in titer in the first 3 days p.i. Subsequently, infection patterns diverged and two definable groups became apparent. Arm, E350, and WE2.2 viral titers were dramatically reduced after this time point and were cleared from the spleen by day 7 (Arm), day 14 (E350), or day 30 (WE2.2) p.i. By contrast, viral titers of Cl 13, Traub, and WE54 continued to increase until day 7 p.i. After this time point, viral titers were reduced, but virus still persisted at day 30 p.i., the last time point studied. In summary, Cl 13, Traub, and WE54 persisted in adult mice following i.v. inoculation of 2 × 106 PFU (P+) while Arm, E350 and WE2.2 were cleared (P−).
FIG. 1.
Viral persistence is associated with a loss of LCMV-specific CTL activity by day 7 p.i. BALB/cByJ were infected with 2 × 106 PFU of LCMV i.v. (A) Virus titers were determined by plaque assay at a series of time points p.i. in mice infected with Cl 13 (⧫), WE54 (■), Traub (▴), Arm (□), E350 (○), or WE2.2 (▵). Results represent the mean viral titers for two mice per group per time point. These data are representative of at least two similar experiments. (B) LCMV-specific CTL responses were determined at day 7 p.i., using a standard 51Cr release assay. Results are representative of three similar experiments.
Strains that establish persistent infection compromise virus-specific CTL responses.
As previously reported, LCMV Arm elicits an efficient virus-specific CD8+ CTL response that effectively controls acute infection, with clearance of virus from the spleen by day 7 to 10 p.i. in BALB/cByJ mice. In contrast, although a CTL response is detected at day 5 p.i. following infection with Cl 13, this response is ablated at day 7 p.i. (4). Accordingly, at day 7 p.i. in mice infected with P− (Arm, E350, or WE2.2) and P+ (Cl 13, Traub, or WE54) viruses, distinct immune phenotypes again emerged (Fig. 1B). Mice infected with Arm or E350 mounted a considerable antiviral CTL response. Mice infected with WE2.2 also demonstrated a good antiviral CTL response, although it was lower than that seen in mice infected with Arm or E350. However, this response was higher than the response by Cl 13-, Traub-, or WE54-infected animals, which had severely compromised or ablated CTL responses. These data confirmed our grouping of the viruses into two phenotypes: CTL+ P− (Arm, E350, and WE2.2) and CTL− P+ (Cl 13, Traub, and WE54).
Viral tropism in the spleen correlates with viral persistence.
Earlier studies indicated that by day 3 following i.v. inoculation of 2 × 106 PFU of LCMV Cl 13 into immunocompetent mice, virus had localized predominantly within the T-cell-dependent white pulp area of the spleen (4). In contrast, similar infection with Arm led to localization of the virus almost exclusively within the red pulp (4). Therefore, we studied the tropism of LCMV E350, Traub, WE54, and WE2.2 in the spleen by using immunofluorescence and MAb 113, which detects expression of LCMV NP equivalently in all these strains. Figure 2 shows the two resulting patterns of infectivity. Initial characteristics of infection were similar for all viruses tested in that 1 day following i.v. injection, infection was focused predominantly within cells of the marginal zone (MZ) (data not shown). However, by day 3 p.i., the pattern of viral spread had diverged. In mice infected with a CTL+ P− virus (Arm, E350, or WE2.2), the focus of infection shifted primarily to cells of the red pulp, with minimal white pulp infiltration (WP−). In contrast, in those mice infected with a CTL− P+ virus (Cl 13, Traub, or WE54), heavy infection was visible within the inner white pulp of the spleen (WP+). Thus, the two groups of viruses can be further defined as CTL+ P− WP− (Arm, E350, and WE2.2) or CTL− P+ WP+ (Cl 13, Traub, and WE54).
FIG. 2.
CTL− P+ and CTL+ P− viruses exhibit distinct tropisms within the spleens of adult BALB/cByJ mice. In the spleens of mice infected with the CTL− P+ virus Cl 13, Traub, or WE54, virus localized primarily to the white pulp (solid white arrow); in contrast, the CTL+ P− viruses Arm, E350, and WE2.2 localized primarily to the red pulp (open arrow) by 3 days p.i. Virus was detected by an immunofluorescence assay using a guinea pig anti-LCMV antibody. Results are representative of three similar experiments using three mice per group per experiment. Magnification, ×10.
LCMV strains differ in their affinity of binding to α-DG.
