Abstract
Simple Summary
Prostate cancer is the most common cancer in men and a major cause of cancer-related deaths around the world. Prostate cancer that has spread to other parts of the body (advanced prostate cancer) is often treated with a drug called enzalutamide, which is a type of hormone therapy. Enzalutamide works by blocking the effect of the testosterone hormone on prostate cancer cells to stop them from growing. While this can be effective for several years, unfortunately, many patients being treated with enzalutamide eventually go on to become resistant to treatment, and the therapy stops working. Here, we show that prostate cancer cells that have become resistant to enzalutamide have increased levels of a type of sugar (known as sialic acid) on their surfaces. We set out to test whether stripping sialic acid from the surface of prostate cancer cells could help keep enzalutamide working for longer. Excitingly, our experiments show that treating prostate cancer cells with a drug to block sialic acid can partially reverse enzalutamide resistance. These findings suggest that drugs targeting sialic acid could be used in combination with enzalutamide therapy to disarm drug resistance and provide urgently needed new treatment options for men with prostate cancer.
Abstract
Prostate cancer is a lethal solid malignancy and a leading cause of cancer-related deaths in males worldwide. Treatments, including radical prostatectomy, radiotherapy, and hormone therapy, are available and have improved patient survival; however, recurrence remains a huge clinical challenge. Enzalutamide is a second-generation androgen receptor antagonist that is used to treat castrate-resistant prostate cancer. Among patients who initially respond to enzalutamide, virtually all acquire secondary resistance, and an improved understanding of the mechanisms involved is urgently needed. Aberrant glycosylation, and, in particular, alterations to sialylated glycans, have been reported as mediators of therapy resistance in cancer, but a link between tumour-associated glycans and resistance to therapy in prostate cancer has not yet been investigated. Here, using cell line models, we show that prostate cancer cells with acquired resistance to enzalutamide therapy have an upregulation of the sialyltransferase ST6 beta-galactoside alpha-2,6-sialyltransferase 1 (ST6GAL1) and increased levels of α2,6-sialylated N-glycans. Furthermore, using the sialyltransferase inhibitor P-SiaFNEtoc, we discover that acquired resistance to enzalutamide can be partially reversed by combining enzalutamide therapy with sialic acid blockade. Our findings identify a potential role for ST6GAL1-mediated aberrant sialylation in acquired resistance to enzalutamide therapy for prostate cancer and suggest that sialic acid blockade in combination with enzalutamide may represent a novel therapeutic approach in patients with advanced disease. Our study also highlights the potential to bridge the fields of cancer biology and glycobiology to develop novel combination therapies for prostate cancer.
Keywords: prostate cancer, castrate resistance, enzalutamide, sialic acid, sialyltransferase inhibitor, combination therapies
1. Introduction
Prostate cancer is the second most common cancer in men worldwide and is a significant cause of morbidity and mortality in the global male population [1]. Although localised prostate cancer is largely curable and has a five-year survival rate of >99%, the mortality rate for advanced prostate cancer is high, and only 32% of advanced prostate cancer patients will be alive after 5 years [2]. Primary prostate cancer is largely driven through androgen signalling via interactions with the androgen receptor (AR) and is commonly therapeutically managed with androgen deprivation therapies (ADT) [3,4]. Although ADT achieves initial success, the majority of patients will develop resistance to these therapies within 5 years of diagnosis and will go on to develop castrate-resistant prostate cancer (CRPC), where tumours persist despite low androgen conditions due to the acquisition of resistance mechanisms [5,6]. Patients with CRPC are managed with second-generation androgen receptor inhibitors (enzalutamide, abiraterone, apalutamide, or darolutamide), radium-233 for bone metastases, immunotherapy (sipuleucel-T, pembrolizumab), poly-ADP ribose polymerase (PARP) inhibitors (olaparib, rucaparib), or chemotherapy (most commonly docetaxel) [2,7,8,9,10,11]. However, resistance to these interventions is commonplace, culminating in the dire median survival of 9–30 months for CRPC patients [2,12].
Enzalutamide (MDV3100, Xtandi®) is a commonly used second-generation AR inhibitor that demonstrates a higher affinity for binding to the AR in comparison to its predecessors, such as bicalutamide, and has the capacity to prevent AR nuclear translocation, DNA-binding, and recruitment of co-activators [13,14,15,16,17,18]. Clinical trial data have highlighted that the inclusion of enzalutamide in the clinical regimen for treating patients with CRPC could significantly improve overall survival and progression-free survival [13,15,19,20,21]. However, both primary and acquired resistance are observed in relation to enzalutamide treatment. Primary resistance to enzalutamide (defined as treatment failure within the first 3 months following treatment initiation) occurs in 25% of CRPC patients, and acquired resistance is typically observed 9–15 months following treatment initiation [14,22,23,24]. How CRPC tumours develop resistance to enzalutamide remains to be fully understood, but it likely includes AR amplification, AR mutations, the generation of AR splice variants (AR-v), alterations to steroidogenesis, overexpression of glucocorticoid and progesterone receptors, and neuroendocrine differentiation [14,22,25]. As most patients treated with enzalutamide eventually develop resistance and disease progression, there is a critical need to identify mechanisms of resistance as targets for future therapies [26,27]
Glycosylation is a post translational modification where glycan structures are added to proteins and lipids [28,29,30]. Altered glycosylation is a hallmark of cancer and can mediate critical events in tumour development and progression [31,32]. Even though aberrant glycosylation is a potentially druggable hallmark of cancer [28,33,34,35], to date, it has been relatively underexplored particularly in the context of prostate cancer. Studies have sought to understand the molecular mechanisms underpinning prostate cancer progression and resistance to therapy [36,37,38,39,40], but only a few have studied glycans. Historically, this has been due to technological limitations, but this is now changing. For example, the development of N-glycan imaging mass spectrometry [41,42,43] has enabled the profiling of glycosylation changes throughout prostate cancer evolution [44]. An understanding of the role glycans play in prostate cancer will be crucial to the identification of new targets that can be exploited to treat advanced disease and prolong patient survival [45,46,47,48]. Recent studies have highlighted the potential to target aberrant glycosylation in combination with existing therapies to boost treatment response and potentially overcome therapy resistance [34,49,50] and this area of research is beginning to show promise for prostate cancer [51,52,53,54].
A common change in tumour glycosylation is alterations to sialylated glycans, and this can actively drive aggressive disease [55,56,57,58]. Aberrant sialylation has been linked to paclitaxel and cisplatin resistance in ovarian cancer [59,60,61], chemotherapy resistance in gastric cancer [62], sensitivity to docetaxel in hepatocarcinoma [63], multi-drug resistance in leukaemia [64,65], sensitivity to tyrosine kinase inhibition in lung cancer [66], and bortezomib resistance in myeloma [67]. A correlation between radiotherapy resistance and increased sialylation is also well established, particularly for colorectal cancer [68,69,70,71]. The contribution of sialylated glycans to chemotherapy resistance in cancer could be due to the physical barrier of extra sialic acid on the surface of cells which can potentially modify key receptors or block the uptake of drugs into the cell. For example, sialylation of the oncogenic receptor Erb2 can mask an epitope recognised by the anti-cancer antibody trastuzumab and promote resistance [72]. Sialylated glycans may also promote therapy resistance by absorbing ionising radiation [56], and radiation exposure can enhance the sialylation of membrane glycoproteins to promote radiation resistance [68,73]. Taken together, these studies raise the possibility of targeting aberrant sialylation in combination with existing cancer therapies to improve patient outcomes.
In prostate cancer, a rewiring of the tumour glycome is associated with disease progression, and glycans play important roles in tumour growth, metastasis, and immune evasion [45,48,74,75,76]. Recently, we showed that ST6 beta-galactoside alpha-2,6-sialyltransferase 1 (ST6GAL1) and larger branched sialylated N-glycans are upregulated in men with prostate cancer, and this can promote tumour growth and the spread of tumours to bone [52,77]. Aberrant sialylation is causally linked to therapy resistance in cancer [55], and ST6GAL1 has been identified as a mediator of treatment resistance in several tumour types, including pancreatic cancer [78], colorectal cancer [73,79], leukaemia [80,81], and gastric cancer [72]. A previous study showed resistance to hormonal therapy in prostate cancer is associated with an upregulation of complex larger-branched N-glycans [74]. However, to date, studies investigating the role of ST6GAL1 and its associated glycans in therapy-resistant prostate cancer are lacking.
Here, using VCaP and LNCaP cell line models, we show that prostate cancer cells with acquired resistance to enzalutamide have upregulated ST6GAL1 and significantly higher levels of α2-6 sialylated N-glycans. Our findings identify ST6GAL1-mediated aberrant sialylation as a potential mediator of acquired resistance to enzalutamide therapy in prostate cancer. Furthermore, using the newly developed C-5 carbamate sialyltransferase inhibitor P-SiaFNEtoc [82,83], we show that acquired resistance to enzalutamide by prostate cancer cells can be partially reversed by sialic acid blockade. These results suggest that inhibiting sialylation in combination with enzalutamide may represent a novel therapeutic approach in patients with advanced prostate cancer.
