Abstract
Dendritic cells are pivotal antigen-presenting cells for generating adaptive T-cell responses. Here, we show that dendritic cells belonging to either the myeloid-related or lymphoid-related subset are permissive for infection by mouse polyomavirus and, when loaded with a peptide corresponding to the immunodominant anti-polyomavirus CD8+ T-cell epitope or infected by polyomavirus, are each capable of driving expansion of primary polyomavirus-specific CD8+ T-cell responses in vivo.
Dendritic cells (DC) are bone marrow-derived antigen-presenting cells (APC) uniquely suited to induce primary T-cell responses (2). In peripheral nonlymphoid tissues, immature DC are highly phagocytic cells having low cell surface expression of major histocompatibility complex (MHC) and costimulatory molecules. Upon encounter with inflammatory cytokines or bacterial products, or following tissue damage, DC migrate to regional lymphoid organs and mature into professional APC (6). This maturation process involves upregulated expression of cell surface MHC and costimulatory molecules, loss in capacity to capture antigen, and morphological changes (31).
Murine DC can be divided into at least two subsets that arise from distinct lineages and differ in phenotype and anatomical localization (24, 26, 27). Although both DC subsets are CD11c+, lymphoid-related DC express CD8α homodimers and express little or no CD11b, a marker of myeloid differentiation. Conversely, DC of the myeloid-related lineage express high levels of CD11b but no CD8α (19, 23). As done by others (3, 24), we have applied the designations “MDC” to the CD11bhigh CD8α− myeloid-related DC subset and “LDC” to the CD11blow/− CD8α+ lymphoid-related DC subset. MDC are thought to originate from a common DC and myeloid cell precursor and constitute the majority of DC in lymphoid and nonlymphoid tissues (30); LDC appear to develop from a lymphoid progenitor population (28), although recent work suggests that intrathymic T cells and lymphoid-related DC arise from different precursors (25). MDC and LDC also reside in distinct microenvironments; the former reside in marginal zones in the spleen, and the latter localize to the thymic medulla and T-cell-rich areas of secondary lymphoid organs (23, 32).
There is conflicting evidence for the role of LDC and MDC in the generation of adaptive T-cell responses. A number of reports indicated that MDC were the principal APC for inducing primary T-cell responses, whereas LDC were the predominant APC responsible for negative selection in the thymus and induction of peripheral T-cell tolerance (5, 11). More recent studies, however, suggest that both DC subsets are capable of generating primary T-cell responses but produce distinct signals that differentially regulate T helper cell lineage commitment. For example, LDC produce high levels of interleukin-12 and gamma interferon (IFN-γ) that promote the development of Th1 CD4+ T cells (17, 21, 24). MDC, which secrete much lower levels of these cytokines, preferentially promote differentiation of CD4+ T lymphocytes toward Th2 effectors (24). LDC and MDC pulsed with a class I MHC-restricted viral peptide have also been shown to be equally capable of priming antigen-specific CD8+ T cells in vivo, as demonstrated by development of antiviral cytotoxic activity upon in vitro restimulation (27). Whether peptide-loaded or infected DC of these subsets differ in the capacity to expand virus-specific CD8+ T-cell responses in vivo has not been investigated.
Polyomavirus is a highly oncogenic pathogen in the mouse, its natural host. The virus induces a broad array of tumors when inoculated into immunocompromised adult mice or neonatal mice of particular inbred strains (7). A number of studies, including our own, document that virus-specific CD8+ T lymphocytes are essential components of protective antipolyomavirus tumor immunity (4, 9, 13, 14, 16). We recently showed that both macrophages and DC are permissive for polyomavirus infection and present the immunodominant Dk-restricted CD8+ T-cell epitope of polyomavirus (amino acids 389 to 397 of the nonstructural middle T [MT] protein, designated MT389-397). Administration of unfractionated DC infected by polyomavirus or pulsed with MT389-397 peptide efficiently induced antigen-specific CD8+ T cells (10). Here, we investigated whether LDC and MDC subsets are capable of driving polyomavirus-specific CD8+ T-cell expansion in vivo.