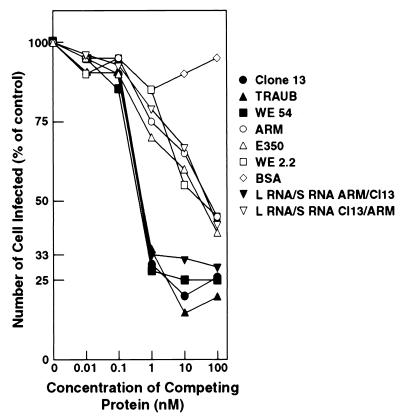
Arm and Cl 13 both bind to purified α-DG in VOPBAs (9). We quantitated binding of the LCMV strains by two assays: binding of viruses to α-DG immobilized on nitrocellulose membranes (VOPBA), and a competitive entry inhibition assay using soluble α-DG. Two sources of α-DG were used in the VOPBAs. First, we studied binding of virus to purified rabbit α-DG that was log serially diluted from 1 μg of protein/lane. As seen in Fig. 3A, the CTL− P+ WP+ viruses Cl 13 and Traub bound to 0.01 μg of α-DG, a binding affinity 2 to 3 logs higher than that of Arm or E350 (CTL+ P− WP−). Similarly, WE54 (CTL− P+ WP+) demonstrated intense binding to the purified protein (data not shown). In stark contrast, binding of WE2.2 (CTL+ P− WP−) to α-DG was not detected (data not shown). Thus, either binding was below detection levels in this assay or no interaction occurred between WE2.2 and α-DG. We also studied virus binding to Jacalin-Sepharose-purified proteins from α-DG+/+ and α-DG−/− murine ES cells (Fig. 3B). This purification procedure enriches for α-DG. Cl 13, Traub, and WE54 bound to the jacalin-Sepharose-purified protein from α-DG+/+ cells, but not to that from α-DG−/− ES cells. In contrast, no binding of Arm, E350, or WE2.2 was evident in this assay, suggesting that the α-DG concentration in these preparations was too low for detectable levels of binding to occur. The second assay used soluble α-DG to competitively inhibit binding of LCMV to α-DG expressed on mouse 3T6 fibroblasts. Cao et al. previously demonstrated that infection of 3T6 cells by Cl 13 could be blocked by addition of soluble α-DG (9). Soluble α-DG was used in our assay at 0.01, 0.1, 1, 10, and 100 nM, because these concentrations routinely had no effect on the viability of the 3T6 cells while soluble α-DG concentrations above 400 nM were often toxic. As seen in Fig. 4, only <1 to 4 nM α-DG was needed for a 33% inhibition of infection of 3T6 cells by the CTL− P+ WP+ viruses Cl 13, Traub, and WE54, but for the CTL+ P− WP− viruses Arm, E350, and WE2.2, an α-DG concentration of over 100 nM was required to achieve this level of inhibition. Thus, the LCMV virus isolates could now be grouped into the distinct phenotypes CTL+ P− WP− α-DGlow (Arm, E350, and WE2.2) and CTL− P+ WP+ α-DGhigh (Cl 13, Traub, and WE54).
FIG. 3.
CTL− P+ WP+ viruses bind vigorously to immobilized α-DG. α-DG purified from rabbit skeletal muscle (A) or α-DG−/−(−) and α-DG−/−(+) ES cells (B) was separated by SDS-PAGE and immobilized on a nitrocellulose membrane. Membranes were incubated with 107 PFU of virus/ml overnight at 4°C. Bound virus was detected with the MAbs WE33 and WE36, which are specific for LCMV glycoproteins 1 and 2, respectively, followed by incubation with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin. A positive signal was detected by chemiluminescence. 2° ab, secondary antibody.
FIG. 4.
Infection of 3T6 mouse fibroblasts by CTL− P+ WP+ viruses is inhibited by preincubation with soluble α-DG. 3T6 mouse fibroblast cells were infected with LCMV strains and reassortants which were preincubated with 0 to 100 nM soluble α-DG. Percentages of infected cells were determined 16 h later by quantitating the percengage of cells expressing viral NP, Using MAb 113, and the number of infected cells was determined by fluorescence microscopy, with at least 200 cells being counted per sample. BSA, bovine serum albumin.
LCMV strains differ in their dependence on α-DG for cellular entry.
The variability in affinity of binding to α-DG suggested that the LCMV strains might differ in their dependence on α-DG for cell entry. To formally test this possibility, α-DG+/+ (wild-type) and α-DG−/− (knockout) ES cell lines were incubated with virus at various MOIs and productive infection was assessed by expression of LCMV NP at 24 and 48 h p.i. (Fig. 5). At 24 h p.i., few DG−/− ES cells were infected by any viral strain tested, with the exception of WE2.2, which infected 70 to 80% of both wild-type and knockout cells. Yet, even at this early time point, CTL+ P− WP− α-DGlow viruses achieved higher levels of infection of the knockout cells than did CTL− P+ WP+ α-DGhigh viruses. By 48 h p.i., a marked divergence of the viral groups became apparent. Wild-type cells were infected by all viruses to similar high levels, whereas knockout ES cells still remained largely refractory to infection by CTL− P+ WP+ α-DGhigh viruses. Indeed, only approximately 10 to 15% of the cells were infected by Cl 13, Traub, or WE54, even at an MOI of 5, indicating a strong dependence on α-DG for cell entry and productive infection by these viruses. In contrast, CTL+ P− WP− α-DGlow viruses (Arm and E350) showed a greatly reduced dependence on α-DG. At 48 h p.i., at an MOI of 5, Arm and E350 showed high levels of infection of α-DG−/− cells, comparable to those for wild-type cells. The ability of virus to infect the α-DG−/− cells was still somewhat compromised by the absence of α-DG and appeared to be viral dose dependent, since at a lower MOI (1 or 0.1) both Arm and E350 infected fewer knockout mutant than wild-type cells. These data suggested that although the presence of α-DG was not an absolute requirement for cell entry by CTL+ P− WP− α-DGlow viruses, its absence impeded optimal virus uptake. Interestingly, and in contrast to the other five viruses, WE2.2 (CTL+ P− WP− α-DGlow) infected similarly high numbers of α-DG+/+ and α-DG−/− cells at 24 and 48 h p.i., demonstrating minimal reliance on α-DG for cell entry by this virus.
