Abstract
In this study, we examined the effects of murine chemokine DNA, as genetic adjuvants given mucosally, on the systemic and distal mucosal immune responses to plasmid DNA encoding gB of herpes simplex virus (HSV) by using the mouse model. The CC chemokines macrophage inflammatory protein 1β (MIP-1β) and monocyte chemotactic protein 1 (MCP-1) biased the immunity to the Th2-type pattern as judged by the ratio of immunoglobulin isotypes and interleukin-4 cytokine levels produced by CD4+ T cells. The CXC chemokine MIP-2 and the CC chemokine MIP-1α, however, mounted immune responses of the Th1-type pattern, and such a response rendered recipients more resistant to HSV vaginal infection. In addition, MIP-1α appeared to act via the upregulation of antigen-presenting cell (APC) function and the expression of costimulatory molecules (B7-1 and B7-2), whereas MIP-2 enhanced Th1-type CD4+ T-cell-mediated adaptive immunity by increasing gamma interferon secretion from activated NK cells. Our results emphasize the value of using the mucosal route to administer DNA modulators such as chemokines that function as adjuvants by regulating the activity of innate immunity. Our findings provide new insight into the value of CXC and CC chemokines, which act on different innate cellular components as the linkage signals between innate and adaptive immunity in mucosal DNA vaccination.
Mucosal surfaces represent the primary site for the transmission of several viruses including human immunodeficiency virus and herpes simplex virus (HSV). In consequence, immunity at mucosal sites represents an important issue in vaccine development. Mucosae have numerous innate defenses, some of which serve to alert and direct the nature of subsequent acquired immune events (1, 10). Interest has recently focused on cytokines and chemokines and the role they appear to play as modulators of the adaptive immune responses (12, 31). Accordingly, members of both types of molecules induced nonspecifically upon infection are involved in regulating the inflammatory reaction and the subsequent adaptive Th1 or Th2 type of T-cell reaction that occurs in the draining lymphoid tissue. Consequently, manipulating the expression of cytokines and chemokines during exposure to infectious agents or vaccine represents a valuable approach to achieve optimal protection.
Most information on immunomodulatory effects of immune mediators has emphasized cytokines (34, 40). However, various chemokines may represent even more useful innate modulators since these molecules are involved in the recruitment and activation of cells related to innate immunity (35, 39). Currently, little is known about the nonspecific adjuvant effect of chemokines, and the two previous studies which used chemokine DNA given systemically with antigen (Ag) provided conflicting data (13, 18). In the present study, we investigated whether genetic cotransfer of certain chemokines along with plasmid DNA encoding viral Ag to a mucosal site can affect the efficiency of subsequent acquired mucosal and systemic immune responses. Our results show that mucosal genetic cotransfer of chemokines affects the nature of both systemic and distal mucosal acquired immune responses. The CC chemokines monocyte chemotactic protein 1 (MCP-1) and macrophage inflammatory protein 1β (MIP-1β) biased the response toward Th2-type immunity, whereas the CC chemokine MIP-1α and the CXC chemokine MIP-2 emphasized a Th1-type response. Mice with this later pattern were more resistant to HSV mucosal infection. MIP-1α appeared to act via up-regulation of antigen-presenting cell (APC) function and expression of the costimulatory molecules B7-1 and B7-2, whereas MIP-2 appeared to increase the function of NK cells. Our results emphasize the value of CXC or CC chemokines as mucosal adjuvants and indicate that they function by influencing the interaction between innate and adaptive immunity.
MATERIALS AND METHODS
Mice and viruses.
Female 4- to 5-week old BALB/c (H-2d) and C57BL/6 (H-2b) mice were purchased from Harlan Sprague-Dawley (Indianapolis, Ind.) and housed in the animal facilities at the University of Tennessee. DO11.10-SCID Tg mice were obtained by crossing DO11.10 (kindly provided by Casey Weaver, University of Alabama, Birmingham, Ala.) and SCID mice as described previously (11). All investigations followed guidelines of the Committee on the Care of Laboratory Animals Resources, Commission on Life Science, National Research Council. HSV-1 KOS and McKrae were grown on Vero cells obtained from the American Type Culture Collection (Rockville, Md.).
Plasmid DNA preparation.
Plasmid DNA encoding gB (gB DNA) of HSV-1 KOS under the cytomegalovirus promoter has been described in detail elsewhere (19). Plasmids encoding the CXC chemokine murine MIP-2 and CC chemokines including murine MIP-1α and murine MIP-1β were kindly donated by Barbara Sherry (Picower Institute, Manhasset, N.Y.) and then inserted into the pCI-neo mammalian expression vector (Promega Corp., Madison, Wis.) (Fig. 1). Another CC chemokine, human MCP-1, inserted into the pCDNA3 expression vector (Invitrogen, Inc., San Diego, Calif.) was kindly provided by David Weiner (University of Pennsylvania, Philadelphia, Pa.). The plasmid DNAs were purified by polyethylene glycol precipitation by the method of Sambrook et al. (32) with some modifications. Cellular proteins were precipitated with 1 volume of 7.5 M ammonium acetate followed by isopropanol precipitation of the supernatant. After polyethylene glycol precipitation, the plasmids were phenol-chloroform extracted three times and precipitated with pure ethanol. The quality of DNA was checked by electrophoresis on 1% agarose gels. The expression of each plasmid DNA was identified by reverse transcriptase PCR and a chemotaxis assay (38) after in vitro transfection into human fibroblast cells (Fig. 1). The amount of endotoxin was determined by the Limulus amebocyte lysate test. The effect of endotoxin in vivo was addressed in parallel by administration of control vector.
FIG. 1.
Diagram of the mammalian expression vector for chemokines and identification of chemokine expression. The expression of chemokines was identified by a chemotaxis assay and mRNA reverse transcriptase (RT) PCR following in vitro transfection into human fibroblast cells. SV40, simian virus 40; CMV, cytomegalovirus; I.E, immediate-early.
Immunization and sample collection.
Groups of 3- to 4-week old female mice were coimmunized intranasally (i.n.) with 100 μg of gB DNA plus 200 μg of chemokine DNA formulated in phosphate-buffered saline (PBS) (pH 7.2). Coadministration of various gene expression cassettes involved mixing the chosen plasmid before administration. Immunization was performed three times at 5-day intervals. The control mice were immunized i.n. with 200 μg of pCI-neo control vector. In some experiments mice were immunized intramuscularly with 106 PFU of HSV-1 KOS or 100 μg of gB DNA. Serum samples from the mice were collected by retro-orbital bleeding. Vaginal lavage fluid was obtained by introducing 100 μl of PBS (pH 7.2) into the vaginal canals and then recovering it with a micropipette. Fecal samples were weighed and suspended at 100 mg/ml in PBS containing 0.1% sodium azide. Each sample was stored at −80°C until used.
ELISA for gB-specific Ab.
