Abstract
Abstract
There have been increasing reports of Klebsiella pneumoniae resistant to β-lactam antibiotics. This study aimed to determine the prevalence of some selected carbapenemase genes among clinical isolates of Klebsiella pneumoniae recovered from patients attending a private tertiary hospital in Southwestern Nigeria. The study was conducted over two months (February-March 2024). A total of 50 clinical isolates of Klebsiella pneumoniae from different clinical specimens were obtained from the Medical Microbiology Department, Babcock University Teaching Hospital (BUTH). The clinical isolates were then characterized using standard microbiological procedures and were tested for susceptibility to meropenem and other classes of antibiotics according to Clinical and Laboratory Standards Institute (CLSI) guidelines. Polymerase Chain Reaction (PCR) detection for OXA-48 and NDM-1 carbapenemase genes was performed on the 50 clinical isolates. PCR analysis showed that 9 (18%) clinical isolates were positive for the OXA-48 gene, 22 (44%) were positive for the NDM-1 gene, 4 (8%) possessed both the OXA-48 and NDM-1 genes, and 23 (46%) possessed neither the OXA-48 nor NDM-1 genes. Antibiotic Susceptibility Testing (AST) revealed that all the clinical isolates were resistant to meropenem. In conclusion, this study demonstrates the presence of OXA-48 and NDM-1 genes in clinical isolates of Klebsiella pneumoniae recovered from patients attending a private tertiary hospital in Southwestern Nigeria, highlighting the role of ESBL (extended-spectrum beta-lactamase) as a major resistance mechanism alongside other mechanisms. Population-based surveillance programs should be implemented to monitor the prevalence and epidemiology of Klebsiella pneumoniae infections at the community level, facilitating early detection of outbreaks and identification of emerging antimicrobial resistance patterns.
Core Tip
This study highlights the significant prevalence of NDM-1 and OXA-48 carbapenemase genes among Klebsiella pneumoniae clinical isolates in a private tertiary hospital in Southwestern Nigeria, with 44% and 18% of isolates harboring these genes, respectively. Notably, 46% of isolates were resistant to carbapenems despite lacking these genes, suggesting alternative resistance mechanisms. The findings underscore the urgent need for enhanced surveillance, infection control measures, and antibiotic stewardship programs to combat the spread of multidrug-resistant Klebsiella pneumoniae in healthcare settings.
Keywords: Carbapenemase genes, Klebsiella pneumoniae, NDM-1, OXA-48, Southwestern nigeria
Introduction
Klebsiella pneumoniae is a Gram-negative pathogen often acquired in the hospital settings despite being part of normal flora. It is commonly present in the environment, such as in soil and water, and has been implicated in pneumonia, urinary tract infections and bloodstream infections [1, 2]. Klebsiella pneumoniae is a significant contributor to healthcare-associated infections, comprising about 8% of all nosocomial infections and up to 14% of primary bacteremia cases [2–5]. The organism plays a substantial role in the spread of antimicrobial resistance among Enterobacteriaceae, posing challenges in clinical settings [6–8]. Over the past decade, the appearance of strains with decreased susceptibility or resistance to carbapenems has become a therapeutic challenge. Identifying its virulence genes is crucial for potential therapies [9–13].
Carbapenem resistance in Klebsiella pneumoniae is primarily driven by the production of carbapenemases, which fall into three categories: (1) metallo-β-lactamases (e.g., NDM-1, IMP, VIM) of molecular class B, which hydrolyze all β-lactams, except monobactams and inhibited by EDTA, but not clavulanic acid; (2) The second type of resistance mechanism involves serine beta-lactamases, including GES and KPC, which are not inhibited by clavulanic acid and (3) OXA-48 carbapenemases which hydrolyze monobactams (and thus monobactam antibiotics are ineffective against bacteria expressing this resistance mechanism).These unique characteristics are used for laboratory identification [14–21]. Carbapenemases, often obtained through mutations or horizontal gene transfer, include common types such as NDM-1 (produced by the blaNDM gene) and OXA-48 (produced by the blaOXA48-like gene) [9, 22–24].