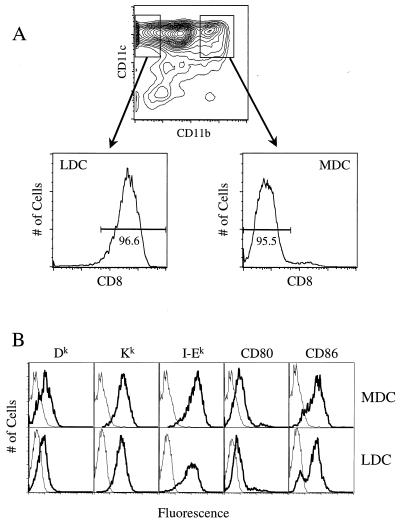
To overcome technical difficulties involved in isolating DC, a rare mononuclear cell population, and to avoid potential artifacts stemming from extensive cytokine-driven expansion in vitro, we expanded DC in vivo by administering Flt3 ligand (FL). FL is a hematopoietic growth factor that dramatically increases the number of DC in lymphoid and nonlymphoid tissues (19). Adult C3H/HeN female mice (Frederick Cancer Research and Development Center of the National Cancer Institute, Frederick, Md.) were injected intraperitoneally with 20 μg of Chinese hamster ovary cell-derived human FL for 10 consecutive days as described elsewhere (10). DC were isolated from collagenase (CLS III; Worthington Biochemical, Lakewood, N.J.)-digested spleens as follows. Spleen cells were resuspended in a 14% (wt/vol) Nycodenz (Accurate Chemical, Westbury, N.Y.) solution containing 74 mM NaCl, 1.5 mM KCl, 2.4 mM Tris, and 145 μM EDTA, overlaid with Hanks' balanced salt solution containing 10 mM EDTA, and then centrifuged at 1,700 × g for 15 min at 4°C. Purified DC were collected at the interface and cultured overnight in DC medium (Iscove's modified Eagle medium containing 10% fetal bovine serum, 4 mM l-glutamine, 50 μM 2-mercaptoethanol, and 10 ng of granulocyte-macrophage colony-stimulating factor [Intergen, Purchase, N.Y.] per ml). Splenic DC purity was typically 85 to 90% after Nycodenz density gradient enrichment, as determined by flow cytometric analysis for CD11c+ cells (data not shown). Flow cytometric analysis of these DC triple stained with phycoerythrin-conjugated anti-CD11c monoclonal antibody (MAb) (PharMingen, San Diego, Calif.), allophycocyanin-conjugated anti-CD11b MAb (PharMingen), and Tri-color-conjugated anti-CD8α MAb (Caltag Laboratories, South San Francisco, Calif.) highlights, first of all, the heterogeneous expression of CD11b by CD11chigh DC (Fig. 1A). As described by Maraskovsky et al. (19) and confirmed as shown in Fig. 1A, nearly all CD11chigh CD11b− cells (left-gated region) express CD8α, while CD11chigh CD11bhigh cells (right-gated region) lack surface CD8α expression; the left- and right-gated regions correspond to populations E and C, respectively, as reported elsewhere (19, 23). This distinct difference in CD8α expression by CD11chigh DC permits using these left- and right-gated regions to cleanly define LDC and MDC subsets, respectively. It should be noted that a proportion of the CD11chigh CD11bdull cells (population D in references 19 and 23) express low levels of CD8α (19, 23; data not shown) and that these CD8αdull DC are excluded in order to clearly distinguish myeloid-related and lymphoid-related DC subpopulations. DC stained as above for CD11c and CD11b expression were additionally stained with fluorescein isothiocyanate (FITC)-conjugated MAbs to Dk and Kk (both from Caltag), to CD80, CD86, and I-Ek (from the American Type Culture Collection, Manassas, Va.), and to CD3ε (PharMingen). As shown in Fig. 1B, both MDC and LDC expressed high levels of CD80, CD86, and I-Ek, which is consistent with results by others (17), as well as similar levels of both class I MHC molecules Kk and Dk. Although freshly isolated FL-expanded DC cells are phenotypically and functionally mature (19), the high levels of MHC and costimulatory molecule expression by the DC shown here may reflect additional DC maturation driven by granulocyte-macrophage colony-stimulating factor during overnight culture (20).
