Abstract
Simple Summary
African swine fever (ASF) presents a significant challenge in Asia, where outbreaks have devastated the pork industry and threatened food security due to high mortality rates in pigs. The disease, caused by the ASF virus genotype II, has spread rapidly across the region since its emergence in China in 2018. This paper discusses the introduction and implications of vaccines such as NAVET-ASFVAC and AVAC ASF Live in Vietnam, emphasizing the necessity for rigorous testing and regulatory oversight. Despite the potential use of vaccines to control the disease, concerns about the safety of live attenuated vaccines (LAVs), including their ability to revert to virulence or create new recombinant strains, highlight the complexity of ASF management. Effective vaccine strategies, alongside strict biosecurity measures, and rapid diagnostics are essential to mitigate the economic and social impacts of ASF and ensure the stability of pig populations in Asia.
Abstract
This paper explores the significance of quality vaccines in managing ASF in Asia, where it poses a substantial threat to the pork industry. It emphasizes the risks associated with substandard vaccines, including the emergence of new virus strains that complicate disease control. Highlighting recent advancements in vaccine deployment in Vietnam, the paper calls for rigorous testing and regulations to guarantee vaccine effectiveness and safety. The authors advocate for the implementation of vaccines with the inclusion of differentiating infected from vaccinated animals (DIVA), which enhances disease management strategies in both endemic and non-endemic regions. The conclusion underscores the necessity of stringent standards in vaccine development and strict adherence to regulatory guidelines to ensure successful ASF management and maintain public trust in the vaccines.
Keywords: vaccine safety, DIVA vaccination, ASF control in endemic countries
1. Introduction to ASF and Current Disease Situation in Asia
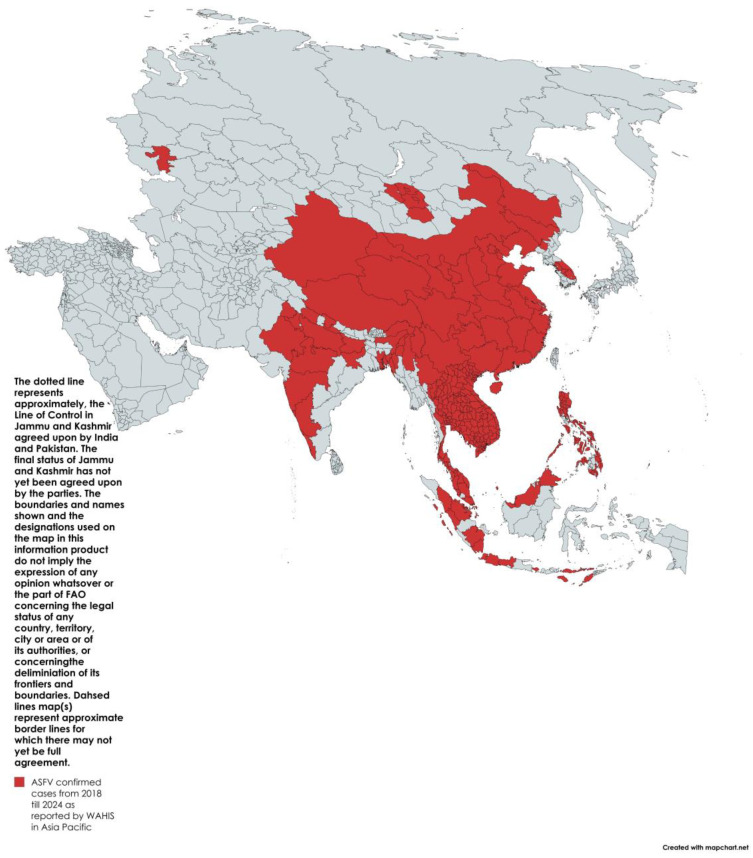
African swine fever virus (ASFV) genotype II, following its emergence and rapid spread in China in 2018, poses a critical threat due to its high fatality rate in infected pigs (domestic and wild). Since then, outbreaks in 19 countries within this region have been reported to the World Animal Health Information System (WAHIS) as of July 2024 (Figure 1). The social, health, and economic impacts on communities due to ASF are severe. For instance, in China, retail pork prices rose by 78%, heavily impacting consumers. It is estimated that by the end of 2019, the national pig herd in China was reduced by half as a consequence of ASFV genotype II outbreaks [1]. Similarly, in Vietnam, a quarter of pig populations succumbed to the diseases or had to be culled during the first year after ASF’s arrival. Low- and middle-income countries suffer severe socio-economic losses, in addition to threats to food security and livelihoods [2]. These impacts are further complicated by the difficulty in assessing the true global impact due to frequent under-reporting in endemic countries, where sick pigs continue to be traded to recover some of the losses [3].
