Abstract
A time course analysis was performed to identify the sites of formation and timing of appearance of polytropic recombinant viruses following infection of NIH/Swiss mice with the murine retrovirus SL3-3 murine leukemia virus (SL3) or with a weakly pathogenic mutant termed SL3ΔMyb5. The results indicated that (i) polytropic recombinant viruses occur initially in the thymus of SL3-infected animals, (ii) the timing of appearance of polytropic recombinants in bone marrow is not consistent with their participation in the previously reported formation of transplantable tumor-forming cells at 3 to 4 week postinoculation, and (iii) the efficient generation of recombinant virus is correlated with efficient tumor induction.
SL3-3 (SL3) is a highly pathogenic ecotropic murine leukemia virus (MuLV) that induces T-cell lymphoma uniformly in mice within 3 months after neonatal inoculation (9). The mechanisms by which this virus induces lymphoma are incompletely understood, although it is apparent that multiple steps are required. As is typical of ecotropic MuLV infection, recombinant polytropic viruses arise in vivo following SL3 inoculation in which the 5′ portion of the env gene encoding the receptor binding domain of gp70 (SU) is acquired from endogenous viral sequences (12, 14). By acquiring a new env gene, the recombinant virus can utilize a different cellular receptor for entry, thus permitting multiple infection events in an individual cell. Repeated viral infection may accelerate the malignant process by increasing the likelihood of long terminal repeat (LTR)-mediated oncogene activation and/or consequent activation of regulatory pathways in cells (2, 5). Two structurally distinct types of polytropic recombinant viruses arise during infection with SL3. Type I env recombinants retain ecotropic virus sequences in the 3′ portion of SU and the p15E (TM) gene. In type II env recombinants, the SU gene and the 5′ portion of TM are replaced by polytropic sequences. The structure of the polytropic recombinant generated during SL3 infection is dependent on the mouse strain serving as host and is influenced by host factors. In HRS/J and CBA/J mice, for example, the dominant selection for type I env recombinants is controlled by a single host gene that is not an endogenous provirus but resides within the major histocompatibility complex (4, 10, 12–14). Both type I and type II env recombinants arise during SL3 infection NIH/Swiss mice (12, 14), the strain examined in the present study.
Initial descriptions of the env recombinants generated during SL3 infection reported that the viruses grow poorly if at all in mink cells (6, 12). These observations suggest the possibility of extensive pseudotyping in ecotropic virions, as described for the early stages of Moloney MuLV (M-MuLV) infection. Unlike M-MuLV infection, however, the later stages of SL3 infection are apparently not characterized by a significant increase in the release of polytropic virions infectious to mink cells (8). The role of polytropic recombinant virus in the SL3-mediated malignant process is not well understood, nor is the biologic significance of the structural differences between type I and type II env genes. Both types of recombinants are identified in SL3-induced tumors (12, 14). Little is known about the tissue distribution or timing of appearance of recombinant viruses during SL3 infection because a time course analysis has not yet been performed. Such an analysis was performed in the present study to identify the sites of formation and timing of appearance of polytropic recombinant viruses following infection of NIH/Swiss mice with SL3 or with a weakly pathogenic enhancer mutant termed SL3ΔMyb5.
Type I and type II SL3 recombinants can be distinguished by PCR amplification and restriction enzyme digestion based on the presence of restriction enzyme sites definitive for each form (Fig. 1) (13). We first used this approach to confirm the presence of polytropic envelope recombinant viruses in T-cell tumors induced by SL3 in NIH/Swiss mice and to determine if recombinants occur in the occasional tumors induced by SL3ΔMyb5. The SL3ΔMyb5 mutant is identical to SL3 other than a three-nucleotide mutation that eliminates binding of the c-Myb protein to its cognate site in the viral LTR enhancer. Ablation of the c-Myb binding site in the enhancer is linked to greatly attenuated pathogenicity. Specifically, SL3ΔMyb5 was shown to induce no tumors during the 100-day period that we typically follow in infected animals and to induce tumors in only 3 of 11 NIH/Swiss mice after prolonged latency (230 days, compared to 70 days with SL3 [9]). Polytropic recombinant env genes were specifically amplified from SL3- and SL3ΔMyb5-induced tumor DNA using PCR primers EnvR+ (5′ GCGAATTCTCTATAGTCCC 3′) and EnvR− (5′ TTCCCGGGTCTCTTGAACTGTTGTTG 3′). These primers are homologous to the endogenous polytropic env and ecotropic LTR regions, respectively, of SL3 recombinants (13). The resulting 1.5-kb amplification product was digested with either XbaI or PstI and was hybridized to radiolabeled probes specific for type I or type II recombinants (13).