FIG. 5.
α-DG−/− ES cells are highly refractory to infection with CTL− P+ WP+ viruses but are permissive to infection with CTL+ P− WP− viruses. α-DG−/− (white bars) or α-DG-+/+ (black bars) ES cells were infected with LCMV at MOIs of 5, 1, and 0.1 for 24 or 48 h. Infection levels were assessed by immunofluorescence staining with NP-specific MAb 113. Duplicate samples were counted for each virus and cell line at each MOI and time point. Data are mean percentages of infected cells ± standard deviations. These data are representative of three similar experiments.
The ability of viruses to bind at high affinity to α-DG, initiate a persistent infection, and localize to the MZ or white pulp of the spleen maps to genes encoded on the viral S RNA segment.
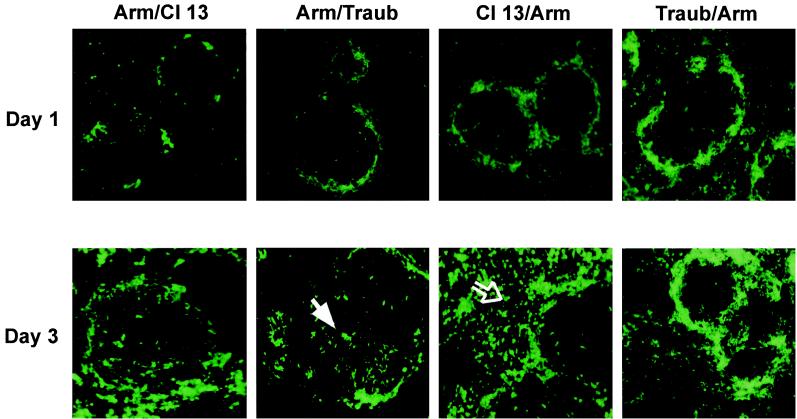
The last series of experiments utilized CTL+ P− WP− α-DGlow-CTL− P+ WP+ α-DGhigh virus reassortants to determine where these phenotypes mapped genetically. L RNA/S RNA reassortants of Cl 13/Arm, Arm/Cl 13, Traub/Arm, and Arm/Traub were analyzed initially for in vivo tropism and disease kinetics. Figure 6 shows that, as was seen with the parental viruses, at day 1 p.i. the main focus of virus infection in the spleen was within the MZ. However, as for the parental viruses, by day 3 p.i. there was a divergence of infectivity patterns, with reassortant viruses showing movement from the MZ into either the white pulp or the red pulp of the spleen. The foci of Cl 13/Arm and Traub/Arm were found predominantly in the splenic red pulp. In addition, however, Traub/Arm still demonstrated infection of the MZ, which may indicate a delay in its movement into the red pulp, perhaps because of a reduction in replication kinetics of this reassortant. Importantly, however, the white pulp was virtually uninfected by either of these reassortant viruses. In contrast, the Arm/Cl 13 and Arm/Traub localized primarily within the white pulp of the spleen, indicating that the tropism for this area of the spleen maps to the S RNA of Traub and Cl 13. We then evaluated reassortant viral titers in the spleen at day 30 p.i. At this time point, mice infected with reassortants containing the S RNA of either Cl 13 or Traub displayed low but detectable titers of persisting virus (Arm/Cl 13, 2,266 ± 757 PFU/g of spleen tissue; Arm/Traub, 1,133 ± 643 PFU/g). However, at this time point, spleens of mice infected with Cl 13/Arm or Traub/Arm were completely clear of virus. Binding to α-DG and entry into ES cells also mapped to genes encoded by the viral S RNA. As shown in Fig. 7, in VOPBAs on jacalin-Sepharose-purified proteins from α-DG+/+ and α-DG−/− ES cells, reassortant viruses containing an S RNA originating from Cl 13 or Traub (i.e., Arm/Cl 13 or Arm/Traub) bound effectively to jacalin-Sepharose-purified proteins from α-DG+/+, but not α-DG−/−, ES cells. In contrast, binding by reassortants containing an S RNA from Arm (i.e., Cl 13/Arm or Traub/Arm) was undetectable. Similar results were obtained in VOPBAs of α-DG from rabbit skeletal muscle (data not shown). Using these reassortants, we found that only 8 to 9 nM soluble α-DG was necessary to competitively inhibit the binding of viruses containing S RNA from Cl 13 (i.e., Arm/Cl 13) or Traub (Arm/Traub) (data not shown) to 3T6 cells, while >100 nM soluble α-DG was required to competitively inhibit binding of those viruses containing an S RNA from Arm (Fig. 4).
FIG. 6.
