Abstract
Purpose of Review
Addiction may be characterized along three functional domains: Approach Behavior, Executive Function, and Negative Emotionality. Constructs underlying impulsivity thought to be relevant in addiction map on to these three functional domains. The purpose of the present review was to evaluate the extant research regarding sex/gender differences in the multi-dimensional domains of addiction using human neuroimaging and discuss their relevance to impulsivity.
Recent Findings
Few papers over the past two decades have used human neuroimaging to test sex/gender differences in addiction. There is therefore a significant gap in the literature regarding sex/gender differences in the neurobiological mechanisms driving the multi-dimensionality of addiction and their implications to impulsivity.
Summary:
Of the 34 reviewed papers, the orbitofrontal cortex/ventromedial prefrontal cortex (OFC/vmPFC) was the most frequently reported brain region to evidence a sex/gender difference during fMRI tasks probing Approach Behavior and Negative Emotionality. This finding suggests potential sex/gender-specific patterns of subjective valuation in substance misuse, driven by OFC/vmPFC dysregulation.
Keywords: Addiction, substance use disorder, functional neuroimaging, fMRI, sex differences, gender differences
Introduction
Substance Use Disorders (SUDs) remain one of the leading causes of preventable death across the United States [1]. According to the most recent National Survey on Drug Use and Health, over 43 million people aged 12 and over met DSM-5 criteria for a SUD in 2021, of whom only 6% of whom received treatment [2]. One challenge in both the study and treatment of SUDs is its complex dimensionality; a breadth of neurobiological, behavioral, and social factors likely contribute to both the etiology and maintenance of addiction [3–5]. Impulsivity has emerged as a key neurobehavioral component of SUDs [6]. Indeed, the now widely-accepted medical model of SUD describes addiction as a chronic health condition, characterized by ‘uncontrollable, compulsive drug craving, seeking, and use, even in the face of negative health and social consequences’ [7,8]. This role of impulse control in addiction is bolstered by preclinical literature suggesting that impulsivity is a key process in the development of compulsive drug-seeking behavior in rodent models [9–11]. Impulsivity itself has also been defined as a multi-dimensional construct, with different facets such as impulsive decision-making and impulsive behavior elicited by an emotional or stressful context [12,13]. Importantly, growing evidence supports sex/gender-specific patterns in different addiction-relevant mechanisms, such as stress reactivity, craving, and risky decision-making [14–17]. For example, one meta-analysis of 741 effect sizes from 277 studies found that men demonstrated greater task-related risk-taking and self-reported sensation-seeking relative to women [18]. Substance-dependent women are also more likely to report greater stress response and less stress tolerance, and are more likely to relapse to a stressful trigger relative to substance-dependent men [14,19–22]. Despite these behavioral sex/gender differences, current reviews regarding sex/gender differences in the neurobiology underlying SUDs in humans are limited, inconclusive, and, to our knowledge, restricted to a single drug of misuse [23,24]. Therefore, a systematic review of the neurobiology underlying sex/gender differences across multiple substances of misuse, and the implications for these differences in neurobehavioral processes like impulsivity, is warranted. Moreover, although historically men have endorsed SUDs more frequently than women, this gender gap is closing, driven primarily by an increase in substance use in women [25–27]. This shift further emphasizes the urgent need for such a review.
Here, we reviewed studies that assessed function across three domains that have been shown to underlie human behavior: ‘Approach-related Behavior” (also referred to as ‘Incentive Salience’ or ‘Reward Processing’ in addiction neuroscience), ‘Executive Function’, and ‘Negative Emotionality’ [28]. Indeed, the National Institute of Health’s Research Domain Criteria (RDOC) includes these three domains among its six key neurobehavioral domains [29]. We and others have empirically demonstrated the relevance of all three functional domains to addiction [30–32]. Kwako and colleagues provided evidence supporting the importance of these domains in Alcohol Use Disorder (AUD) [31], and we extended these findings beyond AUD to all SUDs [30]. Impulsivity, defined as ‘predisposition toward rapid, unplanned reactions to internal or external stimuli without regard to the negative consequences of these reactions to the impulsive individual or to others’, has also been shown to be a multi-dimensional construct [6,33]. Substantial work has been done to understand the constructs underlying impulsivity, leading to (among other measures), the development of the Urgency, Premeditation (lack thereof), Perseverance (lack of), and Sensation Seeking (UPPS) Impulsive Behavior Scale, and later the UPPS-Positive Urgency (UPPS-P) scale [34,35]. The UPPS-P conceptualizes impulsivity as five constructs: (1) lack of premeditation, (2) lack of perseverance, (3) sensation-seeking,(4) negative urgency, and (5) positive urgency, and its construct validity has been confirmed in substance misuse [36,37]. Importantly, these constructs parallel the three functional domains we have previously demonstrated in SUDs. Positive urgency (i.e., the tendency to act rashly under positive affect) and sensation-seeking (i.e., openness to and pursuing exciting activities) map onto the ‘Approach-related Behavior’ functional domain of addiction [13,38]. In contrast, the ‘Executive Function’ domain captures the (lack of) premeditation (i.e., the tendency to think through potential consequences of a behavior) and (lack of) perseverance (the ability to focus on tedious activities) factors of impulsivity [13,38]. Finally, negative urgency (i.e., the tendency to act rashly under negative affect) maps onto the ‘Negative Emotionality’ domain [13,36,38].
Previous work has further evidenced that task-based measures that capture states converge onto self-reported trait-based processes [39–41]) for all three functional domains. For example, trait-level components of neuroticism are highly predictive of symptom changes in depression and anxiety [39]. In another report, 99% of the variance attributed to a common latent factor underlying task-based executive function was explained by trait-level genetic influence [40]. Correspondingly, ecological momentary assessments of impulsive states mapped onto the heterogeneity observed for trait-level impulsivity [41]. Moreover, in a report of heavy drinkers, alcohol-related cue reactivity was positively associated with increased trait-level sensation-seeking [42]. In an independent sample of heavy alcohol drinkers, self-reported negative urgency moderated the effect of a stress induction on the reinforcing value of alcohol, which was related to increased alcohol misuse [43]. These associations between state-level task-based measures (cue reactivity and stress induction) and trait-level dimensions of impulsivity (e.g., sensation-seeking and negative urgency) evidence the convergence of state- and trait-level impulsivity on substance misuse.
Neuroimaging approaches (e.g., functional magnetic resonance imaging [fMRI]), have primarily used task-based measures to investigate function on the three domains in SUD populations. A recent systematic review of task-related fMRI in addiction found that dysregulation in brain regions underlying reward processing (e.g., nucleus accumbens [NAcc], orbitofrontal cortex [OFC], among others), during reward and socio-emotional processing tasks is related to craving, addiction severity, elevated use, and relapse across various substances of misuse [44]. Correspondingly, another review emphasized the importance of the prefrontal cortex (PFC), OFC, and amygdala as common brain regions across SUDs implicated in negative urgency [45]. Taken together, these reviews highlight the potential role of brain regions involved in the representation of subjective value (e.g., OFC, among other regions) in both Approach Behavior and Negative Emotionality in SUDs. Reviews of fMRI studies using Executive Function tasks have most consistently found hypoactivation in the anterior cingulate cortex (ACC), inferior frontal gyrus, and dorsolateral prefrontal cortex (dlPFC) in substance-dependent samples, together suggesting that downregulation of regions involved in the executive network may be implicated in inhibitory processing across SUD populations [44,46,47].
In sum, fMRI approaches probing the processes underlying Approach Behavior, Executive Function, and Negative Emotionality in SUDs have been key in understanding the unique neurobiological mechanisms of this condition. Importantly, however, literature regarding the neurobiological mechanisms underlying sex/gender differences in these domains is scarce. In fact, across 105 task-related fMRI studies in SUD populations, only 7% conducted gender comparisons [44]. Thus, understanding sex/gender differences in the neurobiological mechanisms of these domains is essential for understanding the multi-dimensionality driving sex/gender-related patterns of behavior in addiction and their relevance for impulsivity.
In this review, we examine the extant literature in human fMRI research regarding sex/gender differences across the multi-dimensional functional domains of Approach Behavior, Executive Function, and Negative Emotionality underlying SUDs in adults and discuss their implications for impulsivity. The term ‘sex/gender’ here is used to represent the complex relationship between biological sex and the social construct ‘gender’ in brain and behavior [23,48–50]. We operationalize sex/gender as binary to be congruent with the reviewed literature. We conclude our review with providing recommendations, informed by our findings, for future work to fill the gaps in the literature.
Methodology
We performed a systematic review of studies published between 2000 and February 8, 2023. The literature search (conducted on PubMED, Embase, and PsycINFO) combined variations of keywords related to functional neuroimaging, substance dependence or Use Disorders, the 10 drug classes included in the DSM-5, and sex and/or gender differences (for exact search syntax see the Supplemental Material). We included all empirical studies that conducted sex and/or gender comparisons in adults with heavy/problematic use, abuse, dependence (DSM-IV), or Use Disorders (DSM-5) using task- or resting-state-based functional neuroimaging. The original search yielded 593 records. AMM examined each title and abstract. After removing those with exclusionary criteria, AMM and LRB reviewed the full text of all papers. After full review, 34 studies met our inclusion and exclusion criteria (Figure 1). AMM extracted data from the remaining studies, including data on both empirical findings and study design (e.g., sample size, task duration, analytic approach). Full inclusion/exclusion criteria and data extraction methods are available in the Supplemental Material. Studies were categorized into either Approach Behavior, Executive Function, or Negative Emotionality domains based on the fMRI paradigm used (Figure 2). To synthesize across studies, brain regions from each paper were grouped under a naming convention based on functional neuroanatomy, using coordinates when reported (see Supplemental Table 1). Primary findings across all reviewed studies were tallied by the grouped naming conventions, accounting for whole brain or region of interest (ROI) approach. Study quality was evaluated considering sample size, scan length duration, and statistical analysis. The rationale and full evaluation criteria are available in the Supplemental Materials.