Figure 1.
Distribution of confirmed ASF cases in Asia–Pacific from 2018 to 2024 as reported by WAHIS (red).
ASFV is a stable double-stranded DNA virus that is classified within the family Asfarviridae and the genus Asfivirus. Genotyping of ASFV involves amplifying and sequencing the variable 3′ end of the B646L gene [4]. This gene encodes for p72, the primary capsid protein, which is crucial for tracing and identifying different strains of ASFV [5,6]. More than 20 genotypes of ASFV have been identified in Africa; however, only genotypes I and II have been detected outside the continent. ASFV genotype II is currently responsible for global ASF outbreaks, while genotype I, largely eradicated from regions outside of Africa, was recently detected in China [7]. Notably, the last reported cases of genotype I in Europe occurred in Sardinia, with one detection in a wild boar in 2019 and one in a domestic pig in 2018 [8,9,10]. The genotype I viruses identified in China to date do not resemble those found in Sardinia, but are more closely related to two attenuated viruses obtained in Portugal in 1968 and 1988 [7].
ASFV primarily infects pig macrophages, altering signaling pathways and disrupting the expression of genes associated with both innate and acquired immunity. These cells are also widely utilized in vitro for virus detection and research, given their susceptibility to ASFV infection [4,11]. Transmission occurs through direct contact and bodily excretions such as blood, urine, feces, and saliva, although airborne transmission is unlikely. ASFV can also be transmitted by Ornithodoros ticks. If Ornithodoros ticks and pigs co-exist in an ecological niche situation, this presents a significant risk in virus persistence in the field [12,13]. Infected animals are most contagious during the late stages of the disease, when clinical signs are evident and often only post-mortem. However, viral shedding in oronasal and lachrymal secretions, urine, and feces can occur as early as 1 to 7 days post-infection (dpi), with shedding from the oral cavity occurring prior to systemic dissemination of the virus. Even low amounts of the virus are sufficient to cause efficient in vivo infection, highlighting the importance of early detection and control measures [14]. Blood is particularly significant in ASFV transmission, and like other arboviruses, ASFV can also be transmitted through ticks.
2. Vaccine as a Tool to Control Disease and Limit Economic Losses—The Example of Vietnam
To combat the threat of ASF, Vietnam has taken proactive measures by licensing two vaccines: NAVET-ASFVAC, manufactured by NAVETCO, and AVAC ASF Live, produced by AVAC, in June 2022 for field trials. Subsequently, on 24 July 2023, the Ministry of Agriculture and Rural Development (MARD) approved the nationwide use of these vaccines, emphasizing the need for vaccine quality and safety standards in the field. NAVET-ASFVAC is based on ASFV-G-ΔI177L, demonstrating stability and attenuation following five passages in domestic pigs [15]. AVAC ASF Live is based on the ASFV-G-∆MGF strain, featuring six deletions and propagated in Diep’s macrophage cell (DMAC) line. Moreover, the Philippines recently announced that its ASFV vaccination campaign with AVAC will commence in 2024 (Table 1) [16]. However, farmers in Vietnam are cautious in using these vaccines, echoing concerns similar to those faced during vaccine trials in the Philippines [2]. The long-term safety and efficacy data of these two LAVs in the field are not publicly available, raising concerns about potential reversion to virulence. This cautious approach highlights the critical need for stringent vaccine regulation and thorough testing to ensure safety and efficacy in the field. Indeed, the World Organization for Animal Health (WOAH) warned that current ASF vaccines need more testing [17]. At the same time, vaccines have been exported to the Philippines, Indonesia, Malaysia, India, and Myanmar for limited trials [2,18]. Issues such as shedding of vaccine virus, vertical and horizontal transmission, immunogenicity to various field strains, reversion to virulence, potential for recombination with field virus, and post-vaccine complications, among others, are unresolved issues in vaccine development, which are particularly critical in LAVs. These challenges are addressed in the new draft WOAH standard, which advocates for the development and evaluation of ASF vaccine candidates to ensure they meet regulatory approval criteria [19]. Table 1 shows the distribution of ASFV vaccine doses by NAVETCO and AVAC up to June 2024, with NAVETCO distributing a total of 667,000 doses and AVAC distributing 3,601,710 doses across Vietnam, the Dominican Republic, the Philippines, and Nigeria.
