Abstract
We examined the prevalence of cleavage site mutations, both within and outside the gag region, in 28 protease inhibitor (PI) cross-resistant patients treated with indinavir, ritonavir, and/or saquinavir compared to control patients treated with reverse transcriptase inhibitors. Three human immunodeficiency virus protease cleavage sites within gag (p2/NC, NC/p1, and NC/TFP) showed considerable in vivo evolution before and after therapy with indinavir, ritonavir, and/or saquinavir. Another gag cleavage site (p1/p6gag) showed a trend compared to matched controls. The other eight recognized cleavage sites showed relatively little difference between PI-resistant cases and controls. An A→V substitution at the P2 position of the NC/p1 and NC/TFP cleavage sites was the most common (29%) change selected by the PIs used in this study.
The human immunodeficiency virus (HIV) protease must recognize and cleave up to 12 sites, each with a different amino acid sequence, in the Gag and Gag-Pol precursor polypeptides (18) and in Nef (9, 10). Protease inhibitor (PI)-resistant or cross-resistant isolates (1, 5) with impaired replication or Gag and Gag-Pol processing (3, 17, 23) can partially compensate by acquiring amino acid substitutions at gag cleavage sites in HIV culture (2, 8) and in vivo (17, 24). There is surprisingly little data (17, 24) published on the frequency and nature of cleavage site mutations in the clinical HIV population, particularly for those mutations situated away from the protease gene. This case control study is an attempt to determine the prevalence of mutations at all 12 recognized protease cleavage sites within a population of highly PI cross-resistant HIV-infected patients (n = 28) compared to a matched control group who received antiretroviral therapy that did not include PIs.
MATERIALS AND METHODS
Selection and characteristics of study and control groups.
Clinical isolates (n = 28) with >4-fold increases in the 50% inhibitory concentration (IC50) for indinavir, ritonavir, saquinavir, and nelfinavir were identified by Virco Antivirogram (11). All patients with an available pre-PI therapy sample which could be amplified by PCR (n = 28) were included in this study. No patient had experience with PIs other than saquinavir, ritonavir, or indinavir at the time of this study or with non-nucleoside reverse transcriptase (RT) inhibitors. Each case was individually matched with a control patient treated with nucleoside analogue RT inhibitors (NRTIs), but not PIs or non-nucleosides. Matching was based upon (i) length of time on therapy, (ii) plasma viral load prior to PI or NRTI therapy, and (iii) plasma viral load of the post-therapy sample. For each control, a sample collected prior to any antiretroviral therapy was also retrieved.
Genotypic analysis of protease cleavage site mutations.
Plasma HIV RNA was amplified by nested reverse transcription-PCR and analyzed by automated sequence analysis using conditions described elsewhere (14) with primers chosen to amplify regions surrounding the protease cleavage sites (primer sequences available upon request). For the purpose of this analysis, a “mutation” is defined as any change in the HIV RNA nucleotide sequence between the pre- and post-therapy samples resulting in an inferred amino acid substitution.
Statistical analyses.
The frequency of patients developing at least one HIV mutation resulting in an amino acid change within the 10 codons surrounding the 12 protease cleavage sites was compared by using the Fisher's exact test adjusted for multiple comparisons (a significant association was found if P < 0.004). The association of the A→V substitution with protease mutations at positions 10, 36, 46, 48, 82, 84, and 90 was compared similarly, with an adjustment for multiple comparison. In this instance, patient 19 (with the valine present at baseline) (see Table 3) was included in the analysis.
TABLE 3.
