Abstract
Dependence of the primary antiviral immune response on costimulatory interactions between CD28/CD80-86 and between CD40/CD154 (CD40 ligand) has been correlated with the extent of viral replication in two models of systemic infection, lymphocytic choriomeningitis virus and vesicular stomatitis virus. To determine the role of these costimulatory interactions in the context of an acute cytolytic, but locally replicating viral infection, herpes simplex virus (HSV) infection was assessed in mice that had the CD28/CD80-86 or CD40/CD154 interactions disrupted either genetically or with blocking reagents (CTLA4Ig and MR1, respectively). CTLA4Ig treatment greatly reduced paralysis-free survival during primary acute HSV infection. This reflected an almost total ablation of the anti-HSV CD4+ and CD8+ T-cell responses due to anergy and reduced cell numbers, respectively. Disruption of CD40/CD154 interactions impaired survival, but the effect was less severe than that observed in CTLA4Ig-treated mice, with reductions observed in the CD4+ T-cell but not CD8+ T-cell responses. These two costimulatory pathways functioned in part independently, since disruption of both further impaired survival. The dependence on these costimulatory interactions for the control of primary HSV infection may represent a more widespread paradigm for nonsystemic viruses, which have restricted sites of replication and which employ immunoevasive measures.
Herpes simplex virus (HSV), a member of the alphaherpesvirus family, has a complex life cycle involving both lytic and latent phases, ultimately resulting in lifelong infection of the host. Replication of HSV occurs in the target tissues, e.g., epithelial cells and the nervous system, rather than systemically. This property, in combination with its ability to disrupt antigen presentation in fibroblasts (2, 21, 59, 63) and to impair cell maturation and migration in infected dendritic cells (DC) (48), presumably enables HSV to impede detection by the immune system. A protective immune response to HSV is critical in resolving the highly lytic primary HSV infection, since failure to do so can result in encephalitis and ultimately death, a condition observed in newborns and immunocompromised hosts (13). Although roles exist for cells of the innate immune system in controlling initial viral spread (1, 37, 58) and for the CD8+ cytotoxic T-lymphocyte (CTL) response in controlling viral infection in the central nervous system (22, 44, 51, 52), CD4+ T cells appear to be the most important cells in protection against primary HSV infection based on studies in CD4+ T-cell-depleted or -deficient mice (32, 38, 39, 56). Since the magnitude of the memory response correlates with that of the primary immune response (40), understanding the requirements for the initial protective response may contribute to understanding long-term immunity to HSV.
The antigen-specific response to a viral pathogen is initiated when a T cell recognizes a viral peptide presented in the context of major histocompatibility complex (MHC) on antigen-presenting cells (APC). This primary signal results in the activation of the T cell, the extent of activation being a function of both the affinity and duration of this interaction (23, 27). Low affinity or brief primary signals can result in insufficient T-cell activation unless augmented by secondary interactions called costimulatory signals (CS). The best-characterized CS determined to be important in the initiation of the immune response are the CD28/CD80-86 and CD40/CD154 (CD40 ligand) receptor-ligand interactions (9, 19, 41). In addition to their essential role in the development of the antigen-specific humoral response, they appear to facilitate T-cell activation in response to low-affinity or low-abundance antigens by lowering the threshold required for activation and by promoting survival of activated T cells (5, 6, 27, 53, 60).
A number of groups have studied the roles of these two CS in the immune responses to viral pathogens by exploring the effects of blocking CS in the well-characterized lymphocytic choriomeningitis virus (LCMV) and vesicular stomatitis virus (VSV) models in mice. Both of these models result in systemic infections, but the extents to which these viruses replicate differ greatly: LCMV replicates to high titers, while VSV replicates poorly. The dependency of the antiviral CD8+ T-cell responses on costimulation parallels the differences in titers. The primary CD8+ T-cell response to LCMV is largely intact, but the CD8+ T-cell response to VSV is impaired when these CS are blocked (3, 11, 27, 43). Antiviral CD4+ T-cell responses to both viruses are moderately dependent on these CS (62); however, the reduction in CD4+ T cells has a greater effect on the protective immune response to VSV, which is heavily dependent on antibody, compared to that of LCMV, which is solely dependent on CD8+ T cells.
To determine the relevance of costimulatory interactions in the context of an acute cytolytic but locally replicating viral infection, the roles of these two receptor-ligand interactions were assessed in mice infected with HSV. Using reagents which block the CD28/CD80-86 and CD40/CD154 interactions in combination with mice with genetic deficits in either CD28 or CD154, we observed the following: treatment of mice with CTLA4Ig greatly reduced paralysis-free survival during primary acute HSV infection, primarily due to an almost total ablation of the anti-HSV responses by both CD4+ and CD8+ T cells over the first 10 days of infection; and disruption of CD40/CD154 interactions had a less severe effect on outcome and primarily impaired the CD4+ T-cell response. Our results indicate that these CS are required for successful resolution of the primary HSV infection in mice and further highlight their roles in the generation of antiviral CD4+ and CD8+ T-cell responses.
MATERIALS AND METHODS
Reagents.
Soluble murine CTLA4Ig and a hamster immunoglobulin G (IgG) monoclonal antibody (MAb) to murine CD154 (MR1) were generously provided by Bristol-Myers Squibb, Inc. (31). Mice were treated intraperitoneally with 200 μg of CTLA4Ig, 200 μg of control isotype-matched antibody L6, 500 μg of MR1, or 500 μg control hamster IgG 24 h before and 48 and 96 h after infection. These doses are equal to or greater than those previously described to be effective at blocking the corresponding receptor-ligand interactions in vivo (16, 26, 31).
Mice.
All mice were of the H-2b haplotype. Wild-type C57BL/6 (B6) mice were obtained from Taconic or Jackson Laboratories. CD154−/− mice on a B6 × 129 background were obtained from Richard Flavell (Yale University) and compared to CD154 wild-type or CD154+/− littermates. B6 congenic CD28−/− mice were obtained from Jackson Laboratories. CD28−/− CD154−/− mice were generated by intercrossing of CD28−/− and CD154−/− mice and were compared to heterozygous littermate controls. Genotypes of mice were determined via PCR analysis of tail DNA. All mice were housed under specific-pathogen-free conditions.
Virus.
HSV type 1 (HSV-1; KOS strain) was grown and titered in Vero cells as described elsewhere (22). HSV antigen consisted of virus inactivated with UV light, and control antigen (mock) consisted of lysate of uninfected Vero cells (22). Virus stocks were kept at −80°C and thawed immediately prior to use.
Analysis of paralysis free-survival from HSV infection.
Mice were infected with 2.5 × 106 PFU/foot as described elsewhere (22, 55) and were evaluated daily for evidence of footpad lesions and hind limb paralysis. Mice that developed bilateral hind limb paralysis were immediately euthanized, as we have previously found that >80% die within 24 h (22). Statistical differences between groups were determined using life table analysis and log rank tests.