Table 1.
ASFV vaccines distributed (doses) till June 2024 [20].
| NAVETCO | AVAC | |
|---|---|---|
| Vietnam | 660,000 | 3,296,710 |
| Dominican Republic | 7000 | - |
| Philippines | - | 300,000 |
| Nigeria | - | 5000 |
| Total | 667,000 | 3,601,710 |
3. The Types of Vaccines Applied in Asia, Field Vaccine-Related Viruses, and Their Impact on Disease Surveillance and Control
Recent research has identified the emergence of attenuated genotype I ASFV strains, HeN/ZZ-P1/21 and SD/DY-I/21, in China. These strains are characterized by reduced virulence, but high transmissibility, leading to chronic conditions such as necrotic skin lesions and joint swelling [21]. Phylogenetic analysis indicates that these strains are closely related to isolated genotype I viruses, namely, strains NH/P68 and OURT88/3, which were isolated in Portugal [22]. In 2023, reports suggested potential recombination of these genotype I strains with pandemic genotype II viruses, creating a highly virulent recombinant strain [23,24]. This recombinant virus exhibits high lethality and rapid transmission akin to genotype II, complicating the ASF landscape further. Given the absence of evidence for the persistent circulation of NH/P68 and OURT88/3in other endemic areas, its emergence is particularly alarming. A plausible hypothesis is the introduction of this genotype I strain through illicit vaccine trials aiming to develop new live attenuated or genetically modified vaccines. This genotype I/II recombinant might have been created naturally in the field as well, given the co-circulation of both genotypes. Thus, both laboratory and field origins of the recombinant are possible, with no evidence that can conclusively exclude one or the other. However, no recombinant mosaic virus has so far been detected outside Asia or in Africa, where more than 20 genotypes are simultaneously circulating. The illicit application of vaccines further complicates the epidemiological landscape, exemplified by the case of ASFV-GUS-Vietnam, identified in Northern Vietnam in 2021. It is important to clarify that ASFV-GUS-Vietnam is the same virus as the AVAC vaccine virus (ASFV-G-∆MGF). [25]. Notably, this genetically modified LAV initially caused only mild clinical signs, but displayed increased virulence in subsequent trials [26]. While it provided complete protection against a homologous challenge, the increased virulence raises serious safety concerns and underscores the necessity for stringent regulation and careful oversight in deploying live attenuated recombinant ASF vaccines in field settings. The occurrence of clinical signs with the disease patterns observed after 7 days post-inoculation with GUS-Vietnam in a recent study from 2024 contrasts with a previous study from 2015, where no clinical signs were observed when ASFV-G-∆MGF was inoculated into domestic pigs [26,27]. Notably, ASFV-GUS-Vietnam was found prior to the release of licensed ASF vaccines in Vietnam. These examples show that the illicit use of improper vaccines can not only spread the disease but also facilitate the emergence of new variants in the regions, in addition to the risk of recombination between different ASFV genotypes.
