Abstract
Study Design
Literature Review.
Objective
Myelopathy affecting the thoracic spinal cord can arise secondary to several aetiologies which have similar presentation and management. Consequently, there are many uncertainties in this area, including optimal terminology and definitions. Recent collaborative cervical spinal research has led to the proposal and subsequent community adoption of the name degenerative cervical myelopathy(DCM), which has facilitated the establishment of internationally-agreed research priorities for DCM. We put forward the case for the introduction of the term degenerative thoracic myelopathy(DTM) and degenerative spinal myelopathy(DSM) as an umbrella term for both DCM and DTM.
Methods
Following PRISMA guidelines, a systematic literature search was performed to identify degenerative thoracic myelopathy literature in Embase and MEDLINE.
Results
Conditions encompassed within DTM include thoracic spondylotic myelopathy, ossification of the posterior longitudinal ligament, ossification of the ligamentum flavum, calcification of ligaments, hypertrophy of ligaments, degenerative disc disease, thoracic osteoarthritis, intervertebral disc herniation, and posterior osteophytosis. The classic presentation includes girdle pain, gait disturbance, leg weakness, sensory disturbance, and bladder or bowel dysfunction, often with associated back pain. Surgical management is typically favoured with post-surgical outcomes dependent on many factors, including the causative pathology, and presence of additional stenosis.
Conclusion
The clinical entities encompassed by the term DTM are interrelated, can manifest concurrently, and present similarly. Building on the consensus adoption of DCM in the cervical spine and the recent proposal of degenerative cervical radiculopathy(DCR), extending this common nomenclature framework to the terms degenerative spinal myelopathy and degenerative thoracic myelopathy will help improve recognition and communication.
Keywords: thoracic, myelopathy, ossification of the posterior longitudinal ligament, spondylosis, disc herniation, stenosis
Introduction
Myelopathy refers to a symptomatic spinal cord injury resulting from multiple causes, including degeneration, tumours, inflammation, infection, and vascular anomalies. It may occur at any spinal cord level, albeit most commonly at the cervical level. Myelopathies arise secondary to a range of aetiologies, however those resulting from degenerative spinal conditions are the most common. This review focuses on thoracic myelopathy and, in particular, myelopathy caused by degenerative conditions of the spine, which precipitate mechanical stress on the spinal cord.
The thoracic spine consists of 12 vertebrae from T1 to T12. Important distinguishing factors of the thoracic vertebrae include a body size that is larger than the cervical vertebrae but smaller than the lumbar vertebrae, pointed and downward-angled spinous processes, and articulation with the ribs. The latter is responsible for the reduced mobility of the thoracic spine compared to the cervical spine, which is thought to contribute to the lower prevalence of degenerative spinal myelopathy at the thoracic compared to the cervical level.1,2
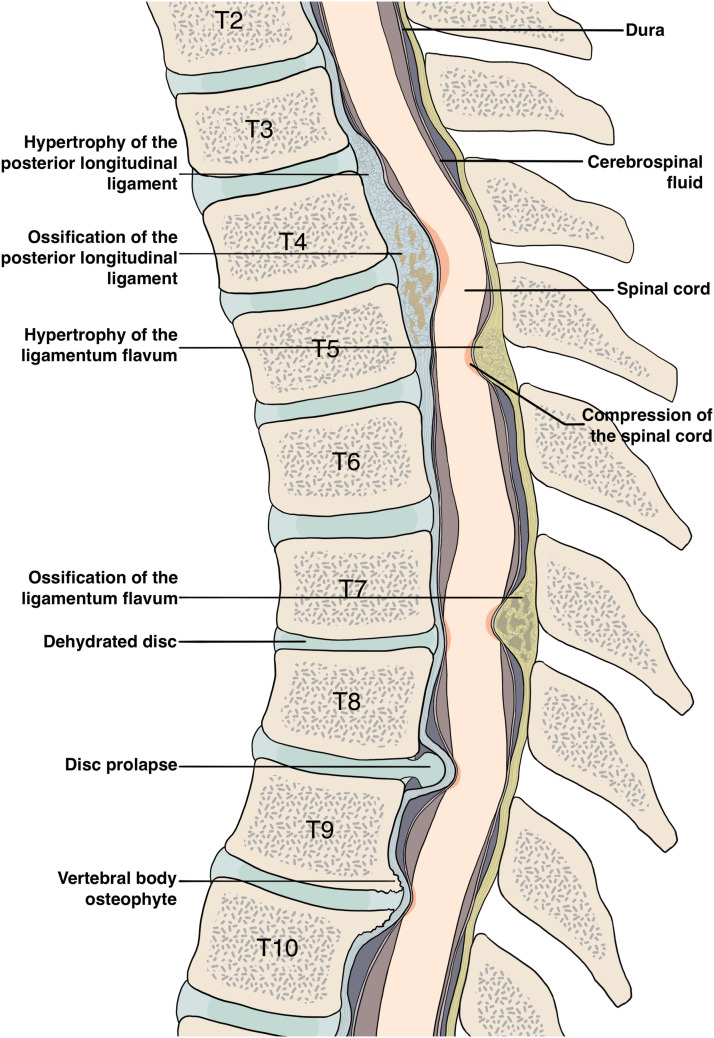
Myelopathy from thoracic spondylotic myelopathy, and other forms of thoracic spine degeneration, including ossification of the posterior longitudinal ligament (OPLL), 3 ossification of the ligamentum flavum (OLF),4-6 calcification of ligaments,5,7 hypertrophy of ligaments, 5 degenerative disc disease (DDD), thoracic osteoarthritis, intervertebral disc herniation (with the exception of acute herniation)8,9 and posterior osteophytes10,11 (Figure 1) share similarities in presentation and management. They trigger an uncommon, but disabling form of ‘slow motion’ spinal cord injury.
Figure 1.
A sagittal view of the thoracic spine demonstrates several pathologies that can cause DTM, including spondylosis, degenerative disc disease, and ossification of the posterior longitudinal ligament.
Many uncertainties challenge clinical care and research in this area, including significant heterogeneity in the use of terminology and definitions. Recent work in the cervical spine has resolved classification uncertainties with the proposal and subsequent community adoption of the term degenerative cervical myelopathy (DCM). DCM recognises that multiple degenerative spine pathologies converge on a common neurological phenotype, which is diagnosed and managed with similar approaches. This improvement in classification has also facilitated the establishment of common data elements and the definition of National Insitute for Health Research (NIHR) James Lind Alliance (JLA) research priorities.12,13
In this review we put forward the case for the introduction of the term degenerative thoracic myelopathy (DTM) to depict degenerative spinal pathologies affecting the thoracic spinal cord. A list of conditions that can be considered as constituents of DTM is provided in Table 1. We draw upon a structured search of the evidence and expert opinion to consolidate current knowledge and propose critical knowledge gaps. The choice of DTM is based on the framework used in the cervical spine (DCM and degenerative cervical radiculopathy (DCR)). We also propose a broader nomenclature of degenerative spinal myelopathy to capture all degenerative conditions of the spine associated with myelopathy, including DCM and DTM. Adopting this standardized terminology throughout the spine will help augment understanding, raise awareness and promote research efficiency.
Table 1.
List of conditions From the International Classification of Diseases 11th Revision (ICD-11) That may be Considered Under the Umbrella Term DTM.
| Classification Group | Specific Category |
|---|---|
| 8B42 myelopathy | |
| FA37 certain joint disorders, not elsewhere classified | FA37.0 osteophyte |
| FA37.Y other specified certain joint disorders, not elsewhere classified | |
| FA80 intervertebral disc degeneration | FA80.4 intervertebral disc degeneration of thoracic spine without prolapsed disc |
| FA80.5 intervertebral disc degeneration of thoracic spine with prolapsed disc | |
| FA80.6 intervertebral disc degeneration of thoracic spine with bony spur at the vertebra | |
| FA80.7 intervertebral disc degeneration of thoracic spine with nervous system involvement | |
| FA80.Y other specified intervertebral disc degeneration | |
| FA80.Z intervertebral disc degeneration, unspecified | |
| FA81 Spondylolysis | FA81.0 Spondylolysis with slippage |
| FA81.1 Spondylolysis without slippage | |
| FA81.Z Spondylolysis, unspecified | |
| FA82 spinal stenosis | |
| FA83 ossification of spinal ligaments | |
| FA84 spondylolisthesis | FA84.0 spondylolisthesis with pars defect |
| FA84.1 spondylolisthesis without pars defect | |
| FA84.Z spondylolisthesis, unspecified | |
| FA8Y other specified degenerative condition of spine | |
| FA8Z degenerative condition of spine, unspecified | |
| FB1Y other specified conditions associated with the spine | |
| FB1Z conditions associated with the spine, unspecified | |
Methods
A systematic literature search was performed in Embase and MEDLINE using the Ovid platform (Ovid Technologies, New York, USA), using an adapted version of a published search strategy for degenerative cervical myelopathy14,15 and following PRISMA guidelines 16 from inception to 27th July 2022. Tables 2 and 3 outline the search strategy. The results of this search underwent title and abstract screening. Exclusion criteria included letters, editorials, opinion articles, corrections, and papers not relevant to DTM or the scope of the review. Neither informed consent nor institutional review board approval were required due to the nature of the study.