2. Methods
2.1. Cell Culture
Cell culture was carried out as described previously [84]. VCaP (CRL-2876) and LNCaP (CRL-1740) cells were purchased from ATCC. Enzalutamide-resistant VCaP (VCaPEnzR) and LNCaP (LNCaPEnzR) cell lines were generated as described previously [85]. Briefly, LNCaP and VCaP cells were grown in 10 μM enzalutamide in long-term culture (>6 months). Resistant cells were pooled and maintained. Once resistant, cells were continually cultured in 10 μM enzalutamide. No changes in phenotype were observed, and we did not detect any genomic changes. The cell lines were authenticated using DNA STR analysis and tested every 3 months for mycoplasma contamination.
2.2. Inhibitors
The enzalutamide (MDV3100, Xtandi®) was purchased from MedChemExpress® (HY-70002). The sialyltransferase inhibitor P-SiaFNEtoc was synthesised as described previously [82] (compound 10).
2.3. Western Blotting
Western blotting was performed as previously described [86]. Immunoblots were probed with antibodies for ST6GAL1 at 1:1000 dilution (Abgent, San Diego, CA, USA, AP19891c) or GAPDH at 1:2000 dilution (Abgent, AP7873b), followed by incubation with appropriate fluorescent secondary antibodies at 1:10,000 dilution, (anti-mouse 680, Cell Signalling, Leiden, The Netherlands, 5470S) or anti-rabbit 800 (Cell Signalling, 5151S)).
2.4. ELISA Assays
Conditioned media samples were prepared from cell lines as described previously [86]. ST6GAL1 protein levels were monitored using sandwich ELISA assays, which have previously been validated (Cambridge Bioscience, Cambridge, UK, ELH-ST6GAL1-1) [77].
2.5. Immunocytochemistry
The cells were cultured in a Nunc™ 4 well plate (Thermo Scientific™, Oxford, UK, 176740) on top of a sterilised 10 mm-round coverslip (VWRTM, 631-1340) in complete media. Treatments with P-SiaFNEtoc were performed for either for 3 days (LNCaP cells) or 6 days (VCaP cells) with the indicated concentrations. Cells treated with DMSO were used as controls. The cells were washed with PBS before permeabilization and fixation with ice-cold absolute methanol for 10 min at −20 °C. Next, the cells were washed with PBS and blocked with 10% goat serum (Abcam, Cambridge, UK, ab7481) for 1 h at room temperature. The cells were incubated overnight at 4 °C with a ST6GAL1 antibody at 1:200 (Abgent, AP19891c), followed by goat anti-rabbit IgG H and L (Alexa Fluor® 594) (Abcam, ab150080), diluted 1:500. Finally, the cells were washed with PBS and stained with Hoechst (Thermo Scientific, 62249) for 15 min at room temperature. Images were acquired and processed with a ZEISS Axio Imager 4.
2.6. Lectin Immunofluorescence
The cells were cultured in a Nunc™ 4-well plate (Thermo Scientific™, 176740) on top of a sterilised 10 mm-round coverslip (VWRTM, 631-1340) in complete media. Treatments with P-SiaFNEtoc were performed for either for 3 days (LNCaP cells) or 6 days (VCaP cells) with the indicated concentrations. Cells treated with DMSO were used as controls. P-SiaFNEtoc concentrations were optimised to 2 µM for 3 days and 20 µM for 6 days for the LNCaP and VCaP cells, respectively. For neuraminidase treatment, α2-3,6,8 neuraminidase (NEB, UK, P0720) was used as a negative control to strip sialic acid from the surface of the cells. The cells were cultured in 100 units/mL of neuraminidase as described previously [44]. To monitor Sambucas nigra (SNA) lectin binding, the cells were washed with PBS before permeabilization and fixation with ice-cold absolute methanol for 10 min at −20 °C. Next, the cells were washed with PBS and blocked with 1X Carbo-Free™ Blocking Solution (1X CFB) (Vector Laboratories, Cambs, UK, SP-5040-125) for 1 h at room temperature. The cells were incubated overnight at 4 °C with FITC-conjugated SNA lectin (Vector Laboratoriesabs, FL-1301-2). Finally, the cells were washed with PBS and stained with Hoechst (Thermo Scientific, 62249) for 15 min at room temperature. The cells were mounted using ProLongTM gold antifade mountant (Thermo Scientific™, P36930), and images were acquired and processed with the ZEISS Axio Imager 4.
2.7. CellTiter-Glo® Assays
CellTiter-Glo® Luminescent cell viability assays (Promega, UK, G9682) were performed as described previously [51]. Cell viability was assessed at the times indicated, and luminescence was recorded with the Varioskan™ LUX microplate reader.
VCaP cells: The VCaP control and VCaPEnzR cells were seeded at a density of 7.0 × 103 cells/well in 96-well plates (Thermo cientific, 130188). After 24 h, the cells were treated with a range of concentrations of enzalutamide with or without concurrent treatment with 20 µM P-SiaFNEtoc for 6 days (144 h).
LNCaP cells: The LNCaP control and LNCaPEnzR cells were seeded at a density of 3.0 × 103 cells/well in a 96-well plate. After 24 h, the cells were treated with a range of enzalutamide concentrations with or without concurrent treatment with 2 µM P-SiaFNEtoc for 3 days (72 h).
2.8. Statistical Analyses
Statistical analyses were conducted using the GraphPad Prism software (version Prism 9.4.1). Data are presented as the mean of three independent samples ± standard error of the mean (SEM). Statistical significance is denoted as * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
3. Results
3.1. Prostate Cancer Cells with Acquired Enzalutamide Resistance Have Upregulated ST6GAL1
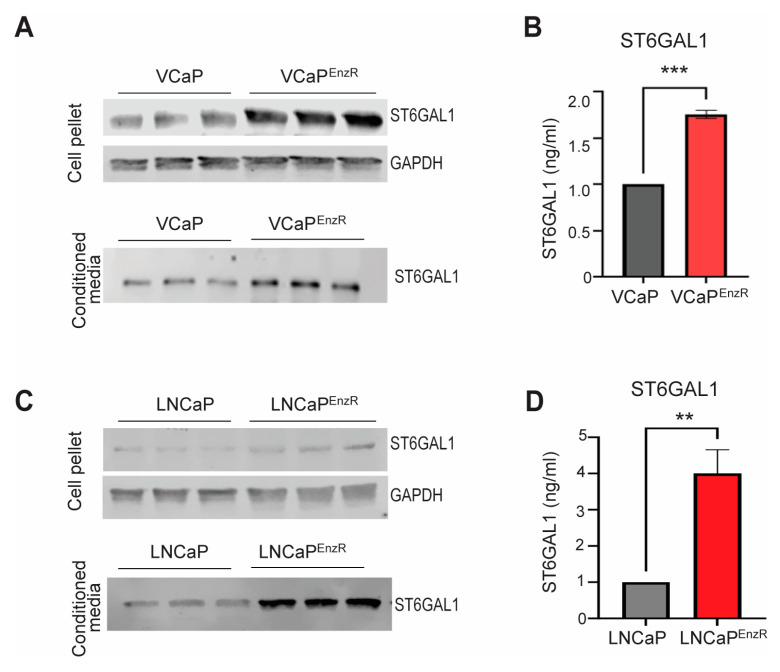
The sialyltransferase ST6GAL1 has been previously identified to be upregulated in prostate cancer and linked with tumour growth, metastasis, and poor overall survival [52,77,87,88]. ST6GAL1 has been reported as a mediator of therapy resistance in other cancer types [72,73,78,79,80,81], but a link between ST6GAL1 and resistance to therapy has not yet been investigated for prostate cancer. To address this gap, we used western blotting and pre-validated sandwich ELISA assays [77] to monitor ST6GAL1 protein levels in VCaP and LNCaP prostate cancer cell line models with acquired resistance to enzalutamide. Our findings show intra-cellular and secreted ST6GAL1 levels are upregulated in the enzalutamide-resistant VCaP cells (VCaPEnzR), compared to the enzalutamide-sensitive control VCaP cells (Figure 1A,B). Upregulation of ST6GAL1 was also detected in the enzalutamide-resistant LNCaP cells (LNCaPEnzR), where levels of ST6GAL1 are increased compared to the enzalutamide-sensitive control LNCaP cells (Figure 1C,D). Taken together, these findings suggest that the sialyltransferase ST6GAL1 is upregulated in prostate cancer cells with acquired resistance to enzalutamide therapy.
Figure 1.
