Abstract
Menopause leads to decreased estradiol levels affecting tissue health and causing local inflammation in the genital organs and urinary tract. The rise of blood C-reactive protein (CRP) levels in menopausal women may indicate systemic inflammation associated with estradiol decline. The aim of this study was to determine the relationship between serum estradiol and CRP levels on genitourinary syndrome in menopausal women. A cross-sectional study was conducted among menopausal women who had not experienced menstruation for at least 12 consecutive months at Prof. dr. Chairuddin P. Lubis Hospital, Medan, Indonesia, in 2023. Estradiol and CRP levels were measured using enzyme-linked immunosorbent assay (ELISA) and the presence of genitourinary syndrome was assessed using the Menopause-Specific Quality of Life (MENQOL) questionnaire. The mean levels of estradiol and CRP were compared to menopausal women with and without genitourinary syndrome with the Mann-Whitney test. To assess the correlation between estradiol and CRP levels, and between their levels with the presence of genitourinary symptoms, the Spearman correlation test was used. The genitourinary syndrome was reported in 25% of the total included menopausal women. Our data indicated that the mean estradiol levels were not significantly different between menopausal women with and without genitourinary syndrome (9.13±2.47 pg/mL vs 18.96±31.23 pg/mL, p=0.881). The mean serum CRP level of menopausal women with genitourinary syndrome (9.72±6.30 mg/L) was higher than that of women without the syndrome (2.09±1.26 mg/L) with p<0.001. In addition, serum CRP level, not estradiol, was correlated with the symptom score of genitourinary syndrome. This study highlights that to identify and manage genitourinary syndrome, monitoring of CRP levels is essential in menopausal women.
Keywords: Menopause, genitourinary syndrome, estradiol, CRP, MENQOL
Introduction
Menopause is characterized by the decreased production of estrogen and inhibin hormones by the ovarian follicles, causing various changes in organ structure and function, potentially increasing the likelihood of chronic diseases in women. Each year, about 1.5 million women enter the menopausal transition, experiencing distressing symptoms, including vasomotor symptoms, vaginal dryness, urinary disorders, decreased libido, sleep disturbances, fatigue, and joint pain [1]. The prevalence of genitourinary syndrome in postmenopausal women is approximately 27– 84% and it often leads to a range of symptoms [2].
Certain symptoms of genitourinary syndromes are known to be closely linked to hormonal shifts during menopause, with many women directly associating menopause with common symptoms like hot flashes, vaginal dryness, and disrupted sleep (with or without night sweats) [1]. Estradiol deficiency induces hormonal alterations in the vaginal canal, resulting in reduced elasticity, leading to shortening and narrowing, and the loss of vaginal rugae. This condition causes a reduction in the epithelial layer, vaginal secretions, muscles, and vascularization, thus leading to genitourinary syndrome [3]. In genitourinary syndrome, menopause is also associated with inflammation of the genital tissue and urinary tract, especially due to a decrease in estrogen levels. Inflammation, including in the genitourinary tissue, can increase C-reactive protein (CRP) levels as a response to the immune system’s inflammation. Therefore, high CRP levels in the blood may reflect the level of inflammation in the genitourinary tissue [4].
The relationship between estradiol and CRP is multifaceted. Reduced estradiol levels in menopause can affect tissue health, which can trigger inflammation in the genital organs and urinary tract. However, elevated CRP levels in the blood may indicate systemic inflammation, which is not always directly linked to estradiol levels. When assessing menopausal health, monitoring estradiol and CRP levels can be beneficial, yet comprehensive diagnosis and management of menopausal symptoms necessitate thorough evaluation and additional testing by healthcare providers [5]. The aim of this study was to determine the relationship between serum estradiol and CRP levels in menopausal women with genitourinary syndrome.
Methods
Study design and setting
A cross-sectional study was conducted at Prof. dr. Chairuddin P. Lubis Hospital, Medan, Indonesia, in 2023 among menopausal women. This study included any menopausal patient who came to the gynecology clinic, met the inclusion and exclusion criteria, and was willing to participate.
Sample and sampling method
A consecutive sampling method was employed in this study to recruit the samples. Sampling is carried out in accordance with research criteria in the order of arrival until the sample size is reached. The number of samples was calculated based on a numerical-numeric correlative analytical formula, resulting in a minimum of 32.