The ability to infiltrate the splenic white pulp of BALB/cByJ mice maps to the S RNA of LCMV. Mice (three/group) were infected with 2 × 106 PFU of reassortant virus i.v. Spleens were harvested at day 1 and day 3 p.i. At day 1, all reassortant virus was localized in the MZ of the spleen. By day 3 p.i., mice infected with reassortants containing S RNA from the CTL− P+ viruses had localized primarily in the white pulp (solid white arrow) whereas mice infected with reassortants containing S RNA from CTL+ P− viruses had localized to the red pulp (open arrow). Virus was detected by immunofluorescence staining, using a guinea pig anti-LCMV antibody. Data are representative of two similar experiments. Magnification, ×10.
FIG. 7.
Affinity of binding to α-DG in vitro maps to the S RNA segment of LCMV. Reassortant virus (107 PFU/ml) was incubated with jacalin-Sepharose-purified protein (α-DG) from α-DG−/− and α-DG+/+ ES cells. Virus was detected as described in the legend to Fig. 3. Infection of 3T6 mouse fibroblast cells by LCMV reassortants preincubated with 1 to 100 nM soluble α-DG is depicted in Fig. 4.
α-DG+/+ and α-DG−/− ES cells were used to study the dependence of the reassortant viruses on α-DG for cellular entry (Fig. 8). Reassortants containing an S RNA from Cl 13 or Traub were markedly inhibited from establishing a productive infection in α-DG−/− cells at 24 h p.i., and at 48 h these cells remained largely refractory to infection, with 15% or fewer becoming infected at an MOI of 5. In contrast, reassortants containing an S RNA originating from Arm (Cl 13/Arm and Traub/Arm) demonstrated a low but reproducible level of infection of the α-DG−/− ES cells at 24 h p.i., although at this time point α-DG+/+ ES cells were much more readily infected. However, at 48 h p.i. with an MOI of 5, Cl 13/Arm infected wild-type and α-DG−/− ES cells at comparable levels and Traub/Arm infected more than 75% of α-DG−/− ES cells. As was seen with Arm and E350, at lower MOIs these reassortants demonstrated titratable levels of infection of the knockout stem cells, suggesting that α-DG remained the favored receptor.
FIG. 8.
Dependence on α-DG for cell entry maps to the S RNA segment of LCMV. α-DG−/− (white bars) and α-DG+/+ (black bars) ES cells were incubated with LCMV reassortants at MOIs of 5, 1, and 0.1 for 24 or 48 h. Expression of viral NP was detected by immunofluorescence staining with MAb 113. Duplicate samples were counted for each virus and cell line at each MOI and time point. Data are the mean percentages of infected cells ± standard deviations. These data are representative of two similar experiments.
DISCUSSION
Our results have established that LCMV strains and variants can be divided into two functional groups. The first group demonstrates a high affinity of binding to α-DG and dependence on this protein for cell entry. These viruses invariably cause a persistent infection, correlating with an ability to infect cells within the white pulp of the spleen and a subsequent loss of the virus-specific CTL response. We designated this group CTL− P+ WP+ α-DGhigh. The second group demonstrates low-level or no binding to α-DG and a reduced dependence on this protein for cell entry. These viruses replicate mainly within cells of the splenic red pulp, and infection is rapidly resolved. We designated this group CTL+ P− WP− α-DGlow. These data strongly suggest the involvement of α-DG in the in vivo tropism and pathogenesis of LCMV.
Infections of mice with Cl 13, Arm, Traub, E350, WE54, and WE2.2 progressed similarly during the first 3 days p.i., during which viral titers increased dramatically (Fig. 1A). However, beyond this time point, the courses of infection with different LCMV isolates diverged. In mice infected with Cl 13, Traub, or WE54, viral titers continued to rise over the first week p.i. Between day 3 and day 7 p.i., when peak titers were reached, Cl 13, Traub, and WE54 demonstrated 10-, 7-, and 18-fold increases in titer, respectively. Afterward, a degree of control was attained so that viral titers decreased. However, sterile immunity was not achieved, and considerable viral loads persisted at day 30 p.i. A very different series of events occurred following infection of mice with Arm or E350. Viral titers were rapidly reduced after day 3 p.i. In mice infected with Arm, viral titers were reduced approximately 5,000-fold between day 3 and day 5 p.i. and were below detectable levels by day 7 p.i. Similarly, but with a slight delay, E350 viral loads dropped approximately 47-fold between days 3 and 5 p.i. However, a 4,800-fold reduction from peak (day 3 p.i.) viral titers was seen by day 7 p.i., and E350 was cleared by day 14 p.i. WE2.2 titers were also reduced dramatically after day 3 of infection. Between day 3 and day 5 p.i., viral titers dropped fivefold, and between days 3 and 7 p.i. they decreased ninefold. Clearance of WE2.2 was slower than that of Arm and E350; however, virus was below detectable levels at day 30 p.i.
