Abstract
Expansins are cell wall (CW) proteins that mediate the CW loosening and regulate salt tolerance in a positive or negative way. However, the role of Populus trichocarpa expansin A6 (PtEXPA6) in salt tolerance and the relevance to cell wall loosening is still unclear in poplars. PtEXPA6 gene was transferred into the hybrid species, Populus alba × P. tremula var. glandulosa (84K) and Populus tremula × P. alba INRA ‘717-1B4’ (717-1B4). Under salt stress, the stem growth, gas exchange, chlorophyll fluorescence, activity and transcription of antioxidant enzymes, Na+ content, and Na+ flux of root xylem and petiole vascular bundle were investigated in wild-type and transgenic poplars. The correlation analysis and principal component analysis (PCA) were used to analyze the correlations among the characteristics and principal components. Our results show that the transcription of PtEXPA6 was downregulated upon a prolonged duration of salt stress (48 h) after a transient increase induced by NaCl (100 mM). The PtEXPA6-transgenic poplars of 84K and 717-1B4 showed a greater reduction (42–65%) in stem height and diameter growth after 15 days of NaCl treatment compared with wild-type (WT) poplars (11–41%). The Na+ accumulation in roots, stems, and leaves was 14–83% higher in the transgenic lines than in the WT. The Na+ buildup in the transgenic poplars affects photosynthesis; the activity of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT); and the transcription of PODa2, SOD [Cu-Zn], and CAT1. Transient flux kinetics showed that the Na+ efflux of root xylem and leaf petiole vascular bundle were 1.9–3.5-fold greater in the PtEXPA6-transgenic poplars than in the WT poplars. PtEXPA6 overexpression increased root contractility and extensibility by 33% and 32%, indicating that PtEXPA6 increased the CW loosening in the transgenic poplars of 84K and 717-1B4. Noteworthily, the PtEXPA6-promoted CW loosening was shown to facilitate Na+ efflux of root xylem and petiole vascular bundle in the transgenic poplars. We conclude that the overexpression of PtEXPA6 leads to CW loosening that facilitates the radial translocation of Na+ into the root xylem and the subsequent Na+ translocation from roots to leaves, resulting in an excessive Na+ accumulation and consequently, reducing salt tolerance in transgenic poplars. Therefore, the downregulation of PtEXPA6 in NaCl-treated Populus trichocarpa favors the maintenance of ionic and reactive oxygen species (ROS) homeostasis under long-term salt stress.
Keywords: Populus, expansin, cell wall loosening, root contractility, root extensibility, Na+ flux, photosynthesis, SOD, POD, CAT
1. Introduction
The cell wall proteins expansins (EXPs) mediate cell wall loosening by breaking the hydrogen bonds between cellulose microfibrils and matrix polymers [1,2]. In addition to regulating wall extension during plant growth, expansins are also involved in plant responses to various environmental stresses such as cold, heat, drought, salt, heavy metal, and osmotic stress [3,4,5,6,7,8]. Much attention has been paid to the role of expansins in stress physiology, and there is now considerable experimental evidence that expansins may mediate the plant response to salinity. Salt induced the upregulation of wheat expansin genes in leaves (TaEXPA3-A, TaEXPB2-A, TaEXPB4-A, TaEXPB10-A, and TaEXPA9-A) and roots (TaEXPB4-A, TaEXPB10-A, and TaEXPA6-A) [9]. The overexpression of tobacco NtEXPA4 was shown to confer salt and drought tolerance, while RNAi mutants exhibited increased hypersensitivity to salinity [10]. The grass AstEXPA1 gene improved the performance of transgenic tobacco plants under salt stress [11]. Similarly, AtEXP2 overexpression may confer greater tolerance to salt and osmotic stress in Arabidopsis seed germination [12]. Recently, the expansin gene SmEXPA23 from Salix matsudana and the expansin gene PttEXPA8 from Populus tomentosa also increased the salt tolerance of plants [13,14].
There is increasing evidence that expansins modulate morphological and anatomical features to confer salt tolerance in various species like Nicotiana tabacum (Nt), Rosa hybrida (Rh), and Oryza sativa (Os). NtEXPA11-overexpressed plants have significantly larger pith and parenchyma cells compared to the wild type (WT) [15]. The overexpression of RhEXPA4 leads to several changes in the epidermal structure of leaves that respond to abiotic stress, e.g., smaller, compact cells, fewer stomata, and midvein vascular patterning in leaves [16]. In addition, OsEXPA7 overexpression increases cell size and number in the leaf and the elongation of metaxylem cells in the root, which may be involved in improving CW loosening and salt tolerance [17]. Similarly, RhEXPA4-transgenic plants had more lateral roots and longer primary roots under salt stress [16]. It is assumed that the expansin-mediated CW loosening and cell elongation reduces Na+ concentration in the cytoplasm and vacuoles due to the increased water uptake [17]. Expansins have been shown to increase salt tolerance by improving the plant water status, ionic relationships, and ROS homeostasis under saline conditions. For example, the overexpression of TaEXPB23 in tobacco improved salt tolerance by decreasing osmotic potential and increasing water retention ability [18]. The ectopic expression of wheat expansin gene TaEXPA2 in tobacco improved salt tolerance by regulating water status, antioxidant defense, and Na+/K+ balance [19]. Chenopodium quinoa expansin 50 (CqEXPA50) could promote the accumulation of photosynthetic pigment and activate enzymatic and non-enzymatic antioxidant systems [20]. Similarly, OsEXPA7 overexpression resulted in a reduction in Na+ and ROS, and an accumulation of K+ in the leaves and roots under salt stress [17].
In contrast to the increase in salinity tolerance, there is also evidence that expansins negatively influence the response of plants to salinity. The overexpression of two typical Arabidopsis α- and β-expansin genes, AtEXP3 and AtEXPβ1, resulted in increased sensitivity to salt stress. Although an increased peroxidase activity was observed in both AtEXP3- and AtEXPβ1-overexpressed seedlings, their overexpression can lead to deleterious defects in growth and development [21]. It has also been shown that excessive AtEXPA1 expression could disrupt cell wall organization and lead to growth reduction, which facilitates plant adaptation to environmental stress [22]. In addition, the expression of Festuca arundinacea EXPA7 decreased by 74–82% in the fast-growing genotype ‘K-310’ and the slow-growing genotype ‘Bonsai’ under salt stress [23]. It is, therefore, possible that salinized plants downregulate the transcription of expansins to reduce CW loosening and plant growth under unfavorable conditions. However, this needs to be clarified by further investigations.
In this study, we report a downregulation of expansin A6 (EXPA6) in long-term salt-stressed Populus trichocarpa. The purpose of this study was to investigate whether Populus trichocarpa PtEXPA6 mediates the salt tolerance of poplars and its relevance to cell wall loosening. PtEXPA6 gene was transferred into the hybrid species, Populus alba × P. tremula var. glandulosa (84K) and Populus tremula × P. alba INRA ‘717-1B4’. Under salt stress, the stem growth, gas exchange, chlorophyll fluorescence, activity and transcription of antioxidant enzymes, Na+ content, and Na+ flux of root xylem and petiole vascular bundle were investigated in wild-type and transgenic poplars. The correlation analysis and principal component analysis (PCA) were used to analyze the correlations among the characteristics and principal components. Our results showed that the overexpression of PtEXPA6 reduced plant growth, photosynthetic capacity, and the ability for ROS scavenging under salt stress, which was related to the excessive Na+ transported from root to shoots. The PtEXPA6-promoted cell wall loosening and the relevance to apoplastic Na+ transport were explored in the root tips of the WT and transgenic poplars. We hypothesize that the overexpression of PtEXPA6 leads to loosening of the cell walls, facilitating the radial translocation of Na+ into the root xylem and the subsequent Na+ translocation from roots to leaves, resulting in an excessive Na+ accumulation and reduced salt tolerance in the transgenic poplars of 84K and 717-1B4.
2. Results
2.1. Expression of PtEXPA6 in Root and Shoot of Populus trichocarpa
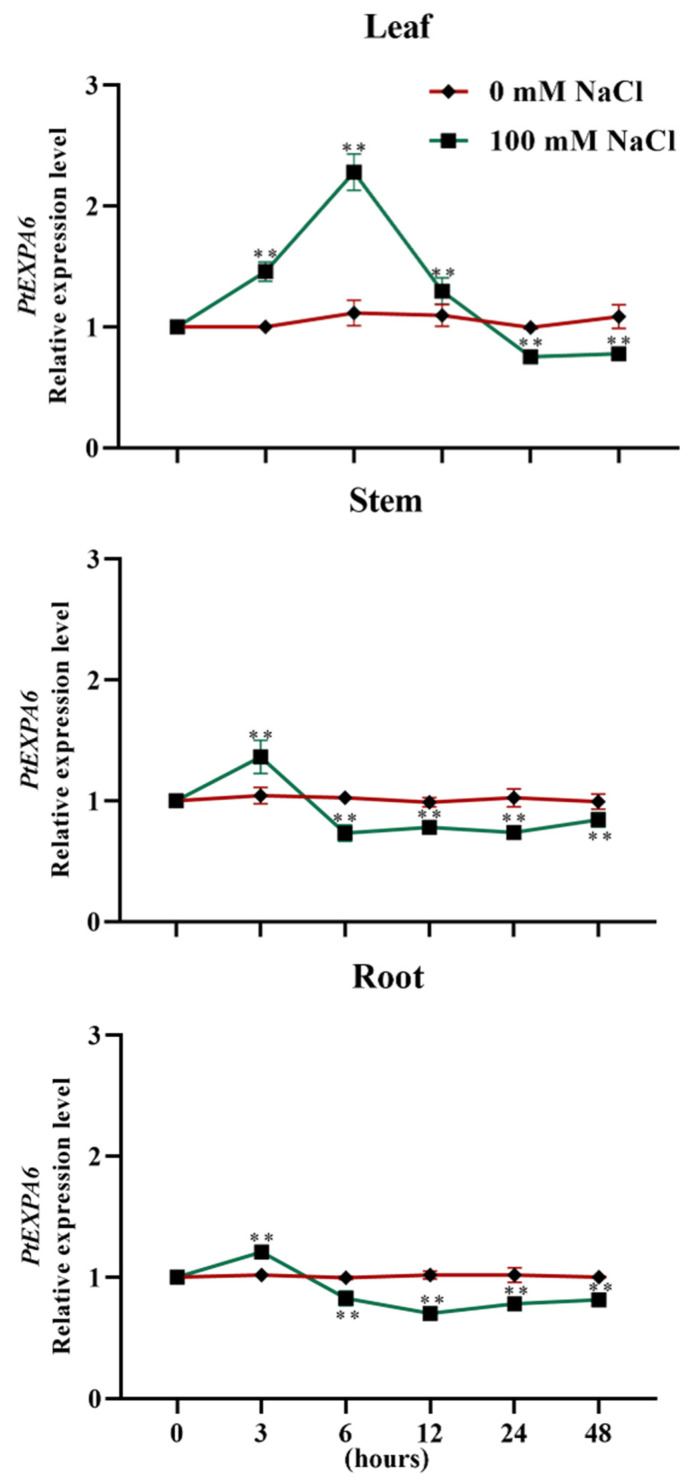
The transcription level of PtEXPA6 in P. trichocarpa fluctuated during the observation period of salt stress (100 mM NaCl, 48 h). The expression of PtEXPA6 was transiently upregulated in the leaves and peaked after 6 h of salt treatment, followed by a rapid decrease to a level below that of the controls after 24 h (Figure 1). The PtEXPA6 expression then stabilized at the end of the salt treatment (48 h) and remained below the pre-treatment level (Figure 1). In the roots and stems, the NaCl-altered transcription of PtEXPA6 was similar to that in the leaves, with an initial upregulation at 3 h, followed by a significant decrease at 6 h, and stabilized at a level lower than that of the controls (Figure 1).