Protease genotypes before and after PI therapy from the nine patients with virus harboring the A→V substitution within the NC/p1 cleavage site
| Patient | Genotype before therapy | PIc | Genotype after therapy |
|---|---|---|---|
| 5 | L63P | RIT/SAQ | L10I, M46M/L, I62V, L63P, I66L, G73S, V77I, I84V, I93L |
| 9 | L63P | SAQ→IND→RIT/SAQ | M36I, I54V, L63P, A71V, V82A |
| 12 | R41K, I62V, A71T, I93L | IND→RIT/SAQ→IND | L10I, M46M/L, I62V, L63P, I66L, G73S, V77I, I84V, I93L |
| 14 | None | IND | V32I, M46I, A71V, V82A |
| 15a | K14R, I15V, G17D, L24I, L63P, A71V, V82T | IND | L10I, K14R, I15V, G17D, K20T, L24I, K29T, M46I, I54V, L63P, A71V, V82T |
| 18 | None | IND | V32I, M46L, L63P, A71V, V82A |
| 19b | L24I, M46I, Q93E, I93L | RIT→RIT/SAQ | L10F, L24I, M36I, K45K/E, M46I, I54V, L63P, I84V, Q92K |
| 23 | L19I, I93L | SAQ→IND | L10I, L19I, G48V, I62V, I93L |
| 27 | L10I, A71T, V77I, I93L | SAQ→IND→RIT/SAQ | L10I, M46I, G48V, A71T, V77I, V82A, L90M, I93L |
Patient had a mixture of A/V in NC/p1 before PI.
Patient HIV harbored the V prior to PI therapy.
Sequence of PI experienced by patient during therapy. RIT, ritonavir; SAQ, saquinavir; IND, indinavir. Differences from HIV-1 HXB2 at amino acids 35 and 37 are not listed.
RESULTS
Patient and viral characteristics.
The PI-resistant and control groups were very similar for most parameters, except CD4 counts (Tables 1 and 2). The duration on PI or NRTI regimens ranged between 3 and 22 months and, within a matched pair, the length of time on this therapy differed by a maximum of 3 months (Table 2). By definition, all viruses had >4-fold increased IC50s for indinavir, ritonavir, saquinavir, and nelfinavir, with a very high median fold resistance (29-, 33-, 30-, and 41-fold, respectively). In fact, all but one PI sample showed >10-fold resistance to at least three of these four PIs.
TABLE 1.
Characteristics of the highly PI-resistant patients (PI group) versus the NRTI-treated patients (control group)
| Parameter | PI group (n = 28) median (IQR)a | Control group (n = 28) median (IQR)a |
|---|---|---|
| Pretherapy pVL (log) | 5.04 (4.42–5.56) | 4.86 (4.59–5.36) |
| Post-therapy pVL (log) | 4.84 (4.40–5.28) | 4.56 (4.27–4.95) |
| Change in pVL (log) | 0.28 (0.09–0.63) | 0.28 (0.09–0.63) |
| Time on therapy (mo) | 15 (12–17) | 16 (12–18) |
| Minimum CD4 countb | 140 (90–145) | 400 (190–480) |
| Maximum CD4 countb | 380 (245–630) | 410 (310–640) |
| Gender (% male) | 89 | 75 |
IQR, interquartile range.
Maximum and minimum CD4 counts observed over the whole treatment within the drug treatment program.
TABLE 2.
Protease inhibitors received, cleavage site mutations, and number of protease gene mutations appearing during PI therapy (PI) or NRTI therapy (control)
| Case | Time (mo) on therapy (PI/control) | PI receiveda | Cleavage site(s) with at least one amino acid substitution
|
No. of PR mutations
|
||
|---|---|---|---|---|---|---|
| PI | Control | “Primary” (PI/control) | Total (PI/control) | |||
| 1 | 22/22 | S, I, R/S, S | p2/NC, TFP/p6pol, p66/INT | None | 2/0 | 7/0 |