Table 2.
Search Strategy for MEDLINE.
| Ovid MEDLINE(R) and Epub Ahead of Print, In-Process, In-Data-Review & Other Non-Indexed Citations, Daily and Versions | ||
|---|---|---|
| # | Searches | Number of Results |
| 1 | myelopath*.mp. or exp spinal cord diseases/or (spinal cord adj3 (diseas* or disorder*)).mp. or myeloradiculopath*.mp. or spondylomyelopath*.mp. or spondylomyeloradiculopath*.mp. or (spinal cord adj3 Compress*).mp. or exp spinal cord compression/or exp ossification of posterior longitudinal ligament/or exp ligamentum flavum/or ((“Japanese Orthop?edic association" adj2 score*) or (joa adj2 score*)).mp. | 160243 |
| 2 | exp Thoracic Vertebrae/or exp Thoracic cord/or thoracic.tw. | 273334 |
| 3 | 1 and 2 | 28558 |
| 4 | exp Atlanto-Occipital Joint/or exp Arteriovenous Fistula/or exp Radiotherapy/or exp Vitamin B 12/or exp Radiation/or exp Radiation Injuries/or exp Re-Irradiation/or exp Craniospinal Irradiation/or exp Whole-body Irradiation/or exp motor Neuron disease/or exp Amyotrophic lateral Sclerosis/or exp neoplasm/or exp metastasis/or exp Nervous system Malformations/or exp “autoimmune diseases of the nervous system”/or exp “congenital, hereditary, and neonatal diseases and abnormalities"/or exp virus diseases/or exp tuberculosis/or exp cyst/or exp hematoma/or exp infection/or hemangioma.mp. or cancer.mp. or meningioma.mp. or tumo*r.mp. or cyst.mp. or h*ematoma.mp. or exp trauma/or exp vascular diseases/or web.ti. or acute.ti. or exp Myelin Sheath/or plexus.ti. or tuberculosis.mp. or myelitis.mp. or exp dog/or exp cat/or glioma.mp. or deficiency.ti. or exp multiple sclerosis/ | 12067042 |
| 5 | 3 not 4 | 2641 |
| 6 | Limit 5 to english language | 2248 |
Table 3.
Search Strategy for Embase.
| Embase | ||
|---|---|---|
| # | Searches | Number of Results |
| 1 | Myelopath*.mp. or exp myelopathy/or exp spondylosis/or spondylotic thoracic myelopathy.mp. or exp *spinal cord disease/or thoracic spinal cord injury.ti,ab. or exp *myelography/or exp *myeloradiculopathy/or myeloradiculopath*.ti,ab. or (spinal cord adj3 (diseas* or disorder*)).ti,ab. or spondylomyelopath*.ti,ab. or (spinal cord adj3 Compress*).ti,ab. or exp *spinal cord compression/or exp *Japanese Orthopaedic association score/or Japanese Orthop?edic Association.mp. or (Japanese Orthop?edic association adj2 scor*).mp. or (joa adj2 scor*).mp. or exp ligamentum flavum/or ossification of posterior longitudinal ligament.ti,ab. or exp *ligament calcinosis/or (exp posterior longitudinal ligament/and (exp *ossification/or ossifi*.ti,ab.)) | 308383 |
| 2 | exp *thoracic vertebra/or exp *thoracic spinal cord/or thoracic.tw. or exp *thoracic spine/ | 230149 |
| 3 | 1 and 2 | 16449 |
| 4 | exp atlantooccipital joint/or exp arteriovenous fistula/or exp radiotherapy/or exp cyanocobalamin/or exp radiation injury repair/or exp radiation injury/or exp *radiation/or exp re-irradiation/or exp irradiation/or exp craniospinal irradiation/or exp whole body radiation/or exp *motor neuron disease/or exp *amyotrophic lateral sclerosis/or neoplasm metastasis.mp. or exp metastasis/or exp *neoplasm/or exp malignant neoplasm/or exp radiation induced neoplasm/or exp myeloproliferative neoplasm/or exp vertebra hemangioma/or exp hemangioma/or exp nervous system malformation/or autoimmune diseases of the nervous system.mp. or autoimmune nervous system.mp. or (congenital, hereditary, and neonatal diseases and abnormalities).mp. or congenital disorder.mp. or exp genetic disorder/or newborn disease.mp. or exp virus infection/or exp tuberculosis/or exp cyst/or exp hematoma/or exp infection/or hemangioma.mp. or cancer.mp. or meningioma.mp. or tumo*r.mp. or cyst.mp. or h*ematoma.mp. or exp trauma/or exp vascular diseases/or web.ti. or acute.ti. or exp Myelin Sheath/or plexus.ti. or tuberculosis.mp. or myelitis.mp. or exp dog/or exp cat/or glioma.mp. or deficiency.ti. or exp multiple sclerosis/ | 15244318 |
| 5 | 3 not 4 | 1607 |
| 6 | Limit 5 to medline | 228 |
| 7 | 5 not 6 | 1379 |
| 8 | Limit 7 to english language | 1232 |
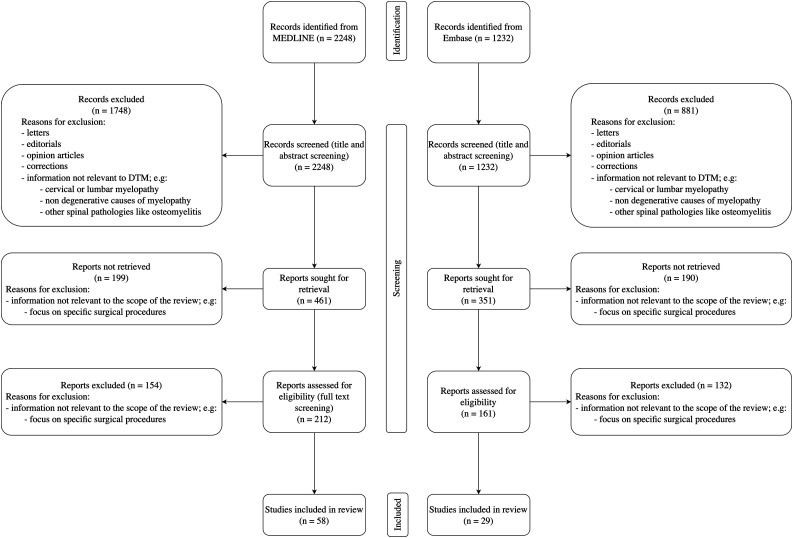
The majority of the screened papers had information irrelevant to DTM with a focus on topics such as cervical myelopathy, non-degenerative causes of myelopathy, and other spinal pathologies, such as osteomyelitis. Additionally, many papers focused on specific surgical procedures, which was outside the scope of this review. Papers meeting the inclusion criteria of a focus on degenerative pathology of the thoracic spinal column published in the English language were sought for retrieval. Retrieved papers were assessed for eligibility using a full-text review, and those that contained relevant information were included. The reference lists of included papers were also hand-searched to identify additional relevant studies. The search methodology is summarised in Figure 2. MEDLINE was searched first, with duplicate results from Embase automatically excluded from the search results. Included papers are listed in Supplementary Table 1. Most papers were published after 2010; 11 papers were published in 2021 and 8 in the first 6 months of 2022. A narrative synthesis of the identified literature is presented; no statistical analysis was performed.
Figure 2.
Search methodology flowchart based on PRISMA 2020 statement. 16 .
Discussion
Aetiology of DTM
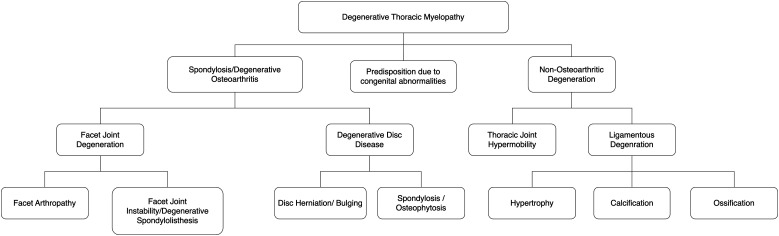
Degenerative pathology in the thoracic spine that causes DTM can be divided into spondylotic (or osteoarthritic) and non-osteoarthritic (Figure 3). Both subcategories can compromise the spinal cord via exerting mechanical stress. In addition, certain congenital disorders can predispose to DTM. For example, Ehler-Danlos syndrome predisposes to thoracic instability and subsequent myelopathy 17 ; Scheuermann’s disease is associated with thoracic disc herniation and OLF and is thus associated with at increased risk of DTM in affected individuals.18,19 Additionally, there may be a link between DTM and scoliosis, with a reported case of a patient with Klippel-Trenaunay-Weber syndrome having spinal stenosis and suffering from myelopathy. 20 However, due in part to the low incidence of thoracic myelopathy, research on predisposing factors is sparse.