Figure 1: Study identification:
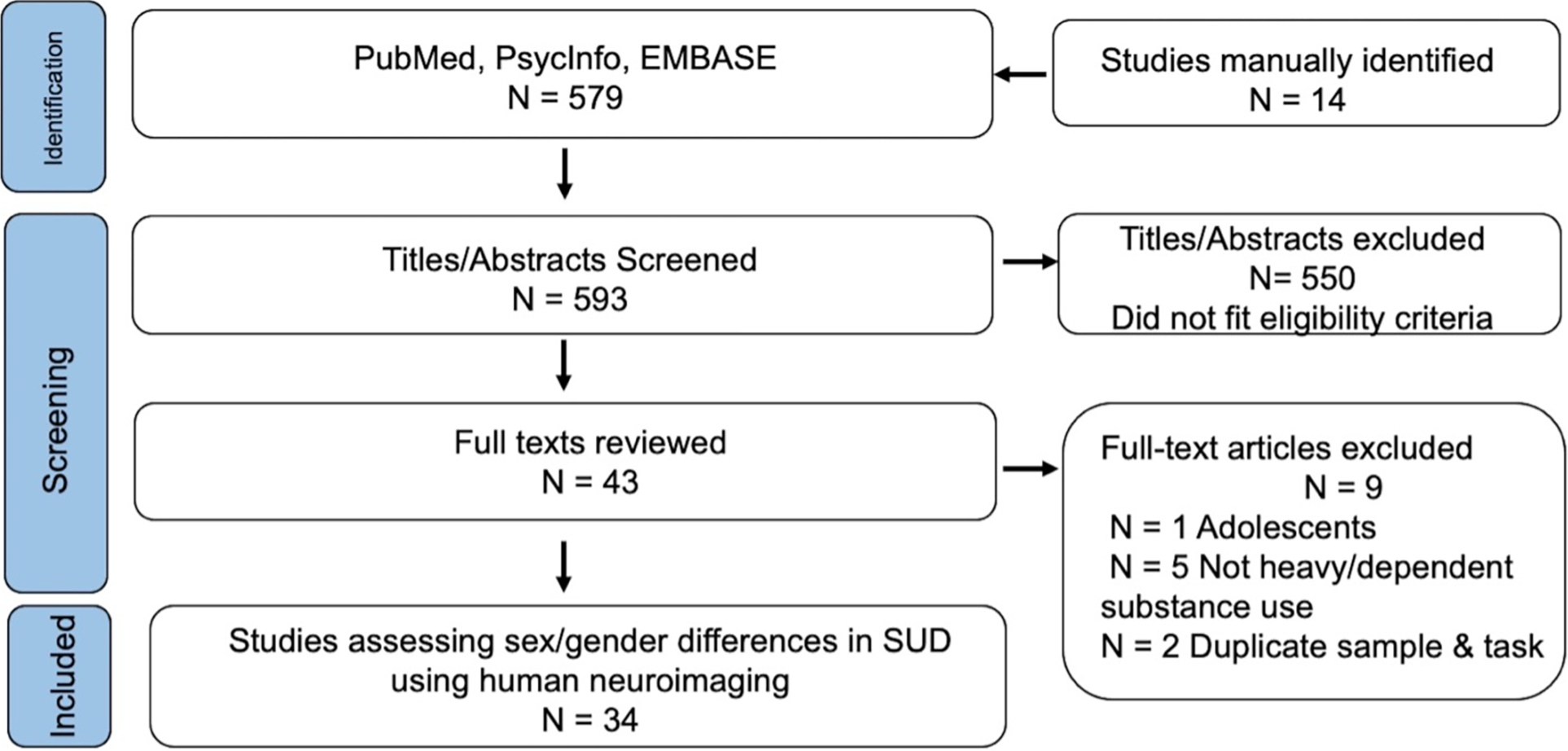
Flow diagram of paper identification, screening, and selection.
Figure 2: Investigated Populations and Task Paradigms:
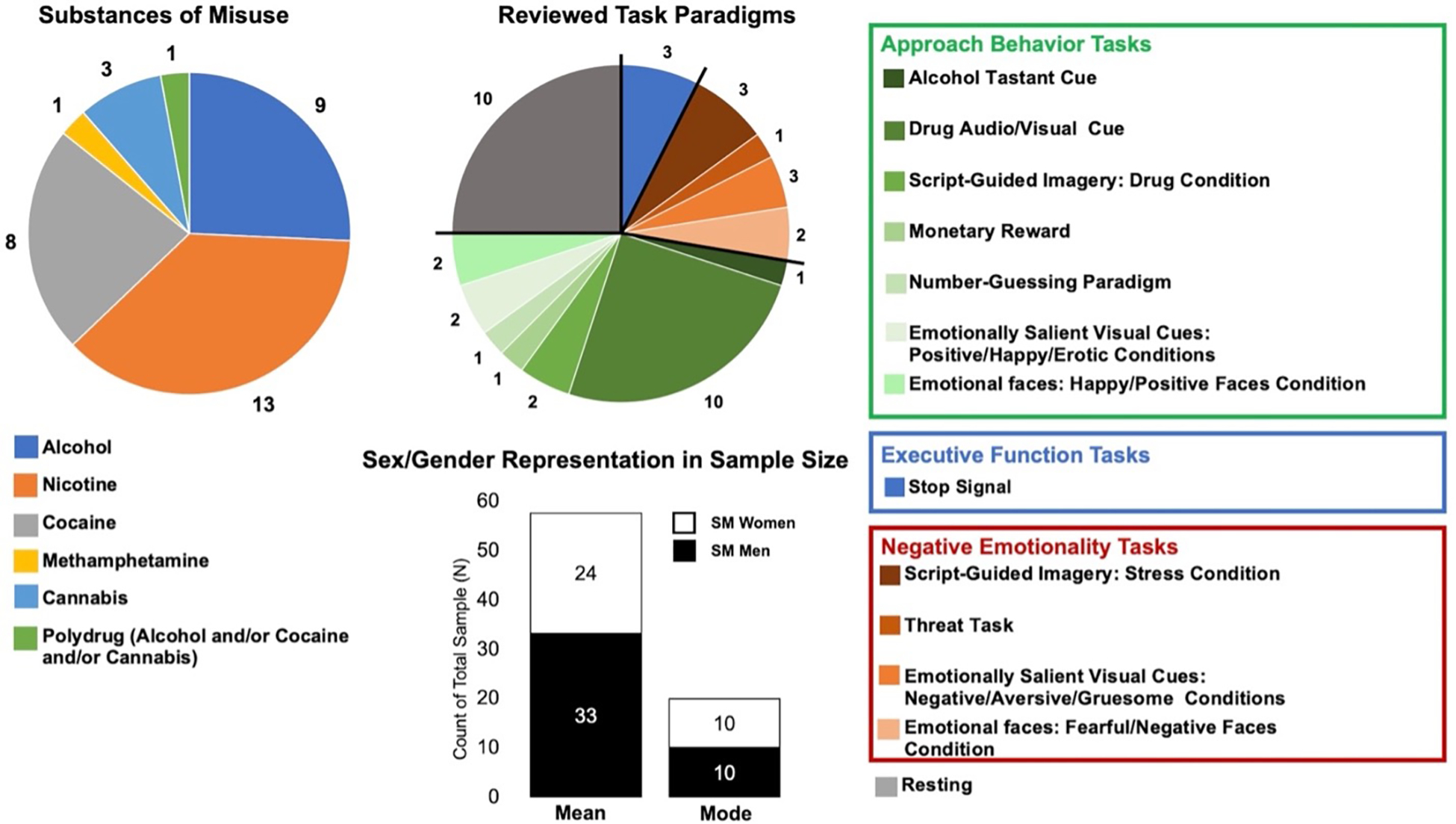
The majority of studies investigated nicotine dependence, and the most frequently used task paradigm was drug-related audio/visual cues. The mean sample size of men with misuse was greater than that of women, and the most frequently reported sample size of both men and women with misuse was small at N=10. SM = substance misuse.
Results
Study Design and Methods.
A summary of the design and methods implemented in all reviewed studies is listed in Table 1; see Supplemental Material for the full reference list of all reviewed papers. Of the 34 studies reviewed, the average sample size of those with substance misuse was N = 58 (± 58). Sixteen studies also included a healthy control sample with an average sample size of N = 42 (± 39). The average number of men and women with substance misuse per sample was 33 (± 39) and 25 (± 20), respectively. The most frequently reported sample size (i.e., mode) for both men and women with substance misuse was 10 (Figure 2). The largest number of studies were conducted in people with nicotine dependence, and the most frequently used task paradigm was a drug-related cue reactivity task (Figure 2). Of the studies that reported the task or resting scan length (N=32), the average length was 19.72 ± 14.61 minutes. The majority of papers were published after 2015, the year the NIH mandated sex as a biological variable of interest in preclinical research (Supplemental Material Figure 1) [51].
Table 1.
A summary of papers included in this review
| Paper | Domains | Drug | Use level | Signal | Total N | SM Men N | SM Women N (% of SM) | HC Men | HC Women N (% of HC) | Task/Resting | WB/ROI | Scan Duration (mins) | Sex or Gender Measurement |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| McClernon 2008 | Approach | Nicotine | ≥15 CPD past 2 yrs | BOLD | 30 | 7 | 23 (77) | -- | --- | Drug Cue | WB | 52 | NR |
| Claus 2011 | Approach | Alcohol | AUDIT & ADS ‘Heavy’ | BOLD | 326 | 226 | 100 (31) | -- | -- | Taste Cue Task | WB & ROI | 18 | NR |
| Potenza 2012 | Approach & Neg Emotion | Cocaine | DSM IV Dependence | BOLD | 66 | 14 | 16 (53) | 18 | 18 (50) | Stress & Drug Cue | WB | 332 | NR |
| Nikolova 2013 | Approach | Alcohol | AUDIT ‘Mild–Heavy’ | BOLD | 138 | 66 | 72 (52) | -- | -- | Number-guessing | ROI | 62 | NR |
| Wetherill 2013 | Approach | Nicotine | FTND Dependence | CBF | 51 | 31 | 20 (39) | -- | -- | Drug Cue | ROI | 20 | NR |
| Mendrek 2014 | Approach | Nicotine | FTND Dependence | BOLD | 34 | 15 | 19 (56) | -- | -- | Drug Cue | WB | 42 | NR |
| Padula 2015 | Approach & Neg Emotion | Alcohol | DSM IV Dependence | BOLD | 28 | 6 | 8 (57) | 5 | 9 (64) | Emotional Faces | WB | 14 | NR |
| Wetherill 2015 | Approach | Cannabis | DSM IV Dependence | BOLD | 44 | 27 | 17 (39) | -- | -- | Drug Cue | ROI | 9 | Bio Sex3 |
| Canterberry 2016 | Approach & Neg Emotion | Cocaine | DSM IV Dependence | BOLD | 40 | 10 | 10 (50) | 10 | 10 (50) | Emotional Stimuli | WB | 92 | |
| Konova 2016 | Approach | Cocaine | DSM IV Dependence | BOLD | 53 | 14 | 14 (50) | 14 | 11(44) | Monetary Reward | WB | 192 | NR |
| Zanchi 2016 | Approach | Nicotine | ≥10 CPD past 2 yrs | BOLD | 521 | 8 | 10 (56) | 8 | 9 (53) | Drug Cue | WB | 202 | NR |
| Dumais 2017–1 | Approach | Nicotine | FTND Dependence | CBF | 40 | 17 | 23 (58) | -- | -- | Drug Cue | ROI | 18 | NR |
| Dumais 2017–2 | Approach | Nicotine | FTND Dependence | BOLD | 32 | 14 | 18 (56) | -- | -- | Drug Cue | ROI | 122 | NR |
| Sawyer 2019 | Approach & Neg Emotion | Alcohol | DSM IV Abuse or Dependence | BOLD | 88 | 17 | 25 (60) | 22 | 24 (52) | Emotional Stimuli | WB | 22 | NR |
| Molokotos 2020 | Approach | Nicotine | DSM IV Dependence | BOLD | 24 | 10 | 14 (58) | -- | -- | Drug Cue | ROI | 25 | NR |
| Prashad 2020 | Approach | Cannabis | ≥5000 lifetime uses | BOLD | 112 | 58 | 54 (48) | -- | -- | Drug Cue | ROI | 28 | NR5 |
| Zhang 2020 | Approach | Cocaine | DSM IV Dependence | BOLD | 52 | 42 | 10 (19) | --- | -- | Drug Cue | WB, ROI,& ROI-to-WB | 18 | NR |
| Oscar-Berman 2021 | Approach & Neg Emotion | Alcohol | DSM IV Abuse or Dependence | BOLD | 84 | 21 | 21 (50) | 21 | 21 (50) | Emotional Faces | WB & ROI | 54 | Self-Report4 |
| Claus 2022 | Approach & Neg Emotion | Alcohol | AUDIT ‘Hazardous/Harmful’ | BOLD | 112 | 63 | 49 (44) | -- | -- | Drug & Neg Cues | WB & ROI-to-WB | 25 | NR |
| Smith 2023 | Approach & Neg Emotion | Alcohol Cocaine Cannabis | DSM IV Dependence | BOLD | 72 | 46 | 26 (36) | -- | --- | Stress & Drug Cue | WB | 302 | NR |
| Luo 2013 | Exec Funct | Cocaine | DSM IV Dependence | BOLD | 97 | 60 | 37 (38) | -- | -- | SST | WB | 40 | NR |
| Zhang 2014 | Exec Funct | Cocaine | DSM IV Dependence | BOLD | 108 | 35 | 19 (35) | 29 | 25 (46) | SST | ROI-to-WB | 40 | NR |
| Swartz 2021 | Exec Funct | Alcohol | AUDIT ‘Hazardous’ | BOLD | 153 | 89 | 64 (42) | --- | -- | SST | WB | 6 | Bio Sex3 |
| Li 2005 | Neg Emotion | Cocaine | DSM IV Dependence | BOLD | 27 | 17 | 10 (37) | -- | -- | Stress Cue | WB | 142 | NR |
| Gorka 2019 | Neg Emotion | Alcohol | DSM-5 curr/past AUD | BOLD | 65 | 22 | 16 (42) | 15 | 12 (44) | Threat Startle | ROI | 72 | Bio Sex3 |
| Chang 2002 | Resting | Meth | DSM IV Dependence | CBF | 40 | 10 | 10 (50) | 10 | 10 (50) | NA | WB | NR | NR |
| Tanabe 2008 | Resting | Nicotine | FTND Dependence | CBF | 12 | 5 | 7 (58) | -- | -- | NA | ROI | NR | NR |
| Wetherill 2014 | Resting | Nicotine | FTND Dependence | CBF | 51 | 31 | 20 (39) | -- | -- | NA | ROI | 5 | NR |
| Beltz 2015 | Resting | Nicotine | ≥10 CPD past 1 yr | BOLD | 50 | 27 | 23 (46) | -- | -- | NA | ROI | 5 | NR |
| Li 2016 | Resting | Nicotine | FTND Dependence | BOLD | 134 | 35 | 33 (49) | 33 | 33 (50) | NA | WB | 52 | NR |
| MS Maria 2017 | Resting | Nicotine | FTND Dependence | BOLD | 44 | 13 | 14 (52) | 11 | 6 (35) | NA | ROI | 11 | NR |
| Zhang 2017 | Resting | Nicotine | FTND Dependence | BOLD | 213 | 20 | 20 (50) | 72 | 101 (58) | NA | ROI-to-WB | 10 | NR |
| Manza 2018 | Resting | Cannabis | DSM IV Dependence | BOLD | 60 | 22 | 8 (27) | 20 | 10 (33) | NA | ROI | 54 | NR |
| Zhang 2018 | Resting | Cocaine | DSM IV Dependence | BOLD | 140 | 52 | 18 (26) | 46 | 24 (34) | NA | ROI-to-WB | 10 | NR |
| Canessa 2021 | Resting | Alcohol | DSM 5 AUD | BOLD | 41 | 13 | 9 (41) | 11 | 8 (42) | NA | WB | 82 | NR |
ADS = Alcohol Dependence Scale; AUD = Alcohol Use Disorder; AUDIT = Alcohol Use Disorder Identification Test; BOLD = Blood Oxygenation Level Dependent; CBF = Cerebral Blood Flow; CPD = Cigarettes per day; curr = Current DSM = Diagnostic and Statistical Manual; Exec = Executive; FTND = Fagerstrom Test for Nicotine Dependence; Funct = Function; HC = Healthy controls; Neg = Negative; NR = Not reported; Resting = Resting-state fMRI; ROI = Region of Interest; SST = Stop Signal Task; SM = Substance misuse; WB = Whole Brain; yr(s) = Year(s)
Ex-smokers included in the Total N but not included in the percentage calculation as analyses from these participants are not reported here
Manually estimated scan duration
Reported categorization by biological sex but did not report measurement of biological sex (e.g., self-reported sex assigned at birth, chromosomal assay, reproductive anatomy)
Reported that the term ‘gender’ was used because they did not measure biological sex.
Approach Behavior Functional Domain:
Nineteen reviewed papers used a task-based design to measure Approach Behavior (Table 1). Overall, the OFC/ventromedial prefrontal cortex (vmPFC) was the most frequently reported brain region to indicate any sex/gender difference, with a significant finding in eight of the 18 studies (44%) that examined this area (Supplemental Figure 2, Supplemental Table 4) [52–58]. Although the OFC and vmPFC are distinct neural regions, they are functionally connected and, because the OFC is often defined as encompassing the vmPFC, both regions have been combined here for interpretation across reviewed studies [59,60]. Five of the seven studies that found a sex/gender difference in the OFC/vmPFC conducted a direct comparison between men and women with substance misuse, and the most consistent finding (80%) was increased reactivity in men relative to women in response to drug-related or positively arousing stimuli (Table 2) [53,54,57]. Generally, studies of higher quality, specifically studies with the largest sample sizes, longest scan duration, and/or most rigorous statistical analyses, most commonly reported a sex/gender difference in the OFC/vmPFC (see Supplemental Table 2 for details on the quality assessment), further bolstering this region as a potential key location for sex/gender differences. For example, Potenza and colleagues used a drug-related script imagery paradigm to assess whole-brain reactivity in 66 individuals (30 abstinent cocaine-dependent; of which 14 were women) [55]. They found a three-way interaction of sex, diagnostic group (cocaine-dependent or healthy control), and cue conditions (drug and stress scripts conditions) in the putamen, caudate, NAcc, amygdala, hippocampus, dlPFC, insula, dorsal ACC (dACC), posterior cingulate cortex (PCC), OFC, Broca’s Area, and the fusiform, supplemental motor area(SMA)/motor, and temporal cortices [55]. To further explore this interaction effect, they analyzed men and women separately. They found that men with cocaine dependence demonstrated increased reactivity in the bilateral OFC and vmPFC in response to drug-related imagery compared to control men, while women did not show this pattern [55]. Correspondingly, Study 1 of Dumais and colleagues analyzed 40 smokers (N = 23 females) using perfusion fMRI and found that men demonstrated increased reactivity to smoking (relative to non-smoking) cues compared to women in the NAcc and vmPFC [53]. Finally, Claus and colleague’s 2022 analysis of 112 individuals reporting hazardous/harmful drinking (N = 49 women) found that women evidenced greater reactivity in the vmPFC, bilateral somatosensory cortex/precuneus, and left dlPFC relative to men in response to alcohol-related cues [52]. Together, these findings highlight the OFC/vmPFC as a potential key region for sex/gender differences across different substances of misuse.