FIG. 1.
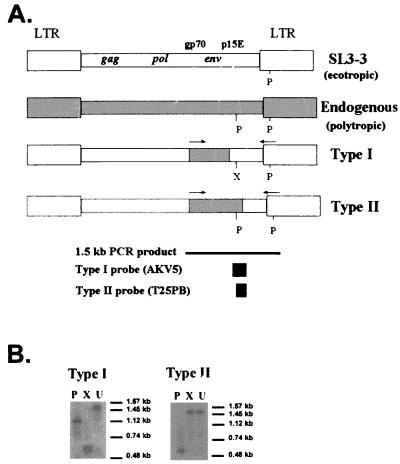
(A) Diagrammatic representation of proviral DNA of SL3, endogenous polytropic virus, and polytropic recombinant virus generated during SL3 infection in NIH/Swiss mice, adapted from reference 10. Type I and type II recombinant viruses differ in the source of the 5′ portion of the p15E gene and can be distinguished by restriction digestion following PCR amplification of the env region. The indicated 1.5-kb envelope region was amplified with 5′ and 3′ primers (indicated by arrows) homologous to polytropic envelope sequences and ecotropic LTR sequences, respectively. Indicated are restriction enzyme sites for PstI (P) and XbaI (X). Type I-specific (AKV5) or type-II specific (T25PB) hybridization probes are indicated. (B) Representative hybridization analysis of type I and type II polytropic recombinants from SL3-infected tissue, indicating the distinctive patterns generated by restriction enzyme digestion of amplification products with PstI (P) or XbaI (X). Also indicated is the size of the undigested amplification product (U). Molecular size markers electrophoresed in parallel are indicated.
As described by others (13), the AKV5 probe specific for type I recombinants was generated by PCR amplification of a portion of the p15E gene from ecotropic SL3. The T25PB probe specific for type II recombinants, originally isolated from polytropic sequences of the CWN-T-25 provirus (13), was generated by PCR amplification of endogenous polytropic envelope sequences in NIH/Swiss mouse DNA and was verified by nucleotide sequencing. Type-specific probes were hybridized as previously described (1), and hybridization signals were visualized by autoradiography or phosphorimaging for various periods of time. Using this approach, the restriction patterns generated by XbaI and PstI digestion are readily distinguishable and characteristic of type I or type II recombinants (Fig. 1B). A tissue was considered negative for polytropic recombinants if no hybridization signal was detected after 10 days of autoradiography at −70°C or after phosphorimaging for 2 days. No attempt was made to quantify the amount of recombinant virus present; rather, the approach was used to identify the presence or absence of recombinants. Using this approach, no recombinants were detected in tissues from uninfected NIH/Swiss mice (data not shown).