Analysis of HSV-specific cellular immune responses.
Mice were infected by intradermal injection into the hind footpads of 5 × 105 PFU of HSV in 50 μl of serum-free RPMI. Draining popliteal lymph node (LN) cells from HSV-infected mice were collected on days 4 to 8 and 10 after inoculation. Cells were cultured in Iscove's medium (Life Technologies) containing 10% fetal bovine serum 5 × 10−5 M 2-mercaptoethanol, and antibiotics (complete Iscove's medium). Intracellular gamma interferon (IFN-γ) staining was performed by the method of Flynn et al. (17). Briefly, cells were cultured for 6 h in the presence of brefeldin A (10 μg/ml) with or without 1 μM HSV gB peptide comprising amino acids 498 to 505 (SSIEFARL) (HSVgB498–505; United Biochemical Research, Inc., Seattle, Wash.). Parallel cultures of cells were stimulated for 6 to 8 h with UV-inactivated whole HSV (UVHSV), mock antigen, or anti-murine CD3 (1452C11 culture supernatant) plus 5 ng of phorbol myristate acetate per ml (αCD3+PMA). Brefeldin A (10 μg/ml) was added for the final 3 h. These cells were then analyzed for expression of CD4, CD8, and intracellular IFN-γ using three-color flow cytometric analysis. Anti-CD4, anti-IFN-γ, and anti-CD8α antibodies were from Caltag. Cells were also stained with MHC class I (MHC-I) Kb/HSVgB498–505 tetramer (NIAID Tetramer Core Facility, Emory University). Staining with tetramers was carried out at 37°C for 30 min on unstimulated cells. Profiles were acquired on a FACScan flow cytometer, and the data were analyzed using CELLQuest software (Becton-Dickinson Immunocytometry Systems, San Jose, Calif.). ANOVA (analysis of variance) was used for statistical analysis.
In vitro culture conditions and CD8 CTL assays.
LN cells were isolated from HSV-infected mice at the indicated days. Cells were cultured for 3 days in complete Iscove's medium with the presence or absence of recombinant interleukin-2 (IL-2; 5 ng/ml), CTLA4Ig (20 μg/ml), or rat anti-mouse IL-2 MAb (Pharmingen clone JES6-5H4; 10 μg/ml). After the culture period, cells were analyzed by FACScan or were tested for lytic function in CTL assays. A standard 5.5-h CTL assay was performed using 51Cr (100 μCi)-labeled EL4 or EL4-HSVgB (EL4 cells stably expressing HSV gB) as target cells. Targets were plated at 5 × 103 cells/well. All variables were tested in triplicate. Values indicated were derived as follows: 100 × (experimental release − spontaneous release)/(maximum release-spontaneous release). Spontaneous release was less than 12% in all assays.
RESULTS
Disruption of costimulatory interactions impairs the survival of mice infected with HSV-1.
To investigate the roles of the CD40/CD154 and CD28/CD80-86 receptor-ligand interactions in the control of a primary HSV-1 infection, we tested mice in which one or both of these costimulatory interactions were disrupted either genetically or via treatment with blocking reagents. Mice were infected in the hind footpads. In this model of infection, virus spreads from the footpad to the spinal ganglia and central nervous system. Infection is controlled by a T-cell-dependent mechanism that controls active viral replication between days 5 and 10 postinoculation. Failure to control infection leads to paralysis and death.
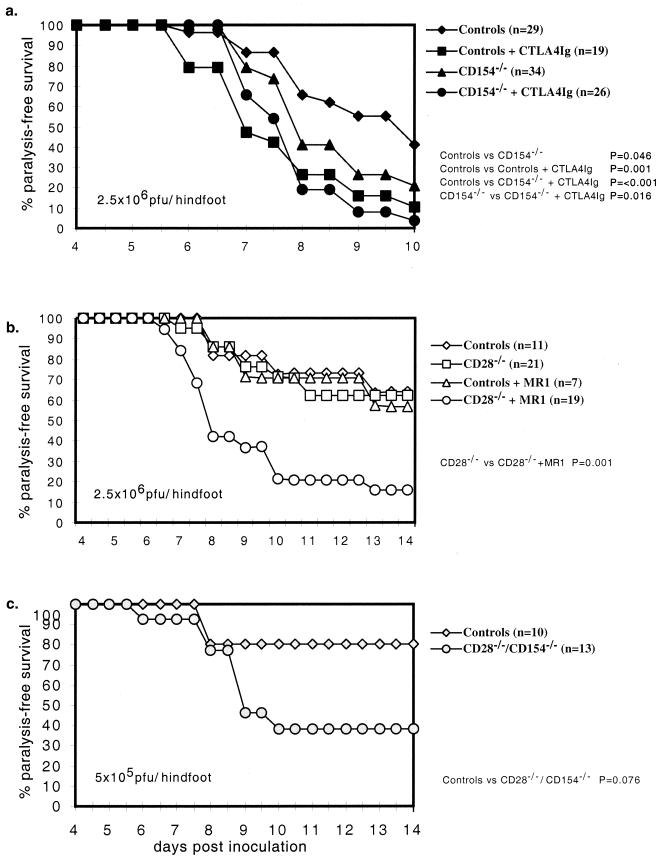
Paralysis-free survival was modestly impaired in CD154−/− mice compared to control mice (P = 0.046) (Fig. 1a). Treatment with murine CTLA4Ig, which efficiently blocks CD80 and CD86 interactions with CD28 (Fig. 1a), clearly impaired paralysis-free survival of wild-type (WT) mice (P = 0.001) and further impaired paralysis-free survival in CD154−/− mice (P = 0.016). As a complementary approach, we also evaluated CD28−/− mice treated with either control hamster Ig or MR1, a blocking MAb to CD154 (Fig. 1b). By contrast to the results for CTLA4Ig-treated mice, results for CD28−/− mice did not differ from controls (Fig. 1b). Nonetheless, the incidence of paralysis and death in CD28−/− mice treated with MR1 was 40% greater than in CD28−/− mice treated with hamster Ig (Fig. 1b, P = 0.001), whereas treatment of WT mice with MR1 did not impair their ability to control HSV infection. The failure of MR1 treatment to impair outcome in WT mice differs from the findings in CD154−/− mice. These results suggest that inhibition of CD154/CD40 interactions by MR1 was incomplete and insufficient to affect outcome in control mice but sufficient to impair outcome in CD28−/− mice. These findings also suggest that CD28−/− mice compensate for the genetic deficiency of CD28 by relying more heavily than WT mice on alternative CD154-mediated costimulatory pathways. To further test this notion, CD28−/− CD154−/− mice were infected with HSV (Fig. 1c). Compared to CD28+/− CD154+/− littermate control mice, paralysis-free survival was reduced >40% in CD28−/− CD154−/− mice (P = 0.076).
FIG. 1.