Table 2:
Findings from sex/gender comparisons in reviewed papers
| Study | Domains; Task | Behavioral Comparisons & Covariates | Direct Comparison SM Men v. SM Women | Interaction Effect and/or Group Differences | Brain-Behavior Associations |
|---|---|---|---|---|---|
| McClernon 2008 | Approach; Smoking-related visual cues | Sex/gender differences not reported Covariate(s): Age, FTND, SJWQ craving & negative affect |
Smoking > Control Cue W > M: R Putamen, BL cuneus, L temporal cortex, L Motor/SMA M > W: L Hippocampus and L OFC |
--- |
M: Cue reactivity in the hippocampus was positively associated with negative affect W: No brain-behavior associations |
| Claus 2011 | Approach; Taste Cue Task | Sex/gender differences not reported Covariate(s): ADS |
Alcohol > Control Beverage Whole brain analysis: No main effect of sex/gender ROI Analysis: M > W in L Amygdala |
--- | Not reported |
| Potenza 2012 | Approach & Negative Emotionality; Stress & Drug Imagery Script |
No sex/gender difference in cocaine use or cue-elicited craving, anxiety, heart rate Covariate(s): None |
---- |
3-way interaction of sex, diagnostic group, and condition: R temporal cortex, putamen, amygdala, hippocampus, insula, PCC, Fusiform cortex, L caudate, NAcc, dlPFC, SMA, BL Broca’s Area, OFC Directions explored separately: Drug Condition: M: CUD > HC in ACC, PCC, L thalamus, BL insula, BL caudate, BL hippocampus, BL putamen, BL dlPFC, BL vmPFC, BL OFC W: CUD < HC: Cerebellum, BL ACC, BL PCC, L Primary Sensory & Motor, Parietal Cortex Stress Condition: M: CUD > HC in cerebellum, ACC, NAcc, thalamus, insula; CUD < HC in precuneus W: CUD > HC in ACC, PCC, BL vmPFC, BL dmPFC, L dlPFC, BL insula, BL OFC, BL amygdala, BL hippocampus, R NAcc |
Drug > Neutral: M: Positive association between craving and reactivity in the hippocampus, insula, PCC, dlPFC, dmPFC, temporal and parietal cortices, cerebellum W: Positive association between craving and reactivity in the midbrain, hippocampus, vlPFC, temporal cortex, cerebellum, thalamus Stress Condition > Neutral: M: Positive association between craving and reactivity in the BL occipital cortex W: No brain-behavior associations |
| Nikolova 2013 | Approach; Number-guessing |
M > W problem drinking Covariate(s): Current Axis 1 Diagnosis, Age |
Positive > Negative Feedback CA haplotype carriers only: M > W in BL NAcc reactivity |
--- |
M: Increased NAcc reactivity mediated an association between CA haplotype and increased problematic drinking W: Decreased R NAcc reactivity mediated an association between CA haplotype and decreased problematic drinking |
| Wetherill 2013 | Approach; Smoking-related audio/visual cues |
M > W: CPD & Pack-years W > M: Cue-elicited craving Covariate(s): CPD |
Smoking > Neutral Cue M > W: Activity in BL clusters in the hippocampus and amygdala to smoking cues |
--- | Craving was not associated with brain reactivity in either men or women |
| Mendrek 2014 | Approach; Smoking-related visual cues |
No sex/gender differences in basal or cue-induced craving, depressive symptoms Covariate(s): None |
Smoking-related > Smoking Unrelated Cues No sex/gender differences |
---- | Not reported |
| Padula 2015 |
Approach & Negative Emotionality; Emotional Faces |
Sex/gender differences not reported Covariate(s): Age, Handedness, Task Accuracy |
Not reported | Interaction effect: Fearful > Neutral faces: M AUD > M HC while W AUD < W HC in the BL OFC Happy > Neutral Faces: M AUD > M HC while W AUD < W HC in L caudate, L OFC, L Parietal cortex and R Occipital cortex |
Not reported |
| Wetherill 2015 | Approach; Backward-Masked Cue Task |
No sex/gender differences in cannabis use behaviors or craving M > W # weekly drinkers Covariate(s): Age, weekly quantity and frequency of substance use; HAM-D/A |
No sex/gender differences | ---- |
M: Baseline cannabis craving positively associated with striatum activation to cannabis cues W: Baseline cannabis craving positively correlated with bilateral anterior insula activation to cannabis cues; inversely correlated with the L lateral OFC activity |
| Canterberry 2016 |
Approach & Negative Emotionality; Emotional Stimuli |
No sex/gender differences in demographics, cocaine use behavior, or other clinical variables; No sex/gender differences on arousal rating to cues Covariate(s): Depression symptomology, education, state anxiety, CPD |
Arousing Positive > Neutral Cues M > W in the R OFC Arousing Negative > Neutral Cues No sex/gender differences |
Arousing Positive > Neutral M: No significant differences W: CUD < HC in OFC, vACC, PCC, angular gyrus Arousing Negative > Neutral M: No significant differences W: CUD < HC OFC, ACC, fusiform cortex, L temporal cortex, putamen |
Not reported |
| Konova 2016 | Approach; Monetary Reward |
M < W task reaction time; No sex/gender differences in cue-elicited cocaine craving or cocaine use behaviors; Female cocaine users more likely to report lifetime mood disorders relative to male cocaine users Covariate(s): Age, BDI scores, and reaction times |
W < M in R Hippocampus, BL PCC, L temporal cortex |
SUD W < SUD M, HC W in R primary Motor SUD W > SUD M, HC W in L Broca’s Area |
No sex/gender differences in brain-behavior associations |
| Zanchi 2016 | Approach; Smoking-related audio-visual cues |
No sex/gender differences in smoking behavior or cue-elicited craving Covariate(s): None |
Smoking > Neutral Cue W > M: Activity in the dlPFC, Broca’s Area, dACC, and temporal and occipital cortices |
Sex/gender difference in dlPFC, Broca’s Area, dACC, and precuneus was greater in smokers relative to non-smokers | Craving was not associated with brain reactivity in either M or W |
|
Dumais 2017 Study 1 |
Approach; Smoking-related audio-visual cues |
M > W pack-years No sex/gender differences in basal or cue-induced raving Covariate(s): Pack-years |
Smoking > Neutral M > W reactivity in the NAcc and vmPFC |
---- |
M: Change in pre- to post- scan craving was positively correlated with NAcc reactivity W: No brain-behavior associations |
|
Dumais 2017 Study 2 |
Approach; Smoking-related visual cues |
M > W cigarettes per day No sex/gender differences in basal or cue-induced craving Covariate(s): CPD |
Smoking > Neutral M > W in bilateral vmPFC |
--- | M: Positive association between post-SC craving in and entire ROI mask (encompassing all listed regions) W: No brain-behavior associations |
| Sawyer 2019 |
Approach & Negative Emotionality; Emotional Stimuli |
AUD W < AUD M average # daily alcoholic drinks & sensation-seeking AUD W > AUD M delayed memory scores & Wechsler Memory Scale Covariate(s): None |
Tested but region-specific results not reported |
Happy and Erotic > Neutral: AUD M < HC M in BL OFC, BL dlPFC, L parietal cortex, BL SMA, L Occipital cortex, L precuneus, L cuneus, L dACC, L cerebellum; W either did not exhibit these decreases or exhibited increases Aversive and Gruesome > Neutral: AUD M < HC M in L dmPFC, R OFC and L dlPFC, BL SMA, L parietal cortex, L temporal cortex, L cerebellum; W either did not exhibit these decreases or exhibited increases |
Not reported |
| Molokotos 2020 |
Approach; Smoking-related visual cues |
No sex/gender differences in smoking behavior or demographics Covariate(s): Discriminability on PRT task |
Not reported | --- |
M: Out-of-scanner PRT response bias positively associated with L caudate reactivity W: No association between PRT and brain reactivity |
| Prashad 2020 | Approach; Visual Drug Cue |
M > W number of alcohol drinking days and number of drinks W > M subjective cannabis craving Covariate(s): None |
No sex/gender differences in neural response | --- | Not reported |
| Zhang 2020 | Approach; Cocaine-related visual cues |
No sex/gender differences in cocaine use measures or craving CUD W > CUD M on BDI score, age Covariate(s): Age, Years of drinking, BDI scores |
Whole brain analysis: No sex/gender differences ROI: PAG Activity: No sex/gender differences PAG Connectivity No sex/gender differences |
--- |
PAG Activity: No sex/gender differences PAG Connectivity M: PAG -- vmPFC connectivity positively associated with craving W: PAG – vmPFC connectivity negatively associated with craving Granger Causality analysis: M: PAG → vmPFC W: vmPFC → PAG |
| Oscar-Berman 2021 |
Approach & Negative Emotionality; Emotional Faces |
AUD W < AUD M Duration of Heavy Drinking in Years AUD W > AUD M HAM-D/A Covariate(s): None |
Whole brain analysis: No sex/gender differences ROI analysis No sex/gender differences |
Whole brain analysis: Positive > Fixation HC W > AUD W, differences in L temporal cortex, but not between in M Negative > Fixation AUD M > HC M, differences in L FEF, but not in W ROI analysis: Emotion X Group X Sex/Gender: In HC M and AUD W: Greater reactivity to positive faces (vs. fixation) relative to negative and neutral faces compared to AUD men and HC women in L hippocampus |
Not reported |
| Claus 2022 |
Approach & Negative Emotionality; Alcohol & Negative Cues |
No sex/gender differences in alcohol use behaviors W > M in craving, sadness, fear affect, and perceived stress; M > W in self-efficacy Covariate(s): None |