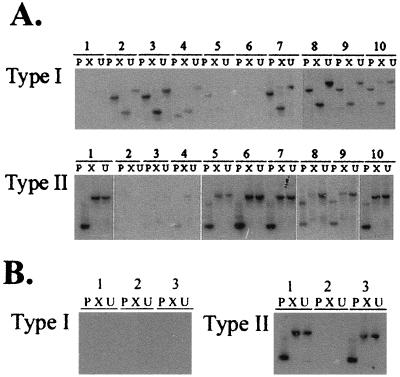
Of 10 SL3-induced tumors examined (Fig. 2A), 7 (samples 2, 3, 4, 7, 8, 9, and 10) were shown to contain type I recombinants and 7 (samples 1, 5, 6, 7, 8, 9, and 10) were shown to contain type II recombinants. Each tumor contained at least one type of recombinant, and 4 of 10 contained both types. The restriction enzyme pattern of type I recombinant viruses detected in sample 4 appears somewhat anomalous, although the precise structure of the recombinant has not been further examined. The endogenous viruses that serve as recombination partners in NIH/Swiss mice have not been identified; thus, it is possible that variant env gene structures may represent interactions with distinct recombination partners. Relatively weak hybridization signals were detected in samples 5 (type I), 3 (type II), and 4 (type II) after prolonged autoradiographic exposure; thus, these tissues were not considered positive for recombinant viruses. These findings clearly demonstrate that all SL3-induced tumors contain polytropic recombinant viruses and that many tumors (40%) contain representatives of both type I and type II. In contrast, of the three SL3ΔMyb5-induced tumors available for examination, none was observed to contain type I recombinant viruses. Type II recombinant viruses were detectable in the DNA of two of three SL3ΔMyb5-induced tumors (Fig. 2B). As described below, type I recombinants are generated during the course of SL3ΔMyb5 infection in the thymus and other tissues but are apparently not selected in tumors. It is noteworthy that all SL3-induced lymphomas examined in this study contained clonal, somatic rearrangement of the T-cell receptor beta locus (data not shown), but only one of the three SL3ΔMyb5-induced tumors did so (Fig. 2B, sample 1) (9). Thus, the absence of type I recombinant viruses in SL3ΔMyb5-induced tumors may reflect a difference in the target cell for transformation compared to SL3.
FIG. 2.
Type I or type II recombinant viruses in SL3- and SL3ΔMyb5-induced lymphomas in NIH/Swiss mice. Recombinant envelope gene sequences were specifically amplified from lymphomas of 10 SL3-infected animals (A) and 3 SL3ΔMyb5-infected animals (B). Amplification products were digested with PstI (P) or XbaI (X) or were undigested (U) and resolved by agarose gel electrophoresis. Southern blots were prepared and hybridized to probes specific for type I or type II recombinants. (A) Autoradiographic exposures at −70°C for 18 h (type I, samples 7 to 10; type II, samples 1, 5 to 7, and 10) or 10 days (type I, samples 1 to 6; type II, samples 2 to 4, 8, and 9). (B) Autoradiographic exposures at −70°C for 10 days (type I) or 1 h (type II).
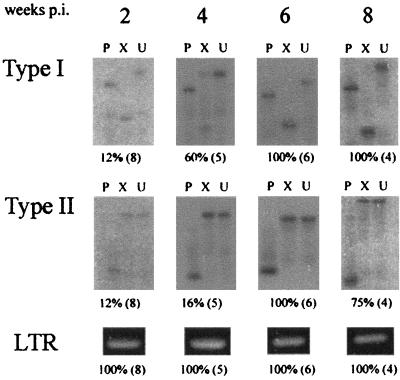
A time course analysis was then performed to determine the timing of appearance and tissue distribution of polytropic recombinant viruses during the preleukemic time period. Neonatal NIH/Swiss mice (Harlan Sprague Dawley, Inc.) were inoculated intraperitoneally (i.p.) with 106 infectious units of SL3 (or SL3ΔMyb5) and were sacrificed at 2, 4, 6, and 8 weeks postinoculation (p.i.). Thymus, bone marrow, and spleen were retrieved at each collection, and genomic DNA was prepared from the tissues. Virus infection was confirmed in each tissue by PCR using oligonucleotide primers SL3A and SL3C, previously described to amplify a portion of the proviral LTR. These primers do not amplify endogenous viral sequences from NIH/Swiss mice and can therefore be used as an indicator of SL3 (or SL3ΔMyb5) replication in target tissues (3). Polytropic recombinant viruses were then specifically amplified using primers EnvR+ and EnvR− as described above. Although no attempt was made to quantify the amount of proviral DNA in tissues, identical mass amounts of genomic DNA (1 μg) were used as template in each reaction, and the results were analyzed within the linear range of amplification. As depicted in Fig. 3, SL3 proviral DNA was detected in the thymus at all time points examined beginning as early as 2 weeks p.i. Polytropic recombinant virus was detectable in the thymus of one of eight SL3-infected animals (12%) as early as 2 weeks p.i., at which point that animal exhibited infection with both type I and type II. Type I recombinants were generated rapidly in the thymus, with 60% of animals infected at 4 weeks and 100% of animals infected thereafter. Type II recombinants were generated only slightly less frequently, with 16% of animals infected at 4 weeks and 100% infected at later times (Fig. 3). In the bone marrow, in contrast, neither type I nor type II envelope recombinants were detectable until 6 weeks p.i., at which time both types were detected in all animals examined. The incidence of recombinant virus infection in the bone marrow was observed to decline rapidly thereafter, to 25% at the 8-week time point (Table 1). A similar pattern was detected in the spleen, except that type I recombinants were detected as early as 4 weeks p.i. (Table 1). The basis for the steep decline observed after 6 weeks is not known but may represent the migration of recombinant-infected cells to a different tissue compartment. Neither SL3 nor recombinant virus was detected in tissues from age-matched, uninfected animals at any time point examined (data not shown). Furthermore, SL3 replication was apparently restricted to hematopoietic tissues, since no provirus was detected by PCR in liver or kidney at any time point examined (data not shown).