In the case of ASF, a pig infected with the highly virulent strain ASFV genotype II typically develops severe hemorrhagic disease, often resulting in death within a few days. A targeted vaccine against this genotype should prevent the animal from dying. However, the presence of recombinant viral strains alters the disease pattern, potentially undermining efforts like partial culling [28]. The slow spread of ASF through a farm (low contagiousness) can usually be leveraged to implement a partial or selective culling strategy effectively, but this approach may not work if the disease pattern changes due to strains such as ASFV-GUS-Vietnam. In several Asian countries, partial culling has been adopted as a strategy to manage ASF’s spread while minimizing economic losses. For example, Vietnam officially adopted partial culling in July 2019, which helped preserve over 50% of the pig population and extended control measures by only eight days, although the long-term impact on livelihoods remains uncertain [29]. Similarly, South Africa successfully eliminated ASF in specific areas using this method [30]. Other countries, including Laos, Cambodia, India, and China, have also implemented partial culling strategies [17]. Understanding the high-risk period (HRP)—the duration that the virus is present before detection—is essential to prevent spread and minimize losses in partial culling. Early detection is crucial for controlling secondary outbreaks and managing the spread through animal movements, contaminated products, and other vectors. However, challenges arise with the introduction of uncontrolled vaccines or illicit LAVs, which can disrupt existing control strategies. Furthermore, the absence of a serological DIVA vaccine complicates the rapid identification of infected versus vaccinated animals, necessitating whole-genome sequencing for definitive differentiation. Although Brake (2022) argues that in endemic regions where ASF is widespread, DIVA vaccines might not be essential since vaccination can still reduce disease spread by diminishing viral shedding and alleviating clinical symptoms [31], DIVA vaccines remain crucial for effective disease management in both endemic and non-endemic areas. They help accurately distinguish between vaccinated and naturally infected animals, which can be isolated and culled. The licensed ASFV-G-∆I177L vaccine and other candidates contain molecular markers that facilitate the monitoring of vaccinated populations [32]. Given these complexities, countries must weigh the expected vaccine efficacy, achievable population coverage, and likely compliance when considering ASF vaccination programs.
4. The Importance of Development and Application of Safe, Effective Vaccines
The situation in Asia with ASF mirrors challenges faced in the recent past with other diseases, such as lumpy skin disease (LSD). In Asia, the LSDV vaccine (Neethling strain) encountered several critical safety and effectiveness issues, including contamination of vaccine batches with wild-type LSDV and goat-pox virus (GTPV) [33]. This contamination introduced more strains into the vaccinated population, including recombinant virulent strains. These recombinant strains exhibited unpredictable behavior and increased virulence, complicating clinical outcomes in vaccinated animals. Furthermore, the presence of both vaccine and wild-type strains, as well as recombinant strains, led to significant diagnostic confusion, making it difficult to differentiate between vaccinated and naturally infected animals. These challenges were documented in various studies highlighting the complexity and risks associated with using contaminated or recombinant vaccine strains [33,34,35]. Moreover, molecular characterization revealed that the Bangladeshi LSDV strains were genetically distinct from contemporary field strains. Indeed, whole-genome sequencing showed that Bangladeshi LSDV strains had unique genetic markers distinguishing them from other contemporary strains. These strains shared similarities with historical isolates from Kenya and other parts of Africa from the 1950s, suggesting an older strain’s reintroduction into the region [36,37]. Pre-analysis of vaccines could have shown that LAVs against LSDV are not safe for use in animals. Similarly, for the genotype I ASFV strain, as observed in the abovementioned case of LSDV, there is a possibility that the strain was introduced through vaccines. The provided examples show that vaccines not rigorously tested can even introduce new variants into regions that were previously free of such strains.
5. Addressing Current Vaccine Challenges
Building on the critical importance of developing and applying safe, effective vaccines, as discussed, it is imperative to address the ongoing challenges and potential risks associated with vaccine quality and safety. In Asia, the experiences with diseases like ASF and LSD underscore the necessity for rigorous testing, regulation, and the strategic development of vaccines to control high-impact animal diseases effectively. Ensuring vaccine integrity not only supports disease management but also prevents the exacerbation of disease spread due to inadequate vaccine quality. Recently developed ASF vaccines can potentially be used to limit the economic losses and disease spread [38]. However, it is essential to examine the context of vaccine use and disease impact in Asia. The importance of these ASF vaccines is underscored by the critical role pigs play in food security and the economy. WOAH (2023) [17] warns against the use of non-compliant and poor-quality vaccines, which, as described in this communication, may have caused recombination between the vaccine virus and either the field virus or another virus used in other vaccines. This recombination can result in new viral strains capable of causing acute, chronic, or persistent infections.
