Abstract
Simple Summary
Germline mutation rates and spectra across three cohorts of Chinese patients: a high-risk breast cancer cohort, an unselected breast cancer cohort, and a benign breast lesion cohort were compared. The high-risk cohort had the highest mutation rate at 11.9%, while the unselected cancer and benign lesion cohorts had rates of 6.5% and 8.1%, respectively. Notably, 29.3% of the unselected breast cancer patients met genetic testing criteria, and this subgroup had a mutation rate similar to the high-risk cohort. High-penetrance gene mutations were only found in the high-risk and unselected cancer cohorts. Unexpectedly, a 2% mutation rate was observed in the benign lesion cohort. These findings highlight the need for broader genetic testing of all breast cancer patients, not just those deemed high-risk, to identify more individuals harboring clinically relevant germline mutations.
Abstract
Mutation study for high-risk breast and ovarian cancer (HBOC) has been extensively studied in patients of different ethnicities. Here we compared the germline mutation rate and mutation spectrum of patients (n = 4341) with benign breast diseases or breast cancers, with and without other risk factors. Three cohorts of Chinese patients were recruited. The first cohort, high-risk cohort (HR, n = 3935) included high-risk breast cancer patients fulfilling high-risk HBOC criteria and who are recruited at our genetics clinic. The second cohort, unselected cancer cohort (CC, n = 307) was from general recruitment of patients with breast cancer at breast surgery clinics. The third cohort, benign breast lesion cohort (NC, n = 99) comprised 99 patients with benign breast diseases such as fibroadenoma, fibroadenomatoid hyperplasia, and intraductal papilloma. Thirty HBOC related genes were sequenced on the above-mentioned patient cohorts. The germline mutation rates of HR, CC, and NC cohort were 11.9%, 6.5%, and 8.1%, respectively. In the CC cohort, 29.3% (90/307) of patients fulfilled the National Comprehensive Cancer Network (NCCN) high-risk genetic test criteria 2022 v.2. The mutation rate for this group of patients was 11.1%, similar to that of the HR cohort, while the mutation rate for those not fulfilling testing criteria was 4.6%, like that of the NC cohort. High penetrance genes (BRCA1/2, CDH1, PALB2, PTEN, and TP53) mutations were only found in the HR (10.6%) and CC (3.3%) cohorts but were not found in the NC cohort. ATM, BRIP1, RAD51C, and RAD51D mutations were identified in all cohorts. RAD51C and RAD51D mutations showed conflicting penetrance. An unexpectedly high mutation rate of total 2% was found in the NC cohort but it was only 0.3% and 0.5% in the HR cohort and CC cohort, respectively. Our results show a clinical need to enhance genetic testing of unselected breast cancer patients to identify the high-risk patients.
Keywords: BRCA, hereditary breast cancers, Chinese, germline mutation
1. Introduction
PARP inhibitors, olaparib and talazoparib, have been approved for the treatment of metastatic breast cancer in germline BRCA1/2 mutation (gBRCAm) carriers [1,2]. The recent OlympiA trial showed improved progression-free survival (PFS) and distant disease-free survival with adjuvant olaparib for gBRCAm carriers with HER2-negative high-risk early-stage breast cancer [3]. The use of PARP inhibitor resulted in a significant improvement in PFS and health-related quality of life in gBRCAm carriers compared to non-platinum based single-agent chemotherapy [4,5]. Germline mutations in homologous recombination repair (HRR) genes were also listed as an inclusion criterion for the trials evaluating the effectiveness of talazoparib in HER2-negative breast cancer [6]. Advances in molecular genetics have identified pathogenic or likely pathogenic (P/LP) variants in several high to moderate-penetrance genes involved in regulating cell growth and/or DNA repair [7,8,9] that are associated with inherited susceptibility to breast, ovarian, prostate, colon, and pancreatic cancers (e.g., BRCA1/2, TP53, CDH1, PTEN, and PALB2), characterized by an early disease onset and exhibiting an autosomal dominant inheritance pattern [10,11,12]. With the technological development in next-generation sequencing (NGS), the availability of NGS has been extended to many developing countries and the cost of testing has been substantially reduced [12]. Multigene panel testing has become a common option to detect disease associated genetic mutation in hereditary breast cancer patients and their relatives. In 2014, King and her co-workers were the first group to advocate for population-based germline BRCA1/2 screening for all women [13], but they met with controversy. In 2019, the American Society of Breast Surgeons published a consensus statement on genetic testing for all patients with a personal history of breast cancer [14]. The American College of Medical Genetics and Genomics (ACMG) also suggested subsequently evaluating the need for germline genetic testing on all patients with breast cancer [15]. In 2020, a Mayo Clinic study proposed a fusion approach for genetic testing in breast cancer patients. The study recommended testing all women diagnosed before the age of 65 and following NCCN testing criteria for those diagnosed after 65 [16]. However, the NCCN guideline for germline genetic testing has favored a more restricted approach, recommending testing only for high-risk patients due to the low rate of positive detections [17]. It was not until 2021, in guideline 2021 v.1, that the criteria were loosened by NCCN to include more breast cancer patients for genetic screenings to aid in PARP inhibitor treatment decisions [17]. In the 2023 v.1 update, NCCN further changed their testing criteria, expanding them to include even more breast cancer patients for genetic screenings based on diagnosis age and family histories [18]. A more recent update by ASCO Society of Surgical Oncology also recommend testing all newly diagnosed patients with breast cancer ≤65 years (with stage I–III or de novo stage IV/metastatic disease), and the testing criteria only apply for patients >65 years based on personal cancer history, family history, and ancestry [19].
Here, we conducted a local examination of germline mutations in consecutive patients recruited from three different specialist centers (high-risk genetic clinic, breast surgery center, and general breast clinic) to compare mutation detection rates and spectra in Chinese populations. Our results show a clinical need to enhance genetic testing for all patients with breast cancer to identify high-risk mutation carriers.
