Abstract
Parvovirus B19 is a common human pathogen which can cause severe syndromes, including aplastic anemia and fetal hydrops. The mapping of the first parvovirus B19-derived CD8+ T-lymphocyte epitope is described. This HLA-B35-restricted peptide derives from the nonstructural (NS1) protein and is strongly immunogenic in B19 virus-seropositive donors.
Parvovirus B19 is a widespread pathogen, which normally causes a self-limiting illness in immunocompetent individuals (26). It is a short linear DNA virus which infects eythroid precursors by binding to its cellular receptor, the P antigen (5). Most of those infected will undergo a mild illness, associated typically in childhood with a facial rash (erythema infectiosum). In the fetus or those with hemolytic anaemias with rapid red cell turnover, however, the infection can induce fatal aplastic anemia (26). In some patients, a chronic infection may ensue, associated with milder persistent anemia (11, 13, 14, 22).
Despite its prevalence and potential clinical significance, little is known about cellular immune responses against B19 virus (18, 32). A primary B19 virus infection is usually associated with clearance of viraemia, and lifelong immunoglobulin G (IgG) antibodies are the hallmark of immunity (3). Lack of neutralizing antibodies has been associated with chronic B19 virus infection (18). However, chronic B19 virus infections can also occur in the presence of B19 virus-specific neutralizing antibodies (22).
We initiated studies of the CD8+ lymphocyte response against B19 virus by screening healthy laboratory volunteers for prior exposure to B19 virus. They were also tissue typed by sequence-specific primer PCR (6). The methods for analysis of immunoglobulin G avidity and epitope type specificity have been described elsewhere: an epitope type specificity ratio of >5 and an avidity score of >25 were used to define past infection (>6 months) previously (27, 28). B19 virus in serum samples was detected according to a previously described method (4), with the exception of the use of a different outer forward primer, GGC AGC ATG TGT TAA AGT GG. The nested PCR amplified a 284-bp fragment in the NS1 gene.
A total of 146 15-mer peptides overlapping by 10 amino acids were used to cover the entire length of the B19 virus NS1 protein, produced in a multiple peptide synthesizer (SyRo; Multisyntech, Bochum, Germany). Short-term stimulation of peripheral blood mononuclear cells (PBMC) using pools of overlapping peptides to amplify specific cytotoxic T-lymphocyte precursors (CTLp) was performed as previously described (17). After 8 to 10 days, assays for cytolysis were performed using conventional chromium-51 release assays (24), with targets of autologous or matched B-cell lines (BCL) prepulsed with peptides or peptide pools at concentrations of up to 10 μg/ml per peptide.
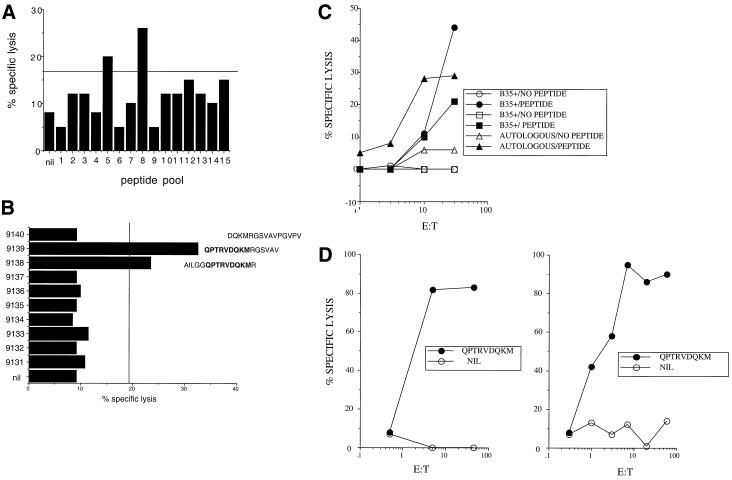
In initial screening experiments, PBMC from a normal volunteer (donor 1; HLA A2, A26, B35, and B62) were stimulated using 15 pools of up to 10 15-mer NS1 peptides. Lytic activity against autologous BCL prepulsed with the same peptide pools was first tested (Fig. 1A). Subsequently, the same expanded CTL population was tested for lytic activity against the individual peptides from the pool (Fig. 1B). These experiments identified a reproducible response against a peptide within pool 8 (peptides 9138 and 9139). Further CTL lines were directly stimulated from PBMC using a 1:1 mixture of these two peptides. These lines were highly active against autologous BCL prepulsed with the peptide 9138-9139 mix, and peptide-pulsed targets matched at HLA-B35 (Fig. 1C) but not HLA-A2 (data not shown).