Figure 3.
A conceptual differentiation of the constituents of degenerative thoracic myelopathy. DTM can be due to either osteoarthritic or non-osteoarthritic degeneration. In addition, certain congenital abnormalities can predispose individuals to DTM. Figure drawn with reference to Nouri et al 2015. 21
As recently outlined for DCM, 22 the pathophysiology of degenerative spinal myelopathy can be considered a function of mechanical stress, vulnerability and time, wherein mechanical stress consists of multiple mechanisms of loading, not just compression. Spinal cord vulnerability is influenced by cellular processes, and systemic factors, such as genetic and adaptive protective mechanisms, including autoregulation of spinal cord perfusion and nutritional status. 22 The association between obesity and smoking and both DCM and DTM, suggests similar pathogenesis may be exist for both DCM and DTM. 23 Moreover, the co-existence of DCM and DTM, such as by cervical and thoracic OLF and/or OPLL occurring concurrently, reinforces this hypothesis. 24
The aetiology of the mechanical stress that triggers DSM, i.e., whether it is due to static or dynamic mechanisms, can be used to subcategorise DTM. 25 Static spinal cord compression or spinal canal stenosis is the result of degenerative changes indenting the spinal cord, including osteophyte formation and ligament hypertrophy, calcification, and ossification. 25 Dynamic compression refers to compression as a result of movement, whether physiological or pathological. Due to the relatively reduced mobility of the thoracic spine, dynamic mechanisms are likely to be less important than in the cervical spine. Nevertheless, dynamic mechanisms may explain the increased rate of DTM in individuals with increased mobility and laxity of the thoracic spine. 17 Importantly, static and dynamic mechanisms of spinal cord stress may occur concomitantly. 22 Since dynamic mechanisms likely play a smaller role in DTM, a greater degree of static compression may well be needed compared to DCM to precipitate myelopathy.22,26 However, comparative analysis of the MRIs of patients with DCM and DTM and correlation with their presentations are needed to evaluate this hypothesis.
DTM Conditions
The most common aetiology of DTM is dependent on the population. In Japanese populations, in which most of the included studies were conducted, OLF and OPLL are the commonest causes, followed by disc herniation, with rare causes including calcification of the ligamentum flavum and degenerative spondylolisthesis.11,27-31 A recent systematic review found that in Japan, 45% of cases were OPLL, 36.4% OLF, 16.5% OPLL with OLF and 2.1% disc herniation, 31 whilst in China, 75.8% of cases were OLF, 13.8% disc herniation, 6.6% OPLL and 3.8% OPLL with OLF. 31 That same study found that in the US, disc herniation is the commonest aetiology with 95.7% of cases, with OLF making up 3.6% and OPLL .5%. 31 Overall, 41.5% of cases were OLF, 18.7% OPLL, 7.4% OPLL with OLF and 32.4% disc herniation. 31 Another systematic review of surgical procedures on thoracic myelopathy found that of 2183 patients, 69.8% had OLF, 20.0% OPLL and 9.3% disc herniation. 30 Table 4 provides a summary of the prevalence of the different causes of DTM. Equivalent conditions affect the cervical spine, with the prevalence of each being similarly population-dependent. 21
Table 4.
Comparison of Prevalence of Difference Causes of DTM
Furthermore, multiple degenerative changes often occur simultaneously. 32 For example, OLF and OPLL may present concomitantly, 31 and coexistent OPLL, OLF, and thoracic disc herniation have also been reported.29,31
Osteoarthritic Degenerative Conditions
Due to repetitive use, ageing, and environmental factors such as smoking, the proteoglycan composition of the nucleus pulposus changes, altering hydrostatic pressure, disc height, and subsequently force distribution.33-35 This can lead to a cascade of degenerative changes, which lead to osteophyte formation and intervertebral disc failure, such as disc bulging and herniation.25,36 Polymorphisms in several genes, such as those associated with cartilage and collagen formation like AGC1 and COL9A, have been associated with this degenerative process, 37 indicating a possible genetic vulnerability.
Thoracic disc herniation is a cause of myelopathy with an estimated frequency between 1 per 1000 and 1 per million.38-40 It is less common than cervical disc herniation, 41 which has an annual incidence of 18.6 per 100,000. 42 In fact, thoracic disc herniation accounts for only .15-4% of all disc operations.43,44 In approximately 40% of cases, the herniated disc is calcified. Typically, thoracic disc herniation occurs in middle-aged or older men and at levels below T8.9,41,45 The levels affected are classically at mechanical inflection points; increased mobility at these spinal levels is postulated to explain the increased prevalence of herniations. 41
Spondylosis is another osteoarthritic degenerative condition that can cause myelopathy. Here, protruding osteophytes, formed by the degenerative processes outlined above, compress the spinal cord. 46 This condition is infrequent in the thoracic spine compared to the cervical and lumbar regions 46 ; the low prevalence is partly explained by the high rate of misdiagnosis due to its co-occurrence with cervical and lumbar spondylosis. 46
In addition, another cause of thoracic myelopathy secondary to osteoarthritic pathology is degenerative spondylolisthesis. In this condition, an increased pedicle–facet joint angle and facet joint disruption has been observed. 47 Whilst far more common in the cervical and lumbar regions, 48 it can also occur in the thoracic region, often secondary to intervertebral disc degeneration. 48 Thoracic degenerative spondylolisthesis tends to occur in combination with lumbar spondylosis, which can lead to misdiagnosis and subsequently under-reporting. 48 Furthermore, it has also been associated with degenerative scoliosis. 49
Non-Osteoarthritic Degenerative Conditions
Changes in the spinal ligaments, notably the ligamentum flavum and the posterior longitudinal ligament, can lead to DTM. Although genetics may play a role in calcification, hypertrophy, and ossification of these ligaments, the fact that these phenomena usually manifest in older age implies a link with ageing and a likely degenerative aetiology.50-53
OLF is a condition whereby the ligamentum flavum undergoes progressive endochondral ossification.54,55 Ossification at multiple different spinal levels, i.e., tandem ossification, is a frequently recognised occurrence.3,25,31 OLF is more common in the thoracic spine compared to the cervical or lumbar spine, which may be due to the reduced mobility of this segment.56-58 The lower thoracic spine (T10-T12) is typically most affected.56,59-62 Thoracic OLF typically presents before 60 years of age 59 and is thought to be more prevalent in men,59-61,63,64 although this is inconsistent across studies.6,65,66 Genetic factors are believed to play a role in the development of OLF; an altered genome-wide DNA methylation profile has been reported in individuals with thoracic OLF. 67 Furthermore, overexpression of genes and transcription factors associated with the Notch and Wnt signaling pathways such as LGR5, ANGPT2, CX48, Runx2, and Osterix have also been associated with OLF in overexpression and knockdown experiments.54,68-71 Polymorphisms in the COL6A1 gene, which plays an important role in forming collagen, also appear to be associated with OLF. 72 Nonetheless, environmental factors are likely to play a role; mechanical stress has been shown to promote OLF by inducing the Notch and Wnt pathways. 73 Diet appears to be another important factor, 74 and fluoride intake is associated with risk of OLF. 75 There is also an association with obesity. 76
OPLL is another non-osteoarthritic degenerative precipitant of DTM. This is a condition whereby the posterior longitudinal ligament undergoes progressive thickening and endochondral ossification. 77 Similar to OLF, tandem ossification is frequent.3,25,31 OPLL is more common in the cervical spine. 78 In the thoracic spine, the mid-levels are typically affected.29,31 Thoracic OPLL is less common than OLF and is most commonly seen in Asian populations,31,79,80 with most studies conducted in Japan.80,81 The prevalence is much lower in North America and Europe. 80 Moreover, thoracic OPLL seems to be more often reported in males,31,82 although reports are inconsistent. 83 Similar to OLF, both genetic and environmental factors play a role in the development of the condition. Increased expression of IL17RC and COL6A1, two osteogenic genes, have been associated with OPLL.84-87 IGF-1 has been associated with ligament ossification, 88 which may explain the association between acromegaly and OPLL. 89 Hyperleptinemia and hyperinsulinemia are other factors involved in the development of OPLL, 90 as is obesity.78,90
Prevalence
DTM is less common than DCM. This may be due to the reduced range of motion in the thoracic spinal segment. 27 Nonetheless, accurate estimation of DTM prevalence is currently a challenge due to the heterogeneity in the classification of DTM as separate clinical entities, the paucity of literature on the topic, and the fact that studies have been mainly performed in Asian populations. Underdiagnosis is another challenge. 11 The incidence of surgical interventions is an important estimate of the prevalence of thoracic myelopathy. However, this is likely to be a substantial underestimate. Surgical intervention for thoracic myelopathy has a reported prevalence of approximately .9 per 100,000 population, which is less 10% of that of cervical myelopathy, according to a retrospective study in Japan. 91 These values were reported in studies of highly specific populations and are thus are not likely to be representative of the wider global epidemiology.