2. Methods
2.1. Participants and Selection Criteria
Germline mutation screening was performed on 4341 Chinese individuals recruited through the Hong Kong Hereditary Breast Cancer Family Registry from March 2007 to August 2022. These individuals were from three cohorts of Chinese patients. The first cohort, the HBOC high-risk cohort (HR, n = 3935), comprised high-risk breast cancer patients who fulfilled the testing criteria described in our previous paper [20], including patients meeting any of the following criteria: (1) they had been diagnosed with breast cancer at any age and had at least one first- or second-degree relative with breast and/or ovarian cancer, regardless of age; (2) they had been diagnosed with breast cancer at or before 45 years of age; (3) they had bilateral breast cancer; (4) they had triple-negative breast cancer; or (5) they were male with breast cancer. The second cohort, the unselected cancer cohort (CC, n = 307), consisted of patients with breast cancer who were recruited from general breast surgery clinics, regardless of any selection criteria. The third cohort, the benign breast lesion cohort (NC, n = 99), included 99 patients with benign breast diseases or abnormalities such as fibroadenoma, fibroadenomatoid hyperplasia, and intraductal papilloma. All participants recruited gave written informed consent for the study. The research was conducted in accordance with the Declaration of Helsinki.
2.2. DNA Extraction and Sequencing
Genomic DNA extraction from the peripheral blood was performed by the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) or the QIAsymphony DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Genomic DNA were sequenced by Color Genomics with a 30 HBOC gene panel or in-house with 93-genes DHS-001Z human breast cancer panel by the Qiagen breast cancer panel (Qiagen, Hilden, Germany) on MiSeq/NextSeq/NovaSeq (Illumina, San Diego, CA, USA) instruments, and the sequencing results of 30 HBOC genes were analyzed. The minimum sequencing depth was 50× and median coverage was 200–300×. All detected pathogenic variants were further validated by conventional Sanger bi-directional DNA sequencing.
2.3. Variant Interpretation and Annotation
Variants calling bioinformatics was performed as previously described [20,21]. Paired sequencing reads were mapped to human reference genome sequence GRCh37/hg19. Variants with a minor allele frequency of at least 1% reported by the 1000 Genomes Project [22] were excluded from manual variant curation. Variants were described according to the recommendations of the Human Genome Variation Society (HGVS) nomenclature (http://www.HGVS.org/varnomen). The variant descriptions were further cross-checked with Mutalyzer Name Checker (http://mutalyzer.nl).
2.4. Statistical Analysis
Multiple comparisons were conducted among cohorts. One-way analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) were applied to compare continuous variables. Fisher’s exact test was used to study the independence of categorical variables between cohorts. The limit of significance for all analyses was defined as a p-value of <0.05. Data analyses were performed using the statistical software R (version 3.4.2) [23].
3. Results
3.1. Patient Characteristics of the Cohorts
We recruited 3935 HBOC high-risk (HR) patients (n = 3935) from our genetic clinic. These patients fulfilled the NCCN 2022.v2 testing criteria during their first referral, with a median age of diagnosis of 47.6 years, ranging from 19.3 to 95.8. Over 70% of the breast cancers from these patients were hormonal positive invasive ductal carcinoma. Of these patients, 40.8% had a family history of breast cancer, and 30.7% had a family history of BRCA-related cancers.
Our unselected breast cancer control cohort (CC, n = 307) consisted of patients randomly recruited from our breast surgery cancer center. Among these patients, 29.3% (90/307) fulfilled the NCCN 2022.v2 testing guidelines, while 70.7% (217/307) did not meet any high-risk criteria. The median age of diagnosis for those fulfilling the testing criteria was 55.3 years, ranging from 32.1 to 90.6, while those not fulfilling the criteria had an older median age of diagnosis at 59.8 years, ranging from 45.0 to 82.5. The histology of both groups was predominantly invasive ductal carcinoma, at 90% and 82.5%, respectively. The major differences between those who did or did not fulfill the NCCN 2022.v2 testing guideline were in TNBC subtype and family history of breast or ovarian cancers (Table 1). In the CC cohort who fulfilled the NCCN criteria, 22.5% of the patients had TNBC, 36.7% had a family history of breast cancer, and 5.6% had a family history of ovarian cancer.
Table 1.
Clinicopathological characteristics of patient cohorts.
| High-Risk (HR) Cancer Patient | Unselected Cancer Control (CC) Patient | Benign Disease (NC) Patient | p-Value | |||
|---|---|---|---|---|---|---|
| Fulfill NCCN Testing Criteria | Not Fulfill NCCN Testing Criteria | |||||
| n = 3935 | n = 90 | n = 217 | n = 99 | |||