A standard enzyme-linked immunosorbent assay (ELISA) as described previously (23) was used to determine gB-specific antibodies (Ab) in the samples. Briefly, ELISA plates were coated with gB protein (kindly provided by Chiron, Emeryville, Calif.) and goat anti-mouse immunoglobulin G IgG (Southern Biotechnology Associate Inc., Birmingham, Ala.) or rabbit anti-mouse IgA (Zymed, San Francisco, Calif.) and then incubated overnight at 4°C. The plates were washed with PBS-Tween (PBST) three times and blocked with 3% dehydrated milk. Samples were serially diluted twofold, incubated for 2 h at 37°C, and then incubated with goat anti-mouse IgG-conjugated horseradish peroxidase for 1 h. To measure the IgA level in vaginal lavage fluid and fecal samples, biotinylated goat anti-mouse IgA was added for 2 h at 37°C, followed by peroxidase-conjugated streptavidin (Jackson ImmunoResearch Laboratories, West Grove, Pa.). The color was developed by adding the substrate solution (11 mg of 2,2-azinobis-3-ethylbenzothiazoline-6-sulfonic acid in 25 ml of 0.1 M citric acid–25 ml of 0.1 M sodium phosphate–10 μl of hydrogen peroxide). Ab concentrations were calculated with an automated ELISA reader (Spectra MAX340; Molecular Devices, Sunnyvale, Calif.).
HSV-specific Th-cell proliferation.
HSV-specific Th-cell proliferation was evaluated after sacrificing coimmunized mice 12 days after the last immunization. The spleens and cervical draining lymph nodes (DLN) of coimmunized mice were excised. Immune splenocytes were then enriched for T cells on a nylon wool column. Enriched splenic T cells and unfractionated cervical DLN lymphocytes were individually restimulated in vitro with an irradiated syngeneic dendritic cell (DC) population (stimulators) pulsed with UV-inactivated HSV-1 KOS (multiplicity of infection [MOI], 1.5 before UV inactivation) for 5 days as described previously (5). [3H]thymidine (1 μCi/well) was added to each well 18 h before harvest. Harvested cells were measured for radioactivity using a beta scintillation counter (Inotech, Lancing, Mich.). Concanavalin A at 5 μg/ml was used as a polyclonal-stimulator positive control for the lymphoproliferation assay.
Cytokine ELISA.
Cytokine levels in culture supernatants from immune T cells (responder cells) that had been restimulated in vitro with irradiated syngeneic enriched DC pulsed with UV-inactivated HSV (MOI, 5.0 before UV inactivation) or irradiated naive enriched DC were determined by ELISA. Similar number of cells were stimulated with 1 μg of concanavalin A as a polyclonal positive stimulator for 48 h. ELISA plates were coated with interleukin-2 (IL-2), IL-4 and gamma interferon (IFN-γ) anti-mouse Ab (Pharmingen, San Diego, Calif.) and incubated overnight at 4°C. The plates were washed three times with PBST and blocked with 3% nonfat dry milk for 2 h at room temperature. Culture supernatant and standards were added to the plates in duplicate, and the plates were incubated overnight at 4°C. Biotinylated IL-2, IL-4, and IFN-γ Ab were then added, and the plates were incubated for 2 h at 37°C. The plates were washed and incubated with peroxidase-conjugated streptavidin for 1 h, and then the color was developed. The cytokine concentration was calculated with an automated ELISA reader.
Quantification of cytokine-producing cells (ELISPOT).
The enzyme-linked immunospot (ELISPOT) assay was performed as described previously (14). Briefly, ELISPOT plates (Millipore, Molsheim, France) were previously coated with IFN-γ anti-mouse Ab. The enriched immune T cells (responder cells) were mixed with an enriched DC population pulsed with UV-inactivated HSV (MOI, 5.0 before UV inactivation). The responder cells and stimulator DC (naive or pulsed) were mixed at responder-to-stimulator ratios of 10:1, 5:1, 2.5:1, and 1.25:1 for 96 h at 37°C. The ELISPOT plates were washed three times with PBS and three times with PBST, and then biotinylated IFN-γ Ab was added to the plates for 1 h at 37°C. The spot was developed using nitroblue tetrazolium (Sigma, St. Louis, Mo.) and 5-bromo-4-chloro-3-indolylphosphate (Sigma) as a substrate following incubation with alkaline phosphatase-conjugated streptavidin (Jackson ImmunoResearch) for 1 h and counted 24 h later under a stereomicroscope.
FACS analysis.
The following monoclonal Abs obtained from Pharmingen were used for fluorescence-activated cell sorter analysis: phycoerythrin (PE)–anti-CD4, PE–anti-CD11c, fluorescein isothiocyanate (FITC)–anti-B7-1 (anti-CD80), FITC–anti-B7-2 (anti-CD86), anti-mouse α4β7, biotinylated anti-rat IgG2a, and streptavidin-FITC. For staining, cells were resuspended at a concentration of 106 to 107 cells/ml in PBS containing 1% bovine serum albumin and 0.05% NaN3 and incubated at 4°C for 30 min with properly diluted monoclonal Ab. For the detection of integrin α4β7, biotinylated anti-rat IgG2a and streptavidin-FITC were used as secondary reagents for amplification. After being stained, the cells were washed twice at 4°C and 1,200 rpm for 5 min. Following refixation, the cells were resuspended in PBS and analyzed using a FACScan analyzer (Becton Dickinson, Mountain View, Calif.).
Vaginal challenge.
The mice were previously treated with progesterone to synchronize their estrous cycles, as described earlier (30). Briefly, BALB/c mice were injected subcutaneously with Depo-Provera (DP) (Upjohn Co., Kalamazoo, Mich.) at 2 mg per mouse. Five days following the injection of DP, the mice were challenged intravaginally with 104 PFU (5 50% lethal doses [5 LD50]) of HSV-1 McKrae. The mice were examined daily for vaginal inflammation, neurological illness, and death, as described previously (14). They were scored 1 to 5 depending on the clinical severity of disease (0, no change; 1, mild inflammation; 2, moderate swelling; 3, severe inflammation; 4, paralysis; 5, death).
Determination of vaginal IFN-γ secretion.
Vaginal lavage fluid for IFN-γ secretion was collected daily by introducing 100 μl of PBS (pH 7.2) into the vaginal canals and then recovering it with a micropipette following infection of synchronized mice with HSV-1 McKrae. The vaginal mucus was subsequently removed from the fluid by centrifugation at 10,000 rpm for 1 min. IFN-γ concentrations in vaginal lavages were determined by ELISA using IFN-γ anti-mouse Ab and biotinylated IFN-γ Ab. Each concentration was adjusted for the vaginal protein content as determined using a protein assay reagent (Bio-Rad Laboratories, Hercules, Calif.).
Virus titer determination.
Vaginal washings were collected at different time points after intravaginal challenge, by micropipetting 100 μl of PBS into the vaginal cavity and then recovering it. The samples were stored at −80°C until used. Individual subsamples (50 μl from each sample) were further diluted, and viral titers were determined by a plaque assay performed on Vero cells as described elsewhere (36).
Preparation of vaginal T lymphocytes and iliac LN cells.
Vaginal T lymphocytes were prepared as previously described (9) with some modifications. Briefly, the vaginas were excised, cut longitudinally, and minced with a sterile scalpel in Hanks buffer without calcium and magnesium (HBSS) (Life Technologies, Rockville, Md.). After four washes with HBSS containing 1 mM EDTA, the minced tissues were digested in RPMI medium containing 1 mg of collagenase type VIII (Sigma) per ml and 1 mg of Dispase II (Boehringer Mannheim, Indianapolis, Ind.) per ml. Digestion was performed with stirring (1 h at 37°C). The cells were filtered through a sterile gauze mesh and washed with RPMI medium. Additional tissue debris was excluded by low-speed centrifugation (200 × g for 10 min). The cells were collected by an additional centrifugation (400 × g for 10 min), resuspended in RPMI medium, and enriched on a nylon-wool column. Vaginal cells for NK cell-mediated lysis were used before application to the nylon-wool column. Approximately 2 × 106 to 3 × 106 cells were collected from seven mice. The vaginal T lymphocytes passed through the nylon-wool column usually showed 40 to 60% CD4+ T cells by flow-cytometric analysis. Iliac LN cells were isolated from excised iliac LN, and then contaminating erythrocytes were lysed by hypotonic shock with a 0.83% ammonium chloride solution.