FIG. 1.
Cell surface phenotypic analysis of LDC and MDC. (A) CD11c and CD11b expression by Nycodenz gradient-enriched, FL-expanded spleen cells. Cells in the left- and right-gated regions differentially express CD8α. (B) Plots represent cell number versus log fluorescence intensity of CD11c+ cells in the left-gated region (LDC) and in the right-gated region (MDC). Thick lines represent staining with FITC-conjugated MAbs against the indicated molecules; thin lines represent staining by FITC-conjugated isotype control MAbs.
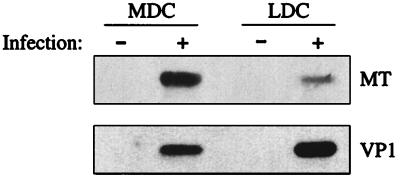
To investigate whether MDC and LDC are permissive for polyomavirus infection, DC were infected by polyomavirus strain A2 at a multiplicity of infection (MOI) of 2 and cultured in DC medium for 20 h. LDC and MDC were then sorted by fluorescence-activated cell sorting (FACS) in a FACSVantage (Becton Dickinson, San Jose, Calif.) using the gates illustrated in Fig. 1A and analyzed for expression of polyomavirus proteins by Western immunoassay as described elsewhere (10). Figure 2 shows that both DC subsets express the early region nonstructural MT protein and late region VP1 capsid protein; VP1 expression indicates productive infection (1). Since expression of the late region transcripts negatively regulates early region transcription (12), the higher amount of VP1 and lower level of MT in infected LDC than in infected MDC may indicate that the polyomavirus life cycle had progressed further in the LDC. No changes in expression of MHC and costimulatory molecules by MDC and LDC were seen after polyomavirus infection (data not shown).
FIG. 2.
MDC and LDC are permissive for polyomavirus infection. Whole cell protein lysates (50 μg/lane) from uninfected or infected (MOI of 2), FACS-sorted CD11c+ CD11b− LDC or CD11c+ CD11bhigh MDC were electrophoresed on a 10% reducing sodium dodecyl sulfate-polyacrylamide gel, transferred to nitrocellulose membranes, and immunoblotted with the anti-MT protein MAb F4 (22) (top) or rabbit anti-VP1 antiserum (33) (bottom).
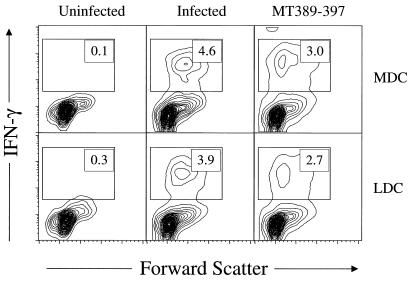
We previously showed that polyomavirus-infected, FL-expanded DC elicit primary virus-specific CD8+ T-cell responses in vivo (10). To determine whether MDC and LDC differ in the capacity to generate polyomavirus-specific CD8+ T lymphocyte responses in vivo, Nycodenz gradient-enriched DC were either infected by polyomavirus (MOI of 2), pulsed with MT389-397 peptide (10 μM), or left untreated for ∼20 h in DC medium at 37°C. CD11c+ cells were then FACS sorted into CD11b− and CD11bhigh subpopulations, using the gates shown in Fig. 1A, and 5 × 105 cells of each sorted population were injected subcutaneously (s.c.) into naïve syngeneic adult C3H/HeN female mice. Six days later, CD8+ T cells in freshly explanted spleens were quantitatively assayed by flow cytometry for MT389-397 peptide-specific stimulation of intracellular IFN-γ production as described previously (10). As shown in Fig. 3, both virus-infected and MT389-397 peptide-loaded, but not untreated, MDC and LDC efficiently induced expansion of IFN-γ effector function-competent, primary polyomavirus-specific CD8+ T-lymphocyte responses in vivo. The possibility that recipient APC infected by virus released from donor DC were responsible for inducing virus-specific CD8+ T cells is unlikely because no infectious virus was detected in the spleen by plaque assay (detection limit, 1 PFU/mg) at day 6 after s.c. transfer of infected, FACS-sorted CD11c+ DC (data not shown). Furthermore, induction of polyomavirus-specific CD8+ T cells during acute infection is associated with high levels of infectious virus in the spleen (15).