| Age at recruitment (Median/Range) | ||||||
| Mean | 49.4 | 55.7 | 60.9 | 45.9 | - | |
| Median | 47.6 | 55.3 | 59.8 | 47.0 | - | |
| Range | 19.3–95.8 | 32.1–90.6 | 45.0–82.5 | 20.8–84.9 | - | |
| No. of patients identified P/LP (30 genes) | 463 ^ | 10 | 10 * | 8 | - | |
| Overall mutation % | 11.9% (469/3935) |
6.5% (20/307) | 8.1% (8/99) |
0.0174 | ||
| 11.1% | 4.6% | |||||
|
Mutation % in high penetrance genes (BRCA1/2, PALB2, CDH1, PTEN, TP53) |
10.4% (410/3935) |
6.7% (6/90) |
1.8% (4/217) |
0% | <0.0001 | |
| Mutation % in moderate and low penetrance genes | 1.5% (59/3935) |
4.4% (4/90) |
2.8% (6/217) |
8.1% (8/99) |
<0.0001 | |
| Personal breast disease | ||||||
| Breast cancer | 3935 | 90 | 217 | - | - | |
| Fibro-epithelial tumors | - | - | - | 55 (55.6%) | - | |
| Other benign tumors | 14 (14.1%) | |||||
| Fibrocystic changes | 11 (11.1%) | |||||
| Other non-neoplastic | 8 (8.1%) | |||||
| Inflammatory | 6 (6.1%) | |||||
| Undefined | 3 (3.0%) | |||||
| Congenital anomalies | 2 (2.0%) | |||||
| Histology | ||||||
| Ductal | 3312 (70.4%) | 81 (90%) | 179 (82.5%) | - | - | |
| In situ | 771 (16.4%) | 1 (1.1%) | 2 (0.9%) | - | ||
| Other | 446 (9.5%) | 8 (8. 9%) | 35 (16.1%) | - | ||
| Unclassified | 174 (3.7%) | 0 (0%) | 1 (0.5%) | - | ||
| Molecular subtypes | ||||||
| Luminal A (Her2−) | 2080 (52.9%) | 51 (57.3%) | 202 (94%) | - | - | |
| Luminal B (Her2+) | 476 (12.1%) | 0 (0%) | 9 (4.2%) | - | ||
| Luminal A/B (Her2 unknown/equivocal) | 221 (5.6%) | 0 (0%) | 1 (0.5%) | - | ||
| TNBC | 593 (15.1%) | 20 (22.5%) | - | - | ||
| Histology (invasive) grade | ||||||
| Low | 2078 (63.9%) | 49 (57.0%) | 151 (73.7%) | - | - | |
| High | 1174 (36.1%) | 37 (43.0%) | 54 (26.3%) | - | ||
| No information | 680 | 3 | 9 | - | ||
| Stage of breast | ||||||
| 0 | 824 (18.6%) | 0 (0%) | 0 (0%) | - | - | |
| 1 | 1643 (37.1%) | 40 (45.5%) | 83 (38.6%) | - | ||
| 2 | 1332 (30.0%) | 31 (35.2%) | 104 (48.4%) | - | ||
| 3 | 483 (10.9%) | 17 (19.3%) | 24 (11.2%) | - | ||
| 4 | 151 (3.4%) | 0 (0%) | 4 (1.9%) | - | ||
| No information | 270 | 2 | 2 | - | ||
| Family history (1st or 2nd degree) | ||||||
| Breast cancer | 1604 (40.8%) | 33 (36.7%) | 15 (6.9%) | 6 (6.1%) | <0.0001 | |
| Ovarian cancer | 195 (5.0%) | 5 (5.6%) | 0 (0.0%) | 1 (1.1%) | 0.0047 | |
| Other BRCA related cancer $ | 1208 (30.7%) | 13 (14.4%) | 29 (13.4%) | 19 (19.2%) | <0.0001 | |
^ 7 probands & * 1 proband carried double pathogenic mutations. $ Other BRCA related cancer: prostate cancer, pancreatic cancer, colorectal cancer, stomach cancer, melanoma, cholangiocarcinoma.
Of the 99 benign breast patients in the NC cohort, the median age of diagnosis of breast benign disease was 47.0 years, ranging from 20.8 to 84.9. Most of the breast diseases in these patients were fibro-epithelial tumors (55.6%), such as fibroadenoma, phyllodes tumor, and fibroadenomatoid change. Patients numbering 14.1% had intraductal papilloma, ductal adenoma, or fibromatosis, and were classified as other benign tumors. Patients numbering 11.1% had fibrocystic changes in their breasts, and 8.1% had other non-neoplastic benign breast problems. Only 6.1% and 1.1% of these NC patients had family history of breast cancer or ovarian cancer, respectively. Clinicopathological characteristics of the patient cohorts are listed in Table 1.
3.2. Germline Mutations between Cohorts
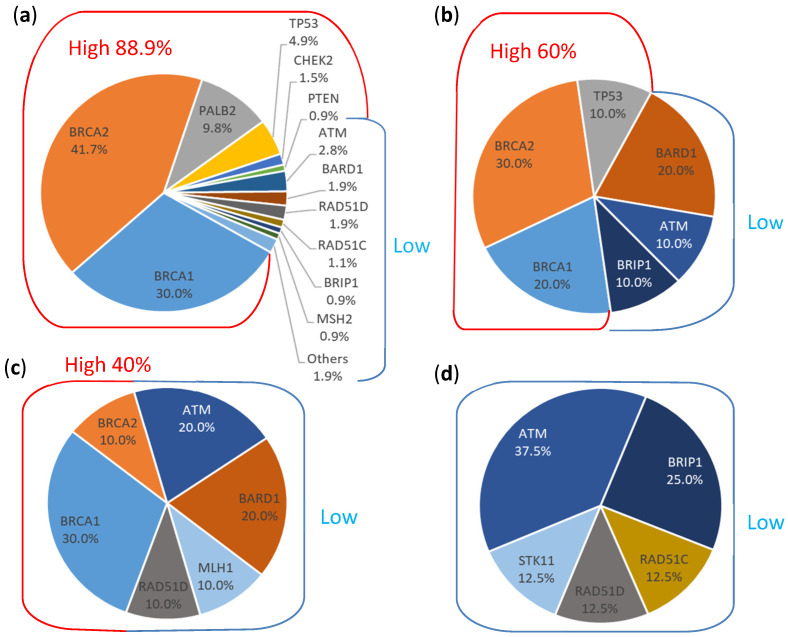
The germline mutation rates of having pathogenic or likely pathogenic mutation variant among 30 genes of the HR, CC, and NC cohort were 11.9%, 6.5%, and 8.1%, respectively. In the CC cohort, the mutation rate for patients who fulfilled the NCCN genetic test criteria was 11.1%, similar to that of the HR cohort, while the mutation rate for those who did not fulfill the testing criteria was 4.6%, like that of the NC cohort. High penetrance gene mutations (BRCA1/2, CDH1, PALB2, PTEN, and TP53) were found in 10.6% of HR patients and in 3.3% of the CC cohort (with mutation rates of 6.7% and 1.8% for those who did and did not fulfill the NCCN criteria, respectively), but none were found in the NC cohort (Table 2). The most frequently mutated gene was BRCA2 in the HR and CC (met criteria) cohorts, with the mutation rates of 5% and 3.3%, respectively. BRCA2 mutations were also identified in the CC non-high-risk cohort but the mutation rate was only 0.5%, and no BRCA2 mutation was found in the NC cohort. In other moderate and low penetrance genes, mutations were found in only 1.3% of HR patients and in 3.3% of the CC cohort (with mutation rates of 4.4% and 2.8% for those who did and did not fulfill the NCCN criteria, respectively), but in 8.1% of the NC cohort (Table 2). Mutations in ATM, BRIP1, RAD51C, and RAD51D were identified in all cohorts. RAD51C and RAD51D mutations showed conflicting penetrance. An unexpectedly high mutation rate of 2% was found in the NC cohort, but it was only 0.3% and 0.5% in the HR cohort and CC non-high-risk cohorts, respectively (see Table 2 and Figure 1). Detailed mutation spectra from each cohort are listed in Supplementary Table S1.
