Abstract
Background: Rheumatoid arthritis-associated interstitial lung disease (RA-ILD) is an important extra-articular manifestation of rheumatoid arthritis (RA). Identifying patients at risk of progression and death is crucial for improving RA-ILD management and outcomes. This paper explores current evidence on prognostic factors in RA-ILD. Methods: We conducted a systematic literature review to examine the impact of clinical, radiological, and histological factors on lung function decline and the survival of RA-ILD patients. We searched electronic databases, including Medline and EMBASE, from inception to date. The incidence and prognosis of predictors were qualitatively analyzed, and univariate results were combined when feasible. Following the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)” guidelines, our systematic literature review involved a five-step algorithm. Out of 2217 records, 48 studies were eligible. These studies reported various prognostic factors, including demographic variables, clinical risk factors, serum markers, and preexisting treatments. Results: Lung function declined over time in 1225 subjects, with significant variability in smoking history and radiological/pathological UIP patterns. Severe lung fibrosis and abnormal pulmonary function tests (PFTs) were key univariate prognostic indicators, while age at initial presentation, RA disease activity, predicted DLCO percentage, and UIP pattern were the most reliable multivariate risk factors for ILD progression. Age, male gender, disease duration, RA activity, acute phase reactants, and specific serum biomarkers (Krebs vin den Lungen 6, surfactant protein D, and interleukin 6) were significantly associated with all-cause mortality. Conclusions: RA-ILD is a severe complication of RA characterized by significant prognostic variability. Key prognostic factors include extensive fibrosis observed on imaging, a marked decline in lung function, high RA disease activity, and specific biomarkers. These factors can guide treatment strategies and improve patient outcomes.
Keywords: rheumatoid arthritis, interstitial lung disease, progression
1. Introduction
Rheumatoid arthritis (RA) affects up to 1% of the global population, primarily women, with a female/male ratio of 3:1 to 4:1 [1]. Rheumatoid arthritis-associated interstitial lung disease (RA-ILD) is a severe and potentially life-threatening manifestation of rheumatoid arthritis (RA), ranking as the second leading cause of death in RA patients after cardiovascular disease [2,3,4,5]. The prevalence of RA-ILD among RA patients ranges from 3.6 to 58%, reflecting variability due to differences in detection methods and thresholds used in various studies [6,7]. The clinical course of RA-ILD is highly variable and generally associated with a worse prognosis compared to RA without lung involvement [8,9]. Notably, patients with the usual interstitial pneumonia (UIP) subtype of RA-ILD have survival rates comparable to those with idiopathic pulmonary fibrosis (IPF) [3].
Recent studies highlight the need for more research to confidently characterize the prognosis and mortality associated with RA-ILD. For instance, a comprehensive review by the European Respiratory Society discussed the epidemiology, risk factors, and diagnostic approaches for RA-ILD, emphasizing the complexity of managing this condition [3]. Additionally, Yang et al. explored new trends and potential hotspots in the research of RA-ILD and provided an in-depth understanding of the development of RA-ILD publications throughout the previous two decades [10]. Despite the growing body of literature, the natural history of RA-ILD remains insufficiently characterized, necessitating further research to better understand prognosis and mortality.
Identifying patients at risk of near-term progression and death is crucial for informed treatment decisions [5,6]. The predictors of disease progression and survival include physiological, radiological, and histopathological features, along with demographic factors and RA severity indicators such as baseline pain, disease activity, and health-assessment scores [3,9,11,12,13,14,15,16,17,18,19,20,21]. Although multifaceted scoring systems exist to estimate prognosis, they are often cumbersome and focused mainly on idiopathic pulmonary fibrosis (IPF), limiting their clinical use [15,22,23]. Relying on the most reliable prognostic indicators—abnormal pulmonary function tests (PFTs), fibrotic extent on imaging, and symptom worsening—often delay treatment initiation until overt fibrotic progression occurs [24]. Additionally, recent evidence suggest that ultrasound, a low-cost point-of-care tool with a high negative predictive value, is becoming valuable for monitoring RA patients [25]. It may also help discriminate ILD severity between males and females, alongside rheumatoid factors, though these findings are still preliminary [26]. Therefore, to tailor treatment with a personalized approach, we need to understand which groups of RA-ILD patients will benefit most from immunosuppression, antifibrotics, or a combination of both as the initial therapeutic strategy [27]. This paper reviews clinical, radiological, and histological data on RA-ILD prognosis and mortality to enhance treatment predictions and optimize care strategies.
2. Materials and Methods
This review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA: we did not register for this research) [28]. The objective of the systematic literature review (SLR) was to evaluate the current evidence on the risk factors linked to disease progression and mortality in RA-ILD patients, focusing on previously identified poor outcomes. Specifically, the review aimed to consider any clinical information—such as demographic features, symptoms, PFTs, radiological findings, laboratory tests, and prior treatments—that could influence these outcomes.
2.1. Eligibility
The included studies were randomized controlled trials, cohort studies, case/control studies of adults with RA-ILD, and conference abstracts with relevant outcomes. Exclusion were editorial letters, studies without a clear definition of RA-ILD, non-English language studies, and duplicates.
The participants were adults over 18, meeting either the 1987 American College of Rheumatology classification criteria [29] or 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for RA [30] with no restrictions on gender, ethnicity, or disease duration/severity. ILD was characterized by interstitial inflammatory and fibrotic changes in pulmonary parenchyma, diagnosed through symptomatic, functional, radiological, and/or pathological findings [31]. We focused on the most common high-resolution computed tomography (HRCT) pattern of RA-ILD, classified according to the standard criteria of the American Thoracic Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias [32], as follows: (1) UIP; (2) nonspecific interstitial pneumonia (NSIP); and (3) other (bronchiolitis, obliterans organizing pneumonia, lymphocytic interstitial pneumonitis, and mixed patterns). Progressive fibrosing ILD was defined according to the INBUILD criteria [33] and survival was assessed using data from Kaplan/Meier estimates, Cox Proportional Hazard models, Hazard Ratios (HRs), and Log-Rank Tests where applicable.
2.2. Search Strategy
Electronic databases, i.e., PubMed Medline and EMBASE, were searched using subject headings and text words related to the study population such as ‘interstitial lung disease’, ‘rheumatoid arthritis’, ‘prognosis’, ‘outcome’, and ‘mortality’. Methodology filters were not used to avoid limiting the sensitivity of the search. The Science Citation Index Expanded was also consulted using terms adapted from the previous search of Medline and EMBASE. Although no time limit was set, nearly all the articles were published from 1990 onwards. The references lists of the included studies and relevant review papers, and www.clinicaltrials.gov were also searched. Subsequently, a secondary manual search was carried out on the basis of the bibliographies of the initially selected articles. To address the risk of missing results, we evaluated study quality and examined the impact of incomplete data, although some biases were inevitable. To assess the included studies’ methodological quality, we used the Jadad quality scale for clinical trials [34] and the Oxford levels of evidence for observational studies [35]. The quality was then classified according to an asterisk system (* = low quality, ** = intermediate quality, and *** = high quality). The results were synthesized by the prognostic factor.
2.3. Data Extraction
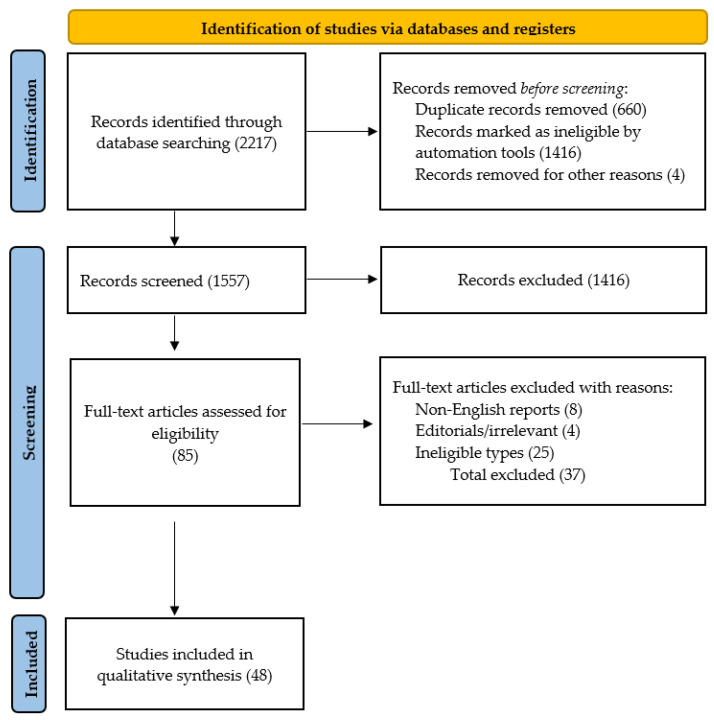
Two reviewers (LG and CN) independently extracted relevant papers using a data extraction form that was modified from a previously published protocol paper for a systematic literature review (SLR) [22]. Any uncertainty or disagreement between the reviewers arising from these processes was resolved by discussion. The following data were extracted from each eligible study: the first author’s name, year of publication, study design, sample size and its demographic features, outcomes of interest, risk and prognostic factors, methods of statistical analysis, and summary statistics (Figure 1).