Alcohol > Neutral Cues Whole brain analysis: W > M in the bilateral somatosensory cortex/precuneus, L dlPFC, and vmPFC ROI analysis: No main effect of sex/gender Negative > Neutral Cues Whole brain analysis: No main effect of sex/gender ROI analysis: W > M in L NAcc – vACC and L NAcc – medial OFC connectivity |
--- | Not reported |
| Smith 2023 |
Approach & Negative Emotionality; Stress & Drug Imagery Script |
SUD M > SUD W years of substance use SUD W > SUD M history of trauma, lifetime PTSD, anxiety ratings to drug cues with same pattern at the trend-level for stress cues; No sex/gender main effect on craving, heart rate, anxiety Covariate(s): CTQ and significant sex differences in any demographic variable included as a covariate in all analyses |
Drug Cues (Drug > Neutral): No sex/gender differences Stress Cues (Stress > Neutral): M > W in the BL caudate, thalamus, hypothalamus, R hippocampus, and L putamen |
--- |
Drug Cues: M: Positive association between reactivity in the putamen and caudate and # of follow-up days of substance use W: Inverse association between reactivity in the L dlPFC and L insula and future number of days used Stress Cues: M: No brain-behavior associations W: Inverse association between reactivity in the vmPFC and future days of substance use |
| Luo 2013 |
Executive Function; Stop Signal Paradigm |
No sex/gender differences in craving or task performance W > M # days used cocaine M > W # years of alcohol use Covariate(s): Years of cocaine and alcohol use, amount of cocaine and alcohol use in the month before admission |
Not reported | --- |
Stop Error > Stop Success M: Decreased left insula and dACC activation predicted relapse and earlier time to relapse W: Decreased thalamic and dACC activation predicted relapse and earlier time to relapse |
| Zhang 2014 |
Executive Function; Stop Signal Paradigm |
Sex/gender differences in cocaine use measures, craving, and task performance not assessed
Covariate(s): Days of abstinence |
Stop Error > Stop Success Not reported |
Not reported |
M: No brain-behavior associations W: Thalamus – vmPFC connectivity was positively correlated with the amount of cocaine use in the month before admission |
| Swartz 2021 | Executive Function; Stop Signal Paradigm |
No significant first-order correlation between sex/gender and AUDIT scores or age Sex/gender differences in other clinical measures (anxiety, mood) not statistically tested M < W RT on ‘Go’ Trials Covariate(s): Diagnosis of mood or anxiety disorder, self-reported use of cannabis in the previous 90 days |
Correct Stop > Correct Go No sex/gender differences Incorrect Stop > Correct Go: M > W reactivity in the primary motor cortex/somatosensory cortex, parietal cortex, precuneus, lateral occipital cortex Correct stop > Incorrect stop No sex/gender differences |
--- |
M: Negative association between reactivity in the somatosensory and lateral occipital cortex and AUDIT score W: Positive association between reactivity and AUDIT score in the somatosensory and lateral occipital cortex |
| Li 2005 |
Negative Emotionality; Stress Imagery Script |
No sex/gender differences in cue-elicited heart rate, anxiety, craving, or vividness rating of imagery No sex/gender differences in cocaine measures Covariate(s): None |
W > M: L FEF, L vACC, R PCC, L Broca’s Area, L insula |
--- |
M: No brain-behavior associations W: R PCC reactivity inversely correlated with cocaine craving and positively correlated with change in heart rate |
| Gorka 2019 |
Negative Emotionality; Threat Startle Response |
Sex/gender differences in demographics, alcohol use behaviors, craving, or task performance not reported Covariate(s): None |
No main effect of sex/gender | No interaction effect | No Brain X Sex/Gender Interaction of behavioral measures |
| Chang 2002 | Resting | Sex/gender differences in methamphetamine use behavior, demographics, or behavior not reported Covariate(s): None |
Not reported | Interaction Effect: W: MD > HC R occipital cortex and midline structure (pineal gland) M: MD < HC R occipital cortex and midline structure (pineal gland) |
Not reported |
| Tanabe 2008 | Resting |
No sex/gender differences in smoking behavior or age Covariate(s): None |
No main effect of sex/gender | ---- | Not reported |
| Wetherill 2014 | Resting |
M > W CPD Covariate(s): None |
W > M connectivity between the hippocampus/amygdala and bilateral AI, vACC, & L parietal cortex | --- | Not reported |
| Beltz 2015 | Resting |
No sex/gender differences in smoking behavior Covariate(s): Age, CO in analyses related to nicotine dependence/tolerance |
W > M in Default Mode Network (PCC, dACC, BL parietal cortex) | --- |
M: No brain-behavior associations W: Positive correlation between nicotine tolerance and connectivity within the reward network (bilateral striatum, OFC) |
| Li 2016 | Resting | Sex/gender differences in alcohol use measures, craving, and behavior not reported Covariate(s): Age |
W > M BEN in L primary motor, BL somatosensory, BL temporal, L parietal, and R occipital cortices, precuneus, R cerebellum | Smokers had less widespread resting BEN difference between men and women relative to HC |
M: Positive association between BEN and years-smoking in R premotor/SMA; Negative association with R cuneus, L occipital cortex, R OFC; Further correlations between BEN and CPD, FTND) (see Li et al
supplemental material) W: Positive association between BEN and years-smoking in R amygdala, BL Broca’s area, R primary motor, BL temporal, parietal, occipital cortices; further correlations between BEN and CPD, FTND) (see Li et al supplemental material) |
| MS Maria 2017 | Resting |
No sex/gender differences in demographics, smoking behavior or dependence Covariate(s): None |
No sex/gender differences | Not reported |
W: No brain-behavior associations M: Positive association between EVC and FTND in R insula; trend-level positive association between DIV and FTND in the L NAcc |
| Zhang 2017 | Resting |
M > F years of tobacco use Compared to HC, W but not men smokers reported greater narcotics and marijuana use Covariate(s): Age, Years of marijuana use, Days of marijuana use in the prior month |
Not reported |
No interaction effect. M and W analyzed separately: M: Smokers > Nonsmokers: None Nonsmokers > Smokers: None W: Smokers > Nonsmokers: SMA, somatosensory and parietal cortices, anterior insula; Smokers < Nonsmokers: PCC/precuneus |
M: BNM-SMA connectivity negatively correlated with FTND W: No brain-behavior associations |
| Manza 2018 | Resting | Sex/gender effects on cannabis use measures and craving not reported Covariate(s): None |
No sex/gender differences | No sex/gender differences | Not reported |
| Zhang 2018 | Resting | Sex/gender effects on methamphetamine use, craving, task performance not reported Covariate(s): Age, Smoking status |
Not reported | Interaction effect: M: CD < HC MH-dmPFC connectivity W: CD > HC MH-dmPFC connectivity |
M: No brain-behavior associations W: MH-dmPFC connectivity is negatively associated with craving |
| Canessa 2021 | Resting |
No sex/gender differences in alcohol use behavior No sex/gender differences in out-of-scanner task performance; Covariate(s): Age, smoking status |
Not reported | No Sex X Group effects | No Sex X Group X Decision-latency effects on resting-state metrics |
ADS = Alcohol Dependence Scale; AUD = Alcohol Use Disorder; AUDIT = Alcohol Use Disorders Identification Test; BDI = Beck Depression Inventory; BEN = Brain Entropy; BL = Bilateral; BNM = Basal Nucleus of Meynert; CA = haplotype of GAL gene; CD = Cocaine-dependent; CO = Carbon monoxide CPD = Cigarettes smoked per day; CTQ.= Childhood Trauma Questionnaire; CUD = Cocaine Use Disorder; dACC = dorsal anterior cingulate cortex; DIV = Diversity coefficient; dlPFC = dorsolateral prefrontal cortex; dmPFC; = dorsomedial prefrontal cortex; EVC = Eigenvector centrality; FTND= Fagerstrom Test for Nicotine Dependence; FEF = Frontal eye field; HAM-D/A = Hamilton Depression Rating scale; Hamilton Anxiety Rating Scale; HC = healthy control L = Left; M = Men; MD = Methamphetamine-dependent; MH = Medial hypothalamus; NAcc = Nucleus Accumbens; OFC = Orbitofrontal cortex; R = Right; Pack-years = Quantification of the amount an individual has smoked, calculated by the number of packs smoked per day multiplied by the number of years the individual has smoked; PAG = Periaqueductal gray; PCC= Posterior cingulate cortex; PTSD = Post-Traumatic Stress Disorder; PRT = Probabilistic reward task; SJWQ = Shiffman-Jarvik Withdrawal Questionnaire; ROI = Region of Interest; RT = Reaction Time; SM = Substance misuse; SMA = Supplemental motor area; SUD = Substance use disorder; vACC= Ventral anterior cingulate cortex; vlPFC = Ventrolateral prefrontal corte; vmPFC= Ventromedial prefrontal cortex; W = Women
Executive Control Functional Domain
Three reviewed papers used a task-based design to measure Executive Function, all assessing response inhibition by implementing a Stop Signal Task (Table 1) [61–63]. Overall, the thalamus was the most frequently reported brain region to indicate any sex/gender differences, with two of the three studies reporting a significant finding in this region (Supplemental Figure 3, Supplemental Table 5) [61,62]. Only one study conducted a direct comparison between men and women with substance misuse [63]. This study found that when measuring error processing, men exhibited increased reactivity in the precuneus and primary motor, somatosensory, parietal, and lateral occipital cortices relative to women [63]. Two studies reported sex/gender differences in brain-behavior associations involving the thalamus, such that there were sex/gender-specific relationships between thalamic connectivity and substance use behavior measures (Table 2) [61,62].