FIG. 3.
Incidence of type I or type II recombinant virus infection in the thymus of SL3-infected animals collected at 2, 4, 6, and 8 weeks p.i. Genomic DNA isolated from each sample was PCR amplified with primers specific for recombinant envelope sequences or for the SL3 LTR. Amplification products representing recombinant envelope sequences were digested with PstI (P) or XbaI (X) or were undigested and resolved by agarose gel electrophoresis. Southern blots were prepared and hybridized to probes specific for type I or type II recombinants. Indicated below each representative autoradiograph is the percentage of animals testing positive for recombinant virus, and the total number of animals examined (in parentheses).
TABLE 1.
Percentage of SL3-infected mice positive for polytropic recombinant virus or LTR sequencea
| Sample | % of mice positive for recombinant at indicated wk p.i.b
|
|||
|---|---|---|---|---|
| 2 | 4 | 6 | 8 | |
| Bone marrow | ||||
| Type I | 0 (2) | 0 (2) | 100 (4) | 25 (4) |
| Type II | 0 (2) | 0 (2) | 100 (4) | 25 (4) |
| LTR | 100 (2) | 100 (2) | 100 (4) | 100 (4) |
| Spleen | ||||
| Type I | 0 (3) | 50 (2) | 100 (4) | 50 (4) |
| Type II | 0 (3) | 0 (2) | 100 (4) | 25 (4) |
| LTR | 100 (3) | 100 (2) | 100 (4) | 100 (4) |
Recombinant polytropic virus was detected using a PCR-Southern blot approach as described in the text. LTR sequence was detected by PCR.
NIH/Swiss mice were inoculated i.p. with SL3 as neonates (<24 h of age). Tissues were collected at periodic intervals thereafter as indicated. Data show percentages of animals examined that exhibited recombinant virus. Indicated in parentheses is the number of animals examined at each time point.
Taken together, these data demonstrate that polytropic recombinant viruses are generated initially in the thymus during SL3 infection and that they occur uniformly in bone marrow and spleen only weeks later. The absence of detectable SL3 polytropic recombinants in the bone marrow before 6 weeks p.i. suggests that they may play little role in the initial transforming events reported to occur in that tissue. Specifically, previous studies described transplantable preleukemic cells in the bone marrow of SL3-infected AKR mice between 3 and 4 weeks p.i. (11). Despite the implication that recombinant viruses are therefore unlikely to be involved in the initial transforming event in bone marrow, it is important to note that differences in mouse strains used in the previous and present studies prevent a firm conclusion on this point. A recent study of M-MuLV infection similarly showed that M-MuLV polytropic recombinants are unlikely to be involved in the bone marrow and spleen changes characteristic of the early preleukemic period (5, 7). The influence of M-MuLV and SL3 polytropic recombinants in tumor development may therefore occur in the late stages of the malignant process.