Presentation and Management
DTM may present with back or girdle pain, gait difficulty, leg weakness, sensory disturbance, and bladder or bowel dysfunction.27,92 Symptom burden usually increases over time. Initial symptoms typically include leg numbness, leg weakness, gait difficulty, and bladder or bowel disturbance. 27 Gait disturbance is particularly common in OPLL,27,93 and back pain at initial diagnosis is particularly associated with myelopathy at the upper or middle thoracic levels. 27 Neurological signs may include hyperreflexic patellar and ankle tendon reflexes, ankle clonus, and positive Babinski sign, although with lower frequency if spinal cord compression is at lower levels of the thoracic spine.27,92 The severity of the disease can be scored using an adapted version of the modified Japanese Orthopedic Association (mJOA) score, assessing lower limb motor function, sensory dysfunction, and bladder and bowel issues.21,27,94 Table 5 provides a summary of the adapted mJOA score that can be used for DTM.
Table 5.
mJOA for DTM. Adapted From Hilton et al, 2019. 95
| Score | mJOA | |
|---|---|---|
| Lower limb motor dysfunction | 0 | Complete loss of motor and sensory function |
| 1 | Sensory preservation without ability to move legs | |
| 2 | Able to move legs but unable to walk | |
| 3 | Able to walk on flat floor with a walking aid | |
| 4 | Able to walk up and/or down stairs with a handrail | |
| 5 | Moderate to significant lack of stability but able to walk up and/or downstairs without handrail | |
| 6 | Mild lack of stability but walk unaided with smooth reciprocation | |
| 7 | No dysfunction | |
| Sensory dysfunction | 0 | Complete loss of sensation |
| 1 | Severe sensory loss or pain | |
| 2 | Mild sensory loss | |
| 3 | No sensory loss | |
| Sphincter dysfunction | 0 | Inability to urinate voluntarily |
| 1 | Marked difficulty with micturition | |
| 2 | Mild to moderate difficulty with micturition | |
| 3 | Normal micturition |
Furthermore, there are some rarer presentations of DTM. For example, high thoracic OLF may present with Horner’s syndrome, 96 whilst foot drop can be a symptom of calcified thoracic disc herniation at T11–L1 level. 97
The symptoms described are also often present when cervical or lumbar spinal disorders compress neural elements, which can result in misdiagnosis and delayed treatment.27,98 False localising levels, whereby the symptoms appear to emanate from a different anatomical location than their true origin,99,100 are another challenge that complicates diagnosis. For instance, cervical cord compression may present with a thoracic sensory level, and thoracic cord compression may present with a lumbar sensory level.92,99 Furthermore, it is relatively common for other neurological problems, such as peripheral neuropathy or lumbar and cervical spine disease, to occur concurrently with thoracic myelopathy, adding further diagnostic challenge.92,101 Radiculopathy and myelopathy also often occur simultaneously. 102 Finally, it is important always to consider the many non-degenerative conditions that can cause myelopathy, including autoimmune, inflammatory, and idiopathic causes.
Thoracic myelopathy tends to respond poorly to conservative management.103,104 Consequently, surgery is often required. Posterior decompressive laminectomy or laminoplasty and circumferential decompression via a posterior approach are the favoured approaches for posterior pathologies. 30 Posterior decompression is the most common operation for thoracic disc herniation, OLF, and OPLL, 30 whilst circumferential decompression is rarer and most commonly used in OPLL. 30 The preference for posterior decompression over circumferential decompression is related to factors such as reduced blood loss, lower complication rates, post-operation recovery rate, and less immediate neurologic deterioration. 30 Similar to the surgical approach for DCM, 105 there does not appear to be a clear difference between approaches with regard to long-term outcomes, 30 however conclusions are limited by a paucity of high quality comparative studies. Instrumented fusion may also be utilised alongside posterior decompression in OPLL, which has been associated with overall favourable outcomes. 106 Moreover, there appears to be a trend towards posterior instrumented fusion surgery for OLF, with 48.9% of cases in one prospective multicentre study being posterior decompression with instrumented fusion. 107
Anterior or lateral surgical approaches are performed less commonly but may be required for anterior compressive pathology; these come with the additional risks of damage to structures such as the aorta and oesophagus. 108 Nonetheless, anterior decompression for calcified discs may be associated with improved neurological outcomes. 109 Minimally invasive surgery has become increasingly common, particularly for disc herniations affecting a single thoracic level110,111; however, data on minimally invasive surgery is sparse.
Post-surgical outcomes in DTM depend on many factors, such as the operative approach, causative pathology, symptom duration, and presence of additional stenosis.30,104 For OLF, statistically significant improvements of JOA scores are reported, although recovery is typically incomplete.57,59,112,113 Recovery is dependent on several factors, such as preoperative neurological status, duration of symptoms, imaging findings, age, sex, number of levels involved, and type of OLF.52,57,66,113,114 However, the preoperative severity of myelopathy appears to be the most important factor.57,66,113 Outcomes for surgical management of thoracic disc herniations are generally poorer than those for cervical disc herniation. 115
Conclusion
Myelopathy of the thoracic spinal cord can be triggered by several interrelated, degenerative conditions that affect the thoracic spine, such as disc herniation, OLF, and OPLL. The paucity of published literature in this area is partly due to the relatively low prevalence of these conditions. The heterogeneity and inconsistency in the classification of DTM conditions as separate clinical entities further hinders synthesis of published data. Similar to the recent consensus process for DCM, we propose the introduction of the term degenerative thoracic myelopathy as an umbrella term for thoracic myelopathies triggered by degenerative conditions of the thoracic spine. Whilst there may be early hurdles in adoption, this terminology will improve recognition and communication as well as promote research efficiency and accelerate understanding of DTM. The present proposal represents current evidence-based expert consensus; future wider consultation may be necessary to refine it further.
To further standardise the classification of myelopathies triggered by spinal degenerative pathologies, we propose the term degenerative spinal myelopathy (DSM) as overarching nomenclature for all degenerative spinal pathology triggering myelopathy. DSM as the umbrella term for DCM and DTM, will further aid communication and enable synergies, such as for studies investigating molecular and cellular changes underpinning myelopathies triggered by spine conditions, as well as their risk factors. Nevertheless, further research is needed both on DTM itself and on better defining how it compares to DCM. Table 6 provides a summary of the current understanding of DTM and current uncertainties.
Table 6.
Summary of the Current Understanding of DTM and Uncertainties.
| Current Understanding | Uncertainty | |
|---|---|---|
| Classification of DTM | Conditions causing DTM can be divided into spondylotic, non-spondylotic, and predisposition due to congenital conditions. Another classification is via pathophysiology whereby spinal cord stress can be due to static and dynamic mechanisms. | - Both classifications are based mainly on DCM research. Research is needed to assess the validity of the classifications for DTM. - Which congenital conditions predispose to DTM? |
| DTM conditions | The most common cause of DTM is OLF, followed by posterior osteophytosis, OPLL and disc herniation with rare causes being calcification of the ligamentum flavum and degenerative spondylolisthesis. | - The most is known about OLF and disc herniation. Research on the other conditions is lacking. - What are the genetic and environmental associations for each of the conditions? |
| Prevalence | Surgical intervention is the most accurate source of prevalence estimates. Approximately .9 per 100,000 population are affected by DTM. | - Studies investigating prevalence have been mainly carried out in specific Asian populations. Exploring prevalence in other populations is an important topic for future research. |
| Presentation | DTM may present with back or girdle pain, gait difficulty, leg weakness, sensory disturbance and bladder or bowel dysfunction. The burden of symptoms usually increases over time. Neurological signs may include hyperreflexic patellar and ankle tendon reflexes; ankle clonus and positive Babinski sign may also be present. | - The frequency, sensitivity, specificity and positive predictive value of the symptoms and signs are current uncertainties. - What are the differences in how each of the DTM pathologies present? - What is the best scoring system for grading the severity of DTM? |
| Management | Surgery is the first line of management and involves decompression of the spinal cord, with posterior decompression typically the favoured approach. Post-surgical outcome depends on many factors such as the specific causative pathology, symptom duration and presence of additional stenosis. | - How important is the timing of intervention for outcomes? - What is the role of novel therapies in DTM? - Which surgical technique works best for each pathology? |
Supplemental Material
Supplemental Material for Degenerative Thoracic Myelopathy: A Scoping Review of Epidemiology, Genetics, and Pathogenesis by Tanzil Rujeedawa BA, Oliver D. Mowforth MA, MB, BChir, MSt, MRCS, Benjamin M. Davies BSc, MPhil, MRCS, Cylene Yang BSc MBBS, Aria Nouri MD, MSc, Jibin J. Francis MBBCh, FCNeurosurg FRCS, Bizhan Aarabi, MD, Brian K. Kwon, MD, PhD FRCSC, James Harrop MD, Jefferson R. Wilson, MD PhD, Allan R. Martin MD PhD, Vafa Rahimi-Movaghar, MD, James D. Guest, MD, PhD, Michael G. Fehlings, MD PhD FRCSC, Mark R. Kotter MD MPhil PhD in Global Spine Journal
Author Contributions: TR – conceptualization, manuscript drafting, and preparation, ODM – conceptualization, manuscript drafting, and preparation, BMD – conceptualization and manuscript review, CY – illustration and manuscript review, JF, BA, BKK, JH, MGF, JRW, ARM, VRM, JDG – manuscript review, MRK – conceptualization and manuscript preparation and review
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Research in the MRK’s laboratory is supported by a core support grant from the Wellcome Trust and MRC to the Wellcome Trust-Medical Research Council Cambridge Stem Cell Institute. MRNK is supported by a NIHR Clinician Scientist Award, CS-2015-15-023. BMD is supported by an NIHR Clinical Doctoral Fellowship. ODM is supported by an Academic Clinical Fellowship at the University of Cambridge.