Table 2.
Mutation distributions among cohorts.
| High-Risk Breast Cancer Patient (HR) | Unselected Cancer Cohort (CC) | Patient with Benign Breast Disease (NC) | ||
|---|---|---|---|---|
| High-Risk Breast Cancer Patient (Met NCCN 2022 v2 Criteria) |
Non High-Risk Breast Cancer Patient (Not Met NCCN 2022 v2 Criteria) |
Not in NCCN 2022 v2 Criteria Based on FH |
||
| n | 3935 | 90 | 217 | 99 |
| Identified P/LP | 463 ^ | 10 | 10 * | 8 |
| Overall mutation (%) | 11.9% (469/3935) |
6.5% (20/307) | 8.1% | |
| 11.1% | 4.6% | |||
| High penetrance (%) | 10.6% (417/3935) |
3.3% (10/307) | 0% | |
| 6.7% (6/90) | 1.8% (4/217) | |||
| BRCA1 | 3.6% | 2.2% | 1.4% | 0% |
| BRCA2 | 5.0% | 3.3% | 0.5% | 0% |
| CDH1 | 0% | 0% | 0% | 0% |
| PALB2 | 1.2% | 0% | 0% | 0% |
| PTEN | 0.1% | 0% | 0% | 0% |
| TP53 | 0.6% | 1.1% | 0% | 0% |
| Moderate & low penetrance (%) | 1.3% (52/3935) | 3.3% (10/307) | 8.1% | |
| 4.4% (4/90) | 2.8% (6/217) | |||
| RAD51C | 0.1% | 0% | 0% | 1% |
| RAD51D | 0.2% | 0% | 0.5% | 1% |
^ 7 probands & * 1 proband carried double pathogenic mutations.
Figure 1.
Distributions of pathogenic or likely pathogenic mutations identified in different cohorts. (a) High-risk cohort (HR, n = 3935) comprised high-risk breast cancer patients fulfilling the NCCN genetic testing criteria. (b) Unselected breast cancer cohort (n = 90) who fulfilled the NCCN genetic testing criteria. (c) Unselected breast cancer cohort (n = 217) who did not fulfill the NCCN genetic testing criteria. (d) Benign breast lesion cohort (NC, n = 99) comprised of patients with benign breast diseases. Red line: high penetrance genes; blue line: moderate to low penetrance genes.
4. Discussion
NGS multiple-gene panel testing has improved the detection rates of mutations and provided a cost-effective cancer risk assessment. The identification of germline pathogenic or likely pathogenic mutations in cancer susceptibility genes has considerable significant implications for cancer prevention, early detection, treatment (such as with poly ADP ribose polymerase inhibitors, PARPi), and management, both for patients and for their relatives. Despite the benefits of molecular screening, many breast cancer patients never undergo testing due to the current adopted testing criteria, such as England NICE test criteria, which predominantly rely on family history and pathology of the tumors. In a study of 35,000 patients from multiple ancestries with unselected breast cancer, a mutation rate of 9.3% was found using a 25-gene panel, and the positive rate ranged from 7.2% to 11.5% based on ancestry [24]. In another study of a consecutive series of 10,000 cancer patients and unaffected individuals undergoing 29-genes NGS testing, with 82% of patients being Caucasian, 9.0% of patients were found to carry at least one pathogenic or likely pathogenic variant, with 51.2% of these mutations being in high penetrance genes [25]. In another multicenter study involving 2984 patients with unselected personal cancer history and family history who underwent an 80-genes panel test, P/LP variant was found in 13.3% of patients, with 5% of the mutations being identified from highly penetrant genes, and more than half of the identified variants from genes with moderate or low penetrance [26]. In Asian populations, studies were done on unselected breast cancer germline mutation spectra, the mutation rates ranged from 1.5% to 2.7% for BRCA1 and 2.4% to 3.8% for BRCA2 [27]. For Asian HBOC patients, mutation rates ranged from 1.3% to 14.6% for BRCA1 and 3.2% to 10.8% for BRCA2 [27]. In another study of 13,129 cancer-free Chinese individuals, the BRCA1 and BRCA2 mutation rates were 0.2% and 0.4%, respectively [28]. At least 10% of unselected breast cancer patients in China carry pathogenic variants in cancer susceptibility genes [29]. These findings demonstrate that at least 1.5% to 3.8% of BRCA1/2 mutations were found in breast cancer patients without criteria selection. In our study, we clearly showed a 1.8% mutation rate of high penetrance genes, with 1.4% from BRCA1 and 0.5% from BRCA2 (Table 2), concealed in non-high-risk breast cancer patients, who needed alternations in management during their ongoing healthcare.
Among the mutation spectra in moderate and low penetrance genes, ATM, BRIP1, RAD51C, and RAD51D mutations were identified in all cohorts. A systemic review showed that the prevalence of the deletion, insertion, substitution mutation variants in ATM are associated with breast cancer [30]. In United States, a high prevalence of 6.6% was found in ATM [31], and many ATM mutations have been described and associated with a moderate risk of developing breast cancer [32,33,34,35]. In Asian populations, the pathogenic mutation frequency of ATM was around 1% for HBOC patients and similar frequencies were seen in unselected breast cancer patients. However, the mutation percentage increased to 3% for those with benign breast disease (Table 3).
Table 3.