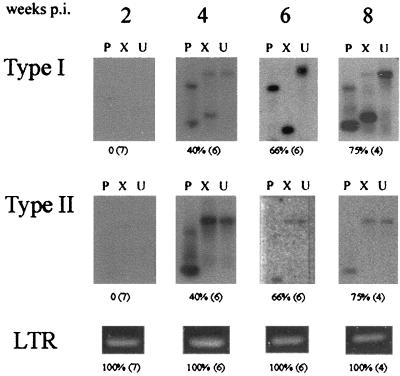
In SL3ΔMyb5-infected mice, PCR amplification of ecotropic LTR sequences using primers SL3A and SL3C demonstrated proviral DNA in the thymus throughout the preleukemic time course (Fig. 4). The use of polytropic recombinant virus-specific primers EnvR+ and EnvR− demonstrated type I and/or type II recombinants in the thymus of 40% of animals by 4 weeks p.i. The incidence of recombinant virus infection increased to 75% by 8 weeks p.i. (Fig. 4). The identification of recombinant virus in the thymus of most SL3ΔMyb5-infected mice between 4 and 8 weeks p.i. indicates that the generation of recombinant virus in that tissue does not ensure tumor formation. In the bone marrow and spleen of SL3ΔMyb5-infected animals, recombinants of both types were detected only infrequently beginning at 4 weeks p.i. (Table 2). Thus, polytropic recombinant viruses of types I and II are generated in SL3ΔMyb5-infected NIH/Swiss mice, but with a lower frequency and delayed timing. The basis for the reduced frequency of formation and delayed appearance of polytropic recombinants in SL3ΔMyb5-infected mice is not known but may reflect a diminished replicative capacity of SL3ΔMyb5, a differential ability to infect cells that express transcripts of the appropriate endogenous recombination partners, or reduced infection of a cell type that ferries the virus between compartments.
FIG. 4.
Incidence of type I or type II recombinant virus infection in the thymus of SL3ΔMyb5-infected animals collected at 2, 4, 6, and 8 weeks p.i. Genomic DNA isolated from each sample was PCR amplified with primers specific for recombinant envelope sequences or for the SL3ΔMyb5 LTR. Amplification products representing recombinant envelope sequences were digested with PstI (P) or XbaI (X) or were undigested and resolved by agarose gel electrophoresis. Southern blots were prepared and hybridized to probes specific for type I or type II recombinants. Indicated below each representative autoradiograph is the percentage of animals testing positive for recombinant virus and the total number of animals examined (in parentheses).
TABLE 2.
Percentage of SL3ΔMyb5-infected mice positive for polytropic recombinant virus or LTR sequencea
| % of mice positive for recombinant at indicated wk p.i.b
|
||||
|---|---|---|---|---|
| 2 | 4 | 6 | 8 | |
| Bone Marrow | ||||
| Type I | 0 (3) | 33 (3) | 0 (4) | 0 (4) |
| Type II | 0 (3) | 0 (3) | 25 (4) | 0 (4) |
| LTR | 100 (3) | 100 (3) | 100 (4) | 100 (4) |
| Spleen | ||||
| Type I | 0 (3) | 0 (4) | 25 (4) | 0 (4) |
| Type II | 0 (3) | 0 (4) | 25 (4) | 0 (4) |
| LTR | 100 (3) | 100 (4) | 100 (4) | 100 (4) |
Recombinant polytropic virus was detected using a PCR-Southern blot approach as described in the text. LTR sequence was detected by PCR.
NIH/Swiss mice were inoculated i.p. with SL3ΔMyb5 as neonates (<24 h of age). Tissues were collected at periodic intervals thereafter as indicated. Data show percentages of animals examined that exhibited recombinant virus. Indicated in parentheses is the number of animals examined at each time point.
The results of these studies indicate that (i) polytropic recombinant viruses occur initially in the thymus of SL3-infected animals and (ii) the timing of appearance of polytropic recombinants in bone marrow is not consistent with their participation in the initial transforming event in that tissue. Our studies further link the efficient generation of polytropic recombinant viruses in SL3-infected animals with efficient tumor induction but do not reveal the basis for the weak oncogenic potential of SL3ΔMyb5. Recombinant viruses are generated in the thymus of most SL3ΔMyb5-infected animals (Fig. 4) but are detected in the bone marrow and spleen of only 25 to 33% of infected animals (Table 2). Considering that tumors were previously observed to arise in a comparable proportion of SL3ΔMyb5-infected animals (27%) (9), it is possible that the diminished ability to form and/or propagate polytropic env recombinants is responsible for attenuated pathogenicity. Alternatively, the weak oncogenic potential of SL3ΔMyb5 may reflect differences at one or more other steps in the malignant process such as (i) quantitative differences in the levels of virus replication in target tissues, (ii) differences in the target cell types that support infection within tissues, and/or (iii) differences in the abilities of LTR enhancer sequences to activate the expression of adjacent cellular proto-oncogenes.