Patients and criteria
This study included menopausal women who had not experienced menstruation for at least 12 consecutive months, had never undergone a hysterectomy or oophorectomy (either unilaterally or bilaterally), never received hormone replacement therapy, had no history of metabolic, cardiovascular, or malignant disease, had no undergone radiotherapy or cytostatic therapy, free form infection and have not taken antibiotics for at least two weeks before blood sample collection. The exclusion criteria were withdrawal from research and damaged blood samples that could not be examined.
Data collection and variables
Samples’ characteristics, including age, parity, occupation, education, smoking history, weight, height, and duration of menopause, were collected. The Menopause-Specific Quality of Life (MENQOL) questionnaire was used to conduct assessments related to the genitourinary syndrome of menopause consisting of five items in the physical and sexual domains (urinary frequency, stress incontinence, decreased libido, vaginal dryness, and sexual intercourse avoidance). Each question had alternative responses on a seven-point Likert scale ranging from 0 to 6. A score of 0 meant that the patient did not experience the specific symptom in the previous month, while a score of 1 indicated that the woman was experiencing the symptom, but it was not disturbing; scores of 2 to 6 indicated increasing levels of discomfort with the symptoms. A comprehensive physical and gynecological examination was performed after evaluating the symptoms.
Following that, a total of 1.5 mL of blood was collected from the antecubital vein of each patient and transported to the Prodia laboratory, Medan, Indonesia, for further process. The levels of estradiol and CRP levels were measured using the enzyme-linked immunosorbent assay (ELISA) method. The normal range for estradiol levels is between 20 and 400 pg/mL, while the normal range for CRP levels is under 0.3 mg/L.
Statistical analysis
The Mann-Whitney test was used to compare the levels of serum estradiol and CRP between menopausal women with and without genitourinary syndrome. The Spearman correlation test was used to assess the correlation between estradiol and CRP levels and between the levels of estradiol and CRP with the presence of genitourinary symptoms. Statistical significance was considered at p<0.05. All data were analyzed with the SPSS version 26 program (IBM SPSS, Chicago, USA).
Results
Characteristics of the patients
A total of 32 menopausal women were included in the study, and their characteristics are presented in Table 1. The vast majority of the women were ≥50 years old (93.8%), and most were multiparous (71.9%). Based on education level, more than half of the women had junior high school education (56.3%) and were obese (59.4%). None of the women smoked and all of them were housewives. The average length of menopause was 7.59±5.24 years.
Table 1.
Characteristics of the menopausal women included in the study (n=32)
| Characteristics | Frequency (%) |
|---|---|
| Age | |
| <50 years | 2 (6.3) |
| ≥50 years | 30 (93.8) |
| Parity | |
| Nulliparous | 3 (9.4) |
| Primiparous | 4 (12.5) |
| Multiparous | 23 (71.9) |
| Grande multiparous | 2 (6.3) |
| Occupation | |
| Employees | 0 (0) |
| Housewives | 32 (100) |
| Education | |
| Elementary school | 7 (21.9) |
| Junior high school | 18 (56.3) |
| Senior high school | 7 (21.9) |
| Smoking | |
| Yes | 0 (0) |
| No | 32 (100) |
| Body mass index (BMI) | |
| Underweight | 0 (0) |
| Normal | 10 (31.3) |
| Overweight | 3 (9.4) |
| Obesity | 19 (59.4) |
| Duration of menopause (years) | |
| Mean±standard deviation (SD) | 7.59±5.24 |
| Median (min-max) | 6 (2–20) |
| Estradiol (ng/mL), mean±SD | 14.05±10.32 |
| C-reactive protein (pg/mL), mean±SD | 6.03±3.66 |
Prevalence of genitourinary syndrome of menopausal women
Our data indicated that 8 (25%) of the samples classified had genitourinary syndrome, while 24 (75%) had no genitourinary syndrome. Among those who had the genitourinary syndrome, the primary reported complaints included frequent urination, vaginal dryness during sexual intercourse, and avoidance of sexual intercourse. All of them experienced urinary frequency, vaginal dryness during sexual intercourse and avoided sexual intercourse (Table 2).