Disclaimer: The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Tanzil Rujeedawa https://orcid.org/0000-0002-7089-1684
Oliver D. Mowforth https://orcid.org/0000-0001-6788-745X
Benjamin M. Davies https://orcid.org/0000-0003-0591-5069
Aria Nouri https://orcid.org/0000-0002-4965-3059
Jefferson R. Wilson https://orcid.org/0000-0001-5965-0305
Michael G. Fehlings https://orcid.org/0000-0002-5722-6364
References
- 1.El-Khoury GY, Whitten CG. Trauma to the upper thoracic spine: Anatomy, biomechanics, and unique imaging features. Am J Roentgenol. 1993;160(1):95-102. doi: 10.2214/ajr.160.1.8416656. [DOI] [PubMed] [Google Scholar]
- 2.Andriacchi T, Schultz A, Belytschko T, Galante J. A model for studies of mechanical interactions between the human spine and rib cage. J Biomech. 1974;7(6):497-507. doi: 10.1016/0021-9290(74)90084-0. [DOI] [PubMed] [Google Scholar]
- 3.Yamazaki M, Mochizuki M, Ikeda Y, et al. Clinical results of surgery for thoracic myelopathy caused by ossification of the posterior longitudinal ligament: Operative indication of posterior decompression with instrumented fusion. Spine. 2006;31(13):1452-1460. doi: 10.1097/01.brs.0000220834.22131.fb [DOI] [PubMed] [Google Scholar]
- 4.Akhaddar A, Mansouri A, Zrara I, et al. Thoracic spinal cord compression by ligamentum flavum ossifications. Jt Bone Spine. 2002;69(3):319-323. doi: 10.1016/S1297-319X(02)00400-1. [DOI] [PubMed] [Google Scholar]
- 5.Barnett GH, Hardy RW, Little J, Bay J. Thoracic spinal canal stenosis. J Neurosurg. 1988;68(1):160-161. doi: 10.3171/jns.1988.68.1.0160. [DOI] [PubMed] [Google Scholar]
- 6.Mo L, Wang Z, Du J. Thoracic myelopathy caused by ossification of the ligamentum flavum. J Korean Neurosurg Soc. 2013;46(3):189-194. doi: 10.3340/jkns.2009.46.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiguematsu FY, de Souza ECC, Zimmermann AF, Castro GRW, Pereira IA, Neves FS. Thoracic myelopathy due to calcification of the ligamentum flavum with hyperproteinorachia and responsive to steroid therapy: Case report. Rev Bras Reumatol. 2012;52(3):438-446. [PubMed] [Google Scholar]
- 8.Teufack S, Campbell P, Sharma P, et al. Thoracic myelopathy due to an intramedullary herniated nucleus pulposus: First case report and review of the literature. Neurosurgery. 2012;71(1):199-202. doi: 10.1227/NEU.0b013e3182582cf1. [DOI] [PubMed] [Google Scholar]
- 9.Cornips EMJ, Janssen MLF, Beuls EAM. Thoracic disc herniation and acute myelopathy: Clinical presentation, neuroimaging findings, surgical considerations, and outcome: Clinical article. J Neurosurg Spine. 2011;14(4):520-528. doi: 10.3171/2010.12.SPINE10273. [DOI] [PubMed] [Google Scholar]
- 10.Seidenwurm DJ, Wippold FJ, Cornelius RS, et al. ACR appropriateness criteria® myelopathy. J Am Coll Radiol. 2012;9(5):315-324. doi: 10.1016/j.jacr.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Girardi FP, Sax OC, Salzmann S, Shue J. JSM neurosurgery and spine literature review of thoracic myelopathy: Causes of acute worsening. JSM Neurosurg Spine. 2016;4(4):4-7. [Google Scholar]
- 12.Davies B, Mowforth O, Sadler I, et al. Recovery priorities in degenerative cervical myelopathy: A cross-sectional survey of an international, online community of patients. BMJ Open. 2019;9(10):e031486. doi: 10.1136/bmjopen-2019-031486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies BM, Khan DZ, Mowforth OD, et al. RE-CODE DCM (REsearch objectives and Common data elements for degenerative Cervical myelopathy): A Consensus process to improve research efficiency in DCM, through establishment of a standardized dataset for Clinical research and the definition of the Re. Glob spine J. 2019;9(1 Suppl):65S-76S. doi: 10.1177/2192568219832855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan MA, Mowforth OM, Kuhn I, Kotter MRN, Davies BM. Development of a validated search filter for Ovid Embase for degenerative cervical myelopathy. Health Info Libr J. 2021;1:1-9. doi: 10.1111/hir.12373 [DOI] [PubMed] [Google Scholar]
- 15.Davies BM, Goh S, Yi K, Kuhn I, Kotter MRN. Development and validation of a MEDLINE search filter/hedge for degenerative cervical myelopathy. BMC Med Res Methodol. 2018;18(1):1-8. doi: 10.1186/s12874-018-0529-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021:372. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson FC, Austin C, Benzel E, et al. Neurological and spinal manifestations of the Ehlers–Danlos syndromes. Am J Med Genet Part C Semin Med Genet. 2017;175(1):195-211. doi: 10.1002/ajmg.c.31549. [DOI] [PubMed] [Google Scholar]
- 18.Ding Y, Lv S, Dong S, Cui J, Cao Z, Chen Y. Relationship between scheuermann disease and symptomatic thoracic spinal stenosis: A retrospective study. Acta Orthop Traumatol Turc. 2021;55(3):253-257. doi: 10.5152/j.aott.2021.20022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapetanos GA, Hantzidis PT, Anagnostidis KS, Kirkos JM. Thoracic cord compression caused by disk herniation in Scheuermann’s disease: A case report and review of the literature. Eur Spine J. 2006;15(SUPPL. 5):553-558. doi: 10.1007/s00586-005-0053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arai Y, Takagi T, Matsuda T, Kurosawa H. Myelopathy due to scoliosis with vertebral hypertrophy in Klippel-Trenaunay-Weber syndrome. Arch Orthop Trauma Surg. 2002;122(2):120-122. doi: 10.1007/s004020100334. [DOI] [PubMed] [Google Scholar]
- 21.Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: Epidemiology, genetics, and pathogenesis. Spine. 2015;40(12):E675-E693. doi: 10.1097/BRS.0000000000000913 [DOI] [PubMed] [Google Scholar]
- 22.Davies BM, Mowforth O, Gharooni AA, et al. A new Framework for investigating the biological basis of degenerative Cervical myelopathy [AO spine RECODE-DCM research priority number 5]: mechanical stress, vulnerability and time. Glob Spine J. 2022;12(1_suppl):78S-96S. doi: 10.1177/21925682211057546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rujeedawa T, Mowforth OD, Brannigan J, et al. A single centre service evaluation of degenerative cervical and thoracic myelopathy. J Clin Neurosci. 2023;117(August):168-172. doi: 10.1016/j.jocn.2023.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Park JY, Chin DK, Kim KS, Cho YE. Thoracic ligament ossification in patients with cervical ossification of the posterior longitudinal ligaments: tandem ossification in the cervical and thoracic spine. Spine. 2008;33(13):E407-E410. doi: 10.1097/BRS.0b013e318175c276 [DOI] [PubMed] [Google Scholar]
- 25.Badhiwala JH, Ahuja CS, Akbar MA, et al. Degenerative cervical myelopathy — update and future directions. Nat Rev Neurol. 2020;16(2):108-124. doi: 10.1038/s41582-019-0303-0. [DOI] [PubMed] [Google Scholar]
- 26.Davies BM, Mowforth OD, Smith EK, Kotter MRN. Degenerative cervical myelopathy. BMJ. 2018;360(February):8-11. doi: 10.1136/bmj.k186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ando K, Imagama S, Kobayashi K, et al. Clinical Features of thoracic myelopathy: A single-Center study. JAAOS Glob Res Rev. 2019;3(11):e18. doi: 10.5435/jaaosglobal-d-18-00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato T, Kokubun S, Tanaka Y, Ishii Y. Thoracic myelopathy in the Japanese: Epidemiological and clinical observations on the cases in Miyagi Prefecture. Published online. 1998;184(1):1-11. [DOI] [PubMed] [Google Scholar]