FIG. 3.
MT389-397 peptide-pulsed or polyomavirus-infected DC of either subset induce antipolyomavirus CD8+ T cells in vivo. Uninfected, MT389-397 peptide-pulsed (10 μM), or infected LDC and MDC were injected s.c. in hind footpads. Six days later, spleen cells were stimulated directly ex vivo with MT389-397 peptide (10 μM), the non-polyomavirus Dk-binding Gag88-96 peptide (10 μM) (8), or no peptide for 6 h in the presence of brefeldin A and then stained for surface CD8 and intracellular IFN-γ. Plots are gated on CD8+ cells, and values represent the percentage of cells in the indicated region. No IFN-γ+ CD8+ cells were detected in the absence of peptide or presence of Gag88-96 peptide (data not shown). Results are representative of two experiments using two mice per group.
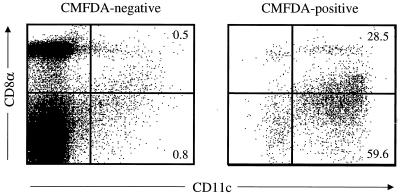
The capacity of infected and peptide-pulsed MDC and LDC to prime polyomavirus-specific CD8+ T cells in vivo implies that s.c.-administered DC of both subsets traffic to secondary lymphoid organs. This conclusion is further supported by a previous report showing that either peptide-pulsed MDC or LDC from FL-treated mice primed antigen-specific CD4+ T cells after s.c. transfer (24). To directly test whether MDC and LDC migrate to lymph nodes, we s.c. injected CMFDA (Molecular Probes, Eugene, Oreg.)-labeled, Nycodenz-enriched spleen cells from FL-treated donors into syngeneic mice and, 24 h later, analyzed the draining lymph nodes for donor CD11c+ cells nodes that expressed surface CD8α. As shown in Fig. 4, flow cytometric analysis of CMFDA+ CD11c+ cells showed that both CD8α+/dull (i.e., LDC) and CD8α− (i.e., MDC) DC migrate to regional lymph nodes; heterogeneity in CD8α expression levels by lymphoid-related DC has been previously documented (19). In addition, there appears to be preferential homing of the MDC to the draining lymph nodes, since in FL-treated mice, LDC outnumber MDC by 3 to 1 (24). Other groups using DC isolated from untreated mice reported that only MDC migrated to draining lymph nodes (27, 29). The possibility that FL-expanded DC and directly isolated DC differ in trafficking behavior is unlikely in light of evidence that LDC and MDC from either untreated or FL-treated mice prime comparable antigen-specific T-helper cell responses (18). Another explanation may lie in differences in experimental design; whereas we adoptively transferred unfractionated DC and then phenotyped the donor DC subpopulations in the draining lymph nodes, other groups transferred sorted DC subsets. An interesting possibility is that the trafficking behavior of donor LDC is affected by donor MDC.
FIG. 4.
LDC and MDC migrate to draining lymph nodes after s.c. inoculation. Nycodenz gradient-enriched, FL-expanded splenic DC (2.5 × 106) were labeled with CMFDA and injected s.c. into each hind footpad of syngeneic mice; 24 h later, single-cell suspensions of popliteal lymph nodes were stained for surface CD11c and CD8α expression and analyzed by flow cytometry. Plots are gated on CMFDA+ and CMFDA− cells, and quadrants are assigned based on staining for CD11c and CD8α expression in popliteal lymph nodes from naïve mice (data not shown). Values represent the percentage of cells in the indicated region. Axes are log scale.
This study shows that both myeloid-related and lymphoid-related DC are permissive for polyomavirus infection and contribute to priming antipolyomavirus CD8+ T-cell responses in vivo, with an efficiency comparable to that of unfractionated infected DC (10). Thus, at least as an immunization strategy for inducing antigen-specific CD8+ T cells, there may not be a need to use a particular DC subpopulation. Furthermore, the capacity of polyomavirus to infect DC affords the opportunity for DC to present a variety of viral class I MHC epitopes and induce a polyspecific antipolyomavirus CD8+ T-cell response. Finally, this study, together with our previous report (10), supports the use of DC vaccination to drive expansion of protective polyomavirus-specific CD8+ T cells in mice susceptible to polyomavirus tumorigenesis.