Infections caused by carbapenem-resistant Klebsiella pneumoniae present significant complications associated with various hospital-related risk factors. The prevalence of CRKP isolates in invasive Klebsiella pneumoniae infections varies globally. For example, in India, carbapenem resistance rates increased from 9% in 2008 to 44% in 2010, a surge of 35% in two years, while in Italy, the rates dramatically rose to 60% in 2013 from non-detectable levels in 2008 [25–27]. In a multicenter retrospective study in India, involving 344 Klebsiella pneumoniae isolates from eight centers, the blaOXA48-like gene was found in 73.2% of the isolates, followed by blaNDM-1/5 in 24.4%. However, in several other studies, NDM was identified as the primary cause of carbapenem resistance, with the OXA-48-like group following [9, 28]. NDM is recognized as the most prevalent carbapenemase, and co-production of NDM-1 with other carbapenemases has been observed in Enterobacteriaceae isolates in various regions [26–30].
The rise of carbapenem-resistant K. pneumoniae poses a significant obstacle for antimicrobial treatment [31, 32]. In Nigeria, the epidemiology of antibiotic resistance has become alarming, with a notable increase in carbapenem-resistant infections. Several studies have reported the prevalence of carbapenemase-producing Enterobacteriaceae (CPE) in various regions of the country, underscoring the critical public health threat posed by these pathogens. For instance, in Jos (North-Central Nigeria), a study by Nkup et al. [33] reported a CRKP prevalence of 42.1% from clinical specimens, with the highest occurrence in urine samples (62.5%). In another study carried out in Enugu (South-eastern Nigeria) by Maduakoret al. [34]. , 27.7% of the Klebsiella pneumoniae isolates were resistant to imipenem, with a significant portion producing metallo-beta-lactamases (MBLs). Similarly, in Minna (North-Central of Nigeria), 20.9% of isolates from wound surfaces were carbapenem-resistant [35]. Studies from Lagos and other southwestern regions of Nigeria also indicate high resistance rates, underscoring a national concern [36–38]. The resistance mechanisms of CRKP in Nigeria are diverse, involving various carbapenemase genes and other determinants. In a study carried out in Enugu State (South-eastern, Nigeria), the presence of MBL-producing K. pneumoniae indicates a significant role of MBLs in carbapenem resistance [34]. Whole-genome sequencing in South-West Nigeria identified multiple resistance genes, including blaOKP-B-1 and fosA5 [39], highlighting the complexity of the resistance landscape.
CRKP isolates in Nigeria exhibit high levels of multidrug resistance, complicating treatment options. In Jos (North-Central Nigeria), isolates showed high resistance to gentamicin, ceftriaxone, amoxicillin/clavulanic acid, ciprofloxacin, and cefepime, with some resistance to meropenem [33]. Studies from Lagos (South-West Nigeria) [37] and Kano (North-West Nigeria) [40, 41], also reported significant resistance rates to meropenem and imipenem, with co-production of carbapenemase and extended-spectrum β-lactamases being common. This resistance extends to other antibiotic classes, limiting available treatment options. A national perspective by Olalekan et al. [36] noted a high proportion of carbapenemase-producing K. pneumoniae among ESBL-producing Enterobacteriaceae, with 27.4% being carbapenem-resistant. A systematic review and meta-analysis by Irekeola et al. [42] revealed a progressive increase in carbapenem resistance across Nigeria, with pooled prevalence estimates for resistance to various carbapenems ranging from 11.2 to 37.9%. These findings highlight the growing challenge of carbapenem-resistant K. pneumoniae in Nigeria and call for urgent, coordinated efforts.
Rationale of the study
The OXA-48 and NDM-1 carbapenemase genes are critically important due to their capacity to rapidly disseminate and induce high-level resistance to carbapenems, which significantly limit treatment options for infections caused by Klebsiella pneumoniae. Surveillance data from tertiary hospitals in Nigeria indicate a rising trend in carbapenem resistance, highlighting the urgent need for effective monitoring and control strategies to address the spread of these resistant strains. Despite the growing concerns, there is a notable absence of published research focused on the molecular detection of these carbapenemase genes in Klebsiella pneumoniae clinical isolates from patients at Babcock University Teaching Hospital, Ilishan-Remo, Ogun State, Nigeria. This gap in the literature underscores the necessity of this study to provide critical insights into the prevalence and distribution of OXA-48 and NDM-1 genes in this specific healthcare setting, ultimately contributing to more targeted and effective infection control measures.