- 29.Hou X, Sun C, Liu X, et al. Clinical features of thoracic spinal stenosis-associated myelopathy. J Spinal Disord Tech. 2016;29(2):86-89. doi: 10.1097/BSD.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 30.Zhu S, Wang Y, Yin P, Su Q. A systematic review of surgical procedures on thoracic myelopathy. J Orthop Surg Res. 2020;15(1):1-10. doi: 10.1186/s13018-020-02081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen G, Fan T, Yang X, Sun C, Fan D, Chen Z. The prevalence and clinical characteristics of thoracic spinal stenosis: A systematic review. Eur Spine J. 2020;29(9):2164-2172. doi: 10.1007/s00586-020-06520-6. [DOI] [PubMed] [Google Scholar]
- 32.Yan C, Tan HY, Ji CL, et al. The clinical value of three-dimensional measurement in the diagnosis of thoracic myelopathy caused by ossification of the ligamentum flavum. Quant Imaging Med Surg. 2021;11(5):2040-2051. doi: 10.21037/qims-20-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nixon J. Intervertebral disc mechanics: A review. J R Soc Med. 1986;79(2):100-104. doi: 10.1177/014107688607900211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferguson SJ, Steffen T. Biomechanics of the aging spine. Eur Spine J. 2003;12(SUPPL. 2):97-103. doi: 10.1007/s00586-003-0621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palepu V, Kodigudla M, Goel VK. Biomechanics of disc degeneration. Adv Orthop. 2012;2012:1-17. doi: 10.1155/2012/726210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galbusera F, Van Rijsbergen M, Ito K, Huyghe JM, Brayda-Bruno M, Wilke HJ. Ageing and degenerative changes of the intervertebral disc and their impact on spinal flexibility. Eur Spine J. 2014;23(SUPPL 3):31. doi: 10.1007/s00586-014-3203-4. [DOI] [PubMed] [Google Scholar]
- 37.Chan D, Song Y, Sham P, Cheung KMC. Genetics of disc degeneration. Eur Spine J. 2006;15(SUPPL 3):317-325. doi: 10.1007/s00586-006-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshihara H. Surgical treatment for thoracic disc herniation: An update. Spine. 2014;39(6):315. doi: 10.1097/BRS.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 39.Court C, Mansour E, Bouthors C. Thoracic disc herniation: Surgical treatment. Orthop Traumatol Surg Res. 2018;104(1):S31-S40. doi: 10.1016/j.otsr.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 40.Quint U, Bordon G, Preissl I, Sanner C, Rosenthal D. Thoracoscopic treatment for single level symptomatic thoracic disc herniation: a prospective followed cohort study in a group of 167 consecutive cases. Eur Spine J. 2012;21(4):637-645. doi: 10.1007/s00586-011-2103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stino AM, Lorusso SJ. Myelopathies due to structural Cervical and thoracic disease. Contin Lifelong Learn Neurol. 2018;24(2):567-583. doi: 10.1212/CON.0000000000000594. Spinal Cord Disorders). [DOI] [PubMed] [Google Scholar]
- 42.Radhakrishnan K, Litchy WJ, O’Fallon WM, Kurland LT. Epidemiology of cervical radiculopathy: a population-based study from Rochester, Minnesota, 1976 through 1990. Brain. 1994;117(2):325-335. doi: 10.1093/brain/117.2.325. [DOI] [PubMed] [Google Scholar]
- 43.Schimel S, Deeb ZL. Herniated thoracic intervertebral disks. J Comput Tomogr. 1985;9(2):141-143. doi: 10.1016/0149-936X(85)90009-8. [DOI] [PubMed] [Google Scholar]
- 44.Hott JS, Feiz-Erfan I, Kenny K, Dickman CA. Surgical management of giant herniated thoracic discs: analysis of 20 cases. J Neurosurg Spine. 2005;3(3):191-197. doi: 10.3171/spi.2005.3.3.0191. [DOI] [PubMed] [Google Scholar]
- 45.Gay CW, Bishop MD, Beres JL. Clinical presentation of a patient with thoracic myelopathy at a chiropractic clinic. J Chiropr Med. 2012;11(2):115-120. doi: 10.1016/j.jcm.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marzluff JM, Hungerford GD, Kempe LG, Rawe SE, Trevor R, Perot PL. Thoracic myelopathy caused by osteophytes of the articular processes. Thoracic spondylosis. J Neurosurg. 1979;50(6):779-783. doi: 10.3171/jns.1979.50.6.0779. [DOI] [PubMed] [Google Scholar]
- 47.Shimada Y, Kasukawa Y, Miyakoshi N, Hongo M, Ando S, Itoi E. Spondylolisthesis of the thoracic spine. Case report. J Neurosurg Spine. 2006;4(5):415-418. doi: 10.3171/spi.2006.4.5.415. [DOI] [PubMed] [Google Scholar]
- 48.Hsieh PC, Lee ST, Chen JF. Lower thoracic degenerative spondylithesis with concomitant lumbar spondylosis. Clin Neurol Neurosurg. 2014;118:21-25. doi: 10.1016/j.clineuro.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 49.Ploumis A, Transfledt EE, Denis F. Degenerative lumbar scoliosis associated with spinal stenosis. 2007;7:428-436. doi: 10.1016/j.spinee.2006.07.015 [DOI] [PubMed] [Google Scholar]
- 50.Stapleton CJ, Pham MH, Attenello FJ, Hsieh PC. Ossification of the posterior longitudinal ligament: genetics and pathophysiology. Neurosurg Focus. 2011;30(3):22-25. doi: 10.3171/2010.12.FOCUS10271. [DOI] [PubMed] [Google Scholar]
- 51.Stetler WR, La Marca F, Park P. The genetics of ossification of the posterior longitudinal ligament. Neurosurg Focus. 2011;30(3):40-42. doi: 10.3171/2010.12.FOCUS10275. [DOI] [PubMed] [Google Scholar]
- 52.Inamasu J, Guiot BH, Sachs DC. Ossification of the posterior longitudinal ligament: An update on its biology, epidemiology, and natural history. Neurosurgery. 2006;58(6):1027-1038. doi: 10.1227/01.NEU.0000215867.87770.73. [DOI] [PubMed] [Google Scholar]
- 53.Ning S, Chen Z, Fan D, et al. Genetic differences in osteogenic differentiation potency in the thoracic ossification of the ligamentum flavum under cyclic mechanical stress. Int J Mol Med. 2017;39(1):135-143. doi: 10.3892/ijmm.2016.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daniels AH, Mcdonald CL, Basques BA, Kuris EO. Ossified ligamentum Flavum: epidemiology, treatment, and Outcomes. J Am Acad Orthop Surg. 2022;30(12):E842-E851. doi: 10.5435/JAAOS-D-21-01253. [DOI] [PubMed] [Google Scholar]
- 55.Yayama T, Mori K, Okumura N, et al. Wnt signaling pathway correlates with ossification of the spinal ligament: A microRNA array and immunohistochemical study. J Orthop Sci. 2018;23(1):26-31. doi: 10.1016/j.jos.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 56.Maigne JY, Ayral X, Guérin-Surville H. Frequency and size of ossifications in the caudal attachements of the ligamentum flavum of the thoracic spine. Role of rotatory strains in their development - an anatomic study of 121 spines. Surg Radiol Anat. 1992;14(2):119-124. doi: 10.1007/BF01794886. [DOI] [PubMed] [Google Scholar]
- 57.Miyakoshi N, Shimada Y, Suzuki T, et al. Factors related to long-term outcome after decompressive surgery for ossification of the ligamentum flavum of the thoracic spine. J Neurosurg. 2003;99(3 Suppl):251-256. doi: 10.3171/spi.2003.99.3.0251. [DOI] [PubMed] [Google Scholar]
- 58.Liao CC, Chen TY, Jung SM, Chen LR. Surgical experience with symptomatic thoracic ossification of the ligamentum flavum. J Neurosurg Spine. 2005;2(1):34-39. doi: 10.3171/spi.2005.2.1.0034. [DOI] [PubMed] [Google Scholar]
- 59.Li Z, Ren D, Zhao Y, et al. Clinical characteristics and surgical outcome of thoracic myelopathy caused by ossification of the ligamentum flavum: a retrospective analysis of 85 cases. Spinal Cord. 2016;54(3):188-196. doi: 10.1038/sc.2015.139. [DOI] [PubMed] [Google Scholar]
- 60.Yu S, Wu D, Li F, Hou T. Surgical results and prognostic factors for thoracic myelopathy caused by ossification of ligamentum flavum: posterior surgery by laminectomy. Acta Neurochir (Wien). 2013;155(7):1169-1177. doi: 10.1007/s00701-013-1694-0. [DOI] [PubMed] [Google Scholar]