Figure 1.
Transcription profile of PtEXPA6 in the leaves, stems, and roots of Populus trichocarpa during the period of salt stress. Uniform plants of P. trichocarpa were treated with NaCl saline (0 or 100 mM) for 48 h. The fine roots, stems, and upper leaves (3rd to 8th from shoot tip) were sampled at 0, 3, 6, 12, 24, and 48 h, respectively. For the RT-qPCR analysis, the primer sequences for PtEXPA6 and the reference gene, PtUBQ, are shown in Supplementary Table S1. The data are means ± SD (n = 3), and the bars with asterisks indicate significant differences, **: p < 0.01.
2.2. Homologous Sequence Analysis of PtEXPA6
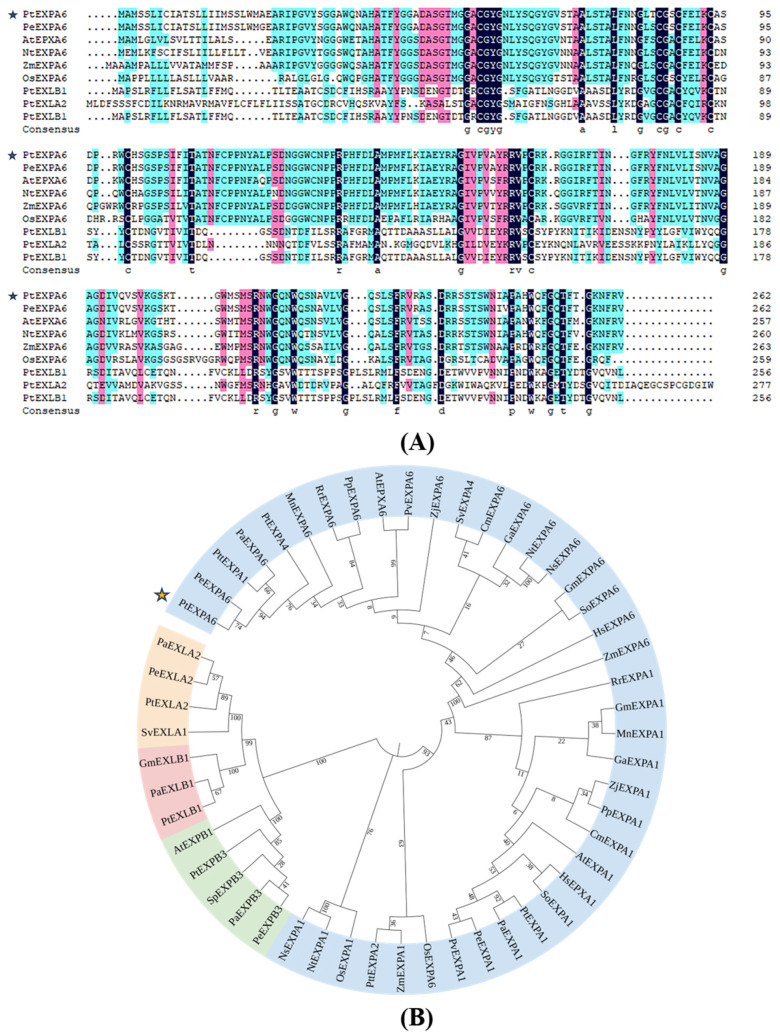
The coding sequence (CDS) of PtEXPA6 is 789 bp in length, encoding 266 amino acids. The translated protein sequence has a molecular weight of 28.38 kDa and an isoelectric point of 9.79 (Figure 2A). The phylogenetic analysis revealed that PtEXPA6 is evolutionarily closely related to PeEXPA6 in P. euphratica, but more distantly related to AtEXPA6 in Arabidopsis thaliana (Figure 2B).
Figure 2.
Sequence and phylogenetic analysis of Populus trichocarpa PtEXPA6. (A) The multiple sequence alignment of EXPA and expansin family from Populus and other species. The black shading indicates identical amino acid residues, and the blue and pink shadings indicate conserved amino acids, respectively. The lower-case letters represent the same amino acids in different species. (B) The phylogenetic analysis of expansin from various species. Populus euphratica (Pe), Populus trichocarpa (Pt), Populus tremula × Populus tremuloides (Ptt), Populus alba (Pa), Arabidopsis thaliana (At), Zea mays (Zm), Nicotiana tabacum (Nt), and Oryza sativa (Os), Glycine max (Gm), Salix viminalis (Sv), Morus notabilis (Mn), Rosa rugosa (Rr), Prunus persica (Pp), Pistacia vera (Pv), Ziziphus jujuba (Zj), Cucumis melo (Cm), Gossypium arboreum (Ga), Nicotiana sylvestris (Ns), Syzygium oleosum (So), Hibiscus syriacus (Hs), Pistacia vera (Pv), and Salix purpurea (Sp). Supplementary Table S2 lists the accession numbers of the EXPA orthologs. The blue, yellow, pink and green shadings indicate expansin orthologs of EXPA, EXLA, EXLB, and EXPB, respectively. The PtEXPA6 is labelled with star symbols in (A,B).
2.3. Overexpression of PtEXPA6 in Poplars
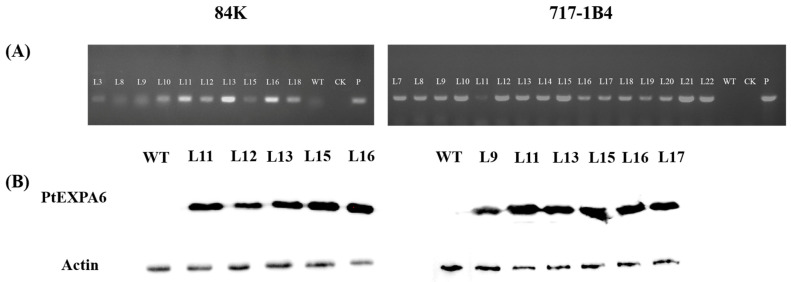
The downregulation of PtEXPA6 following the NaCl treatment suggests that it is involved in the response of P. trichocarpa to salt stress. To investigate the regulatory effects of PtEXPA6 under saline conditions, we overexpressed PtEXPA6 in the hybrid poplars of 84K and 717-1B4. PCR assay revealed different expressions of PtEXPA6 in the transgenic lines of 84K and 717-1B4 (Figure 3A). The Western blot analysis confirmed five transgenic lines of 84K and six transgenic lines of 717-1B4 with higher expression levels, three of which were selected for further investigation, i.e., L11, L12, and L13 for the 84K poplar and L9, L15, and L16 for the 717-1B4 poplar (Figure 3).
Figure 3.
Molecular verification of the transgenic lines overexpressing P. trichocarpa PtEXPA6 in 84K and 717-1B4. (A) The PCR assay of the transgenic poplars. The primer sequences for PtEXPA6 are shown in Supplementary Table S1. WT: negative control (wild type); CK: blank control; P: positive control. (B) The Western blot of the transgenic lines. The Western blot analysis performed with an anti-MYC-specific antibody for PtEXPA6.
2.4. Effect of Salinity on Shoot Growth of PtEXPA6-Overexpressed Poplars
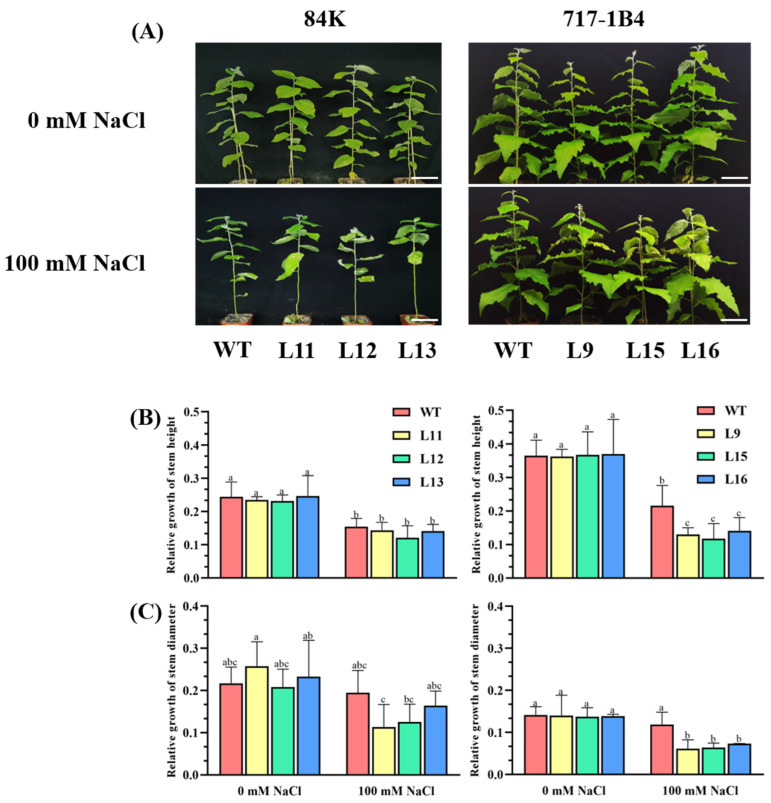
The NaCl effects on stem height and diameter growth were investigated by exposing the WT and PtEXPA6-overexpressed poplars to 100 mM NaCl for 15 days. Under normal growth conditions, there was no significant difference in the plant height and ground diameter between wild-type and transgenic lines (Figure 4A−C). However, the WT and transgenic lines of 84K and 717-1B4 showed significantly reduced growth after the salt treatment (p < 0.05), with the growth of the transgenic lines being more suppressed (Figure 4B,C). In comparison, the transgenic poplars of 717-1B4 showed a greater reduction in the plant height and ground diameter (52–65%) under salt stress than the transgenic poplars of 84K (42–43%, Figure 4).
Figure 4.
Phenotypic tests of the wild-type (WT) and PtEXPA6-overexpressing lines of 84K and 717-1B4 under long-term salt stress. The PtEXPA6-overexpressing lines of 84K (L11, L12, and L13) and 717-1B4 (L9, L15, and L16), and wild-type (WT) were exposed to NaCl with 0 or 100 mM for 15 days. The stem height and diameter of the no-salt control and salinized plants were measured after 15 days of the salt treatment. The relative growth of the stem height and diameter during the observation period are shown. (A) Representative images showing plant performance after the salt treatment. Scale bars = 5 cm. (B) Relative growth of the stem height. (C) Relative growth of the stem diameter. The data are means ± SD (n = 3), and the bars with different letters indicate significant differences (p < 0.05).