Clearance of acute LCMV infection is dependent on the virus-specific CD8+ CTL response (15, 39; reviewed in reference 5). Mice infected with LCMV Arm mount a strong, sustained LCMV-specific CTL response that peaks at 7 to 8 days p.i. Likewise, in mice infected with another P− LCMV strain, E350, strong LCMV-specific CTL responses were also detected on day 7 p.i. Mice infected with WE2.2 generated a good CTL response, better than that generated by mice infected with a P+ virus (Cl 13, Traub, or WE54). However, WE2.2-specific CTL activity was lower than that seen in mice infected with either of the other two P− viruses (Arm or E350). This relatively reduced level of CTL activity likely explains the delay in WE2.2 clearance (i.e., day 30 p.i.). However, the absence of virus at this time point indicates that the mice generated a CTL response capable of clearing the virus. Although a CTL response is initially mounted in mice infected i.v. with Cl 13, this response is ablated by day 7 p.i. When the CTL response to other P+ LCM viruses was measured, we observed that a loss of CTL activity by day 7 p.i. was common in mice infected with any P+ strain (Fig. 1B).
The ability of LCMV Cl 13 to establish a persistent infection and induce a state of generalized immune suppression after i.v. inoculation into adult mice has been associated with its ability to infect cells within the inner white pulp of the spleen by day 3 p.i. It has been reported that both interdigitating dendritic cells and follicular dendritic cells are infected by Cl 13 and that these cells are subsequently destroyed by virus-specific CTL-mediated lysis (4, 24; reviewed in reference 42). In contrast, LCMV Arm, which does not establish a persistent infection after i.v. inoculation into adult mice, has a strikingly different tropism within the spleen, replicating predominantly within the red pulp rather than white pulp areas by day 3 p.i. We investigated whether the correlation between virus tropism within the spleen and the ability to establish a persistent infection in adult mice extended to other LCMV strains. At day 1 p.i., all the viruses studied showed similar foci of infection within the MZ. However, by day 3 p.i., in adult BALB/c mice infected with Arm, E350, or WE2.2, infection shifted to cells within the red pulp, whereas Traub and WE54 shared with Cl 13 the ability to infect cells of the white pulp.
Although viral tropism within the spleen is believed to play a key role in the viral persistence and generalized immunosuppression seen following i.v. infection of adult mice with Cl 13, the molecular mechanisms underlying the divergence of persistent and nonpersistent strain distribution within the spleen remain unclear. Since the initial characteristics of infection appear to be shared by all the LCMV strains studied here, events occurring between day 1 and day 3 p.i. within the MZ must determine the disparate tropisms, immune responses, and disease kinetics that follow. The MZ contains a highly heterogeneous population of cell types (reviewed in reference 21). At day 1 p.i., virus may be present in different populations of MZ cells following uptake either by phagocytosis (e.g., by the highly phagocytic MZ macrophages or dendritic cells in this zone) or by receptor-mediated entry. Subsequent sites of viral replication in the spleen will be determined by the migration pattern of initially infected cell populations and/or spread of infection to new cells, the latter again being influenced by viral receptor binding properties. We therefore chose to study the interaction of the different viruses with the newly identified arenavirus receptor α-DG (9). All LCMV strains and reassortants tested here (with the exception of WE2.2) demonstrated binding to purified α-DG (Fig. 3 and 7). However, marked differences in binding affinity were noted. Those viruses able to infect cells of the white pulp and establish a persistent infection (Cl 13, Traub, WE54, Arm/Traub, and Arm/Cl 13) exhibited high affinities of binding to α-DG. In marked contrast, those viruses replicating mainly within the red pulp (Arm, E350, Traub/Arm, and Cl 13/Arm) exhibited binding affinities 2 to 3 logs lower. WE2.2, which is also red pulp tropic, did not bind to α-DG.
α-DG was found to be critically involved in cellular entry by all LCMV strains and reassortants able to persist in vivo. ES cells lacking α-DG were highly refractory to infection by these viruses (Fig. 5 and 8), and infection was blocked by 1 to 4 nM soluble α-DG (Fig. 4). These data confirm our earlier finding that α-DG is a major functional receptor for LCMV (3, 9). However, the observations that nonpersistent strains entered cells by an additional, α-DG-independent mechanism and that far higher concentrations (>100 nM) of soluble receptor (α-DG) were required to block infection highlight another important implication of this study, i.e., that additional cell surface receptors or cofactors exist and can be utilized by these viruses. Nevertheless, despite the existence of an alternative uptake pathway, α-DG remained the favored receptor for the nonimmunosuppressive strains. That is, although levels of infection of α-DG+/+ and α-DG−/− cells by Arm, E350, Cl 13/Arm, and Traub/Arm were comparable at an MOI of 5 at 48 h p.i., α-DG−/− cells remained less susceptible to infection by these viruses than α-DG+/+ cells at lower MOIs (of 1 and 0.1). A possible explanation is that an alternative receptor requires a threshold level of virus attachment and receptor clustering to be reached before the downstream events of viral uptake can commence. Binding of virus at this threshold level may alter the structure of the receptor, enabling it to interact with a cofactor(s) required for viral uptake. Alternatively, a threshold level of virus binding may be required to trigger cell signaling events that culminate in uptake of virus into the cell. At 48 h p.i., even at an MOI of 5, viruses exhibiting a CTL− P+ WP+ α-DGhigh phenotype (Cl 13, Traub, WE54, Arm/Cl 13, and Arm/Traub) exhibited less than 20% infection of α-DG−/− ES cells. The low level of infection of α-DG−/− cells at this time point may result when these high titers of virus achieve threshold-level binding on just a small percentage of cells. Further compelling evidence for the existence of an additional receptor(s) or coreceptor(s) for LCMV comes from the observation that binding of WE2.2 to α-DG from two sources (rabbit skeletal muscle and mouse ES cells) was undetectable in our assays. Furthermore, WE2.2 infected α-DG−/− and α-DG+/+ ES cells comparably at 24 and 48 h p.i. at all MOIs used, suggesting a completely α-DG-independent mechanism of viral uptake by this virus.