Acknowledgments
This work was supported by National Institutes of Health grant CA71971 (to A.E.L.) and the Immunex Corporation.
We thank Robert Karaffa for expertise in flow cytometry.
REFERENCES
- 1.Atencio I A, Shadan F F, Zhou X J, Vaziri N D, Villarreal L P. Adult mouse kidneys become permissive to acute polyomavirus infection and reactivate persistent infections in response to cellular damage and regeneration. J Virol. 1993;67:1424–1432. doi: 10.1128/jvi.67.3.1424-1432.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y-J, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 3.Bauman S K, Nichols K L, Murphy J W. Dendritic cells in the induction of protective and nonprotective anticryptococcal cell-mediated immune responses. J Immunol. 2000;165:158–167. doi: 10.4049/jimmunol.165.1.158. [DOI] [PubMed] [Google Scholar]
- 4.Berke Z, Palmer S, Bergman T, Wester D, Svedmyr J, Linder S, Jornvall H, Dalianis T. A short peptide eluted from the H-2Kb molecule of a polyomavirus-positive tumor corresponds to polyomavirus large T antigen peptide at amino acids 578 to 585 and induces polyomavirus-specific immunity. J Virol. 1996;70:3093–3097. doi: 10.1128/jvi.70.5.3093-3097.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brocker T, Riedinger M, Karjalainen K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J Exp Med. 1997;185:541–550. doi: 10.1084/jem.185.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation, and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 7.Dawe C J, Freund R, Mandel G, Ballmer-Hofer K, Talmage D A, Benjamin T L. Variations in polyoma virus genotype in relation to tumor induction in mice: characterization of wild type strains with widely differing tumor profiles. Am J Pathol. 1987;127:243–261. [PMC free article] [PubMed] [Google Scholar]
- 8.de Bergeyck V, De Plaen E, Chomez P, Boon T, Van Pel A. An intracisternal A-particle sequence codes for an antigen recognized by syngeneic cytolytic T lymphocytes on a mouse spontaneous leukemia. Eur J Immunol. 1994;24:2203–2212. doi: 10.1002/eji.1830240941. [DOI] [PubMed] [Google Scholar]
- 9.Drake D R, III, Lukacher A E. β2-Microglobulin knockout mice are highly susceptible to polyoma virus tumorigenesis. Virology. 1998;252:275–284. doi: 10.1006/viro.1998.9455. [DOI] [PubMed] [Google Scholar]
- 10.Drake D R, III, Moser J M, Hadley A, Altman J D, Maliszewski C, Butz E, Lukacher A E. Polyomavirus-infected dendritic cells induce antiviral CD8+ T lymphocytes. J Virol. 2000;74:4093–4101. doi: 10.1128/jvi.74.9.4093-4101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fazekas de St. Groth B. The evolution of self tolerance: a new cell is required to meet the challenge of self-reactivity. Immunol Today. 1998;19:448–454. doi: 10.1016/s0167-5699(98)01328-0. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Batt D B, Carmichael G G. Targeted nuclear antisense RNA mimics natural antisense-induced degradation of polyoma virus early RNA. Proc Natl Acad Sci USA. 1994;91:4258–4262. doi: 10.1073/pnas.91.10.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ljunggren G, Ljunggren H-G, Dalianis T. T cell subsets involved in immunity against polyoma virus-induced tumors. Virology. 1994;198:714–716. doi: 10.1006/viro.1994.1084. [DOI] [PubMed] [Google Scholar]
- 14.Lukacher A E, Ma Y, Carroll J P, Abromson-Leeman S R, Laning J C, Dorf M E, Benjamin T L. Susceptibility to tumors induced by polyoma virus is conferred by an endogenous mouse mammary tumor virus superantigen. J Exp Med. 1995;181:1683–1692. doi: 10.1084/jem.181.5.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukacher A E, Moser J M, Hadley A, Altman J D. Visualization of polyoma virus-specific CD8+ T cells in vivo during infection and tumor rejection. J Immunol. 1999;163:3369–3378. [PubMed] [Google Scholar]