Enzalutamide-resistant prostate cancer cells have upregulation of ST6GAL1. (A) Western blot analysis of ST6GAL1 in the VCaP control and enzalutamide-resistant VCaP (VCaPEnzR) cells. ST6GAL1 levels are increased in both cell pellet and conditioned media samples from the VCaPEnzR cells. GAPDH is included as a loading control. (B) Analysis of ST6GAL1 levels in conditioned media samples from the VCaP control and VCaPEnzR cells using pre-validated sandwich ELISA assays [77]. The levels of ST6GAL1 are significantly higher in conditioned media from VCaPEnzR cells (n = 3, unpaired t-test, *** p = 0.0002). (C) Western blot analysis of ST6GAL1 in the LNCaP control and enzalutamide-resistant LNCaP (LNCaPEnzR) cells. GAPDH is used as a loading control. The levels of ST6GAL1 are increased in both cell pellet and conditioned media samples (D) Analysis of ST6GAL1 levels in conditioned media samples from the LNCaP control and LNCaPEnzR cells using pre-validated sandwich ELISA assays [77]. The levels of ST6GAL1 are significantly higher in conditioned media from the LNCaPEnzR cells compared to the control LNCaP cells (n = 6, unpaired t-test, ** p = 0.0014). Results are representative of three biological repeats and are presented as the mean ± standard error. Original western blots are presented in File S1.
3.2. Enzalutamide-Resistant Prostate Cancer Cells Have Increased Levels of α2,6-Sialylated N-Glycans
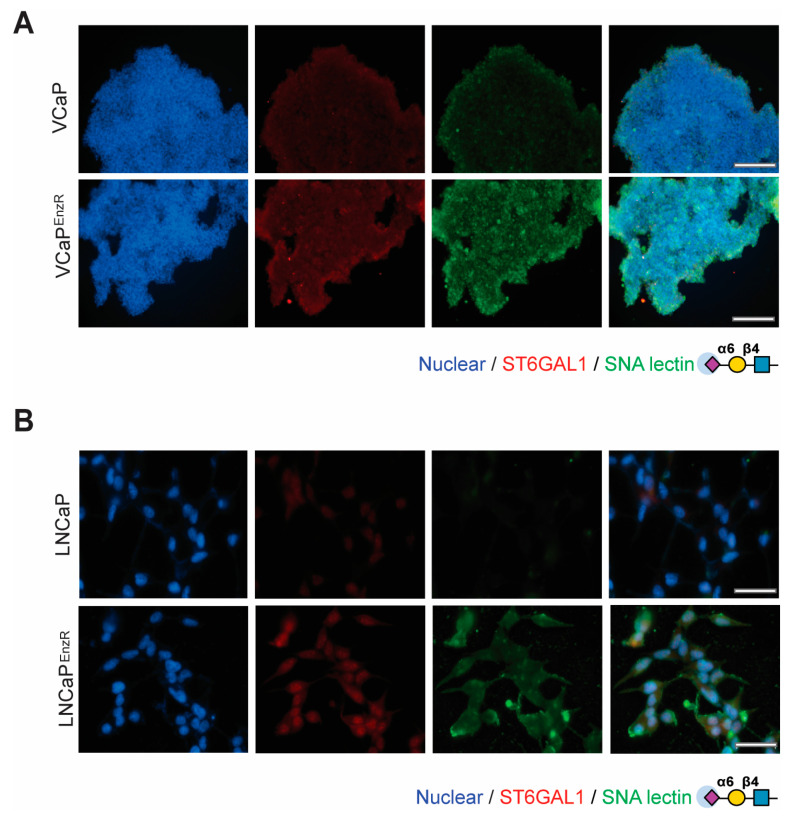
ST6GAL1 adds α2,6-linked sialic acid to N-glycosylated proteins that are destined for the cell membrane or secretion [55,89,90]. In previous studies, we detected increased levels of α2,6 sialylation in prostate cancer cells with an upregulation of ST6GAL1 [52,77]. We thus hypothesised that the upregulation of ST6GAL1 in enzalutamide-resistant prostate cancer cells will alter the levels of α2,6 sialylated N-glycans. To test this, we used immunofluorescence to monitor recognition by the SNA lectin (which recognises α2,6-linked sialylated N-glycans [91]) in prostate cancer cells with acquired resistance to enzalutamide. Our findings show that the VCaPEnzR cells have increased binding of SNA lectin relative to the control enzalutamide-sensitive VCaP cells (Figure 2A). Similarly, the LNCaPEnzR cells also show increased recognition by SNA lectin, indicating increased levels of α2,6 sialylation in these cells compared to the control enzalutamide-sensitive LNCaP cells (Figure 2B). Confirming the specificity of our results, SNA binding was eliminated when cells were treated with neuraminidase (which removes terminal sialic acids of glycans) (Supplementary Figure S1). In summary, these findings suggest enzalutamide resistance correlates with an upregulation of α2,6-sialylated N-glycans in prostate cancer cell line models.
Figure 2.
Enzalutamide-resistant prostate cancer cells have upregulation of ST6GAL1 and increased levels of α2,6-sialylated glycans. (A) SNA lectin immunofluorescence shows VCaPEnzR cells have increased levels of ST6GAL1 and α2-6 sialylation (SNA, the lectin from Sambucus nigra, recognises α2-6-linked sialylated N-glycans [91]). (B) SNA lectin immunofluorescence shows LNCaPEnzR cells have increased levels of ST6GAL1 and α2-6 sialylation compared to control LNCaP cells. DNA is stained with Hoechst. Scale bar = 200 µM.
3.3. The Sialyltransferase Inhibitor P-SiaFNEtoc Blocks α2,6 Sialylation in Enzalutamide-Resistant Prostate Cancer Cells
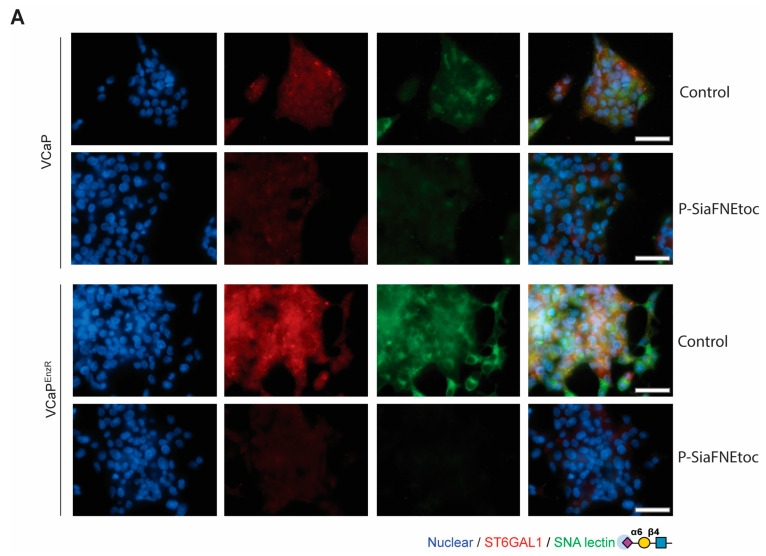
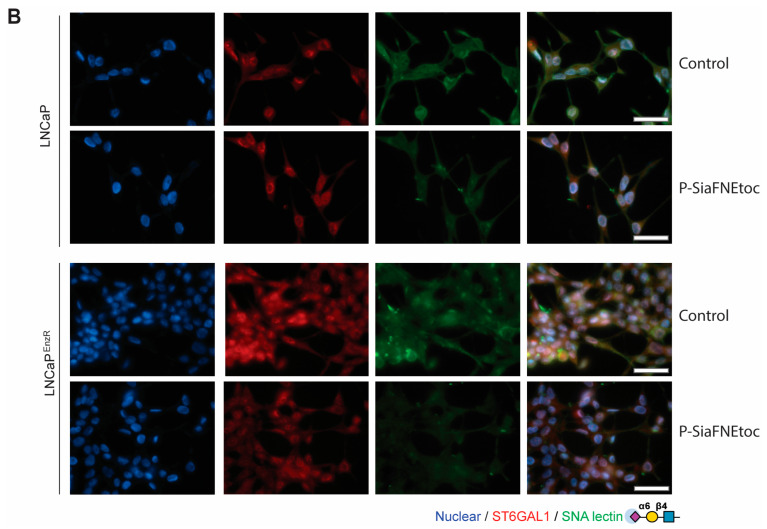
We previously showed that the newly developed C-5 carbamate sialyltransferase inhibitor P-SiaFNEtoc [82] can effectively inhibit the sialylation of prostate cancer cells with only minor effects on other glycan types [51]. Next, to test whether P-SiaFNEtoc can inhibit α2,6-sialylated N-glycans in enzalutamide-resistant prostate cancer cells, we treated VCaPEnzR and LNCaPEnzR cells with P-SiaFNEtoc and monitored α2,6-sialylation using SNA lectin immunofluorescence after 3 or 6 days (Supplementary Figure S2). For the VCaPEnzR cells, treatment with 20 µM P-SiaFNEtoc for 6 days suppressed recognition by SNA lectin, suggesting a reduction in α2,6-sialylated N-glycans (Figure 3A). For the LNCaPEnzR cells, treatment with 2 µM P-SiaFNEtoc for 3 days reduced levels of levels of α2,6 sialylation (Figure 3B). As VCaP cells have a significantly longer doubling time than LNCaP cells [92,93], and have higher endogenous levels of ST6GAL1 [87], we hypothesised that this may explain why the VCaP cells required increased concentrations of P-SiaFNEtoc to reduce α2,6-sialylation levels, but we did not investigate this further. Together with previously published findings [51], our data show that treatment with the sialyltransferase inhibitor P-SiaFNEtoc can block α2,6 sialylation in enzalutamide-resistant prostate cancer cells.