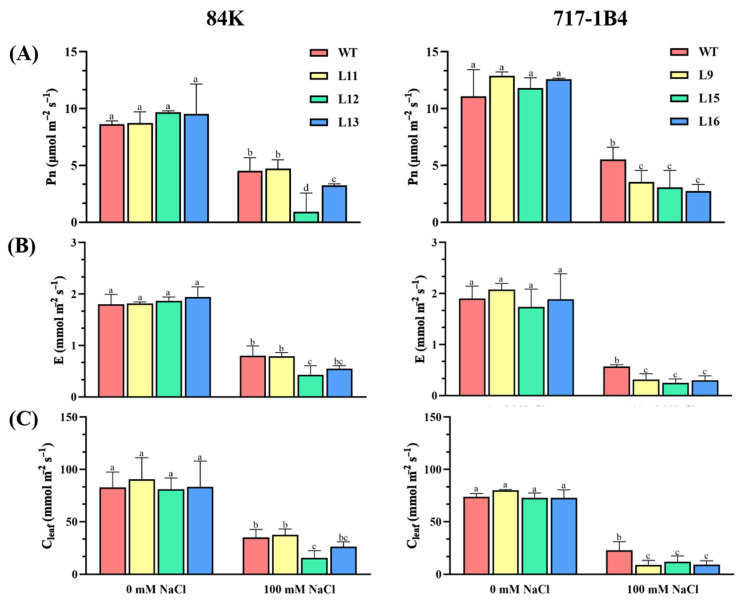
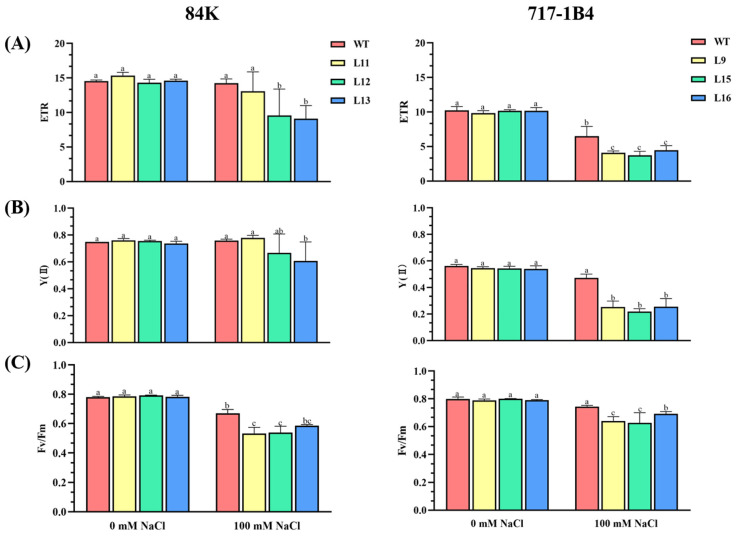
2.5. Effect of Salinity on Photosynthesis of PtEXPA6-Overexpressing Poplars
The salt-restricted growth of the transgenic poplars was related to the reduced photosynthetic capacity. Leaf gas exchange and chlorophyll fluorescence were analyzed in the WT and transgenic lines of 84K and 717-1B4. After 15 days of salt stress, the net photosynthetic rate (Pn) decreased more in the transgenic lines of 84K (67%) and 717-1B4 (74%) compared to the WT poplars (46%, Figure 5A). The decrease in transpiration rate and stomatal conductance were also more pronounced in the transgenic lines than in the WT (Figure 5). Chlorophyll fluorescence measurements showed a decrease in the relative electron transport rate (ETR), actual photosynthetic quantum yield (YII), and maximum photochemical PSII efficiency (Fv/Fm) due to NaCl stress; however, the decrease was less pronounced in the WT (13%) compared to the transgenic poplars (16–44%), in particular, the 717-1B4 (Figure 6).
Figure 5.
Effect of NaCl on leaf gas exchange in the wild-type and PtEXPA6-overexpressing lines of 84K and 717-1B4. The PtEXPA6-overexpressing lines of 84K (L11, L12, and L13) and 717-1B4 (L9, L15, and L16), and wild-type (WT) were exposed to NaCl with 0 or 100 mM for 15 days. Leaf gas exchange, i.e., net photosynthetic rate, transpiration rate, and stomatal conductance were measured in the leaves of the no-salt control and salinized plants after 15 days of the salt treatment. (A) Net photosynthetic rate (Pn). (B) Transpiration rate (E). (C) Stomatal conductance (Cleaf). The data are means ± SD (n = 3), and the bars with different letters indicate significant differences (p < 0.05).
Figure 6.
Effect of NaCl on chlorophyll fluorescence in the wild-type and PtEXPA6-overexpressing lines of 84K and 717-1B4. The PtEXPA6-overexpressing lines of 84K (L11, L12, and L13) and 717-1B4 (L9, L15, and L16), and wild-type (WT) were exposed to NaCl with 0 or 100 mM for 15 days. Chlorophyll fluorescence, i.e., the relative electron transport rate, the actual photosynthetic quantum yield, and the maximum photochemical efficiency of PSII were measured in the leaves of the no-salt control and salinized plants after 15 days of the salt treatment. (A) The relative electron transport rate (ETR). (B) The actual photosynthetic quantum yield (YII). (C) The maximum photochemical efficiency of PSII (Fv/Fm). The data are means ± SD (n = 3), and the bars with different letters indicate significant differences (p < 0.05).
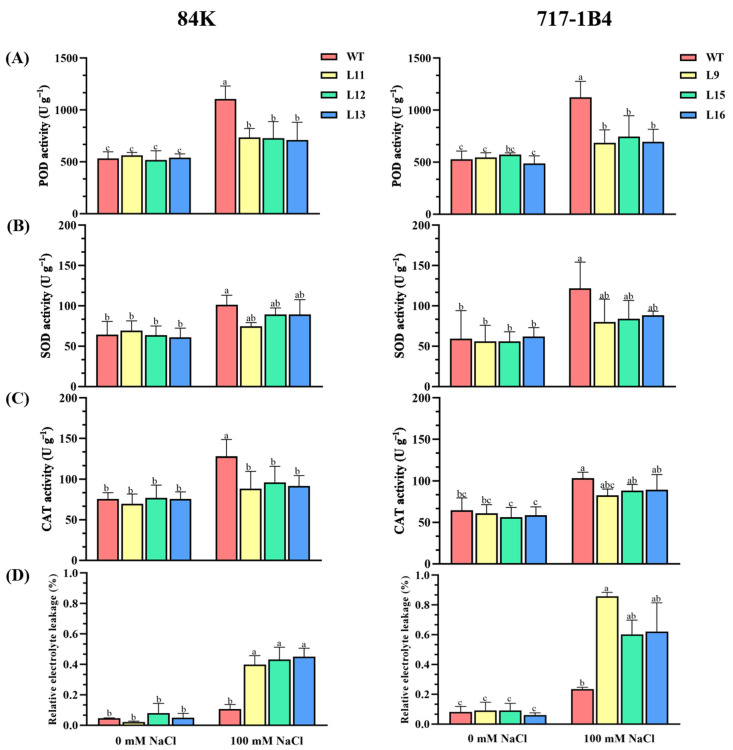
2.6. The Activity and Transcription of Antioxidant Enzymes
The ability to control ROS is critical for plant adaptation to saline environments. The activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) were examined in the WT and transgenic lines of 84K and 717-1B4 under salt stress. The activities of the antioxidant enzymes increased significantly in all the genotypes tested under salt stress (p < 0.05), although the increase was 29–71% lower in the transgenic lines than in the WT (Figure 7A–C). In addition, the relative electrolyte leakage (REL) of each genotype was evaluated under control and salt treatments. The REL showed no significant difference between the genotypes tested under normal conditions, but a significant increase in the transgenic lines compared to WT after the salt treatment (p < 0.05, Figure 7D).
Figure 7.
Effect of NaCl on antioxidant enzyme activity and relative electrolyte leakage in the wild-type and PtEXPA6-overexpressing lines of 84K and 717-1B4. The PtEXPA6-overexpressing lines of 84K (L11, L12, and L13) and 717-1B4 (L9, L15, and L16), and wild-type (WT) were exposed to NaCl with 0 or 100 mM for 15 days. The activity of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), and relative electrolyte leakage were measured in the leaves of the no-salt control and salinized plants after 15 days of the salt treatment. (A) POD activity. (B) SOD activity. (C) CAT activity. (D) Relative electrolyte leakage. The data are means ± SD (n = 3), and the bars with different letters indicate significant differences (p < 0.05).
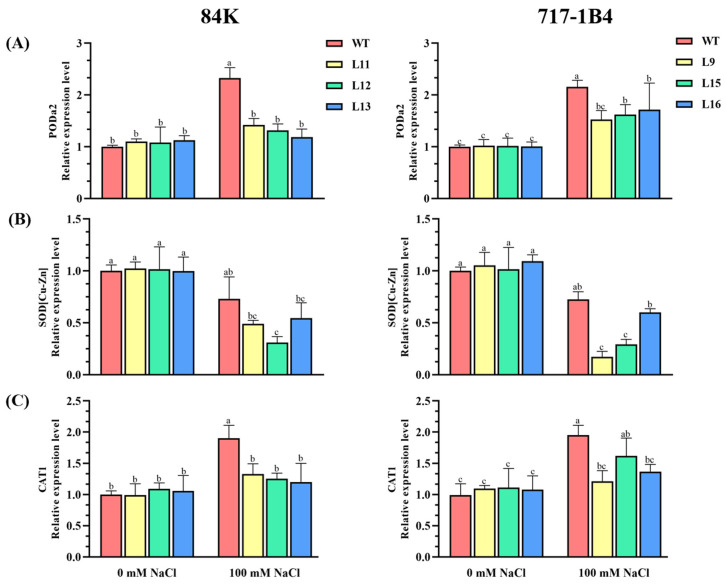
In this study, we analyzed the transcription of SOD [Cu-Zn], CAT1, and PODa2 in the leaves of the WT and transgenic poplars. After the salt treatment, the transcription levels of CAT1 and PODa2 increased significantly in the WT poplars and the transgenic lines of 84K and 717-1B4 (p < 0.05), and the upregulation of CAT1 and PODa2 was 55–92% greater in the WT than the transgenic poplars (Figure 8). Conversely, the transcription level of SOD [Cu-Zn] was decreased in the salt-exposed poplars (Figure 8). And, the transgenic lines 84K and 717-1B4 showed a 29–38% greater decrease than the WT under the salt treatment (Figure 8).
Figure 8.
Effect of NaCl on the transcription levels of antioxidant enzymes in the wild-type and PtEXPA6-overexpressing lines of 84K and 717-1B4. The PtEXPA6-overexpressing lines of 84K (L11, L12, and L13), 717-1B4 (L9, L15, and L16), and wild-type (WT) were exposed to NaCl with 0 or 100 mM for 15 days. The relative expression of the antioxidant enzyme genes such as peroxidase a2 (PODa2), superoxide dismutase [Cu-Zn] (SOD [Cu-Zn]), and catalase 1 (CAT1) were examined in the WT and PtEXPA6-overexpressing poplars after 15 days of the salt treatment. (A) PODa2. (B) SOD [Cu-Zn]. (C) CAT1. The primer sequences of PODa2, SOD [Cu-Zn], and CAT1 and the reference actin gene, PtUBQ, are shown in Supplementary Table S1. The data are means ± SD (n = 3), and the bars with different letters indicate significant differences (p < 0.05).