FIG. 1.
Mapping of an immunodominant epitope in parvovirus B19 virus NS1 protein. (A) Chromium-51 release assay using CTL lines from donor 1. Peptide-stimulated PBMC lines were tested for lytic activity against autologous BCL prepulsed with pools. Each pool contained 10 15-mers overlapping by 5 except for pool 15, which contained 6 peptides. (B) The same PBMC from positive pool 8 were tested against the 10 individual peptides tested from the pool in an identical assay against autologous BCL. (C) Fresh PBMC from donor 1 were stimulated using only peptides 9138 and 9139 (in a 1:1 mix at 10 μM each) from pool 8, and after 8 days, cytolysis was tested against autologous and two different HLA-B35-matched targets. (D) PBMC from two HLA-B35-positive, B19 virus-seropositive donors were restimulated for 8 days with optimized peptide QPTRVDQKM, and cytolysis was tested as previously against HLA-B35-matched peptide-pulsed and control targets at various effector-to-target cell (E:T) ratios.
The region of NS1 within the overlapping peptides 9138 and 9139 contained a peptide with a clear HLA-B35 motif (QPTRVDQKM; amino acids 391 to 399 of NS1), which was obtained from Research Genetics (Huntsville, Ala.). PBMC from the same donor and a second HLA-B35-positive B19 virus-seropositive donor (donor 2) were restimulated in vitro with the optimal peptide alone, and a cytolysis assay was performed against autologous targets prepulsed with the same optimal peptide. After 8 days, very strong CTL responses were observed for both individuals (Fig. 1D).
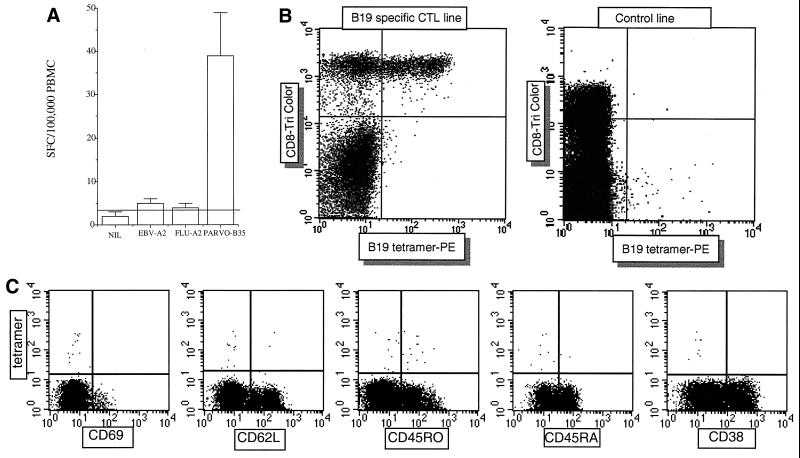
We next measured CD8+ T lymphocyte responses directly ex vivo using a gamma interferon (IFN-γ) Elispot. This assay was performed as previously described using nitrocellulose plates from Millipore (Bedford, Mass.) and IFN-γ antibodies (Mabtech AB, Stockholm, Sweden) followed by chromogenic substrate (Bio-Rad) (19). CTL responses to the optimized peptide were reproducibly measured at approximately 300 spot-forming cells (SFC) per 106 PBMC. Interestingly, at each time point tested, the CTL frequency as obtained by Elispot in this individual was higher for parvovirus than the immunodominant HLA-A2-restricted influenza virus matrix response (60 to 100 SFC/106 PBMC) (19), and also an HLA-A2-restricted Epstein-Barr virus epitope (100 to 120 SFC/106 PBMC) (7) (Fig. 2A).
FIG. 2.
Ex vivo detection of parvovirus (PARVO)-specific CTL responses by Elispot and tetramer staining. (A) Fresh PBMC from donor 1 were tested for overnight release of IFN-γ after peptide stimulation. Peptides were used at 10 μM, and cells were plated at 300,000 per well. Results are expressed as SFC/105 PBMC. FLU-A2 and EBV-A2 are HLA-A2-restricted peptides derived from influenza virus (GILGFVFTL) and Epstein-Barr virus (GLCTVAML), respectively. Donor 1 is HLA-A2 positive. (B) A CTL line from donor 1 specific for the optimized peptide QPTRVDQKM was stained with the HLA-B35 QPTRVDQKM tetramer and anti-CD8 (left-hand panel). CD8 staining (FL3) is on the y axis, and tetramer staining (FL2) is on the x axis. Staining of peptide-stimulated PBMC from a separate HLA-B35-positive B19 virus-seronegative donor, where no peptide-specific responses were obtained, is shown for comparison (right-hand panel). PE, phycoerythrin. (C) Fresh PBMC from donor 1 were stained with tetramer and CD8 as in panel B, but in addition phenotypic markers were conjugated with FITC, as shown. Representative results from assays of samples from three individuals stained at separate time points are shown. Tetramer staining is on the y axis, and the population shown is that contained within the CD8 high gate.