Figure 1.
PRISMA flowchart: study selection process for RA-ILD prognosis and mortality review.
3. Results
3.1. Selection of Studies
Out of a total of 2217 records identified through a search of the two electronic databases, 85 records were retrieved as full-text after excluding 660 duplicates, 8 non-English reports, 1416 reports ineligible types, and 4 irrelevant articles, and finally, 48 studies were eligible for this review.
3.2. Demographic Features of Eligible Studies
Fifteen studies reported ILD progression, while the rest provided overall mortality data. The full results are available in Table A1. Among the selected studies, seven included subgroup analysis based on baseline disease activity [12,36,37,38], thirteen examined serological markers (rheumatoid factor-RF, anticitrullinated antibodies-ACPA, Kerbs von der Lungen-KL-6, interleukin-6-IL-6, and surfactant protein-D-SP-D) [9,38,39,40,41,42,43,44,45,46,47,48], eleven analyzed trajectories of pulmonary function tests [44,46,47,49,50,51,52,53,54,55,56], twenty-one studied the radiological pattern of ILD [9,10,16,39,43,44,48,50,52,53,57,58,59,60,61,62,63,64,65,66,67], and nine mentioned immunomodulatory treatments before ILD diagnosis [9,16,40,51,54,58,68,69,70]. A total of 1225 subjects with RA-ILD showed worsening lung function over time. Smoking history, reported in 37 studies, ranged from 5 to 85%, while the radiological and/or pathological UIP pattern, reported in 22 studies, ranged from 1.5 to 73%. The frequency of acute RA-ILD exacerbations ranged from 5.2% to 26.4% in three studies [58,71,72]. Subgroup analysis results should be interpreted with caution, as they were not the primary study focus, and many lacked p-values, making it difficult to draw firm conclusions about the observed effect.
3.3. Risk Factors of Lung Function Decline
Thirteen factors, including demographic variables, clinical risk factors (such as arthritis onset, clinical disease activity index—CDAI, and disease activity score—DAS28 scores), serum proteins (ACPA, KL-6, matrixmetaloproteinase 13-MMP13, and C-X-C motif chemokine 11-CSCL11/I-TAC), and preexisting treatments (corticosteroid and disease-modifying antirheumatic drugs—DMARDSs), were associated with RA-ILD progression. These factors were identified through univariate analyses (Table A2). Multivariate analyses most frequently identified age at initial presentation, RA disease activity, the percentage of the predicted diffuse capacity of the lungs for carbon monoxide—DLCO, and UIP pattern as significant risk factors for ILD prediction (Table 1).
Table 1.
Risk factors for ILD progression by multivariate analysis.
| Potential Risk Factors | Study | Effect Estimate |
|---|---|---|
| Age at ILD diagnosis ** | 44, 70 | HR 2.18; OR 1.7 |
| Male gender ** | 70 | OR 2.2 |
| Smoking history *** | 37, 70 | OR 1.7–6.13 |
| DAS28 *** | 37 | OR 1.71 |
| Arthritis onset ** | 44 | HR 1.87 |
| KL-6 ** | 74 | HR 3.37 |
| DLCO ** | 53 | OR 3.02 |
| UIP pattern ** | 53 | OR 3.47 |
| Combined pulmonary fibrosis and emphysema ** | 59 | OR 6.12 |
| Preexisting rheumatic airway disease ** | 95 | OR 7.40 |
DAS28: disease activity score 28; KL-6: Krebs von der Lungen; DLCO: diffusion capacity of the lung for carbon monoxide; UIP-usual interstitial pneumonia; **: moderate-quality evidence; ***: good-quality evidence.
3.4. Prognostic Factors for All-Cause Mortality of RA-ILD
Clinical data on all-cause mortality in RA-ILD were more prevalent than those on ILD progression, most of them reporting high mortality rates. Univariate analysis identified about 32 risk factors (Table A3), mainly related to RA disease activity (DAS28, CDAI, multidimensional health assessment questionnaire—MDHAQ, pain visual analoque scale-pain VAS, physician global assessment—PGA, and patient global assessment—PtGA), and HRCT pattern. In the multivariate analysis (Table 2), almost half of the prognostic factors remained significant. Age at initial presentation, male gender, disease duration, RA disease activity, elevated acute phase reactants, RF, PFTs, and a UIP pattern were significantly associated with all-cause mortality. The additional independent predictors of mortality were final oxygen saturation in the 6 min walking tests (6MWTs) [(HR 0.62, (95% CI 0.39–0.99)] [54], long-term exposure to particulate matter with an aerodynamic diameter of ≤10 µm (PM10) [HR 1.67 (95% CI 1.10–2.52)] [73], radiomics [HR 9.35 (95% 1.56–55.86)] [62], serum lactate dehydrogenase-LDH [HR 1.05 (95% CI 1.00–1.01)] [9], and specific serum biomarkers such as KL-6 [HR 3.23 (95% 1.39–7.51)], SP-D [HR 1.00 (95% CI 1.000–1.006)] [8,74], and IL-6 [HR 1.04 (95% 1.002–1.080)] [74].
Table 2.
Prognostic factors for mortality in RA-ILD by multivariate analysis.
| Prognostic Factor | References | Effect Estimate |
|---|---|---|
| Age ** | [10,38,43,44,48,50,52,54,65,67] | HR 1.04–5.02 |
| Male gender ** | [70] | OR 2.5–18.13 |
| Female gender ** | [53] | HR 6.8 |
| Smoking history * | [67] | HR 1.06–3.89 |
| Disease duration of RA ** | [50] | HR 1.3 |
| ESR ** | [44] | HR 5.35 |
| HAQ disability ** | [70] | OR 2.3 |
| Steinbrocker class 3 or 4 ** | [67] | HR 2.1 |
| FVC% pred * | [44] | HR 2.52 |
| DLCO *** | [49,54] | HR 0.85–0.97 |
| TLCO ** | [55] | HR 0.98 |
| Final oxygen saturation in the 6MWT ** | [54] | HR 0.62 |
| UIP pattern ** | [43,60,65,66,67] | HR 2.3–10.3 |
| Non-UIP pattern ** | [67] | HR 4.9 |
| ILD extent *** | [52,53] | HR 2.40–9.01 |
| Radiological honeycombing ** | [48] | HR 3.69 |
| Combined pulmonary fibrosis and emphysema ** | [57] | HR 2.16 |
| Pleural effusion ** | [66] | HR 14.4 |
| Corticosteroid * | [70] | HR 2.5 |
| Immunosuppressive agents ** | [70] | HR 3.0 |
| Withdrawal of MTX or LFN after ILD diagnosis ** | [54] | HR 2.18 |
| Diagnostic delay of ILD ** | [54] | HR 1.11 |
| PM10 * | [73] | HR 1.67 |
| History of acute ILD exacerbations *** | [48,75] | HR 2.42–6.48 |
RA—rheumatoid arthritis; ESR—erythrocyte sedimentation rate; HAQ—health assessment questionnaire; FVC—forced vital capacity; TLCO—transfer factor for carbon monoxide; DLCO—diffusion capacity of the lung for carbon monoxide; 6MWT—6 min walking test; UIP—usual interstitial pneumonia; ILD—interstitial lung disease; MTX—methotrexate; LFL—leflunomide; PM10—particulate matter with an aerodynamic diameter of ≤10 µm; HR: Hazard Ratio; OR: Odds Ratio; CI: Confidence Interval; *: low-quality evidence; **: moderate-quality evidence; ***: good-quality evidence.