Ongoing global research focuses on developing live attenuated, subunit, inactivated, and vector-based vaccines, which are still under extensive testing and not yet widely available. Traditional inactivated vaccines, including chemically inactivated or irradiated ones, have shown limited protective effects against ASF [39,40]. Research has revealed reduced viral loads and an immune response, but these vaccines did not prevent severe symptoms, leading to the euthanasia of vaccinated and control animals. A multi-epitope subunit vaccine using immunoinformatics, analyzing 18,858 proteins from 100 ASFV proteomes, has shown high antigenicity and immunogenicity, but remains untested in animals [40]. Challenges include identifying protective antigens, defining immune correlates, and scaling production in cell culture [30,41]. LAV candidates for ASF, focusing on the p72 genotype II strain, use naturally attenuated and recombinant viruses with gene deletions [31,42,43]. Pigs vaccinated with ASFV-G-ΔMGF exhibited mild, transient symptoms without significant virulence reversion. However, there are ongoing concerns about the genetic stability of the virus due to the “ΔMGFnV” mutation, which involves deletions and duplications of ASFV genes [44]. Notably, the development of mutant strains and the occurrence of mild symptoms and prolonged viremia were evident after just five passages. This raises significant concerns about field applications where thousands of pigs would be vaccinated, potentially leading to more severe issues [44]. Thus, it cannot be ruled out that vaccinated animals are shedding the virus, plus there is the concern of reversion to virulence in LAVs.
6. Conclusions
In conclusion, the examples described in this paper demonstrate the role that vaccines can play in controlling animal diseases and limiting economic losses, while simultaneously highlighting the negative impacts that can arise from improper vaccine application. The types of vaccines applied in Asia and the emergence of field vaccine-related viruses highlight the need for stringent biosecurity measures and comprehensive surveillance. The importance of developing and applying safe, effective vaccines cannot be overstated, as illustrated by WOAH’s concerns and the challenges faced in similar situations, such as LSD in Asia. The use of an unsafe vaccine in endemic areas in Africa where multiple genotypes circulate would likely increase the challenges faced by poor people whose pigs provide a much-needed source of income. Ensuring vaccine quality and safety through rigorous testing and regulation is essential for effective disease control and management. To improve trust and support decision-making regarding ASF vaccines, a standard for long-term field monitoring of vaccine impact is needed, which vaccine developers and manufacturers can follow. Additionally, the establishment of independent bodies to evaluate vaccine efficacy and safety in Asia is essential.
Acknowledgments
Special thanks to the Laboratory Unit, Animal Health Service (NSAH-CJW), Food and Agriculture Organization of the United Nations (FAO), Rome, Italy.
Author Contributions
Conceptualization, A.A., G.C., P.P., C.E.L. and A.R.; methodology, A.A.; investigation, all authors; resources, A.R.; data curation, A.A.; writing—original draft preparation, A.A. and G.C.; writing—review and editing, A.A., G.C., P.P., C.E.L., Y.O., S.J. and A.R.; supervision, A.R.; project administration, A.R., P.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Disclaimer
© FAO, 2024; Agathe Auer; Food and Agriculture Organization of the United Nations. The views expressed in this publication are those of the author(s) and do not necessarily reflect the views or policies of the Food and Agriculture Organization of the United Nations.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Berthe F. Panorama 2020-1: African Swine Fever: Responding to the Global Threat. WOAH; Online: 2020. [(accessed on 2 May 2024)]. Global Economic Impact of African Swine Fever. WOAH Bull. 2020, 24/07, 2 p; p. 2. Available online: https://oiebulletin.com/?panorama=02-2-2-2020-1-economic. [DOI] [Google Scholar]