Negative Emotionality Functional Domain:
Nine papers used a task paradigm to investigate Negative Emotionality (Table 1). Overall, the most frequently reported region with observed sex/gender differences was the OFC/vmPFC, with four of the nine studies (44%) reporting a significant finding (Supplemental Figure 4, Supplemental Table 6) [52,55,56,58]. Only one of the studies that found a sex/gender effect in the OFC/vmPFC conducted a direct comparison between men and women with substance misuse (Table 2) [52]. Similar to those that examined Approach Behavior, the higher quality studies reported sex/gender differences in the OFC/vmPFC (see Supplemental Table 2 for details). Potenza and colleagues found a three-way interaction between diagnosis (cocaine dependence or healthy control), sex/gender, and condition (stress- or drug-related imagery) that evidenced a significant interaction effect in the OFC, among other regions [55]. After examining men and women with cocaine dependence separately under the stress condition, they found that women with cocaine dependence demonstrated increased OFC reactivity to stressful imagery (relative to neutral imagery) compared to women without cocaine dependence (Table 2) [55]. This pattern was not observed in men with cocaine dependence. Although Claus and colleagues (2022) did not observe sex/gender differences in reactivity to negative cues in a whole-brain analysis, they did find that women with alcohol misuse exhibited increased connectivity between the NAcc, the ventral ACC and medial OFC relative to men with alcohol misuse [52]. Finally, Smith and colleagues found that in women with Alcohol and/or Cocaine and/or Cannabis Use Disorder, but not men, there was an inverse association between reactivity in the vmPFC and future days of substance use [64]. Together, similar to Approach Behavior, the findings from these more rigorous studies bolster the evidence that the OFC/vmPFC may be a key region of neurobiological sex/gender difference underlying Negative Emotionality in addiction.
Resting-state
Ten reviewed studies used a resting-state paradigm (Table 1). The most frequently reported region to demonstrate sex/gender differences was the parietal cortex, with three of eight (38%) studies examining this region reporting a significant finding (Supplemental Figure 5; Supplemental Table 7) [65–67]. All three of these studies performed a direct comparison between nicotine-dependent men and women and found that women with nicotine dependence demonstrated increased connectivity in regions within the parietal cortices relative to men. Specifically, Li and colleagues found that women demonstrated increased brain entropy in the superior parietal lobule, Wetherill and colleagues found that women demonstrated increased cerebral blood flow between the hippocampus/amygdala and the inferior parietal lobule, and Beltz and colleagues found that women had increased connectivity between key regions of the Default Mode Network, specifically the PCC, dACC, and bilateral lateral parietal lobules [65–67].
Discussion
In this review, we characterized the current state of the literature regarding sex/gender differences in the neurobiological mechanisms underlying substance misuse in adults, as assessed by fMRI. We examined the addiction-related functional domains of Approach Behavior, Executive Function, and Negative Emotionality that parallel the multi-dimensionality observed in impulsivity. We now discuss both our synthesized findings of neurobiological mechanisms of addiction as well as broader patterns of the reviewed literature and their implications.
We found that in studies that investigated Approach Behavior and Negative Emotionality across a variety of substances, the OFC/vmPFC was the most frequently reported region to exhibit a difference between men and women with substance misuse. The most consistent finding in our review was increased reactivity in men relative to women in response to drug-related or positively-arousing stimuli, whereas women, relative to men, demonstrated increased reactivity to negative stimuli. Similarly, previous research in the general population suggested that women, in comparison to men, demonstrate selective recruitment of the OFC and amygdala to negative, threatening cues (e.g., angry faces) [68]. Furthermore, reactivity of the OFC in response to appetitive cues in a food craving task was related to levels of hunger in men but not women [69]. Both the OFC and vmPFC play key roles in computing subjective value and reward expectations [44,70,71]. Taken together, our and other’s findings suggest sex/gender differences in the OFC-mediated subjective valuation of both appetitive and negative cues.
Both preclinical and human research also support a role of the OFC as a key mediator of impulsive decision-making. Individuals with OFC lesions have demonstrated increased impulsive behavior in gambling tasks [72–75]. Rodents with excito-toxic lesions of the OFC have demonstrated increased preference to larger but delayed rewards, suggesting an inability to alter preference for a reward despite a decrease in value [75]. These findings support that the OFC may facilitate updating the incentive salience of a reward under dynamic conditions (i.e., adaptive decision-making) [75,76]. Interestingly, dissociable effects have been observed within the OFC, where lesions of the medial OFC (often equated with the vmPFC) resulted in increased preference for a larger, delayed reward, whereas lesions to the lateral OFC resulted in decreased preference for the larger, delayed reward [77]. These findings further corroborate the role of the OFC in adaptative valuation of reward.
Moreover, the OFC is considered a key brain region that may contribute to the impairment of impulse control associated with substance misuse [78,79]. Preclinical work found that increased impulsivity during withdrawal from cocaine is driven by OFC dysfunction [80]. Cocaine-induced changes in the OFC may impair adaptive behavioral control, thus lending vulnerability to less flexible, maladaptive behavior characteristic of SUDs [81,82]. Importantly, the sex/gender differences observed in OFC function may extend to addiction, as previous work suggests that men with cocaine dependence demonstrate bilateral OFC hypoperfusion while women demonstrate more limited medial OFC hypoperfusion [83]. One study examining internet gaming disorder (a behavioral addiction) found decreased vmPFC reactivity to risky decisions in affected men, while there were no group differences in women [84]. Thus, our current review contributes to the extant literature by synthesizing the evidence that supports sex/gender-based neurobiological differences in OFC/vmPFC function, particularly concerning Approach Behavior and Negative Emotionality. These findings have considerable implications for our understanding of impulsivity in addiction.
While only three of the reviewed studies investigated Executive Function, precluding any formal conclusions, previous literature supports sex/gender differences in the neural networks underlying this domain, namely response inhibition [85]. One review of 21 studies examining sex/gender differences in executive function tasks identified differences during response inhibition in the OFC, middle frontal gyrus, superior frontal gyrus, and inferior frontal gyrus [85]. Thus, it is plausible that, given more comprehensive investigation, the neurobiological sex/gender differences we find in the OFC/vmPFC may also extend to the Executive Function domain, bolstering the differential role of OFC-mediated impulsive behavior in men and women with substance misuse.
Finally, resting-state studies did not capture this OFC/vmPFC effect that we observed in the task-based fMRI studies, suggesting that task-based paradigms may be needed to investigate neurobiological sex/gender differences in SUDs linked to behavior. During resting-state, the most consistent sex/gender differences observed were found in the parietal cortex, which forms the fronto-parietal resting-state network underlying attentionally-driven executive function, a key component of cognitive control. Fronto-parietal network activity has been shown to be modulated in addiction, but recent work suggests a lack of sex differences in resting-state networks in healthy populations [86,87]. These disparate findings suggest that dysregulation in these networks may be a key sex/gender neurobiological difference in substance misuse at rest.
Importantly, these sex/gender differences in the neurobiological mechanisms in populations with substance misuse parallel work investigating the divergent patterns of impulsivity in men and women. A growing body of literature suggests that the impulsivity dimensions captured by Approach Behavior (i.e., sensation-seeking and positive urgency) may be more pertinent to substance misuse in men, while impulsivity dimensions captured by Negative Emotionality (i.e., negative urgency) may be more pertinent to women [14,88]. For example, recent work demonstrated that higher levels of sensation-seeking was linked to driving under the influence of alcohol in men, but not in women [89]. In contrast, substance-dependent women demonstrated increased psychological and physical reactivity to stressors relative to substance-dependent men [22]. Understanding these sex/gender differences in impulse control in populations with SUDs is important because there is evidence, albeit limited, that suggests sex/gender-specific efficacy of both pharmacological and psychosocial treatments [90–93].