| 2 | 6/6 | R/S | p2/NC, p1/p6gag, NC/TFP, TFP/p6pol | NEF | 1/0 | 8/0 |
| 3 | 8/9 | S | p1/p6gag | None | 1/0 | 6/0 |
| 4 | 21/22 | I | p2/NC, p1/p6gag, p6pol/PR | TFP/p6pol | 1/0 | 10/0 |
| 5 | 12/12 | R/S | NC/p1, NC/TFP, TFP/p6pol, p6pol/PR, p66/INT, NEF | p6pol/PR | 0/0 | 8/0 |
| 6 | 16/17 | I | CA/p2 | p6pol/PR | 1/0 | 3/0 |
| 7 | 17/16 | S, I, R/S | None | None | 1/0 | 4/3 |
| 8 | 18/17 | S, I | MA/CA, TFP/p6pol | None | 3/0 | 8/1 |
| 9 | 17/16 | S, I, R/S | p2/NC, NC/p1, NC/TFP | None | 1/0 | 4/0 |
| 10 | 15/16 | I | None | None | 2/0 | 7/1 |
| 11 | 20/22 | I | p2/NC, TFP/p6pol | TFP/p6pol | 1/0 | 8/2 |
| 12 | 13/11 | I, R/S, I | NC/p1, NC/TFP, p66/INT | NEF | 2/0 | 10/1 |
| 13 | 16/18 | S, I, R/S | p2/NC | TFP/p6pol, NEF | 2/0 | 9/1 |
| 14 | 5/5 | I | p2/NC, NC/p1, NC/TFP, TFP/p6pol | NC/TFP, TFP/p6pol | 2/0 | 6/1 |
| 15 | 3/5 | I | p2/NC, NC/p1, NC/TFP, TFP/p6pol, p51/p66, NEF | None | 1/0 | 5/1 |
| 16 | 17/20 | S, I | MA/CA, NC/p1, NC/TFP, TFP/p6pol, NEF | p2/NC | 2/0 | 6/1 |
| 17 | 21/21 | S, R/S, I | p2/NC, p1/p6gag, p6pol/PR | NC/TFP, TFP/p6pol | 1/0 | 7/0 |
| 18 | 14/15 | I | p2/NC, NC/p1, NC/TFP, NEF | TFP/p6pol | 2/0 | 6/1 |
| 19 | 12/13 | R, R/S | p1/p6gag | NC/TFP, TFP/p6pol | 0/−1b | 10/2 |
| 20 | 14/15 | S, R, R/S | p2/NC | None | 2/0 | 5/0 |
| 21 | 16/18 | S, I, R/S | p2/NC, p1/p6gag | p2/NC, TFP/p6pol | 1/0 | 5/0 |
| 22 | 15/15 | S, I, R/S | p2/NC, NC/TFP, TFP/p6pol | p6pol/PR | 1/0 | 4/1 |
| 23 | 3/3 | S, I | p2/NC, NC/p1, NC/TFP, TFP/p6pol, p66/INT | p2/NC | 1/0 | 3/1 |
| 24 | 14/16 | S, R/S | p66/INT | PR/RT, p66/INT | 2/0 | 7/3 |
| 25 | 12/13 | I, R/S | None | p2/NC, p66/INT, NEF | 0/0 | 4/2 |
| 26 | 6/5 | S, I | TFP/p6pol | None | 1/0 | 6/2 |
| 27 | 17/16 | S, I, R/S | NC/p1, NC/TFP, TFP/p6pol | None | 4/0 | 5/0 |
| 28 | 16/18 | S, I | PR/RT | None | 3/0 | 6/2 |
Sequence of PIs experienced during treatment; a comma indicates a change in PI, and a slash indicates concurrent PIs. I, indinavir; R, ritonavir; S, saquinavir.
A “primary” protease mutation (as defined in reference 14) was present before but reverted to wild-type during therapy. Because the NC-TFP and TFP-p6pol sites overlap, a single amino acid substitution can affect both sites. For 18 of the 672 cleavage site sequences analyzed in this study, data could not be generated.
Prevalence and patterns of cleavage site mutations.
Mutations at protease cleavage sites were selected far more often during PI therapy than during NRTI therapy (Fig. 1). Following therapy, 93% (26 of 28) of the patients in the PI group harbored HIV with a mutation(s) at one or more cleavage sites compared to 61% of the patients from the control group (Table 2). The majority of patients (61% [17 of 28]) on PI therapy exhibited mutations at ≥2 cleavage sites (including 11 instances where two or more amino acids were substituted within a given cleavage site) versus only 14% in the control group. Mutations at up to six different cleavage sites could be selected together after as little as 3 months of PI therapy (Table 2).