Acknowledgments
We gratefully acknowledge Chris Thomas for help in developing the experimental plan and Chandtip Chandhasin and Audrey Glynn for assistance in examining some of the tissue specimens.
This work was supported by PHS grant CA83823 and Development Funds of the Tulane Cancer Center. A.T. was supported by PHS MSTP grant CA13330. J.L. was supported by PHS grant CA44822.
REFERENCES
- 1.Athas G B, Choi B, Prabhu S, Lobelle-Rich P A, Levy L S. Genetic determinants of feline leukemia virus-induced multicentric lymphomas. Virology. 1995;214:431–438. doi: 10.1006/viro.1995.0053. [DOI] [PubMed] [Google Scholar]
- 2.Athas G B, Starkey C R, Levy L S. Retroviral determinants of leukemogenesis. Crit Rev Oncog. 1994;5:169–199. doi: 10.1615/critrevoncog.v5.i2-3.40. [DOI] [PubMed] [Google Scholar]
- 3.Beaty R M, Rulli K, Bost K L, Pantginis J, Lenz J, Levy L S. High levels of IL-4 and IL-10 mRNA and low levels of IL-2, IL-9 and IFN-gamma mRNA in MuLV-induced lymphomas. Virology. 1999;261:253–262. doi: 10.1006/viro.1999.9846. [DOI] [PubMed] [Google Scholar]
- 4.Coppola M A, Thomas C Y. A host gene regulates the structure of the transmembrane envelope protein of murine leukemia viruses. J Exp Med. 1990;171:1739–1752. doi: 10.1084/jem.171.5.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan H. Leukemogenesis by Moloney murine leukemia virus: a multistep process. Trends Microbiol. 1997;5:74–82. doi: 10.1016/S0966-842X(96)10076-7. [DOI] [PubMed] [Google Scholar]
- 6.Hays E F, Levy J A. Differences in lymphomagenic properties of AKR mouse retroviruses. Virology. 1984;138:49–57. doi: 10.1016/0042-6822(84)90146-6. [DOI] [PubMed] [Google Scholar]
- 7.Lander J K, Chesebro B, Fan H. Appearance of mink cell focus-inducing recombinants during in vitro infection by Moloney murine leukemia virus (M-MuLV) or the Mo+PyF101 M-MuLV enhancer variant: implications for sites of generation and roles in leukemogenesis. J Virol. 1999;73:5671–5680. doi: 10.1128/jvi.73.7.5671-5680.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavignon M, Evans L. A multistep process of leukemogenesis in Moloney murine leukemia virus-infected mice that is modulated by retroviral pseudotyping and interference. J Virol. 1996;70:3852–3862. doi: 10.1128/jvi.70.6.3852-3862.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nieves A, Levy L S, Lenz J. Importance of a c-Myb binding site for lymphomagenesis by the retrovirus SL3–3. J Virol. 1997;71:1213–1219. doi: 10.1128/jvi.71.2.1213-1219.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nuckols J D, Thomas C Y. The mouse H-2A region influences the envelope gene structure of tumor-associated murine leukemia viruses. J Virol. 1998;72:3973–3979. doi: 10.1128/jvi.72.5.3973-3979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeuchi H, Kato A, Hays E F. Presence of prelymphoma cells in bone marrow of the lymphomagenic virus-treated AKR mouse. Cancer Res. 1984;44:1008–1011. [PubMed] [Google Scholar]
- 12.Thomas C Y. AKR ecotropic murine leukemia virus SL3–3 forms envelope gene recombinants in vivo. J Virol. 1986;59:23–30. doi: 10.1128/jvi.59.1.23-30.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas C Y, Coppola M A, Nuckols J D, Lawrenz-Smith S C, Massey A C. An increase in disease latency is associated with a host-dependent selection for recombinant murine leukemia viruses with substitutions in the p15E (TM) gene. J Virol. 1993;67:294–304. doi: 10.1128/jvi.67.1.294-304.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas C Y, Nuckols J D, Murphy C, Innes D. Generation and pathogenicity of an NB-tropic SL3–3 murine leukemia virus. Virology. 1993;193:1013–1017. doi: 10.1006/viro.1993.1218. [DOI] [PubMed] [Google Scholar]