51Cr release assay of NK cells and CTL.
NK cell- and cytotoxic T lymphocyte (CTL)-mediated lysis in the iliac LN and vaginal tract was determined in 5-h 51Cr release assays with labeled target cells (YAC-1 for NK cells and EL-4 [H-2b] for CTL) as previously described (16). Spontaneous release of 51Cr was determined by incubating the target cells with medium alone, and maximum release was determined by adding Triton X-100 to a final concentration of 5%. The percent specific lysis was calculated as follows: 100 × ([experimental release − spontaneous release]/[maximum release − spontaneous release]). Each experiment was performed twice using triplicate samples.
Statistical analysis.
Significant differences between groups were determined using Student's t test.
RESULTS
Mucosal genetic cotransfer of chemokines modulates acquired systemic and mucosal immunity.
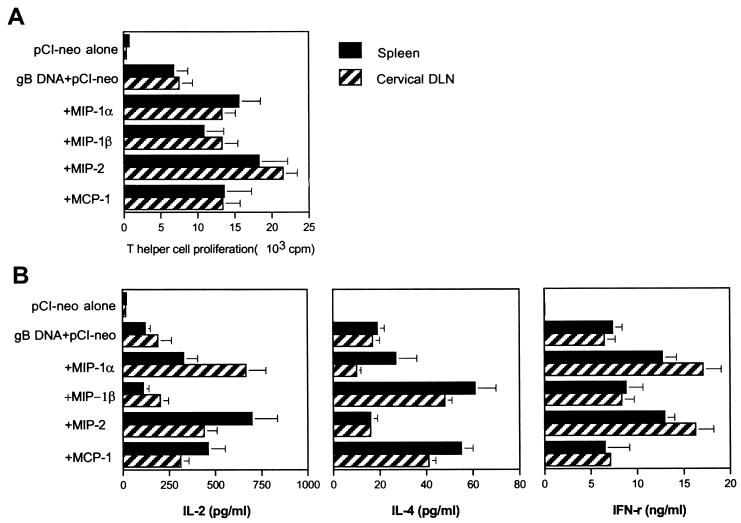
To investigate the immunomodulatory function of chemokines following mucosal genetic immunization, mice were coimmunized i.n. on three occasions with various chemokine-encoding plasmid DNA plus plasmid DNA encoding gB of HSV. Subsequently, serum and distal mucosal Ab responses were analyzed as described in Table 1. Mucosal genetic cotransfer of CXC or CC chemokines influenced gB-specific serum IgG and mucosal IgA response levels. The CC chemokines MIP-1α and MCP-1 strongly augmented serum gB-specific IgG Ab and distal mucosal gB-specific IgA Ab levels, but MIP-1β and the CXC chemokine MIP-2 provided only modest effects. The chemokines MIP-1α and MIP-2 also affected the ratio of Ig isotypes produced. Both pushed the response to the Th1-type pattern (Table 1). Interestingly, MIP-2 coexpression also induced the shift to the Th1 type but had no effect on the overall level of IgG Ab induced. In contrast, the CC chemokines MIP-1β and MCP-1 both caused an isotype response pattern of the Th2 type (Table 1).
TABLE 1.
Serum and distal mucosal antibody responses following mucosal genetic cotransfer of gB DNA plus chemokinesa
| Immunization | Serum gB-specific antibody response
|
Mucosal gB-specific antibody response
|
||||
|---|---|---|---|---|---|---|
| IgG (ng/ml) | IgG2a/IgG1 ratio | Vaginal IgA
|
Fecal IgA
|
|||
| gB specific (pg/ml) | % gB IgA | gB specific (ng/ml) | % gB IgA | |||
| pCI-neo alone | 10 ± 3 | 18 ± 17 | 2.1 ± 0.6 | |||
| gB DNA + pCI-neo | 181 ± 67 | 5.3 | 160 ± 86 | 0.01 | 7.5 ± 4.4 | 0.04 |
| + MIP-1α | 436 ± 75b | 14.6 | 582 ± 152b | 0.04 | 11.2 ± 3.7 | 0.08 |
| + MIP-1β | 142 ± 92 | 4.9 | 141 ± 74 | 0.01 | 7.5 ± 3.7 | 0.04 |
| + MIP-2 | 321 ± 158 | 18.5 | 100 ± 75 | 0.01 | 6.0 ± 3.2 | 0.05 |
| + MCP-1 | 538 ± 124b | 2.6 | 457 ± 88b | 0.03 | 8.0 ± 2.2 | 0.06 |
| HSV i.n. | 20,982 ± 2,755b | 117.0 | 5,666 ± 752b | 0.56 | 23.0 ± 3.8b | 0.16 |
BALB/c mice (seven mice per group) were coimmunized i.n. with 100 μg of gB DNA plus 200 μg of chemokine DNA. The immunization was repeated three times at 5-day intervals. Ten days following the last immunization, serum samples, vaginal lavage fluid, and fecal samples were collected and analyzed individually. The levels of gB-specific antibody were measured by ELISA. Vaginal IgA responses were measured from pooled samples, and fecal samples were resuspended at 100 mg/ml in PBS–0.1% sodium azide. Individual samples were tested for gB-specific as well as total IgA responses. % gB IgA ([gB-specific IgA/total IgA] × 100) was determined from fecal and vaginal samples of mice immunized with HSV and gB DNA.
Significantly different from values obtained for mice coimmunized i.n. with gB DNA plus control vector pCI-neo (P < 0.05).
To analyze the influence of mucosal genetic cotransfer of chemokines on cell-mediated immunity, the effect of chemokine coexpression on Th-cell proliferative responses and the production of Th1- or Th2-type cytokines in splenic and cervical DLN cells was examined following in vitro stimulation with UV-inactivated HSV. As shown in Fig. 2A, coexpression of MIP-1α (P < 0.001), MIP-2 (P < 0.001), and MCP-1 (P = 0.003), but not MIP-1β (P = 0.120 for spleen and P = 0.039 for DLN) significantly increased the HSV-specific proliferative response, with MIP-2 having the greatest effect. With regard to cytokine production by stimulated splenic and DLN cells (Fig. 2B), the chemokines MIP-2 and MIP-1α both induced increased IFN-γ responses beyond those in control gB immunized animals (P < 0.05) and poor IL-4 responses (Th1-type pattern). In contrast, MIP-1β and MCP-1 induced strong IL-4 responses (P < 0.05) but did not increase the levels of IFN-γ produced. The IL-2 response pattern was more complicated but was most highly elevated in the MIP-1α and MIP-2 recipients (those which also induced elevated IFN-γ responses). Thus, both the results of the Ig isotype profile and the cytokine responses indicate that mucosal genetic cotransfer of MIP-1α and MIP-2 drives T cells to predominantly a Th1-type pattern. Conversely, MIP-1β and MCP-1 coexpression resulted in a dominant Th2-type response.
FIG. 2.