Table 2.
Distribution of syndromes of menopausal women experienced genitourinary syndrome (n=8)
| Symptoms | n (%) |
|---|---|
| Urinary frequency | 8 (100) |
| Stress incontinence | 6 (75) |
| Decreased libido | 7 (87.5) |
| Vaginal dryness during sexual intercourse | 8 (100) |
| Sexual intercourse avoidance | 8 (100) |
Comparison of estradiol and CRP levels between menopausal women with and without genitourinary syndrome
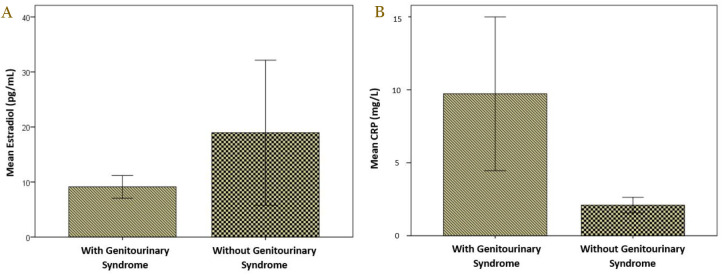
The mean of serum estradiol level in menopausal women with genitourinary syndrome was 9.13±2.47 pg/mL and this was lower compared to those without genitourinary syndrome (18.96±31.23 pg/mL), although no significant difference (p=0.881) (Table 3). The mean CRP levels among menopausal women with the genitourinary syndrome were significantly higher compared to those without the genitourinary syndrome, 9.72±6.30 mg/L vs 2.09±1.26 mg/L, respectively, with p<0.001 (Table 3). The comparisons of mean estradiol and CRP levels between menopausal women with and without genitourinary syndrome are presented in Figure 1.
Table 3.
Comparison of serum estradiol and C-reactive protein (CRP) levels in menopausal women with and without genitourinary syndrome
| Variables | Genitourinary syndrome | p-value* | |
|---|---|---|---|
| Yes | No | ||
| Mean±SD | Mean±SD | ||
| Estradiol (pg/mL) | 9.13±2.47 | 18.96±31.23 | 0.881 |
| CRP (mg/L) | 9.72±6.30 | 2.09±1.26 | <0.001 |
Analyzed with Mann-Whitney test
Figure 1.
Comparison of mean serum estradiol (A) and CRP level (B) in menopausal women with and without genitourinary syndrome.
Correlation between estradiol and CRP levels in menopausal women
Our analysis indicated that the levels of serum estradiol and CRP in menopausal women without genitourinary syndrome were not correlated (r=0.327 and p=0.119) (Table 4). Similarly, the level of serum estradiol was not correlated with CRP level in menopausal women with genitourinary syndrome (r=0.062 and p=0.883) (Table 4).
Table 4.
Correlation between serum estradiol and C-reactive protein (CRP) levels in menopausal women
| Groups | Variables | CRP | |
|---|---|---|---|
| r | p-value* | ||
| With genitourinary syndrome | Estradiol | 0.062 | 0.883 |
| Without genitourinary syndrome | Estradiol | 0.327 | 0.119 |
Analyzed with Spearman correlation test
Correlation between the levels of estradiol and CRP with each genitourinary symptom
Our data indicated that there was no correlation between serum estradiol and the score of any syndrome genitourinary symptom in menopausal women (Table 5). Meanwhile, there was a significant relationship (p<0.001) between CRP levels and each genitourinary symptom with r values ranged from 0.6 to 0.7, indicating a strong correlation (Table 5).
Table 5.