The observations that the two groups of viruses exhibit markedly different affinities of binding to α-DG and that α-DG-independent mechanisms of cellular entry exist for some LCMV strains in vitro suggest a mechanism to explain altered tropism in vivo. Differential initial infection by the two groups of viruses of cell types exhibiting inherently disparate functions and trafficking patterns may provide these viruses with access to different splenic microenvironments. Indeed, preliminary evidence suggests that altering dendritic cell migration within the spleen by blockade of the lymphotoxin-β pathway markedly alters Cl 13, but not Arm, tropism within this organ (S. C. Smelt, Y.-X. Fu, S. Kunz, and M. B. A. Oldstone, Abstr. Am. Assoc. Immunol. Clin. Immunol. Soc. Joint Annu. Meet., abstr. 91.15, 2000).
LCMV, like other RNA viruses, has a high mutation frequency of approximately 10−3 to 10−5 misincorporations per nucleotide site and round of copying (reviewed in reference 12). Viral variants are thus continually generated in vivo and in vitro. As is evident from studies with a number of different types of viruses, such as human immunodeficiency virus, polio virus, and herpes simplex virus, sequence variations can significantly alter the interaction between a virus and its receptor, which may lead to changes in tropism and in receptor or cofactor usage (2, 31, 40). Of the LCMV strains analyzed here, Arm and Cl 13 are highly homologous: Cl 13, a variant isolated from the spleen of a neonatally infected mouse, differs from the parental virus (Arm) by only 2 amino acids, amino acid 260 in GP-1 (encoded in the S RNA) and amino acid 1079 in the viral polymerase (encoded in the L RNA). Traub and E350 exhibit a lower degree of homology with Arm; however, both Cl 13 and Traub have a leucine at amino acid position 260, whereas a phenylalanine occupies this position in Arm and E350. Our earlier studies suggested that the mutation in GP-1 was responsible for the phenotypic differences seen following Cl 13 or Arm infection (4, 11, 34). Supporting these results are studies presented herein, utilizing Cl 13-Arm and Traub-Arm reassortants, that also mapped these phenotypic differences to the S RNA and implicated amino acid 260. Our data also suggest that the presence of a leucine at amino acid 260 confers a high affinity of binding to α-DG while, conversely, a phenylalanine at this position impedes binding to this receptor but enables Arm and E350 to interact with a second, as-yet-unidentified receptor, enabling α-DG-independent viral uptake. Moreover, the leucine at amino acid 260 in Cl 13 and Traub may impede binding to the alternative receptor.
We chose also to study a second pair of highly genetically related LCMV strains, WE54 and WE2.2. WE2.2 differs from WE54 by only one amino acid, encoded in the S RNA. This mutation results in a serine-to-phenylalanine change at amino acid 153 of GP-1. Despite their high degree of amino acid homology, WE54 and WE2.2 elicit distinct disease phenotypes in vivo. When injected intracerebrally into neonatal C3H/St mice, WE2.2, but not WE54, caused growth hormone deficiency syndrome, which mapped to the substitution at amino acid 153 of GP-1 (10, 26, 37). In the present study, these two viruses differed in their splenic tropism, binding to α-DG, and ability to infect α-DG−/− ES cells. These viruses both have a leucine at amino acid 260. WE54 shared its characteristics with the immunosuppressive viruses in accordance with the leucine at position 260. In contrast, WE2.2 did not bind to α-DG at a detectable level and did not depend on α-DG for cell entry. Therefore, the mutation at amino acid 153 (also within the glycoprotein) may directly enable WE2.2 to utilize another receptor while preventing WE2.2 from binding to α-DG. Alternatively, the F260L mutation may represent an allosteric mutation, affecting the overall structure and stability of the GP, resulting in altered receptor binding. Additionally, CTL responses were greatly reduced at day 7 p.i. in mice infected with reassortants containing the S RNA from WE54 compared to those containing the S RNA from Arm (H. A. Lewicki and M. B. A. Oldstone, unpublished observation).
In conclusion, our observations suggest a correlation between affinity of viral binding to α-DG, viral tropism, and disease outcome. These data also imply the existence of at least one other receptor for LCMV and provide a basis for understanding the cause of persistent as opposed to acute viral infection.
ACKNOWLEDGMENTS
This research was supported by U.S. Public Health Service grants AI09484 and AI45927 from the National Institutes of Health. S.K. is in receipt of an award from the Swiss National Science Foundation. K.P.C. is an investigator of the Howard Hughes Medical Institute.