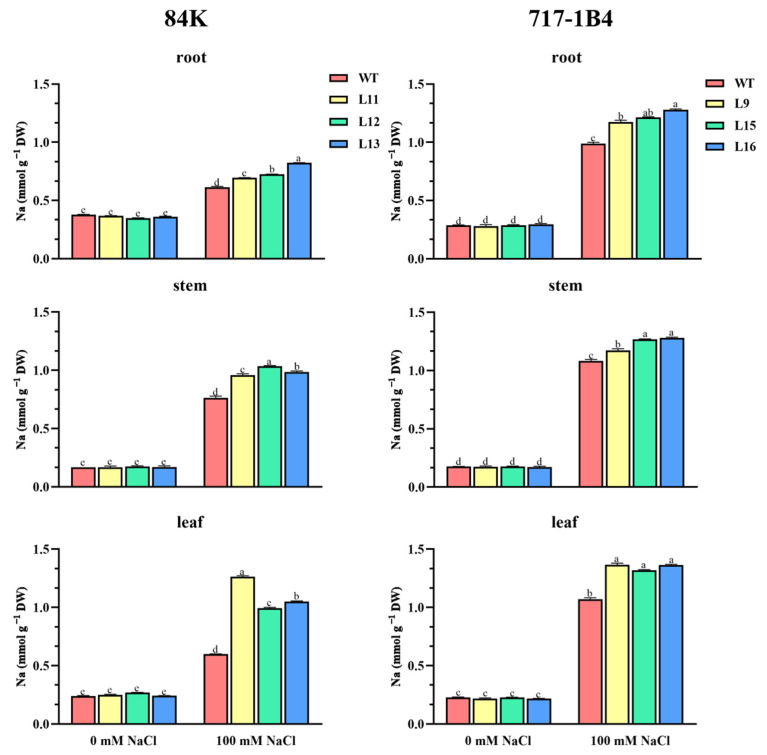
2.7. Na+ Content in Roots, Stems, and Leaves of Transgenic Poplars
Maintaining the Na+ homeostasis is crucial for poplar adapting to salt stress [24]. The content of Na+ ions in roots, stems, and leaves was analyzed in the WT and transgenic poplars of 84K and 717-1B4. Under salt-free conditions, the Na+ content in the roots, stems, and leaves of the 84K poplar was low, with no significant difference between the WT and transgenic lines (Figure 9). However, the Na+ content increased significantly in the roots, stems, and leaves of 84K poplars after the salt treatment, and the PtEXPA6-trangenic lines, L11, L12, and L13, had 22–83% higher Na+ content than the WT (Figure 9). Similar trends were observed in the 717-1B4 poplar, where the Na+ content in the roots, stems, and leaves of the PtEXPA6-trangenic lines L9, L15, and L16 was 14–26% higher than that of the WT under salt stress (Figure 9). In comparison, the Na+ accumulation in the transgenic 717-1B4 poplar was 22–63% higher than in the transgenic poplar of 84K (Figure 9).
Figure 9.
Na+ content in roots, stems, and leaves of wild-type and PtEXPA6-overexpressing lines of 84K and 717-1B4 under long-term salt stress. PtEXPA6-overexpressing lines of 84K (L11, L12, and L13) and 717-1B4 (L9, L15, and L16), and wild-type (WT) were exposed to NaCl with 0 or 100 mM for 15 days. Data are means ± SD (n = 3), and bars with different letters indicate significant differences (p < 0.05).
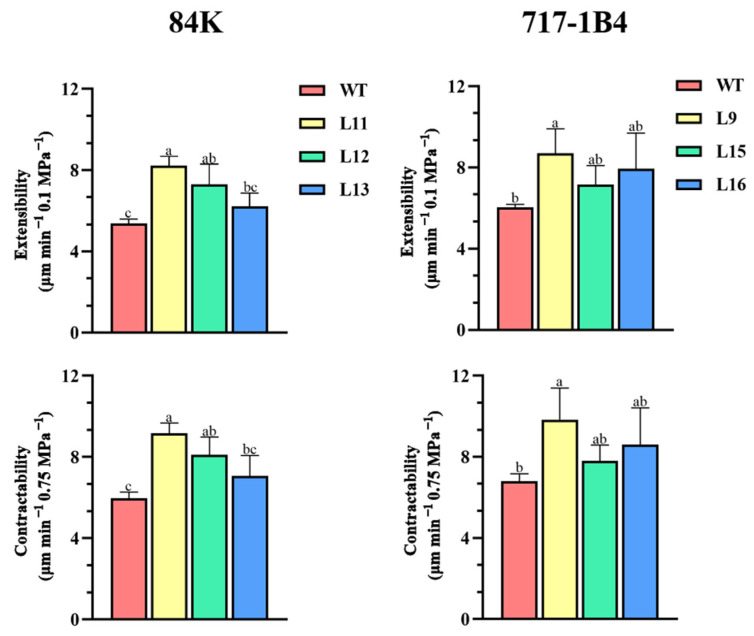
2.8. Comparative Contractability and Comparative Extensibility of Root Cell Walls
Expansins are cell wall (CW) proteins to mediate CW loosening by breaking the hydrogen bonds between cellulose microfibrils and matrix polymers [1,2]. The PtEXPA6-promoted CW loosening was determined by measuring comparative contractability and comparative extensibility using the intact root tips of the WT and transgenic poplars [25,26]. The comparative contractility of the intact root tips was measured after exposure to 300 mOsmol kg−1 mannitol treatment (−0.75 MPa−1) and expressed in µm min−1 0.75 MPa−1. The comparative root extensibility values were obtained for the intact roots by determining the initial (1 min) increase in the root elongation rate induced by a 0.1 MPa osmotic jump [25,26]. Our results show that the overexpression of PtEXPA6 in 84K and 717-1B4 led to an increased root contractability (8.1–8.7 μm min−1 0.75 MPa−1) compared to the WT (6.0–6.8 μm min−1 0.75 MPa−1). Moreover, PtEXPA6 overexpression also increased the root comparative extensibility (7.3–7.9 μm min−1 0.1 MPa−1) compared to the WT (5.4–6.0 μm min−1 0.1 MPa−1) (Figure 10). Therefore, the elevated comparative contractability and comparative extensibility indicate PtEXPA6 increased cell wall loosening in the transgenic poplars.
Figure 10.
Comparative contractability and comparative extensibility of the intact root tip sites in the wild-type and PtEXPA6-overexpressing lines of 84K and 717-1B4. The comparative contractability of the PtEXPA6-overexpressing lines of 84K (L11, L12, and L13) and 717-1B4 (L9, L15, and L16), and wild-type (WT) was measured after the intact root tips were exposed to 300 mOsmol kg−1 mannitol (−0.75 MPa). Then, comparative extensibility of the intact root tip was measured after a 0.10 MPa osmotic jump. The data are means ± SD (n = 3), and the bars with different letters indicate significant differences (p < 0.05).
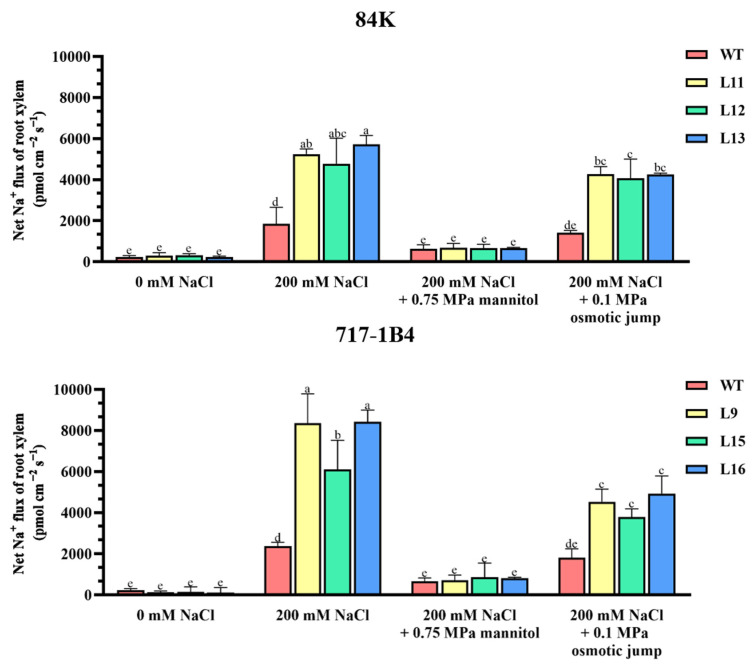
2.9. Na+ Flux of Root Xylem and the Response to Osmotic Jump
The greater Na+ accumulation in the transgenic poplars mainly results from the salt uptake and transport in roots and the root-to-shoot salt transport [24]. The restriction of salt radial translocation in roots is crucial to control the root-to-shoot salt transport [27,28]. Using selective microelectrodes, the Na+ flux of the root xylem was measured in the salt-stressed poplars, as the Na+ flow in the root xylem can reflect the real-time radial transport of Na+ in roots. After a short-term salt exposure, the xylem Na+ efflux of the two poplars drastically increased with a 3.1–3.5-fold higher flux rate in the PtEXPA6-overexpressing lines (Figure 11). Under the no-salt control conditions, the Na+ flux in the root xylem of 84K and 717-1B4 was very low or undetectable, and there is no significant difference between the WT and transgenic lines (Figure 11). The greater Na+ efflux in the xylem indicates that the transgenic poplars exhibited a lower capacity to restrict the radial translocation of Na+ salt to the xylem under saline conditions. Noteworthily, we observed that the PtEXPA6-promoted CW loosening facilitated the radial translocation of Na+ in roots. The PtEXPA6-promoted radial translocation of Na+ in roots was markedly restricted by the addition of 300 mOsmol kg−1 mannitol (osmotic potential is −0.75 MPa, Figure 11), which induces contraction and hardens the cell walls (Figure 10) [29,30]. We noticed that the Na+ flux of the xylem in the contracted roots was resumed after an 0.1 MPa osmotic jump (Figure 11), which induces the extension of the cell walls (Figure 10) [29,30]. This Na+ kinetics upon an osmotic jump demonstrated that CW loosening favors the radial Na+ transport, while a CW contraction restricts the Na+ transport in poplar roots.
Figure 11.
Na+ flux of the root xylem and the response to an osmotic jump in the wild-type and PtEXPA6-overexpressing lines of 84K and 717-1B4. After exposure to 200 mM NaCl for 4 h, the intact root tips of the PtEXPA6-trangenic lines of 84K (L11, L12, and L13) and 717-1B4 (L9, L15, and L16), and wild-type (WT) were exposed to 300 mOsmol kg−1 mannitol (−0.75 MPa), followed by a 0.1 MPa osmotic jump. The net Na+ flux of the root xylem was measured before and after the addition of mannitol and the subsequent 0.1 MPa osmotic jump. The data are means ± SD (n = 3), and the bars with different letters indicate significant differences (p < 0.05).