Correlation between estradiol and C-reactive protein (CRP) with each genitourinary symptom score
| Symptom | Estradiol | CRP | ||
|---|---|---|---|---|
| r | p-value* | r | p-value* | |
| Urinary frequency | -0.002 | 0.993 | 0.752 | <0.001 |
| Stress incontinence | 0.045 | 0.805 | 0.642 | <0.001 |
| Decreased libido | 0.047 | 0.798 | 0.688 | <0.001 |
| Vaginal dryness during sexual intercourse | -0.016 | 0.929 | 0.751 | <0.001 |
| Sexual intercourse avoidance | -0.011 | 0.954 | 0.754 | <0.001 |
Spearman correlation test
Discussion
This study revealed that menopausal women with genitourinary syndrome most commonly complain of urinary frequency, vaginal dryness during sexual intercourse, and avoidance of sexual intercourse. This is related to the decline in estrogen function during menopause. Estrogen helps increase cellular tropism in the epithelial lining of the vagina, urethra, and bladder, as well as in the trigone and puborectalis muscles, due to the presence of hormone receptors in these areas. Therefore, estrogen may cause urinary tract symptoms through different mechanisms [6]. Bladder overactivity is one factor associated with increased urination frequency [7]. A study reported that postmenopausal women often avoid sexual intercourse to reduce vaginal burning, a symptom linked to the estrogen decline that causes urogenital atrophy, vaginal dryness, decreased tissue elasticity, and dyspareunia, all of which affect sexual behavior [8]. A similar study reported that postmenopausal women were more likely to avoid sexual activity than non-menopausal women due to reduced natural vaginal secretions, which cause pain and discomfort during intercourse [9].
This study demonstrated that the average serum estradiol level of menopausal women with genitourinary syndrome was lower than the serum estradiol level of menopausal women with no genitourinary syndrome; however, it was not significant. A study reported that after menopause, estradiol levels fall to prepubertal levels, with average serum estradiol levels usually 10 to 20 pg/mL [10]. Our findings are in line with a study that showed menopausal women with genitourinary syndrome have lower serum estradiol levels than menopausal women without genitourinary syndrome [11]. According to a study, mean estradiol levels did not decline until 2.03 years before the last menstrual period, as measured using a sensitive immunoassay with a lower detection limit of 1 pg/mL, whereas most commercial estradiol tests cannot accurately measure estradiol levels in postmenopausal women (<20 pg/mL), and the varying sensitivity and specificity of these tests make epidemiological comparisons difficult [12]. This study also used a commercial estradiol test which can only detect estradiol with a detection limit of 9 pg/mL; therefore, it was impossible to determine the value for estradiol levels below 9 pg/mL.
Our finding showed that the average serum CRP level of menopausal women with genitourinary syndrome was significantly higher than the serum CRP level of menopausal women with no genitourinary syndrome. In healthy individuals, CRP levels are around 1–3 mg/L, and during inflammation, plasma CRP levels deviate by at least 25%, causing an increase in circulating CRP levels up to 1,000-fold due to bacterial infection [13]. The results of this study indicate that menopausal women without genitourinary syndrome have normal serum CRP levels. In contrast, postmenopausal women with genitourinary syndrome have serum CRP levels that increase from normal values, which indicates inflammation. A study reported that menopausal women had higher concentrations of cytokines such as tumor necrosis factor (TNF)-alpha, interleukin (IL)-6, and CRP [14]. High levels of CRP in the blood can indicate systemic inflammation or inflammation in the body [15]. This inflammation can affect various body systems, including the genitourinary system, which includes the genital organs and urinary tract [16]. Premenopausal women who show higher levels of CRP and IL-6 are associated with lower levels of libido, arousal, and sexual pleasure [17]. The reason for this is that inflammation can disrupt sexual desire and arousal and can lead to sexual reluctance as a form of defensive response to reduce the possibility of infection. Chronic inflammation can result in endothelial dysfunction and hinder the ability of nitric oxide to vasodilate, which is essential for causing sexual stimulation [17]. Pain is also a sign of inflammation that can reduce sexual performance and desire during sexual intercourse [17].