Mutation frequencies in different cohorts (HBOC, unselected breast cancer patient, benign breast disease patient, and normal control) from Asian countries on commonly tested HBOC related genes besides BRCA1/2.
| HBOC | Unselected Breast Cancer | Benign Breast | Normal Control | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Japan [41] |
Taiwan [42] |
China [43] |
Singapore [44] |
Korea [45] |
Hong Kong | China [28] |
Hong Kong | Hong Kong | China [28] | |
| Panel | 30 Genes | 20 Genes | 22 Genes | Mixed | 35 Genes | Mixed | 15 Genes | 30 Genes | 30 Genes | 15 Genes |
| N = | 568 | 480 | 481 | 460 | 120 | 3935 | 8067 | 307 | 101 | 13,129 |
| % | % | % | % | % | % | % | % | % | % | |
| BRCA1 | Neg | 1.25 | 14.6 | 14.1 | Neg | 3.58 | 1.81 | 1.63 | 0 | 0.19 |
| BRCA2 | Neg | 7.08 | 5 | 9.3 | Neg | 4.98 | 3.52 | 1.3 | 0 | 0.35 |
| PALB2 | 1.23 | 1.88 | 1.7 | 0.4 | 2.5 | 1.2 | 0.71 | 0 | 0 | 0.14 |
| TP53 | 0 | 0.21 | 0.6 | 1.3 | 1.7 | 0.6 | 0.38 | 0.33 | 0 | 0.02 |
| PTEN | 0 | 0 | 0.4 | 0 | 0 | 0.1 | 0.06 | 0 | 0 | 0 |
| CDH1 | 0 | 0 | 0 | 0.22 | 0 | 0 | 0.01 | 0 | 0 | 0 |
| ATM | 0.88 | 0.63 | 1 | 0.4 | 0 | 0.3 | 0.38 | 0.98 | 3 | 0.18 |
| BARD1 | 0.88 | 0.21 | 0 | 0 | 1.7 | 0.2 | 0.19 | 1.3 | 0 | 0.06 |
| RAD51D | 0.7 | 1.25 | 0.4 | 0.22 | 0 | 0.2 | 0.38 | 0.33 | 1 | 0.18 |
| BRIP1 | 0.53 | 0.21 | 0.8 | 0.4 | 1.7 | 0.1 | 0.14 | 0.33 | 2 | 0.22 |
| RAD51C | 0.53 | 0.42 | 0 | 0.22 | 0 | 0.1 | 0.02 | 0 | 1 | 0.17 |
| CHEK2 | 0.18 | 0 | 0 | 0.22 | 0 | 0.2 | 0.32 | 0 | 0 | 0.13 |
| NBN | 0 | 0 | 0.4 | 0.22 | 0 | 0 | 0.07 | 0 | 0 | 0.04 |
| STK11 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 1 | 0.01 |
| RAD50 | 0.18 | 0.21 | 0.4 | 0.22 | 0 | NT | 0.26 | NT | NT | 0.24 |
| PMS2 | 0 | 0.21 | 0 | 0 | 0 | 0.03 | NT | 0 | 0 | NT |
| MSH2 | 0 | 0 | 0.4 | 0.22 | 0 | 0.1 | NT | 0 | 0 | NT |
| MLH1 | 0 | 0 | 0 | 0.7 | 0 | 0 | NT | 0.33 | 0 | NT |
| MSH6 | 0 | 0 | 0 | 0 | 0 | 0.03 | NT | 0 | 0 | NT |
| MUTYH | NT | NT | 0.6 | 1.5 | 0 | 0.13 | NT | 0 | 0 | NT |
| BLM | 0.7 | NT | NT | NT | 0 | NT | NT | NT | NT | NT |
NT: Not tested.
Germline mutations were also commonly found in patients with breast fibroadenomas. An ATM c.8246A > T; p. (Lys2749Ile) germline mutation carrier was identified in one out of 12 fibroadenoma patients in the Chinese population [36]. Most studies from Sweden, Finland, and Denmark reported no association between common variants in the ATM gene and breast cancer susceptibility [30]. This observation was also seen in our Chinese population.
RAD51C and RAD51D mutations showed conflicting penetrance. An unexpectedly high mutation rate of 2.0% was found in the NC cohort, but it was only 0.36% and 0.33% in the HR and CC cohorts, respectively. Most of our RAD51D mutation carriers carried c.270_271dupTA; p. (Lys91Ilefs*13), which is a well-known mutation, especially in the Asian population [37,38]. In the Genome Aggregation Database (gnomAD), this variant was observed in 14/18, 394 (0.076%) individuals in the East Asian population but not in other populations. Management of RAD51C and RAD51D mutation carriers has been controversial. In ACMG published guidance for reporting secondary findings in the context of clinical exome and genome sequencing, RAD51C and RAD51D mutations were not listed in SF v3.1 and were no longer being reported as secondary findings, with comments on moderate risk of primarily later-onset breast cancer and low penetrance for ovarian cancer [39]. In the NCCN management guidelines 2023 v.3 [17], the absolute risk of breast cancer for RAD51C and RAD51D mutation carriers was revised form 15–40% to 20–40%, and management was also revised to include annual mammograms and consideration of breast MRI with contrast starting at age 40. For ovarian cancer, management on RAD51C and RAD51D mutation carriers was also revised, with patients now being recommended for risk-reducing salpingo-oophorectomy (RRSO) at age 45–50, rather than just being considered for it. The incomplete penetrance of RAD51D pathogenic variants has been demonstrated from a clinical, molecular pathology, and in vitro perspective (manuscript under review). Given the incomplete penetrance and high mutation rate observed in the NC cohort, a population screening approach is particularly important.