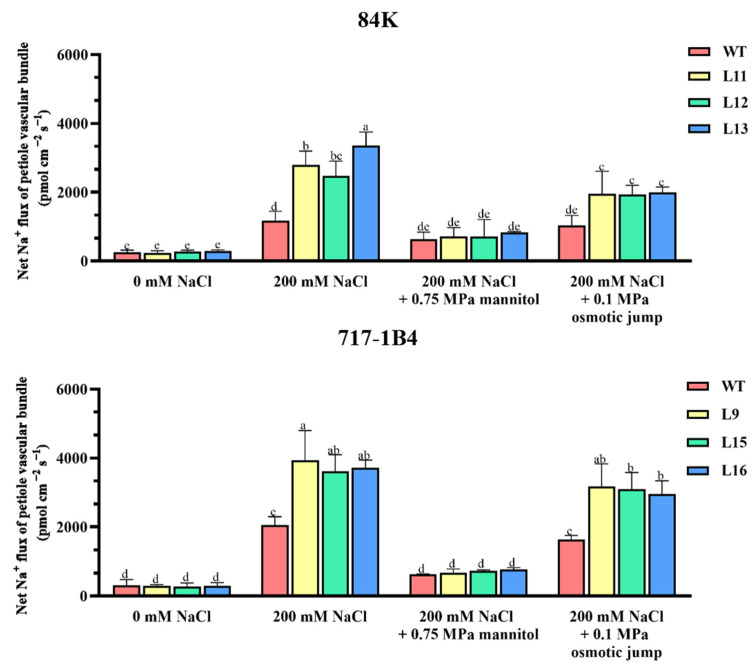
2.10. Na+ Flux of Leaf Petiole Vascular Bundles and the Response to Osmotic Jump
The radial translocation of Na+ salt to the xylem would result in a higher rate of salt transport to the shoots. Thus, the Na+ flux of the vascular bundles in the leaf petiole was examined to determine the real-time Na+ translocation kinetics from the roots to the leaves [31]. The roots of intact plants were subjected to short-term salt exposure, the leaf petiole was immobilized in a measuring chamber after the leaf blade was removed. The Na+ flux of the vascular bundles in the leaf petiole was immediately measured with selective microelectrodes. The xylem Na+ efflux of the vascular bundles drastically increased in the two salinized poplars with a 1.9–2.9-fold higher flux rate in the PtEXPA6-overexpressing lines (Figure 12). Under no-salt control conditions, the Na+ flux in the leaf vascular bundles of 84K and 717-1B4 was very low or undetectable, and there is no significant difference between the WT and transgenic lines. We also found that the PtEXPA6-promoted cell wall loosening facilitated the root-to-shoot Na+ transport. The Na+ flux of the vascular bundles in the leaf petiole were restricted by 300 mOsmol kg−1 mannitol (−0.75 MPa), while the Na+ flux was markedly recovered upon an 0.1 MPa osmotic jump (Figure 12), which is similar to the Na+ flux of the root xylem (Figure 11). The real-time Na+ translocation kinetics upon an osmotic jump demonstrated that CW loosening favors the Na+ transport from the roots to the leaves, while a CW contraction restricts the root-to-shoot Na+ transport.
Figure 12.
Na+ flux of the petiole vascular bundle and the response to an osmotic jump in the wild-type and PtEXPA6-overexpressing lines of 84K and 717-1B4. After exposure to 200 mM NaCl for 4 h, the intact root tips of the PtEXPA6-trangenic lines of 84K (L11, L12, and L13) and 717-1B4 (L9, L15, and L16), and wild-type (WT) were exposed to 300 mOsmol kg−1 mannitol (−0.75 MPa), followed by a 0.1 MPa osmotic jump. The net Na+ flux of the petiole vascular bundle was measured before and after the addition of mannitol, and the subsequent 0.1 MPa osmotic jump. The data are means ± SD (n = 3), and the bars with different letters indicate significant differences (p < 0.05).
2.11. The Correlation Analysis and Principal Component Analysis (PCA)
The correlation analysis and principal component analysis (PCA) of the measured traits showed that there are species differences in the correlations among the characteristics and principal components (Tables S3–S6). The correlation analysis showed significant positive and negative correlations among the growth, physiological, and biochemical measurements, in particular the clone 717-1B4. For example, stem growth, gas exchange, chlorophyll fluorescence, activity, and the transcription of antioxidant enzymes had high positive correlations (ca. 0.800–0.999), with a few exceptions (e.g., CAT activity and SOD [Cu-Zn] expression). Significant negative correlations were also noted between Na+ content, Na+ fluxes, relative electrolyte leakage, and salt-resistant characteristics, such as stem growth, gas exchange, chlorophyll fluorescence, activity, and the transcription of antioxidant enzymes (Table S3). Compared with the clone 717-1B4, the 84K poplar showed relatively lower correlations among the measured characteristics. In general, leaf gas exchange and chlorophyll fluorescence had low correlations with the antioxidant enzymes, Na+ content and fluxes, and relative electrolyte leakage, although there are significant positive correlations between gas exchange, chlorophyll fluorescence, and stem height growth (Table S4). For the 84K poplar, significant negative correlations were always observed between the Na+ content, Na+ fluxes, and antioxidant enzymes, which is similar to the clone 717-1B4 (Tables S3 and S4).
The PCA revealed variations among the traits and determined three main factors that explained almost 100% of the total variance for the two hybrid poplars, 717-1B4 and 84K (Tables S5 and S6). It is shown that the three PCs displayed eigenvalues higher than significant although the eigenvalues varied within species (Tables S5 and S6). For the clone 717-1B4, the first principal component (PC1), second principal component (PC2), and third principal component (PC3) accounted for 84.737%, 8.120%, and 7.144% of the observed variations, respectively (Table S5). For the 84K poplar, PC1, PC2, and PC3 accounted for 62.782%, 26.080%, and 11.138% of the observed variations, respectively (Table S6). There are also species differences in the traits included in PC1 and PC2. For clone 717-1B4, the PC1 covered the stem growth, gas exchange, chlorophyll fluorescence, activity and transcription of antioxidant enzymes, and Na+ content and fluxes (Table S5). But the gas exchange, i.e., net photosynthetic rate (Pn), transpiration rate (E), and stomatal conductance (Cleaf), fell into PC2 for the 84K poplar (Table S6). The PC2 also contained traits like relative electrolyte leakage, SOD activity, and Na+ flux of petiole at −0.75 MPa mannitol (Table S6).
3. Discussion
3.1. PtEXPA6 Negatively Regulates Salt Tolerance in Transgenic Poplars
The PtEXPA6 transgenic lines of 84K and 717-1B4 showed a more pronounced reduction in stem height and diameter growth compared to the WT poplars after 15 days of exposure to 100 mM NaCl (Figure 4). The salt-reduced growth was associated with a drastic reduction in the photosynthetic capacity of the transgenic poplars (Figure 5 and Figure 6), as there are significant positive correlations between gas exchange, chlorophyll fluorescence, and stem growth for the two poplars (Tables S3 and S4). Thus, PtEXPA6 increases the salt sensitivity of the transgenic poplars of both 84K and 717-1B4. This is consistent with the Arabidopsis expansin genes, AtEXP3 and AtEXPβ1, which resulted in increased sensitivity to salt stress [21]. However, these results are inconsistent with the finding that the overexpression of NtEXPA4 [10], AstEXPA1 [11], AtEXP2 [12], SmEXPA23 [13], PttEXPA8 [14], and CqEXPA50 [20] increased the salt tolerance of the transgenic plants. These contrasting results suggest that expansin proteins play different roles in regulating plant responses to salinity. The positive and negative effects of expansins in salt tolerance are probably related to the function of expansin in mediating water and ionic relations that are associated with the expansin-modified morphological structures. RhEXPA4 leads to smaller, compact cells, fewer stomata, and vascular patterning in the center of the leaf epidermis [16]. This suggests that RhEXPA4 might confer salt tolerance by reducing water loss. In contrast, the size of the leaves in AtEXP3- and AtEXPβ1-overexpressed plants was larger than those of the wild type [21]. The increased transpiration surface would lead to greater water consumption under salt stress, resulting in an increased salt sensitivity in AtEXP3- and AtEXPβ1-overexpressed plants. There are increasing evidence showing that expansins confer salt tolerance by improving primary root length and root architecture. RhEXPA4, OsEXPA7, NtEXPA11, and TaEXPB23 have been shown to enhance the root growth and overall development of the root system [15,16,17,18]. This could enhance the uptake of more water and nutrients, resulting in better plant growth and development under salt stress. Moreover, wheat TaEXPA2 and rice OsEXPA7 have been shown to reduce Na+ in leaves and roots under salt stress [17,19]. It is hypothesized that the longer metaxylem cells in OsEXPA7-overexpressed plants can take up more water than the WT plants, reducing the Na+ concentration both in the cytoplasm and in the vacuoles [17]. However, our results show that the overexpression of PtEXPA6 leads to a loosening of the cell walls but does not promote root growth under long-term salt stress. At present, it is unknown whether PtEXPA6 affects the length of the metaxylem cells in poplar roots. Therefore, the PtEXPA6-modified cell wall loosening and morphological structures and the significance for water and Na+ transport need to be further investigated.
The pattern of PtEXPA6 transcription contrasts with that of salt-induced expansins in wheat leaves and roots, such as TaEXPB2-A, TaEXPA3-A, TaEXPB4-A, TaEXPA6-A, TaEXPA9-A, and TaEXPB10-A (Figure 1) [9]. Thus, the downregulation of PtEXPA6 favors the adaptation of Populus trichocarpa to saline conditions (Figure 1). The suppression of salt tolerance by PtEXPA6 was mainly due to excessive Na+ accumulation, which resulted in a reduced ability to maintain photosynthesis and ROS homeostasis in the transgenic poplars (Figure 5, Figure 6, Figure 7 and Figure 8).
3.2. PtEXPA6 Increases Na+ Transport from Root to Shoot under Salt Stress
The PtEXPA6-overexpressing lines of 84K and 717-1B4 showed greater Na+ accumulation in roots, stems, and leaves compared to the WT poplars (Figure 9). The accumulation of Na+ ions was due to the increased radial salt translocation in the roots and the subsequent salt transport from the root to the shoot, as the Na+ content was positively correlated to the Na+ fluxes in roots and leaf petiole (Tables S3 and S4). The flux data showed that the Na+ efflux of the root xylem increased dramatically in the PtEXPA6-overexpressing lines of both poplars (Figure 11). This indicates that the transgenic poplars had a reduced ability to limit the radial translocation of Na+ into the xylem under salt stress. The increased radial translocation of Na+ salt into the xylem would lead to a higher rate of salt transport to the shoots, as the Na+ flux of the vascular bundles in the petiole increased significantly under salt conditions (Figure 12). The PtEXPA6-stimulated Na+ translocation in roots and shoots was probably due to the CW loosening, which facilitated Na+ transport in the apoplast. In accordance, the overexpression of PtEXPA6 increased the CW extensibility in poplar roots (Figure 10). The CW loosening would lead to an increase in CW volume and hydraulic conductivity, stimulating the flow of solution with high Na+ concentration. This probably increases the radial translocation of Na+ salt into the xylem and the subsequent salt transport to the shoots. The apoplastic pathway has been shown to be responsible for up to 50% of the total Na+ and Cl− uptake by the root [32,33]. It is noteworthy that the PtEXPA6-promoted radial translocation of Na+ in roots and root-to-shoot transport were both restricted by the application of 300 mOsmol kg−1 mannitol (Figure 11 and Figure 12), which induces the contraction and hardening of the cell walls (Figure 10) [29,30]. Furthermore, the Na+ transport was resumed when the contracted roots were exposed to a 0.1 MPa osmotic jump (Figure 11 and Figure 12), which induces the extension of the cell walls (Figure 10). Collectively, this transient Na+ kinetics in response to a 0.1 MPa osmotic jump demonstrated that CW loosening favors the apoplastic Na+ transport, while a CW contraction restricts the radial translocation of Na+ in roots and root-to-shoot transport. The wild-type 84K and 717-1B4 showed a lower contractibility and extensibility than the transgenic lines, this could benefit the salt-stressed poplars to restrict the radial and longitudinal transport of sodium ions (Figure 10, Figure 11 and Figure 12). Consistent with this, the long-term salt-stimulated CW stiffening, observed at high solution ionic strength, contributes to the decrease in CW swelling capacity [34]. The resulting decrease in CW volume and hydraulic conductivity restricts the flow of solution with high Na+ concentration. This probably allows the root cells to adapt to the stress conditions and prevents Na+ and Cl− from entering the xylem [35]. In our study, the Na+ transport stimulated by PtEXPA6 contradicts the findings that wheat TaEXPA2 and rice OsEXPA7 lead to a reduction in Na+ in the leaves and roots under salt stress [17,19]. These contrasting results suggest that expansin proteins play different roles in regulating the ionic relations and salt tolerance of salinized plants. It has been suggested that expansins confer this ability to remodel cell wall composition and maintain cell wall flexibility in roots under NaCl stress, contributing to improved root architecture and salt tolerance [16]. In the OsEXPA7-OX rice plants, the longer metaxylem cells in the primary roots showed that cell elongation occurs through expansin-mediated cell wall loosening [17]. Similarly, the transgenic RhEXPA4 plants had longer primary roots and more lateral roots under salt stress [16]. It is assumed that the longer metaxylem cells can take up more water than the WT plants, thus reducing Na+ concentration in both the cytoplasm and vacuoles [17]. However, our results show that the overexpression of PtEXPA6 leads to a loosening of the cell wall, which facilitates the radial translocation of Na+ into the root xylem and the subsequent Na+ translocation from the roots into the leaves (Figure 10, Figure 11 and Figure 12). The correlation analysis revealed that in the two poplars, particularly 717-1B4, Na+ content and flux showed a positive correlation to relative electrolyte leakage but are usually negatively correlated with stem growth, gas exchange, chlorophyll fluorescence, and activity and transcription of antioxidant enzymes (Tables S3 and S4). Therefore, the downregulation of PtEXPA6 would result in the restriction of Na+ transport and favor Populus trichocarpa in maintaining growth, photosynthesis, and ROS homeostasis under saline conditions (Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8).