Figure 3.
The sialyltransferase inhibitor P-SiaFNEtoc blocks α2,6 sialylation in the VCaPEnzR and LNCaPEnzR prostate cancer cells. (A) Detection of immunofluorescent staining of ST6GAL1 and α2,6-sialylation of N-glycans in the VCaP control and VCaPEnzR cells treated with 20 µM of the sialyltransferase inhibitor P-SiaFNEtoc for 6 days. Treatment of both cell lines with P-SiaFNEtoc inhibits α2,6-sialylation of N-glycans (detected using SNA lectin). Control cells were treated with DMSO. Scale bar = 50 µm. The images are representative of three biological repeats. (B) Detection of immunofluorescent staining of ST6GAL1 and α2,6-sialylation of N-glycans in LNCaP control and LNCaPEnzR cells treated with 2 µM of the sialyltransferase inhibitor P-SiaFNEtoc for 3 days. Treatment of both cell lines with P-SiaFNEtoc inhibits α2,6-sialylation of N-glycans (detected using SNA lectin). The control cells were treated with DMSO. Scale bar = 50 µm. The images are representative of three biological repeats.
3.4. Sialic Acid Blockade Partially Reverts Acquired Resistance to Enzalutamide in Prostate Cancer Cells
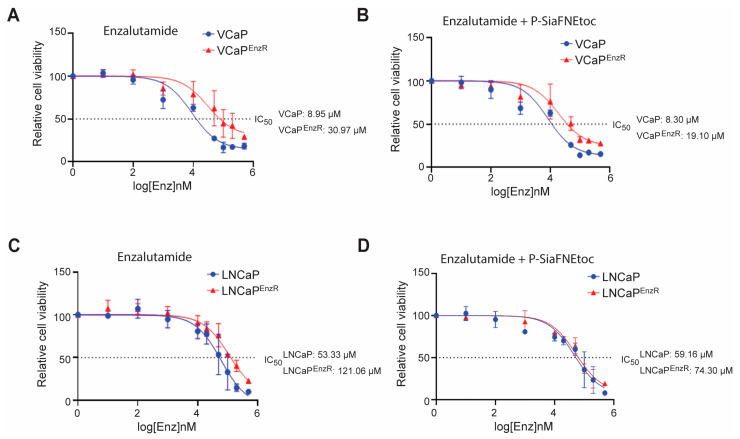
Together with the literature [59,60,61,62,63,64,65,66,67,68,69,70,71,72], the above data raises the possibility that aberrant sialylation could play a functional role in the acquired resistance of prostate cancer to the second-generation AR antagonist enzalutamide. This led to the hypothesis that therapies targeting sialylation might have the potential to re-sensitise prostate cancer cells to treatment with enzalutamide. To investigate this, we first treated control VCaP and VCaPEnzR cells with a range of enzalutamide concentrations (up to 500 µM) and measured cellular viability using CellTiter-Glo® luminescence assays and IC50 analyses. As expected, the IC50 value for the VCaPEnzR cells treated with enzalutamide was 3.46-fold higher relative to the control VCaP cells (VCaP control IC50: 8.95 µM, VCaPEnzR IC50: 30.97 µM) (Figure 4A). Next, to test if sialic acid blockade can impact the resistance of prostate cancer cells to enzalutamide, we treated the VCaPEnzR cells with enzalutamide in combination with the sialylation inhibitor P-SiaFNEtoc (treatments for VCaP cells were carried out with 20 µM P-SiaFNEtoc for 6 days to match our findings in Figure 3A). Excitingly, this revealed that although sialic acid blockade in combination with enzalutamide therapy did not alter the IC50 value for the VCaP control cell line, for the VCaPEnzR cells, there was a 38.33% reversion in the IC50 value in comparison to enzalutamide treatment alone (VCaP control IC50: 8.30 µM, VCaPEnzR IC50: 19.10 µM) (Figure 4B).
Figure 4.
Sialic acid blockade using P-SiaFNEtoc partially re-sensitises prostate cancer cells to the second-generation androgen receptor antagonist enzalutamide. (A) The VCaPEnzR cells have increased resistance to enzalutamide compared to the control VCaP cells. The control VCaP and VCaPEnzR cells were treated with a range of enzalutamide concentrations (0–500 µM). Cell viability was measured after 6 days using a CellTitrer-Glo® luminescence assay. The IC50 values (the concentration of enzalutamide which reduced cellular viability by 50% relative to the DMSO control) was 3.46-fold greater in the VCaPEnzR cells (8.95 µM for VCaP control cells and 30.97 µM for the VCaPEnzR cells). (B) Treatment of the VCaPEnzR cells with P-SiaFNEtoc partially reverts resistance to enzalutamide. The control VCaP and VCaPEnzR cells were treated with 20 µM P-SiaFNEtoc and a range of concentrations of enzalutamide (0–500 µM) for 6 days. Inhibiting sialylation in the VCaPEnzR cells reduced the IC50 value from 30.97 µM to 19.10 µM, indicating a partial reversion of their resistance to enzalutamide. (C) The LNCaPEnzR cells have increased resistance to enzalutamide compared to the control LNCaP cells. The control LNCaP and LNCaPEnzR cells were treated with a range of enzalutamide concentrations (0–500 µM). Cell viability was measured after 3 days using a CellTitre-Glo® luminescence assay. The IC50 value was 2.27-fold higher in the LNCaPEnzR cells (53.33 µM for LNCaP control cells and 121.06 µM for the LNCaPEnzR cells). (D) Treatment of the LNCaPEnzR cells with P-SiaFNEtoc partially reverts their resistance to enzalutamide. The control LNCaP and LNCaPEnzR cells were treated with 2 µM P-SiaFNEtoc and a range of concentrations of enzalutamide (0–500 µM) for 3 days. A DMSO-only control arm was included for each cell line. Inhibiting sialylation in the LNCaPEnzR cells reduced the IC50 value from 121.06 µM to 74.30 µM, indicating a partial reversion of their resistance to enzalutamide. A line of best fit was utilised for interpolating the IC50 value. Results are presented as the cell viability (luminescence) relative to the respective DMSO control against the log of the enzalutamide concentration (nM) used for each cell line. Results are presented as the mean ± standard error and are representative of three biological repeats.
The IC50 value for the LNCaPEnzR cells was 2.27-fold higher relative to the control LNCaP cells when treated with enzalutamide alone (LNCaP control: 53.33 µM, LNCaPEnzR: 121.06 µM) (Figure 4C). When the LNCaPEnzR cells were treated with both P-SiaFNEtoc and enzalutamide (treatments for the LNCaP cells were carried out with 2 µM P-SiaFNEtoc for 3 days to match our findings in Figure 3B), there was a 38.64% reversion in the IC50 value in comparison to enzalutamide treatment alone (LNCaP control IC50: 59.16 µM, LNCaPEnzR IC50: 74.30 µM) (Figure 4C,D). These data indicate that that sialic acid blockade, in combination with enzalutamide treatment, can partially revert acquired enzalutamide resistance in cell line models of prostate cancer.
4. Discussion
Enzalutamide is an orally administered small-molecule inhibitor of the AR that is designed to overcome resistance to anti-androgens and can improve overall survival in CRPC [19,20,94]. Unfortunately, some patients have primary resistance to enzalutamide, and others eventually develop acquired resistance and continue to progress [27,95]. Numerous studies have sought to characterise the molecular mechanisms underlying how prostate cancer becomes resistant to enzalutamide therapy, with AR amplification, AR variants, altered expression of AR co-regulators, upregulation of glucocorticoid receptor (GR), and metabolic alterations believed to play a role [95,96]. ST6GAL1 and its associated glycans are likely to be an important target in cancer cells [89,90]. ST6GAL1 is upregulated in numerous types of cancer, including pancreatic, ovarian, breast, and prostate cancer, and its expression is associated with aggressive tumours and reduced survival times [52,89,97,98]. Upregulation of ST6GAL1 impacts oncogenic cell behaviours and can play a key role in tumour growth, survival, metastasis, immune evasion, and resistance to therapy [89,97,99]. Specifically, ST6GAL1 can mediate resistance to chemoradiation in rectal cancer [73], the sensitivity of gastric cancer cells to trastuzumab [72], and resistance to the EGFR inhibitor, gefitinib, in ovarian cancer [100]. In prostate cancer, the upregulation of ST6GAL1 promotes the growth and metastatic spread of prostate tumours to bone [52,77], and targeting aberrant sialylation in prostate cancer cells can inhibit the metastatic spread of tumours to bone [51,52]. The data presented in this manuscript indicate that ST6GAL1-mediated aberrant sialylation could also be an important mediator of the acquired resistance of prostate tumours to enzalutamide therapy (which is a major clinical issue potentially affecting all men who develop CRPC [101]. Furthermore, our study provides proof-of-concept data for a therapeutic strategy to target aberrant sialylation that has the potential to partially revert enzalutamide resistance and prolong the clinical efficacy of anti-androgen therapies. It should be noted that the sialyltransferase inhibitor used in this study (P-SiaFNEtoc) is a global sialyltransferase inhibitor that targets all sialyltansferase enzymes (including ST6GAL1), meaning we cannot rule out that that the effects of P-SiaFNEtoc on the enzalutamide-resistant cells may not be solely due to ST6GAL1 inhibition. Inhibitors specifically targeting ST6GAL1 are being developed [56,102] and, once available, will be highly relevant to prostate cancer.