An HLA-B35 tetramer was constructed using the optimized epitope, exactly as previously described (25). Staining of lymphocytes was then performed according to the protocol of Whelan et al. (33). Antibodies used for cell surface staining were as follows: CD8-PerCP (Caltag); CD38-fluorescein isothiocyanate (FITC) conjugate (Becton Dickinson); CD28-FITC (Immunotech); CD57-FITC, CD69-FITC, CD62L-FITC, and CD45RA-FITC (Becton Dickinson); and CD3 allophycocyanin conjugate (APC) (Pharmingen). Samples were analyzed by four-color flow cytometry and analyzed using CellQuest software (Becton Dickinson). This tetramer bound well to an in vitro-stimulated CTL line from donor 1 (Fig. 2B) but not to PBMC from B19 virus-seronegative or HLA-B35-mismatched donors (Fig. 2B and data not shown).
Direct analysis of phenotype and frequency of CD8+ T lymphocyte responses was tested ex vivo in donor 1 (Fig. 2C). Tetramer-binding cells were present at a frequency of approximately 0.3% of CD8+ lymphocytes, in good agreement with the Elispot, assuming, as previously, approximately 10% CD8+ lymphocytes in the PBMC preparation (20). These cells were of phenotypes CD69lo, CD38lo, CD62Llo, and mainly CD45ROhi and CD45RAlo. This phenotype was observed in a further two individuals (data not shown), who were additionally found to be CD28hi, CD56lo, CD57lo, and HLA-DRlo, in keeping with a resting memory phenotype.
The HLA-B35-restricted epitope identified stimulates CD8+ T-lymphocyte responses which are readily detectable ex vivo in B19-seropositive donors. These virus-specific T lymphocytes show rapid expansion in vitro, cytolysis, and, importantly, rapid effector function ex vivo. They are also present at high frequencies, comparable to those seen after infection with viruses such as cytomegalovirus and Epstein-Barr virus (7, 20).
The role of these responses in control of B19 virus infection is not yet clear. Since the virus appears to be cytopathic (2, 30), one view would hold that neutralizing antibodies are likely to be the significant mediator of protection (34). However, in the longer term, there is no doubt that B19 virus can persist (23, 29, 31) and there are also clearly demonstrated instances where the presence of neutralizing antibodies does not correlate with clearance of viremia (22). The role of CTL in controlling tissue infection, where antibody might be expected to be less efficient, has been well demonstrated for persistent, poorly cytopathic viruses (35).
The high levels of virus-specific CD8+ T lymphocytes seen may result from a very large burst size (15) or be potentially maintained by continuous or intermittent exposure to antigen (12, 34). This antigen may be endogenously presented, perhaps at a very low level (10), or could result from reexposure due to the high levels of virus in the community.
The fact that these CD8+ T-lymphocyte responses are long-lived and possess appropriate effector function also suggests that parvovirus-based vectors might be considered in vaccine strategies for other infections (8, 9, 21). The identification of B19 epitopes for CD8+ T lymphocytes also opens opportunities to analyze the potential role of such effector cells in chronic arthritides, where it has been speculated that B19 virus may play a role in pathogenesis (1, 16). Parvovirus B19 virus may prove to be a valuable model for analyzing immunological memory, immunodominance, and the interplay between cellular and humoral immune responses to a clinically relevant human pathogen.
Acknowledgments
We thank the Wellcome Trust, NIH (grants AI41534, AI44595, and HD34336), the Swedish Medical Research Council, and the Swedish Children's Cancer Foundation for grant support. Douglas F. Nixon is an Elizabeth Glaser Scientist, and Hans M. L. Spiegel is a Scholar of the Elizabeth Glaser Pediatric AIDS Foundation.
We thank Rodney Phillips and Gillian Harcourt for longstanding advice and encouragement in the laboratory. Thanks also to Ken Welsh and Mike Bunce in tissue typing and to the various lab members who have given blood in support of this study. We also thank Martin Markowitz, Bill Borkowsky, and Henry Pollack for clinical samples.
REFERENCES
- 1.Altschuler E L. Parvovirus B19 and the pathogenesis of rheumatoid arthritis: a case for historical reasoning. Lancet. 1999;354:1026–1027. doi: 10.1016/S0140-6736(98)12312-7. [DOI] [PubMed] [Google Scholar]
- 2.Anderson L J. Human parvoviruses. J Infect Dis. 1990;161:603–608. doi: 10.1093/infdis/161.4.603. [DOI] [PubMed] [Google Scholar]
- 3.Anderson M J, Higgins P G, Davis L R, Willman J S, Jones S E, Kidd I M, Pattison J R, Tyrrell D A. Experimental parvoviral infection in humans. J Infect Dis. 1985;152:257–265. doi: 10.1093/infdis/152.2.257. [DOI] [PubMed] [Google Scholar]
- 4.Broliden K, Tolfvenston T, Ohlssen S, Henter J. Persistence of B19 parvovirus in pediatric malignancies. Med Paediatr Oncol. 1998;31:66–72. doi: 10.1002/(sici)1096-911x(199808)31:2<66::aid-mpo4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Brown K, Anderson S M, Young N S. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science. 1993;262:114–117. doi: 10.1126/science.8211117. [DOI] [PubMed] [Google Scholar]
- 6.Bunce M, O'Neill C M, Barnardo M C, Krausa P, Browning M J, Morris P J, Welsh K I. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers. Tissue Antigens. 1995;46:355–367. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 7.Callan M, Tan L, Annels N, Ogg G, Wilson J, O'Callaghan C, Steven N, McMichael A, Rickinson A. Direct visualisation of antigen specific CD8+ T cells during the primary immune response to EBV in vivo. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casal J I. Use of parvovirus-like particles for vaccination and induction of multiple immune responses. Biotechnol Appl Biochem. 1999;29:141–150. [PubMed] [Google Scholar]
- 9.Casal J I, Rueda P, Hurtado A. Parvovirus-like particles as vaccine vectors. Methods. 1999;19:174–186. doi: 10.1006/meth.1999.0843. [DOI] [PubMed] [Google Scholar]
- 10.Ciurea A, Klenerman P, Hunziger L, Horvath E, Hengartner H, Zinkernagel R. Low level persistence of LCMV in immunocompetent mice. Proc Natl Acad Sci USA. 1999;96:11964–11969. doi: 10.1073/pnas.96.21.11964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crook T W, Rogers B B, McFarland R D, Kroft S H, Muretto P, Hernandez J A, Latimer M J, McKenna R W. Unusual bone marrow manifestations of parvovirus B19 infection in immunocompromised patients. Hum Pathol. 2000;31:161–168. doi: 10.1016/s0046-8177(00)80215-4. [DOI] [PubMed] [Google Scholar]
- 12.Doherty P C, Hou S, Tripp R A. CD8+ T-cell memory to viruses. Curr Opin Immunol. 1994;6:545–552. doi: 10.1016/0952-7915(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 13.Faden H, Gary G W, Jr, Anderson L J. Chronic parvovirus infection in a presumably immunologically healthy woman. Clin Infect Dis. 1992;15:595–597. doi: 10.1093/clind/15.4.595. [DOI] [PubMed] [Google Scholar]
- 14.Frickhofen N, Abkowitz J L, Safford M, Berry J M, Antunez-de-Mayolo J, Astrow A, Cohen R, Halperin I, King L, Mintzer D, et al. Persistent B19 parvovirus infection in patients infected with human immunodeficiency virus type 1 (HIV-1): a treatable cause of anemia in AIDS. Ann Intern Med. 1990;113:926–933. doi: 10.7326/0003-4819-113-12-926. [DOI] [PubMed] [Google Scholar]
- 15.Hou S, Hyland L, Ryan K W, Portner A, Doherty P C. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 16.Ishii K K, Takahashi Y, Kaku M, Sasaki T. Role of human parvovirus B19 in the pathogenesis of rheumatoid arthritis. Jpn J Infect Dis. 1999;52:201–207. [PubMed] [Google Scholar]
- 17.Klenerman P, Luzzi G, McIntyre K, Phillips R, McMichael A J. Identification of a novel HLA A-25 restricted epitope in a conserved region of p24 gag (positions 71–80) AIDS. 1996;10:348–350. doi: 10.1097/00002030-199603000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Kurtzman G J, Cohen B J, Field A M, Oseas R, Blaese R M, Young N S. Immune response to B19 parvovirus and an antibody defect in persistent viral infection. J Clin Investig. 1989;84:1114–1123. doi: 10.1172/JCI114274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lechner F, Wong D K, Dunbar P R, Chapman R, Chung R T, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker B D. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo-Man R, Rueda P, Sedlik C, Deriaud E, Casal I, Leclerc C. A recombinant virus-like particle system derived from parvovirus as an efficient antigen carrier to elicit a polarized Th1 immune response without adjuvant. Eur J Immunol. 1998;28:1401–1407. doi: 10.1002/(SICI)1521-4141(199804)28:04<1401::AID-IMMU1401>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 22.Lundqvist A, Tolfvenstam T, Bostic J, Soderlund M, Broliden K. Clinical and laboratory findings in immunocompetent patients with persistent parvovirus B19 DNA in bone marrow. Scand J Infect Dis. 1999;31:11–16. doi: 10.1080/00365549950161817. [DOI] [PubMed] [Google Scholar]
- 23.Lundqvist A, Tolfvenstam T, Brytting M, Stolt C M, Hedman K, Broliden K. Prevalence of parvovirus B19 DNA in bone marrow of patients with haematological disorders. Scand J Infect Dis. 1999;31:119–122. doi: 10.1080/003655499750006128. [DOI] [PubMed] [Google Scholar]
- 24.Nixon D F, Townsend A R M, Elvin J G, Rizza C R, Gallwey J, McMichael A J. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature. 1988;336:484–487. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- 25.Ogg G, Jin X, Bonhoeffer S, Dunbar P, Nowak M, Monard S, Segal J, Cao Y, Rowland-Jones S, Cerundolo V, Hurley A, Markowitz M, Ho D, Nixon D, McMichael A. Quantitation of HIV-1 specific CTL and plasma load of HIV-1. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 26.Rogers B. Parvovirus B19: twenty-five years in perspective. Pediatr Dev Pathol. 1999;2:296–315. doi: 10.1007/s100249900128. [DOI] [PubMed] [Google Scholar]
- 27.Soderlund M, Brown C S, Cohen B J, Hedman K. Accurate serodiagnosis of B19 parvovirus infections by measurement of IgG avidity. J Infect Dis. 1995;171:710–713. doi: 10.1093/infdis/171.3.710. [DOI] [PubMed] [Google Scholar]
- 28.Soderlund M, Brown C S, Spaan W J, Hedman L, Hedman K. Epitope type-specific IgG responses to capsid proteins VP1 and VP2 of human parvovirus B19. J Infect Dis. 1995;172:1431–1436. doi: 10.1093/infdis/172.6.1431. [DOI] [PubMed] [Google Scholar]
- 29.Soderlund M, von Essen R, Haapasaari J, Kiistala U, Kiviluoto O, Hedman K. Persistence of parvovirus B19 DNA in synovial membranes of young patients with and without chronic arthropathy. Lancet. 1997;349:1063–1065. doi: 10.1016/S0140-6736(96)09110-6. [DOI] [PubMed] [Google Scholar]
- 30.Sol N, Le Junter J, Vassias I, Freyssinier J M, Thomas A, Prigent A F, Rudkin B B, Fichelson S, Morinet F. Possible interactions between the NS-1 protein and tumor necrosis factor alpha pathways in erythroid cell apoptosis induced by human parvovirus B19. J Virol. 1999;73:8762–8770. doi: 10.1128/jvi.73.10.8762-8770.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stahl H D, Hubner B, Seidl B, Liebert U G, van Der Heijden I M, Wilbrink B, Kraan M C, Emmrich F, Tak P P. Detection of multiple viral DNA species in synovial tissue and fluid of patients with early arthritis. Ann Rheumatic Dis. 2000;59:342–346. doi: 10.1136/ard.59.5.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Poblotzki A, Gerdes C, Reischl U, Wolf H, Modrow S. Lymphoproliferative responses after infection with human parvovirus B19. J Virol. 1996;70:7327–7330. doi: 10.1128/jvi.70.10.7327-7330.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whelan J A, Dunbar P R, Price D A, Purbhoo M A, Lechner F, Ogg G S, Griffiths G, Phillips R E, Cerundolo V, Sewell A K. Specificity of CTL interactions with peptide-MHC class I tetrameric complexes is temperature dependent. J Immunol. 1999;163:4342–4348. [PubMed] [Google Scholar]
- 34.Zinkernagel R M. Immunology taught by viruses. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]
- 35.Zinkernagel R M, Haenseler E, Leist T, Cerny A, Hengartner H, Althage A. T cell-mediated hepatitis in mice infected with lymphocytic choriomeningitis virus: liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? J Exp Med. 1986;164:1075–1092. doi: 10.1084/jem.164.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]