Outcome of HSV-1 (KOS) infection in mice which CD28/B7, CD40/CD154, or both interactions have been disrupted. Outcome was measured as the fraction of mice surviving without neurological impairment (paralysis or gross motor ataxia) over time in days after mice were inoculated via dermal abrasion with 2.5 × 106 (a and b) or 5 × 105 (c) PFU of HSV/hindfoot.
Together these findings indicate important and partly independent roles for these two costimulatory pathways in the control of primary HSV-1 infection in mice. Because genetic deficiency of CD154 and treatment with CTLA4Ig more effectively revealed the roles for CD40/CD154 and CD28/CD80-86 interactions in protection from primary HSV infection, these two approaches for disrupting CS were used to further explore the mechanisms by which they acted.
Costimulatory interactions play an important role in the CD4+ and CD8+ T-cell responses to HSV.
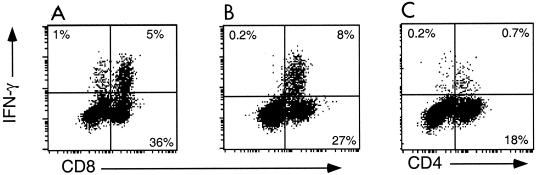
The HSV-specific T-cell response in the draining LN of mice peaks around days 5 to 6 postinfection and wanes dramatically by day 10 (14). To assess the CD4+ and CD8+ T-cell responses, cells from the draining LN were collected on the indicated days and analyzed for cell surface markers and intracellular IFN-γ production after stimulation in vitro. The HSV-specific CD8+ T-cell response in C57BL/6 mice, which are of the H-2b MHC haplotype, is almost solely directed against HSVgB498–505 and mediated by T cells that utilize the Vβ10 T-cell receptor (14, 15). The HSV antigens recognized by CD4+ T cells are not defined. Accordingly, we used HSVgB498–505 to activate HSV-specific CD8+ T cells and UVHSV to activate HSV-specific CD4+ T cells. αCD3+PMA was used to activate IFN-γ-producing, effector CD4+ and CD8+ T cells in an antigen-nonspecific manner. Comparison of the fraction of cells producing IFN-γ in response to antigen versus αCD3+PMA was used to gauge the fraction of effector cells that were HSV specific (Fig. 2).
FIG. 2.
Representative IFN-γ intracellular staining of large activated cells from the draining LN of day 5 HSV-infected B6 mice in response to various stimuli. IFN-γ staining of cells stimulated in vitro with αCD3+PMA (A) or HSVgB498–505 (B) are plotted in relation to CD8+ staining; cells stimulated with UVHSV (C) are shown with respect to CD4+ staining.
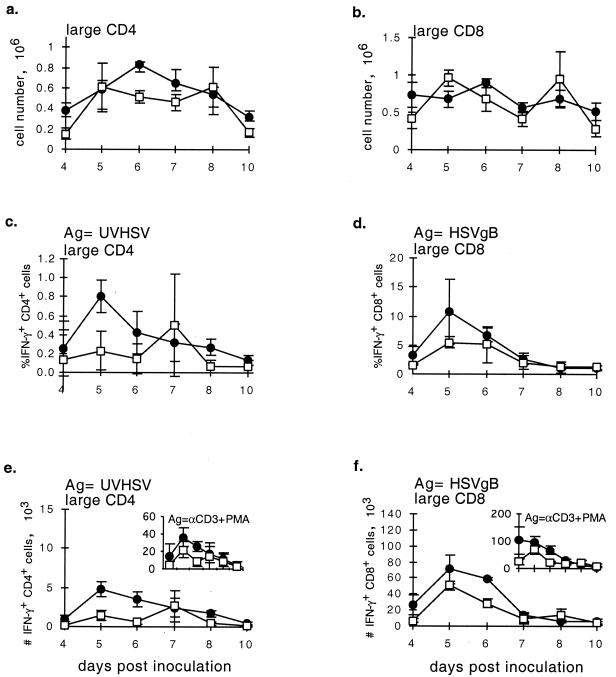
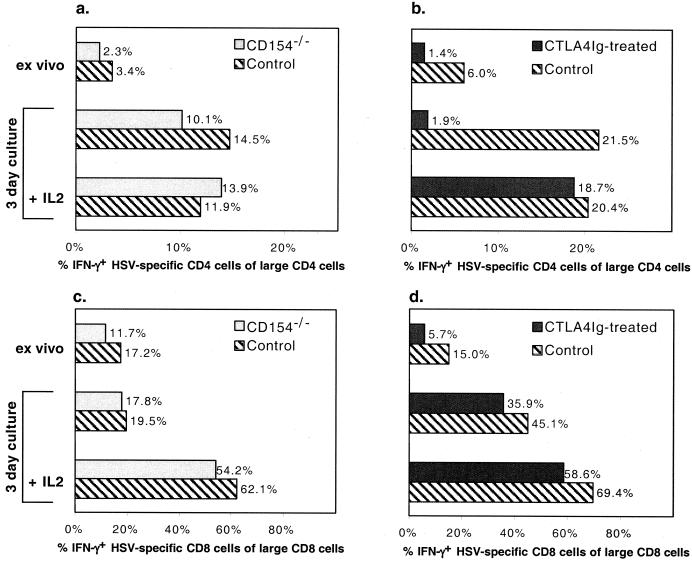
CD154 deficiency appeared to have little effect on the numbers of total (data not shown) and large, activated (CD44+) CD4+ and CD8+ T cells recovered from the LN of HSV-infected mice (Fig. 3a and b). There was also little difference between CD154−/− mice and controls in the fraction or numbers of CD8+ T cells that produced IFN-γ in response to HSVgB peptide (Fig. 3d and f). However, in CD154−/− mice, the fraction and numbers of CD4+ T cells that produced IFN-γ in response to UVHSV or αCD3+PMA were substantially reduced at the time of the peak response (Fig. 3c and e).
FIG. 3.
Characterization of the cellular immune response to HSV over days 4 to 10 postinoculation in CD154−/− (□) and control (●) mice. Mice were inoculated via intradermal injection with 5 × 105 PFU of HSV/hindfoot, and draining LN cells were collected from three mice per group on each day. Absolute numbers of large activated CD4+ (a) and CD8+ (b) cells were determined via fluorescence-activated cell sorting analysis. The fraction and absolute number of HSV-specific CD4+ cells were determined via IFN-γ intracellular staining of cells in response to UVHSV as antigen (Ag) (c and e). The fraction and absolute number of HSV-specific CD8+ cells were determined from IFN-γ production of cells stimulated with HSVgB498–505 (d and f). Insets in panels e and f depict IFN-γ staining of either CD4+ or CD8+ cells in response to maximal αCD3+PMA stimulus. ANOVA single-variant statistical analysis was used for determining significance between groups. P values: a, 0.055; b, 0.506; c, 0.004; d, 0.036; e, 0.010; f, 0.013.