3.5. Additional Analysis
Subgroup analysis was not undertaken due to the small number of the included studies. Sensitivity analysis could not be conducted because no studies were deemed as low risk of bias. Small study bias such as publication bias could not be assessed because the designated minimum number of studies was not available for any meta-analysis in this review.
4. Discussion
The current SLR underscores that ILD significantly impacts prognosis, contributing to increased morbidity and mortality in patients with RA. Clinical outcomes in RA-ILD patients showed considerable variation, with poor lung function outcomes observed in 1.5 to 85% of the cases and all-cause mortality ranging from 1.3 to 75%.
The multivariate analysis identified several key factors for ILD progression: age at RA diagnosis, male gender, smoking, high RF, serum KL-6, DAS28 score, lower baseline DLCO, and the presence of a UIP pattern on high-resolution computed tomography (HRCT). While RA-ILD often accompanies high RF and ACPA levels, only one study has demonstrated an association between ACPA and long-term function decline [39]. DLCO has been frequently associated with the acute exacerbations of lung disease and poorer outcomes compared to forced vital capacity—FVC. Several studies have indicated that DLCO serves as an early indicator of worsening lung function in RA-ILD patients [56,58,76]. However, there is notable heterogeneity among these studies, underscoring the need for further research into the relationship between PFTs and ILD progression.
Although five studies [16,40,51,57,68] linked corticosteroids and DMARDs to impaired lung function, these findings were based on small groups without comparators. Post hoc analyses suggest that certain poor prognostic factors may worsen ILD with methotrexate, leflunomide, or TNF inhibitors (TNFis). While some studies associated RA activity (DAS28) with ILD progression [36,67], a three-year study found no such link [77], hinting that immunosuppression could be beneficial, though more trials are needed. An analysis of 16 registries showed that seropositivity improves drug retention and response rates for non-TNFi biologic DMARDs, especially abatacept and rituximab, over tocilizumab [78]. Consequently, abatacept and rituximab are preferred as first-line biologics, with studies since 2018 suggesting they may stabilize or improve lung function in progressive ILD. Early research suggests JAK inhibitors could also be beneficial for RA-ILD, but further research is needed to confirm their safety and efficacy [62,79]. Additionally, Cano Jiménez et al. and Matson et al. found that discontinuing DMARDs negatively impacts survival, while immunosuppression improves lung function, even in patients with a UIP pattern on HRCT [54,80].
Equally important, numerous studies have identified significant predictors of mortality in RA-ILD patients: older age, male gender, reduced PFTs, and the presence of a UIP pattern on HRCT. Other factors linked to poorer survival include lower socioeconomic status, higher disease activity scores, and elevated ESR, although findings across studies have been variable [37,38,46,50]. The acute exacerbations of RA-ILD contribute directly to 10–20% of the mortality in RA, with a standardized mortality ratio of 2.5–5.0 compared to control populations [43]. A decline in the FVC of 10% consistently predicts death in retrospective RA-ILD cohorts and other non-IPF ILD groups, where radiologic progression of fibrosis also strongly indicates subsequent FVC decline [81]. In multivariable models, features like honeycombing [HR 2.49 (95% CI 1.09–5.69)] and combined pulmonary fibrosis and emphysema [HR 2.16 (95% 1.01–4.62)] independently predict mortality. A visual HRCT staging shows that ILD extent ≥20% increases mortality risk 3.78-fold in RA-ILD cohorts [57]. Similarly, in a Korean cohort of 153 RA-ILD patients, a visual scoring indicating ≥20% fibrosis is associated with a 4.5-fold risk of death in multivariable analysis [76]. Oh et al.’s quantitative lung fibrosis scoring on the HRCT images of 144 RA-ILD patients predicts worse 5-year mortality, with scores ≥12% of the total lung volume correlating with survival similar to IPF patients [50]. Radiomics, which quantifies computed tomography imaging features, was identified as a significant predictor for mortality [HR 9.35 (1.56–55.86)] but only in univariate analysis [62]. Regarding HRCT patterns, studies suggest that RA patients with a UIP pattern may experience poorer survival compared to those with NSIP or indeterminate patterns. Initial reports noted a 24% prevalence of UIP pattern in RA-ILD, associated with a disease trajectory similar to IPF and higher mortality rates [60]. However, in adjusted multivariable models considering baseline lung function and other confounders, the UIP pattern no longer independently correlates with increased mortality, suggesting complex relationships that warrant further investigation [44,51,82].
These findings carry significant implications for clinical practice, healthcare policy, and research. Clinicians should incorporate the identified factors into the assessment and management of RA-ILD patients, enabling more personalized treatment strategies. There is also a critical need for standardized guidelines to reduce variability in diagnosis and management. Research should prioritize the early detection of disease progression, particularly lung function decline, and focus on validating these prognostic factors.
The main limitation of this SLR is the inconsistency of the results, which limits confidence in the pooled estimates. Most studies were retrospective, with only 14 out of the 48 being prospective. Among these, six focused on the clinical course of RA-ILD, while the rest examined mortality predictors. The clinical diversity among the subjects, influenced by varying study objectives and criteria for RA-ILD diagnosis and classification, likely contributed to this inconsistency. Additionally, the inability to perform a meta-analysis due to significant heterogeneity in the study designs, patient populations, follow-up periods, and outcome definitions further limits our findings. This variability, along with the inconsistent reporting of prognostic factors, would have compromised the validity of a pooled analysis.
5. Conclusions
It is evident that ILD is a serious complication for patients with RA, and its mortality rate is significantly higher than that of patients with RA without ILD. Therefore, it is very important to know the prognostic factors of RA-ILD in advance for better treatment. This leads to a chance for early therapy and attentive follow-up, which could stop the progression of ILD and enhance the long-term result.
Appendix A
Table A1.
Characteristics of included studies.