Ag-specific Th-cell proliferation of spleen and cervical DLN in mice following mucosal genetic cotransfer of chemokines and gB DNA. Mice coimmunized i.n. with gB DNA plus chemokine DNA were sacrificed 12 days following the last immunization. Enriched splenic T cells and cervical DLN lymphocytes were used as responder cells for HSV-specific Th-cell proliferation (A) and Th1- or Th2-type cytokine production (B). The responder cells were mixed with irradiated syngeneic enriched DC that had been stimulated with UV-inactivated HSV-1 KOS and then incubated for 5 days. The test was done in quadruplicate wells. Each bar represents the mean cpm or concentration and standard deviation from three independent experiments.
The Th1-type CD4+ T-cell pattern provides more effective protection against HSV vaginal infection.
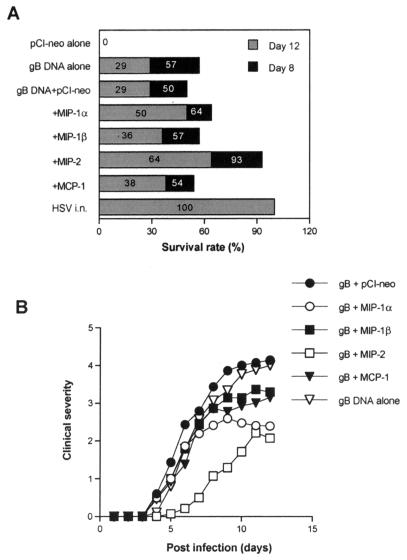
To investigate whether Th1- or Th2-type immunity enhanced by mucosal genetic cotransfer of chemokines could influence the level of protective immunity to distal mucosal challenge with lethal HSV, groups of progesterone-synchronized mice from the various test groups were challenged intravaginally with HSV-1 McKrae. As shown in Fig. 3A, immunization with gB DNA alone protected 50 and 29% of animals on days 8 and 12, respectively. Mucosal genetic cotransfer of MIP-1α and MIP-2 (type 1 pattern inducers) increased the level of protection (64 and 93% on day 8 and 50 and 64% on day 12 for MIP-1α and MIP-2, respectively). However, coexpression of MIP-1β and MCP-1 (type 2 pattern inducers) had no significant effect on levels of protection. Similarly, the average day of death in groups of mice coimmunized with gB DNA plus MIP-1α or MIP-2 was later than in mice coimmunized with gB DNA plus control vector pCI-neo (P = 0.05 for MIP-1α and P = 0.02 for MIP-2), as is evident in Table 2.
FIG. 3.
Induction of protective immunity against vaginal infection with HSV following mucosal genetic cotransfer of chemokines and gB DNA. Each group of mice (n = 14) coimmunized i.n. with gB DNA plus chemokine DNA was challenged intravaginally with HSV-1 McKrae 2 weeks the last immunization. Mice were previously injected with DP to synchronize their estrus cycles. Mice immunized i.n. with HSV-1 KOS (106 PFU) were included as positive controls. (A) The surviving mice were counted 8 and 12 days following vaginal challenge. (B) Clinical severity was graded as follows: 0, no inflammation; 1, mild inflammation; 2, moderate swelling and redness; 3, severe inflammation; 4, paralysis; 5, death.
TABLE 2.
Prolonged survival of challenged mice following mucosal genetic cotransfer of gB DNA plus chemokinesa
| Immunization | Mean time of death ± SD (days) |
|---|---|
| pCI-neo alone | 5.3 ± 1.0 |
| gB+PCI-neo | 5.9 ± 1.6 |
| gB+MIP-1α | 8.9 ± 4.3b |
| gB+MIP-1β | 6.7 ± 2.0c |
| gB+MIP-2 | 12.1 ± 6.4d |
| gB+MCP-1 | 6.6 ± 1.3e |
| HSV i.n. | NDf |
Each mouse (n = 14) coimmunized i.n. with gB DNA plus chemokine DNA was challenged with HSV-1 McKrae 2 weeks following the last immunization.
Significantly prolonged survival compared with gB DNA plus control vector pCI-neo (P = 0.05).
Not significantly prolonged survival compared with gB DNA plus control vector pCI-neo (P = 0.19).
Significantly prolonged survival compared with gB DNA plus control vector pCI-neo (P = 0.02).
Not significantly prolonged survival compared with gB DNA plus control vector pCI-neo (P = 0.21).
ND, not done.
Animals challenged with virus were also assessed for clinical signs of inflammation in the vaginal track. As shown in Fig. 3B, the inflammatory reactions (intensity and duration) were significantly reduced in recipients of the Th1-type-inducing chemokines. Animals with the Th2-type pattern had inflammatory reactions approximately the same as those in control gB DNA immunized mice. Thus, this result supports the idea that the Th1-type immunity provides more effective protection against HSV mucosal infection than the Th2-type pattern does.
MIP-1α but not MIP-2 may enhance immunity by exerting effects on APC function.
Since chemokines are known to recruit and affect APC activity (6, 35), we considered if the Th1-type immune enhancing effect of MIP-1α and MIP-2 might be the consequence of effects on APC function. To measure such effects, mice were given i.n. doses of one of the two Th1-type-inducing chemokines or control plasmids on two occasions and APCs were prepared from spleens. These APCs were used to present either UV-inactivated HSV Ag to gB- or HSV-primed T cells or OVA323–339 peptide to T cells isolated from DO11.10 mice transgenic for the T-cell receptor (TCR) that recognizes the OVA323–339 peptide. Whereas the former UV-inactivated HSV Ag system would require Ag processing as well as presentation, the latter OVA323–339 peptide system would not require processing. Responses were measured by Ag-specific proliferation as well as by Ag-specific cytokine production.
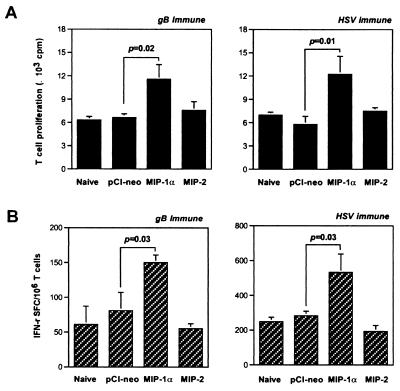
As shown in Fig. 4A, APC taken from mice given the CC chemokine MIP-1α, but not the CXC chemokine MIP-2, significantly enhanced proliferation of gB- or HSV-primed T cells following stimulation with UV-inactivated HSV Ag. In addition, the number of HSV-specific IFN-γ-producing T cells measured by ELISPOT was increased (Fig. 4B). Additionally, proliferation of OVA323–339 peptide-specific T cells and production of IL-2 and IFN-γ were enhanced when APC from MIP-1α-treated mice were used to present peptide (Table 3). The latter result indicates that the effect of MIP-1α on APC probably involved presentation rather than processing. In a parallel experiment, MIP-2-treated APC failed to enhance the proliferation of OVA323–339 peptide-specific T cells or the production levels of the cytokines IL-2 and IFN-γ (Table 3).
FIG. 4.
APC from mice given MIP-1α DNA but not MIP-2 DNA enhance Ag-specific T-cell proliferation and IFN-γ spot-forming cells (SFC). Naive BALB/c mice were given 200 μg of MIP-1α or MIP-2 DNA i.n. twice at a 5-day interval. APC were isolated from the spleens 7 days later and irradiated, pulsed with UV-inactivated HSV-1 KOS, and used as stimulators for enriched immune T cells obtained from the spleens of mice immunized with gB DNA or HSV-1 KOS. The test was performed in quadruplicate wells for T-cell proliferation (A) and in duplicate wells for IFN-γ SFC measurements (B). The graph shows the means of cpm or SFC and standard deviation from three independent experiments.