This study had some limitations. Multiple comparisons were made using one-way ANOVA with LSD tests between age distributions in the four cohorts, which showed a significant difference (p < 0.001). The HR cohort was found to be relatively younger than the CC (fulfilled NCCN) cohort, resulting in a higher mutation frequency in high penetrance genes, even though they had the same grouping criteria. Additionally, the family history of BRCA-related cancers in these two cohorts also showed a p-value of 0.0007. In the HR cohort, 30.7% of patients had family members with BRCA-related cancers other than breast and ovarian cancer (such as prostate cancer, pancreatic cancer, colorectal cancer, stomach cancer, melanoma, and cholangiocarcinoma), while only 14.4% of CC (fulfilled NCCN) patients had such family histories. This may suggest that these BRCA-related cancers are often missed by clinicians, and patients are not referred to high-risk genetic clinics for genetic testing. This reveals a problem in the referral system, as patients in the CC (fulfilled NCCN) cohort should have been referred for genetic testing. In one of our previous studies, we interviewed medical specialists in breast and ovarian cancer in Hong Kong and found that 8.5% of them may hesitate to refer patients for genetic test, and 9.9% of them do not have the related information and resources to refer patients to such testing [40].
Given that mutations from high penetrance genes were identified not only in the high-risk cohort but also in unselected breast cancer control, genetics screening on unselected breast cancer approach is particularly important, especially on identifying patients who are suitable for use of PARPi [4]. However, financial support is another concern of limitation for genetic tests; many counties often require a patient to meet stringent genetic testing criteria before reimbursement while the test is not supported by governments in many well-developed Asian countries including Hong Kong, Singapore, Malaysia, and Taiwan, while Korea and Japan only support patients suspected for HBOC. To balance between the limitations between strategic and universal screening, the Mayo Clinic suggested a hybrid approach of testing all women diagnosed with breast cancer before the age of 65 years and using NCCN criteria for older patients [16]. In 19 of our identified pathogenic mutation carriers from non-high-risk breast cancer patients, 10 of them carry mutation from high penetrance genes, 3 out of 10 were aged over 65, and among them two of them are BCRA1 mutations carriers. From this finding, the hybrid approach of testing is not applicable in the Chinese population. Thus, we propose performing universal germline mutation screening on all patients with breast cancer as a favorable approach to implement in the Chinese population.
5. Conclusions
In summary, our study across these three clinical settings revealed the necessity to broaden genetic testing protocols for breast cancer patients in the Chinese population. Implementing more comprehensive testing strategies could lead to better detection of high-risk germline mutations and improve clinical management for these individuals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16173066/s1, Table S1: Mutation Spectrum from different cohort.
Author Contributions
The study was designed by A.K., C.-H.A. and E.S.K.M., A.K. designed and coordinated prospective data collection for the Hong Kong Hereditary Breast Cancer Family Registry. H.C.M.L. and A.W.S.L. conducted the bioinformatics analysis. C.Y.S.H. retrieved and collected data for this study, interpreted the results, and drafted the manuscript. A.K., C.-H.A. and E.S.K.M. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was performed in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority West Cluster and respective authorities of other contributing hospitals in Hong Kong. The reference numbers and dates of approval are listed: UW 06-274 T/1299 (HKW Cluster), 7/8/2006; HKEC-2006-156 (HKE Cluster), 5/12/2006; KW/EX/06-088 (KWC Cluster), 21/12/2006; KC/KE 06-0135/ER-1 (KWC/E Cluster), 1/11/2006; CRE-2007.423 (NTE Cluster), 4/12/2007; NTWC/CREC/473/06 (NTW Cluster), 20/10/2006; RC-2006-01 (HKSH), 15/8/2006; SPH16/REC0001 (SPH), 21/9/2016.
Informed Consent Statement
Written informed consent was obtained from all participants recruited in this study.
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article and its Supplementary Files.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the Dr. Ellen Li Charitable Foundation; the Kerry Kuok Foundation; the Health and Medical Research Fund (03143406); the Asian Fund for Cancer Research and the Hong Kong Hereditary Breast Cancer Family Registry.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Robson M., Goessl C., Domchek S. Olaparib for Metastatic Germline BRCA-Mutated Breast Cancer. N. Engl. J. Med. 2017;377:1792–1793. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 2.Litton J.K., Rugo H.S., Ettl J., Hurvitz S.A., Gonçalves A., Lee K.H., Fehrenbacher L., Yerushalmi R., Mina L.A., Martin M., et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tutt A.N.J., Garber J.E., Kaufman B., Viale G., Fumagalli D., Rastogi P., Gelber R.D., de Azambuja E., Fielding A., Balmaña J., et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021;384:2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geyer C.E., Jr., Garber J.E., Gelber R.D., Yothers G., Taboada M., Ross L., Rastogi P., Cui K., Arahmani A., Aktan G., et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann. Oncol. 2022;33:1250–1268. doi: 10.1016/j.annonc.2022.09.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robson M.E., Tung N., Conte P., Im S.A., Senkus E., Xu B., Masuda N., Delaloge S., Li W., Armstrong A., et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann. Oncol. 2019;30:558–566. doi: 10.1093/annonc/mdz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruber J.J., Afghahi A., Timms K., DeWees A., Gross W., Aushev V.N., Wu H.T., Balcioglu M., Sethi H., Scott D., et al. A phase II study of talazoparib monotherapy in patients with wild-type BRCA1 and BRCA2 with a mutation in other homologous recombination genes. Nat. Cancer. 2022;3:1181–1191. doi: 10.1038/s43018-022-00439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fearon E.R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-I. [DOI] [PubMed] [Google Scholar]