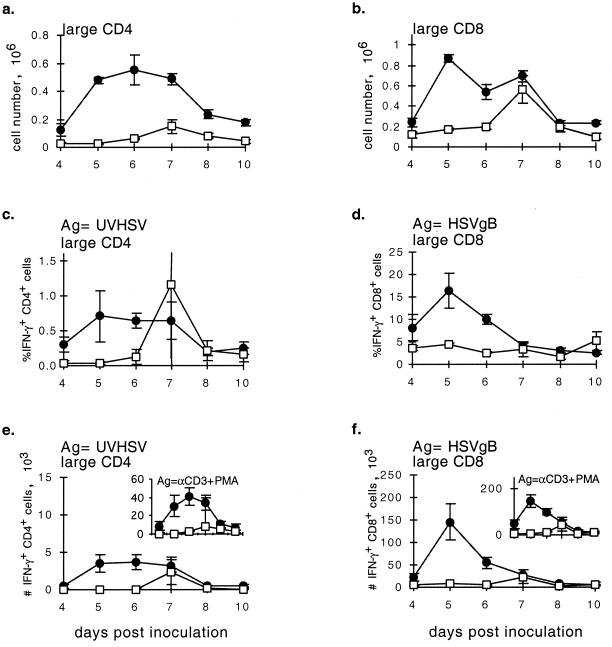
Consistent with its more marked effect on survival, CTLA4Ig treatment profoundly impaired all aspects of the CD4+ and CD8+ T-cell response. CTLA4Ig-treated mice had markedly decreased numbers of total (data not shown) and large, activated CD4+ and CD8+ cells in the draining LN at the peak of the response (Fig. 4a and b). This was closely paralleled by a reduction in the numbers of CD4+ and CD8+ T cells that produced IFN-γ in response to HSV antigens or to αCD3+PMA (Fig. 4c to f).
FIG. 4.
Characterization of the cellular immune response to HSV over days 4 to 10 postinoculation in CTLA4Ig-treated (□) and control (●) mice. Mice were treated and data are plotted as described for Fig. 3. ANOVA single-variant statistical analysis was used for determining significance between groups. P values: a, <0.0001; b, <0.0001; c, 0.17 (the variable CD4+ T-cell response at day 7 reflected one CTLA4Ig-treated mouse with high numbers of IFN-γ-producing cells [P < 0.0001 with censoring of this data point]; d, 0.0002; e, 0.0020; f, <0.0001.
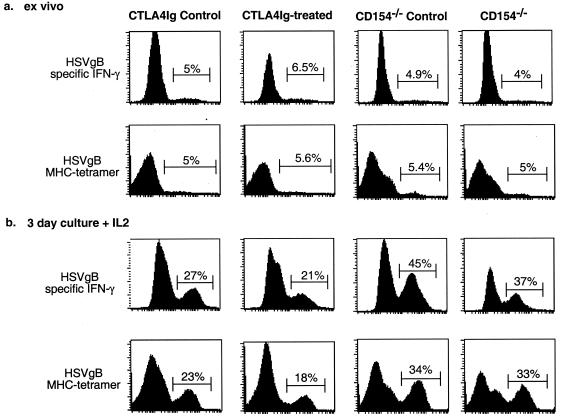
The reduction in IFN-γ-producing CD8+ T cells in CTLA4Ig-treated mice could reflect a reduction in HSV-specific effector T-cell numbers or an impairment in the ability of these cells to produce IFN-γ in response to stimulation in vitro. To evaluate these possibilities, we compared the fraction of cells isolated from the draining LN that stained with MHC-I Kb/HSVgB498–505 tetramers to the fraction that produced IFN-γ in response to HSVgB498–505 peptide. Tetramer staining and IFN-γ production closely paralleled each other for cells from CD154−/−, CTLA4Ig-treated, and the corresponding control mice (Fig. 5a).
FIG. 5.
Fractions of cells from day 5 HSV-infected mice which produced IFN-γ in response to HSVgB498–505 and which stained positive for MHC-I Kb/HSVgB498–505 tetramer closely paralleled each other directly ex vivo (a) and after 3 days in culture with or without IL-2 (b). Tetramer staining was performed on unstimulated cells after 6 h of culture. IFN-γ staining was done on cells incubated with HSVgB498–505 for 6 h.
In vitro assessment of the roles of CD28/CD80-86 interactions and IL-2 in CD4+ and CD8+ T-cell responses to HSV.
Previous studies have shown that culturing of cells explanted from draining LN of HSV-infected mice results in their expansion and in the generation of cytolytic CD8+ CTL, which cannot be detected directly ex vivo (42). To explore the basis for the differences in HSV-specific CD4+ and CD8+ T-cell responses, we explanted cells from the draining LN and cultured them for 3 days in vitro under various conditions.
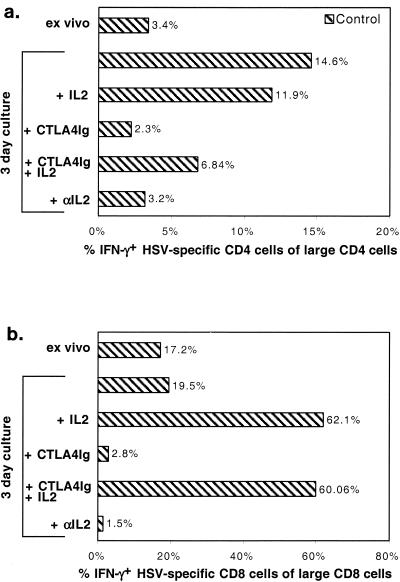
When cells from control and CD154−/− mice were cultured in medium alone, the number and fraction of CD4+ and CD8+ T cells that produced IFN-γ in response to UVHSV and HSVgB498–505 increased (Fig. 6a and c). Addition of IL-2 did not result in an increase in IFN-γ-producing CD4+ T cells compared to medium alone, whereas IFN-γ-producing CD8+ T cells were increased by the addition of IL-2. By contrast, when cells from CTLA4Ig-treated mice were cultured in vitro, there was little or no expansion of IFN-γ-producing CD4+ T cells compared to controls (Fig. 6b). However, addition of IL-2 largely overcame this deficit. In control and CTLA4Ig-treated mice that produced IFN-γ in response to HSVgB498–505 after culture in vitro, the fractions of CD8+ T cells increased in parallel upon the addition of IL-2 (Fig. 6d).
FIG. 6.
CTLA4Ig treatment causes anergy in HSV-specific CD4+ T cells but not in CD8+ T cells. Day 5 draining LN cells from CD154−/− mice ( ), CTLA4Ig-treated mice (■), and corresponding controls (
), CTLA4Ig-treated mice (■), and corresponding controls ( ) were analyzed directly ex vivo or after 3 days in culture with or without IL-2 (5 ng/ml) for HSV-specific IFN-γ production in response to UVHSV or HSVgB498–505. Plots represent fractions of IFN-γ-producing HSV-specific CD4+ or CD8+ cells in large CD4+ or CD8+ populations in CD154−/− (a) or CTLA4Ig-treated (b) mice.