We are grateful to Michael Henry for generous gifts of reagents and valuable discussion and to Phyllis Minick for critical reading of the manuscript.
Footnotes
Manuscript 13229-NP of the Scripps Research Institute.
REFERENCES
- 1.Ahmed R, Salmi A, Butler L D, Chiller J M, Oldstone M B A. Selection of genetic variants of LCMV in spleens of persistently infected mice. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 3.Borrow P, Oldstone M B A. Characterization of lymphocytic choriomeningitis virus-binding protein(s): a candidate cellular receptor for the virus. J Virol. 1992;66:7270–7281. doi: 10.1128/jvi.66.12.7270-7281.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow P, Evans C F, Oldstone M B A. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow P, Oldstone M B A. Lymphocytic choriomeningitis virus. In: Nathanson N, editor. Viral pathogenesis. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 593–627. [Google Scholar]
- 6.Buchmeier M J, Oldstone M B A. Virus-induced immune complex disease: identification of specific viral antigens and antibodies deposited in complexes during chronic lymphocytic choriomeningitis virus infection. J Immunol. 1978;120:1297–1304. [PubMed] [Google Scholar]
- 7.Buchmeier M J, Lewicki H A, Tomori O, Oldstone M B A. Monoclonal antibodies to lymphocytic choriomeningitis and Pichinde viruses: generation, characterization, and cross-reactivity with other arenaviruses. Virology. 1981;113:73–85. doi: 10.1016/0042-6822(81)90137-9. [DOI] [PubMed] [Google Scholar]
- 8.Burnet F M. A modification of Jerne's theory of antibody production using the concept of clonal selection. CA Cancer J Clin. 1976;26:119–121. doi: 10.3322/canjclin.26.2.119. [DOI] [PubMed] [Google Scholar]
- 9.Cao W, Henry M D, Borrow P, Yamada H, Elder J H, Ravkov E V, Nichol S T, Compans R W, Campbell K P, Oldstone M B A. Identification of α-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 10.de la Torre J C, Oldstone M B A. Selective disruption of growth hormone transcription machinery by viral infection. Proc Natl Acad Sci USA. 1992;89:9939–9943. doi: 10.1073/pnas.89.20.9939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dockter J, Evans C F, Tishon A, Oldstone M B A. Competitive selection in vivo by a cell for one variant over another: implications for RNA virus quasispecies in vivo. J Virol. 1996;70:1799–1803. doi: 10.1128/jvi.70.3.1799-1803.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domingo E, Menendez-Arias L, Holland J J. RNA virus fitness. Rev Med Virol. 1997;7:87–96. doi: 10.1002/(sici)1099-1654(199707)7:2<87::aid-rmv188>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Durbeej M, Henry M D, Ferletta M, Campbell K P, Ekblom P. Distribution of dystroglycan in normal adult mouse tissues. J Histochem Cytochem. 1998;46:449–457. doi: 10.1177/002215549804600404. [DOI] [PubMed] [Google Scholar]
- 14.Dutko F J, Oldstone M B. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J Gen Virol. 1983;64:1689–1698. doi: 10.1099/0022-1317-64-8-1689. [DOI] [PubMed] [Google Scholar]
- 15.Fung-Leung W P, Kundig T M, Zinkernagel R M, Mak T W. Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J Exp Med. 1991;174:1425–1429. doi: 10.1084/jem.174.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemler M E. Dystroglycan versatility. Cell. 1999;97:543–546. doi: 10.1016/s0092-8674(00)80764-3. [DOI] [PubMed] [Google Scholar]
- 17.Henry M D, Campbell K P. Dystroglycan: an extracellular matrix receptor linked to the cytoskeleton. Curr Opin Cell Biol. 1996;8:625–631. doi: 10.1016/s0955-0674(96)80103-7. [DOI] [PubMed] [Google Scholar]
- 18.Henry M D, Campbell K P. A role for dystroglycan in basement membrane assembly. Cell. 1998;95:859–870. doi: 10.1016/s0092-8674(00)81708-0. [DOI] [PubMed] [Google Scholar]
- 19.Henry M D, Campbell K P. Dystroglycan inside and out. Curr Opin Cell Biol. 1999;11:602–607. doi: 10.1016/s0955-0674(99)00024-1. [DOI] [PubMed] [Google Scholar]
- 20.Ibraghimov-Beskrovnaya O, Ervasti J M, Leveille C J, Slaughter C A, Sernett S W, Campbell K P. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- 21.Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- 22.Lee K J, Novella I S, Teng M N, Oldstone M B A, de La Torre J C. NP and L proteins of lymphocytic choriomeningitis virus (LCMV) are sufficient for efficient transcription and replication of LCMV genomic RNA analogs. J Virol. 2000;74:3470–3477. doi: 10.1128/jvi.74.8.3470-3477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matloubian M, Kolhekar S R, Somasundaram T, Ahmed R. Molecular determinants of macrophage tropism and viral persistence: importance of single amino acid changes in the polymerase and glycoprotein of lymphocytic choriomeningitis virus. J Virol. 