| Study | Design | Subjects (n) | Smoking (n, %) | UIP Pattern (n, %) | Frequency of Lung Function Decline (n, %) | All-Cause Mortality (n, %) |
|---|---|---|---|---|---|---|
| Akiyama et al., 2016 [72] | Case/control retrospective (2008–2014) | 395 | 69 (20.3) | 78 (19.7) | 6 (1.5) | |
| Franzen et al., 2016 [83] | Observational retrospective (2013–2015) | 33 | 17 (51) | 6 (22) | ||
| Md Yusof et al., 2017 [84] | Observational retrospective (2004–2015) | 56 | 32 (57) | 20 (36) | 14 (25) | 9 (16) |
| Mochizuki et al., 2018 [40] | Observational retrospective, 47.8 months | 131 | ||||
| Kim et al., 2010 [60] | Observational retrospective (2001–2008) | 82 | 59 (72) | 20 (24) | 8 (9.7) | |
| Zamora-Legoff et al., 2016 [51] | Observational retrospective (1998–2014) | 167 | 105 (63) | 89 (53) | 33 (19) | |
| Dawson et al., 2015 [55] | Observational prospective, 2 years | 29 | 10 (34) | 10 (35) | 4 (14) | |
| Solomon et al., 2015 [44] | Observational retrospective (1995–2013) | 137 | 87 (64) | 108 (79) | 54 (39) | |
| Dixon et al., 2010 [37] | Observational registry-based cohort study, 3.8 years | 14.113 | 10799 (76) | 160 (1.13) | ||
| Wolfe et al., 2007 [70] | Case/control prospective, 3.6 years | 17498 | 100 (27) | |||
| Kurata et al., 2019 [85] | Observational retrospective (2008–2017) | 49 | 14 (35) | 6 (12) | ||
| Chen et al., 2020 [43] | Observational retrospective (2008–2017) | 241 | 88 (36) | 66 (27) | 39 (16) | |
| Hyldgaard et al., 2017 [86] | Case/control prospective (2004–2015) | 679 | 26 (3.8) | |||
| Koduri et al., 2010 [38] | Prospective cohort study (1986–1998) |
52 | 19 (36) | 39 (75) | ||
| Tsuchiya et al., 2011 [63] | Observational retrospective (1996–2006) | 144 | 54 (37.5) | 7 (5) | 71 (49) | |
| Song et al., 2012 [87] | Observational prospective (2002–2011) | 51 | 21 (41) | |||
| Izuka et al., 2021 [58] | Observational retrospective (2007–2019) | 165 | 73 (44) | 70 (42) | 30 (18) | 13 (8) |
| Nurmi et al., 2017 [49] | Observational retrospective (2000–2014) | 59 | 30 (51) | 31 (52) | 24 (41) | 27 (46) |
| Oh et al., 2022 [50] | Observational retrospective (1999–2015) | 144 | 63 (44) | 53 (37) | 44 (30) | |
| Hozumi et al., 2022 [56] | Observational retrospective (2007–2019) | 58 | 39 (67) | 34 (59) | 43 (74) | |
| Cano-Jiménez et al., 2021 [54] | Observational retrospective (2013–2018) | 106 | 63 (60) | 55(61) | 53 (50) | 18 (17) |
| Jacob et al., 2018 [57] | Observational retrospective (1995–2015) | 90 | 65 (72) | |||
| Kelly et al., 2014 [88] | Observational retrospective (1987–2012) | 230 | 135 (59) | 103 (65) | 90 (57) | |
| Ng et al., 2022 [69] | Observational retrospective (1997–2013) | 214 | 48 (22) | |||
| Li et al., 2019 [52] | Observational retrospective (2008–2017) | 278 | 106 (38) | 91 (33) | 83 (29) | 53 (69) |
| Juge et al., 2023 [89] | Observational retrospective (2013–2018) | 4330 | ||||
| Brooks et al., 2022 [46] | Prospective cohort study, 2 years | 227 | 192 (85) | 147 (65) | 108 (47) | |
| Rojas-Serrano et al., 2022 [90] | Observational prospective | 37 | ||||
| Kim et al., 2020 [47] | Observational retrospective (1995–2018) | 84 | 37 (44) | 34 (40) | 33 (39) | |
| Lee et al., 2016 [74] | Retrospective cohort study | 62 | 62 (100) | |||
| Avouac et al.2020 [42] | Observational prospective study | 147 | 52 (35) | 21 (14) | 7 (4.7) | |
| Ito et al., 2017 [53] | Observational retrospective (2007–2016) | 65 | 16 (24) | |||
| Font et al., 2017 [64] | Longitudinal prospective (2007–2017) | 37 | 26(72) | 24(66) | 7 (19) | |
| Nieto et al., 2021 [65] | Longitudinal prospective (2005–2018) | 47 | 25 (54) | 26 (55) | 10 (21) | 16 (34) |
| Yang et al., 2019 [10] | Longitudinal prospective (1991–2011) | 77 | 4 (5) | 32 (48) | 27 (36) | |
| Mena-Vázquez et al., 2024 [36] | Observational prospective (2015–2023) | 148 | 13 (18) | 46 (66) | 21 (30) | 1 (1.4) |
| Kim et al., 2024 [73] | Observational retrospective (1995–2018) | 313 | 139 (45) | 202 (65) | 125 (40) | |
| Chen et al., 2022 [43] | Observational prospective, 5 years | 60 | 3 (16) | 19 (49) | 4 (7) | |
| Kelly et al., 2021 [88] | Observational retrospective (1990–2015) | 290 | 174 (60) | 200 (69) | 75 (28) | |
| Venerito et al., 2022 [62] | Observational retrospective (2021–2022) | 30 | 18 (60) | 13 (43) | ||
| Yamakawa et al., 2019 [91] | Observational retrospective (2012–2017) | 96 | 45 (47) | 21 (20) | 11 (11) | 25 (26) |
| Ekici et al., 2021 [66] | Observational retrospective (2010–2018) | 156 | 67 (42) | 74 (47) | 40 (26) | |
| Kakutani et al., 2020 [67] | Observational retrospective (2009–2014) | 261/2702 | 120 (46) | 120 (46) | 19 (7) | |
| Wang et al., 2019 [39] | Observational retrospective (2016–2019) | 96 | 9 (20) | 18 (40) | 25 (56) | 4 (9) |
| Tanaka et al., 2021 [71] | Observational retrospective (2010–2019) | 125 | 59 (50) | 32 (25) | 37 (29) | |
| Kwon et al., 2022 [75] | Observational retrospective (2016–2022) | 310 | 87 (28) | 89 (29) | ||
| Kang et al., 2020 [68] | Retrospective cohort (2006–2015) | 1999 | 759 (38) | 415 (21) | ||
| Farquhar et al., 2024 [59] | Observational retrospective (2006–2008, 2011–2013) | 100 | 64 (64) | 38 (43) | 68 (73) | 26 (26) |
| Marcoux et al., 2023 [79] | Observational prospective (2015–2018) | 181 | 112 (70) | 66 (73) | 39 (24) | |
| Tyker et al., 2021 [45] | Observational retrospective (2006–2019) | 70 | 45 (64) | 47 (67) | 29 (70) |
Table A2.
Risk factors of lung function decline in RA-ILD patients by univariate analysis.
| Category | Potential Risk Factors | References | Effect Estimate |
|---|---|---|---|
| Demographic features | Age * | [6,43,58] | OR 0.55–2.91, AUC 0.74 |
| CDAI score * | [72] | OR 4.7 | |
| Laboratory findings | ACPA ** | [6] | HR 3.94 |
| KL-6 * | [40,41,42,71] | OR 1.00–72.7 | |
| MMP13 ** | [43] | AUC 0.71 | |
| CXCL11/I-TAC ** | [43] | AUC 0.67 | |
| MUC5B mutation ** | [6] | HR 2.30 | |
| Pulmonary function | FVC% pred ** | [51] | HR 3.42 |
| DLCO% pred ** | [51] | HR 1.72 | |
| Underlying radiological features | UIP pattern on HRCT * | [6,58] | OR 2.29–4.11 |
| Pre-treatment | Corticosteroid * | [51] | HR 15.0 |
| Nonbiologic DMARDs * | [40,58] | OR 1.75–.75 | |
| Biologic DMARDs ** | [16,68] | HR 0.44–2.33 |
CDAI: clinical disease activity index; ACPA: anticitrullinated protein autoantibodies; KL-6: serum Krebs von den Lungen-6; MMP13: matrix metalloproteases 13; FVC: forced vital capacity; DLCO: diffusion capacity of the lung for carbon monoxide; UIP: usual interstitial pneumonia; DMARDs: disease-modifying antirheumatic drugs; HR: Hazard Ratio; OR: Odds Ratio; AUC: Area Under Curve; rho: Correlation Coefficient; *: low-quality evidence; **: moderate-quality evidence.
Table A3.
Prognostic factors of all-cause mortality in RA-ILD patients by univariate analysis.