TABLE 3.
APC from mice given MIP-1α but not MIP-2 augment DO11.10 TCR T-cell proliferation and cytokine production following stimulation of OVA323–339
| Treatment | Lymphoproliferation (cpm)a of cells after stimulation with OVA323–339 at (μg/ml):
|
Cytokine production (pg/ml)b
|
|||
|---|---|---|---|---|---|
| 0.0 | 5.0 | 10.0 | IL-2 | IFN-γ | |
| pCI-neo | 379 ± 257 | 16,345 ± 1,115 | 21,401 ± 4,317 | 4,307 ± 677 | 243 ± 93 |
| MIP-1α | 572 ± 301 | 27,454 ± 2,469c | 39,362 ± 5,276c | 8,603 ± 532d | 615 ± 218e |
| MIP-2 | 457 ± 291 | 21,936 ± 3,827 | 25,006 ± 4,192 | 4,793 ± 1,238 | 353 ± 120 |
DO11.10 TCR T cells were enriched from the spleens of DO11.10-Tg mice using a nylon wool column and used as responder cells. APC taken from mice given MIP-1α or MIP-2 DNA were irradiated and pulsed with OVA323–339 in serum-free medium for 60 min. The OVA323–339-treated APCs were extensively washed and used as stimulator cells. OVA323–339-pulsed APCs were mixed with enriched DO11.10 TCR T cells and incubated for 3 days. [3H]thymidine was added to each well 18 h before harvest. The results show mean cpm ± standard deviation for three independent experiments.
Following in vitro stimulation of DO11.10 TCR T cells with OVA323–339 (5.0 μg/ml)-pulsed APCs, the cytokine concentration in the culture supernatant was determined by ELISA. The data represent mean ± standard deviation for three independent experiments.
Statistically significant between control vector pCI-neo and MIP-1α (P = 0.004).
Statistically significant between control vector pCI-neo and MIP-1α (P < 0.001).
Statistically significant between control vector pCI-neo and MIP-1α (P = 0.01).
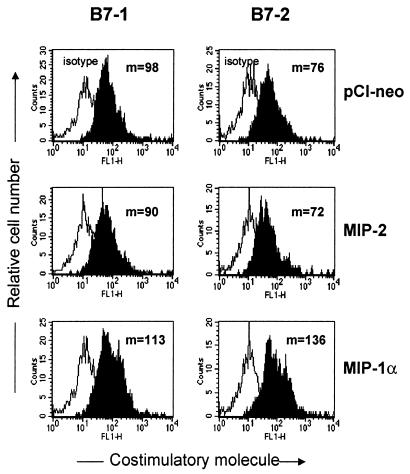
To investigate the hypothesis that the enhancement of peptide presentation provided by MIP-1α to APCs is related primarily to the induction of costimulatory molecules, the expression level of costimulatory molecules B7-1 and B7-2 on CD11c+ DC of APCs following genetic transfer of MIP-1α or MIP-2 was tested. As shown in Fig. 5, MIP-1α exhibited a significant increase in the expression of both costimulatory molecules, particularly in B7-2. Conversely, MIP-2 failed to influence the expression of costimulatory molecules. The expression of other molecules, such as CD40 and major histocompatibility complex (MHC) class II, was also addressed, but MIP-1α and MIP-2 did not alter the expression of those molecules (data not shown). Similar findings were obtained in the CD11b+ population of APCs (data not shown). Thus, these results indicate that mucosal genetic cotransfer of the CC chemokine MIP-1α enhances APC function by affecting costimulatory molecules (such as B7-1 and B7-2) involved in Ag presentation.
FIG. 5.
Enhanced expression of costimulatory molecules B7-1 and B7-2 in APC from the spleens of mice given MIP-1α but not MIP-2 DNA. Naive BALB/c mice given MIP-1α or MIP-2 DNA i.n. were sacrificed 7 days later. APC were then isolated from the spleens, and fluorescence-activated cell sorter analysis was performed for B7-1 and B7-2 molecule expression. The figure shows costimulatory molecule expression in the CD11c+ cell population. Results representative of those in four mice are given. Values (m) in the figure denote mean fluorescence.
How does MIP-2 provide more effective protection against HSV mucosal infection?
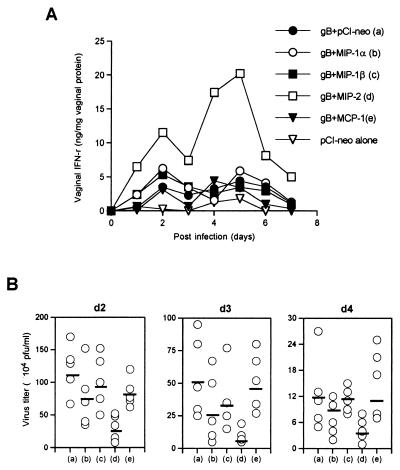
To investigate how MIP-2 provides the effective protective immunity against distal mucosal infection with lethal HSV, the effects on vaginal IFN-γ secretion and viral clearance were measured in coimmunized mice at various times after challenge with HSV. The results are shown in Fig. 6. The recipients of MIP-2 plus gB DNA had high levels of IFN-γ in vaginal washes, with two peaks of activity observed (the first peak on day 2 postchallenge and the second peak on day 5 postchallenge). IFN-γ was no longer detectable in vaginal washes after day 7 postchallenge (Fig. 6A). In addition, virus titers were measured on days 2, 3, and 4 postchallenge to determine the relationship between vaginal IFN-γ secretion and viral clearance. MIP-2 coexpression resulted in early viral clearance from the vaginal tract (Fig. 6B), showing especially significant clearance on days 2 (P = 0.05) and 3 (P = 0.01) postchallenge in comparison to the group treated with gB DNA plus control vector pCI-neo. The increase of IFN-γ secretion following MIP-2 coexpression probably caused the early clearance of HSV from vaginal tract. However, other CC chemokines failed to result in early viral clearance. These results probably mean that IFN-γ produced locally in the vaginal tract enhances virus clearance and may ultimately determine the outcome of HSV vaginal infection.
FIG. 6.
Vaginal IFN-γ secretion and viral clearance following vaginal infection with HSV in mice coimmunized with gB DNA plus chemokine DNA. (A) Vaginal IFN-γ secretion following vaginal infection with HSV. Each group of mice (n = 5) coimmunized i.n. with gB DNA plus chemokine DNA was intravaginally challenged, and vaginal lavage fluid was collected every day. Vaginal IFN-γ concentrations were determined by ELISA, and each concentration was adjusted for the vaginal protein content. The results are expressed as a mean for five mice. (B) Influence of chemokine expression on viral clearance. Coimmunized mice (n = 5) were intravaginally challenged 2 weeks after immunization. Vaginal lavage fluid was then collected on days 2, 3, and 4 postchallenge, and the virus titer was determined by a plaque assay. The open circles represent individual virus titers of mice, and the black lines represent the average virus titer in each group.