3.3. PtEXPA6 Influences Photosynthesis under Salt Stress
The long-term salinity resulted in a more pronounced reduction in Pn in the transgenic lines of 84K (L11, L12, and L13) and 717-1B4 (L9, L15, and L16) compared to the WT (Figure 5). The salt-decreased photosynthetic capacity partly resulted from the decrease in stomatal conductance as a positive correlation was observed between Pn and stomatal conductance (Figure 5, Tables S3 and S4). Additionally, the salt-reduced activity of the PSII reaction center and decrease in ETR might also affect the photosynthetic process in the PtEXPA6-transgenic poplars as Pn positively correlated with chlorophyll fluorescence (Figure 6, Tables S3 and S4). We have previously shown that the excessive accumulation of salt ions leads to decreased stomatal conductance and photosynthetic capacity [36,37]. It is possible that PtEXPA6 enhanced salt transport from roots to shoots, and the great buildup of Na+ in the chloroplasts of the transgenic poplars of 84K and 717-1B4 exhibit direct and indirect restrictions on dark and light reactions, as Na+ content and fluxes are negatively correlated with gas exchange and chlorophyll fluorescence, in particular clone 717-1B4 (Figure 9, Tables S3 and S4).
3.4. PtEXPA6 Influences ROS Scavenging Capacity under Salt Stress
NaCl caused significant activation of antioxidant enzymes, POD, SOD, and CAT, in the WT and transgenic poplars (Figure 7). This was mainly due to the salt-increased transcripts of CAT1 and PODa2 (Figure 8), as the enzymic activity was positively correlated with the transcript abundance of the antioxidant enzymes (Tables S3 and S4). However, in the salt-stressed poplars, the activity and transcription of the antioxidant enzymes were typically lower in the PtEXPA6-overexpressing lines (Figure 7 and Figure 8). The less stimulated activity and transcription of antioxidant enzymes resulted in the inability to efficiently remove salt-induced ROS during long-term salinity. In accordance, REL was significantly higher in the transgenic 84K and 717-1B4 than in the WT poplars (Figure 7). Our results are consistent with the finding that the overexpression of wheat TaEXPA2 or Chenopodium quinoa CqEXPA50 improves the salt tolerance of transgenic plants by enhancing the enzymatic antioxidant system [19,20]. Apparently, the lower activation of the antioxidant enzymes in the PtEXPA6-overexpressing lines was at least partly the result of the excessive accumulation of salt ions, which led to increased ROS production and decreased ROS scavenging capacity [36,37]. Here, Na+ content and fluxes also showed a negative correlation with the activity and transcription of antioxidant enzymes (Tables S3 and S4). Consistent with this, we previously found that salt exposure in P. popularis leaves increased the activity of ascorbate peroxidase (APX), CAT, and glutathione reductase (GR) [36,37]. However, the salt-produced ROS exceeded the antioxidant capacity of the enzymatic system, leading to oxidative damage in the salt-sensitive poplars [36,37].
4. Materials and Methods
4.1. Total RNA Isolation, PtEXPA6 Cloning, and Sequence Analysis
Total RNA was isolated from the leaves of Populus trichocarpa using the E.Z.N.A.TM Plant RNA Kit (Omega Bio-Tek, Guangzhou, China). The upper leaves (3rd to 8th from the top) were collected from the soil-cultured plants. The removal of genomic DNA and the synthesis of the first strand of cDNA were performed using the reverse transcriptase kit HiFiScript gDNA Removal RT Master Mix (CoWin Biotech, Taizhou, China). PtEXPA6 was cloned by PCR amplification and the reaction mixture (50 µL) contained 2 µL cDNA product, 25 µL KOD One™ PCR Master Mix (TOYOBO, OSAKA, JAPAN) and 1 µL specific primers (10 µM), 5′-ATGGCAATGAGCAGTTTAA-3′ (forward), and 5′-GACCCTGAAATTCTTGCCGG-3′ (reverse). The multiple sequence alignments of the EXPA proteins were performed using ClustalW (http://www.genome.jp/tools/clustalw/, EMBL-EBI, Hinxton, Cambridgeshire, UK, accessed on 18 August 2020). The phylogenetic tree was created using the software MEGA11 (http://www.megasoftware.net, the Centre for Evolutionary Medicine and Informatics, Tempe, AZ, USA, accessed on 16 February 2023). The accession numbers of the expansin orthologs used for the multiple sequence alignment and phylogenetic analysis are listed in Supplementary Table S1.
4.2. PtEXPA6 Overexpression in Poplars
The coding sequence of PtEXPA6 was ligated into the pART-CAM-FLAG vector, in which the Xho I and Xba I sites were driven by the cauliflower mosaic virus (CaMV) 35S promoter. Subsequently, the PtEXPA6 overexpression construct was transferred into Agrobacterium tumefaciens (strain GV3101) for plant transformation. A total of 10 transgenic lines of Populus alba × P. tremula var. glandulosa (84K) and 16 lines of Populus tremula × P. alba INRA ‘717-1B4’ were obtained and used for PCR detection. Five transgenic lines of 84K and six transgenic lines of 717-1B4 were confirmed with a Western blot analysis. The 1/2MS solid medium was used to propagate regenerated plantlets, and the medium was supplemented with 6 g of agar, 30 g of sucrose, 0.05 mg/L of IBA, and 0.02 mg/L of NAA, and pH was adjusted to 5.8. The light intensity in the tissue culture room was 100–200 μmol m−2s−1 with the photoperiod being 16 h light/8 h dark. The relative humidity was 75%, and the temperature was 22 ± 1 °C. Poplar plantlets (5–10 cm high) were transplanted into individual pots (1000 mL) for soil culture. The matrix was a mixture of peat soil and quartz sand (peat soil/quartz sand = 1:1). The soil culture conditions were the same as those in the culture room.
4.3. Western Blotting
The Western blotting of PtEXPA6 was performed as previously described [38]. In brief, total protein was isolated from the leaf samples with an extraction buffer (50 mM Tris-MES, pH 7.5, 80 mM NaCl, 10 mM MgCl2, 10% glycerol, 0.2% NP-40, 1 mM EDTA, 1 mM PMSF, and protease inhibitor cocktail [Roche (China) Holding Ltd. Shanghai, China]). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and the subsequent immunoblotting were performed according to standard procedures. PtEXPA6 protein immunoblots were performed using mouse monoclonal anti-FLAG antibody (Abclonal, Wuhan, China, cat no: AE005) at 1:5000 dilution. Actin (26F7) mAb for PLANTs (Abmart, Shanghai, China, cat no: M20009L) was used to detect endogenous actin protein, which served as a loading control. Secondary HRP-conjugated Goat anti-Mouse IgG (H + L) (Abclonal, Wuhan, China, cat no: AS003) was used at 1:5000 for detection via the eECL Western Blot Kit (CoWin Biosciences, Beijing, China, cat no: CW0049S). Protein gel blots were imaged by the ChemiDoc MP system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The Western blotting was repeated three times and similar results were obtained.
4.4. Comparative Extensibility and Contractility of Root Tips
To determine the loosening of the cell wall promoted by PtEXPA6, the comparative extensibility and contractility of intact root tip tissue were measured in wild-type and transgenic poplars [25,26]. The comparative contractility was determined by assessing the initial root contraction elongation rate (1 min) induced by 300 mOsmol kg−1 mannitol (−0.75 MPa). The comparative elongation capacity of the growing tip tissue was then directly determined by an osmotic jump method [25,26]. Briefly, the wild-type and PtEXPA6-overexpressing lines of 84K (L11, L12, and L13) and 717-1B4 (L9, L15, and L16) were grown hydroponically in 1/2 MS culture medium for one week. The primary root of an intact plant was fixed on a Petri dish before the addition of 300 mM mannitol (−0.75 MPa). Osmotically induced changes in the position of the growing tip began within 20 s of the addition of mannitol, and the determination of the initial rate of mannitol-induced contraction was completed after 20–30 s to minimize deviation from initial conditions. The comparative contractility is the difference between the root apex length and the initial root apex length per unit time after mannitol treatment and is expressed in µm min−1 0.75 MPa−1. Comparative extensibility was then determined by assessing the 1 min initial increase in root elongation rate induced by an osmotic jump of 0.1 MPa [25]. The comparative elongation rate is the difference between the length of the apical site per unit time of elongation and the length of the initial apical site, expressed in µm min−1 0.1 MPa−1. Changes in the root tip position were observed using an ML31 biomicroscope (Mshot, Guangzhou, China) and determined using an Mshot Image Analysis System (Mshot, Guangzhou, China). Three root tips were measured for each plant, and three independent individuals were biologically replicated for each treatment.
4.5. Phenotype Test of Salt Tolerance
4.5.1. Measurement of Growth
Uniform plants of the wild-type (WT) and PtEXPA6-overexpressing lines of 84K (L11, L12, and L13) and 717-1B4 (L9, L15, and L16) were treated with NaCl saline (0 or 100 mM) for 15 days. The light intensity in the tissue culture room was 100–200 μmol m−2s−1 with the photoperiod being 16 h light/8 h dark. The relative humidity was 75%, and the temperature was 22 ± 1 °C. Shoot height and stem diameter were measured at the end of the experiment. The relative growth of the stem height and diameter during the observation period was calculated as folllows: (H1−H0)/H0 or (D1−D0)/D0. H1 (cm) and H0 (cm) are the stem height at the end and beginning of the salt treatment. D1 (mm) and D0 (mm) are the stem diameter at the end and beginning of the salt treatment. For the WT and transgenic lines of two poplars, six individual plants were established for the control and salt treatment.