Our results showed that there was no significant relationship between serum estradiol levels and CRP in menopausal women, both in the group of menopausal women with genitourinary syndrome and those without genitourinary syndrome. This study’s results align with a previous study demonstrating that serum estradiol levels are inversely related to CRP levels in premenopausal women but not in postmenopausal women [18]. Another study reported an inverse relationship between estradiol levels and CRP in 259 women of childbearing age in the United States [19]. In contrast, serum estradiol levels were positively associated with CRP levels in 513 Italian postmenopausal women [20]. The mechanisms underlying the menopause-specific association between serum estradiol and CRP levels remain unclear. Estrogen inhibits the production of inflammatory cytokines and migration of inflammatory cells in non-reproductive tissues, and estrogen receptors are highly expressed on vascular smooth muscle and endothelial cells throughout the human body. The anti-inflammatory effects of estrogen are partly mediated by producing nitric oxide, decreasing TNF-α levels, and also reactive oxygen species in mitochondria [18]. However, the effects of estradiol may differ depending on menopausal status. The lack of an inverse relationship between estradiol and CRP levels in postmenopausal women may be explained by the observation that 80% of circulating estradiol in such individuals comes from the aromatization of testosterone, primarily in adipose tissue [18]. Aromatase, an enzyme that converts testosterone to estradiol, is stimulated by inflammatory cytokines and CRP [18]. In addition, it has been reported that an increase in CRP can occur through non-inflammatory pathways and through inflammatory pathways influenced by progestogens [21].
This study found a significant relationship between serum CRP levels and each genitourinary symptom, revealing an increased serum CRP is associated with overall genitourinary symptoms. According to a study, higher serum CRP levels were associated with lower levels of sexual desire, arousal, and pleasure, which is in line with our findings [22]. CRP is a marker of systemic inflammation where inflammation can disrupt women’s sexual desire and arousal through direct (nervous) and indirect (endocrine, vascular, and social/behavioral) pathways. The liver produces CRP in response to cytokine signals throughout the body, making it a useful system-level inflammation index [22]. Cytokines can exert powerful effects on the central nervous system, contributing to behavioral and sexual responses. Brain areas that are relevant for the coordination of sexual desire and arousal, such as the mesolimbic reward system, cingulate cortex, and thalamus, have been shown to respond to cytokine signals, both directly at neuronal receptors and indirectly through interactions with dopamine and other neurotransmitters [22,23]. Inflammation may act on the central nervous system to contribute to female sexual desire and arousal dysfunction through interference with reward processing (decreased motivation to have sex) and increased disgust (increased avoidance and decreased sexual arousal) [22]. Meanwhile, regarding urological symptoms, it has been reported that there is a relationship between inflammation and bladder overactivity [24]. There was a positive association between increased levels of inflammatory markers, including CRP, TNF-α, and IL-6, with urinary continence [25]. Inflammatory cytokines play an important role in modulating the expression of connexins and the pathogenesis of bladder dysfunction; they are involved in the interaction of overactive bladder parasympathetic and peptide/sensory innervation with local immune cells [25].
Conclusion
Our study found that serum estradiol levels were not associated with genitourinary syndrome of menopausal women. The average serum CRP level of menopausal women with genitourinary syndrome was significantly higher than those without genitourinary syndrome. Additionally, CRP levels were related to genitourinary syndrome of menopausal women. Therefore, it is recommended to monitor CRP levels in menopausal women as a potential marker for identifying and managing genitourinary syndrome.
Acknowledgments
The authors would like to acknowledge everyone who helped with the research.
Ethics approval
This research has received approval from the Health Research Ethics Committee of the University of North Sumatra No.110/KEPK/USU/2023.
Competing interests
The authors declare that there is no conflict of interest.
Funding
This study received no external funding.
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
How to cite
Siregar MFG, Terauchi M, Lubis RB, et al. Role of estradiol and C-reactive protein levels on genitourinary syndrome in menopausal women. Narra J 2024; 4 (2): e626 - http://doi.org/10.52225/narra.v4i2.626.