Significance of the study
The molecular detection of carbapenemase genes in K. pneumoniae isolates will guide the empirical treatment of infections caused by this drug-resistant pathogen. Moreover, the findings will be invaluable for governmental and non-governmental agencies, the academic community, healthcare workers, and other stakeholders in the health sector, providing diagnostic and treatment services for patients suffering from K. pneumoniae infections. The significance of this study lies in its focus on the molecular detection of OXA-48 and NDM-1 carbapenemase genes among clinical isolates of K. pneumoniae from patients in a private tertiary hospital in Southwestern Nigeria. By identifying the prevalence and distribution of these carbapenemase genes, the study will provide valuable epidemiological data that can inform local and national antimicrobial resistance strategies. The findings can guide infection control policies and antibiotic stewardship programs to curb the spread of these formidable pathogens within healthcare settings. Given the global nature of antimicrobial resistance, data from this study will contribute to the broader understanding of resistance patterns and facilitate international efforts to combat the spread of carbapenem-resistant Klebsiella pneumoniae (CRKP).
Aim of the study
The aim of this study is to determine the prevalence of selected carbapenemase genes among clinical isolates of Klebsiella pneumoniae recovered from patients attending a private tertiary hospital in Southwestern Nigeria.
Methodology
Study design
This is an analytical and epidemiological study.
Study area
This study was carried out at the Medical Microbiology Laboratory, as well as the Molecular and Tissue Culture Laboratory at Babcock University Teaching Hospital (BUTH), Ilishan-Remo, Ogun State. BUTH is a private hospital with over 400-bed capacity and is the only tertiary hospital within the community, whereas Ilishan-Remo is one of the geo-political wards in Ikenne Local Government Area of Ogun State.
Study duration
The study lasted for a period of 2 months (February-March, 2024).
Sample size
A total of 50 non-duplicate clinical isolates of Klebsiella pneumoniae (chosen based on meropenem-resistance within the duration of the study) recovered from the specimens (urine, blood, sputum, pus, tracheal aspirate, and ascitic fluid) of patients attending Babcock University Teaching Hospital (BUTH), Ilishan-Remo, Ogun state, Nigeria, who presented with pneumonia, urinary tract infection, bloodstream infection, and meningitis, stored in separate sterile Bijou bottle containing nutrient agar slant were used for the study.
Laboratory analysis
Culture of clinical isolates of Klebsiella pneumoniae
The clinical isolates were sub-cultured from different media onto differential media, including MacConkey agar (MCA), Eosin Methylene Blue (EMB) agar and Cystine Lactose Electrolyte-Deficient (CLED) agar (Oxoid, LabMal Malaysia), and were re-identified biochemically using standard methods.
Identification of clinical isolates of Klebsiella pneumoniae
Standard methods (macroscopy and microscopy) for identifying Klebsiella pneumoniae isolates were adopted. Colony morphological features such as size, shape, texture, elevation, pigmentation, margins and opacity were recorded. Gram staining was performed to illustrate their morphology. Their biochemical characteristics were determined by carrying out catalase test, coagulase test, oxidase test, indole test, citrate test and urease test. Also, the isolates were placed on Chromogenic agars to help further identification. ESBL-producing Klebsiella pneumoniae formed red and metallic blue colonies on CHROMagar ESBL and CHROMagar Klebsiella (Sigma-Aldrich, United States of America), respectively.
DNA extraction
Genomic DNA isolated from bacterial cells was used for epidemiological or diagnostic purposes. Genomic DNA was extracted using a DNA pure extraction kit (Zymo) according to the manufacturer’s recommendations. The amount and purity of the extracted DNA was measured using a NanodropNDJacoby, 20,090 spectrophotometer (Thermo Fisher Scientific, Waltham, MA USA).
DNA extraction procedure
Sample preparation
A colony of each clinical isolates of Klebsiella pneumoniae was inoculated into a Brain Heart infusion broth contained in a properly labelled Bijou bottle.
Reagent preparation
1,040 µl of Proteinase K storage buffer was added to 20 mg of proteinase K tube prior to use. It was stored at -20 °C after mixing.