- 2.Fernandez-Colorado C.P., Kim W.H., Flores R.A., Min W. African Swine Fever in the Philippines: A Review on Surveillance, Prevention, and Control Strategies. Animals. 2024;14:1816. doi: 10.3390/ani14121816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costard S., Mur L., Lubroth J., Sanchez-Vizcaino J.M., Pfeiffer D.U. Epidemiology of African Swine Fever Virus. Virus Res. 2015;173:191–197. doi: 10.1016/j.virusres.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Galindo I., Alonso C. African Swine Fever Virus: A Review. Viruses. 2017;9:103. doi: 10.3390/v9050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ankhanbaatar U., Auer A., Ulziibat G., Settypalli T.B.K., Gombo-Ochir D., Basan G., Takemura T., Tseren-Ochir E.-O., Ouled Ahmed H., Meki I.K., et al. Comparison of the Whole-Genome Sequence of the African Swine Fever Virus from a Mongolian Wild Boar with Genotype II Viruses from Asia and Europe. Pathogens. 2023;12:1143. doi: 10.3390/pathogens12091143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boshoff C.I., Bastos A.D.S., Gerber L.J., Vosloo W. Genetic Characterisation of African Swine Fever Viruses from Outbreaks in Southern Africa (1973–1999) Vet. Microbiol. 2007;121:45–55. doi: 10.1016/j.vetmic.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Qu H., Ge S., Zhang Y., Wu X., Wang Z. A Systematic Review of Genotypes and Serogroups of African Swine Fever Virus. Virus Genes. 2022;58:77–87. doi: 10.1007/s11262-021-01879-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavone S., Iscaro C., Dettori A., Feliziani F. African Swine Fever: The State of the Art in Italy. Animals. 2023;13:2998. doi: 10.3390/ani13192998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loi P., Casu S., Cappai S., Ladu S., Desini P., Mereu M., Coccollone A., Fiori M.S., Sanna M.L., Di Gennaro A., et al. Eradication of African Swine Fever in Sardinia: A Successful Result of Regional Programme Implementation. Vaccines. 2022;10:799. doi: 10.3390/vaccines10050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franzoni G., Dei Giudici S., Loi P., Sanna D., Fiori M.S., Zinellu S., Sanna M.L., Coradduzza E., Amadori M., Falchi G., et al. Intracellular Cytokine Staining and Transcriptomic Analysis Provide Insights into the Swine Immune Response Following the Infection with Two Low and High Pathogenic Genotype I African Swine Fever Virus Isolates. Viruses. 2021;13:1502. doi: 10.3390/v13081502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sánchez E.G., Pérez-Núñez D., Revilla Y. Mechanisms of Entry and Endosomal Pathway of African Swine Fever Virus. Vaccines. 2017;5:42. doi: 10.3390/vaccines5040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olesen A.S., Lohse L., Boklund A., Halasa T., Gallardo C., Pejsak Z., Belsham G.J., Rasmussen T.B., Bøtner A. Transmission of African Swine Fever Virus from Infected Pigs by Direct Contact and Aerosol Routes. Vet. Microbiol. 2017;211:92–102. doi: 10.1016/j.vetmic.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Beltrán-Alcrudo D., Arias M., Gallardo C., Kramer S., Penrith M.L. African Swine Fever: Detection and Diagnosis—A Manual for Veterinarians. FAO; Rome, Italy: 2017. 88p FAO Animal Production and Health Manual No. 19. [Google Scholar]
- 14.Sánchez-Cordón P.J., Vidaña B., Neimanis A., Núñez A., Wikström E., Gavier-Widén D. Understanding and Combatting African Swine Fever. Wageningen Academic Publishers; Wageningen, The Netherlands: 2021. pp. 87–139. [Google Scholar]
- 15.Tran X.H., Le T.T.P., Nguyen Q.H., Do T.T., Nguyen V.D., Gay C.G., Borca M.V., Gladue D.P. African Swine Fever Virus Vaccine Candidate ASFV-G-ΔI177L Efficiently Protects European and Native Pig Breeds against Circulating Vietnamese Field Strain. Transbound. Emerg. Dis. 2022;69:e497–e504. doi: 10.1111/tbed.14329. [DOI] [PubMed] [Google Scholar]
- 16.FAO Asia&Pacific Update 8 August 2024; ASF Situation in Asia & Pacific Update. [(accessed on 8 August 2024)]. Available online: https://www.fao.org/animal-health/situation-updates/asf-in-asia-pacific/en.
- 17.World Organisation for Animal Health (WOAH) African Swine Fever: WOAH Warns Veterinary Authorities and Pig Industry of Risk from Use of Sub-Standard Vaccines. WOAH. 2023. [(accessed on 2 May 2024)]. Available online: https://www.woah.org/en/african-swine-fever-woah-warns-veterinary-authorities-and-pig-industry-of-risk-from-use-of-sub-standard-vaccines/
- 18.Senate of the Philippines (2023, October 25). Opening Remarks During the Public Hearing on the Proliferation of the Unauthorized Sale of African Swine Fever (ASF) in the Market While the Same is Still Undergoing Trial and the Role of the BAI and the FDA with Regards to Importation of Vaccine. Laurel Hall, 2nd Floor, Senate of the Philippines, at 10:00 AM October 25, 2023. [(accessed on 2 May 2024)]; Available online: https://legacy.senate.gov.ph/press_release/2023/1025_villar1.asp.