1993;67:7340–7349. doi: 10.1128/jvi.67.12.7340-7349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odermatt B, Eppler M, Leist T P, Hengartner H, Zinkernagel R M. Virus-triggered acquired immunodeficiency by cytotoxic T cell-dependent destruction of antigen-presenting cells and lymph follicle structure. Proc Natl Acad Sci USA. 1991;88:8252–8256. doi: 10.1073/pnas.88.18.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oldstone M B, Tishon A, Chiller J M, Weigle W O, Dixon F J. Effect of chronic viral infection on the immune system. I. Comparison of the immune responsiveness of mice chronically infected with LCM virus with that of noninfected mice. J Immunol. 1973;110:1268–1278. [PubMed] [Google Scholar]
- 26.Oldstone M B, Ahmed R, Buchmeier M J, Blount P, Tishon A. Perturbation of differentiated functions during viral infection in vivo. I. Relationship of lymphocytic choriomeningitis virus and host strains to growth hormone deficiency. Virology. 1985;142:158–174. doi: 10.1016/0042-6822(85)90430-1. [DOI] [PubMed] [Google Scholar]
- 27.Oldstone M B. Virus-lymphoid cell interactions. Proc Natl Acad Sci USA. 1996;93:12756–12758. doi: 10.1073/pnas.93.23.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oldstone M B. How viruses escape from cytotoxic T lymphocytes: molecular parameters and players. Virology. 1997;234:179–185. doi: 10.1006/viro.1997.8674. [DOI] [PubMed] [Google Scholar]
- 29.Parekh B S, Buchmeier M J. Proteins of lymphocytic choriomeningitis virus: antigenic topography of the viral glycoproteins. Virology. 1986;153:168–178. doi: 10.1016/0042-6822(86)90020-6. [DOI] [PubMed] [Google Scholar]
- 30.Peters C J, Buchmeier M, Rollin P E, Ksiazek T G. Arenaviruses. In: Fields B N, Knipe D L, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1521–1551. [Google Scholar]
- 31.Racaniello V R. Early events in poliovirus infection: virus-receptor interactions. Proc Natl Acad Sci USA. 1996;93:11378–11381. doi: 10.1073/pnas.93.21.11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riviere Y, Ahmed R, Southern P, Oldstone M B. Perturbation of differentiated functions during viral infection in vivo. II. Viral reassortants map growth hormone defect to the S RNA of the lymphocytic choriomeningitis virus genome. Virology. 1985;142:175–182. doi: 10.1016/0042-6822(85)90431-3. [DOI] [PubMed] [Google Scholar]
- 33.Salvato M, Shimomaye E, Souther P, Oldstone M B. Virus-lymphocyte interactions. IV. Molecular characterization of LCMV Armstrong (CTL+) small genomic segment and that of its variant, clone 13 (CTL−) Virology. 1988;164:517–522. doi: 10.1016/0042-6822(88)90566-1. [DOI] [PubMed] [Google Scholar]
- 34.Salvato M, Borrow P, Shimomaye E, Oldstone M B A. Molecular basis of viral persistence: a single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with suppression of the antiviral cytotoxic T-lymphocyte response and establishment of persistence. J Virol. 1991;65:1863–1869. doi: 10.1128/jvi.65.4.1863-1869.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Southern P J, Bishop D H L. SEquence comparison among arenaviruses. Curr Top Microbiol Immunol. 1987;133:19–39. doi: 10.1007/978-3-642-71683-6_3. [DOI] [PubMed] [Google Scholar]
- 36.Southern P J. Arenaviridae: the viruses and their replication. In: Fields B N, Knipe D L, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1505–1519. [Google Scholar]
- 37.Teng M N, Borrow P, Oldstone M B A, de la Torre J C. A single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with the ability to cause growth hormone deficiency syndrome. J Virol. 1996;70:8438–8443. doi: 10.1128/jvi.70.12.8438-8443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tishon A, Borrow P, Evans C, Oldstone M B. Virus-induced immunosuppression. 1. Age at infection relates to a selective or generalized defect. Virology. 1993;195:397–405. doi: 10.1006/viro.1993.1389. [DOI] [PubMed] [Google Scholar]
- 39.Tishon A, Lewicki H, Rall G, Von Herrath M, Oldstone M B. An essential role for type 1 interferon-gamma in terminating persistent viral infection. Virology. 1995;212:244–250. doi: 10.1006/viro.1995.1477. [DOI] [PubMed] [Google Scholar]
- 40.Warner M S, Geraghty R J, Martinez W M, Montgomery R I, Whitbeck J C, Xu R, Eisenberg R J, Cohen G H, Spear P G. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 41.Zinkernagel R M, Doherty P C. Restriction of in vitro cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248:701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]
- 42.Zinkernagel R M, Planz O, Ehl S, Battegay M, Odermatt B, Klenerman P, Hengarter H. General and specific immunosuppression caused by antiviral T-cell responses. Immunol Rev. 1999;168:305–315. doi: 10.1111/j.1600-065x.1999.tb01300.x. [DOI] [PubMed] [Google Scholar]