4.5.2. Measurement of the Relative Electrolyte Leakage (REL)
The upper leaves of the shoot (3rd to 8th from the top) were sampled from the WT and PtEXPA6-overexpressing lines of 84K (L11, L12, and L13) and 717-1B4 (L9, L15, and L16) after 15 days of NaCl treatment (0 or 100 mM). The REL was calculated from the initial relative conductivity (EC1) before boiling and the final conductivity (EC2) after boiling: REL (%) = (EC1/EC2) × 100%. Three to four individual plants of each genotype were used for each treatment.
4.5.3. Measurement of Leaf Gas Exchange and Chlorophyll Fluorescence
Leaf gas exchange and chlorophyll fluorescence were measured after the WT poplar and the PtEXPA6-overexpressing lines of 84K (L11, L12, and L13) and 717-1B4 (L9, L15, and L16) were treated with NaCl (0 or 100 mM) for 15 days. The net photosynthetic rate (Pn, μmol m−2s−1), transpiration rate (E, mmol m−2s−1), and stomatal conductance (Cleaf, mmol m−2s−1) of the upper mature leaves (6th–8th from the top) were measured using a portable open gas exchange system, the LI-6400 (Li-Cor, Inc., Lincoln, NE, USA). The gas exchange was measured with light intensity at 150 μmol m−2s−1, relative humidity 75%, and temperature was 22 ± 1 °C. The maximum photochemical efficiency of PSII (Fv/Fm), the actual photosynthetic quantum yield (YII), and the relative electron transport rate (ETR) were analyzed with a pulse-amplitude-modulated (PAM) chlorophyll fluorometer, the JUNIOR-PAM (HeinzWalz GmbH, Effeltrich, Germany) [39]. The leaves were dark-adapted for 20 min, and fluorescence was measured at 22 ± 1 °C.
4.6. Determination of Antioxidant Enzyme Activity
The WT and PtEXPA6-overexpressing lines of 84K (L11, L12, and L13) and 717-1B4 (L9, L15, and L16) were salinized with NaCl (0 or 100 mM) for 15 days. Samples were taken from the leaves (3rd to 8th from the top) and used to measure the total activity of antioxidant enzymes. POD, SOD, and CAT activity (U g−1) was analyzed using assay kits for POD (BC0090), CAT (BC0205), and SOD (BC0175), respectively (Beijing Solarbio Science & Technology, Beijing, China) [39]. One unit of SOD was defined as the amount of enzyme causing 50% inhibition of the reaction compared with a blank sample. The unit of CAT activity was defined as the consumption of 1 μmol H2O2 per minute per gram of plant tissue. One unit of enzyme activity of POD was defined as the change in absorbance at 470 nm by 0.01 in a milliliter of the reaction system per minute per gram of tissue.
4.7. Na+ Concentration in the Roots, Leaves and Stems
Roots, stems, and leaves were collected from the soil-cultured WT and PtEXPA6-overexpressing lines of 84K (L11, L12, and L13) and 717-1B4 (L9, L15, and L16) after 15 days of salt treatment (0 or 100 mM NaCl). The oven-dried samples (60 °C, 5 days) were digested with H2SO4-H2O2 and used for the Na+ content (mmol g−1 DW) determination with an atomic absorption spectrometer (Varian SpectrAA 220FS, Palo Alto, CA, USA) [39]. Three to four individual plants of each genotype were used for each treatment.
4.8. Flux Records of Na+ in the Root Xylem and Leaf Petiole Vascular Bundle
The net Na+ flux (pmol cm−2s−1) in the root xylem was recorded using a non-invasive micro-test system (NMT-YG-100, Younger USA, LLC, Amherst, MA, USA) [40]. After short-term salt exposure (200 mM, 4 h), the roots with tips and mature zones of approximately 10–15 cm in length were collected from the control and salt-stressed poplars of WT and PtEXPA6-overexpressing lines 84K (L11, L12, and L13) and 717-1B4 (L9, L15, and L16). The roots were equilibrated for 30 min in a measuring solution (0.1 mM NaCl, 0.1 mM CaCl2, 0.1 mM MgCl2, and 0.5 mM KCl, pH 5.7). The selective microelectrodes for Na+ were calculated and used to monitor the net flux of Na+ in the xylem of the maturation zone. Continuous recordings were made at each measurement point for 5–8 min and the average flux was calculated. Three to four individual plants of each genotype were used for flux recording. The response of the root xylem Na+ flux to osmotic jump was also examined in WT and transgenic poplars. After the NaCl treatment as described above, 300 mOsmol kg−1 mannitol (−0.75 MPa) was added to a measuring solution, and real-time Na+ flux was recorded in the root xylem. Thereafter, the concentration of mannitol in the measuring solution was routinely diluted with an 0.10 MPa increase in external osmotic potential, and Na+ flux kinetics was immediately measured in the root xylem.
Real-time translocation kinetics of Na+ flux from vascular bundles in the petiole was performed as described previously [31]. In brief, the entire root system of intact plants was exposed to a short-term salt stress (200 mM NaCl, 4 h). Then, the petiole was immobilised in the measurement chamber after the leaf blade was removed. The Na+ flux of the vascular bundles in the petiole was immediately measured with NMT microelectrodes as described above. The response of the Na+ flux in the leaf petiole vascular bundles to osmotic jump was also examined in the WT and transgenic poplars. After 300 mOsmol kg−1 mannitol was added to the NaCl-treated roots, Na+ flux kinetics was observed in the leaf petiole vascular bundle. Thereafter, the concentration of mannitol was diluted with an 0.10 MPa increase in external osmotic potential, and the Na+ flux kinetics was immediately measured in the leaf petiole vascular bundles. Three to four individual plants of each genotype were used for the flux recording of the petiole vascular bundles.
4.9. RT-qPCR Analysis
P. trichocarpa was subjected to treatment with 0 or 100 mM NaCl for 48 h. The roots, stem, and mature leaves in the upper shoot were collected after 0, 3, 6, 12, 24, and 48 h of NaCl treatment and used for the RT-qPCR analysis of PtEXPA6. The transcripts of PODa2, SOD [Cu-Zn], and CAT1 in the WT and transgenic poplars were analyzed under the control and NaCl treatments (100 mM, 15 days). RNA isolation from P. trichocarpa, 84K, and 717-1B4 was performed using the E.Z.N.A.TM Plant RNA Kit (Omega Bio-Tek, Guangzhou, China). Subsequently, the RNA (1 µg) was used for reverse transcription with Moloney murine leukemia virus (M-MLV) reverse transcriptase and an oligo (dT) primer (Promega, Madison, WI, USA) according to the manufacturer’s recommended protocol. RT-qPCR was performed using the LineGene 9600 Plus (Bioer Technology, Hangzhou, China) and UBQ served as an internal control for P. trichocarpa, 84K and 717-1B4 [41]. Three individual biological replicates were generated for each treatment.
4.10. Data Analysis
Na+ fluxes were calculated using JCal V3.2.1, a free MS Excel spreadsheet developed by Yue Xu (http://www.xuyue.net/, accessed on 5 May 2024). All the experimental data were statistically analyzed using SPSS version 19.0 (IBM Corporation, Armonk, NY, USA). The correlation analysis and principal component analysis (PCA) were also performed using the SPSS software (version 19.0). The one-way ANOVA method was used to compare mean values between the treatments. p < 0.05 or p < 0.01 was considered a significant difference unless otherwise stated.
5. Conclusions
In summary, the overexpression of PtEXPA6 reduces plant growth, photosynthetic capacity, and ROS scavenging capacity under salt stress, which is due to the excessive Na+ accumulation in roots and shoots. The PtEXPA6-transgenic poplars exhibited a more pronounced increase in the radial translocation of Na+ salt into the root xylem and the translocation of Na+ from roots to leaves under salt stress. Moreover, PtEXPA6 increased the root contractability and extensibility in transgenic poplars. Therefore, we hypothesize that the overexpression of PtEXPA6 results in cell wall loosening, which leads to an increase in CW volume and hydraulic conductivity, stimulating the flow of solution with high Na+ concentration. As a result, the radial translocation of Na+ into the root xylem and the subsequent Na+ translocation from roots to leaves were consequently increased under salt stress, resulting in excessive Na+ accumulation and reduced salt tolerance. Therefore, the downregulation of PtEXPA6 in the NaCl-treated Populus trichocarpa would lead to a restriction of Na+ accumulation, thus favoring the maintenance of photosynthesis and ROS homeostasis under saline conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25179354/s1.