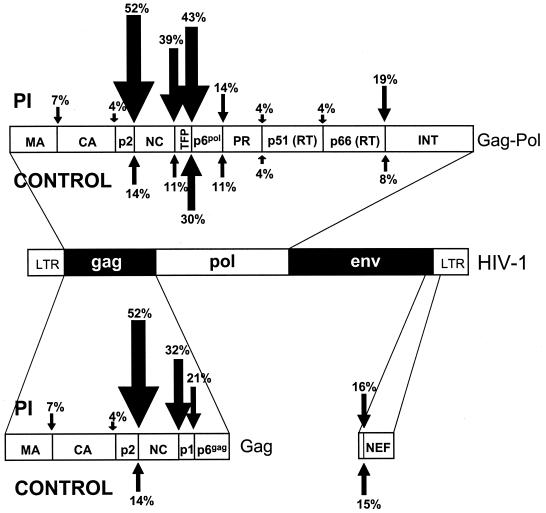
FIG. 1.
Frequency of amino acid substitution at all the potential protease cleavage sites within HIV-1 during PI therapy (above) versus NRTI therapy (below).
In the PI group, mutations were observed in both HIV reading frames at all potential cleavage sites (Fig. 1 and 2). For the control group, mutations occurred at TFP/p6pol, NEF, p2/NC, NC/TFP, p6pol/protease, and protease/RT, with no substitutions observed at other sites. A total of 52, 32, or 39% of PI cases had mutations at the p2/NC, NC/p1, or NC/TFP sites, versus 14, 0, or 11%, respectively, in the controls (P < 0.004). Mutations at a fourth site, p1/p6gag, were present in 6 of 28 of the PI-treated group versus 0 of 28 in the control group, though this was not statistically significant after an adjustment was made for multiple comparisons (P = 0.01). Mutations at other cleavage sites were either relatively uncommon or observed at nearly equal frequencies in controls (Fig. 1 and 3).
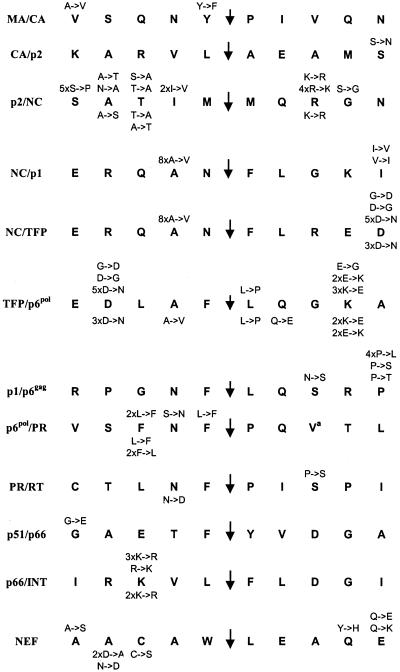
FIG. 2.
Amino acid substitutions observed during therapy. The identity and the number of times each substitution occurred is indicated above the HIV-1 HXB2 “consensus” cleavage site sequence for the PI group (n = 28) and below the sequence for the control group (n = 28).
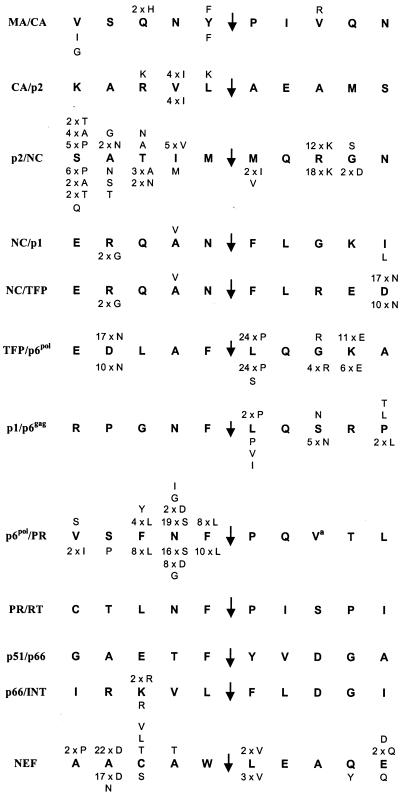
FIG. 3.