| Category | Potential Risk Factors | References | Effect Estimate |
|---|---|---|---|
| Demographic features | Age ** | [9,37,38,44,48,49,50,57,58,60,65,69] | HR 1.04–4.8 |
| Male gender ** | [17,65,86] | HR 2.83–14.5 | |
| Female gender ** | [17] | HR 3.6 | |
| Smoking history ** | [44,60] | HR 2.58–3.17 | |
| Low socioeconomic status * | [38] | HR 2.07 | |
| The onset of ILD before RA onset | [17] | HR 8.4 | |
| Disease activity | DAS28 score ** | [37] | HR 1.21–1.43 |
| CDAI score * | [90] | HR 1.07 | |
| MDHAQ score ** | [46] | HR 1.85 | |
| Pain VAS * | [38] | HR 1.01 | |
| Patient global assessment ** | [46] | HR 1.16 | |
| Laboratory findings | RF * | [9,44,45] | HR 1.00–2.08 |
| ESR ** | [38,46,50] | HR 1.01–1.15 | |
| CRP ** | [46] | HR 1.12 (1.06–1.18) | |
| LDH * | [9] | HR 1.05 | |
| KL-6 ** | [47,74] | HR 1.00–3.23 | |
| IL-6 ** | [74] | HR 1.04 | |
| SP-D * | [48] | HR 1.0 | |
| Pulmonary function | DLCO % pred ** | [44,46,49,50] | HR 0.97–1.77 |
| FVC % pred ** | [44,46,47,50,53,56] | HR 0.97–4.43 | |
| TLCO % pred ** | [50] | HR 0.97 | |
| PaO2/FiO2 * | [56] | HR 0.94 | |
| Underlying radiological features | ILD extent ** | [9,16,57,59] | HR 1.03–4.47 |
| UIP ** | [10,44,47,50,58,73,79] | HR 2.44–5.84 | |
| Honeycombing * | [48] | HR 2.49 | |
| Radiomics * | [62] | HR 9.35 | |
| DAD * | [63] | HR 2.88 | |
| Emphysema * | [50,57] | HR 3.43–6.84 | |
| Comorbidities | COPD ** | [69] | HR 2.12 |
| Diabetes mellitus ** | [69] | HR 1.09 | |
| Pre-treatment | Corticosteroid ** | [69] | HR 1.09 |
| Nonbiologic DMARDs * | [9,58] | HR 0.16–5.53 | |
| Biologic DMARDs ** | [49] | HR 0.44–2.33 | |
| Acute exacerbations of ILD ** | [48] | HR 1.12–3.19 |
CDAI: clinical disease activity index; MDHAQ: multidimensional health assessment questionnaire; RF: rheumatoid factor; ESR: erythrocyte sedimentation rate; CRP: C-Reactive Protein; LDH: lactate dehydrogenase; KL-6: serum Krebs von den Lungen-6; SP-D: surfactant protein-D; FVC: forced vital capacity; DLCO: diffusion capacity of the lung for carbon monoxide; UIP: usual interstitial pneumonia; DAD: diffuse alveolar damage; COPD: chronic obstructive pulmonary disease; DMARDs: disease-modifying antirheumatic drugs; HR: Hazard Ratio; OR: Odds Ratio; AUC: Area Under Curve; rho: Correlation Coefficient; *: low-quality evidence; **: moderate-quality evidence.
Author Contributions
Conceptualization, L.G. and C.N.; methodology, C.N.; software, C.N.; validation, L.G.; formal analysis, C.N.; investigation and resources, C.N.; data curation, L.G.; writing—original draft preparation, C.N.; writing—review and editing, L.G.; visualization, L.G.; supervision, L.G.; project administration, L.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were not required for this study because it was conducted retrospectively.
Informed Consent Statement
Patient consent was not required for this study because it was conducted retrospectively.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Aletaha D., Smolen J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA. 2018;320:1360–1372. doi: 10.1001/jama.2018.13103. [DOI] [PubMed] [Google Scholar]
- 2.Almutairi K., Nossent J., Preen D., Keen H., Inderjeeth C. The global prevalence of rheumatoid arthritis: A meta-analysis based on a systematic review. Rheumatol. Int. 2021;41:863–877. doi: 10.1007/s00296-020-04731-0. [DOI] [PubMed] [Google Scholar]
- 3.Kadura S., Raghu G. Rheumatoid arthritis-interstitial lung disease: Manifestations and current concepts in pathogenesis and management. Eur. Respir. Rev. 2021;30:210011. doi: 10.1183/16000617.0011-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang S., Kronzer V.L., Dellaripa P.F., Deane K.D., Bolster M.B., Nagaraja V., Khanna D., Doyle T.J., Sparks J.A. Rheumatoid Arthritis–Associated Interstitial Lung Disease: Current Update on Prevalence, Risk Factors, and Pharmacologic Treatment. Curr. Treat. Options Rheumatol. 2020;6:337–353. doi: 10.1007/s40674-020-00160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fazeli M.S., Khaychuk V., Wittstock K., Han X., Crocket G., Lin M., Ferri L. Rheumatoid Arthritis-Associated Interstitial Lung Disease: Epidemiology, Risk/Prognostic Factors, and Treatment Landscape. Clin. Exp. Rheumatol. 2021;39:1108–1118. doi: 10.55563/clinexprheumatol/h9tc57. [DOI] [PubMed] [Google Scholar]
- 6.Wang H.F., Wang Y.Y., Li Z.Y., He P.J., Liu S., Li Q.S. The prevalence and risk factors of rheumatoid arthritis-associated interstitial lung disease: A systematic review and meta-analysis. Ann. Med. 2024;56:2332406. doi: 10.1080/07853890.2024.2332406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albrecht K., Strangfeld A., Marschall U., Callhoff J. Interstitial lung disease in rheumatoid arthritis: Incidence, prevalence and related drug prescriptions between 2007 and 2020. RMD Open. 2023;9:e002777. doi: 10.1136/rmdopen-2022-002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laria A., Lurati A.M., Zizzo G., Zaccara E., Mazzocchi D., Re K.A., Marrazza M., Faggioli P., Mazzone A. Interstitial Lung Disease in Rheumatoid Arthritis: A Practical Review. Front. Med. 2022;9:837133. doi: 10.3389/fmed.2022.837133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H., Lee S.I., Kim H.O. Recent Advances in Basic and Clinical Aspects of Rheumatoid Arthritis-associated Interstitial Lung Diseases. J. Rheum. Dis. 2022;29:61–70. doi: 10.4078/jrd.2022.29.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y., Zhang Z., Zhang X., Zhang X., Zhi K., Zhao X., Zhao J., Cao W. Rheumatoid arthritis-associated interstitial lung disease hotspots and future directions: A Web-of-Science based scientometric and visualization study. Immun. Inflamm. Dis. 2023;11:e944. doi: 10.1002/iid3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer A., Du Bois R. Interstitial lung disease in connective tissue disorders. Lancet. 2012;380:689–698. doi: 10.1016/S0140-6736(12)61079-4. [DOI] [PubMed] [Google Scholar]
- 12.Kolb M., Vašáková M. The natural history of progressive fibrosing interstitial lung diseases. Respir. Res. 2019;20:57. doi: 10.1186/s12931-019-1022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duarte C., Ferreira R.J.O., Santos E.J.F., Da Silva J.A.P. Treating-to-target in rheumatology: Theory and practice. Best. Pract. Res. Clin. Rheumatol. 2022;36:101735. doi: 10.1016/j.berh.2021.101735. [DOI] [PubMed] [Google Scholar]
- 14.Challa D.N.V., Crowson C.S., Davis J.M. The Patient Global Assessment of Disease Activity in Rheumatoid Arthritis: Identification of Underlying Latent Factors. Rheumatol. Ther. 2017;4:201–208. doi: 10.1007/s40744-017-0063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.du Bois R.M., Weycker D., Albera C., Bradford W.Z., Costabel U., Kartashov A., King T.E., Jr. Ascertainment of Individual Risk of Mortality for Patients with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2011;184:459–466. doi: 10.1164/rccm.201011-1790OC. [DOI] [PubMed] [Google Scholar]
- 16.Kelly C.A., Saravanan V., Nisar M., Arthanari S., Woodhead F.A., Price-Forbes A.N., Dawson J., Sathi N., Ahmad Y., Koduri G., et al. Rheumatoid arthritis-related interstitial lung disease: Associations, prognostic factors and physiological and radiological characteristics—A large multicentre UK study. Rheumatology. 2014;53:1676–1682. doi: 10.1093/rheumatology/keu165. [DOI] [PubMed] [Google Scholar]
- 17.Juge P.A., Crestani B., Dieudé P. Recent advances in rheumatoid arthritis-associated interstitial lung disease. Curr. Opin. Pulm. Med. 2020;26:477–486. doi: 10.1097/MCP.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 18.Akiyama M., Kaneko Y. Pathogenesis, clinical features, and treatment strategy for rheumatoid arthritis-associated interstitial lung disease. Autoimmun. Rev. 2022;21:103056. doi: 10.1016/j.autrev.2022.103056. [DOI] [PubMed] [Google Scholar]