Author Contributions
Z.L.: investigation, data curation, validation, visualization, and writing—original draft. K.Y.: investigation, validation, data curation, and visualization. Y.Z.: investigation, methodology, and visualization. C.Y.: investigation, methodology, and visualization. Z.Z.: investigation, methodology, and visualization. J.L. (Jing Li): investigation and methodology. Y.L.: investigation and methodology. B.F.: investigation and methodology. R.Z.: investigation and methodology. J.L. (Jian Liu): investigation and methodology. K.D.: investigation and methodology. J.Y.: investigation and methodology. N.Z.: methodology and resources. X.Z.: resources. S.C.: conceptualization, supervision, funding acquisition, project administration, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
The research was supported jointly by the National Natural Science Foundation of China (grant nos. 32371828, 32071730, and 31770643).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.McQueen-Mason S.J., Durachko D.M., Cosgrove D.J. Two endogenous proteins that induce cell wall extension in plants. Plant Cell. 1992;4:1425–1433. doi: 10.1105/tpc.4.11.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McQueen-Mason S.J., Cosgrove D.J. Disruption of hydrogen- bonding between plant cell wall polymers by proteins that induce wall extension. Proc. Natl. Acad. Sci. USA. 1994;91:6574–6578. doi: 10.1073/pnas.91.14.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tenhaken R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015;5:771. doi: 10.3389/fpls.2014.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li F., Xing S.C., Guo Q.F., Zhao M.R., Zhang J., Gao Q., Wang G.P., Wang W. Drought tolerance through over-expression of the expansin gene TaEXPB23 in transgenic tobacco. J. Plant Physiol. 2011;168:960–966. doi: 10.1016/j.jplph.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Narayan J.A., Chakravarthi M., Nerkar G., Manoj V.M., Dharshini S., Subramonian N., Premachandran M.N., Kumar R.A., Surendar K.K., Hemaprabha G., et al. Overexpression of expansin EaEXPA1, a cell wall loosening protein enhances drought tolerance in sugarcane. Ind. Crops Prod. 2021;159:113035. doi: 10.1016/j.indcrop.2020.113035. [DOI] [Google Scholar]
- 6.Zhang H., Ding Y.N., Zhi J.K., Li X.Y., Liu H.B., Xu J.C. Over-expression of the poplar expansin gene PtoEXPA12 in tobacco plants enhanced cadmium accumulation. Int. J. Biol. Macromol. 2018;116:676–682. doi: 10.1016/j.ijbiomac.2018.05.053. [DOI] [PubMed] [Google Scholar]
- 7.İncili Ç.Y., Arslan B., Çelik E.N.Y., Ulu F., Horuz E., Çağlıyan M.C.B.E., Burcu G., Bayarslan A.U., Altunoglu Y.C. Comparative bioinformatics analysis and abiotic stress responses of expansin proteins in Cucurbitaceae members: Watermelon and melon. Protoplasma. 2023;260:509–527. doi: 10.1007/s00709-022-01793-8. [DOI] [PubMed] [Google Scholar]
- 8.Liu H.B., Li H.Y., Zhang H., Li J., Xie B.L., Xu J.C. The expansin gene PttEXPA8 from poplar (Populus tomentosa) confers heat resistance in transgenic tobacco. Plant Cell Tissue Organ Cult. 2016;126:353–359. doi: 10.1007/s11240-016-1003-8. [DOI] [Google Scholar]
- 9.Han Z.S., Liu Y.L., Deng X., Liu D.M., Hu Y.K., Yan Y.M. Genome-wide identification and expression analysis of expansin gene family incommon wheat (Triticum aestivum L.) BMC Genom. 2019;20:101. doi: 10.1186/s12864-019-5455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L.J., Zou W.S., Fei C.Y., Wu G., Li X.Y., Lin H.H., Xi D.H. α-Expansin EXPA4 positively regulates abiotic stress tolerance but negatively regulates pathogen resistance in Nicotiana tabacum. Plant Cell Physiol. 2018;59:2317–2330. doi: 10.1093/pcp/pcy155. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H., Xu Q., Xu X., Liu H.B., Zhi J.K., Xu J.C. Transgenic tobacco plants expressing grass AstEXPA1 gene show improved performance to several stresses. Plant Biotechnol. Rep. 2017;11:331–337. [Google Scholar]
- 12.Yan A., Wu M.J., Yan L.M., Hu R., Ali I., Gan Y.B. AtEXP2 is involved in seed germination and abiotic stress response in Arabidopsis. PLoS ONE. 2014;9:e85208. doi: 10.1371/journal.pone.0085208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang R.X., Yang L.H., Wang X., Wang Y., Zhang J.K., Xu J.C. Over-expression of the Salix matsudana expansin gene SmEXPA23 enhances plant salt tolerance. Plant Cell Tissue Organ Cult. 2023;152:309–316. doi: 10.1007/s11240-022-02407-0. [DOI] [Google Scholar]
- 14.Zhang H., Liu H.B., Yang R.X., Xu X., Liu X., Xu J.C. Over-expression of PttEXPA8 gene showed various resistances to diverse stresses. Int. J. Biol. Macromol. 2019;130:50–57. doi: 10.1016/j.ijbiomac.2019.02.115. [DOI] [PubMed] [Google Scholar]
- 15.Marowa P., Ding A.M., Xu Z.C., Kong Y.Z. Overexpression of NtEXPA11 modulates plant growth and development and enhances stress tolerance in tobacco. Plant Physiol. Biochem. 2020;151:477–485. doi: 10.1016/j.plaphy.2020.03.033. [DOI] [PubMed] [Google Scholar]
- 16.Lü P.T., Kang M., Jiang X.Q., Dai F.W., Gao J.P., Zhang C.Q. RhEXPA4, a rose expansin gene, modulates leaf growth and confers drought and salt tolerance to Arabidopsis. Planta. 2013;237:1547–1559. doi: 10.1007/s00425-013-1867-3. [DOI] [PubMed] [Google Scholar]
- 17.Jadamba C., Kang K., Paek N.C., Lee S.I., Yoo S.C. Overexpression of rice expansin7 (OsEXPA7) confers enhanced tolerance to salt stress in rice. Int. J. Mol. Sci. 2020;21:454. doi: 10.3390/ijms21020454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han Y.Y., Li A.X., Li F., Zhao M.R., Wang W. Characterization of a wheat (Triticum aestivum L.) expansin gene, TaEXPB23, involved in the abiotic stress response and phytohormone regulation. Plant Physiol. Biochem. 2012;54:49–58. doi: 10.1016/j.plaphy.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y.H., Han Y.Y., Kong X.Z., Kang H.H., Ren Y.Q., Wang W. Ectopic expression of wheat expansin gene TaEXPA2 improved the salt tolerance of transgenic tobacco by regulating Na+/K+ and antioxidant competence. Physiol. Plant. 2017;159:161–177. doi: 10.1111/ppl.12492. [DOI] [PubMed] [Google Scholar]
- 20.Sun W.J., Yao M., Wang Z., Chen Y., Zhan J.Y., Yan J., Jiang S.Q., Jian S.S., Chen H., Bu T.L., et al. Involvement of auxin-mediated CqEXPA50 contributes to salt tolerance in Quinoa (Chenopodium quinoa) by interaction with auxin pathway genes. Int. J. Mol. Sci. 2022;23:8480. doi: 10.3390/ijms23158480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon Y.R., Lee H.J., Kim K.H., Hong S.W., Lee S.J., Lee H. Ectopic expression of Expansin3 or Expansinβ1 causes enhanced hormone and salt stress sensitivity in Arabidopsis. Biotechnol. Lett. 2008;30:1281–1288. doi: 10.1007/s10529-008-9678-5. [DOI] [PubMed] [Google Scholar]
- 22.Gao X., Liu K., Lu Y.T. Specific roles of AtEXPA1 in plant growth and stress adaptation. Russ. J. Plant Physl. 2010;57:254–259. doi: 10.1134/S1021443710020111. [DOI] [Google Scholar]
- 23.Xu Q., Burgess P., Xu J.C., Meyer W., Huang B.R. Osmotic stress- and salt stress-inhibition and gibberellin-mitigation of leaf elongation associated with up-regulation of genes controlling cell expansion. Environ. Exp. Bot. 2016;131:101–109. doi: 10.1016/j.envexpbot.2016.06.001. [DOI] [Google Scholar]
- 24.Polle A., Chen S.L. On the salty side of life: Molecular, physiological and anatomical adaptation and acclimation of trees to extreme habitats. Plant Cell Environ. 2015;38:1794–1816. doi: 10.1111/pce.12440. [DOI] [PubMed] [Google Scholar]
- 25.Neumann P.M., Leon D. Physical restraints underlying short-term inhibition by auxin of root elongation in intact maize seedlings. J. Plant Growth Regul. 1992;11:119–123. doi: 10.1007/BF00198024. [DOI] [Google Scholar]
- 26.Neumann P., Azaizeh H., Leon D. Hardening of root cell walls: A growth inhibitory response to salinity stress. Plant Cell Environ. 1994;17:303–309. doi: 10.1111/j.1365-3040.1994.tb00296.x. [DOI] [Google Scholar]
- 27.Chen S.L., Li J.K., Fritz E., Wang S.S., Hüttermann A. Sodium and chloride distribution in roots and transport in three poplar genotypes under increasing NaCl stress. For. Ecol. Manag. 2002;168:217–230. doi: 10.1016/S0378-1127(01)00743-5. [DOI] [Google Scholar]
- 28.Chen S.L., Li J.K., Wang S.S., Fritz E., Hüttermann A., Altman A. Effects of NaCl on shoot growth, transpiration, ion compartmentation, and transport in regenerated plants of Populus euphratica and Populus tomentosa. Can. J. For. Res. 2003;33:967–975. doi: 10.1139/x03-066. [DOI] [Google Scholar]
- 29.Kuzmanoff K.M., Evans M.L. Kinetics of adaptation of osmotic stress in lentil (Lens culinaris Med.) roots. Plant Physiol. 1981;68:244–247. doi: 10.1104/pp.68.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsaio T.C., Jing J. Leaf and root expansive growth in response to water deficits. In: Cosgrove D.J., Knievel D.P., editors. Physiology of Cell Expansion During Plant Growth. American Society for Plant Physiology; Rockville, MD, USA: 1987. pp. 180–192. [Google Scholar]
- 31.Sun J., Wang R.G., Liu Z.Q., Ding Y.Z., Li T.Q. Non-invasive microelectrode cadmium flux measurements reveal the spatial characteristics and real-time kinetics of cadmium transport in hyperaccumulator and nonhyperaccumulator ecotypes of Sedum alfredii. J. Plant Physiol. 2013;170:355–359. doi: 10.1016/j.jplph.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Kronzucker H.J., Britto D.T. Sodium transport in plants: A critical review. New Phytol. 2011;189:54–81. doi: 10.1111/j.1469-8137.2010.03540.x. [DOI] [PubMed] [Google Scholar]
- 33.Shi Y., Wang Y., Flowers T.J., Gong H. Silicon decreases chloride transport in rice (Oryza sativa L.) in saline conditions. J. Plant Physiol. 2013;170:847–853. doi: 10.1016/j.jplph.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Meychik N.R., Yermakov I.P., Khonarmand S.D., Nikolaeva Y.I. Ion-exchange properties of cell walls in chickpea cultivars with different sensitivities to salinity. Russ. J. Plant Physl. 2010;57:620–630. doi: 10.1134/S1021443710050043. [DOI] [Google Scholar]
- 35.Meychik N., Nikolaeva Y., Kushunina M. The significance of ion-exchange properties of plant root cell walls for nutrient and water uptake by plants. Plant Physiol. Biochem. 2021;166:140–147. doi: 10.1016/j.plaphy.2021.05.048. [DOI] [PubMed] [Google Scholar]
- 36.Wang R.G., Chen S.L., Zhou X.Y., Shen X., Deng L., Zhu H.J., Shao J., Shi Y., Dai S.X., Fritz E., et al. Ionic homeostasis and reactive oxygen species control in leaves and xylem sap of two poplars subjected to NaCl stress. Tree Physiol. 2008;28:947–957. doi: 10.1093/treephys/28.6.947. [DOI] [PubMed] [Google Scholar]
- 37.Wang R.G., Chen S.L., Deng L., Fritz E., Hüttermann A., Polle A. Leaf photosynthesis, fluorescence response to salinity and the relevance to chloroplast salt compartmentation and anti-oxidative stress in two poplars. Trees. 2007;21:581–591. doi: 10.1007/s00468-007-0154-y. [DOI] [Google Scholar]
- 38.Zhang H.L., Deng C., Wu X., Yao J., Zhang Y.L., Zhang Y.N., Deng S.R., Zhao N., Zhao R., Zhou X.Y. Populus euphratica remorin 6.5 activates plasma membrane H+-ATPases to mediate salt tolerance. Tree Physiol. 2020;40:731–745. doi: 10.1093/treephys/tpaa022. [DOI] [PubMed] [Google Scholar]
- 39.Li J., Zhao R., Liu J., Yao J., Ma S., Yin K., Zhang Y., Liu Z., Yan C., Zhao N., et al. Populus euphratica GRP2 interacts with target mRNAs to negatively regulate salt tolerance by interfering with photosynthesis, Na+, and ROS homeostasis. Int. J. Mol. Sci. 2024;25:2046. doi: 10.3390/ijms25042046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun J., Dai S.X., Wang R.G., Chen S.L., Li N.Y., Zhou X.Y., Lu C.F., Shen X., Zheng X.J., Hu Z.M., et al. Calcium mediates root K+/Na+ homeostasis in poplar species differing in salt tolerance. Tree Physiol. 2009;29:1175–1186. doi: 10.1093/treephys/tpp048. [DOI] [PubMed] [Google Scholar]
- 41.Brunner A.M., Yakovlev I.A., Strauss S.H. Validating internal controls for quantitative plant gene expression studies. BMC Plant Biol. 2004;4:14. doi: 10.1186/1471-2229-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.