- 8.Vogelstein B., Kinzler K.W. The multistep nature of cancer. Trends Genet. 1993;9:138–141. doi: 10.1016/0168-9525(93)90209-Z. [DOI] [PubMed] [Google Scholar]
- 9.Espinel W., Champine M., Hampel H., Jeter J., Sweet K., Pilarski R., Pearlman R., Shane K., Brock P., Westman J.A., et al. Clinical Impact of Pathogenic Variants in DNA Damage Repair Genes beyond BRCA1 and BRCA2 in Breast and Ovarian Cancer Patients. Cancers. 2022;14:2426. doi: 10.3390/cancers14102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graffeo R., Rana H.Q., Conforti F., Bonanni B., Cardoso M.J., Paluch-Shimon S., Pagani O., Goldhirsch A., Partridge A.H., Lambertini M., et al. Moderate penetrance genes complicate genetic testing for breast cancer diagnosis: ATM, CHEK2, BARD1 and RAD51D. Breast. 2022;65:32–40. doi: 10.1016/j.breast.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung K., Lee S., Na H.Y., Kim J.W., Lee J.C., Hwang J.H., Kim J.W., Kim J. NGS-based targeted gene mutational profiles in Korean patients with pancreatic cancer. Sci. Rep. 2022;12:20937. doi: 10.1038/s41598-022-24732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwong A., Shin V.Y., Chen J., Cheuk I.W.Y., Ho C.Y.S., Au C.H., Chan K.K.L., Ngan H.Y.S., Chan T.L., Ford J.M., et al. Germline Mutation in 1338 BRCA-Negative Chinese Hereditary Breast and/or Ovarian Cancer Patients: Clinical Testing with a Multigene Test Panel. J. Mol. Diagn. 2020;22:544–554. doi: 10.1016/j.jmoldx.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 13.King M.C., Levy-Lahad E., Lahad A. Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA. 2014;312:1091–1092. doi: 10.1001/jama.2014.12483. [DOI] [PubMed] [Google Scholar]
- 14.Manahan E.R., Kuerer H.M., Sebastian M., Hughes K.S., Boughey J.C., Euhus D.M., Boolbol S.K., Taylor W.A. Consensus Guidelines on Genetic Testing for Hereditary Breast Cancer from the American Society of Breast Surgeons. Ann. Surg. Oncol. 2019;26:3025–3031. doi: 10.1245/s10434-019-07549-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pal T., Agnese D., Daly M., La Spada A., Litton J., Wick M., Klugman S., Esplin E.D., Jarvik G.P., Professional Practice and Guidelines Committee Points to consider: Is there evidence to support BRCA1/2 and other inherited breast cancer genetic testing for all breast cancer patients? A statement of the American College of Medical Genetics and Genomics (ACMG) Genet. Med. 2020;22:681–685. doi: 10.1038/s41436-019-0712-x. [DOI] [PubMed] [Google Scholar]
- 16.Desai N.V., Yadav S., Batalini F., Couch F.J., Tung N.M. Germline genetic testing in breast cancer: Rationale for the testing of all women diagnosed by the age of 60 years and for risk-based testing of those older than 60 years. Cancer. 2021;127:828–833. doi: 10.1002/cncr.33305. [DOI] [PubMed] [Google Scholar]
- 17.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Genetic/Familial High-risk Assessment: Breast and Ovarian. Version 2.2021, 1.2022, 2.2022, 1.2023 and 3.2023. [(accessed on 17 April 2023)]. Available online: https://www.nccn.org/home.
- 18.Kwong A., Ho C.Y.S., Luk W.-P., Fung L.-H., Au C.-H., Ma E.S.K. Effect on Germline Mutation Rate in a High-Risk Chinese Breast Cancer Cohort after Compliance with The National Comprehensive Cancer Network (NCCN) 2023 v.1 Testing Criteria. Cancers. 2023;15:2635. doi: 10.3390/cancers15092635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedrosian I., Somerfield M.R., Achatz M.I., Boughey J.C., Curigliano G., Friedman S., Kohlmann W.K., Kurian A.W., Laronga C., Lynce F., et al. Germline Testing in Patients With Breast Cancer: ASCO-Society of Surgical Oncology Guideline. J. Clin. Oncol. 2024;42:584–604. doi: 10.1200/JCO.23.02225. [DOI] [PubMed] [Google Scholar]
- 20.Kwong A., Shin V.Y., Au C.H., Law F.B., Ho D.N., Ip B.K., Wong A.T., Lau S.S., To R.M., Choy G., et al. Detection of Germline Mutation in Hereditary Breast and/or Ovarian Cancers by Next-Generation Sequencing on a Four-Gene Panel. J. Mol. Diagn. 2016;18:580–594. doi: 10.1016/j.jmoldx.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Neben C.L., Zimmer A.D., Stedden W., van den Akker J., O’Connor R., Chan R.C., Chen E., Tan Z., Leon A., Ji J., et al. Multi-Gene Panel Testing of 23,179 Individuals for Hereditary Cancer Risk Identifies Pathogenic Variant Carriers Missed by Current Genetic Testing Guidelines. J. Mol. Diagn. 2019;21:646–657. doi: 10.1016/j.jmoldx.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 22.1000 Genomes Project Consortium. Auton A., Brooks L.D., Durbin R.M., Garrison E.P., Kang H.M., Korbel J.O., Marchini J.L., McCarthy S., McVean G.A., et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. [(accessed on 2 February 2024)]. Available online: https://www.R-project.org/
- 24.Buys S.S., Sandbach J.F., Gammon A., Patel G., Kidd J., Brown K.L., Sharma L., Saam J., Lancaster J., Daly M.B. A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer. 2017;123:1721–1730. doi: 10.1002/cncr.30498. [DOI] [PubMed] [Google Scholar]