) were analyzed directly ex vivo or after 3 days in culture with or without IL-2 (5 ng/ml) for HSV-specific IFN-γ production in response to UVHSV or HSVgB498–505. Plots represent fractions of IFN-γ-producing HSV-specific CD4+ or CD8+ cells in large CD4+ or CD8+ populations in CD154−/− (a) or CTLA4Ig-treated (b) mice.
When cells were cultured in the presence of CTLA4Ig in vitro, the expansion of HSV-specific CD4+ and CD8+ T cells was blocked in CD154−/− and CTLA4Ig-treated mice (data not shown) and in controls (Fig. 7). Addition of IL-2 to cultures partially and fully overcame this inhibition for CD4+ T cells and CD8+ T cells, respectively, while addition of anti-mouse IL-2 (10 μg/ml) reproduced the effects of adding CTLA4Ig to the cultures (Fig. 7). Under each of the conditions noted above, the fraction of cells that stained with MHC-I Kb/HSVgB498–505 tetramers was similar to the fraction that produced IFN-γ in response to HSVgB498–505 peptide (Fig. 5b).
FIG. 7.
The effect of CTLA4Ig on HSV-specific cells is mediated through IL-2. Cells from day 5 HSV-infected mice were analyzed either directly or after 3 days in culture with or without (5 ng/ml), IL-2, CTLA4Ig, (20 μg/ml), and IL-2-specific blocking antibody (20 μg/ml). Plots represent fractions of IFN-γ-positive CD4+ or CD8+ cells in large CD4+ and CD8+ populations in response to UVHSV or HSVgB498–505.
IFN-γ production by CD8+ T cells correlates with lytic function after culture in vitro.
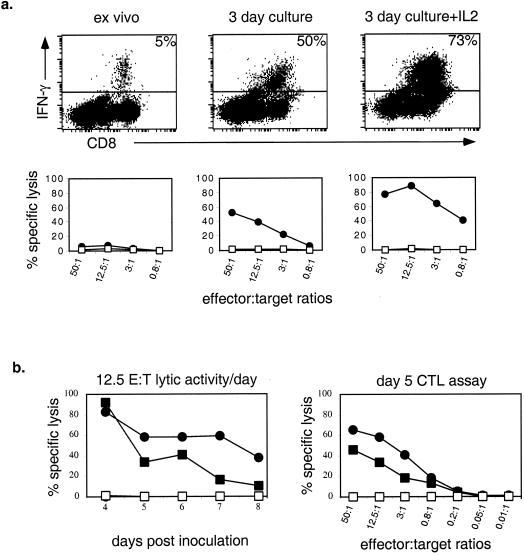
In HSV-infected mice, CD8+ T cells acquire cytolytic activity after they are cultured for 3 days in vitro in medium alone (33). Furthermore, the acquisition of cytolytic activity is CD4+ T-cell dependent and resides in the CD25 (IL-2Rα)+ subset of CD8+ T cells (33). Consistent with this and the results noted above, acquisition of cytolytic activity was enhanced by the addition of IL-2 to cultures of cells from the draining LN of infected mice (Fig. 8a). HSV gB-specific cytolytic activity of CTLA4Ig-treated cells was also enhanced by IL-2 but consistently less than in controls except at day 4, which suggests that CTL precursors were reduced at later time points.
FIG. 8.
(a) HSVgB498–505-specific IFN-γ production correlates with HSV gB-specific lytic activity after 3 days in culture with or without IL-2 (5 ng/ml). Effector cells were from day 5 draining LN cells from HSV-infected control mice. EL4-HSVgB (●) and control EL4 (□) cells were used as targets in the CTL assay. (b) HSV gB-specific lytic activity of cells from CTLA4Ig-treated mice is reduced at later time points during infection compared to controls (left panel). Lytic activity at an effector/target (E:T) ratio of 12.5:1 was determined in day 5 cells. Symbols represent effector cells from CTLA4Ig-treated mice (squares), control effector cells (circles), EL4-HSVgB targets (solid symbols), and EL4 control targets (open symbols).
DISCUSSION
This study demonstrates that CD40/CD154 and CD28/CD80-86 receptor-ligand interactions contribute to host defense against primary HSV infection. Disruption of either of these interactions reduced paralysis-free survival, and disruption of both had an even greater effect. Both costimulatory pathways contributed to the generation of HSV-specific CD4+ T-cell and T-cell-dependent antibody responses, whereas the CD28/CD80-86 pathway also played a critical role in generation of the HSV-specific CD8+ T-cell response.
We used complementary approaches to disrupt CD40/CD154 and CD28/CD80-86 interactions, which allowed us to study the functional interrelationship between these two costimulatory pathways in the response to HSV infection. Survival was impaired in WT mice treated with CTLA4Ig and in CD154−/− mice. CD154 may act in antiviral defense in part by up-regulation of CD80 and CD86 expression on APC. However, we found that treatment of CD154−/− mice with CTLA4Ig or of CD28−/− mice with MR1 further impaired survival, indicating that the CD28/CD80-86 and CD40/CD154 interactions act, at least in part, independently. By contrast to the results in WT mice treated with CTLA4Ig, CD28−/− mice did not demonstrate impaired survival to HSV, suggesting that these mice were able to compensate for this deficiency. This compensation, however, was mediated through CD40/CD154-dependent costimulatory pathways that did not act through CD28; such pathways may include the induction of 4-1BBL, OX40L, and integrins (5, 28, 35, 49, 57, 61).
Analysis of the HSV-specific cellular immune response during the first 10 days after infection revealed a role for both CS in the development of T-cell-mediated immunity to HSV. Our results provide further evidence that both CD40/CD154 and CD28/CD80-86 interactions are important for the generation of an effective antiviral CD4+ T-cell response. Similar results have been obtained by others in studies of mice with LCMV and VSV infection (3, 57, 62). Our studies extend those previously reported by evaluating the response to HSV infection and by directly comparing the magnitude of and mechanisms for impaired CD4+ T-cell responses when these costimulatory pathways are blocked.
Activation of naive CD4+ T cells through the T-cell receptor in the absence of a CD28-mediated CS induces anergy, as indicated by an inability of these cells to produce IL-2 and to proliferate. Culturing such cells in IL-2 induces proliferation and reverses the anergic state (45). In the context of antiviral responses, previous reports indicate that CD28/CD80-86 interactions help to activate CD4+ T cells that are specific for weak antigenic peptides, thereby increasing the overall diversity of responding cells (5, 6, 10, 25). This would imply that CTLA4Ig treatment might impair the initial priming and survival of HSV-specific CD4+ T cells and do so in part by inducing anergy. We tested this hypothesis by culturing cells in vitro with and without exogenous IL-2 and then identifying IFN-γ-producing CD4+ effector T-cells after stimulation with UVHSV or αCD3+PMA. CTLA4Ig treatment affected the ability of effector CD4+ T cells to expand in vitro, resulting in cells which failed to expand or gain effector function in the absence of exogenous IL-2. Addition of IL-2 fully restored the development of IFN-γ-producing effector T cells, indicating that the antigen-specific CD4+ T cells that were present were anergic. In contrast to the results in CTLA4Ig-treated mice, IFN-γ-producing effector CD4+ T cells in CD154−/− mice were only marginally reduced when tested directly ex vivo and expanded substantially when cultured for 3 days with or without IL-2. Thus, unlike the results in CTLA4Ig-treated mice, CD154 deficiency did not result in CD4+ T-cell anergy.