- 16.Lukacher A E, Wilson C S. Resistance to polyoma virus-induced tumors correlates with CTL recognition of an immunodominant H-2Dk-restricted epitope in the middle T protein. J Immunol. 1998;160:1724–1734. [PubMed] [Google Scholar]
- 17.Maldonado-Lopez R, De Smedt T, Michel P, Godfroid M, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maldonado-Lopez R, De Smedt T, Pajak B, Heirman C, Thielemans K, Oberdan L, Urbain J, Maliszewski C R, Moser M. Role of CD8α+ and CD8α− dendritic cells in the induction of primary immune responses in vivo. J Leukoc Biol. 1999;66:242–246. doi: 10.1002/jlb.66.2.242. [DOI] [PubMed] [Google Scholar]
- 19.Maraskovsky E, Brasel K, Teepe M, Roux E R, Lyman S D, Shortman K, McKenna H J. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connell P J, Morelli A E, Logar A J, Thomson A W. Phenotypic and functional characterization of mouse hepatic CD8α+ lymphoid-related dendritic cells. J Immunol. 2000;165:795–803. doi: 10.4049/jimmunol.165.2.795. [DOI] [PubMed] [Google Scholar]
- 21.Ohteki T, Fukao T, Suzue K, Maki C, Ito M, Nakamura M, Koyasu S. Interleukin 12-dependent interferon γ production by CD8α+ lymphoid dendritic cells. J Exp Med. 1999;189:1981–1986. doi: 10.1084/jem.189.12.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pallas D C, Schley C, Mahoney M, Harlow E, Schaffhausen B S, Roberts T M. Polyomavirus small t antigen: overproduction in bacteria, purification, and utilization for monoclonal and polyclonal antibody production. J Virol. 1986;60:1075–1084. doi: 10.1128/jvi.60.3.1075-1084.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulendran B, Lingappa J, Kennedy M K, Smith J, Teepe M, Rudensky A, Maliszewski C R, Maraskovsky E. Developmental pathways of dendritic cells in vivo: distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand-treated mice. J Immunol. 1997;159:2222–2231. [PubMed] [Google Scholar]
- 24.Pulendran B, Smith J L, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski C R. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radtke F, Ferrero I, Wilson A, Lees R, Aguet M, MacDonald H R. Notch1 deficiency dissociates the intrathymic development of dendritic cells and T cells. J Exp Med. 2000;191:1085–1093. doi: 10.1084/jem.191.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid S D, Penna G, Adorini L. The control of T cell responses by dendritic cell subsets. Curr Opin Immunol. 2000;12:114–121. doi: 10.1016/s0952-7915(99)00059-x. [DOI] [PubMed] [Google Scholar]
- 27.Ruedl C, Bachmann M F. CTL priming by CD8+ and CD8− dendritic cells in vivo. Eur J Immunol. 1999;29:3762–3767. doi: 10.1002/(SICI)1521-4141(199911)29:11<3762::AID-IMMU3762>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 28.Shortman K, Vremec D, Corcoran L M, Georgopoulos K, Lucas K, Wu L. The linkage between T-cell and dendritic cell development in the mouse thymus. Immunol Rev. 1998;165:39–46. doi: 10.1111/j.1600-065x.1998.tb01228.x. [DOI] [PubMed] [Google Scholar]
- 29.Smith A L, Fazekas de St. Groth B. Antigen-pulsed CD8α+ dendritic cells generate an immune response after subcutaneous injection without homing to the draining lymph node. J Exp Med. 1999;189:593–598. doi: 10.1084/jem.189.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinman R M, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 31.Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, Zimmermann V S, Davoust J, Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu L, Li C-L, Shortman K. Thymic dendritic cell precursors: relationship to the lymphocyte lineage and phenotype of the dendritic cell progeny. J Exp Med. 1996;184:903–911. doi: 10.1084/jem.184.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi X, Peterson J, Freund R. Transformation and tumorigenic properties of a mutant polyomavirus containing a middle T antigen defective in Shc binding. J Virol. 1997;71:6279–6286. doi: 10.1128/jvi.71.9.6279-6286.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]