Polymorphism observed in both groups. The identity and the number of patients harboring HIV-1 with each polymorphism is indicated above the HIV-1 HXB2 “consensus” cleavage site sequence for the PI group (n = 28) and below the sequence for the control group (n = 28).
The substitution QAN/F→QVN/F at the P2 position of the NC/p1 and NC/TFP sites was the single most common and specific adaptation observed, occurring in 8 of 28 (29%) of PI-resistant cases but in none of the control group. Surprisingly, the L→F substitution at position P1′ of the p1/p6gag cleavage site (8, 17, 24) was not detected in this study, although proline, valine, and isoleucine were all observed at that position (Fig. 3).
Prevalence and patterns of cleavage site polymorphisms.
In this report, we define a “polymorphism” as an amino acid that differed from the HXB2 sequence at baseline and did not change during the course of treatment (Fig. 3). Several were common: a V→I change at position P3′ of the p6pol/PR cleavage site, an R→K change at position P3′ of the p2/NC cleavage site, and an L→P change at position P1′ of the TFP/p6pol site, as well as a D→N change at position P4 (which is also the P5′ position of NC/TFP), an N→S change at position P2′ of the p6pol/PR site, and an A→D change at position P4 of the NEF cleavage site. Two positions within the transframe (TFP) region of Gag-Pol (16) were highly polymorphic: the aspartate shared between the NC/TFP and the TFP/p6pol sites and the lysine in position P4′ of the TFP/p6pol site (Fig. 3). In addition, the results showed that several positions were consistently occupied by an amino acid different from that found in HXB2.
Prevalence of protease mutations.
Consistent with their phenotypes, genotypic analysis indicated that the highly PI-resistant isolates had far more “primary” and “secondary” protease mutations (as defined in reference 12) than the control group (43 versus 0 and 110 versus 35, respectively [data not shown]). The number of protease mutations remained relatively stable during treatment within the control group but increased significantly under PI therapy, a result consistent with the large body of literature on PI resistance development (for a review, see reference 12). Two individuals (one in each group) had virus with an M46I change at baseline, and one from the PI group had a V82T mutation at the onset. The M46I mutation from the control group reverted to M46 during the study.
Relationship between protease and cleavage site mutations.
There was no obvious relationship between the number of protease mutations and the number of cleavage site changes observed during PI therapy (data not shown), nor was there an obvious relationship between specific cleavage site mutations and the degree of PI resistance or the duration of PI therapy (data not shown). However, the A→V substitution in NC/p1 was associated with the M46I or L substitution in the protease (P < 0.007) (Table 3). In fact, the only patient with HIV harboring a valine residue at that position prior to PI therapy was also the only one to show a M46I mutation before therapy. V82A/T mutations were present in six of nine patients with A→V-substituted HIV (including two of the three that did not have a mutation at M46) but were also found in six of the other patients of the PI group (P = 0.243).
DISCUSSION
The data presented are consistent with previous observations that mutations in the HIV protease and at Gag protease cleavage sites are specifically selected during PI therapy. The inclusion in this study of a control group of patients who did not receive any PI therapy enabled the assessment of the contribution of PI exposure (and resistance) to the selection of cleavage site mutations versus nonspecific changes. The Gag p2/NC and NC/p1 and the Gag-Pol NC/TFP cleavage sites were the only protease cleavage sites for which a statistically significant association between the mutations and the development of high-level PI cross-resistance could be demonstrated in this study. Changes at sites outside Gag were either rare or not significantly more common in those patients who had received PI therapy than in those who did not.
The sites observed to mutate most frequently flanked the nucleocapsid protein (NC). The NC/p1 and NC/TFP are cleaved most slowly within the Gag and Gag-Pol precursors (7, 15, 16, 18, 22). This likely represents a rate-limiting step in the processing of NC, a protein required for the formation of mature infectious particles. These sites may therefore evolve relatively quickly under small selective pressures. Interestingly, the amino acids in positions P1 and P1′ of these two sites also differ considerably from the more common pattern of an aromatic amino acid opposite a leucine or a proline.