Consistent with the impaired CD4+ T-cell response, the T-cell-dependent IgG anti-HSV antibody response was substantially impaired in CTLA4Ig-treated and CD154−/− mice (data not shown). However, it is unlikely that impaired antibody production was the major factor in the impaired outcome of these mice, since mice in which B-cell development has been suppressed control acute HSV-1 infection with normal kinetics (50). Furthermore, while antibody responses were equally impaired in CD154−/− and CTLA4Ig-treated mice, the outcomes were not.
Unlike the CD4+ T-cell response, the CD8+ response was substantially impaired in CTLA4Ig-treated mice but not in CD154−/− mice. Under all conditions, the percentage of cells which produced IFN-γ in response to HSVgB498–505 were comparable to the percentage of cells which stained positive for the MHC-I Kb /HSVgB498–505 tetramers. This suggests that the reduction of IFN-γ-responsive CD8+ T cells in the CTLA4Ig-treated mice was due not to impaired development of effector function but rather to a reduction in the overall numbers of responding HSV-specific CD8+ T cells.
The impaired CD8+ T-cell response in CTLA4Ig-treated mice may reflect both direct and indirect roles for the CD28-CD80/86 interaction in the generation of CD8+ T cells. CD28-mediated enhancement of T-cell proliferation is largely but not solely IL-2 dependent (4). By contrast to CD4+ T cells, HSV-specific CD8+ T cells from CTLA4Ig-treated mice expanded in culture in the absence of exogenous IL-2. This expansion was both CD28 and IL-2 dependent, which is consistent with the observation that HSV-specific CD8+ CTL precursors express high levels of CD25 (24, 34). Therefore, our in vitro results suggest that CD28-mediated CD8+ T-cell expansion was mediated largely by enhancement of endogenous IL-2 production.
The CD8+ T-cell response to HSV is at least in part CD4+ T-cell dependent (24, 36, 56); thus, impairment of the CD4+ T-cell response in CTLA4Ig-treated mice may indirectly retard the ability of primed CD8+ T cells to expand and develop optimal effector function. These CD8+ T cells do not appear anergic, given their ability to expand in the absence of exogenous IL-2. However, IL-2 was a limiting factor for the expansion of these cells in vitro, and so the decreased HSV-specific CD4+ helper T-cell response may limit the potential expansion of the responding CD8+ T cells in vivo. Alternatively, impairment of the CD4+ T-cell response could result in inefficient APC activation and thereby reduce the generation of CD8+ T cells (29, 46).
The primary anti-HSV immune response is similar to the anti-VSV response in its dependency on costimulation mediated both by CD28/CD80-86 and CD40/CD154 interactions. Unlike the case for LCMV infection, the antiviral responses to VSV and HSV depend heavily on robust CD4+ T-cell responses to promote both humoral and CD8+ T-cell responses (12, 30, 32, 54, 56). The essential role of CS in the response to VSV but limited role in response to LCMV is thought to reflect the very limited replication of the VSV in mice and the limited ability of this virus to drive the maturation of DC in vivo (27), which contrasts with the extensive replication and efficient priming of DC in LCMV-infected mice (3, 47). Unlike VSV, HSV replicates to high titers in vivo. However, HSV replicates locally rather than systemically, and so the amounts of antigen reaching the secondary lymphoid organs may be limited. Further, HSV impairs the maturation and migration of infected DC (48). These effects of HSV may impede the delivery and presentation of viral antigens to T cells in the secondary lymphoid organs and place a greater reliance on cross-presentation of viral antigens by uninfected DC, a process requiring costimulation (7, 8, 20). Finally, the role for costimulation in the human anti-HSV immune response might be even more important, considering that HSV-mediated downregulation of antigen presentation in humans occurs much more efficiently than in mice (2, 18).
ACKNOWLEDGMENTS
This work was supported in part by grants T32GM07270 (K.H.E.) and HD18184 (K.H.E. and C.B.W.) from the National Institutes of Health.
We thank A. Aruffo and R. Peach (Bristol-Myers Squibb, Inc.) for murine CTLA4Ig and MR1, P. Greenberg and L. Corey (University of Washington) for helpful discussions, and H. K. Jessup and K. Pritchett for technical assistance. The H-2Kb/HSVgB tetramers were provided by the NIAID Tetramer Core Facility at Emory University.