Given the low number of neuroimaging studies that have investigated sex/gender differences, the overall conclusions drawn from this review need to be tentative. The current state of the literature is extremely limited, with only 34 neuroimaging papers since 2000 reporting sex/gender analyses in their study of adults with SUDs. Of the 34 studies included in this review, 19 (55%) were published following the NIH mandate including sex as a biological variable in 2015, indicating that the state of sex/gender difference research in addiction neuroimaging has been evolving but slow. This lack of data is concerning, given that the closing gender gap in addiction is driven primarily by the increase in substance use in women [25–27]. Furthermore, there is significant room for improvement in the measurement of sex/gender. First, all papers implicitly assumed sex and gender as binary. Although three papers categorized participants by ‘biological sex’, these papers did not report how they measured sex (e.g., self-reported or medical records of sex assigned at birth, chromosomal analysis, and/or reproductive anatomy) [63,94,95]. Only one paper rationalized their use of the term ‘gender [96]. Recent work in both the psychological and neuroscience literature suggests that measuring both sex and gender as non-binary, multidimensional constructs may more accurately represent both brain structure/function and uncover stronger brain-behavior associations [50,97,98]. The inclusion of both gender diverse and intersex samples in clinical addiction research is crucial, as there is increased rates of Substance Use Disorders in these communities [99]. Second, only three papers examined the role of sex hormones or menstrual cycle phase (although our search terms were not designed to target neuroimaging studies examining the role of sex hormones in addiction) [55,100,101]. Recent work demonstrates dynamic sex hormone-driven functional reorganization across the menstrual cycle, suggesting a potential role of hormone-driven processes on addiction and other psychopathologies [102]. Finally, none of the studies examined the role of gendered social context, such as social support, on brain function in addiction. Investigating the neurofunctional role of social support in SUD is rare, with only one publication to date using neuroimaging to study social support, negative emotionality, and gender differences in a substance misuse population [103]. Given that our recent work has evidenced the gender-specific role of social support in SUDs, more work in this area of research is warranted [4].
The literature on sex/gender differences in substance misuse is not only limited in general, but also lacks investigations into these effects in populations with different primary drugs of use. We found that nicotine was the most frequent drug of abuse studied, with 13 of the 34 papers focusing on smokers. In contrast, there was not a single study that examined sex/gender differences in opioid misuse. This disparity is particularly concerning, given the drastic impact of the ongoing opioid epidemic in the United States on women. Women have experienced a 642% increase in overdose deaths from 1999–2017 relative to the 439% increase in men over the same time period, yet another indication of the closing addiction gender gap driven by women [104]. The reasons for this closing gender gap are multifactorial, with both social context and sex-specific biological milieu likely playing a role. Thus, it is essential that opioid misuse be incorporated into future work on sex/gender differences in addiction.
Finally, we evaluated the quality of the reviewed studies based on three criteria: sample size, scan length duration, and statistical analyses (see Supplemental Table 2). The samples included in the reviewed papers were small to moderately sized. Only four papers included sample of at least 40 participants per group and were relatively balanced (40–60%) between men and women (Table 1 [52,63,101,105]). On average, most papers had a smaller proportion of women relative to men, and the most frequently reported sample size for both men and women with substance misuse was small at N=10 people (Figure 2). This pattern is particularly problematic given concerns about underpowered analyses, especially in brain-wide analyses of brain-behavior associations that may have small effect sizes [106]. We found that in addition to the small number of studies, challenges with data quality also impede the overall interpretability of our findings. While the average total scan length across both task and resting-state scans was about 20 minutes, the range was substantial (4 – 54 minutes). Twelve papers reported a scan duration of over 20 minutes, although two studies could not be evaluated because they did not report the duration. Twelve papers reported a scan length of less than 10 minutes duration; recent work suggests that anything below 10 minutes of resting-state data leads to low reliability estimates and systematically biased results [107]. Correspondingly, a recent meta-analysis of task fMRI studies highlights the poor test-retest reliability of fMRI tasks and proposes that both increasing sample size and the quantity of data collected per individual may be necessary to improve reliability [108]. To maintain a small to moderate (and thus more feasible) sample size, data quantity could be substantially increased by either increasing scan acquisition time or by implementing high-density sampling within a limited number of individuals [107,109,110]. Finally, just over half of the studies (N = 18) tested sex/gender differences in behavior and/or drug use variables and accounted for these confounders in the brain analyses, as appropriate. Several papers (N = 11) either did not test for potentially confounding variables or found significant sex/gender effects and did not account for these confounds in the brain analyses. There appeared to be a general trend of more consistent findings in higher quality studies, with 52% of moderate or high-quality papers that investigated Approach Behavior or Negative Emotionality reporting a sex/gender effect in the OFC/vmPFC and none of the low-quality papers.
Based on our results, we would like to highlight several opportunities for growth in the field of studying sex/gender differences in the neurobiological mechanisms of substance misuse. First, although sex/gender differences in all functional domains are understudied, research probing the neurobiological processes underlying Executive Function are particularly lacking both in number (i.e., how many papers are published) and breadth (i.e., variety of tasks). Second, future work should not only equally represent men and women in their samples, but also integrate measures probing both gendered social context (e.g., social support) as well as sex-related neuroendocrine variables (e.g., estrogen, testosterone levels), which are woefully understudied [111]. Third, there is a need for more work probing how to accurately operationalize both sex and gender as multidimensional rather than binary constructs, and how to include both gender diverse and intersex participants in clinical addiction neuroscience research. Fourth, there is currently no work on sex/gender differences in neuroimaging of opioid dependence, which is an enormous area of clinical need that has yet to be explored. Finally, particular emphasis on improving data quality through larger sample sizes, longer scan length, higher density sampling, and/or appropriate statistical controls may aid in the reproducibility of results.
Conclusion
In conclusion, the present review describes early evidence for sex/gender differences in key regions of addiction neurocircuitry across three neurobehavioral functional domains, with implications for understanding neurobiological mechanisms underlying the multi-dimensionality of impulsivity. We have reviewed the extant literature and demonstrated that there is (1) early evidence for sex/gender differences in the OFC/vmPFC in both Approach Behavior and Negative Emotionality in addiction, with potential for this to be extended to the Executive Function domain, and (2) this evidence supports a larger role of Approach Behavior-related impulsivity dimensions (sensation-seeking and positive urgency) in men and Negative Emotionality-related impulsivity dimensions (negative urgency) in women. We also found that there is (3) an empirical demand for more nuanced and balanced practices in the inclusion, measurement, and reporting of sex and gender, (4) an urgent need for research in sex/gender differences in the neurobiology underlying opioid dependence, and (5) an increased need for improved data quality. Overall, it is imperative to bolster this scientific body of work given the incredible clinical need, especially in women with substance misuse.
Supplementary Material
Acknowledgements
We thank Laura E. Padilla for her review and edits.
Funding:
This work was supported by the Dr. Warren J. Warwick and Henrietta Holm Warwick Fellowship by the University of Minnesota (to AMM) and by a grant by the National Institute on Alcohol Abuse and Alcoholism (R01AA029406 to AZ).
Footnotes
Conflict of interest: The authors have no relevant financial or non-financial interests to disclose.
Ethics Declarations
Human and Animal Rights and Informed Consent: N/A
References
- 1.Understanding Drug Overdoses and Deaths. [cited 5 Apr 2023]. Available: https://www.cdc.gov/drugoverdose/epidemic/index.html#:~:text=Overdose%20deaths%20remain%20a%20leading,have%20increased%20in%20recent%20years.
- 2.U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. National Survey on Drug Use and Health 2021. 4 Jan 2023. Available: https://www.samhsa.gov/data/report/2021-nsduh-annual-national-report
- 3.Rawls E, Kummerfeld E, Zilverstand A. An integrated multimodal model of alcohol use disorder generated by data-driven causal discovery analysis. Commun Biol. 2021;4: 435. doi: 10.1038/s42003-021-01955-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. ••. Maxwell AM, Harrison K, Rawls E, Zilverstand A. Gender Differences in the Psychosocial Determinants Underlying the Onset and Maintenance of Alcohol Use Disorder. Front Neurosci. 2022;16: 808776. doi: 10.3389/fnins.2022.808776. A perspective piece that provided empirical evidence and a literature review on the gender-specific role of psychosocial factors in alcohol misuse and dependence.
- 5. ••. Niklason GR, Rawls E, Ma S, Kummerfeld E, Maxwell AM, Brucar LR, et al. Explainable machine learning analysis reveals sex and gender differences in the phenotypic and neurobiological markers of Cannabis Use Disorder. Sci Rep. 2022;12: 15624. doi: 10.1038/s41598-022-19804-2. A machine learning analysis that demonstrated that environmental factors such as social support and education level play a larger role in cannabis misuse and dependence in women compared to men.
- 6.Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158: 1783–1793. doi: 10.1176/appi.ajp.158.11.1783 [DOI] [PubMed] [Google Scholar]
- 7.Saitz R, Larson MJ, Labelle C, Richardson J, Samet JH. The case for chronic disease management for addiction. J Addict Med. 2008;2: 55–65. doi: 10.1097/ADM.0b013e318166af74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). 2013. [Google Scholar]
- 9.Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320: 1352–1355. doi: 10.1126/science.1158136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Lääne K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315: 1267–1270. doi: 10.1126/science.1137073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363: 3125–3135. doi: 10.1098/rstb.2008.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torres A, Catena A, Megías A, Maldonado A, Cándido A, Verdejo-García A, et al. Emotional and non-emotional pathways to impulsive behavior and addiction. Front Hum Neurosci. 2013;7: 43. doi: 10.3389/fnhum.2013.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. •. Teese R, Willie C, Jago A, Gill PR. An Investigation of Alternative Factor Models of Impulsivity using the UPPS-P. J Pers Assess. 2021;103: 324–331. doi: 10.1080/00223891.2020.1739059. Validation of the five-factor model of impulsivity through confirmatory factor analysis.
- 14.Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, et al. Sex differences in stress-related alcohol use. Neurobiol Stress. 2019;10: 100149. doi: 10.1016/j.ynstr.2019.100149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ. 2012;3: 14. doi: 10.1186/2042-6410-3-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karmarkar UR. Gender differences in “optimistic” information processing in uncertain decisions. Cogn Affect Behav Neurosci. 2023. doi: 10.3758/s13415-023-01075-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker JB, McClellan ML, Reed BG. Sex differences, gender and addiction. J Neurosci Res. 2017;95: 136–147. doi: 10.1002/jnr.23963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cross CP, Copping LT, Campbell A. Sex differences in impulsivity: a meta-analysis. Psychol Bull. 2011;137: 97–130. doi: 10.1037/a0021591 [DOI] [PubMed] [Google Scholar]
- 19.Oyola MG, Handa RJ. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress. 2017;20: 476–494. doi: 10.1080/10253890.2017.1369523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. ••. Guinle MIB, Sinha R. The Role of Stress, Trauma, and Negative Affect in Alcohol Misuse and Alcohol Use Disorder in Women. Alcohol Res. 2020;40: 05. doi: 10.35946/arcr.v40.2.05. Review about sex/gender differences in the contribution of stress, trauma, and negative affect to alcohol misuse which calls for more research on sex/gender differences.
- 21.Blaine SK, Nautiyal N, Hart R, Guarnaccia JB, Sinha R. Craving, cortisol and behavioral alcohol motivation responses to stress and alcohol cue contexts and discrete cues in binge and non-binge drinkers. Addict Biol. 2019;24: 1096–1108. doi: 10.1111/adb.12665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology. 2005;180: 169–176. doi: 10.1007/s00213-004-2129-7 [DOI] [PubMed] [Google Scholar]
- 23. ••. Verplaetse TL, Cosgrove KP, Tanabe J, McKee SA. Sex/gender differences in brain function and structure in alcohol use: A narrative review of neuroimaging findings over the last 10 years. J Neurosci Res. 2021;99: 309–323. doi: 10.1002/jnr.24625. Recent systematic review of sex/gender differences in alcohol use using neuroimaging that highlights the disparities in sex/gender brain research.