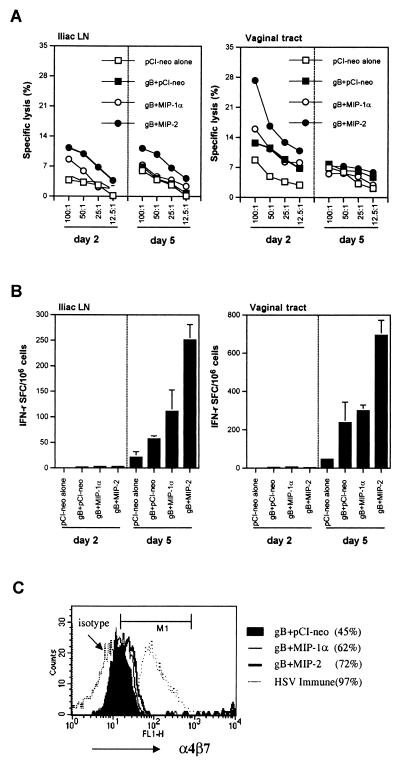
Since the peak level of IFN-γ in vaginal secretions was evident around day 2 postchallenge, the source of initial IFN-γ production was suspected to be NK cells rather than Th1-type CD4+ cells, which are known to cause a later peak (24). To further define the role of NK cells as the likely source of initial IFN-γ secretion in vaginal washes of MIP-2-cotransferred mice, iliac LN cells and vaginal tract cells were isolated on days 2 and 5 postinfection. The cells were then tested for NK-cell-mediated lysis of target cells and Th1-type CD4+ IFN-γ-producing T cells. As shown in Fig. 7A, NK-cell activity was elevated in MIP-2 recipients, particularly on day 2 postchallenge, but had declined to levels comparable to those in controls by day 5 postchallenge. In addition, whereas Th1-type CD4+ IFN-γ producing T-cells were not detectable on day 2 postchallenge, by day 5 postchallenge the number of such cells in MIP-2-treated mice was markedly elevated over the number in mice given other treatments (Fig. 7B). These results indicate that cotransferred MIP-2 initially increased NK-cell activity and subsequently Th1-type CD4+ T-cell activity, both of which were likely sources of the soluble IFN-γ recovered in vaginal washes.
FIG. 7.
NK-cell-mediated lysis of YAC-1 cells and IFN-γ-producing CD4+ T cells in iliac LN and vaginal tracts of mucosally coimmunized BALB/c mice with gB DNA plus MIP-1α or MIP-2 DNA following vaginal infection with HSV. (A) NK-cell-mediated lysis of YAC-1 cells in iliac LN and vaginal tracts. Cells of iliac LN and vaginal tracts were isolated from coimmunized mice on days 2 and 5 postinfection. NK-cell-mediated lysis of target cells was determined in a 5-h 51Cr release assay against labeled YAC-1 cells. (B) Number of IFN-γ-producing CD4+ T cells in iliac LN and vaginal tracts. Iliac LN cells and vaginal tract T lymphocytes were prepared on day 2 and 5 postinfection. The number of IFN-γ-producing CD4+ T cells in the vaginal tracts of the mice was then determined by an ELISPOT assay after in vitro restimulation with enriched DC pulsed with UV-inactivated HSV-1 KOS, while the number of IFN-γ-producing CD4+ T cells in iliac LN cells was determined without in vitro restimulation. (C) Profile of expression of integrin α4β7 on CD4+ T cells of the vaginal tract on day 5 following HSV vaginal infection.
A possible reason for the increased number of CD4+ IFN-γ-producing T cells in the MIP-2-treated mice could be the up-regulation of adhesion molecules essential for migration to various mucosal surfaces such as α4β7 (2). The data in Fig. 7C support this idea since mucosal genetic cotransfer of MIP-2 resulted in enhanced α4β7 integrin expression on CD4+ T cells (72%) comparable to levels in other groups (61% for MIP-1α cotreatment and 42% for control vector cotreatment).
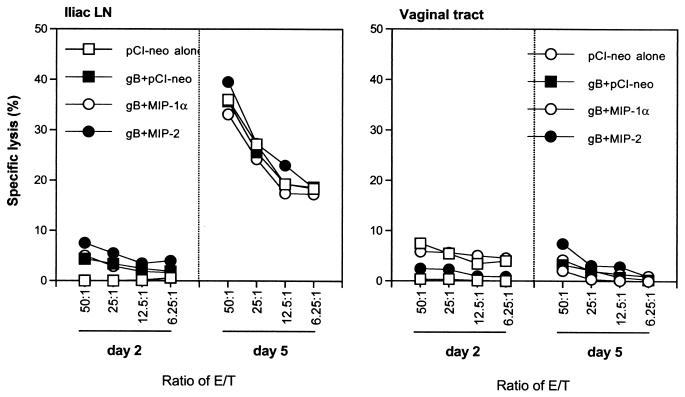
Although Th1-type CD4+ T cells were primarily responsible for the second peak of the IFN-γ levels involved in the early clearance of virus, the role of CD8+ T cells was not examined following HSV vaginal infection. To determine the protective role of CD8+ T cells in the vaginal tracts of coimmunized mice, the CTL activity of iliac LN cells and vaginal tract CD8+ T cells was checked on days 2 and 5 postinfection. Vaginal tract CD8+ T cells were expanded in vitro with SSIEFARL peptide (gB498–505 peptide specific for MHC class I-restricted CD8+ T lymphocytes) before antigen-specific lysis was measured, whereas the CTL activity of iliac LN cells was determined without in vitro stimulation. The targets included 51Cr-labeled MHC-matched EL-4 pulsed with SSIEFARL peptide or not pulsed and MHC-mismatched EMT-6. To calculate the specific lysis of targets, the percent lysis of irrelevant targets was subtracted from the percent lysis of specific targets. In contrast to Th1-type CD4+ T cells, CTL activity did not show significant differences between treated groups (Fig. 8). This result supports the notion that CD8+ T cells do not contribute to the second wave of IFN-γ production on day 5 postchallenge.
FIG. 8.
CTL activity of CD8+ T cells in the iliac LN and vaginal tracts of C57BL/6 mice mucosally coimmunized with gB DNA plus MIP-1α or MIP-2 DNA following vaginal infection with HSV. Iliac LN cells and vaginal tract T lymphocytes were prepared on days 2 and 5 postinfection. The CTL activity of CD8+ T cells in the vaginal tracts was determined following in vitro peptide stimulation with SSIEFARL (gB498–505 peptide specific for MHC class I-restricted CD8+ T lymphocytes), while the CTL activity of CD8+ T cells in iliac LN was determined without in vitro restimulation.
DISCUSSION
This report shows that mucosal genetic cotransfer of plasmid DNA encoding chemokines along with plasmid DNA encoding Ag can enhance and change the nature of acquired systemic and distal mucosal immune responses. Accordingly, the CC chemokines MIP-1β and MCP-1 induced a Th2-type response as judged by the ratio of serum Ig isotypes and cytokine IL-4 production. In contrast, coexpression of the CC chemokine MIP-1α and the CXC chemokine MIP-2 established a Th1-type pattern, and such immunized mice were more resistant to subsequent vaginal challenge with HSV. The CC chemokine MIP-1α, which induced the Th1-type response, appeared to act via up-regulation of APC function and expression of the costimulatory molecules B7-1 and B7-2. The CXC chemokine MIP-2, however, enhanced the subsequent Th1-type CD4+ T-cell adaptive immunity by increasing IFN-γ secretion from NK cells following HSV vaginal infection. This is the first report that documents the value of chemokine DNA given mucosally as an approach to modulate the functional efficacy of systemic and distal mucosal immunity to infection.