REFERENCES
- 1.Adler H, Beland J L, Del-Pan N C, Kobzik L, Sobel R A, Rimm I J. In the absence of T cells, natural killer cells protect from mortality due to HSV-1 encephalitis. J Neuroimmunol. 1999;93:208–213. doi: 10.1016/s0165-5728(98)00236-7. [DOI] [PubMed] [Google Scholar]
- 2.Ahn K, Meyer T H, Uebel S, Sempe P, Djaballah H, Yang Y, Peterson P A, Fruh K, Tampe R. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. EMBO J. 1996;15:3247–3255. [PMC free article] [PubMed] [Google Scholar]
- 3.Andreasen S O, Christensen J E, Marker O, Thomsen A R. Role of CD40 ligand and CD28 in induction and maintenance of antiviral CD8+ effector T cell responses. J Immunol. 2000;164:3689–397. doi: 10.4049/jimmunol.164.7.3689. [DOI] [PubMed] [Google Scholar]
- 4.Appleman L J, Berezovskaya A, Grass I, Boussiotis V A. CD28 costimulation mediates T cell expansion via IL-2-independent and IL-2-dependent regulation of cell cycle progression. J Immunol. 2000;164:144–151. doi: 10.4049/jimmunol.164.1.144. [DOI] [PubMed] [Google Scholar]
- 5.Bachmann M F, McKall-Faienza K, Schmits R, Bouchard D, Beach J, Speiser D E, Mak T W, Ohashi P S. Distinct roles for LFA-1 and CD28 during activation of naive T cells: adhesion versus costimulation. Immunity. 1997;7:549–557. doi: 10.1016/s1074-7613(00)80376-3. [DOI] [PubMed] [Google Scholar]
- 6.Bachmann M F, Sebzda E, Kundig T M, Shahinian A, Speiser D E, Mak T W, Ohashi P S. T cell responses are governed by avidity and co-stimulatory thresholds. Eur J Immunol. 1996;26:2017–2022. doi: 10.1002/eji.1830260908. [DOI] [PubMed] [Google Scholar]
- 7.Bennett S R, Carbone F R, Karamalis F, Flavell R A, Miller J F, Heath W R. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 8.Bennett S R, Carbone F R, Karamalis F, Miller J F, Heath W R. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bluestone J A. New perspectives of CD28–B7-mediated T cell costimulation. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 10.Bonneau R H, Salvucci L A, Johnson D C, Tevethia S S. Epitope specificity of H-2Kb-restricted, HSV-1-, and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology. 1993;195:62–70. doi: 10.1006/viro.1993.1346. [DOI] [PubMed] [Google Scholar]
- 11.Borrow P, Tishon A, Lee S, Xu J, Grewal I S, Oldstone M B, Flavell R A. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response. J Exp Med. 1996;183:2129–2142. doi: 10.1084/jem.183.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brundler M A, Aichele P, Bachmann M, Kitamura D, Rajewsky K, Zinkernagel R M. Immunity to viruses in B cell-deficient mice: influence of antibodies on virus persistence and on T cell memory. Eur J Immunol. 1996;26:2257–2262. doi: 10.1002/eji.1830260943. [DOI] [PubMed] [Google Scholar]
- 13.Burchett S K, Corey L, Mohan K M, Westall J, Ashley R, Wilson C B. Diminished interferon-gamma and lymphocyte proliferation in neonatal and postpartum primary herpes simplex virus infection. J Infect Dis. 1992;165:813–818. doi: 10.1093/infdis/165.5.813. [DOI] [PubMed] [Google Scholar]
- 14.Cose S C, Jones C M, Wallace M E, Heath W R, Carbone F R. Antigen-specific CD8+ T cell subset distribution in lymph nodes draining the site of herpes simplex virus infection. Eur J Immunol. 1997;27:2310–2316. doi: 10.1002/eji.1830270927. [DOI] [PubMed] [Google Scholar]
- 15.Cose S C, Kelly J M, Carbone F R. Characterization of diverse primary herpes simplex virus type 1 gB-specific cytotoxic T-cell response showing a preferential V beta bias. J Virol. 1995;69:5849–5852. doi: 10.1128/jvi.69.9.5849-5852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durie F H, Aruffo A, Ledbetter J, Crassi K M, Green W R, Fast L D, Noelle R J. Antibody to the ligand of CD40, gp39, blocks the occurrence of the acute and chronic forms of graft-vs-host disease. J Clin Investig. 1994;94:1333–1338. doi: 10.1172/JCI117453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flynn K J, Belz G T, Altman J D, Ahmed R, Woodland D L, Doherty P C. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 18.Goldsmith K, Chen W, Johnson D C, Hendricks R L. Infected cell protein (ICP)47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response. J Exp Med. 1998;187:341–348. doi: 10.1084/jem.187.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grewal I S, Borrow P, Pamer E G, Oldstone M B, Flavell R A. The CD40-CD154 system in anti-infective host defense. Curr Opin Immunol. 1997;9:491–497. doi: 10.1016/s0952-7915(97)80100-8. [DOI] [PubMed] [Google Scholar]
- 20.Heath W R, Carbone F R. Cytotoxic T lymphocyte activation by cross-priming. Curr Opin Immunol. 1999;11:314–318. doi: 10.1016/s0952-7915(99)80050-8. [DOI] [PubMed] [Google Scholar]
- 21.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 22.Holterman A X, Rogers K, Edelmann K, Koelle D M, Corey L, Wilson C B. An important role for major histocompatibility complex class I-restricted T cells, and a limited role for gamma interferon, in protection of mice against lethal herpes simplex virus infection. J Virol. 1999;73:2058–2063. doi: 10.1128/jvi.73.3.2058-2063.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 24.Jennings S R, Bonneau R H, Smith P M, Wolcott R M, Chervenak R. CD4-positive T lymphocytes are required for the generation of the primary but not the secondary CD8-positive cytolytic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell Immunol. 1991;133:234–252. doi: 10.1016/0008-8749(91)90194-g. [DOI] [PubMed] [Google Scholar]
- 25.Johnston J V, Malacko A R, Mizuno M T, McGowan P, Hellstrom I, Hellstrom K E, Marquardt H, Chen L. B7-CD28 costimulation unveils the hierarchy of tumor epitopes recognized by major histocompatibility complex class I-restricted CD8+ cytolytic T lymphocytes. J Exp Med. 1996;183:791–800. doi: 10.1084/jem.183.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kay M A, Meuse L, Gown A M, Linsley P, Hollenbaugh D, Aruffo A, Ochs H D, Wilson C B. Transient immunomodulation with anti-CD40 ligand antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc Natl Acad Sci USA. 1997;94:4686–4691. doi: 10.1073/pnas.94.9.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kundig T M, Shahinian A, Kawai K, Mittrucker H W, Sebzda E, Bachmann M F, Mak T W, Ohashi P S. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity. 1996;5:41–52. doi: 10.1016/s1074-7613(00)80308-8. [DOI] [PubMed] [Google Scholar]
- 28.Lane P J, Brocker T. Developmental regulation of dendritic cell function. Curr Opin Immunol. 1999;11:308–313. doi: 10.1016/s0952-7915(99)80049-1. [DOI] [PubMed] [Google Scholar]
- 29.Lanzavecchia A. Immunology. Licence to kill. Nature. 1998;393:413–414. doi: 10.1038/30845. [DOI] [PubMed] [Google Scholar]
- 30.Lefrancois L. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: distinct mechanisms of action in vivo. J Virol. 1984;51:208–214. doi: 10.1128/jvi.51.1.208-214.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linsley P S, Wallace P M, Johnson J, Gibson M G, Greene J L, Ledbetter J A, Singh C, Tepper M A. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 32.Manickan E, Rouse B T. Roles of different T-cell subsets in control of herpes simplex virus infection determined by using T-cell-deficient mouse models. J Virol. 1995;69:8178–8178. doi: 10.1128/jvi.69.12.8178-8179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNally J M, Andersen H A, Chervenak R, Jennings S R. Phenotypic characteristics associated with the acquisition of HSV-specific CD8 T-lymphocyte-mediated cytolytic function in vitro. Cell Immunol. 1999;194:103–111. doi: 10.1006/cimm.1999.1498. [DOI] [PubMed] [Google Scholar]