- 24.Andersen ML, Sawyer EK, Howell LL. Contributions of neuroimaging to understanding sex differences in cocaine abuse. Exp Clin Psychopharmacol. 2012;20: 2–15. doi: 10.1037/a0025219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White AM. Gender Differences in the Epidemiology of Alcohol Use and Related Harms in the United States. Alcohol Res. 2020;40: 01. doi: 10.35946/arcr.v40.2.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grucza RA, Sher KJ, Kerr WC, Krauss MJ, Lui CK, McDowell YE, et al. Trends in adult alcohol use and binge drinking in the early 21st-century United States: A meta-analysis of 6 national survey series. Alcohol Clin Exp Res. 2018;42: 1939–1950. doi: 10.1111/acer.13859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McHugh RK, Nguyen MD, Chartoff EH, Sugarman DE, Greenfield SF. Gender differences in the prevalence of heroin and opioid analgesic misuse in the United States, 2015–2019. Drug Alcohol Depend. 2021;227: 108978. doi: 10.1016/j.drugalcdep.2021.108978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tellegen Waller. Exploring personality through test construction: Development of the Multidimensional Personality Questionnaire. The SAGE handbook of personality. 2008. Available: https://books.google.com/books?hl=en&lr=&id=sdD41qBTJSUC&oi=fnd&pg=PA261&dq=Exploring+Personality+Through+Test+Construction:+Development+of+the+Multidimensional+Personality+Questionnaire%E2%80%99&ots=Xm9q1vdX7P&sig=qzXplo5y4JHTpQWCqmJBwHtIHvU [Google Scholar]
- 29.National Institute of Mental Health. Definitions of the RDoC Domains and Constructs. [cited 25 Sep 2023]. Available: https://www.nimh.nih.gov/research/research-funded-by-nimh/rdoc/definitions-of-the-rdoc-domains-and-constructs
- 30. ••. Drossel G, Brucar LR, Rawls E, Hendrickson TJ, Zilverstand A. Subtypes in addiction and their neurobehavioral profiles across three functional domains. Transl Psychiatry. 2023;13: 127. doi: 10.1038/s41398-023-02426-1. Demonstrated the existence of three subtyes across different Substance use Disorders that each had an impairment on one of three domains: Approach Behavior, Executive Function and Negative Emotionality.
- 31.Kwako LE, Schwandt ML, Ramchandani VA, Diazgranados N, Koob GF, Volkow ND, et al. Neurofunctional Domains Derived From Deep Behavioral Phenotyping in Alcohol Use Disorder. Am J Psychiatry. 2019;176: 744–753. doi: 10.1176/appi.ajp.2018.18030357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yücel M, Oldenhof E, Ahmed SH, Belin D, Billieux J, Bowden-Jones H, et al. A transdiagnostic dimensional approach towards a neuropsychological assessment for addiction: an international Delphi consensus study. Addiction. 2019;114: 1095–1109. doi: 10.1111/add.14424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamilton KR, Mitchell MR, Wing VC, Balodis IM, Bickel WK, Fillmore M, et al. Choice impulsivity: Definitions, measurement issues, and clinical implications. Personal Disord. 2015;6: 182–198. doi: 10.1037/per0000099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Pers Individ Dif. 2001;30: 669–689. doi: 10.1016/S0191-8869(00)00064-7 [DOI] [Google Scholar]
- 35.Cyders MA, Smith GT, Spillane NS, Fischer S, Annus AM, Peterson C. Integration of impulsivity and positive mood to predict risky behavior: development and validation of a measure of positive urgency. Psychol Assess. 2007;19: 107–118. doi: 10.1037/1040-3590.19.1.107 [DOI] [PubMed] [Google Scholar]
- 36.Lynam DR, Smith GT, Whiteside SP, Cyders MA. The UPPS-P: Assessing five personality pathways to impulsive behavior. West Lafayette, IN: Purdue. 2006. Available: https://scholar.google.ca/scholar?cluster=8421730804696653372&hl=en&as_sdt=0,5&sciodt=0,5 [Google Scholar]
- 37. ••. Sánchez-Domínguez R, Benjet C, Marín-Navarrete R, Nicolini H. Validity and reliability of the short version of the UPPS-P impulsive Behavior Scale in patients with substance use disorders. J Subst Use. 2022; 1–9. doi: 10.1080/14659891.2022.2087777. Validation of the construct validity of the five-factor model of impulsivity in substance use disorder.
- 38.Zsila Á, Beáta Bőthe, Demetrovics Z, Billieux J, Orosz G. Further exploration of the SUPPS-P impulsive behavior scale’s factor structure: Evidence from a large Hungarian sample. Curr Psychol. 2020;39: 378–388. doi: 10.1007/s12144-017-9773-7 [DOI] [Google Scholar]
- 39.Naragon-Gainey K, Gallagher MW, Brown TA. Stable “trait” variance of temperament as a predictor of the temporal course of depression and social phobia. J Abnorm Psychol. 2013;122: 611–623. doi: 10.1037/a0032997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. J Exp Psychol Gen. 2008;137: 201–225. doi: 10.1037/0096-3445.137.2.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halvorson MA, Pedersen SL, Feil MC, Lengua LJ, Molina BSG, King KM. Impulsive States and Impulsive Traits: A Study of the Multilevel Structure and Validity of a Multifaceted Measure of Impulsive States. Assessment. 2021;28: 796–812. doi: 10.1177/1073191120939161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burnette EM, Grodin EN, Lim AC, MacKillop J, Karno MP, Ray LA. Association between impulsivity and neural activation to alcohol cues in heavy drinkers. Psychiatry Res Neuroimaging. 2019;293: 110986. doi: 10.1016/j.pscychresns.2019.110986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Owens MM, Amlung MT, Stojek M, MacKillop J. Negative urgency moderates reactivity to laboratory stress inductions. J Abnorm Psychol. 2018;127: 385–393. doi: 10.1037/abn0000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zilverstand A, Huang AS, Alia-Klein N, Goldstein RZ. Neuroimaging Impaired Response Inhibition and Salience Attribution in Human Drug Addiction: A Systematic Review. Neuron. 2018;98: 886–903. doi: 10.1016/j.neuron.2018.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Um M, Whitt ZT, Revilla R, Hunton T, Cyders MA. Shared Neural Correlates Underlying Addictive Disorders and Negative Urgency. Brain Sci. 2019;9. doi: 10.3390/brainsci9020036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bedir D, Reinhardt P, Winterer G, Zacharias N. Investigating Response Inhibition Using the Stop-signal Task and Neuroimaging Methods: A Literature Review. 2022. Available: https://osf.io/vm7wb/download. [Google Scholar]
- 47.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12: 652–669. doi: 10.1038/nrn3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rippon G, Jordan-Young R, Kaiser A, Fine C. Recommendations for sex/gender neuroimaging research: key principles and implications for research design, analysis, and interpretation. Front Hum Neurosci. 2014;8: 650. doi: 10.3389/fnhum.2014.00650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaiser A. Re-conceptualizing “sex” and “gender” in the human brain. Z Psychol. 2012;220: 130–136. doi: 10.1027/2151-2604/a000104 [DOI] [Google Scholar]
- 50.Hyde JS, Bigler RS, Joel D, Tate CC, van Anders SM. The future of sex and gender in psychology: Five challenges to the gender binary. Am Psychol. 2019;74: 171–193. doi: 10.1037/amp0000307 [DOI] [PubMed] [Google Scholar]
- 51.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509: 282–283. doi: 10.1038/509282a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Claus ED, Blaine SK, Witkiewitz K, Ansell EB. Sex moderates effects of alcohol and cannabis co-use on alcohol and stress reactivity. Alcohol Clin Exp Res. 2022;46: 530–541. doi: 10.1111/acer.14797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dumais KM, Franklin TR, Jagannathan K, Hager N, Gawrysiak M, Betts J, et al. Multi-site exploration of sex differences in brain reactivity to smoking cues: Consensus across sites and methodologies. Drug Alcohol Depend. 2017;178: 469–476. doi: 10.1016/j.drugalcdep.2017.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33: 2148–2157. doi: 10.1038/sj.npp.1301618 [DOI] [PubMed] [Google Scholar]
- 55.Potenza MN, Hong K-IA, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry. 2012;169: 406–414. doi: 10.1176/appi.ajp.2011.11020289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Padula CB, Anthenelli RM, Eliassen JC, Nelson E, Lisdahl KM. Gender effects in alcohol dependence: an fMRI pilot study examining affective processing. Alcohol Clin Exp Res. 2015;39: 272–281. doi: 10.1111/acer.12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Canterberry M, Peltier MR, Brady KT, Hanlon CA. Attenuated neural response to emotional cues in cocaine-dependence: a preliminary analysis of gender differences. Am J Drug Alcohol Abuse. 2016;42: 577–586. doi: 10.1080/00952990.2016.1192183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sawyer KS, Maleki N, Urban T, Marinkovic K, Karson S, Ruiz SM, et al. Alcoholism gender differences in brain responsivity to emotional stimuli. Elife. 2019;8. doi: 10.7554/eLife.41723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rudebeck PH, Rich EL. Orbitofrontal cortex. Curr Biol. 2018;28: R1083–R1088. doi: 10.1016/j.cub.2018.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heather Hsu C-C, Rolls ET, Huang C-C, Chong ST, Zac Lo C-Y, Feng J, et al. Connections of the Human Orbitofrontal Cortex and Inferior Frontal Gyrus. Cereb Cortex. 2020;30: 5830–5843. doi: 10.1093/cercor/bhaa160 [DOI] [PubMed] [Google Scholar]
- 61.Luo X, Zhang S, Hu S, Bednarski SR, Erdman E, Farr OM, et al. Error processing and gender-shared and -specific neural predictors of relapse in cocaine dependence. Brain. 2013;136: 1231–1244. doi: 10.1093/brain/awt040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang S, Hu S, Bednarski SR, Erdman E, Li C-SR. Error-related functional connectivity of the thalamus in cocaine dependence. Neuroimage Clin. 2014;4: 585–592. doi: 10.1016/j.nicl.2014.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swartz M, Burton F, Vakamudi K, Al-Khalil K, Witkiewitz K, Claus ED. Age dependent neural correlates of inhibition and control mechanisms in moderate to heavy drinkers. Neuroimage Clin. 2021;32: 102875. doi: 10.1016/j.nicl.2021.102875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith K, Lacadie CM, Milivojevic V, Fogelman N, Sinha R. Sex differences in neural responses to stress and drug cues predicts future drug use in individuals with substance use disorder. Drug Alcohol Depend. 2023;244: 109794. doi: 10.1016/j.drugalcdep.2023.109794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wetherill RR, Jagannathan K, Shin J, Franklin TR. Sex differences in resting state neural networks of nicotine-dependent cigarette smokers. Addict Behav. 2014;39: 789–792. doi: 10.1016/j.addbeh.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beltz AM, Berenbaum SA, Wilson SJ. Sex differences in resting state brain function of cigarette smokers and links to nicotine dependence. Exp Clin Psychopharmacol. 2015;23: 247–254. doi: 10.1037/pha0000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Z, Fang Z, Hager N, Rao H, Wang Z. Hyper-resting brain entropy within chronic smokers and its moderation by Sex. Sci Rep. 2016;6: 29435. doi: 10.1038/srep29435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McClure EB, Monk CS, Nelson EE, Zarahn E, Leibenluft E, Bilder RM, et al. A developmental examination of gender differences in brain engagement during evaluation of threat. Biol Psychiatry. 2004;55: 1047–1055. doi: 10.1016/j.biopsych.2004.02.013 [DOI] [PubMed] [Google Scholar]