- 19.Dai Y., Wang W., Yu Y., Hu S. Rheumatoid arthritis–associated interstitial lung disease: An overview of epidemiology, pathogenesis and management. Clin. Rheumatol. 2021;40:1211–1220. doi: 10.1007/s10067-020-05320-z. [DOI] [PubMed] [Google Scholar]
- 20.Al-Baldawi S., Zúñiga Salazar G., Zúñiga D., Balasubramanian S., Mehmood K.T. Interstitial Lung Disease in Rheumatoid Arthritis: A Review. 5 February 2024. [(accessed on 8 August 2024)]. Available online: https://www.cureus.com/articles/220746-interstitial-lung-disease-in-rheumatoid-arthritis-a-review. [DOI] [PMC free article] [PubMed]
- 21.Xie M., Zhu C., Ye Y. Incidence, risk factors, and prognosis of acute exacerbation of rheumatoid arthritis-associated interstitial lung disease: A systematic review and meta-analysis. BMC Pulm. Med. 2023;23:255. doi: 10.1186/s12890-023-02532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ley B., Ryerson C.J., Vittinghoff E., Ryu J.H., Tomassetti S., Lee J.S., Poletti V., Buccioli M., Elicker B.M., Jones K.D., et al. A Multidimensional Index and Staging System for Idiopathic Pulmonary Fibrosis. Ann. Intern. Med. 2012;156:684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 23.King T.E., Jr., Tooze J.A., Schwarz M.I., Brown K.R., Cherniack R.M. Predicting Survival in Idiopathic Pulmonary Fibrosis: Scoring System and Survival Model. Am. J. Respir. Crit. Care Med. 2001;164:1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 24.Bendstrup E., Møller J., Kronborg-White S., Prior T.S., Hyldgaard C. Interstitial Lung Disease in Rheumatoid Arthritis Remains a Challenge for Clinicians. J. Clin. Med. 2019;8:2038. doi: 10.3390/jcm8122038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otaola M., Paulin F., Rosemffet M., Balcazar J., Perandones M., Orausclio P., Cazenave T., Rossi S., Marciano S., Schneeberger E., et al. Lung ultrasound is a promising screening tool to rule out interstitial lung disease in patients with rheumatoid arthritis. Respirology. 2024;29:588–595. doi: 10.1111/resp.14679. [DOI] [PubMed] [Google Scholar]
- 26.Bandinelli F., Benucci M., Mallia I., Mauro I., Pecani N., Li Gobbi F., Giannasi G. Do Ultrasound Lung Abnormalities Correlate to Biomarkers and Male Gender in Rheumatoid Arthritis Patients? A Monocentric Cross-Sectional Study. J. Clin. Med. 2024;13:3534. doi: 10.3390/jcm13123534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matteson E.L., Kelly C., Distler J.H.W., Hoffmann-Vold A.M., Seibold J.R., Mittoo S., Dellaripa P.F., Aringer M., Pope J., Distler O., et al. Nintedanib in Patients with Autoimmune Disease–Related Progressive Fibrosing Interstitial Lung Diseases: Subgroup Analysis of the INBUILD Trial. Arthritis Rheumatol. 2022;74:1039–1047. doi: 10.1002/art.42075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnett F.C., Edworthy S.M., Bloch D.A., Mcshane D.J., Fries J.F., Cooper N.S., Healey L.A., Kaplan S.R., Liang M.H., Luthra H.S., et al. The american rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 30.Aletaha D., Neogi T., Silman A.J., Funovits J., Felson D.T., Bingham C.O., 3rd, Birnbaum N.S., Burmester G.R., Bykerk V.P., Cohen M.D., et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 31.American Thoracic Society. European Respiratory Society American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This Joint Statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) Was Adopted by the ATS Board of Directors, June 2001 and by the ERS Executive Committee, June 2001. Am. J. Respir. Crit. Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 32.Travis W.D., Costabel U., Hansell D.M., King T.E., Jr., Lynch D.A., Nicholson A.G., Ryerson C.J., Ryu J.H., Selman M., Wells A.U., et al. An Official American Thoracic Society/European Respiratory Society Statement: Update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. Am. J. Respir. Crit. Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flaherty K.R., Wells A.U., Cottin V., Devaraj A., Walsh S.L., Inoue Y., Brown K.K. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019;381:1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 34.Clark H.D., Wells G.A., Huët C., McAlister F.A., Salmi L., Fergusson D., Laupacis A. Assessing the Quality of Randomized Trials. Control Clin. Trials. 1999;20:448–452. doi: 10.1016/S0197-2456(99)00026-4. [DOI] [PubMed] [Google Scholar]
- 35.Explanation of the 2011 OCEBM Levels of Evidence—Centre for Evidence-Based Medicine (CEBM), University of Oxford. [(accessed on 10 August 2024)]. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/explanation-of-the-2011-ocebm-levels-of-evidence.
- 36.Mena-Vázquez N., Redondo-Rodriguez R., Rojas-Gimenez M., Romero-Barco C.M., Fuego-Varela C., Perez-Gómez N., Añón-Oñate I., Castro Pérez P., García-Studer A., Hidalgo-Conde A., et al. Rate of severe and fatal infections in a cohort of patients with interstitial lung disease associated with rheumatoid arthritis: A multicenter prospective study. Front. Immunol. 2024;15:1341321. doi: 10.3389/fimmu.2024.1341321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dixon W.G., Hyrich K.L., Watson K.D., Lunt M., BSRBR Control Centre Consortium. Symmons DPM Influence of anti-TNF therapy on mortality in patients with rheumatoid arthritis-associated interstitial lung disease: Results from the British Society for Rheumatology Biologics Register. Ann. Rheum. Dis. 2010;69:1086–1091. doi: 10.1136/ard.2009.120626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koduri G., Norton S., Young A., Cox N., Davies P., Devlin J., Dixey J., Gough A., Prouse P., Winfield J., et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: Results from an inception cohort. Rheumatology. 2010;49:1483–1489. doi: 10.1093/rheumatology/keq035. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y., Chen S., Zheng S., Lin J., Hu S., Zhuang J., Lin Q., Xie X., Zheng K., Zhang W., et al. The role of lung ultrasound B-lines and serum KL-6 in the screening and follow-up of rheumatoid arthritis patients for an identification of interstitial lung disease: Review of the literature, proposal for a preliminary algorithm, and clinical application to cases. Arthritis Res. Ther. 2021;23:212. doi: 10.1186/s13075-021-02586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mochizuki T., Ikari K., Yano K., Sato M., Okazaki K. Long-term deterioration of interstitial lung disease in patients with rheumatoid arthritis treated with abatacept. Mod. Rheumatol. 2019;29:413–417. doi: 10.1080/14397595.2018.1481566. [DOI] [PubMed] [Google Scholar]
- 41.Fotoh D.S., Helal A., Rizk M.S., Esaily H.A. Serum Krebs von den Lungen-6 and lung ultrasound B lines as potential diagnostic and prognostic factors for rheumatoid arthritis–associated interstitial lung disease. Clin. Rheumatol. 2021;40:2689–2697. doi: 10.1007/s10067-021-05585-y. [DOI] [PubMed] [Google Scholar]
- 42.Avouac J., Cauvet A., Steelandt A., Shirai Y., Elhai M., Kuwana M., Distler O., Allanore Y. Improving risk-stratification of rheumatoid arthritis patients for interstitial lung disease. PLoS ONE. 2020;15:e0232978. doi: 10.1371/journal.pone.0232978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J., Chen Y., Liu D., Lin Y., Zhu L., Song S., Hu Y., Liang T., Liu Y., Liu W., et al. Predictors of long-term prognosis in rheumatoid arthritis-related interstitial lung disease. Sci. Rep. 2022;12:9469. doi: 10.1038/s41598-022-13474-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solomon J.J., Chung J.H., Cosgrove G.P., Demoruelle M.K., Fernandez-Perez E.R., Fischer A., Frankel S.K., Hobbs S.B., Huie T.J., Ketzer J., et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur. Respir. J. 2016;47:588–596. doi: 10.1183/13993003.00357-2015. [DOI] [PubMed] [Google Scholar]