- 34.McNally J M, Dempsey D, Wolcott R M, Chervenak R, Jennings S R. Phenotypic identification of antigen-dependent and antigen-independent CD8 CTL precursors in the draining lymph node during acute cutaneous herpes simplex virus type 1 infection. J Immunol. 1999;163:675–681. [PubMed] [Google Scholar]
- 35.Melero I, Bach N, Hellstrom K E, Aruffo A, Mittler R S, Chen L. Amplification of tumor immunity by gene transfer of the co-stimulatory 4-1BB ligand: synergy with the CD28 co-stimulatory pathway. Eur J Immunol. 1998;28:1116–1121. doi: 10.1002/(SICI)1521-4141(199803)28:03<1116::AID-IMMU1116>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 36.Mercadal C M, Martin S, Rouse B T. Apparent requirement for CD4+ T cells in primary anti-herpes simplex virus cytotoxic T-lymphocyte induction can be overcome by optimal antigen presentation. Viral Immunol. 1991;4:177–186. doi: 10.1089/vim.1991.4.177. [DOI] [PubMed] [Google Scholar]
- 37.Milligan G N. Neutrophils aid in protection of the vaginal mucosae of immune mice against challenge with herpes simplex virus type 2. J Virol. 1999;73:6380–6386. doi: 10.1128/jvi.73.8.6380-6386.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milligan G N, Bernstein D I. Analysis of herpes simplex virus-specific T cells in the murine female genital tract following genital infection with herpes simplex virus type 2. Virology. 1995;212:481–489. doi: 10.1006/viro.1995.1506. [DOI] [PubMed] [Google Scholar]
- 39.Milligan G N, Bernstein D I. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997;229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- 40.Murali-Krishna K, Altman J D, Suresh M, Sourdive D, Zajac A, Ahmed R. In vivo dynamics of anti-viral CD8 T cell responses to different epitopes. An evaluation of bystander activation in primary and secondary responses to viral infection. Adv Exp Med Biol. 1998;452:123–142. doi: 10.1007/978-1-4615-5355-7_14. [DOI] [PubMed] [Google Scholar]
- 41.Noelle R J. CD40 and its ligand in host defense. Immunity. 1996;4:415–419. doi: 10.1016/s1074-7613(00)80408-2. [DOI] [PubMed] [Google Scholar]
- 42.Nugent C T, Wolcott R M, Chervenak R, Jennings S R. Analysis of the cytolytic T-lymphocyte response to herpes simplex virus type 1 glycoprotein B during primary and secondary infection. J Virol. 1994;68:7644–7648. doi: 10.1128/jvi.68.11.7644-7648.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oxenius A, Campbell K A, Maliszewski C R, Kishimoto T, Kikutani H, Hengartner H, Zinkernagel R M, Bachmann M F. CD40-CD40 ligand interactions are critical in T-B cooperation but not for other anti-viral CD4+ T cell functions. J Exp Med. 1996;183:2209–2218. doi: 10.1084/jem.183.5.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pereira R A, Simon M M, Simmons A. Granzyme A, a noncytolytic component of CD8+ cell granules, restricts the spread of herpes simplex virus in the peripheral nervous systems of experimentally infected mice. J Virol. 2000;74:1029–1032. doi: 10.1128/jvi.74.2.1029-1032.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powell J D, Ragheb J A, Kitagawa-Sakakida S, Schwartz R H. Molecular regulation of interleukin-2 expression by CD28 co-stimulation and anergy. Immunol Rev. 1998;165:287–300. doi: 10.1111/j.1600-065x.1998.tb01246.x. [DOI] [PubMed] [Google Scholar]
- 46.Ridge J P, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 47.Ruedl C, Kopf M, Bachmann M F. CD8(+) T cells mediate CD40-independent maturation of dendritic cells in vivo. J Exp Med. 1999;189:1875–1884. doi: 10.1084/jem.189.12.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salio M, Cella M, Suter M, Lanzavecchia A. Inhibition of dendritic cell maturation by herpes simplex virus. Eur J Immunol. 1999;29:3245–3253. doi: 10.1002/(SICI)1521-4141(199910)29:10<3245::AID-IMMU3245>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 49.Shuford W W, Klussman K, Tritchler D D, Loo D T, Chalupny J, Siadak A W, Brown T J, Emswiler J, Raecho H, Larsen C P, Pearson T C, Ledbetter J A, Aruffo A, Mittler R S. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simmons A, Nash A A. Effect of B cell suppression on primary infection and reinfection of mice with herpes simplex virus. J Infect Dis. 1987;155:649–654. doi: 10.1093/infdis/155.4.649. [DOI] [PubMed] [Google Scholar]
- 51.Simmons A, Tscharke D, Speck P. The role of immune mechanisms in control of herpes simplex virus infection of the peripheral nervous system. Curr Top Microbiol Immunol. 1992;179:31–56. doi: 10.1007/978-3-642-77247-4_3. [DOI] [PubMed] [Google Scholar]
- 52.Simmons A, Tscharke D C. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med. 1992;175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sperling A I, Auger J A, Ehst B D, Rulifson I C, Thompson C B, Bluestone J A. CD28/B7 interactions deliver a unique signal to naive T cells that regulates cell survival but not early proliferation. J Immunol. 1996;157:3909–3917. [PubMed] [Google Scholar]
- 54.Steinhoff U, Muller U, Schertler A, Hengartner H, Aguet M, Zinkernagel R M. Antiviral protection by vesicular stomatitis virus-specific antibodies in alpha/beta interferon receptor-deficient mice. J Virol. 1995;69:2153–2158. doi: 10.1128/jvi.69.4.2153-2158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stevens J G, Cook M L. Latent infections induced by herpes simplex viruses. Cancer Res. 1973;33:1399–1401. [PubMed] [Google Scholar]
- 56.Stohlman S A, Bergmann C C, Lin M T, Cua D J, Hinton D R. CTL effector function within the central nervous system requires CD4+ T cells. J Immunol. 1998;160:2896–2904. [PubMed] [Google Scholar]
- 57.Tan J T, Whitmire J K, Ahmed R, Pearson T C, Larsen C P. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J Immunol. 1999;163:4859–4868. [PubMed] [Google Scholar]
- 58.Tanigawa M, Bigger J E, Kanter M Y, Atherton S S. Natural killer cells prevent direct anterior-to-posterior spread of herpes simplex virus type 1 in the eye. Investig Ophthalmol Visual Sci. 2000;41:132–137. [PubMed] [Google Scholar]
- 59.Tigges M A, Leng S, Johnson D C, Burke R L. Human herpes simplex virus (HSV)-specific CD8+ CTL clones recognize HSV-2-infected fibroblasts after treatment with IFN-gamma or when virion host shutoff functions are disabled. J Immunol. 1996;156:3901–3910. [PubMed] [Google Scholar]
- 60.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 61.Watts T H, DeBenedette M A. T cell co-stimulatory molecules other than CD28. Curr Opin Immunol. 1999;11:286–293. doi: 10.1016/s0952-7915(99)80046-6. [DOI] [PubMed] [Google Scholar]
- 62.Whitmire J K, Flavell R A, Grewal I S, Larsen C P, Pearson T C, Ahmed R. CD40-CD40 ligand costimulation is required for generating antiviral CD4 T cell responses but is dispensable for CD8 T cell responses. J Immunol. 1999;163:3194–3201. [PubMed] [Google Scholar]
- 63.York I A, Roop C, Andrews D W, Riddell S R, Graham F L, Johnson D C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]