- 69.Wang G-J, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci U S A. 2009;106: 1249–1254. doi: 10.1073/pnas.0807423106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moeller SJ, Goldstein RZ. Impaired self-awareness in human addiction: deficient attribution of personal relevance. Trends Cogn Sci. 2014;18: 635–641. doi: 10.1016/j.tics.2014.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith DV, Clithero JA, Boltuck SE, Huettel SA. Functional connectivity with ventromedial prefrontal cortex reflects subjective value for social rewards. Soc Cogn Affect Neurosci. 2014;9: 2017–2025. doi: 10.1093/scan/nsu005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, et al. Dissociable Deficits in the Decision-Making Cognition of Chronic Amphetamine Abusers, Opiate Abusers, Patients with Focal Damage to Prefrontal Cortex, and Tryptophan-Depleted Normal Volunteers: Evidence for Monoaminergic Mechanisms. Neuropsychopharmacology. 1999;20: 322–339. doi: 10.1016/S0893-133X(98)00091-8 [DOI] [PubMed] [Google Scholar]
- 73.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19: 5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation Of working memory from decision making within the human prefrontal cortex. J Neurosci. 1998;18: 428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Winstanley CA, Theobald DEH, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24: 4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Torregrossa MM, Quinn JJ, Taylor JR. Impulsivity, compulsivity, and habit: the role of orbitofrontal cortex revisited. Biol Psychiatry. 2008;63: 253–255. doi: 10.1016/j.biopsych.2007.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mar AC, Walker ALJ, Theobald DE, Eagle DM, Robbins TW. Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J Neurosci. 2011;31: 6398–6404. doi: 10.1523/JNEUROSCI.6620-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winstanley CA. The orbitofrontal cortex, impulsivity, and addiction: probing orbitofrontal dysfunction at the neural, neurochemical, and molecular level. Ann N Y Acad Sci. 2007;1121: 639–655. doi: 10.1196/annals.1401.024 [DOI] [PubMed] [Google Scholar]
- 79.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10: 318–325. doi: 10.1093/cercor/10.3.318 [DOI] [PubMed] [Google Scholar]
- 80.Winstanley CA, Bachtell RK, Theobald DEH, Laali S, Green TA, Kumar A, et al. Increased Impulsivity during Withdrawal from Cocaine Self-Administration: Role for ΔFosB in the Orbitofrontal Cortex. Cereb Cortex. 2008;19: 435–444. doi: 10.1093/cercor/bhn094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G. The impact of orbitofrontal dysfunction on cocaine addiction. Nat Neurosci. 2012;15: 358–366. doi: 10.1038/nn.3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lucantonio F, Caprioli D, Schoenbaum G. Transition from ‘model-based’ to ‘model-free’ behavioral control in addiction: Involvement of the orbitofrontal cortex and dorsolateral striatum. Neuropharmacology. 2014;76: 407–415. doi: 10.1016/j.neuropharm.2013.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adinoff B, Williams MJ, Best SE, Harris TS, Chandler P, Devous MD Sr. Sex differences in medial and lateral orbitofrontal cortex hypoperfusion in cocaine-dependent men and women. Gend Med. 2006;3: 206–222. doi: 10.1016/s1550-8579(06)80209-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang L, Zheng H, Wang M, Chen S, Du X, Dong G-H. Sex differences in neural substrates of risk taking: Implications for sex-specific vulnerabilities to internet gaming disorder. J Behav Addict. 2022;11: 778–795. doi: 10.1556/2006.2022.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gaillard A, Fehring DJ, Rossell SL. Sex differences in executive control: A systematic review of functional neuroimaging studies. Eur J Neurosci. 2021;53: 2592–2611. doi: 10.1111/ejn.15107 [DOI] [PubMed] [Google Scholar]
- 86.Weissman-Fogel I, Moayedi M, Taylor KS, Pope G, Davis KD. Cognitive and default-mode resting state networks: do male and female brains “rest” differently? Hum Brain Mapp. 2010;31: 1713–1726. doi: 10.1002/hbm.20968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Costumero V, Barrós-Loscertales A. The Left Frontoparietal Brain Network in Addictions. In: Patel VB, Preedy VR, editors. Handbook of Substance Misuse and Addictions: From Biology to Public Health. Cham: Springer International Publishing; 2021. pp. 1–24. doi: 10.1007/978-3-030-67928-6_27-1 [DOI] [Google Scholar]
- 88.Cyders MA. Impulsivity and the sexes: measurement and structural invariance of the UPPS-P Impulsive Behavior Scale. Assessment. 2013;20: 86–97. doi: 10.1177/1073191111428762 [DOI] [PubMed] [Google Scholar]
- 89.Navas JF, Martín-Pérez C, Petrova D, Verdejo-García A, Cano M, Sagripanti-Mazuquín O, et al. Sex differences in the association between impulsivity and driving under the influence of alcohol in young adults: The specific role of sensation seeking. Accid Anal Prev. 2019;124: 174–179. doi: 10.1016/j.aap.2018.12.024 [DOI] [PubMed] [Google Scholar]
- 90.Amaro H, Spear S, Vallejo Z, Conron K, Black DS. Feasibility, acceptability, and preliminary outcomes of a mindfulness-based relapse prevention intervention for culturally-diverse, low-income women in substance use disorder treatment. Subst Use Misuse. 2014;49: 547–559. doi: 10.3109/10826084.2013.852587 [DOI] [PubMed] [Google Scholar]
- 91.Fox HC, Morgan PT, Sinha R. Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology. 2014;39: 1527–1537. doi: 10.1038/npp.2014.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McKee SA, Smith PH, Kaufman M, Mazure CM, Weinberger AH. Sex Differences in Varenicline Efficacy for Smoking Cessation: A Meta-Analysis. Nicotine Tob Res. 2016;18: 1002–1011. doi: 10.1093/ntr/ntv207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, et al. Substance abuse treatment entry, retention, and outcome in women: a review of the literature. Drug Alcohol Depend. 2007;86: 1–21. doi: 10.1016/j.drugalcdep.2006.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wetherill RR, Jagannathan K, Hager N, Childress AR, Franklin TR. Sex differences in associations between cannabis craving and neural responses to cannabis cues: Implications for treatment. Exp Clin Psychopharmacol. 2015;23: 238–246. doi: 10.1037/pha0000036 [DOI] [PubMed] [Google Scholar]
- 95.Gorka SM, Kreutzer KA, Petrey KM, Radoman M, Phan KL. Behavioral and neural sensitivity to uncertain threat in individuals with alcohol use disorder: Associations with drinking behaviors and motives. Addict Biol. 2020;25: e12774. doi: 10.1111/adb.12774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oscar-Berman M, Ruiz SM, Marinkovic K, Valmas MM, Harris GJ, Sawyer KS. Brain responsivity to emotional faces differs in men and women with and without a history of alcohol use disorder. PLoS One. 2021;16: e0248831. doi: 10.1371/journal.pone.0248831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Joel D Beyond the binary: Rethinking sex and the brain. Neurosci Biobehav Rev. 2021;122: 165–175. doi: 10.1016/j.neubiorev.2020.11.018 [DOI] [PubMed] [Google Scholar]
- 98.Rauch JM, Eliot L. Breaking the binary: Gender versus sex analysis in human brain imaging. Neuroimage. 2022;264: 119732. doi: 10.1016/j.neuroimage.2022.119732 [DOI] [PubMed] [Google Scholar]
- 99.Hughto JMW, Quinn EK, Dunbar MS, Rose AJ, Shireman TI, Jasuja GK. Prevalence and Co-occurrence of Alcohol, Nicotine, and Other Substance Use Disorder Diagnoses Among US Transgender and Cisgender Adults. JAMA Netw Open. 2021;4: e2036512. doi: 10.1001/jamanetworkopen.2020.36512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mendrek A, Dinh-Williams L, Bourque J, Potvin S. Sex differences and menstrual cycle phase-dependent modulation of craving for cigarette: an FMRI pilot study. Psychiatry J. 2014;2014: 723632. doi: 10.1155/2014/723632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prashad S, Hammonds RP, Wiese AL, Milligan AL, Filbey FM. Sex-related differences in subjective, but not neural, cue-elicited craving response in heavy cannabis users. Drug Alcohol Depend. 2020;209: 107931. doi: 10.1016/j.drugalcdep.2020.107931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mueller JM, Pritschet L, Santander T, Taylor CM, Grafton ST, Jacobs EG, et al. Dynamic community detection reveals transient reorganization of functional brain networks across a female menstrual cycle. Netw Neurosci. 2021;5: 125–144. doi: 10.1162/netn_a_00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fogelman N, Hwang S, Sinha R, Seo D. Social Support Effects on Neural Stress and Alcohol Reward Responses. Curr Top Behav Neurosci. 2022;54: 461–482. doi: 10.1007/7854_2021_246 [DOI] [PubMed] [Google Scholar]
- 104.Barbosa-Leiker C, Campbell ANC, McHugh RK, Guille C, Greenfield SF. Opioid Use Disorder in Women and the Implications for Treatment. Psychiatr Res Clin Pract. 2021;3: 3–11. doi: 10.1176/appi.prcp.20190051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nikolova YS, Singhi EK, Drabant EM, Hariri AR. Reward-related ventral striatum reactivity mediates gender-specific effects of a galanin remote enhancer haplotype on problem drinking. Genes Brain Behav. 2013;12: 516–524. doi: 10.1111/gbb.12035 [DOI] [PubMed] [Google Scholar]
- 106.Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, et al. Publisher Correction: Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;605: E11. doi: 10.1038/s41586-022-04692-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gordon EM, Laumann TO, Gilmore AW, Newbold DJ, Greene DJ, Berg JJ, et al. Precision Functional Mapping of Individual Human Brains. Neuron. 2017;95: 791–807.e7. doi: 10.1016/j.neuron.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Elliott ML, Knodt AR, Ireland D, Morris ML, Poulton R, Ramrakha S, et al. What Is the Test-Retest Reliability of Common Task-Functional MRI Measures? New Empirical Evidence and a Meta-Analysis. Psychol Sci. 2020;31: 792–806. doi: 10.1177/0956797620916786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Laumann TO, Gordon EM, Adeyemo B, Snyder AZ, Joo SJ, Chen M-Y, et al. Functional System and Areal Organization of a Highly Sampled Individual Human Brain. Neuron. 2015;87: 657–670. doi: 10.1016/j.neuron.2015.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zuo X-N, Xu T, Milham MP. Harnessing reliability for neuroscience research. Nat Hum Behav. 2019;3: 768–771. doi: 10.1038/s41562-019-0655-x [DOI] [PubMed] [Google Scholar]
- 111.Taylor CM, Pritschet L, Jacobs EG. The scientific body of knowledge - Whose body does it serve? A spotlight on oral contraceptives and women’s health factors in neuroimaging. Front Neuroendocrinol. 2021;60: 100874. doi: 10.1016/j.yfrne.2020.100874 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