References
- 1.Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clin North Am 2015;44(3):497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarmento ACA, Costa APF, Vieira-Baptista P, et al. Genitourinary syndrome of menopause: Epidemiology, physiopathology, clinical manifestation and diagnostic. Front Reprod Heal 2021;3:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lumbanraja IL, Siregar MF, Lumbanraja SN, et al. Association of vaginal maturation index and vaginal pH with the most bothersome symptoms of genitourinary syndrome of menopause. J South Asian Feder Obst Gynae 2021;13(5):288–291. [Google Scholar]
- 4.Morch LS, Lokkegaard E, Andreasen AH, Krüger KS. Hormone therapy and the impact of inflammation on the risk of lung cancer in the Danish Nurse cohort. Menopause 2021;19(4):385–391. [Google Scholar]
- 5.Ridker PM, Hennekens CH, Rifai N, Buring JE. Antiinflammatory properties of aspirin, acetaminophen, and other nonsteroidal anti-inflammatory drugs in men. Circulation 2000;102(8):975–980.10961960 [Google Scholar]
- 6.Quinn SD, Domoney C.. The effects of hormones on urinary incontinence in postmenopausal women. Climacteric 2009;12(2):106–113. [DOI] [PubMed] [Google Scholar]
- 7.Zhu L, Cheng X, Sun J, et al. Association between menopausal symptoms and overactive bladder: A cross-sectional questionnaire survey in China. PLoS One 2015;10(10):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalesi ZB, Bokaie M, Attari SM. Effect of pregnancy on sexual function of couples. Afr Health Sci 2018;18(2):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akman S, Çakici M, Keskindağ B, Karaaziz M.. Analysis of psychological factors and sexual life in postmenopausal women: A cross-sectional study. Klin Psikiyatr Derg 2019;22(1):27–35. [Google Scholar]
- 10.Carmina E, Stanczyk FZ, Lobo RA. Evaluation of hormonal status. 6th ed. In: Strauss JF, Barbieri RL, editors. Yen & Jaffe’s reproductive endocrinology. Amsterdam: Elsevier; 2009. [Google Scholar]
- 11.Palacios S, Castelo-Branco C, Currie H, et al. Update on management of genitourinary syndrome of menopause: A practical guide. Maturitas 2015;82(3):308–313. [DOI] [PubMed] [Google Scholar]
- 12.Rosner W, Hankinson SE, Sluss PM, et al. Challenges to the measurement of estradiol: An endocrine society position statement. J Clin Endocrinol Metab 2013;98(4):1376–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narayan Swamy SN, Jakanur RK, Sangeetha SR. Significance of C-reactive protein levels in categorizing upper and lower urinary tract infection in adult patients. Cureus 2022;14(6):e26432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honour JW. Biochemistry of the menopause. Ann Clin Biochem 2018;55(1):18–33. [DOI] [PubMed] [Google Scholar]
- 15.Didevar N, Rezasoltani P, Pourgholaminejad A, et al. Interleukin-17, C-reactive protein, neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and lipid profiles in healthy menopausal women with or without hot flashes: A cross-sectional study. PLoS One 2023;18(11):e0291804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nehring SM, Goyal A, Bansal P, Patel BC. C reactive protein. Treasure Island (FL: ): StatPearls; 2021. [Google Scholar]
- 17.Haghshenas N, Baharanchi FH, Melekoglu E, et al. Comparison of predictive effect of the dietary inflammatory index and empirically derived food-based dietary inflammatory index on the menopause-specific quality of life and its complications. BMC Womens Health 2023;23(1):349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JM, Lee YJ. Serum oestradiol levels are inversely associated with C-reactive protein levels in premenopausal women, but not postmenopausal women. J Int Med Res 2020;48(10):300060520961228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaskins AJ, Wilchesky M, Mumford SL, et al. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: The BioCycle Study. Am J Epidemiol 2012;175(5):423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maggio M, Ceda GP, Lauretani F, et al. SHBG, sex hormones, and inflammatory markers in older women. J Clin Endocrinol Metab 2011;96(4):1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obstetrical and Gynecological Society of Malaysia (OGSM). Clinical practice guidelines: Management of menopause in Malaysia. Kuala Lumpur; Perpustakaaan Negara Malaysia: 2022. [Google Scholar]
- 22.Lorenz TK. Interactions between inflammation and female sexual desire and arousal function. Curr Sex Heal Rep 2020;11(4):287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng JC, Secondary J, Burke WH, et al. Neuroimaging and sexual behavior: Identification of regional and functional differences. Curr Psychiatry Rep 2015;17(7):55. [DOI] [PubMed] [Google Scholar]
- 24.Heinrich M, Oberbach A, Schlichting N, et al. Cytokine effects on gap junction communication and connexin expression in human bladder smooth muscle cells and suburothelial myofibroblasts. PLoS One 2011;6(6):e20792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Bian H, Qiu S, et al. Associations between the dietary inflammatory index and urinary incontinence among women younger than 65 years. Sci Rep 2021;11(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]