- 61.Lang N, Yuan HS, Wang HL, et al. Epidemiological survey of ossification of the ligamentum flavum in thoracic spine: CT imaging observation of 993 cases. Eur Spine J. 2013;22(4):857-862. doi: 10.1007/s00586-012-2492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ando K, Imagama S, Ito Z, et al. Predictive factors for a poor surgical outcome with thoracic ossification of the ligamentum flavum by multivariate analysis: A multicenter study. Spine. 2013;38(12). doi: 10.1097/BRS.0b013e31828ff736. [DOI] [PubMed] [Google Scholar]
- 63.Miyasaka K, Kaneda K, Sato S, et al. Myelopathy due to ossification or calcification of the ligamentum flavum: Radiologic and histologic evaluations. Am J Neuroradiol. 1983;4(3):629-632. [PMC free article] [PubMed] [Google Scholar]
- 64.Mori K, Kasahara T, Mimura T, et al. Prevalence, distribution, and morphology of thoracic ossification of the yellow ligament in Japanese; results of ct-based cross-sectional study. Spine. 2013;38(19). doi: 10.1097/BRS.0b013e31829e018b [DOI] [PubMed] [Google Scholar]
- 65.Guo JJ, Luk KDK, Karppinen J, Yang H, Cheung KMC. Prevalence, distribution, and morphology of ossification of the ligamentum flavum: A population study of one thousand seven hundred thirty-six magnetic resonance imaging scans. Spine. 2010;35(1):51-56. doi: 10.1097/BRS.0b013e3181b3f779 [DOI] [PubMed] [Google Scholar]
- 66.Kang KC, Lee CS, Shin SK, Park SJ, Chung CH, Chung SS. Ossification of the ligamentum flavum of the thoracic spine in the Korean population: Clinical article. J Neurosurg Spine. 2011;14(4):513-519. doi: 10.3171/2010.11.SPINE10405. [DOI] [PubMed] [Google Scholar]
- 67.Fan T, Meng X, Sun C, et al. Genome-wide DNA methylation profile analysis in thoracic ossification of the ligamentum flavum. J Cell Mol Med. 2020;24(15):8753-8762. doi: 10.1111/jcmm.15509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qu X, Chen Z, Fan D, et al. Notch signaling pathways in human thoracic ossification of the ligamentum flavum. J Orthop Res. 2016;34(8):1481-1491. doi: 10.1002/jor.23303. [DOI] [PubMed] [Google Scholar]
- 69.Chen Q, Wanghan J, Wang Y, et al. Connexin 43 affects thoracic ossification of ligamentum flavum by regulating the p38 MAPK-RUNX2 signaling pathway. Tissue Cell. 2022;76(January):101760. doi: 10.1016/j.tice.2022.101760. [DOI] [PubMed] [Google Scholar]
- 70.Yang X, Chen Z, Meng X, et al. Angiopoietin-2 promotes osteogenic differentiation of thoracic ligamentum flavum cells via modulating the Notch signaling pathway. PLoS One. 2018;13(12):1-11. doi: 10.1371/journal.pone.0209300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang X, Sun C, Meng X, et al. LGR5 regulates osteogenic differentiation of human thoracic ligamentum flavum cells by Wnt signalling pathway. J Cell Mol Med. 2022(February 2021):3862-3872. doi: 10.1111/jcmm.17420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qu X, Hou X, Chen Z, Chen G, Fan T, Yang X. Association analysis and functional study of COL6A1 single nucleotide polymorphisms in thoracic ossification of the ligamentum flavum in the Chinese Han population. Eur Spine J. 2021;30(10):2782-2790. doi: 10.1007/s00586-021-06932-y. [DOI] [PubMed] [Google Scholar]
- 73.Fan D, Chen Z, Wang D, Guo Z, Qiang Q, Shang Y. Osterix is a key target for mechanical signals in human thoracic ligament Flavum Cells. J Cell Physiol. 2007;211:577-584. [DOI] [PubMed] [Google Scholar]
- 74.Mobbs RJ, Dvorak M. Ossification of the ligamentum flavum: Diet and genetics. J Clin Neurosci. Published online 2007:703–705. [DOI] [PubMed] [Google Scholar]
- 75.Wang W, Kong L, Zhao H, et al. Thoracic ossification of ligamentum flavum caused by skeletal fluorosis. Eur Spine J. 2007;16(8):1119-1128. doi: 10.1007/s00586-006-0242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang H, Deng N, Zhang L, Zhang L, Wang C. Clinical risk Factors for thoracic Ossification of the ligamentum Flavum: A Cross-sectional study based on spinal thoracic three-dimensional computerized tomography. Risk Manag Healthc Policy. 2022;15(May):1065-1072. doi: 10.2147/rmhp.s361730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boody BS, Lendner M, Vaccaro AR. Ossification of the posterior longitudinal ligament in the cervical spine: A review. Int Orthop. 2019;43(4):797-805. doi: 10.1007/s00264-018-4106-5. [DOI] [PubMed] [Google Scholar]
- 78.Endo T, Takahata M, Koike Y, Iwasaki N. Clinical characteristics of patients with thoracic myelopathy caused by ossification of the posterior longitudinal ligament. J Bone Miner Metab. 2020;38(1):63-69. doi: 10.1007/s00774-019-01026-8. [DOI] [PubMed] [Google Scholar]
- 79.Matsunaga S, Sakou T. Ossification of the posterior longitudinal ligament of the cervical spine: Etiology and natural history. Spine. 2012;37(5):309-314. doi: 10.1097/BRS.0b013e318241ad33 [DOI] [PubMed] [Google Scholar]
- 80.Zhai J, Guo S, Li J, Chen B. Progression of spinal ligament Ossification in patients with thoracic myelopathy. 2022;3(82072508):1-6. doi: 10.1111/os.13291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baaj AA, Smith DA, Vale FL, Uribe JS. Surgical approaches to thoracic ossification of the posterior longitudinal ligament. J Clin Neurosci. 2012;19(3):349-351. doi: 10.1016/j.jocn.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 82.Ono M, Russell WJ, Kudo S, et al. Ossification of the thoracic posterior longitudinal ligament in a fixed population. Radiological and neurological manifestations. Radiology. 1982;143(2):469-474. doi: 10.1148/radiology.143.2.7071349. [DOI] [PubMed] [Google Scholar]
- 83.Liang H, Liu G, Lu S, et al. Epidemiology of ossification of the spinal ligaments and associated factors in the Chinese population: a cross-sectional study of 2000 consecutive individuals. BMC Musculoskelet Disord. 2019;20(1):1-12. doi: 10.1186/s12891-019-2569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang P, Liu X, Zhu B, et al. Association of IL17RC and COL6A1 genetic polymorphisms with susceptibility to ossification of the thoracic posterior longitudinal ligament in Chinese patients. J Orthop Surg Res. 2018;13(1):1-6. doi: 10.1186/s13018-018-0817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang P, Teng Z, Liu X, Liu X, Kong C, Lu S. A new single nucleotide polymorphism affects the predisposition to thoracic ossification of the posterior longitudinal ligament. J Orthop Surg Res. 2019;14(1):1-8. doi: 10.1186/s13018-019-1481-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang P, Teng Z, Liu X, Liu X, Kong C, Lu S. The COL6A1 rs201153092 single nucleotide polymorphism, associates with thoracic ossification of the posterior longitudinal ligament. Mol Med Rep. 2020;21(1):191-200. doi: 10.3892/mmr.2019.10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang P, Liu X, Zhu B, et al. Identification of susceptibility loci for thoracic ossification of the posterior longitudinal ligament by whole-genome sequencing. Mol Med Rep. 2018;17(2):2557-2564. doi: 10.3892/mmr.2017.8171. [DOI] [PubMed] [Google Scholar]
- 88.Goto K, Yamazaki M, Tagawa M, et al. Involvement of insulin-like growth factor I in development of ossification of the posterior longitudinal ligament of the spine. Calcif Tissue Int. 1998;62(2):158-165. doi: 10.1007/s002239900410. [DOI] [PubMed] [Google Scholar]