One implication of these results is that the specificity of the HIV protease is not greatly altered despite the development of high-level PI cross-resistance or that the amino and carboxyl termini of structural proteins can more easily tolerate amino acid substitutions than can the enzymatic proteins. Indeed, ≤1% of all protease sequences in an HIV database including both PI-naive and -experienced viruses (19) show a difference from the most common residue at amino acids 1 to 5 and amino acids 95 to 99 of protease, residues involved in the dimerization of the enzyme (21). Similarly, the amino termini of the RT, RNase H, and integrase are also highly conserved among different HIV clades and isolates (13). Although cleaved Nef is found in virus particles (20), the proteolysis of Nef does not seem to influence the infectivity of HIV in culture (4). It is unlikely that the few changes observed in the NEF site in this study play a specific role in the development of PI resistance.
To evaluate the relevance of cleavage site mutations, consideration should be given to naturally occurring polymorphisms. Indeed, these clinical isolates showed baseline protease cleavage site sequences that sometimes differed considerably from the “consensus sequences” after which HIV protease peptide substrates are often designed. Some of the most frequently mutated positions under PI therapy also happen to be more polymorphic (e.g., positions P2 to P5 and P3′ of the p2/NC cleavage site or position P3 of the p66/INT site). Polymorphisms and mutations in the p6pol/protease site were strikingly confined to the P side of the site, and several different amino acids could be accommodated in the P2 position, which is normally quite restrictive (18). There was no evidence of PI-selected evolution within the transframe peptide, though almost half of the patients harbored a polymorphism within the E-D-L tripeptide sequence postulated to influence protease activity (15).
The mutation with the highest prevalence in the PI-treated group encoded the A→V substitution shared by the NC/p1 and NC/TFP cleavage sites within Gag and Gag-Pol. This mutation has been observed before in HIV culture (2, 6, 8) and clinical (17, 24) isolates, correcting defects in NC processing by the mutant protease (17) and producing a better substrate for mutant protease (8). Previously, a large genotypic study of PI- resistant HIV-1 found that 16% (55 of 300) of the samples harbored this mutation, in close association with the protease V82 mutations, and that the level of phenotypic resistance was not influenced by the presence or absence of this mutation in recombinant viruses (B. A. Larder, S. Bloor, K. Hertogs, C. Van den Eynde, and R. Pauwels, Abstr. 2nd Int. Workshop on HIV Drug Resist. Treatment Strategies, abstr. 23, 1998). Although much smaller, this study also found this mutation to be the most common and specific to PI therapy, developing in 32% (9 of 28) of the PI cases, usually associated with the M46I/L protease genotype. In contrast to observations made in vitro with another PI, ABT-378 (2), we did not observe the A→V substitution in conjunction with another at the p1/p6gag site or with the I47V-to-A protease genotype.
Isolates in this study are likely more drug resistant than those in most other studies, as we selected the most phenotypically PI-resistant isolates available to us. It should also be noted that these results were obtained only from patients taking indinavir, saquinavir, and/or ritonavir and that results from individuals receiving other, newer PIs could differ. The specific limitations of this study include the fact that only 28 cases and controls could be examined, in part due to the large number of sequencing reactions required to examine all of the sites before and after therapy and to the absence of pretherapy samples in many cases. It is therefore possible that some mutations failed to achieve statistical significance here, even though their effects were biologically significant. Finally, mutant protease could potentially use new cleavage sites altogether (3), which would not be detected here.
ACKNOWLEDGMENTS
We thank Kurt Hertogs and Brendan Larder (Virco) for performing the phenotypic analyses; Mark Whaley, Winnie Dong, and Keith Chan (B.C. Centre for Excellence in HIV/AIDS) for assistance with plasma HIV RNA extractions and data analysis; and Michael Murphy (Department of Microbiology and Immunology, University of British Columbia) for help with the protease structural examinations.
