Abstract
To study which phase of viral infection promotes antigen sensitization via the airway and which type of antigen-presenting cells contributes to antigen sensitization, BALB/c mice were sensitized by inhalation of ovalbumin (OA) during the acute phase or the recovery phase of influenza A virus infection, and then 3 weeks later animals were challenged with OA. The numbers of eosinophils and lymphocytes, the amounts of interleukin-4 (IL-4) and IL-5 in the bronchoalveolar lavage fluid, and the serum levels of OA-specific immunoglobulin G1 (IgG1) and IgE increased in mice sensitized during the acute phase (acute phase group), while a high level of gamma interferon production was detected in those sensitized during the recovery phase (recovery phase group). In the acute phase group, both major histocompatibility complex class II molecules and CD11c were strongly stained on the bronchial epithelium; in the recovery phase group, however, neither molecule was detected. OA-capturing dendritic cells (DCs) migrated to the regional lymph nodes, and a small number of OA-capturing macrophages were also observed in the lymph nodes of the acute phase group. In the recovery group, however, no OA-capturing DCs were detected in either the lungs or the lymph nodes, while OA-capturing macrophages were observed in the lymph nodes. These results indicate that the timing of antigen sensitization after viral infection determines the type of immune response.
Dendritic cells (DCs) play a central role in antigen presentation and induce a primary immune response to exogenous antigens. When exogenous antigens such as inhaled proteins are administered, DCs capture antigens and migrate to the secondary lymphoid organs, where they acquire the ability to stimulate naive T cells via major histocompatibility complex (MHC) class II molecules (13, 26).
In the immune response to viral infection, DCs are the professional antigen-presenting cells (APCs) that activate naive CD8+ T cells and generate virus-specific cytotoxic T lymphocytes, which recognize viral antigens in association with MHC class I molecules (5, 8, 26). It has also been reported that infection with certain types of viruses such as the Sendai virus increases the number of DCs and induces the expression of MHC class II molecules on epithelial cells in rats (18). Meanwhile, DCs have been detected in the bronchial epithelium in asthmatic patients (1, 29) and induce the type 2 immune response (25, 27).
Clinically, respiratory virus infection has been proposed as a common triggering factor in the development of allergy in children (9, 10, 33). Schwarze et al. showed that in mice, inhalation of an antigen after respiratory syncytial virus (RSV) infection increased both airway responsiveness and eosinophil influx to the lung (23). We have recently demonstrated that influenza A virus infection enhances the airway sensitization of a suboptimal concentration of an antigen (28, 32). Influenza A virus infection induces the migration of DCs to the bronchial epithelium, and these migrated DCs are essential for the sensitization (32). Within the respiratory immune system, both DCs and macrophages are able to capture, process, and present antigens (3, 12). In influenza A virus infection, however, it remains unclear which phase of viral infection promotes antigen sensitization via the airway and whether APCs other than DCs contribute to enhancing antigen sensitization. To test this hypothesis, we sensitized mice with inhaled ovalbumin (OA) at two distinct phases of viral infection, i.e., the acute phase (days 3 to 7) and the recovery phase (days 10 to 14), and examined APCs, i.e., DCs, macrophages, and B cells. Further, we analyzed serum immunoglobulin antibodies and cells and cytokines of bronchoalveolar lavage fluid (BALF).
MATERIALS AND METHODS
Animals.
Specific-pathogen-free, male BALB/c mice (Japan SLC, Shizuoka, Japan) 6 to 11 weeks of age were used in all experiments. The animals were fed OA-free diets and kept under special pathogen-free conditions in a laminar flow container. All experimental animals used in this study were maintained under the approved guidelines of the Institutional Animal Care and Use Committee of Yokohama City University School of Medicine.
Experimental groups.
The experimental groups were as follows (in each group, n = 5 to 8). (i) The control group included animals that were inoculated with phosphate-buffered saline (PBS) on day 0, sensitized with PBS on days 3 to 7, and challenged with PBS on days 29 to 33. (ii) The virus group included animals that were inoculated with influenza A virus on day 0, sensitized with PBS on days 7 to 10, and challenged with OA on days 29 to 33. (iii) The OA group included animals that were inoculated with PBS on day 0, sensitized with OA on days 7 to 10, and challenged with OA on days 29 to 33. (iv) The acute phase group included animals that were inoculated with influenza A virus on day 0, sensitized with OA on days 3 to 7, and challenged with OA (days 29 to 33). (v) The recovery phase group included animals that were inoculated with influenza A virus, followed by OA sensitization on days 10 to 14 and OA challenge on days 36 to 40.
Viral infection.
The mouse-adapted strain of influenza A/Guizhou-X (A/Guizhou/54/89 × A/Puerto Rico/8/34) H3N2 virus was prepared as previously described (28). We used the diluted virus solution with a titer of 4.1 × 103 PFU of H3N2 per ml, which was determined to be a sublethal dose in our previous study (28). Male BALB/c mice were inoculated intranasally with 50 μl of the virus solution under anesthesia with diethylene ether.
Antigen.
OA (Sigma Chemical Co., St. Louis, Mo.) was dissolved in PBS to a concentration of 0.1% and mixed with an equivalent dose of alum adjuvant (10 mg/ml). The final concentration of OA for sensitization was adjusted to 0.05%. For the OA challenge, a solution of 2% OA in PBS without the alum was used.
Sensitization and challenge.
Animals were sensitized by the inhalation of aerosolized 0.05% OA or PBS using a DeVilbiss 646 nebulizer (Somerset, Pa.). Animals in a Plexiglas chamber (capacity, 23.5 liters) were exposed to aerosols of OA or PBS for 30 min each day over a 5-day period. For the inhalation challenge, the aerosols of 2% OA or PBS were administered by a DeVilbiss 646 nebulizer for 30 min each day over a 5-day period.
Measurement of IgG1, IgG2a, and IgE levels.
Two days after the final challenge, blood was collected from the postorbital vein or the heart to measure OA-specific immunoglobulin G1 (IgG1), IgG2a, and IgE. OA-specific IgG1, IgG2a, and IgE antibody levels were measured by using an enzyme-linked immunosorbent assay (ELISA) as follows (20). ELISA plates (96-well Immunolon II; Dynatech Laboratories, Chantilly, Va.) were coated with 10 μg of OA/ml in borate buffer saline and then blocked with PBS–1% bovine serum albumin. Tenfold-diluted samples were incubated on these plates for 3 h. The plates were washed, overlaid with a 1-mg/ml concentration of alkaline phosphatase-conjugated polyclonal anti-mouse IgG1, IgG2a, or IgE (PharMingen, San Diego, Calif.), reacted for 2 h, and then incubated with nitrophenylphosphate (p-NPP; Kirkegaard & Perry Laboratories, Gaithersburg, Md.) for 8 to 12 h at room temperature. A colorimetric assay of the plates was performed by using a microplate autoreader (Bio-Rad Laboratories, Hercules, Calif.). The concentrations of specific antibodies were determined by comparison with a standard curve. Anti-OA-specific IgG1, IgG2a, and IgE standard serum was obtained from animals that had been immunized by intraperitoneal injections of 6 mg of OA absorbed in 4 mg of alum (two injections, 14 days apart). The titer of the OA-specific IgG1, IgG2a, or IgE of the standard was arbitrarily defined as 1.0 × 103 units/ml.
Bronchoalveolar lavage.
The animals were subjected to lavage with 0.8 ml of PBS under anesthesia with pentobarbital sodium (40 mg/kg of body weight). The lavage was repeated four times, and the recovered fluid (BALF) was immediately centrifuged. The cells were then smeared onto a glass slide using a cytocentrifuge at 100 × g (Cytobucket SC-2; Tomy Seiki, Tokyo, Japan) and stained with Diff-Quick (International Reagent, Kobe, Japan). Differential cell counts of at least 200 cells were performed according to the standard morphologic criteria.
Cytokine assay in BALF.
BALF was collected at 4, 12, 24, and 48 h after the final challenge. Levels of gamma interferon (IFN-γ), interleukin-4 (IL-4), and IL-5 in BALF were measured using a sandwich ELISA (22). The 96-well plates were coated with the following anticytokine monoclonal antibodies (MAbs) diluted in carbonate buffer: R4-6A2 for IFN-γ (Endogen, Cambridge, Mass.), BVD4-1D11 for IL-4 (PharMingen), or TRFK5 for IL-5 (PharMingen). The plates were incubated with samples or blanks (1% bovine serum albumin) at room temperature for 3 h. A standard curve was constructed for each plate by using recombinant cytokines (Immugenex, Los Angeles, Calif.). After washing, the plates were again incubated with biotinylated anticytokine MAbs, i.e., XMG1.2 for IFN-γ (Endogen), 24G2 for IL-4 (Endogen), or TRFK4 for IL-5 (PharMingen), at room temperature for 2 h. The plates were then treated with alkaline phosphatase-conjugated avidin D (Vector Laboratories, Burlingame, Calif.) at room temperature for 1 h. Finally, the plates were incubated with the phosphatase substrate p-NPP (Kirkegaard & Perry Laboratories) at room temperature for 8 to 12 h and read in a microplate autoreader (Bio-Rad Laboratories). The lower limit of detection for each ELISA was approximately 5 pg/ml.
Histology of the lung.
The lungs were removed 48 h after the final OA challenge and fixed by intratracheal instillation of 10% buffered formaldehyde solution followed by immersion. After fixation, the lung tissue was sectioned every 2 to 5 mm, and 10 blocks were sampled randomly for evaluation of histology. These sections were then embedded in paraffin, cut to a thickness of 5 μm, and stained with hematoxylin-eosin.
Immunohistochemical analysis.
Although there are no DC-unique markers in mice, DCs may be recognized by high-level expression of the integrin CD11c together with high-level expression of MHC class II molecules (19, 26, 30). Therefore, we have identified DCs by the coexistence of high-level expression of MHC class II molecule and CD11c. The expression of MHC class II molecules (I-Ad antigen) and CD11c in the lungs was examined 48 h after the final challenge by using immunohistochemistry (7, 17). The lungs were removed after fixation by intratracheal instillation of an optimal cutting temperature (OCT) compound (Bayer-Pharma, Zürich, Switzerland) in an equivalent volume of PBS, embedded in OCT compound, frozen in isopentane-chilled liquid nitrogen, and stored at −80°C until use. The frozen tissues were cut into 8- to 10-μm sections with a cryostat. The tissue sections were then mounted on silanized slides, air dried, fixed in cold acetone for 20 min, and stored desiccated at −80°C until staining. To inactivate the endogenous peroxidase, the slides were immersed in 0.3% H2O2–methanol at room temperature for 30 min and then incubated with blocking serum (ZYM Histostain SP kit) at room temperature for 10 min. The slides were then incubated at 4°C overnight with primary antibody (anti-mouse MAbs specific for I-Ad [PharMingen] or CD11c [PharMingen]) diluted 100-fold in PBS. After washing, the slides were incubated with fluorescein isothiocyanate (FITC)-labeled anti-rat IgG (PharMingen) at room temperature for 30 min. After a final washing, the slides were mounted with 95% glycerol–12 mM sodium phosphate buffer and examined with a laser scanning microscope (LSM-GB200; Olympus, Tokyo, Japan). Staining with nonspecific IgG was also examined.
Double coloring of the lung and regional lymph nodes.
APCs in the lung and the mediastinal lymph nodes were stained immunohistochemically with appropriate MAbs: CD11c for DCs (7, 17), Mac-2 for macrophages, and B220 for B cells. FITC-labeled OA was inhaled for 30 min on the first day of sensitization (day 3 or day 10). Two or four hours after sensitization, the lungs, including the mediastinal lymph nodes, were removed and fixed by intratracheal instillation of OCT compound. The sections were immersed in 0.3% H2O2–methanol at room temperature for 30 min and incubated with blocking serum (ZYM Histostain SP kit) at room temperature for 10 min. The slides were then incubated at 4°C overnight with primary antibody {anti-mouse MAbs specific for CD11c (PharMingen), Mac-2 (MAb was purified from M3/38.1.2.8.HL.2 [ATCC, Rockville, Md.] cultured supernatant using protein G-Sepharose), or B220 (PharMingen)}. For the secondary MAb, phycoerythrin-labeled anti-rat IgG (PharMingen) was used. These samples were examined by confocal laser scanning microscopy (LSM-GB200; Olympus).
Statistical analysis.
All values are expressed as the mean ± the standard error of the mean or are otherwise specified. A statistical comparison between groups was carried out by means of a two-way analysis of variance with repeated measures (ANOVA), followed by a post hoc comparison using the Newman-Keuls test. Two mean values were compared using the Wilcoxon matched pairs test. A P value of less than 0.05 was considered significant.
RESULTS
Analysis of BALF.
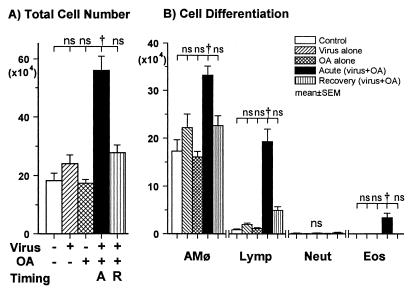
To study the cellular response in the lung, bronchoalveolar lavage was carried out after the final antigen challenge and the BALF was analyzed. The total cell number in the BALF of the mice sensitized during the acute phase of influenza A virus infection (the acute phase group) was significantly higher than that of the other four groups (P < 0.01) (Fig. 1A). In a differential cell count of the acute phase group, the absolute number of lymphocytes was significantly higher than in the control group (P < 0.01), but this parameter in the mice sensitized during the recovery phase (the recovery phase group) was not increased (Fig. 1B). Eosinophils (3.6% of the total cells) were detected in BALF of the acute phase group, whereas eosinophils were not observed in BALF of the other four groups. The number of macrophages was significantly increased in the acute phase group (P < 0.01), compared with that in the control group, the virus group, the OA group, or the recovery phase group.
FIG. 1.
BALF cell analysis 48 h after the final OA challenge. (A) The total cell number in the acute phase group (closed column) was greater than the other four groups (P < 0.01; ANOVA). (B) The numbers of macrophages (AMφ), lymphocytes (Lymp), and eosinophils (Eos) in the acute phase group were greater than those of the other four groups (P < 0.01; ANOVA). The number of lymphocytes in the recovery phase group (vertical lines) was greater than that in the control (open), virus (hatched), and OA (cross-hatched) groups (P < 0.01; ANOVA). There were no differences in cell differentiation among the control, virus, and OA groups. Abbreviations: A, acute phase group; R, recovery phase group; Neut, neutrophils; †, P < 0.01 (comparison among groups); ns, not significant.
OA-specific IgG1, IgG2a, and IgE levels.
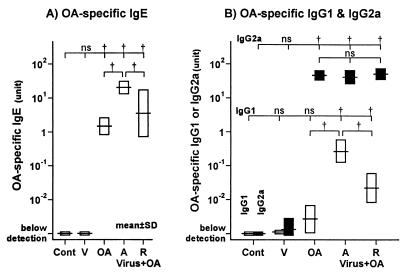
Serum OA-specific IgE was detected in the OA group, the acute phase group, and the recovery phase group (all groups, P < 0.01) (Fig. 2A). The level of OA-specific IgE in the acute phase group was much higher than that in the recovery phase and OA groups (both groups, P < 0.01). OA-specific IgG1 was increased in both the acute phase group and the recovery phase group (both groups, P < 0.01) (Fig. 2B). The level of OA-specific IgG1 was much higher in the acute phase group than in the recovery phase group (P < 0.01) (Fig. 2B). OA-specific IgG2a was increased in the OA group, the acute phase group, and the recovery phase group (P < 0.01) (Fig. 2B). However, the levels of OA-specific IgG2a did not differ significantly among these three groups. Neither OA-specific IgG1, IgG2a, nor IgE was detected in the serum of the control group. Levels of OA-specific IgG1, IgG2a, or IgE in the virus group were not different from the respective control values.
FIG. 2.
Serum levels of OA-specific IgE (A) or IgG1 (open bars) and IgG2a (closed bars) (B). In the acute phase group, the levels of OA-specific IgE and IgG1 were increased (P < 0.01; ANOVA). In the recovery phase group, the levels of OA-specific IgE and IgG1 were increased but lower than that of the acute phase group (both groups, P < 0.01; ANOVA). There were no differences in OA-specific IgG2a between the OA, acute phase, and recovery phase groups. In the control group, neither OA-specific IgG1, IgG2a, nor IgE was detected. Levels of immunoglobulins are expressed on a logarithmic scale. Cont, V, OA, A, and R denote the control, virus, OA, acute, and recovery phase groups, respectively. †, P < 0.01 (comparison among groups); ns, not significant.
Cytokine levels in BALF.
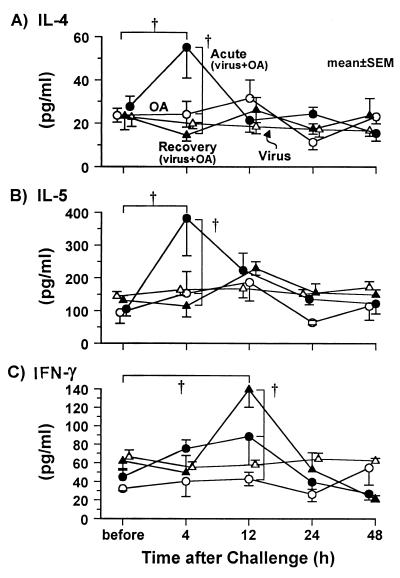
To study the mechanisms involved in the markedly enhanced cellular response, cytokine levels in BALF were measured before the challenge and 4, 12, 24, and 48 h after the final challenge. The levels of IL-4 in the acute phase group were increased by twofold at 4 h after the final challenge (P < 0.01); there was no change, however, in the OA group, the virus group, or the recovery phase group (Fig. 3A). The levels of IL-5 in the acute phase group were increased more than threefold 4 h after the final challenge (P < 0.01) (Fig. 3B); there was no change, however, in the OA group, the virus group, or the recovery phase group. At 12, 24, and 48 h after the final challenge, neither the IL-4 nor IL-5 levels were different from the respective control values before the challenge. The time course of the IL-4 and IL-5 levels were similar in the acute phase group. The levels of IFN-γ in BALF did not change in the acute phase, virus, and OA groups, but IFN-γ levels increased 12 h after the final challenge in the recovery phase group (P < 0.01) (Fig. 3C).
FIG. 3.
Cytokine profiles for IL-4 (A), IL-5 (B), and IFN-γ (C) in BALF before and after the antigen challenge. In the acute phase group (closed circles), the levels of IL-4 and IL-5 were increased by the antigen challenge (both groups, P < 0.01; ANOVA), but the levels of IFN-γ were not changed by the antigen challenge. In the recovery phase group (closed triangles), the levels of IFN-γ were increased by the antigen challenge (P < 0.01; ANOVA), but the levels of IL-4 and IL-5 were not changed. In both the OA group (open circles) and the virus group (open triangles), no changes in the levels of these cytokines were observed. †, P < 0.01 (comparison among groups, or comparison to the value before challenge).
Histological examination.
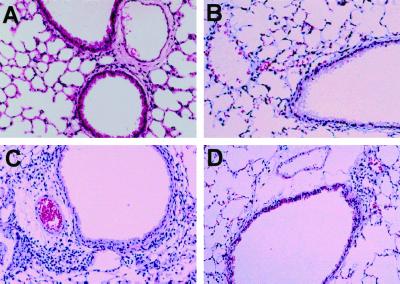
Histological examination of the lungs was performed 48 h after the final challenge (day 35 or day 42). There was no inflammatory infiltration in either the control or the OA group (Fig. 4A and B). In contrast, the acute phase group showed significant peribronchial infiltration of mononuclear cells and eosinophils (Fig. 4C). The recovery phase group, however, showed some peribronchial infiltration of mononuclear cells, but eosinophils were not detected (Fig. 4D).
FIG. 4.
Histology of the lung after the final challenge (hematoxylin-eosin staining). In the acute phase group (C), infiltration of mononuclear cells and eosinophils was observed in the submucosa. In the recovery phase group (D), little infiltration of mononuclear cells was observed in the submucosa and no eosinophils were detected in the lung. No inflammatory cell infiltration was observed in the control (A) and OA (B) groups. Magnification, ×100.
Immunohistochemistry.
To clarify how antigen presentation is affected by viral infection, we examined the expression of CD11c and MHC class II molecules on the bronchial epithelium with immunohistochemistry. In the acute phase group, the expression of MHC class II and CD11c molecules continued on the bronchial epithelium after sensitization on day 7 and continued up to day 35 (after OA challenge, data not shown). The staining of MHC class II and CD11c was quite similar to that of a previous study (32). The staining of CD11c on the bronchial epithelium paralleled that of MHC class II molecules in the acute phase group. In the recovery phase group, expression of neither MHC class II molecules nor CD11c was detected on the bronchial epithelium after sensitization on day 14 and after challenge on day 42 (data not shown).
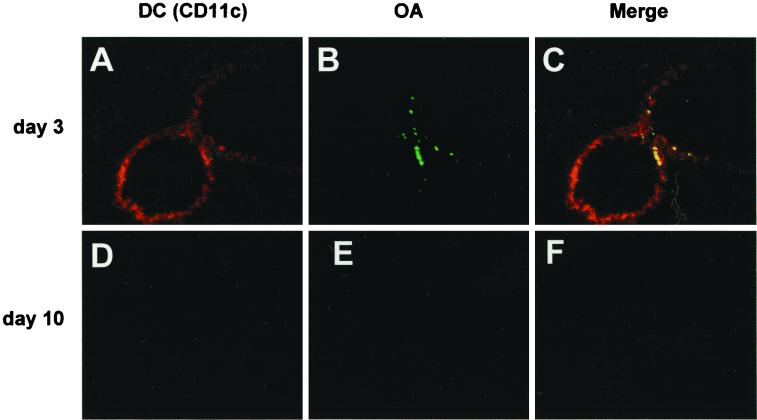
We examined whether APCs captured OA in the lung tissue and the mediastinal lymph nodes. In the acute phase group, DCs identified with the staining of CD11c were observed on the bronchial epithelium (Fig. 5A). OA was captured by DCs on the bronchial epithelium at 2 h after OA inhalation (Fig. 5C). In the recovery phase group, however, DCs were not detected on the bronchial epithelium (Fig. 5D), and inhaled OA was not detected in the lung (Fig. 5E). No phagocytosis of OA was observed (Fig. 5F).
FIG. 5.
Double coloring of the lung 2 h after OA inhalation on day 3 (A to C) or day 10 (D to F) (DCs are stained red; FITC-labeled OA is green). In the acute phase group (day 3), DCs stained with the MAb of CD11c migrated to the bronchial epithelium (A), and OA was observed at the same site of the lung (B). Merging of the two images shows OA-capturing APCs (in yellow) on the bronchial epithelium (C). In the recovery phase group (day 10), neither DCs nor OA was detected anywhere in the lung (D and E). Magnification, ×50.
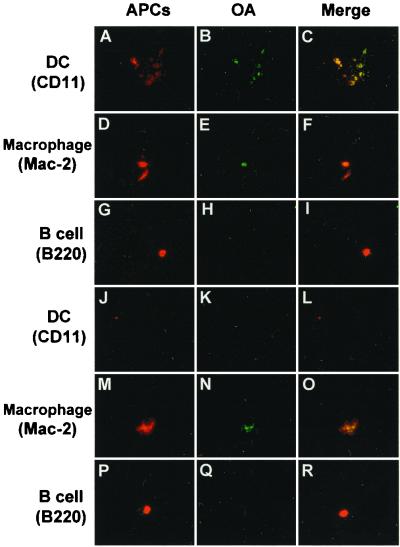
In the mediastinal lymph nodes of the acute phase group, a large number of DCs was observed 4 h after OA inhalation (Fig. 6A) and most of these DCs captured OA (Fig. 6C), although no OA-capturing DCs were observed 2 h after OA inhalation. A substantial number of macrophages were detected (Fig. 6D) and most of these macrophages captured OA (Fig. 6F). In contrast, a small number of B cells were observed (Fig. 6G) but with no phagocytosis of OA (Fig. 6I). In the recovery phase group, few DCs appeared in the lymph nodes and little OA was observed (Figs. 6J and K). Macrophages appeared in the lymph nodes (Fig. 6M) and captured OA (Fig. 6O).
FIG. 6.
Double coloring of the mediastinal lymph nodes 4 h after OA inhalation on day 3 (A to I) or day 10 (J to R). Phagocytosis by APCs (DCs, macrophages, and B cells) expressing MHC class II molecules was examined with immunohistochemistry. DCs (A and J; stained with MAb of CD11c), macrophages (D and M; stained with MAb of Mac-2), and B cells (G and P; stained with MAb of B220) are stained red; FITC-labeled OA is green. In the acute phase group, a large number of DCs (A) and a small number of macrophages (D) were observed in the lymph nodes, and more OA was observed at the site of the DCs (B) compared to that of macrophages (E). DCs captured OA in the lymph nodes (C); in addition, some phagocytosis by macrophages (F) but no phagocytosis by B cells was observed (I). In the recovery phase group, neither DCs nor B cells in the lymph nodes phagocytosed OA (L and R). However, OA-capturing macrophages were observed (O). Magnification, ×400.
DISCUSSION
In the present study, we demonstrated that OA inhalation during the acute phase of influenza A virus infection induces the type 2 immune response, while that during the recovery phase generates the type 1 immune response. Immunohistochemistry showed that infection with influenza A virus induced the migration of DCs to the bronchial epithelium only during the acute phase, but DCs disappeared from the airway on day 7 postinfection without additional stimulation by inhaled OA. In OA inhalation during this acute phase of viral infection, DCs captured the antigens on the bronchial epithelium and then OA-capturing DCs moved to the regional lymph nodes, where OA-capturing macrophages were also observed. However, during the recovery phase, DCs disappeared from the airway; only macrophages captured OA and migrated to the regional lymph nodes. These results indicate that DCs, acting as APCs, play a critical role in the induction of the type 2 immune response in the acute phase group. In the recovery phase group, however, only macrophages act as APCs which seem to induce the type 1 immune response.
From clinical studies (9, 10, 33), respiratory viral infection has been suggested to contribute to the development of asthma. Previous studies (28, 32) demonstrated that infection with influenza A virus enhances airway sensitization with antigen in mice. Schwarze et al. showed that RSV infection enhances the airway sensitization to allergen in a murine model resulting in airway hyperresponsiveness and eosinophilia (23). They started antigen inhalation on day 11 or 21 postinfection, and airway sensitization was enhanced similarly in both phases of RSV infection. In our model, OA inhalation during the acute phase of infection (days 3 to 7) induces airway hyperresponsiveness, eosinophilia, and IgE production, but OA inhalation during the recovery phase (days 7 to 11) does not cause these changes. Thus, there are some differences in airway sensitization between infections of influenza A virus and RSV. Recently, Schwarze et al. demonstrated that CD8+ T cells play a critical role in the development of RSV-induced airway hyperresponsiveness and eosinophilia (24). In a previous study (32), we found an increase in the ratio of CD8+ to CD4+ T cells, which was accompanied with the increased production of Th2 cytokine in BALF. Thus, CD8+ T cells seem to play an essential role in the development of airway sensitization in viral infection-induced airway sensitization with antigen in both models. On the other hand, RSV infection itself increases eosinophils and neutrophils (23). In our preliminary study, influenza A virus infection itself induced significant increases in the number of lymphocytes and neutrophils in BALF on day 7 postinfection, but eosinophils were not found. It has also been reported that the G glycoprotein of RSV promotes a Th2-like immune response and induces an eosinophilic response in lungs of RSV-infected mice (14). Thus, there are some differences between the immune responses to infection by RSV and influenza A virus.
DCs are well known to play a critical role in antigen presentation in vivo. In viral infection, DCs act as the APCs that initiate MHC class I-restricted cytotoxic T-lymphocyte responses (4, 5, 11, 26). On the other hand, certain types of viruses such as Sendai virus and influenza A virus have been shown to induce the migration of DCs to the bronchial epithelium and the expression of MHC class II molecules during the acute phase of viral infection (18, 28). It has also been reported that respiratory DCs preferentially stimulate a Th2 response (27). With exogenous antigen, it is well established that DCs are the main professional APCs for naive CD4+ T cells via MHC class II molecules (2, 13, 26). Resident DCs in nonlymphoid tissues are of the immature type (2, 21). Once the DCs have captured antigens, they mature and their ability to capture antigens rapidly declines, accompanied by the expression of assemble antigen-MHC class II complexes on their cell surfaces (2). Upon maturation, the DCs migrate to the lymphoid tissue. There, DCs may complete their maturation and induce antigen-specific immune responses by CD4+ T cells, which secrete IL-4 and IL-5. In the present study, DCs first migrated to the bronchial epithelium during the acute phase of viral infection and some DCs were retained by simultaneous sensitization with inhaled antigens. Antigen-capturing DCs migrate to the draining lymph nodes and interact with naive T cells (21). Our results indicate that OA inhalation during the acute phase of viral infection is important not only for the induction of a primary immune response initiated by DCs but also for the retention of DCs on the airway, which is necessary for the induction of the secondary immune response by antigen challenge.
During the recovery phase of viral infection, OA inhalation caused the type 1 immune response, and neither nonphagocytic DCs nor OA-capturing DCs were detected in the airway. These results mean that once DCs disappear from the airway on day 7 postinfection, they never come back to the airway even with additional stimulation by inhaled OA during the recovery phase. In the mediastinal lymph nodes, however, OA-capturing macrophages were observed, whereas neither DCs nor B cells were detected. Within the respiratory immune system, both pulmonary macrophages and DCs are able to capture, process, and present particulate antigens (3, 12), although DCs are regarded as the main APCs in vivo, due to their unique ability to stimulate antigen-specific T lymphocytes (2, 26). In our preliminary study, the number of macrophages in BALF after infection with influenza A virus increased 2.5- and 1.8-fold above that of naive animals on days 3 and 14, respectively. Thus, during the recovery phase of viral infection, macrophages are abundant in the airway whereas DCs are depleted, suggesting that macrophages may have acted as the primary APCs with exogenous antigens. The macrophages secrete IL-12 and induce the type 1 immune response (16). Thus, during the recovery phase of viral infection, the macrophages but not DCs can capture the antigens and migrate into the draining lymph nodes, inducing the type 1 immune response.
In the airways of the acute phase group, DCs were shown to capture inhaled antigens on bronchial epithelium. These antigen-capturing DCs moved to the draining lymph nodes at 4 h after the antigen inhalation. DCs in the draining lymph nodes are reported to express antigen-presenting activities at 24 h after the injection of an antigen (31). Inflammatory stimuli are known to induce a rapid formation of peptide-MHC class II complexes (6), and activated DCs travel to the regional lymph nodes (2). Therefore, inflammatory stimuli generated by the influenza virus infection may cause DCs to mature after capturing inhaled antigens and then migrate to the regional lymph nodes within 4 h of antigen exposure. These results, therefore, indicate that the primary immune response (sensitization) induced by antigen-capturing DCs occurs primarily in the regional lymph nodes. On the other hand, some DCs were retained on the bronchial epithelium for at least 5 weeks in the acute phase group, indicating that stimulation by exogenous antigen following viral infection causes the prolonged retention of DCs on the bronchial epithelium and that DCs can be involved in the secondary immune response, i.e., the production of Th2 cytokine and eosinophilic infiltration of the airway. These findings are compatible with the results of a study by Lambrecht et al. (15) showing that DCs are essential for presenting antigens to previously primed Th2 cells in the lung. Thus, the DCs seem to play a critical role not only in the induction of the primary immune response but also in the expanded secondary immune response to antigen challenge.
In summary, we have demonstrated that influenza A virus infection causes the migration of DCs to the bronchial epithelium and enhances airway sensitization during the acute phase of infection. However, antigen sensitization during the recovery phase of viral infection does not affect airway sensitization. Antigen inhalation via the airway in different phases of influenza A virus infection may differentially induce the type 1 or type 2 immune response by different APCs.
ACKNOWLEDGMENT
This research was supported by a grant-in-aid (no. 12670568) from the Japanese Ministry of Education, Science, Sport, and Culture to S.S.
REFERENCES
- 1.Ackerman V, Marini M, Vittori E, Bellini A, Vassali G, Mattoli S. Detection of cytokines and their cell sources in bronchial biopsy specimens from asthmatic patients. Chest. 1994;105:687–696. doi: 10.1378/chest.105.3.687. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Bevan M J. Antigen presentation to cytotoxic T lymphocytes in vivo. J Exp Med. 1995;182:639–641. doi: 10.1084/jem.182.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhardwaj N. Interactions of viruses with dendritic cells: a double-edged sword. J Exp Med. 1997;186:795–799. doi: 10.1084/jem.186.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhardwaj N, Bender A, Gonzalez N, Bui L K, Garret M C, Steinman R M. Influenza virus-infected dendritic cells stimulate strong proliferative and cytolytic responses from human CD8+ T cells. J Clin Investig. 1994;94:797–807. doi: 10.1172/JCI117399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 7.De Camilli P, Cameron R, Greengard P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. 1. Its general distribution in synapses of the central and peripheral nervous system demonstrated by immunofluorescence in frozen and plastic sections. J Cell Biol. 1983;96:1337–1354. doi: 10.1083/jcb.96.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doherty P C, Allan W, Eichelberger M, Carding S R. Roles of αβ and γδ T cell subsets in viral immunity. Annu Rev Immunol. 1992;10:123–151. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- 9.Frick O L, German D F, Mills J. Development of allergy in children: I. Association with virus infections. J Allergy Clin Immunol. 1979;63:228–241. doi: 10.1016/0091-6749(79)90106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurwitz D, Mindorff C, Levison H. Increased incidence of bronchial reactivity in children with a history of bronchiolitis. J Pediatr. 1981;98:551–555. doi: 10.1016/s0022-3476(81)80758-5. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton-Easton A, Eichelberger M. Virus-specific antigen presentation by different subsets of cells from lung and mediastinal lymph node tissues of influenza virus-infected mice. J Virol. 1995;69:6359–6366. doi: 10.1128/jvi.69.10.6359-6366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holt P G, Schon-Hegrad M A. Localization of T cells, macrophages and dendritic cells in rat respiratory tract tissue: implications for immune function studies. Immunology. 1987;62:349–356. [PMC free article] [PubMed] [Google Scholar]
- 13.Inaba K, Metlay J P, Crownley M T, Steinman R M. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990;172:631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson T R, Johnson J E, Roberts S R, Wertz G W, Parker R A, Graham B S. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol. 1998;72:2871–2880. doi: 10.1128/jvi.72.4.2871-2880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambrecht B N, Salomon B, Klatzmann D, Pauwels R A. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J Immunol. 1998;160:4090–4097. [PubMed] [Google Scholar]
- 16.Maldonado-López R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mbow M L, Rutti B, Brossard M. Infiltration of CD4+ and CD8+ T cells, and expression of ICAM-1, Ia antigens, IL-1α and TNF-α in the skin lesion of BALB/c mice undergoing repeated infestations with nymphal Ixodes ricinus ticks. Immunology. 1994;82:596–602. [PMC free article] [PubMed] [Google Scholar]
- 18.McWilliam A S, Marsh A M, Holt P G. Inflammatory infiltration of the upper airway epithelium during Sendai virus infection: involvement of epithelial dendritic cells. J Virol. 1997;71:226–236. doi: 10.1128/jvi.71.1.226-236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metlay J P, Witmer-Pack M D, Agger R, Crownley M T, Lawless D, Steinman R M. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renz H, Smith H R, Henson J E, Ray B S, Irvin C G, Gelfand E W. Aerosolized antigen exposure without adjuvant causes increased IgE production and increased airway responsiveness in the mouse. J Allergy Clin Immunol. 1992;89:1127–1138. doi: 10.1016/0091-6749(92)90296-e. [DOI] [PubMed] [Google Scholar]
- 21.Romani N, Koide S, Crowley M, Witmer-Pack M, Livingstone A M, Fathman C G, Inaba K, Steinman R M. Presentation of exogenous protein antigens by dendritic cells to T cell clones: intact protein is presented best by immature, epidermal Langerhans cell. J Exp Med. 1989;169:1169–1178. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarawar S R, Doherty P C. Concurrent production of interleukin-2, interleukin-10, and gamma interferon in the regional lymph nodes of mice with influenza pneumonia. J Virol. 1994;68:3112–3119. doi: 10.1128/jvi.68.5.3112-3119.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarze J, Hamelmann E, Bradley K, Takeda K, Gelfand E W. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J Clin Investig. 1997;99:226–233. doi: 10.1172/JCI119516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarze J, Mäkelä M J, Cieslewicz G, Dakhama A, Lahn M, Ikemura T, Joetham A, Gelfand E W. Transfer of the enhancing effect of respiratory syncytial virus infection on subsequent allergic airway sensitization by T lymphocytes. J Immunol. 1999;163:5729–5734. [PubMed] [Google Scholar]
- 25.Seder R A, Paul W E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 26.Steinman R M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 27.Stumbles P A, Thomas J A, Pimm C L, Lee P T, Venaille T J, Proksch S, Holt P G. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 Immunity. J Exp Med. 1998;188:2019–2031. doi: 10.1084/jem.188.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki S, Suzuki Y, Yamamoto N, Matsumoto Y, Shirai A, Okubo T. Influenza A virus infection increases IgE production and airway responsiveness in aerosolized antigen-exposed mice. J Allergy Clin Immunol. 1998;102:732–740. doi: 10.1016/S0091-6749(98)70012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Heuvel M M, Vanhee D D C, Postmus P E, Hoefsmit C M, Beelen R H J. Functional and phenotypic differences of monocyte-derived dendritic cells from allergic and nonallergic patients. J Allergy Clin Immunol. 1998;101:90–95. doi: 10.1016/S0091-6749(98)70198-8. [DOI] [PubMed] [Google Scholar]
- 30.Vremec D, Shortman K. Dendritic cell subtypes in mouse lymphoid organs. J Immunol. 1997;159:565–573. [PubMed] [Google Scholar]
- 31.Xia W, Pinto C E, Kradin R L. The antigen-presenting activities of Ia+ dendritic cells shift dynamically from lung to lymph node after an airway challenge with soluble antigen. J Exp Med. 1995;181:1275–1283. doi: 10.1084/jem.181.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto N, Suzuki S, Shirai A, Suzuki M, Nakazawa M, Nagashima Y, Okubo T. Dendritic cells are associated with augmentation of antigen sensitization by influenza A virus infection in mice. Eur J Immunol. 2000;30:316–326. doi: 10.1002/1521-4141(200001)30:1<316::AID-IMMU316>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 33.Zweiman B, Schoenwetter W F, Pappano J E, Jr, Tempest B, Hildreth E A. Patterns of allergic respiratory disease in children with a past history of bronchiolitis. J Allergy Clin Immunol. 1971;48:283–289. doi: 10.1016/0091-6749(71)90029-7. [DOI] [PubMed] [Google Scholar]