The mechanisms underpinning how aberrant sialylation might contribute to enzalutamide resistance in prostate cancer remain unclear but are likely multi-faceted. Aberrant sialylation on the cell surface could potentially inhibit the uptake of enzalutamide into the cell. Another possibility is that alterations to sialylated glycans could interfere with the binding of enzalutamide to the AR to promote resistance. The role of sialic acid in the context of the tumour microenvironment and immune suppression is also an important consideration which was not addressed by the current study. Increased sialylation is common in cancer cells and is associated with an immunosuppressed tumour microenvironment [57,99,103,104]. Sialylated glycans can be engaged by a broad range of immune cell types to promote immune suppression [105,106], and the upregulation of ST6GAL1 in prostate cancer cells has been shown to promote a shift towards an immunosuppressive M2 macrophage phenotype [52]. Anti-androgen therapies are known to remodel the prostate tumour immune microenvironment [107,108], and, recently, it was proposed that this remodelling could include alterations to the Siglec–sialyloglycan axis in prostate tumours, which could contribute to immunosuppression [109]. Moving forward, syngeneic models of prostate cancer could be used to perform pre-clinical evaluation of sialic acid blockade in combination with enzalutamide therapy, alongside investigating the role of altered glycosylation in the resistance to enzalutamide therapy in the context of the tumour-immune microenvironment. Previous studies have identified increased levels of ST6GAL1 in tumours and blood samples from prostate cancer patients [52,77]. In future studies, it will be important to monitor the levels of ST6GAL1 and sialylated glycans in clinical samples from prostate cancer patients with tumours that have become resistant to enzalutamide therapy and investigate whether the detection of ST6GAL1 and/or aberrant sialylation in prostate tumours could be used to predict sensitivity and resistance to treatment strategies.
5. Conclusions
Our findings identify aberrant sialylation as a previously unexplored but clinically relevant contributor to the acquired resistance of prostate tumours to enzalutamide therapy and suggest further research in this area could lead to the development of novel combination therapies to disarm drug resistance in prostate cancer. Recent studies have provided rationale for the use of glyco-immune checkpoint-targeting therapies in advanced prostate cancer [52,109]. Numerous strategies to target sialylated glycans are under development, including sialylation inhibitors and antibody–sialidase conjugates [34,50,110], some of which are currently in clinical trials [34,50] and are likely to be relevant for patients with prostate cancer. Future research should seek to utilise these advancements in the glycobiology field to gain a better understanding of the role which aberrant glycosylation may play in prostate cancer resistance to key therapeutic interventions, such as enzalutamide.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16172953/s1, Figure S1: SNA binding was eliminated when prostate cancer cells were treated with neuraminidase; Figure S2: Detection of α2,6 sialylation in VCaP cells treated with 2–20 µM P-SiaFNEtoc. File S1: Original western blots.
Author Contributions
Conceptualization, E.A.G., J.F.A.P., T.J.B. and J.M. (Jennifer Munkley); formal analysis, E.A.G., M.O.-M., K.H., A.N. and M.M.; funding acquisition, N.W.; investigation, E.A.G., M.O.-M., K.H., A.N., M.M. and Z.P.; methodology, E.A.G. and E.R.; resources, J.M. (Jona Merx), E.R., J.F.A.P. and T.J.B.; supervision, T.J.B., N.W., D.J.E. and J.M. (Jennifer Munkley); writing—original draft, E.A.G. and D.J.E.; writing—review and editing, K.H., N.W. and J.M. (Jennifer Munkley). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials.
Conflicts of Interest
J.M (Jennifer Munkley). is a shareholder of GlycoScoreDx Ltd. J.F.A.P. and E.R. are shareholders of and employed by GlycoTherapeutics B.V. T.J.B. is a shareholder of and scientific advisor of GlycoTherapeutics B.V.; J.F.A.P. and T.J.B. are shareholders of Synvenio B.V.; Radboud University and Radboudumc have filed patent applications related to P-SiaFNEtoc. All other authors declare that there are no potential competing interests.
Funding Statement
This work was funded by the Medical Research Council [MR/N013840/1, MR/R015902/1], Prostate Cancer Research and the Mark Foundation for Cancer Research (grant references 6961 and 6974), Prostate Cancer UK and The Bob Willis Fund through Research Innovation Awards [RIA16-ST2-011 and RIA21-ST2-006] and Newcastle University Hospitals Special Trustees. This work was supported by an ERC-Stg, (GlycoEdit, 758913) awarded to T.J.B.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Archer Goode E., Wang N., Munkley J. Prostate cancer bone metastases biology and clinical management (Review) Oncol. Lett. 2023;25:163. doi: 10.3892/ol.2023.13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujita K., Nonomura N. Role of Androgen Receptor in Prostate Cancer: A Review. World J. Mens Health. 2019;37:288–295. doi: 10.5534/wjmh.180040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narayan V., Ross A.E., Parikh R.B., Nohria A., Morgans A.K. How to Treat Prostate Cancer With Androgen Deprivation and Minimize Cardiovascular Risk: A Therapeutic Tightrope. JACC CardioOncol. 2021;3:737–741. doi: 10.1016/j.jaccao.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirby M., Hirst C., Crawford E.D. Characterising the castration-resistant prostate cancer population: A systematic review. Int. J. Clin. Pract. 2011;65:1180–1192. doi: 10.1111/j.1742-1241.2011.02799.x. [DOI] [PubMed] [Google Scholar]
- 6.Nanda J.S., Koganti P., Perri G., Ellis L. Phenotypic Plasticity-Alternate Transcriptional Programs Driving Treatment Resistant Prostate Cancer. Crit. Rev. Oncog. 2022;27:45–60. doi: 10.1615/CritRevOncog.2022043096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice M.A., Malhotra S.V., Stoyanova T. Second-Generation Antiandrogens: From Discovery to Standard of Care in Castration Resistant Prostate Cancer. Front. Oncol. 2019;9:801. doi: 10.3389/fonc.2019.00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vellky J.E., Ricke W.A. Development and prevalence of castration-resistant prostate cancer subtypes. Neoplasia. 2020;22:566–575. doi: 10.1016/j.neo.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adashek J.J., Jain R.K., Zhang J. Clinical Development of PARP Inhibitors in Treating Metastatic Castration-Resistant Prostate Cancer. Cells. 2019;8:860. doi: 10.3390/cells8080860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aly M., Leval A., Schain F., Liwing J., Lawson J., Vágó E., Nordström T., Andersson T.M.-L., Sjöland E., Wang C., et al. Survival in patients diagnosed with castration-resistant prostate cancer: A population-based observational study in Sweden. Scand. J. Urol. 2020;54:115–121. doi: 10.1080/21681805.2020.1739139. [DOI] [PubMed] [Google Scholar]
- 11.Amaral T.M., Macedo D., Fernandes I., Costa L. Castration-resistant prostate cancer: Mechanisms, targets, and treatment. Prostate Cancer. 2012;2012:327253. doi: 10.1155/2012/327253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran C., Ouk S., Clegg N.J., Chen Y., Watson P.A., Arora V., Wongvipat J., Smith-Jones P.M., Yoo D., Kwon A., et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shore N.D., Chowdhury S., Villers A., Klotz L., Siemens D.R., Phung D., van Os S., Hasabou N., Wang F., Bhattacharya S., et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): A randomised, double-blind, phase 2 study. Lancet Oncol. 2016;17:153–163. doi: 10.1016/S1470-2045(15)00518-5. [DOI] [PubMed] [Google Scholar]
- 14.Dong L., Zieren R.C., Xue W., de Reijke T.M., Pienta K.J. Metastatic prostate cancer remains incurable, why? Asian J. Urol. 2019;6:26–41. doi: 10.1016/j.ajur.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cicero G.D.E.L., Dorangricchia P., Dieli F. The Clinical Efficacy of Enzalutamide in Metastatic Prostate Cancer: Prospective Single-center Study. Anticancer. Res. 2017;37:1475–1480. doi: 10.21873/anticanres.11472. [DOI] [PubMed] [Google Scholar]