The mechanisms by which chemokines can act as adjuvants to boost immune responses remain to be established and are probably multiple. For example, chemokines recruit and can affect the function of many cell types (35, 39). Perhaps most importantly, these include cellular components of innate defense, which in turn influence the nature of the subsequent adaptive immune responses (6, 10). In our study, the Th1-type enhancing effect of the CC chemokine MIP-1α would seem to result from recruitment and activation of innate cellular components involved in Ag presentation such as DC and blood monocytes. Accordingly, from in vitro studies, it is known that immature DC such as those found in local tissue sites and monocytes express the CCR1 and CCR5 receptors, to which MIP-1α binds (35). Conceivably, the binding of CCR1 or CCR5 by chemokines at local tissue sites causes them to migrate to lymphoid tissue and induce appropriate immune responses (35). In our studies, which analyzed the phenotype and function of APC from lymphoid tissue, we observed changes in the expression of costimulatory molecules as well as APC function for Th1-type response. Such effects were evident only for MIP-1α of the four chemokines studied, even though the CC chemokine MIP-1β (type 2 inducer) may also bind to CCR5 (35). Why MIP-1β failed to change APC function requires further investigation.
Interestingly, the effect of MIP-1α on APC function was evident in splenic APC, a location remote from the site of chemokine administration. It is unclear how such an effect is mediated. However, it is likely that the mucosal administration was followed by chemokine DNA access to the bloodstream and dissemination to remote sites that include the spleen. Such events were shown to occur previously with plasmid DNA encoding β-galactosidase or green fluorescence protein (4). Our results also showed that MIP-1α caused an enhanced distal mucosal IgA response. This was probably the consequence of modulatory effects of MIP-1α in the local mucosal DLN followed by migration of effector lymphocytes to the distal mucosal location. In support of this idea, MIP-1α caused an increased population of T lymphocytes expressing integrin α4β7, the mucosal homing receptor, to the vaginal tract (2). Additionally, in vitro studies have shown that the binding of chemokines to their receptor may cause a rapid and robust up-regulation of induction of integrins, including α4β7 (3, 17).
A second chemokine that enhanced the Th1-type CD4+ T-cell response and immunity to vaginal challenge with HSV was the CXC chemokine MIP-2. This chemokine, however, had no detectable modulatory effect on APC function. Instead, the mechanism by which it achieved modulation could have involved effects on NK-cell function. Accordingly, the NK-cell function of cells taken from the vaginal tracts and iliac LN following HSV vaginal infection was enhanced in MIP-2 recipients. Of particular interest, NK-cell activity in the vaginal tracts of mice given MIP-2 greatly increased transiently on day 2 postchallenge. Moreover, since such cells act as an important source of IFN-γ secretion (24), the early enhanced IFN-γ secretion in vaginal washings of MIP-2 recipients challenged with HSV-1 could have been derived from such cells, as described by others (24, 28). In addition, the early IFN-γ produced from NK cells could play an important role in shaping subsequent Th1-type CD4+ T-cell adaptive immunity, as is known to occur in vitro (25, 33). We are currently attempting to support these ideas by comparing the enhancing effects of MIP-2 in normal and NK-cell-depleted mice. An alternative mechanism by which MIP-2 modulates immunity could include effects on neutrophil function. Thus, CXCR2 (the receptor for MIP-2) is expressed on neutrophils as well as on NK cells (26). Moreover, since neutrophils are known to be involved in the control of HSV infection in both ocular and genital sites (21, 37), an enhanced neutrophil function could account for the more effective removal of HSV in MIP-2 recipients after vaginal challenge. The role of neutrophils as the mediators of antiviral immunity requires further investigation.
The issue of which immune defenses are involved in protection against disease following HSV vaginal challenge has not been fully resolved. The present observation and also those of another group favor the hypothesis of the Th1-type CD4+ T cell phenotype as the principal mediator (22, 24). Others, however, advocate that CD8+ T cells act as principal mediators for mucosal defense against HSV infection (27). The present studies, also supported by previous investigations (15), found that immunity correlated best with Th1-type CD4+ T-cell function. In fact, none of the chemokines that caused enhanced immunity had demonstrable effects on CD8+ T-cell function. Curiously, we also failed to demonstrate an apparent effective role for IgA in vaginal immunity. In support of this notion, stimulating the type 2 pattern of reactivity, including an IgA response, appeared not to provide the type of immunity that functions best against HSV mucosal infection whatever the challenge route. It is still curious, however, that the vaginal IgA response appears not to correlate positively with the outcome of HSV vaginal infection, since IgA is an important mediator of defense against several other mucosally infectious agents (7, 20). It appears, in fact, that barrier immunity, such as is mediated by IgA, is ineffective against HSV. Instead, infection control largely involves T-cell immunity, probably by causing an inflammatory response at tissue sites (8). Others have even shown that vaginal immunity to HSV infection proceeds normally in mice genetically unable to produce IgA (29).
In conclusion, we have shown that mucosal genetic cotransfer of chemokines provides a valuable means of changing the quality and effectiveness of mucosal immunity at distal sites. Two chemokines, MIP-1α and MIP-2, caused this to occur by acting by different mechanisms to enhance Th1-type CD4+ T-cell-mediated immunity. This finding supports the hypothesis that MIP-1α and MIP-2 might act synergistically following DNA vaccination. In addition, it remains to be seen if combinations of DNAs encoding other adjuvant activities such as IL-18 can lead to further enhanced levels of immunity. Thus, given the background of past failure with anti-HSV vaccines, novel approaches are needed. DNA vaccines, along with appropriate costimulators perhaps used in a prime-boost combination with other approaches, holds promise as a practical solution for an HSV vaccine.
ACKNOWLEDGMENT
This work was supported by Public Health Service grant AI46462 from the National Institutes of Health.