REFERENCES
- 1.Boden D, Markowitz M. Resistance to human immunodeficiency virus type 1 protease inhibitors. Antimicrob Agents Chemother. 1998;42:2775–2783. doi: 10.1128/aac.42.11.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrillo A, Stewart K D, Sham H L, Norbeck D W, Kohlbrenner W E, Leonard J M, Kempf D J, Molla A. In vitro selection and characterization of human immunodeficiency virus type 1 variants with increased resistance to ABT-378, a novel protease inhibitor. J Virol. 1998;72:7532–7541. doi: 10.1128/jvi.72.9.7532-7541.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carron de la Carrière L C, Paulous S, Clavel F, Mammano F. Effects of human immunodeficiency virus type 1 resistance to protease inhibitors on reverse transcriptase processing, activity, and drug sensitivity. J Virol. 1999;73:3455–3459. doi: 10.1128/jvi.73.4.3455-3459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y-L, Trono D, Camur D. The proteolytic cleavage of human immunodeficiency virus type 1 Nef does not correlate with its ability to stimulate virion infectivity. J Virol. 1998;72:3178–3184. doi: 10.1128/jvi.72.4.3178-3184.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Condra J H, Schleif W A, Blahy O M, Gabryelski L J, Graham D J, Quintero J C, Rhodes A, Robbins H L, Rothy A, Shivaprakash M, Titus D, Yang T, Teppler H, Squires K E, Deutsch P J, Emini E A. In vivo emergence of HIV-1 variants resistant to multiple protease inhibitors. Nature. 1995;374:569–571. doi: 10.1038/374569a0. [DOI] [PubMed] [Google Scholar]
- 6.Croteau G, Doyon L, Thibeault D, McKercher G, Pilote L, Lamarre D. Impaired fitness of human immunodeficiency virus type 1 variants with high-level resistance to protease inhibitors. J Virol. 1997;71:1089–1096. doi: 10.1128/jvi.71.2.1089-1096.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darke P L, Nutt R F, Brady S F, Garsky V M, Ciccarone T M, Leu C H, Lumma P K, Freidinger R M, Veber D F, Sigal I S. HIV-1 protease specificity of peptide cleavage is sufficient for processing of Gag and Pol polyproteins. Biochem Biophys Res Commun. 1988;156:297–303. doi: 10.1016/s0006-291x(88)80839-8. [DOI] [PubMed] [Google Scholar]
- 8.Doyon L, Croteau G, Thibeault D, Poulin F, Pilote L, Lamarre D. Second locus involved in human immunodeficiency virus type 1 resistance to protease inhibitors. J Virol. 1996;70:3763–3769. doi: 10.1128/jvi.70.6.3763-3769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freund J, Kellner R, Konvalinka J, Wolber V, Kräusslich H-G, Kalbitzer H R. A possible regulation of negative factor (Nef) activity of human immunodeficiency virus type 1 by the viral protease. Eur J Biochem. 1994;223:589–593. doi: 10.1111/j.1432-1033.1994.tb19029.x. [DOI] [PubMed] [Google Scholar]
- 10.Gaedigk-Nitschko K, Schön A, Wachinger G, Erfle V, Kohleisen B. Cleavage of recombinant and cell derived human immunodeficiency virus 1 (HIV-1) Nef protein by HIV-1 protease. FEBS Lett. 1995;357:275–278. doi: 10.1016/0014-5793(94)01370-g. [DOI] [PubMed] [Google Scholar]
- 11.Hertogs K, de Béthune M-P, Miller V, Ivens T, Schel P, Van Cauwenberge A, Van Den Eynde C, Van Gerwen V, Azijn H, Van Houtte M, Peeters F, Staszewski S, Conant M, Bloor S, Kemp S, Larder B, Pauwels R. A rapid method for simultaneous detection of phenotypic resistance to inhibitors of protease and reverse transcriptase in recombinant human immunodeficiency virus type 1 isolates from patients treated with antiretroviral drugs. Antimicrob Agents Chemother. 1998;42:269–276. doi: 10.1128/aac.42.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch M S, Conway B, D'Aquila R T, Johnson V A, Brun-Vezinet F, Clotet B, Demeter L M, Hammer S M, Jacobsen D M, Kuritzkes D R, Loveday C, Mellors J W, Vella S, Richman D D. Antiretroviral drug resistance testing in adults with HIV infection: implications for clinical management. International AIDS Society—USA Panel. JAMA. 1998;279:1984–1991. doi: 10.1001/jama.279.24.1984. [DOI] [PubMed] [Google Scholar]
- 13.Kuiken, C. L., B. Foley, B. Hahn, B. Korber, F. McCutchan, P. A. Marx, J. W. Mellors, J. I. Mullins, J. Sodroski, and S. Wolinksy (ed.). Human retroviruses and AIDS 1999: a compilation and analysis of nucleic acid and amino acid sequences. Theoretical Biology and Biophysics Group, Los Alamos National LaboratoryLos Alamos, N.Mex.
- 14.Larder B A, Kemp S, Harrigan P R. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science. 1995;269:696–699. doi: 10.1126/science.7542804. [DOI] [PubMed] [Google Scholar]
- 15.Louis J M, Dyda F, Nashed N T, Kimmel A R, Davies D R. Hydrophilic peptides derived from the transframe region of Gag-Pol inhibit the HIV-1 protease. Biochemistry. 1998;37:2105–2110. doi: 10.1021/bi972059x. [DOI] [PubMed] [Google Scholar]
- 16.Louis J M, Wondrak E M, Kimmel A R, Wingfield P T, Nashed N T. Proteolytic processing of HIV-1 protease precursor, kinetics and mechanism. J Biol Chem. 1999;274:23437–23442. doi: 10.1074/jbc.274.33.23437. [DOI] [PubMed] [Google Scholar]
- 17.Mammano F, Petit C, Clavel F. Resistance-associated loss of viral fitness in human immunodeficiency virus type 1: phenotypic analysis of protease and gag coevolution in protease inhibitor-treated patients. J Virol. 1998;72:7632–7637. doi: 10.1128/jvi.72.9.7632-7637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pettit S C, Michael S F, Swanstrom R. The specificity of the HIV-1 protease. Perspect Drug Discov Des. 1993;1:69–83. [Google Scholar]
- 19.Shafer R W, Stevenson D, Chan B. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res. 1999;27:348–352. doi: 10.1093/nar/27.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welker R, Kottler H, Kalbitzer H R, Krausslich H G. Human immunodeficiency virus type 1 Nef protein is incorporated into virus particles and specifically cleaved by the viral proteinase. Virology. 1996;219:228–236. doi: 10.1006/viro.1996.0240. [DOI] [PubMed] [Google Scholar]
- 21.Wlodawer A, Miller M, Jaskólski M, Sathyanarayana B K, Baldwin E, Weber I T, Selk L M, Clawson L, Schneider J, Kent S B H. Conserved folding in retroviral proteases: crystal structure of a synthetic HIV-1 protease. Science. 1989;245:616–621. doi: 10.1126/science.2548279. [DOI] [PubMed] [Google Scholar]
- 22.Wondrak E M, Louis J M, de Rocquigny H, Chermann J C, Roques B P. The Gag precursor contains a specific HIV-1 protease cleavage site between the NC (p7) and p1 proteins. FEBS Lett. 1993;333:21–24. doi: 10.1016/0014-5793(93)80367-4. [DOI] [PubMed] [Google Scholar]
- 23.Zennou V, Mammano F, Paulous S, Mathez D, Clavel F. Loss of viral fitness associated with multiple Gag and Gag-Pol processing defects in human immunodeficiency virus type 1 variants selected for resistance to protease inhibitors in vivo. J Virol. 1998;72:3300–3306. doi: 10.1128/jvi.72.4.3300-3306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y M, Imamichi H, Imamichi T, Lane H C, Falloon J, Vasudevachari M B, Salzman N P. Drug resistance during indinavir therapy is caused by mutations in the protease gene and in its Gag substrate cleavage sites. J Virol. 1997;71:6662–6670. doi: 10.1128/jvi.71.9.6662-6670.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]