- 16.Linder S., van der Poel H.G., Bergman A.M., Zwart W., Prekovic S. Enzalutamide therapy for advanced prostate cancer: Efficacy, resistance and beyond. Endocr. Relat. Cancer. 2018;26:R31–R52. doi: 10.1530/ERC-18-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schalken J., Fitzpatrick J.M. Enzalutamide: Targeting the androgen signalling pathway in metastatic castration-resistant prostate cancer. BJU Int. 2015;117:215–225. doi: 10.1111/bju.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman-Censits J., Kelly W.K. Enzalutamide: A novel antiandrogen for patients with castrate-resistant prostate cancer. Clin. Cancer Res. 2013;19:1335–1339. doi: 10.1158/1078-0432.CCR-12-2910. [DOI] [PubMed] [Google Scholar]
- 19.Scher H.I., Fizazi K., Saad F., Taplin M.-E., Sternberg C.N., Miller K., De Wit R., Mulders P., Chi K.N., Shore N.D., et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 20.Beer T.M., Armstrong A.J., Rathkopf D.E., Loriot Y., Sternberg C.N., Higano C.S., Iversen P., Bhattacharya S., Carles J., Chowdhury S., et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N. Engl. J. Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penson D.F., Armstrong A.J., Concepcion R., Agarwal N., Olsson C., Karsh L., Dunshee C., Wang F., Wu K., Krivoshik A., et al. Enzalutamide Versus Bicalutamide in Castration-Resistant Prostate Cancer: The STRIVE Trial. J. Clin. Oncol. 2016;34:2098–2106. doi: 10.1200/JCO.2015.64.9285. [DOI] [PubMed] [Google Scholar]
- 22.Tucci M., Zichi C., Buttigliero C., Vignani F., Scagliotti G.V., Di Maio M. Enzalutamide-resistant castration-resistant prostate cancer: Challenges and solutions. Onco Targets Ther. 2018;11:7353–7368. doi: 10.2147/OTT.S153764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Chen J., Wu Z., Ding W., Gao S., Gao Y., Xu C. Mechanisms of enzalutamide resistance in castration-resistant prostate cancer and therapeutic strategies to overcome it. Br. J. Pharmacol. 2020;178:239–261. doi: 10.1111/bph.15300. [DOI] [PubMed] [Google Scholar]
- 24.Lin H.-M., Mak B., Yeung N., Huynh K., Meikle T.G., Mellett N.A., Kwan E.M., Fettke H., Tran B., Davis I.D., et al. Overcoming enzalutamide resistance in metastatic prostate cancer by targeting sphingosine kinase. EBioMedicine. 2021;72:103625. doi: 10.1016/j.ebiom.2021.103625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claessens F., Helsen C., Prekovic S., Broeck T.V.D., Spans L., Van Poppel H., Joniau S. Emerging mechanisms of enzalutamide resistance in prostate cancer. Nat. Rev. Urol. 2014;11:712–716. doi: 10.1038/nrurol.2014.243. [DOI] [PubMed] [Google Scholar]
- 26.Hussain A., Dawson N. Management of advanced/metastatic prostate cancer: 2000 update. Oncology. 2000;14:1677–1688; discussion 1688, 1691–1694. [PubMed] [Google Scholar]
- 27.Blatt E.B., Raj G.V. Molecular mechanisms of enzalutamide resistance in prostate cancer. Cancer Drug Resist. 2019;2:189–197. doi: 10.20517/cdr.2019.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinho S.S., Reis C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 29.Eichler J. Protein glycosylation. Curr. Biol. 2019;29:R229–R231. doi: 10.1016/j.cub.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Reily C., Stewart T.J., Renfrow M.B., Novak J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019;15:346–366. doi: 10.1038/s41581-019-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munkley J., Elliott D.J. Hallmarks of glycosylation in cancer. Oncotarget. 2016;7:35478–35489. doi: 10.18632/oncotarget.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vajaria B.N., Patel P.S. Glycosylation: A hallmark of cancer? Glycoconj. J. 2017;34:147–156. doi: 10.1007/s10719-016-9755-2. [DOI] [PubMed] [Google Scholar]
- 33.Thomas D., Rathinavel A.K., Radhakrishnan P. Altered glycosylation in cancer: A promising target for biomarkers and therapeutics. Biochim. Biophys. Acta Rev. Cancer. 2021;1875:188464. doi: 10.1016/j.bbcan.2020.188464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mereiter S., Balmaña M., Campos D., Gomes J., Reis C.A. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell. 2019;36:6–16. doi: 10.1016/j.ccell.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigues J.G., Duarte H.O., Reis C.A., Gomes J. Aberrant protein glycosylation in cancer: Implications in targeted therapy. Biochem. Soc. Trans. 2021;49:843–854. doi: 10.1042/BST20200763. [DOI] [PubMed] [Google Scholar]
- 36.Sinha A., Huang V., Livingstone J., Wang J., Fox N.S., Kurganovs N., Ignatchenko V., Fritsch K., Donmez N., Heisler L.E., et al. The Proteogenomic Landscape of Curable Prostate Cancer. Cancer Cell. 2019;35:414–427 e6. doi: 10.1016/j.ccell.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng Q., Butler W., Zhou Y., Zhang H., Tang L., Perkinson K., Chen X., Jiang X., McCall S.J., Inman B.A., et al. Pre-existing Castration-resistant Prostate Cancer-like Cells in Primary Prostate Cancer Promote Resistance to Hormonal Therapy. Eur. Urol. 2022;81:446–455. doi: 10.1016/j.eururo.2021.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren S., Shao Y., Zhao X., Hong C.S., Wang F., Lu X., Li J., Ye G., Yan M., Zhuang Z., et al. Integration of Metabolomics and Transcriptomics Reveals Major Metabolic Pathways and Potential Biomarker Involved in Prostate Cancer. Mol. Cell Proteom. 2016;15:154–163. doi: 10.1074/mcp.M115.052381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le T.K., Duong Q.H., Baylot V., Fargette C., Baboudjian M., Colleaux L., Taïeb D., Rocchi P. Castration-Resistant Prostate Cancer: From Uncovered Resistance Mechanisms to Current Treatments. Cancers. 2023;15:5047. doi: 10.3390/cancers15205047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karantanos T., Corn P.G., Thompson T.C. Prostate cancer progression after androgen deprivation therapy: Mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013;32:5501–5511. doi: 10.1038/onc.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powers T.W., Neely B.A., Shao Y., Tang H., Troyer D.A., Mehta A.S., Haab B.B., Drake R.R. MALDI imaging mass spectrometry profiling of N-glycans in formalin-fixed paraffin embedded clinical tissue blocks and tissue microarrays. PLoS ONE. 2014;9:e106255. doi: 10.1371/journal.pone.0106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drake R.R., Powers T.W., Norris-Caneda K., Mehta A.S., Angel P.M. In Situ Imaging of N-Glycans by MALDI Imaging Mass Spectrometry of Fresh or Formalin-Fixed Paraffin-Embedded Tissue. Curr. Protoc. Protein Sci. 2018;94:e68. doi: 10.1002/cpps.68. [DOI] [PubMed] [Google Scholar]
- 43.West C.A., Liang H., Drake R.R., Mehta A.S. New Enzymatic Approach to Distinguish Fucosylation Isomers of N-Linked Glycans in Tissues Using MALDI Imaging Mass Spectrometry. J. Proteome Res. 2020;19:2989–2996. doi: 10.1021/acs.jproteome.0c00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallace E.N., West C.A., McDowell C.T., Lu X., Bruner E., Mehta A.S., Aoki-Kinoshita K.F., Angel P.M., Drake R.R. An N-glycome tissue atlas of 15 human normal and cancer tissue types determined by MALDI-imaging mass spectrometry. Sci. Rep. 2024;14:489. doi: 10.1038/s41598-023-50957-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scott E., Munkley J. Glycans as Biomarkers in Prostate Cancer. Int. J. Mol. Sci. 2019;20:1389. doi: 10.3390/ijms20061389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munkley J., Mills I.G., Elliott D.J. The role of glycans in the development and progression of prostate cancer. Nat. Rev. Urol. 2016;13:324–333. doi: 10.1038/nrurol.2016.65. [DOI] [PubMed] [Google Scholar]
- 47.Kałuża A., Szczykutowicz J., Ferens-Sieczkowska M. Glycosylation: Rising Potential for Prostate Cancer Evaluation. Cancers. 2021;13:3726. doi: 10.3390/cancers13153726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Butler W., Huang J. Glycosylation Changes in Prostate Cancer Progression. Front. Oncol. 2021;11:809170. doi: 10.3389/fonc.2021.809170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costa A.F., Campos D., Reis C.A., Gomes C. Targeting Glycosylation: A New Road for Cancer Drug Discovery. Trends Cancer. 2020;6:757–766. doi: 10.1016/j.trecan.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 50.Smith B.A.H., Bertozzi C.R. The clinical impact of glycobiology: Targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov. 2021;20:217–243. doi: 10.1038/s41573-020-00093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orozco-Moreno M., Visser E.A., Hodgson K., Hipgrave Ederveen A.L., Bastian K., Goode E.A., Öztürk Ö., Pijnenborg J.F.A., Eerden N., Moons S.J., et al. Targeting aberrant sialylation and fucosylation in prostate cancer cells using potent metabolic inhibitors. Glycobiology. 2023;33:1155–1171. doi: 10.1093/glycob/cwad085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hodgson K., Orozco-Moreno M., Goode E.A., Fisher M., Garnham R., Beatson R., Turner H., Livermore K., Zhou Y., Wilson L., et al. Sialic acid blockade inhibits the metastatic spread of prostate cancer to bone. EBioMedicine. 2024;104:105163. doi: 10.1016/j.ebiom.2024.105163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wen R., Stark J., Marti G.E., Riley N., Zhao H., Bertozzi C.R., Pitteri S., Brooks J.D. 517 Blocking Siglec-7/9-sialic acid interactions induces immune cell-mediated suppression of prostate cancer. J. ImmunoTherapy Cancer. 2023;11((Suppl. 1)):A583. [Google Scholar]