- 45.Tyker A., Ventura I.B., Lee C.T., Strykowski R., Garcia N., Guzy R., Jablonski R., Vij R., Strek M.E., Chung J.H., et al. High-titer rheumatoid factor seropositivity predicts mediastinal lymphadenopathy and mortality in rheumatoid arthritis-related interstitial lung disease. Sci. Rep. 2021;11:22821. doi: 10.1038/s41598-021-02066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brooks R., Baker J.F., Yang Y., Roul P., Kerr G.S., Reimold A.M., Kunkel G., Wysham K.D., Singh N., Lazaro D., et al. The impact of disease severity measures on survival in U.S. veterans with rheumatoid arthritis-associated interstitial lung disease. Rheumatology. 2022;61:4667–4677. doi: 10.1093/rheumatology/keac208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim H.C., Choi K.H., Jacob J., Song J.W. Prognostic role of blood KL-6 in rheumatoid arthritis–associated interstitial lung disease. PLoS ONE. 2020;15:e0229997. doi: 10.1371/journal.pone.0229997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamakawa H., Sato S., Tsumiyama E., Nishizawa T., Kawabe R., Oba T., Kamikawa T., Horikoshi M., Akasaka K., Amano M., et al. Predictive factors of mortality in rheumatoid arthritis-associated interstitial lung disease analysed by modified HRCT classification of idiopathic pulmonary fibrosis according to the 2018 ATS/ERS/JRS/ALAT criteria. J. Thorac. Dis. 2019;11:5247–5257. doi: 10.21037/jtd.2019.11.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nurmi H.M., Purokivi M.K., Kärkkäinen M.S., Kettunen H.P., Selander T.A., Kaarteenaho R.L. Are risk predicting models useful for estimating survival of patients with rheumatoid arthritis-associated interstitial lung disease? BMC Pulm. Med. 2017;17:16. doi: 10.1186/s12890-016-0358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oh J.H., Kim G.H.J., Cross G., Barnett J., Jacob J., Hong S., Song J.W. Automated quantification system predicts survival in rheumatoid arthritis-associated interstitial lung disease. Rheumatology. 2022;61:4702–4710. doi: 10.1093/rheumatology/keac184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zamora-Legoff J.A., Krause M.L., Crowson C.S., Ryu J.H., Matteson E.L. Progressive Decline of Lung Function in Rheumatoid Arthritis–Associated Interstitial Lung Disease. Arthritis Rheumatol. 2017;69:542–549. doi: 10.1002/art.39971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li L., Liu R., Zhang Y., Zhou J., Li Y., Xu Y., Gao S., Zheng Y. A retrospective study on the predictive implications of clinical characteristics and therapeutic management in patients with rheumatoid arthritis-associated interstitial lung disease. Clin. Rheumatol. 2020;39:1457–1470. doi: 10.1007/s10067-019-04846-1. [DOI] [PubMed] [Google Scholar]
- 53.Ito Y., Arita M., Kumagai S., Takei R., Noyama M., Tokioka F., Nishimura K., Koyama T., Ishida T. Diffuse Parenchymal Lung Disease. European Respiratory Society; Lausanne, Switzerland: 2017. [(accessed on 13 July 2024)]. Male Gender and Fibrosis Score on High-Resolution Computed Tomography are Independent Poor Prognostic Factors of Survival in Patients with Rheumatoid Arthritis-Related Interstitial Lung Disease; p. PA887. Available online: http://erj.ersjournals.com/lookup/doi/10.1183/1393003.congress-2017.PA887. [Google Scholar]
- 54.Cano-Jiménez E., Rodríguez T.V., Martín-Robles I., Villegas D.C., García J.J., de Miguel E.B., Robles-Pérez A., Galván M.F., Roibas C.M., Lara S.H., et al. Diagnostic delay of associated interstitial lung disease increases mortality in rheumatoid arthritis. Sci. Rep. 2021;11:9184. doi: 10.1038/s41598-021-88734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dawson J.K. Predictors of progression of HRCT diagnosed fibrosing alveolitis in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2002;61:517–521. doi: 10.1136/ard.61.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hozumi H., Kono M., Hasegawa H., Kato S., Inoue Y., Suzuki Y., Karayama M., Furuhashi K., Enomoto N., Fujisawa T., et al. Acute Exacerbation of Rheumatoid Arthritis-Associated Interstitial Lung Disease: Mortality and Its Prediction Model. Respir. Res. 2022;23:1–10. doi: 10.1186/s12931-022-01978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacob J., Hirani N., van Moorsel C.H.M., Rajagopalan S., Murchison J.T., van Es H.W., Bartholmai B.J., van Beek F.T., Struik M.H.L., Stewart G.A., et al. Predicting outcomes in rheumatoid arthritis related interstitial lung disease. Eur. Respir. J. 2019;53:1800869. doi: 10.1183/13993003.00869-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Izuka S., Yamashita H., Iba A., Takahashi Y., Kaneko H. Acute exacerbation of rheumatoid arthritis–associated interstitial lung disease: Clinical features and prognosis. Rheumatology. 2021;60:2348–2354. doi: 10.1093/rheumatology/keaa608. [DOI] [PubMed] [Google Scholar]
- 59.Farquhar H.J., Beckert N., Beckert L., Edwards A.L., Matteson E.L., Frampton C., Stamp L.K. Survival of adults with rheumatoid arthritis associated interstitial lung disease—A systematic review and meta-analysis. Semin. Arthritis Rheum. 2023;60:152187. doi: 10.1016/j.semarthrit.2023.152187. [DOI] [PubMed] [Google Scholar]
- 60.Kim E.J., Elicker B.M., Maldonado F., Webb W.R., Ryu J.H., Van Uden J.H., Lee J.S., King T.E., Jr., Collard H.R. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur. Respir. J. 2010;35:1322–1328. doi: 10.1183/09031936.00092309. [DOI] [PubMed] [Google Scholar]
- 61.Cassone G., Manfredi A., Vacchi C., Luppi F., Coppi F., Salvarani C., Sebastiani M. Treatment of Rheumatoid Arthritis-Associated Interstitial Lung Disease: Lights and Shadows. J. Clin. Med. 2020;9:1082. doi: 10.3390/jcm9041082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Venerito V., Manfredi A., Carletto A., Gentileschi S., Atzeni F., Guiducci S., Lavista M., La Corte L., Pedrollo E., Scardapane A., et al. Evolution of Rheumatoid-Arthritis-Associated Interstitial Lung Disease in Patients Treated with JAK Inhibitors: A Retrospective Exploratory Study. J. Clin. Med. 2023;12:957. doi: 10.3390/jcm12030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsuchiya Y., Takayanagi N., Sugiura H., Miyahara Y., Tokunaga D., Kawabata Y., Sugita Y. Lung diseases directly associated with rheumatoid arthritis and their relationship to outcome. Eur. Respir. J. 2011;37:1411–1417. doi: 10.1183/09031936.00019210. [DOI] [PubMed] [Google Scholar]
- 64.Mortality and Clinical Features in Rheumatoid Arthritis and Interstitial Lung Disease-ACR Meeting Abstracts. [(accessed on 15 July 2024)]. Available online: https://acrabstracts.org/abstract/mortality-and-clinical-features-in-rheumatoid-arthritis-and-interstitial-lung-disease/
- 65.Nieto M.A., Sanchez-Pernaute O., Romero-Bueno F., Leon L., Vadillo C., Freites-Nuñez D.D., Jover J.A., Álvarez-Sala J.L., Abasolo L. Mortality rate in rheumatoid arthritis-related interstitial lung disease: The role of radiographic patterns. BMC Pulm. Med. 2021;21:205. doi: 10.1186/s12890-021-01569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ekici M., Baytar Y., Kardas R.C., Sari A., Akdogan A., Durhan G., Ariyurek M., Kalyoncu U. Predictors of mortality in rheumatoid arthritis-associated lung disease: A retrospective study on ten years. Jt. Bone Spine. 2021;88:105133. doi: 10.1016/j.jbspin.2021.105133. [DOI] [PubMed] [Google Scholar]