REFERENCES
- 1.Biron C A. Initial and innate responses to viral infections—pattern setting in immunity or disease. Curr Opin Microbiol. 1999;2:374–381. doi: 10.1016/s1369-5274(99)80066-6. [DOI] [PubMed] [Google Scholar]
- 2.Brandtzaeg P, Farstad I N, Haraldsen G. Regional specialization in the mucosal immune system: primed cells do not always home along the same track. Immunol Today. 1999;20:267–277. doi: 10.1016/s0167-5699(99)01468-1. [DOI] [PubMed] [Google Scholar]
- 3.Campbell J J, Qin S, Bacon K B, Mackay C R, Butcher E C. Biology of chemokine and classical chemoattractant receptors: differential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. J Cell Biol. 1996;134:255–266. doi: 10.1083/jcb.134.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chun S, Daheshia M, Lee S, Eo S K, Rouse B T. Distribution fate and mechanism of immune modulation following mucosal delivery of plasmid DNA encoding IL-10. J Immunol. 1999;163:2393–2402. [PubMed] [Google Scholar]
- 5.Chun S, Daheshia M, Kuklin N A, Rouse B T. Modulation of viral immunoinflammatory responses with cytokine DNA administered by different routes. J Virol. 1998;72:5545–5551. doi: 10.1128/jvi.72.7.5545-5551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark G J, Angel N, Kato M, Lopez J A, MacDonald K, Vuckovic S, Hart D N J. The role of dendritic cells in the innate immune system. Microbes Infect. 2000;2:257–272. doi: 10.1016/s1286-4579(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 7.Doherty P C, Allan W, Eichilberger M. Roles of antibodies and T cells subsets in viral immunity. Annu Rev Immunol. 1992;10:123–151. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- 8.Doymaz M Z, Rouse B T. Immunopathology of herpes simplex virus infection. Curr Top Microbiol Immunol. 1992;179:121–136. doi: 10.1007/978-3-642-77247-4_8. [DOI] [PubMed] [Google Scholar]
- 9.Dupuy C, Buzoni-Gatel D, Touze A, Bout D, Coursaget P. Nasal immunization of mice with human papillomavirus type 16 (HPV-16) virus-like particles or with the HPV-16 L1 gene elicits specific cytotoxic T lymphocytes in vaginal draining lymph nodes. J Virol. 1999;73:9063–9071. doi: 10.1128/jvi.73.11.9063-9071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fearon D T, Locksley R M. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–54. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 11.Gangappa S, Babu J S, Thomas J, Daheshia M, Rouse B T. Virus-induced immunoinflammatory lesions in the absence of viral antigen recognition. J Immunol. 1998;161:4289–4300. [PubMed] [Google Scholar]
- 12.Gu L, Tseng S, Horner R M, Tam C, Loda M, Rollins B J. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 13.Kim J J, Nottingham L K, Sin J I, Tsai A, Morrison L, Oh J, Dang K, Hu Y, Kazahaya K, Bennett M, Dentchev T, Wilson D M, Chalian A A, Boyer J D, Agadjanyan M G, Weiner D. CD8 positive T cells influence antigen-specific immune responses through the expression of chemokines. J Clin Investig. 1998;102:1112–1124. doi: 10.1172/JCI3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuklin N, Daheshia M, Karem K, Manickan E, Rouse B T. Induction of mucosal immunity against herpes simplex virus by plasmid DNA immunization. J Virol. 1997;71:3138–3145. doi: 10.1128/jvi.71.4.3138-3145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuklin N A, Daheshia M, Chun S, Rouse B T. Role of mucosal immunity in herpes simplex virus infection. J Immunol. 1998;160:5998–6003. [PubMed] [Google Scholar]
- 16.Kumaraguru U, Rouse R J D, Nair S K, Bruce B D, Rouse B T. The involvement of an ATP dependent chaperone in the cross-presentation after DNA immunization. J Immunol. 2000;165:750–759. doi: 10.4049/jimmunol.165.2.750. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd A R, Oppenheim J J, Kelvin D J, Taub D D. Chemokines regulate T cell adherence to recombinant adhesion molecules and extracellular matrix proteins. J Immunol. 1996;156:932–938. [PubMed] [Google Scholar]
- 18.Lu Y, Xin K-Q, Hamajima K, Tsuji T, Aoki I, Yang J, Sasaki S, Fukushima J, Yoshimura T, Toda S, Okada E, Okuda K. Macrophage inflammatory protein-1α (MIP-1α) expression plasmid enhances DNA vaccine-induced immune response against HIV-1. Clin Exp Immunol. 1999;115:335–341. doi: 10.1046/j.1365-2249.1999.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manickan E, Rouse R J D, Yu Z, Wire W, Rouse B T. Genetic immunization against herpes simplex virus. Protection is mediated by CD4 T lymphocytes. J Immunol. 1995;155:259–265. [PubMed] [Google Scholar]
- 20.Mazanec M B, Coudret C L, Fletcher D R. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J Virol. 1995;69:1339–1343. doi: 10.1128/jvi.69.2.1339-1343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milligan G N. Neutrophils aid in protection of the vaginal mucosae of immune mice against challenge with herpes simplex virus type 2. J Virol. 1999;73:6380–6386. doi: 10.1128/jvi.73.8.6380-6386.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milligan G N, Berstein D I. Analysis of herpes simplex virus-specific T cells in the murine female genital tract following genital infection with herpes simplex virus type 2. Virology. 1995;212:481–489. doi: 10.1006/viro.1995.1506. [DOI] [PubMed] [Google Scholar]
- 23.Milligan G N, Bernstein D I. Generation of humoral immune responses against herpes simplex virus type 2 in the murine female genital tract. Virology. 1995;206:234–241. doi: 10.1016/s0042-6822(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 24.Milligan G N, Bernstein D I. Interferon-γ enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997;229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- 25.Mohan K, Moulin P, Stevenson M M. Natural killer cell cytokine production, not cytotoxicity, contributes to resistance against blood-stage Plasmodium chabaudi AS infection. J Immunol. 1997;159:4990–4998. [PubMed] [Google Scholar]
- 26.Morohashi H, Miyawaki T, Nomura H, Kuno K, Murakami S, Matsushima K, Mukaida N. Expression of both types of human interleukin-8 receptors on mature neutrophils, monocytes, and natural killer cells. J Leukoc Biol. 1995;57:180–187. doi: 10.1002/jlb.57.1.180. [DOI] [PubMed] [Google Scholar]
- 27.Parr E L, Parr M B. Immunoglobulin G is the main protective antibody in mouse vaginal secretions after vaginal immunization with attenuated herpes simplex virus type 2. J Virol. 1997;71:8109–8115. doi: 10.1128/jvi.71.11.8109-8115.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parr M B, Parr E L. The role of gamma interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology. 1999;258:282–294. doi: 10.1006/viro.1999.9739. [DOI] [PubMed] [Google Scholar]
- 29.Parr M B, Harriman G R, Parr E L. Immunity to vaginal HSV-2 infection in immunoglobulin A knockout mice. Immunology. 1998;95:208–213. doi: 10.1046/j.1365-2567.1998.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parr M B, Kepple L, McDermott M R, Drew M D, Bozzola J J, Parr E L. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Investig. 1994;70:369–380. [PubMed] [Google Scholar]
- 31.Salazar-Mather T P, Hamilton T A, Biron C A. A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J Clin Investig. 2000;105:985–993. doi: 10.1172/JCI9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Satoskar A R, Stamm L M, Zhang X, Satoskar A A, Okano M, Terhorst C, David J R, Wang B. Mice lacking NK cells develop an efficient Th1 response and control cutaneous Leishimania major infection. J Immunol. 1999;162:6747–6754. [PubMed] [Google Scholar]
- 34.Sin J I, Kim J J, Boyer J D, Ciccarelli R B, Higgins T J, Weiner D B. In vivo modulation of vaccine-induced immune responses toward a Th1 phenotype increases potency and vaccine effectiveness in a herpes simplex virus type 2 mouse model. J Virol. 1999;73:501–509. doi: 10.1128/jvi.73.1.501-509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sozzani S, Allavena P, Vecchi A, Mantovani A. The role of chemokines in the regulation of dendritic cell trafficking. J Leukoc Biol. 1999;66:1–9. doi: 10.1002/jlb.66.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Spear P G, Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972;9:143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas J, Gangappa S, Kanangat S, Rouse B T. On the essential involvement of neutrophils in the immunopathologic disease. J Immunol. 1997;158:1383–1391. [PubMed] [Google Scholar]
- 38.Tsou C-L, Gladue R P, Carroll L A, Paradis T, Boyd J G, Nelson R T, Neote K, Charo I F. Identification of C-C chemokine receptor 1 (CCR1) as the monocyte hemofiltrate C-C chemokine (HCC)-1 receptor. J Exp Med. 1998;188:603–608. doi: 10.1084/jem.188.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward S G, Bacon K, Westwick J. Chemokines and T lymphocytes: more than an attraction. Immunity. 1998;9:1–11. doi: 10.1016/s1074-7613(00)80583-x. [DOI] [PubMed] [Google Scholar]
- 40.Xiang Z, Ertl H C J. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129–135. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]