- 54.Wen R., Marti G.E., Stark J., Marques F.J.G., Riley N., Bermudez A., Zhao H., Bertozzi C., Pitteri S., Brooks J. Abstract 7524: Targeting siglec-7/9 glyco-immune checkpoints in prostate cancer for enhanced immune responses and tumor suppression. Cancer Res. 2024;84((Suppl. 6)):7524. doi: 10.1158/1538-7445.AM2024-7524. [DOI] [Google Scholar]
- 55.Munkley J. Aberrant Sialylation in Cancer: Therapeutic Opportunities. Cancers. 2022;14:4248. doi: 10.3390/cancers14174248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dobie C., Skropeta D. Insights into the role of sialylation in cancer progression and metastasis. Br. J. Cancer. 2020;124:76–90. doi: 10.1038/s41416-020-01126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang J., Huang J., Zhang G. Insights into the Role of Sialylation in Cancer Metastasis, Immunity, and Therapeutic Opportunity. Cancers. 2022;23:5840. doi: 10.3390/cancers14235840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jastrzab P., Narejko K., Car H., Wielgat P. Cell Membrane Sialome: Sialic Acids as Therapeutic Targets and Regulators of Drug Resistance in Human Cancer Management. Cancers. 2023;15:5103. doi: 10.3390/cancers15205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu X., Zhao J., Ruan Y., Sun L., Xu C., Jiang H. Sialyltransferase ST3GAL1 promotes cell migration, invasion, and TGF-beta1-induced EMT and confers paclitaxel resistance in ovarian cancer. Cell Death Dis. 2018;9:1102. doi: 10.1038/s41419-018-1101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schultz M.J., Swindall A.F., Wright J.W., Sztul E.S., Landen C.N., Bellis S.L. ST6Gal-I sialyltransferase confers cisplatin resistance in ovarian tumor cells. J. Ovarian Res. 2013;6:25. doi: 10.1186/1757-2215-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ou L., He X., Liu N., Song Y., Li J., Gao L., Huang X., Deng Z., Wang X., Lin S. Sialylation of FGFR1 by ST6Gal-I overexpression contributes to ovarian cancer cell migration and chemoresistance. Mol. Med. Rep. 2020;21:1449–1460. doi: 10.3892/mmr.2020.10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santos S.N., Junqueira M.S., Francisco G., Vilanova M., Magalhães A., Baruffi M.D., Chammas R., Harris A.L., Reis C.A., Bernardes E.S. O-glycan sialylation alters galectin-3 subcellular localization and decreases chemotherapy sensitivity in gastric cancer. Oncotarget. 2016;7:83570–83587. doi: 10.18632/oncotarget.13192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen X., Wang L., Zhao Y., Yuan S., Wu Q., Zhu X., Niang B., Wang S., Zhang J. ST6Gal-I modulates docetaxel sensitivity in human hepatocarcinoma cells via the p38 MAPK/caspase pathway. Oncotarget. 2016;7:51955–51964. doi: 10.18632/oncotarget.10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patel K.D., De M., Jethva D.D., Rathod B.S., Patel P.S. Alterations in Sialylation Patterns are Significantly Associated with Imatinib Mesylate Resistance in Chronic Myeloid Leukemia. Arch. Med. Res. 2022;53:51–58. doi: 10.1016/j.arcmed.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Ma H., Zhou H., Song X., Shi S., Zhang J., Jia L. Modification of sialylation is associated with multidrug resistance in human acute myeloid leukemia. Oncogene. 2015;34:726–740. doi: 10.1038/onc.2014.7. [DOI] [PubMed] [Google Scholar]
- 66.Yen H.-Y., Liu Y.-C., Chen N.-Y., Tsai C.-F., Wang Y.-T., Chen Y.-J., Hsu T.-L., Yang P.-C., Wong C.-H. Effect of sialylation on EGFR phosphorylation and resistance to tyrosine kinase inhibition. Proc. Natl. Acad. Sci. USA. 2015;112:6955–6960. doi: 10.1073/pnas.1507329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Natoni A., Farrell M.L., Harris S., Falank C., Kirkham-McCarthy L., Macauley M.S., Reagan M.R., O’dwyer M. Sialyltransferase inhibition leads to inhibition of tumor cell interactions with E-selectin, VCAM1, and MADCAM1, and improves survival in a human multiple myeloma mouse model. Haematologica. 2020;105:457–467. doi: 10.3324/haematol.2018.212266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee M., Lee H.J., Bae S., Lee Y.S. Protein sialylation by sialyltransferase involves radiation resistance. Mol. Cancer Res. 2008;6:1316–1325. doi: 10.1158/1541-7786.MCR-07-2209. [DOI] [PubMed] [Google Scholar]
- 69.Lee M., Lee H.J., Seo W.D., Park K.H., Lee Y.S. Sialylation of integrin beta1 is involved in radiation-induced adhesion and migration in human colon cancer cells. Int. J. Radiat. Oncol. Biol. Phys. 2010;76:1528–1536. doi: 10.1016/j.ijrobp.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 70.Park J.J., Lee M. Increasing the alpha 2, 6 sialylation of glycoproteins may contribute to metastatic spread and therapeutic resistance in colorectal cancer. Gut Liver. 2013;7:629–641. doi: 10.5009/gnl.2013.7.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Punch P.R., Irons E.E., Manhardt C.T., Marathe H., Lau J.T.Y. The sialyltransferase ST6GAL1 protects against radiation-induced gastrointestinal damage. Glycobiology. 2020;30:446–453. doi: 10.1093/glycob/cwz108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duarte H.O., Rodrigues J.G., Gomes C., Hensbergen P.J., Ederveen A.L.H., de Ru A.H., Mereiter S., Polónia A., Fernandes E., Ferreira J.A., et al. ST6Gal1 targets the ectodomain of ErbB2 in a site-specific manner and regulates gastric cancer cell sensitivity to trastuzumab. Oncogene. 2021;40:3719–3733. doi: 10.1038/s41388-021-01801-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smithson M., Irwin R., Williams G., Alexander K.L., Smythies L.E., Nearing M., McLeod M.C., Al Diffalha S., Bellis S.L., Hardiman K.M. Sialyltransferase ST6GAL-1 mediates resistance to chemoradiation in rectal cancer. J. Biol. Chem. 2022;298:101594. doi: 10.1016/j.jbc.2022.101594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Butler W., McDowell C., Yang Q., He Y., Zhao Y., Hauck J.S., Zhou Y., Zhang H., Armstrong A.J., George D.J., et al. Rewiring of the N-Glycome with prostate cancer progression and therapy resistance. npj Precis. Oncol. 2023;7:22. doi: 10.1038/s41698-023-00363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wen R., Zhao H., Zhang D., Chiu C.-L., Brooks J.D. Sialylated glycoproteins as biomarkers and drivers of progression in prostate cancer. Carbohydr. Res. 2022;519:108598. doi: 10.1016/j.carres.2022.108598. [DOI] [PubMed] [Google Scholar]
- 76.Hartig J., Young L.E.A., Grimsley G., Mehta A.S., Ippolito J.E., Leach R.J., Angel P.M., Drake R.R. The glycosylation landscape of prostate cancer tissues and biofluids. Adv Cancer Res. 2024;161:1–30. doi: 10.1016/bs.acr.2024.04.005. [DOI] [PubMed] [Google Scholar]
- 77.Scott E., Goode E.A., Garnham R., Hodgson K., Orozco-Moreno M., Turner H., Livermore K., Nangkana K.P., Frame F.M., Bermudez A., et al. ST6GAL1-mediated aberrant sialylation promotes prostate cancer progression. J. Pathol. 2023;261:71–84. doi: 10.1002/path.6152. [DOI] [PubMed] [Google Scholar]