- 19.Report of the Meeting of the Biological Standards Commission. September 2023. [(accessed on 19 June 2024)]. Available online: https://www.woah.org/app/uploads/2023/10/a-bsc-report-sept-2023-7.pdf.
- 20.Department of Animal Health. Work on disease prevention, slaughter control and quarantine in pig farming; Proceedings of the Conference on Sustainable Pig Farming Development, Ministry of Agriculture and Rural Development; Ha Noi, Vietnam. 14 August 2024. [Google Scholar]
- 21.Sun E., Huang L., Zhang X., Zhang J., Shen D., Zhang Z., Bu Z. Genotype I African Swine Fever Viruses Emerged in Domestic Pigs in China and Caused Chronic Infection. Emerg. Microbes Infect. 2021;10:2183–2193. doi: 10.1080/22221751.2021.1999779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vigario J.D., Terrinha A.M., Moura Nunes J.F. Antigenic relationship among strains of African swine fever virus. Archiv für die Gesamte Virusforschung. 1974;45:272–277. doi: 10.1007/BF01249690. [DOI] [PubMed] [Google Scholar]
- 23.Zhao D., Sun E., Huang L., Ding L., Zhu Y., Zhang J., Shen D., Zhang X., Zhang Z., Ren T., et al. Highly Lethal Genotype I and II Recombinant African Swine Fever Viruses Detected in Pigs. Nat. Commun. 2023;14:3096. doi: 10.1038/s41467-023-38868-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le V.P., Jeong D.G., Yoon S.W., Kwon H.M., Trinh T.B.N., Nguyen T.L., Bui N.N., Oh J., Lyoo Y.S., Kim J.B., et al. Outbreak of African Swine Fever, Vietnam, 2019. Emerg. Infect. Dis. 2019;25:1433–1435. doi: 10.3201/eid2507.190303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abrams C.C., Goatley L., Fishbourne E., Chapman D., Cooke L., Oura C.A., Netherton C.L., Takamatsu H.H., Dixon L.K. Deletion of virulence associated genes from attenuated African swine fever virus isolate OUR T88/3 decreases its ability to protect against challenge with virulent virus. Virology. 2013;443:99–105. doi: 10.1016/j.virol.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ambagala A., Goonewardene K., Kanoa I.E., Than T.T., Nguyen V.T., Lai T.N.H., Nguyen T.L., Erdelyan C.N.G., Robert E., Tailor N., et al. Characterization of an African Swine Fever Virus Field Isolate from Vietnam with Deletions in the Left Variable Multigene Family Region. Viruses. 2024;16:571. doi: 10.3390/v16040571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Donnell V., Holinka L.G., Gladue D.P., Sanford B., Krug P.W., Lu X., Arzt J., Reese B., Carrillo C., Risatti G.R., et al. African Swine Fever Virus Georgia Isolate Harboring Deletions of MGF360 and MGF505 Genes Is Attenuated in Swine and Confers Protection against Challenge with Virulent Parental Virus. J. Virol. 2015;89:6048–6056. doi: 10.1128/JVI.00554-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nga B.T.T., Auer A., Padungtod P., Dietze K., Globig A., Rozstalnyy A., Hai T.M., Depner K. Evaluation of Selective Culling as a Containment Strategy for African Swine Fever at a Vietnamese Sow Farm. Pathogens. 2024;13:567. doi: 10.3390/pathogens13070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nga B.T.T., Padungtod P., Depner K., Chuong V.D., Duy D.T., Anh N.D., Dietze K. Implications of partial culling on African swine fever control effectiveness in Vietnam. Front. Vet. Sci. 2022;9:957918. doi: 10.3389/fvets.2022.957918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janse van Rensburg L., Van Heerden J., Penrith M.L., Heath L.E., Rametse T., Etter E.M.C. Investigation of African swine fever outbreaks in pigs outside the controlled areas of South Africa, 2012–2017. J. S. Afr. Vet. Assoc. 2020;91:1–9. doi: 10.4102/jsava.v91i0.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brake D.A. African Swine Fever Modified Live Vaccine Candidates: Transitioning from Discovery to Product Development through Harmonized Standards and Guidelines. Viruses. 