- 67.Kakutani T., Hashimoto A., Tominaga A., Kodama K., Nogi S., Tsuno H., Ogihara H., Nunokawa T., Komiya A., Furukawa H., et al. Related factors, increased mortality and causes of death in patients with rheumatoid arthritis-associated interstitial lung disease. Mod. Rheumatol. 2020;30:458–464. doi: 10.1080/14397595.2019.1621462. [DOI] [PubMed] [Google Scholar]
- 68.Kang E.H., Jin Y., Desai R.J., Liu J., Sparks J.A., Kim S.C. Risk of exacerbation of pulmonary comorbidities in patients with rheumatoid arthritis after initiation of abatacept versus TNF inhibitors: A cohort study. Semin. Arthritis Rheum. 2020;50:401–408. doi: 10.1016/j.semarthrit.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 69.Ng K.H., Chen D.Y., Lin C.H., Chao W.C., Chen H.H. Analysis of risk factors of mortality in rheumatoid arthritis patients with interstitial lung disease: A nationwide, population-based cohort study in Taiwan. RMD Open. 2022;8:e002343. doi: 10.1136/rmdopen-2022-002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolfe F., Caplan L., Michaud K. Rheumatoid arthritis treatment and the risk of severe interstitial lung disease. Scand. J. Rheumatol. 2007;36:172–178. doi: 10.1080/03009740601153774. [DOI] [PubMed] [Google Scholar]
- 71.Tanaka N., Nishimura K., Waki D., Kadoba K., Murabe H., Yokota T. Annual variation rate of KL-6 for predicting acute exacerbation in patients with rheumatoid arthritis-associated interstitial lung disease. Mod. Rheumatol. 2021;31:1100–1106. doi: 10.1080/14397595.2021.1879346. [DOI] [PubMed] [Google Scholar]
- 72.Akiyama M., Kaneko Y., Yamaoka K., Kondo H., Takeuchi T. Association of disease activity with acute exacerbation of interstitial lung disease during tocilizumab treatment in patients with rheumatoid arthritis: A retrospective, case–control study. Rheumatol. Int. 2016;36:881–889. doi: 10.1007/s00296-016-3478-3. [DOI] [PubMed] [Google Scholar]
- 73.Kim S.H., Kim S.Y., Yoon H.Y., Song J.W. PM10 increases mortality risk in rheumatoid arthritis-associated interstitial lung disease. RMD Open. 2024;10:e003680. doi: 10.1136/rmdopen-2023-003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee J.S., Lee E.Y., Ha Y.J., Kang E.H., Lee Y.J., Song Y.W. Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res. Ther. 2019;21:58. doi: 10.1186/s13075-019-1835-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kwon B.S., Lee H.Y., Choe J., Chae E.J., Hong S., Song J.W. Acute Respiratory Deterioration in Rheumatoid Arthritis-Associated Interstitial Lung Disease. Chest. 2022;162:136–144. doi: 10.1016/j.chest.2022.01.007. [DOI] [PubMed] [Google Scholar]
- 76.Habib H.M., Eisa A.A., Arafat W.R., Marie M.A. Pulmonary involvement in early rheumatoid arthritis patients. Clin. Rheumatol. 2011;30:217–221. doi: 10.1007/s10067-010-1492-5. [DOI] [PubMed] [Google Scholar]
- 77.Chang S.H., Lee J.S., Ha Y.-J., Kim M.U., Park C.H., Lee J.S., Kim J.-W., Chung S.W., Pyo J.Y., Lee S.W., et al. Lung function trajectory of rheumatoid arthritis–associated interstitial lung disease. Rheumatology. 2023;62:3014–3024. doi: 10.1093/rheumatology/kead027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Courvoisier D.S., Chatzidionysiou K., Mongin D., Lauper K., Mariette X., Morel J., Gottenberg J.-E., Bergstra S.A., Suarez M.P., Codreanu C., et al. The impact of seropositivity on the effectiveness of biologic anti-rheumatic agents: Results from a collaboration of 16 registries. Rheumatology. 2021;60:820–828. doi: 10.1093/rheumatology/keaa393. [DOI] [PubMed] [Google Scholar]
- 79.Marcoux V., Lok S., Mondal P., Assayag D., Fisher J.H., Shapera S., Morisset J., Manganas H., Fell C.D., Hambly N., et al. Treatment of Rheumatoid Arthritis-Associated Interstitial Lung Disease in A Multi-Center Registry Cohort. J. Thorac. Dis. 2023;15:2517. doi: 10.21037/jtd-22-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matson S.M., Baqir M., Moua T., Marll M., Kent J., Iannazzo N.S., Boente R.D., Donatelli J.M., Dai J., Diaz F.J., et al. Treatment Outcomes for Rheumatoid Arthritis-Associated Interstitial Lung Disease. Chest. 2023;163:861–869. doi: 10.1016/j.chest.2022.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pugashetti J.V., Adegunsoye A., Wu Z., Lee C.T., Srikrishnan A., Ghodrati S., Vo V., Renzoni E.A., Wells A.U., Garcia C.K., et al. Validation of Proposed Criteria for Progressive Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2023;207:69–76. doi: 10.1164/rccm.202201-0124OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singh N., Varghese J., England B.R., Solomon J.J., Michaud K., Mikuls T.R., Healy H.S., Kimpston E.M., Schweizer M.L. Impact of the pattern of interstitial lung disease on mortality in rheumatoid arthritis: A systematic literature review and meta-analysis. Semin. Arthritis Rheum. 2019;49:358–365. doi: 10.1016/j.semarthrit.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 83.Franzen D., Ciurea A., Bratton D.J., Clarenbach C.F., Latshang T.D., Russi E.W., Kyburz D., Kohler M. Effect of rituximab on pulmonary function in patients with rheumatoid arthritis. Pulm. Pharmacol. Ther. 2016;37:24–29. doi: 10.1016/j.pupt.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 84.Yusof Y.M., Kabia A., Darby M., Lettieri G., Beirne P., Vital E.M., Dass S., Emery P. Effect of rituximab on the progression of rheumatoid arthritis–related interstitial lung disease: 10 years’ experience at a single centre. Rheumatology. 2017;56:1348–1357. doi: 10.1093/rheumatology/kex072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kurata I., Tsuboi H., Terasaki M., Shimizu M., Toko H., Honda F., Ohyama A., Yagishita M., Osada A., Ebe H., et al. Effect of Biological Disease-modifying Anti-rheumatic Drugs on Airway and Interstitial Lung Disease in Patients with Rheumatoid Arthritis. Intern. Med. 2019;58:1703–1712. doi: 10.2169/internalmedicine.2226-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hyldgaard C., Hilberg O., Pedersen A.B., Ulrichsen S.P., Løkke A., Bendstrup E., Ellingsen T. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: Comorbidity and mortality. Ann. Rheum. Dis. 2017;76:1700–1706. doi: 10.1136/annrheumdis-2017-211138. [DOI] [PubMed] [Google Scholar]
- 87.Song J.W., Lee H.K., Lee C.K., Chae E.J., Jang S.J., Colby T.V., Kim D.S. Clinical Course and Outcome of Rheumatoid Arthritis-Related Usual Interstitial Pneumonia. Sarcoidosis Vasc. Diffuse. Lung Dis. 2013;30:103–112. [PubMed] [Google Scholar]
- 88.Kelly C.A., Nisar M., Arthanari S., Carty S., Woodhead F.A., Price-Forbes A., Middleton D., Dempsey O., Miller D., Basu N., et al. Rheumatoid arthritis related interstitial lung disease—Improving outcomes over 25 years: A large multicentre UK study. Rheumatology. 2021;60:1882–1890. doi: 10.1093/rheumatology/keaa577. [DOI] [PubMed] [Google Scholar]
- 89.Juge P.A., Hayashi K., McDermott G.C., Vanni K.M.M., Kowalski E., Qian G., Bade K., Saavedra A., Dieudé P., Dellaripa P.F., et al. Effectiveness and tolerability of antifibrotics in rheumatoid arthritis-associated interstitial lung disease. Semin. Arthritis Rheum. 2024;64:152312. doi: 10.1016/j.semarthrit.2023.152312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rojas-Serrano J., Herrera-Bringas D., Pérez-Román D.I., Pérez-Dorame R., Mateos-Toledo H., Mejía M. Rheumatoid arthritis-related interstitial lung disease (RA-ILD): Methotrexate and the severity of lung disease are associated to prognosis. Clin. Rheumatol. 2017;36:1493–1500. doi: 10.1007/s10067-017-3707-5. [DOI] [PubMed] [Google Scholar]
- 91.Yamakawa H., Ogura T., Kameda H., Kishaba T., Iwasawa T., Takemura T., Kuwano K. Decision-Making Strategy for the Treatment of Rheumatoid Arthritis-Associated Interstitial Lung Disease (RA-ILD) J. Clin. Med. 2021;10:3806. doi: 10.3390/jcm10173806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.