- 89.Kamakura D, Fukutake K, Nakamura K, et al. Acromegaly presenting with myelopathy due to ossification of posterior longitudinal ligament: a case report. BMC Musculoskelet Disord. 2021;22(1):1-7. doi: 10.1186/s12891-021-04232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ikeda Y, Nakajima A, Aiba A, et al. Association between serum leptin and bone metabolic markers, and the development of heterotopic ossification of the spinal ligament in female patients with ossification of the posterior longitudinal ligament. Eur Spine J. 2011;20(9):1450-1458. doi: 10.1007/s00586-011-1688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aizawa T, Sato T, Tanaka Y, et al. Thoracic myelopathy in Japan: epidemiological retrospective study in Miyagi Prefecture during 15 years. Tohoku J Exp Med. 2006;210(3):199-208. doi: 10.1620/tjem.210.199. [DOI] [PubMed] [Google Scholar]
- 92.Filatov A, Hammond TC, Swerdloff MA. Compressive thoracic myelopathy. Compressive Thorac Myelopathy Neurol Neurol Sci Open Access. 2021;4(2):1023. https://meddocsonline.org/ [Google Scholar]
- 93.Imagama S, Ando K, Kobayashi K, et al. Factors for a good surgical outcome in posterior decompression and dekyphotic corrective fusion with instrumentation for thoracic ossification of the posterior longitudinal ligament: prospective single-center study. Oper Neurosurg. 2017;13(6):661-669. doi: 10.1093/ons/opx043. [DOI] [PubMed] [Google Scholar]
- 94.Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine. 1981;6(4):354-364. doi: 10.1097/00007632-198107000-00005 [DOI] [PubMed] [Google Scholar]
- 95.Hilton B, Tempest-Mitchell J, Davies B, Kotter M. Route to diagnosis of degenerative cervical myelopathy in a UK healthcare system: a retrospective cohort study. BMJ Open. 2019;9(5):1-9. doi: 10.1136/bmjopen-2018-027000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim DH, Lee SH, Lee JS, Song GS, Son DW. High thoracic ossification of ligamentum flavum causing partial Horner syndrome. Br J Neurosurg. 2021;35(2):231-232. doi: 10.1080/02688697.2018.1441976. [DOI] [PubMed] [Google Scholar]
- 97.Cong M, Si M, Hou Y, Ma H, Nie L. Foot drop as the initial symptom caused by thoracic disc herniation. Eur Spine J. 2022;31(7):1795-1801. doi: 10.1007/s00586-022-07254-3. [DOI] [PubMed] [Google Scholar]
- 98.Toribatake Y, Baba H, Kawahara N, Mizuno K, Tomita K. The epiconus syndrome presenting with radicular-type neurological features. Spinal Cord. 1997;35(3):163-170. doi: 10.1038/sj.sc.3100369. [DOI] [PubMed] [Google Scholar]
- 99.Rousseff RT, Tzvetanov P. False localising levels in spinal cord compression. NeuroRehabilitation. 2006;21(3):219-222. doi: 10.3233/nre-2006-21304. [DOI] [PubMed] [Google Scholar]
- 100.Larner AJ. False localising signs. J Neurol Neurosurg Psychiatry. 2003;74(4):415-418. doi: 10.1136/jnnp.74.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martinez-Del-Campo E, Moon K, Kalb S, Soriano-Baron H, Theodore N. Surgical management of a patient with thoracic spinal cord herniation: technical case report and review. Neurosurgery. 2015;77(3):E492-E498. doi: 10.1227/NEU.0000000000000860. [DOI] [PubMed] [Google Scholar]
- 102.Oyinkan Marquis B, Capone PM. Myelopathy. Handb Clin Neurol. 2016;136:1015-1026. doi: 10.1016/B978-0-444-53486-6.00052-1. [DOI] [PubMed] [Google Scholar]
- 103.Matsuyama Y, Yoshihara H, Tsuji T, et al. Surgical outcome of ossification of the posterior longitudinal ligament (OPLL) of the thoracic spine: Implication of the type of ossification and surgical options. J Spinal Disord Tech. 2005;18(6):492-497. doi: 10.1097/01.bsd.0000155033.63557.9c. [DOI] [PubMed] [Google Scholar]
- 104.Chang UK, Choe WG, Chung CK, Kim HJ. Surgical treatment for thoracic spinal stenosis. Spinal Cord. 2001;39(7):362-369. doi: 10.1038/sj.sc.3101174. [DOI] [PubMed] [Google Scholar]
- 105.Fehlings MG, Barry S, Kopjar B, et al. Anterior versus posterior surgical approaches to treat cervical spondylotic myelopathy: Outcomes of the prospective multicenter AOSpine North America CSM study in 264 patients. Spine. 2013;38(26):2247-2252. doi: 10.1097/BRS.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 106.Maruyama J, Furuya T, Maki S, et al. Posterior decompression and fixation for thoracic spine Ossification: A 10-year follow-up study. J Clin Med. 2023;12(17):9-16. doi: 10.3390/jcm12175701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ando K, Imagama S, Kaito T, et al. Outcomes of surgery for thoracic myelopathy owing to thoracic Ossification of the ligamentum Flavum in a nationwide multicenter prospectively collected study in 223 patients: Is instrumented Fusion necessary? Spine. 2020;45(3):E170-E178. doi: 10.1097/BRS.0000000000003208 [DOI] [PubMed] [Google Scholar]
- 108.Min JH, Jang JS, Lee SH. Clinical results of ossification of the posterior longitudinal ligament (OPLL) of the thoracic spine treated by anterior decompression. J Spinal Disord Tech. 2008;21(2):116-119. doi: 10.1097/BSD.0b013e318060091a. [DOI] [PubMed] [Google Scholar]
- 109.Quraishi NA, Khurana A, Tsegaye MM, Boszczyk BM, Mehdian SMH. Calcified giant thoracic disc herniations: Considerations and treatment strategies. Eur Spine J. 2014;23(SUPPL. 1):76-83. doi: 10.1007/s00586-014-3210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khoo LT, Smith ZA, Asgarzadie F, et al. Minimally invasive extracavitary approach for thoracic discectomy and interbody fusion: 1-Year clinical and radiographic outcomes in 13 patients compared with a cohort of traditional anterior transthoracic approaches - Clinical article. J Neurosurg Spine. 2011;14(2):250-260. doi: 10.3171/2010.10.SPINE09456. [DOI] [PubMed] [Google Scholar]
- 111.Zhao W, Shen C, Cai R, et al. Minimally invasive surgery for resection of ossification of the ligamentum flavum in the thoracic spine. Wideochirurgia I Inne Tech Maloinwazyjne. 2017;12(1):96-105. doi: 10.5114/wiitm.2017.66473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Okada K, Oka S, Tohge K, Ono K, Yonenobu K, Hosoya T. Thoracic myelopathy caused by ossification of the ligamentum flavum: Clinicopathologic study and surgical treatment. Transplantation. 1973;16(3):280-287. doi: 10.1097/00007632-199103000-00005. [DOI] [PubMed] [Google Scholar]
- 113.He S, Hussain N, Li S, Hou T. Clinical and prognostic analysis of ossified ligamentum flavum in a Chinese population. J Neurosurg Spine. 2005;3(5):348-354. doi: 10.3171/spi.2005.3.5.0348. [DOI] [PubMed] [Google Scholar]
- 114.Sung UK, Young SK, Yong EC, et al. Contributing factors affecting the prognosis surgical outcome for thoracic OLF. Eur Spine J. 2006;15(4):485-491. doi: 10.1007/s00586-005-0903-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Patel N. Surgical disorders of the thoracic and lumbar spine: Guide for neurologists. Neurol Pract. 2002;73(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Degenerative Thoracic Myelopathy: A Scoping Review of Epidemiology, Genetics, and Pathogenesis by Tanzil Rujeedawa BA, Oliver D. Mowforth MA, MB, BChir, MSt, MRCS, Benjamin M. Davies BSc, MPhil, MRCS, Cylene Yang BSc MBBS, Aria Nouri MD, MSc, Jibin J. Francis MBBCh, FCNeurosurg FRCS, Bizhan Aarabi, MD, Brian K. Kwon, MD, PhD FRCSC, James Harrop MD, Jefferson R. Wilson, MD PhD, Allan R. Martin MD PhD, Vafa Rahimi-Movaghar, MD, James D. Guest, MD, PhD, Michael G. Fehlings, MD PhD FRCSC, Mark R. Kotter MD MPhil PhD in Global Spine Journal