2022;14:2619. doi: 10.3390/v14122619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gladue D.P., Ramirez-Medina E., Vuono E., Silva E., Rai A., Pruitt S., Espinoza N., Velazquez-Salinas L., Borca M.V. Deletion of the A137R Gene from the Pandemic Strain of African Swine Fever Virus Attenuates the Strain and Offers Protection against the Virulent Pandemic Virus. J. Virol. 2021;95:e0113921. doi: 10.1128/JVI.01139-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandenbussche F., Mathijs E., Philips W., Saduakassova M., De Leeuw I., Sultanov A., Haegeman A., De Clercq K. Recombinant LSDV Strains in Asia: Vaccine Spillover or Natural Emergence? Viruses. 2022;14:1429. doi: 10.3390/v14071429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sprygin A., Pestova Y., Bjadovskaya O., Prutnikov P., Zinyakov N., Kononova S., Ruchnova O., Lozovoy D., Chvala I., Kononov A. Evidence of recombination of vaccine strains of lumpy skin disease virus with field strains, causing disease. PLoS ONE. 2020;15:e0232584. doi: 10.1371/journal.pone.0232584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sendow I., Meki I.K., Dharmayanti N.L.P.I., Hoerudin H., Ratnawati A., Settypalli T.B.K., Ahmed H.O., Nuradji H., Saepulloh M., Adji R.S., et al. Molecular characterization of recombinant LSDV isolates from 2022 outbreak in Indonesia through phylogenetic networks and whole-genome SNP-based analysis. BMC Genom. 2024;25:240. doi: 10.1186/s12864-024-10169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badhy S.C., Chowdhury M.G.A., Settypalli T.B.K., Cattoli G., Lamien C.E., Fakir M.A.U., Akter S., Osmani M.G., Talukdar F., Begum N., et al. Molecular characterization of lumpy skin disease virus (LSDV) emerged in Bangladesh reveals unique genetic features compared to contemporary field strains. BMC Vet. Res. 2021;17:61. doi: 10.1186/s12917-021-02751-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dietze K., Hoffmann B., Tuppurainen E., Stoll M., Probst C., Sauter-Louis C., Conraths F.J. Clinical Epidemiology, Pathology, and Molecular Investigation of Lumpy Skin Disease Outbreaks in Bangladesh during 2020-2021 Indicate the Re-Emergence of an Old African Strain. Viruses. 2022;14:2529. doi: 10.3390/v14112529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vu H.L.X., McVey D.S. Recent progress on gene-deleted live-attenuated African swine fever virus vaccines. npj Vaccines. 2024;9:60. doi: 10.1038/s41541-024-00845-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pikalo J., Zani L., Hühr J., Beer M., Blome S. Pathogenesis of African Swine Fever Virus, ASFV-G-ΔMGF, following Intramuscular Inoculation in Domestic Pigs. Pathogens. 2020;9:746. [Google Scholar]
- 40.Cadenas-Fernández E., Sánchez-Vizcaíno J.M., Pintore A., Denyer M., Laddomada A., Martins C., Kosowska A., von Buttlar H., Dietze K., Ito S., et al. Progress in African swine fever vaccines. Vaccine. 2021;39:7114–7121. [Google Scholar]
- 41.Simbulan V., Gonzales M., Madlangbayan J., Guerrero H. Multi-epitope subunit vaccine design for African swine fever virus. npj Vaccines. 2024;9:42. doi: 10.1038/s41541-024-00823-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meloni D., Franzoni G., Oggiano A. Cell Lines for the Development of African Swine Fever Virus Vaccine Candidates: An Update. Vaccines. 2022;10:707. doi: 10.3390/vaccines10050707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J., Quan Y., Wang H., Wu Z., Lu X. Development of ASF LAV Candidates Using Gene Deletions. Vet. Microbiol. 2021;252:108934 [Google Scholar]
- 44.Deutschmann P., Forth J.H., Sehl-Ewert J., Roszyk H., Probst C., Beer M., Blome S. Assessment of African Swine Fever Vaccine Candidate ASFV-G-ΔMGF in a Reversion to Virulence Study. npj Vaccines. 2023;8:78. doi: 10.1038/s41541-023-00669-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available upon request.