- 25.Susswein L.R., Marshall M.L., Nusbaum R., Vogel Postula K.J., Weissman S.M., Yackowski L., Vaccari E.M., Bissonnette J., Booker J.K., Cremona M.L., et al. Pathogenic and likely pathogenic variant prevalence among the first 10,000 patients referred for next-generation cancer panel testing. Genet. Med. 2016;18:823–832. doi: 10.1038/gim.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samadder N.J., Riegert-Johnson D., Boardman L., Rhodes D., Wick M., Okuno S., Kunze K.L., Golafshar M., Uson P.L.S., Jr., Mountjoy L., et al. Comparison of Universal Genetic Testing vs Guideline-Directed Targeted Testing for Patients With Hereditary Cancer Syndrome. JAMA Oncol. 2021;7:230–237. doi: 10.1001/jamaoncol.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lei H., Zhang M., Zhang L., Hemminki K., Wang X.J., Chen T. Overview on population screening for carriers with germline BRCA mutation in China. Front. Oncol. 2022;12:1002360. doi: 10.3389/fonc.2022.1002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu F., Zhang D., Hu L., Sundaram S., Ying D., Zhang Y., Fu S., Zhang J., Yao L., Xu Y., et al. Association between 15 known or potential breast cancer susceptibility genes and breast cancer risks in Chinese women. Cancer Biol. Med. 2021;19:253–262. doi: 10.20892/j.issn.2095-3941.2021.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun J., Meng H., Yao L., Lv M., Bai J., Zhang J., Wang L., Ouyang T., Li J., Wang T., et al. Germline Mutations in Cancer Susceptibility Genes in a Large Series of Unselected Breast Cancer Patients. Clin. Cancer Res. 2017;23:6113–6119. doi: 10.1158/1078-0432.CCR-16-3227. [DOI] [PubMed] [Google Scholar]
- 30.Moslemi M., Vafaei M., Khani P., Soheili M., Nedaeinia R., Manian M., Moradi Y., Sohrabi E. The prevalence of ataxia telangiectasia mutated (ATM) variants in patients with breast cancer patients: A systematic review and meta-analysis. Cancer Cell Int. 2021;21:474. doi: 10.1186/s12935-021-02172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savitsky K., Bar-Shira A., Gilad S., Rotman G., Ziv Y., Vanagaite L., Tagle D.A., Smith S., Uziel T., Sfez S., et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 32.Matsuoka S., Ballif B.A., Smogorzewska A., McDonald E.R., 3rd, Hurov K.E., Luo J., Bakalarski C.E., Zhao Z., Solimini N., Lerenthal Y., et al. ATM and ATR Substrate Analysis Reveals Extensive Protein Networks Responsive to DNA Damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 33.Thompson D., Duedal S., Kirner J., McGuffog L., Last J., Reiman A., Byrd P., Taylor M., Easton D.F. Cancer risks and mortality in heterozygous ATM mutation carriers. J. Natl. Cancer Inst. 2005;97:813–822. doi: 10.1093/jnci/dji141. [DOI] [PubMed] [Google Scholar]
- 34.Goldgar D.E., Healey S., Dowty J.G., Da Silva L., Chen X., Spurdle A.B., Terry M.B., Daly M.J., Buys S.M., Southey M.C., et al. Rare variants in the ATM gene and risk of breast cancer. Breast Cancer Res. 2011;13:R73. doi: 10.1186/bcr2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angèle S., Hall J. The ATM gene and breast cancer: Is it really a risk factor? Mutat. Res. 2000;462:167–178. doi: 10.1016/S1383-5742(00)00034-X. [DOI] [PubMed] [Google Scholar]
- 36.Xie S.N., Cai Y.J., Ma B., Xu Y., Qian P., Zhou J.D., Zhao F.G., Chen J. The genomic mutation spectrums of breast fibroadenomas in Chinese population by whole exome sequencing analysis. Cancer Med. 2019;8:2372–2379. doi: 10.1002/cam4.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma D., Chen S.Y., Ren J.X., Pei Y.C., Jiang C.W., Zhao S., Xiao Y., Xu X.E., Liu G.Y., Hu X., et al. Molecular Features and Functional Implications of Germline Variants in Triple-Negative Breast Cancer. J. Natl. Cancer Inst. 2021;113:884–892. doi: 10.1093/jnci/djaa175. [DOI] [PubMed] [Google Scholar]
- 38.Yao H., Li N., Yuan H. Clinical characteristics and survival analysis of Chinese ovarian cancer patients with RAD51D germline mutations. BMC Cancer. 2022;22:1337. doi: 10.1186/s12885-022-10456-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller D.T., Lee K., Abul-Husn N.S., Amendola L.M., Brothers K., Chung W.K., Gollob M.H., Gordon A.S., Harrison S.M., Hershberger R.E., et al. ACMG SF v3.1 list for reporting of secondary findings in clinical exome and genome sequencing: A policy statement of the American College of Medical Genetics and Genomics (ACMG) Genet. Med. 2022;24:1407–1414. doi: 10.1016/j.gim.2022.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Kwong A., Cheng K.D., Hsue C.V., Hui S.K., Leung C.R., Leung K.A., Ngan K.R., Soong S.I. BRCA mutation testing for ovarian cancer in the context of available targeted therapy: Survey and consensus of Hong Kong specialists. Asia Pac. J. Clin. Oncol. 2019;15((Suppl. 2)):20–31. doi: 10.1111/ajco.13116. [DOI] [PubMed] [Google Scholar]
- 41.Kaneyasu T., Mori S., Yamauchi H., Ohsumi S., Ohno S., Aoki D., Baba S., Kawano J., Miki Y., Matsumoto N., et al. Prevalence of disease-causing genes in Japanese patients with BRCA1/2-wildtype hereditary breast and ovarian cancer syndrome. npj Breast Cancer. 2020;6:25. doi: 10.1038/s41523-020-0163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y.A., Jian J.W., Hung C.F., Peng H.P., Yang C.F., Cheng H.S., Yang A.S. Germline breast cancer susceptibility gene mutations and breast cancer outcomes. BMC Cancer. 2018;18:315. doi: 10.1186/s12885-018-4229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J., Li W., Shi Y., Huang Y., Sun T., Tang L., Lu Q., Lei Q., Liao N., Jin F., et al. Germline mutation landscape of Chinese patients with familial breast/ovarian cancer in a panel of 22 susceptibility genes. Cancer Med. 2019;8:2074–2084. doi: 10.1002/cam4.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ow S.G.W., Ong P.Y., Lee S.C. Discoveries beyond BRCA1/2: Multigene testing in an Asian multi-ethnic cohort suspected of hereditary breast cancer syndrome in the real world. PLoS ONE. 2019;14:e0213746. doi: 10.1371/journal.pone.0213746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park J.S., Lee S.T., Nam E.J., Han J.W., Lee J.Y., Kim J., Kim T.I., Park H.S. Variants of cancer susceptibility genes in Korean BRCA1/2 mutation-negative patients with high risk for hereditary breast cancer. BMC Cancer. 2018;18:83. doi: 10.1186/s12885-017-3940-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article and its Supplementary Files.