- 78.Chakraborty A., Dorsett K.A., Trummell H.Q., Yang E.S., Oliver P.G., Bonner J.A., Buchsbaum D.J., Bellis S.L. ST6Gal-I sialyltransferase promotes chemoresistance in pancreatic ductal adenocarcinoma by abrogating gemcitabine-mediated DNA damage. J. Biol. Chem. 2018;293:984–994. doi: 10.1074/jbc.M117.808584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smithson M., Al Diffalha S., Irwin R.K., Williams G., McLeod M.C., Somasundaram V., Bellis S.L., Hardiman K.M. ST6GAL1 is associated with poor response to chemoradiation in rectal cancer. Neoplasia. 2024;51:100984. doi: 10.1016/j.neo.2024.100984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang M., Qi T., Yang L., Kolarich D., Heisterkamp N. Multi-Faceted Effects of ST6Gal1 Expression on Precursor B-Lineage Acute Lymphoblastic Leukemia. Front. Oncol. 2022;12:828041. doi: 10.3389/fonc.2022.828041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Q., Ma H., Sun X., Liu B., Xiao Y., Pan S., Zhou H., Dong W., Jia L. The regulatory ZFAS1/miR-150/ST6GAL1 crosstalk modulates sialylation of EGFR via PI3K/Akt pathway in T-cell acute lymphoblastic leukemia. J. Exp. Clin. Cancer Res. 2019;38:199. doi: 10.1186/s13046-019-1208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heise T., Pijnenborg J.F.A., Büll C., van Hilten N., Kers-Rebel E.D., Balneger N., Elferink H., Adema G.J., Boltje T.J. Potent Metabolic Sialylation Inhibitors Based on C-5-Modified Fluorinated Sialic Acids. J. Med. Chem. 2019;62:1014–1021. doi: 10.1021/acs.jmedchem.8b01757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moons S.J., Rossing E., Janssen M.A.C.H., Heise T., Büll C., Adema G.J., Boltje T.J. Structure-Activity Relationship of Metabolic Sialic Acid Inhibitors and Labeling Reagents. ACS Chem. Biol. 2022;17:590–597. doi: 10.1021/acschembio.1c00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Munkley J., Li L., Krishnan S.R.G., Hysenaj G., Scott E., Dalgliesh C., Oo H.Z., Maia T.M., Cheung K., Ehrmann I., et al. Androgen-regulated transcription of ESRP2 drives alternative splicing patterns in prostate cancer. Elife. 2019;8:e47678. doi: 10.7554/eLife.47678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kregel S., Chen J.L., Tom W., Krishnan V., Kach J., Brechka H., Fessenden T.B., Isikbay M., Paner G.P., Szmulewitz R.Z., et al. Acquired resistance to the second-generation androgen receptor antagonist enzalutamide in castration-resistant prostate cancer. Oncotarget. 2016;7:26259–26274. doi: 10.18632/oncotarget.8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scott E., Hodgson K., Calle B., Turner H., Cheung K., Bermudez A., Marques F.J.G., Pye H., Yo E.C., Islam K., et al. Upregulation of GALNT7 in prostate cancer modifies O-glycosylation and promotes tumour growth. Oncogene. 2023;42:926–937. doi: 10.1038/s41388-023-02604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Munkley J., Vodak D., Livermore K.E., James K., Wilson B.T., Knight B., Mccullagh P., Mcgrath J., Crundwell M., Harries L.W., et al. Glycosylation is an Androgen-Regulated Process Essential for Prostate Cancer Cell Viability. EBioMedicine. 2016;8:103–116. doi: 10.1016/j.ebiom.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wei A., Fan B., Zhao Y., Zhang H., Wang L., Yu X., Yuan Q., Yang D., Wang S. ST6Gal-I overexpression facilitates prostate cancer progression via the PI3K/Akt/GSK-3beta/beta-catenin signaling pathway. Oncotarget. 2016;7:65374–65388. doi: 10.18632/oncotarget.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garnham R., Scott E., Livermore K.E., Munkley J. ST6GAL1: A key player in cancer. Oncol. Lett. 2019;18:983–989. doi: 10.3892/ol.2019.10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gc S., Bellis S.L., Hjelmeland A.B. ST6Gal1: Oncogenic signaling pathways and targets. Front. Mol. Biosci. 2022;9:962908. doi: 10.3389/fmolb.2022.962908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bojar D., Meche L., Meng G., Eng W., Smith D.F., Cummings R.D., Mahal L.K. A Useful Guide to Lectin Binding: Machine-Learning Directed Annotation of 57 Unique Lectin Specificities. ACS Chem. Biol. 2022;17:2993–3012. doi: 10.1021/acschembio.1c00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Korenchuk S., Lehr J.E., McLean L., Lee Y.G., Whitney S., Vessella R., Lin D.L., Pienta K.J. VCaP, a cell-based model system of human prostate cancer. In Vivo. 2001;15:163–168. [PubMed] [Google Scholar]
- 93.Horoszewicz J.S., Leong S.S., Kawinski E., Karr J.P., Rosenthal H., Chu T.M., Mirand E.A., Murphy G.P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 94.Davis I.D., Martin A.J., Stockler M.R., Begbie S., Chi K.N., Chowdhury S., Coskinas X., Frydenberg M., Hague W.E., Horvath L.G., et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019;381:121–131. doi: 10.1056/NEJMoa1903835. [DOI] [PubMed] [Google Scholar]
- 95.Culig Z. Molecular Mechanisms of Enzalutamide Resistance in Prostate Cancer. Curr. Mol. Biol. Rep. 2017;3:230–235. doi: 10.1007/s40610-017-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Prekovic S., van den Broeck T., Linder S., van Royen M.E., Houtsmuller A.B., Handle F., Joniau S., Zwart W., Claessens F. Molecular underpinnings of enzalutamide resistance. Endocr. Relat. Cancer. 2018;25:R545–R557. doi: 10.1530/ERC-17-0136. [DOI] [PubMed] [Google Scholar]
- 97.Dorsett K.A., Marciel M.P., Hwang J., E Ankenbauer K., Bhalerao N., Bellis S.L. Regulation of ST6GAL1 sialyltransferase expression in cancer cells. Glycobiology. 2021;31:530–539. doi: 10.1093/glycob/cwaa110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gc S., Tuy K., Rickenbacker L., Jones R., Chakraborty A., Miller C.R., Beierle E.A., Hanumanthu V.S., Tran A.N., Mobley J.A., et al. α2,6 Sialylation mediated by ST6GAL1 promotes glioblastoma growth. J. Clin. Investig. 2022;7:158799. doi: 10.1172/jci.insight.158799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pearce O.M., Laubli H. Sialic acids in cancer biology and immunity. Glycobiology. 2016;26:111–128. doi: 10.1093/glycob/cwv097. [DOI] [PubMed] [Google Scholar]
- 100.Britain C.M., Holdbrooks A.T., Anderson J.C., Willey C.D., Bellis S.L. Sialylation of EGFR by the ST6Gal-I sialyltransferase promotes EGFR activation and resistance to gefitinib-mediated cell death. J. Ovarian Res. 2018;11:12. doi: 10.1186/s13048-018-0385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cerasuolo M., Maccarinelli F., Coltrini D., Mahmoud A.M., Marolda V., Ghedini G.C., Rezzola S., Giacomini A., Triggiani L., Kostrzewa M., et al. Modeling Acquired Resistance to the Second-Generation Androgen Receptor Antagonist Enzalutamide in the TRAMP Model of Prostate Cancer. Cancer Res. 2020;80:1564–1577. doi: 10.1158/0008-5472.CAN-18-3637. [DOI] [PubMed] [Google Scholar]
- 102.Al Saoud R., Hamrouni A., Idris A., Mousa W.K., Izneid T.A. Recent advances in the development of sialyltransferase inhibitors to control cancer metastasis: A comprehensive review. Biomed Pharmacother. 2023;165:115091. doi: 10.1016/j.biopha.2023.115091. [DOI] [PubMed] [Google Scholar]
- 103.Büll C., Brok M.H.D., Adema G.J. Sweet escape: Sialic acids in tumor immune evasion. Biochim. Biophys. Acta (BBA)-Rev. Cancer. 2014;1846:238–246. doi: 10.1016/j.bbcan.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 104.Zhou X., Chi K., Zhang C., Liu Q., Yang G. Sialylation: A Cloak for Tumors to Trick the Immune System in the Microenvironment. Biology. 2023;12:832. doi: 10.3390/biology12060832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Macauley M.S., Crocker P.R., Paulson J.C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014;14:653–666. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lim J., Sari-Ak D., Bagga T. Siglecs as Therapeutic Targets in Cancer. Biology. 2021;10:1178. doi: 10.3390/biology10111178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Long X., Hou H., Wang X., Liu S., Diao T., Lai S., Hu M., Zhang S., Liu M., Zhang H. Immune signature driven by ADT-induced immune microenvironment remodeling in prostate cancer is correlated with recurrence-free survival and immune infiltration. Cell Death Dis. 2020;11:779. doi: 10.1038/s41419-020-02973-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gamat-Huber M., McNeel D.G. Androgen deprivation and immunotherapy for the treatment of prostate cancer. Endocr.-Relat. Cancer. 2017;24:T297–T310. doi: 10.1530/ERC-17-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garnham R., Geh D., Nelson R., Ramon-Gil E., Wilson L., Schmidt E.N., Walker L., Adamson B., Buskin A., Hepburn A.C., et al. ST3 beta-galactoside alpha-2,3-sialyltransferase 1 (ST3Gal1) synthesis of Siglec ligands mediates anti-tumour immunity in prostate cancer. Commun Biol. 2024;7:276. doi: 10.1038/s42003-024-05924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Berois N., Pittini A., Osinaga E. Targeting Tumor Glycans for Cancer Therapy: Successes, Limitations, and Perspectives. Cancers. 2022;14:645. doi: 10.3390/cancers14030645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials.