Abstract
The coronavirus nucleoprotein (N) has been reported to be involved in various aspects of virus replication. We examined by confocal microscopy the subcellular localization of the avian infectious bronchitis virus N protein both in the absence and in the context of an infected cell and found that N protein localizes both to the cytoplasmic and nucleolar compartments.
Coronaviruses are enveloped viruses with positive-stranded, capped and polyadenylated RNA genomes ranging in size between 28 and 32 kb (15). The coronavirus family, Coronaviridae, has been grouped together with the arterivirus family, Arteriviridae, into the order Nidovirales (5). The virus families have many similarities. The 5′ two-thirds of the nidovirus genome encodes the replicase gene producing two polyproteins, Rep1a and Rep1ab, the latter resulting from a -1 frameshift. The remaining proteins are expressed from a nested set of subgenomic mRNAs (sgRNAs) that are produced via a discontinuous transcription mechanism. In the genus Coronavirus, these proteins include the structural proteins, spike (S), envelope (E), membrane (M), and nucleoprotein (N), and several other proteins of unknown function. In both virus families, the virus replication complexes are thought to be membrane associated (24, 28, 29, 35).
While gene function and distribution between the two families are similar, there are some differences that might lead to subtle differences in replication strategies. Rowland et al. (26) recently reported, following the use of confocal microscopy, that the arterivirus porcine reproductive and respiratory syndrome virus (PRRSV) N protein localizes to both the cytoplasm and nucleolus in infected cells. In contrast, the coronavirus N protein has previously been thought to localize only to the cytoplasm (17). Using confocal microscopy, we decided to investigate the intracellular localization of the coronavirus N protein in both the absence and presence of other viral proteins.
Coronavirus N proteins vary from 377 to 455 amino acids in length, are highly basic, and have a high (7 to 11%) serine content, which are potential targets for phosphorylation. Sequence conservation of the N proteins within the genus is low.
Three groups of coronaviruses have been identified. The N proteins of the coronaviruses avian infectious bronchitis virus (IBV; group III) and porcine transmissible gastroenteritis virus (group I) have only 29% identity with that of bovine coronavirus (group II). Within the group II coronaviruses, the N proteins of murine hepatitis virus and bovine coronavirus share only 70% identity (16). Based on sequence comparisons, three structural domains have been identified in the coronavirus N protein (23), of which the middle domain was identified as a potential RNA binding domain (18, 21), capable of binding both coronavirus- and non-coronavirus-derived RNA sequences in vitro (18, 31). No functions have been ascribed to the other two domains.
The coronavirus N protein is the most abundant virus-derived protein produced throughout infection, probably because its template mRNA is the most abundant sgRNA (11, 13) produced during transcription (12). Several functions have been postulated for the coronavirus N protein throughout the virus life cycle (17). Primarily, it complexes with the coronavirus genomic RNA to form a ribonucleocapsid structure (8), and it has been observed, together with the M protein, to be a component of the viral core (25). The N protein has been shown to associate with the leader RNA sequence (2, 22) located at the 5′ end of the genomic RNA and/or to sequences at the 3′ end of the genomic RNA (41). As these regions are believed to be involved in synthesis of coronavirus RNA, the N protein has been postulated to have a role in replication of the genomic RNA (6, 7), in transcription of coronavirus sgRNAs (2, 31), and in translation from the sgRNAs (33). However, replication and transcription have been shown to occur in the absence of N protein in the arterivirus equine arteritis virus (20), although N protein may still have some nonessential involvement in this process.
Expression of N protein in the absence of other viral proteins.
To investigate the intracellular distribution of a coronavirus N protein, the N gene of the Beaudette strain of the avian coronavirus IBV was inserted into the eukaryotic expression vector pCi-Neo (Promega) such that expression of the N gene was under the control of a cytomegalovirus (CMV) polymerase II promoter. The IBV N gene was produced by PCR, using Pfu polymerase (Stratagene), from a plasmid containing an authentic copy of the Beaudette N gene (3). An oligonucleotide (GTCATGGCAAGCGGTAAAGCAGC) corresponding to the 5′ end of the IBV N gene, which contained an optimal Kozak sequence (14), and an oligonucleotide (TCAAAGTTCATTCTCTCCTA) complementary to the 3′ end of the IBV N gene were used for the PCR. The resulting PCR product, corresponding to the IBV N gene, was initially inserted into pTarget (Promega) that had been circularized and then digested with SmaI, creating pT-N. The IBV N gene was excised from pT-N by digestion with XhoI and NotI and directionally inserted into pCi-Neo (Promega), such that transcription of the IBV N gene was under the control of the CMV promoter, thus generating pCi-N; the sequence was confirmed.
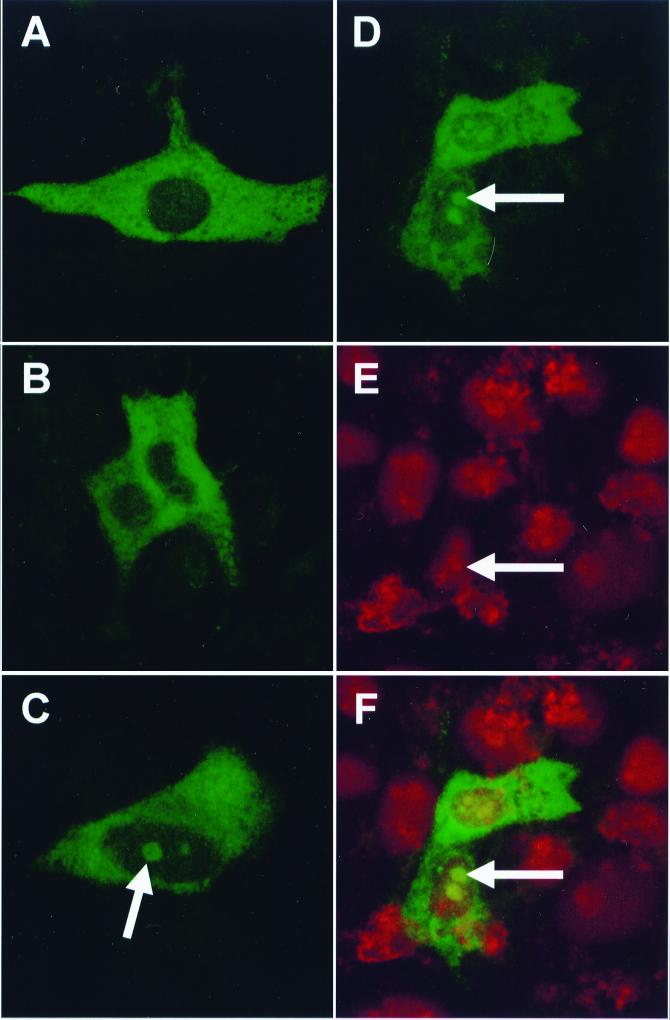
Vero cells (105 per 9.6-cm2 dish) were transfected with 2 μg of pCi-N and 50 μg of Lipofectamine (GibcoBRL). Cells were grown on coverslips and fixed 24 h posttransfection with 50% methanol–50% acetone for analysis by indirect immunofluorescence using rabbit anti-IBV polyclonal sera (19) followed by fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit antibody (Harlan Sera-Lab). The IBV N protein expressed from pCi-N was observed to be distributed throughout the cytoplasm (Fig. 1A and B) and to be colocalized to a structure within the nucleus, tentatively identified as the nucleolus (Fig. 1C). While the number of transfected cells remained constant, the number of cells in which the N protein was colocalized to the nucleus varied each time the experiment was repeated. To confirm that the identified structure was the nucleolus and that N protein colocalized with this structure, cells were stained with propidium iodide to visualize nuclear DNA and nucleoli. Fluorescent images were obtained from the same 0.5-μm optical section by using a confocal microscope (Leica) and the appropriate filters to identify IBV N protein (Fig. 1D) and nuclear DNA (Fig. 1E). The images were digitally superimposed to depict the distribution of N protein and nuclear DNA (Fig. 1F). Nucleoli were identified as distinct regions within the nucleus in images from both optical sections, and localization of the IBV N protein to the nucleolus was confirmed (Fig. 1D and E).
FIG. 1.
Vero cells were transfected with pCi-N, incubated for 24 h, fixed, and analyzed by indirect immunofluorescence using rabbit anti-IBV sera and FITC-labeled goat anti-rabbit antibodies. Additionally, cells were stained with propidium iodide (D to F) to visualize nuclear DNA. For images A to C, the fluorescing protein images were gathered from different 0.5-μm optical sections by using a confocal microscope and the appropriate filter; for images D to F, the differentially fluorescing IBV N protein (D) and nuclear DNA images (E) were gathered separately from the same 0.5-μm optical section by using a confocal microscope and appropriate filters. The two images were digitally superimposed to depict the distribution of the IBV N protein and nuclear DNA (E). Each arrow indicates the position of a nucleolus. Magnification, ×63.
Expression of viral proteins in the context of an IBV-infected cell.
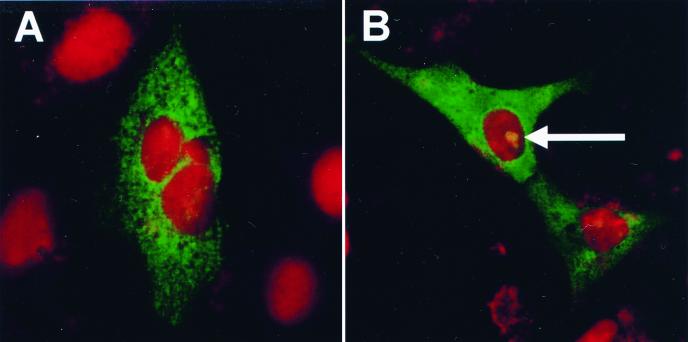
Vero cells (105 per 9.6-cm2 dish) were infected with a Vero-adapted strain of IBV at a multiplicity of infection of 1 and fixed 24 h postinfection. The IBV-infected fixed cells were stained with propidium iodide, to visualize nuclear DNA, and analyzed by indirect immunofluorescence as before to determine whether any of the IBV proteins were localized to the infected cell nucleoli. Confocal images corresponding to the IBV proteins and nuclear DNA were gathered separately from the same 0.5-μm optical section. IBV proteins were observed to localize in only the cytoplasm (Fig. 2A) and in the cytoplasm and nucleolus (Fig. 2B). A similar result was observed in cells infected with PRRSV, up to 75% of which showed localization of the PRRSV N protein to the nucleolus 24 h posttransfection (26). However, in 90% of the IBV-infected cells, no IBV protein was detected in the nucleus. A possible reason for the observation that fewer IBV-infected cells showed localization of an IBV protein(s) to the nucleolus is the number of cells undergoing mitosis. Nucleoli are absent from mitotic cells (1). For example, cells in which no IBV protein was observed in the nucleolus (Fig. 2A) contained two nuclei, indicating that they were undergoing mitosis. In contrast, cells in which IBV protein was found to be localized to the nucleolus (Fig. 2B) contained only one nucleus, indicating that they were in interphase and that rRNA synthesis and assembly were taking place as a consequence of the presence of nucleoli.
FIG. 2.
Vero cells were infected with IBV Beaudette at an multiplicity of infection of 1, incubated for 24 h, fixed, and stained in propidium iodide to visualize nuclear DNA. Indirect immunofluorescence using rabbit anti-IBV sera and FITC-labeled goat anti-rabbit antibodies was used to detect IBV proteins. For each image, the differentially fluorescing protein and nuclear DNA images were gathered separately from the same 0.5-μm optical section by using a confocal microscope and appropriate filters. The arrow indicates the position of a nucleolus. Magnification, ×63.
Based on the expression profile of the IBV N protein from pCi-N, in the absence of other IBV proteins, the most likely IBV protein localizing in the nucleoli of infected cells is N. The other IBV structural proteins, S, M, and E, all contain transmembrane domains and are localized to the virus envelope; they require processing through the endoplasmic reticulum pathway and are not observed in the cytosol. In confirmation, no nucleolar localization of the IBV M protein was observed following expression of the M protein under the control of a CMV promoter (data not shown). The observation that an IBV protein, presumably N, can localize to the nucleolus in virus-infected cells indicated that the nucleolar localization of the IBV N protein expressed from the CMV promoter was not an artifact of the expression system. Localization of the N protein to the nucleoli is not a phenomenon limited to coronaviruses. The observation that in an arterivirus, PRRSV (26), and a coronavirus, IBV, N protein colocalizes to the nucleoli of infected cells is a phenomenon probably common to these two virus families and potentially common to all Nidovirales.
Targeting of an exogenous protein to the nucleolus.
To investigate whether the IBV N protein could be used to transport an exogenous protein to the nucleolus, a reporter gene was fused to the carboxy terminus of the N protein. The IBV N gene was produced by PCR as before except that the oligonucleotide (AAAGTTCATTCTCTCCTA) complementary to the 3′ end of the N gene contained a leucine codon in place of IBV N gene translational stop codon. The resulting PCR product was inserted into pcDNA3.1/CT-GFP-TOPO (Invitrogen); the resulting plasmid, pN-GFP, consisted of a chimeric IBV N-green fluorescent protein (GFP) gene in which GFP fluorescence was dependent on translation of the N-GFP fusion protein.
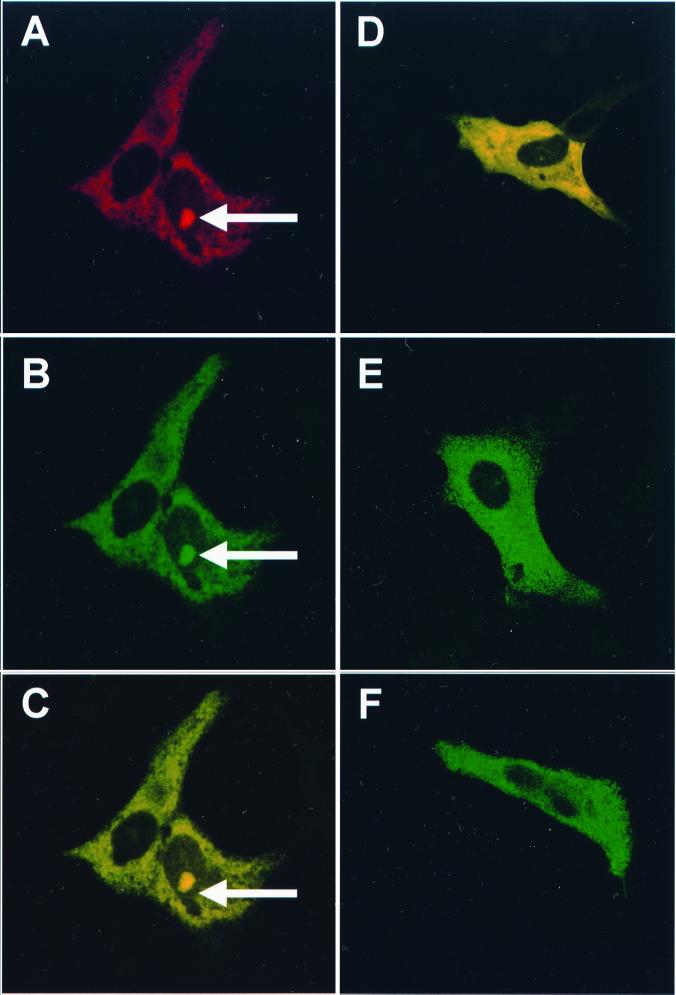
Vero cells (105 per 9.6-cm2 dish) were transfected with 2 μg of pN-GFP and 50 μg of Lipofectamine, incubated for 24 h, fixed, and analyzed by indirect immunofluorescence as before except that rabbit anti-IBV antibodies were detected using an anti-rabbit Alexa Fluor 564 (Molecular Probes). The differentially fluorescing N protein (Fig. 3A) and GFP (Fig. 3B) images were gathered separately as before and then digitally superimposed to depict the relative distributions of the two proteins (Fig. 3C). To investigate whether GFP could localize to the nucleolus without being fused to the IBV N protein, Vero cells were transfected with a plasmid expressing GFP. No nucleolar localization of GFP was observed (Fig. 3E and F). These results demonstrated that the IBV N-GFP fusion protein colocalized to both the cytoplasm and nucleolus, indicating that the IBV N protein moiety directed GFP to the nucleolus. In approximately 90% of the cells transfected with pN-GFP, the IBV N-GFP fusion protein did not colocalize to the nucleolus (Fig. 3D), a result similar to that observed when IBV N protein was expressed either alone or in the context of an IBV-infected cell. Expression of a PRRSV N-GFP fusion protein in transfected cells also led to the colocalization of the fusion protein to both the cytoplasm and nucleolus, although the distribution between the two regions was not detailed (26).
FIG. 3.
Vero cells were transfected with pN-GFP (A to D) or pGFP (E and F) and incubated for 24 h; IBV N protein was detected by indirect immunofluorescence using rabbit anti-IBV sera and anti-rabbit Alexa Fluor 564. The differentially fluorescing IBV N protein (A) and GFP (B) images were gathered separately from the same 0.5-μm section and from different sections (D to F) by using a confocal microscope and appropriate filters; the two images were digitally superimposed (C to F). Each arrow indicates the position of a nucleolus. Magnification, ×63.
Murine hepatitis virus has been shown to replicate in enucleated cells (38), indicating that the nucleus is not required for coronavirus replication. However, our data have shown that the IBV N protein can localize to the nucleolus as well as to the cytoplasm. In each instance, N protein in IBV-infected cells, expressed alone or as a fusion protein with GFP, was localized either in the cytoplasm but not in the nucleoli or in both regions of the cell. These results are in accordance with those described for the expression and colocalization of the arterivirus PRRSV N protein (26). The number and size of nucleoli in a cell varies during the cell cycle (1): multiple nucleoli are present at the beginning of G1 (Fig. 1F), and a single nucleolus is present during the later stages of G1, S, and G2 (e.g., Fig. 1C and 3C). Nucleoli are absent or dissociated from cells during mitosis, which is consistent with the observation that localization of the IBV N protein to the nucleus of cells identified as undergoing mitosis never occurred (Fig. 2A). However, mitosis alone cannot account for the absence of N protein from the nucleoli in the majority of transfected or infected cells, which suggests that localization of the IBV N protein to the nucleoli might be dependent on a particular event(s) or phase of the cell cycle that the transfected or infected cell was in.
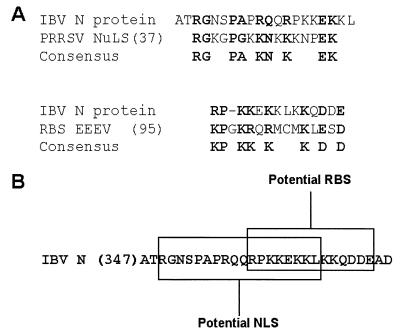
The mechanism by which the IBV N protein was transported to the nucleolus was not elucidated in this study. However, the nucleolus is the site for rRNA synthesis and ribosome assembly, and as such many ribosomal proteins from the cytoplasm are transported to this structure (4). Based on comparison with other virus nucleolar localization signals (NuLSs) and deletion mutagenesis, Rowland et al. (26) identified two separate domains within the N terminus of PRRSV N protein that might target this protein to the nucleolus. Furthermore, experimental evidence indicated that the second region was sufficient to target PRRSV N protein to the nucleolus (26). Amino acid sequence comparison between the putative NuLS of PRRSV and IBV N protein indicated that the C-terminal region of the IBV N protein might contain the IBV homologue of the PRRSV N protein NuLS motif (Fig. 4A). Sequence comparison indicated that the potential NuLS core motif identified in IBV strain Beaudette was conserved in 10 other strains of IBV (GenBank accession numbers are shown in brackets): Ark99 [M85244], DE072 [AF203001], M41 [M28566], VicS [U528566], V5/90 [U52595], N2/75 [U52598], N1/62 [U52596], N9/74 [U52597], QXIBV [AF199412], and KB8523 [M21515] (3, 27, 32, 39).
FIG. 4.
(A) Amino acid sequence alignments to identify a potential NuLS and an RBS on the IBV Beaudette strain N protein, using the PRRSV NuLS (26) and eastern equine encephalitis virus (EEEV) RBS (37) as a basis for comparison. Locations of the two potential motifs on the IBV N protein are also shown (B). Similar amino acids are shown in boldface; the start position of the appropriate amino acid on the full-length protein is indicated in parentheses.
Many other RNA viruses contain RNA binding proteins. For example, the alphavirus RNA binding protein (capsid protein) associates with alphavirus RNA and ribosomes to promote disassembly and assembly of the virus particle (34, 36), and a ribosome binding site (RBS) has been identified (37). Sequence comparison between the RBS of eastern equine encephalitis virus and the IBV N protein identified a similar motif in N protein (Fig. 4A). Interestingly, the potential IBV RBS motif localizes to the same region as the putative NuLS (Fig. 4B), possibly because this is a lysine-rich region. One can speculate that the IBV N protein might associate with ribosomal proteins, possibly as part of a strategy to control coronavirus translation and/or as a by-product of a ribosome-mediated uncoating of the viral core particle.
In coronavirus-infected cells, host cell translation has been reported to be down-regulated (9, 30), while translation of virus-encoded proteins is up-regulated (33). This multifactorial regulation of translation during a coronavirus infection could be due to the coronavirus N protein. First, by localizing to the nucleolus, the coronavirus N protein might interfere with host-cell translation by disrupting the formation of new ribosomes and possibly the cell cycle. Second, by binding to the 5′ end of coronavirus-derived RNA (22), the N protein may be recruiting ribosomes for translation of viral RNAs.
Alternatively, interaction of ribosomes with the coronavirus core could cause destabilization and release of genomic RNA. Similar processes have been observed with virus proteins that bind genomic RNA of alphaviruses (36), tobacco mosaic virus (40), and nodaviruses (10). The coronavirus N protein may therefore traffic to the nucleolus in association with ribosomal proteins resulting from disassembly of the coronavirus cores for the release of the genomic RNA.
Acknowledgments
This work was supported by a BBSRC program grant (45/S12883) and a Royal Society research grant (21651) to J.A.H. and grant CT950064 of the Fourth RTD Framework Program of the European Commission to D.C. and P.B. T.W. was supported by a Reading Endowment Trust Fund awarded to J.A.H.
We thank Steve Poutney for excellent assistance with the confocal microscope.
REFERENCES
- 1.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson J D. Molecular biology of the cell. 3rd ed. New York, N.Y: Garland Publishing; 1994. pp. 381–382. [Google Scholar]
- 2.Baric R S, Nelson G W, Fleming J O, Deans R J, Keck J G, Casteel N, Stohlman S A. Interactions between coronavirus nucleocapsid protein and viral RNAs: implications for viral transcription. J Virol. 1988;62:4280–4287. doi: 10.1128/jvi.62.11.4280-4287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boursnell M E G, Binns M M, Foulds I J, Brown T D K. Sequence of the nucleocapsid genes from two strains of avian infectious bronchitis virus. J Gen Virol. 1985;66:573–580. doi: 10.1099/0022-1317-66-3-573. [DOI] [PubMed] [Google Scholar]
- 4.Carmo-Fonseca M, Mendes-Soares L, Campos I. To be or not to be in the nucleolus. Nat Cell Biol. 2000;2:E107–E112. doi: 10.1038/35014078. [DOI] [PubMed] [Google Scholar]
- 5.Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- 6.Chang R-Y, Brian D A. cis requirement for N-specific protein sequence in bovine coronavirus defective interfering RNA replication. J Virol. 1996;70:2201–2207. doi: 10.1128/jvi.70.4.2201-2207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Compton J R, Rogers D B, Holmes K V, Fertsch D, Remenick J, McGowan J J. In vitro replication of mouse hepatitis virus strain A59. J Virol. 1987;61:1814–1820. doi: 10.1128/jvi.61.6.1814-1820.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies H A, Dourmashkin R R, MacNaughton R. Ribonucleoprotein of avian infectious bronchitis virus. J Gen Virol. 1981;53:67–74. doi: 10.1099/0022-1317-53-1-67. [DOI] [PubMed] [Google Scholar]
- 9.Hilton A, Mizzen L, Macintyre G, Cheley S, Anderson R. Translational control in murine hepatitis virus infection. J Gen Virol. 1986;67:923–932. doi: 10.1099/0022-1317-67-5-923. [DOI] [PubMed] [Google Scholar]
- 10.Hiscox J A, Ball L A. Cotranslational disassembly of flock house virus in a cell-free system. J Virol. 1997;71:7974–7977. doi: 10.1128/jvi.71.10.7974-7977.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiscox J A, Cavanagh D, Britton P. Quantification of individual subgenomic mRNA species during replication of the coronavirus transmissible gastroenteritis virus. Virus Res. 1995;36:119–130. doi: 10.1016/0168-1702(94)00108-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiscox J A, Mawditt K L, Cavanagh D, Britton P. Investigation of the control of coronavirus subgenomic mRNA transcription by using T7-generated negative-sense RNA transcripts. J Virol. 1995;69:6219–6227. doi: 10.1128/jvi.69.10.6219-6227.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keck J G, Hogue B G, Brian D A, Lai M M C. Temporal regulation of bovine coronavirus RNA synthesis. Virus Res. 1988;9:343–356. doi: 10.1016/0168-1702(88)90093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991;115:887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai M M C, Cavanagh D. The molecular biology of coronaviruses. Adv Virus Res. 1997;48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lapps W, Hogue B G, Brian D A. Sequence analysis of the bovine coronavirus nucleocapsid and matrix protein gene. Virology. 1987;157:47–57. doi: 10.1016/0042-6822(87)90312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laude H, Masters P S. The coronavirus nucleocapsid protein. In: Siddell S G, editor. The Coronaviridae. New York, N.Y: Plenum Press; 1995. pp. 141–163. [Google Scholar]
- 18.Masters P S. Localization of an RNA-binding domain in the nucleocapsid protein of the coronavirus mouse hepatitis virus. Arch Virol. 1992;125:141–160. doi: 10.1007/BF01309634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mockett A P A. Envelope proteins of avian infectious bronchitis virus: purification and biological properties. J Virol Methods. 1985;12:271–278. doi: 10.1016/0166-0934(85)90138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molenkamp R, van Tol H, Rozier B C D, van der Meer Y, Spaan W J M, Snijder E J. The arterivirus replicase is the only viral protein required for genome replication and subgenomic mRNA transcription. J Gen Virol. 2000;81:2491–2496. doi: 10.1099/0022-1317-81-10-2491. [DOI] [PubMed] [Google Scholar]
- 21.Nelson G W, Stohlman S A. Localization of the RNA-binding domain of mouse hepatitis virus nucleocapsid protein. J Gen Virol. 1993;74:1975–1979. doi: 10.1099/0022-1317-74-9-1975. [DOI] [PubMed] [Google Scholar]
- 22.Nelson G W, Stohlman S A, Tahara S M. High affinity interaction between nucleocapsid protein and leader/intergenic sequence of mouse hepatitis virus RNA. J Gen Virol. 2000;81:181–188. doi: 10.1099/0022-1317-81-1-181. [DOI] [PubMed] [Google Scholar]
- 23.Parker M M, Masters P S. Sequence comparison of the N genes of 5 strains of the coronavirus mouse hepatitis virus suggests a 3 domain-structure for the nucleocapsid protein. Virology. 1990;179:463–468. doi: 10.1016/0042-6822(90)90316-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedersen K W, van der Meer Y, Roos N, Snijder E J. Open reading frame 1a-encoded subunits of the arterivirus replicase induce endoplasmic reticulum-derived double-membrane vesicles which carry the viral replication complex. J Virol. 1999;73:2016–2026. doi: 10.1128/jvi.73.3.2016-2026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Risco C, Anton I M, Enjuanes L, Carrascosa J L. The transmissible gastroenteritis coronavirus contains a spherical core shell consisting of M and N proteins. J Virol. 1996;70:4773–4777. doi: 10.1128/jvi.70.7.4773-4777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowland R R, Kerwin R, Kuckleburg C, Sperlich A, Benfield D A. The localization of porcine reproductive and respiratory syndrome virus nucleocapsid protein to the nucleolus of infected cells and identification of a potential nucleolar localization signal sequence. Virus Res. 1999;64:1–12. doi: 10.1016/s0168-1702(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 27.Sapats S I, Ashton F, Wright P J, Ignjatovic J. Novel variation in the N protein of avian infectious bronchitis virus. Virology. 1996;226:412–417. doi: 10.1006/viro.1996.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sethna P B, Brian D A. Coronavirus genomic and subgenomic minus-strand RNAs copartition in membrane-protected replication complexes. J Virol. 1997;71:7744–7749. doi: 10.1128/jvi.71.10.7744-7749.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi S T, Schiller J J, Kanjanahaluethai A, Baker S C, Oh J W, Lai M M C. Colocalization and membrane association of murine hepatitis virus gene 1 products and de novo-synthesized viral RNA in infected cells. J Virol. 1999;73:5957–5969. doi: 10.1128/jvi.73.7.5957-5969.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siddell S, Wege H, Barthel A, ter Meulen V. Intracellular protein synthesis and the in vitro translation of coronavirus JHM mRNA. Adv Exp Med Biol. 1981;142:193–207. doi: 10.1007/978-1-4757-0456-3_17. [DOI] [PubMed] [Google Scholar]
- 31.Stohlman S A, Baric R S, Nelson G N, Soe L H, Welter L M, Deans R J. Specific interaction between coronavirus leader RNA and nucleocapsid protein. J Virol. 1988;62:4288–4295. doi: 10.1128/jvi.62.11.4288-4295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutou S, Sato S, Okabe T, Nakai M, Sasaki N. Cloning and sequencing of genes encoding structural proteins of avian infectious bronchitis virus. Virology. 1988;165:589–595. doi: 10.1016/0042-6822(88)90603-4. [DOI] [PubMed] [Google Scholar]
- 33.Tahara S M, Dietlin T A, Bergmann C C, Nelson G W, Kyuwa S, Anthony R P, Stohlman S A. Coronavirus translational regulation: leader affects mRNA efficiency. Virology. 1994;202:621–630. doi: 10.1006/viro.1994.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulmanen I, Soderlund H, Kaarianen L. Semliki Forest virus capsid protein associates with 60S ribosomal subunit in infected cells. J Virol. 1976;20:203–210. doi: 10.1128/jvi.20.1.203-210.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Meer Y, Snijder E J, Dobbe J C, Schleich S, Denison M R, Spaan W J M, Locker J K. Localization of mouse hepatitis virus nonstructural proteins and RNA synthesis indicates a role for late endosomes in viral replication. J Virol. 1999;73:7641–7657. doi: 10.1128/jvi.73.9.7641-7657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wengler G, Wengler G. Identification of a transfer of viral core protein to cellular ribosomes during the early stages of alphavirus infection. Virology. 1984;134:435–442. doi: 10.1016/0042-6822(84)90310-6. [DOI] [PubMed] [Google Scholar]
- 37.Wengler G, Wurkner D, Wengler G. Identification of a sequence element in the alphavirus core protein which mediates interaction of cores with ribosomes and the disassembly of cores. Virology. 1992;191:880–888. doi: 10.1016/0042-6822(92)90263-o. [DOI] [PubMed] [Google Scholar]
- 38.Wilhelmsen K C, Leibowitz J L, Bond C W, Robb J A. The replication of murine coronaviruses in enucleated cells. Virology. 1981;110:225–230. doi: 10.1016/0042-6822(81)90027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams A K, Wang L, Sneed L W, Collisson E W. Comparative analyses of the nucleocapsid genes of several strains of infectious bronchitis virus and other viruses. Virus Res. 1992;25:213–222. doi: 10.1016/0168-1702(92)90135-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson T M A. Cotranslational disassembly of tobacco mosaic virus in vitro. Virology. 1984;137:155–265. doi: 10.1016/0042-6822(84)90217-4. [DOI] [PubMed] [Google Scholar]
- 41.Zhou M L, Williams A K, Chung S I, Wang L, Collisson E W. Infectious bronchitis virus nucleocapsid protein binds RNA sequences in the 3′ terminus of the genome. Virology. 1996;217:191–199. doi: 10.1006/viro.1996.0106. [DOI] [PubMed] [Google Scholar]