Sample processing
The Brain Heart Infusion (BHI) broth containing the sample was dispensed into Eppendorf tube. The sample was centrifuged at 500 xg for 2 min. The supernatant was decanted by sharp inversion. The bottom of the Eppendorf tube was tapped to re-suspend the sediment. 20 µl of DNA elusion buffer was added to the cell pellet (sediment). It was mixed using a vortex machine for 10 s. 200 µl of sample mixture was added to another eppendorf tube. 200 µl of Biofluid and cell buffer and 20 µl of Proteinase K was added to the Eppendorf tube. The content of the tube was mixed using the vortex machine for 10 s and incubated in a heat block at 55 °C for 10 min. 420 µl of Genomic Binding Buffer was added to the digested sample. It was mixed using a vortex for 10 s. The mixture was transferred to a Zymo-spin™ IIC-XLR Columnin a collection tube. It was centrifuged at 12,000 xg for 1 min and the flow through and collection tube was discarded. 400 µl DNA pre-wash buffer was added to the spin column in a new collection tube. It was centrifuged at 12,000 xg for 1 min and the collection tube was emptied. 700 µl of g-DNA wash buffer was added to the spin column. It was centrifuged at 12,000 xg for 1 min and the collection tube was emptied. 200 µl of g-DNA wash buffer was added to the spin column. It was centrifuged at 12,000 xg for 1 min and the collection tube and flow through were discarded. The spin column was transferred to a new Eppendorf tube. 50 µl of DNA elution buffer was added directly on the matrix. It was incubated for 5 min at room temperature. It was centrifuged at maximum speed (14,000 xg) for 1 min to elute the DNA which was used for molecular-based applications.
Primer design
The primer sequences and optimization protocols utilized in this study for the detection of OXA-48 and NDM-1 genes in Klebsiella pneumoniae isolates were adapted from a previously published work by Poirel et al. [43]. These primers have been validated for their efficacy in identifying these genes among clinical isolates of Klebsiella pneumoniae. The primer sequences can be found in Table 1.
Table 1.
Primers sequences used for the molecular detection of Klebsiella pneumoniae carbapenemase genes [43]
| Primer name | Sequence (5’ to 3’) | Product size (bp) | Annealing temperature (°C) |
|---|---|---|---|
|
OXA-48-F OXA-48-R |
GCGTGGTTAAGGATGAACAC CATCAAGTTCAACCCAACCG |
438 | 52 |
|
NDM-1-F NDM-1-R |
GGTTTGGCGATCTGGTTTTC CGGAATGGCTCATCACGATC |
621 | 52 |
PCR amplification with uniplex assays
Briefly, from the extracted genomic DNA of Klebsiella pneumoniae, OXA-48 and NDM-1 genes were amplified using primers listed in Table 1. The amplification reaction was carried out in a 0.5 ml microcentrifuge tubes loaded into a DNA thermocycler (Eppendorf Mastercycler AG 22331, Hamburg, Germany). Each tube contained 20 µl reaction mixture. A known positive sample was used as positive control while asterile distilled water was used as negative control. The samples were subjected to different cycling conditions (Table 2) using a PCR machine supplied by Hangzhou Bioer Technology Company Limited (Zhejiang, China). Afterwards, agarosegel electrophoresis was carried out. At the end of the run, the gel was viewed under UV blue light to determine the band sizes of the amplicons. Using the primers listed belowr, OXA-48 and NDM-1 genes were expected to have a band size of 438 bp and 621 bp, respectively.
Table 2.
PCR thermal cycling conditions [43]
| Gene | Initial denaturation | No of cycles | Denaturation | Annealing | Extension | Final extension |
|---|---|---|---|---|---|---|
| OXA-48 | 94 °C for 10 min | 36 cycles | 94 °C for 30 s | 52 °C for 40 s | 72 °C for 50 s | 72 °C for 5 min |
| NDM-1 | 94 °C for 10 min | 36 cycles | 94 °C for 30 s | 52 °C for 40 s | 72 °C for 50 s | 72 °C for 5 min |
Results
The data presented for this research are in two steps. The first step is the presentation of the primers’ operation with Klebsiella pneumoniae. Results show that the designed primers for the OXA-48 gene and NDM-1 gene operated well with the Klebsiella pneumoniae. The OXA-48 and NDM-1 genes were amplified from the genomic DNA of Klebsiella pneumoniae clinical isolates by uniplex PCR. The generated amplicons for OXA-48 gene and NDM-1 gene were 438 bp and 621 bp respectively (Figs. 1 and 2).
Fig. 1.

PCR amplification of Klebsiellapneumoniae for the detection of OXA-48 gene on gel electrophoresis with a generated amplicon of 438 bp.Uniplex. Lane M: DNA Ladder; (100 bp). Lane P: Positive control. Lane N: Negative control. Lanes 1, 2, 4 and 5 were positive for OXA-48 gene. Lanes 3, 6, 7, 8, 9, 10, 11 and 12 were negative for OXA-48 gene
Fig. 2.
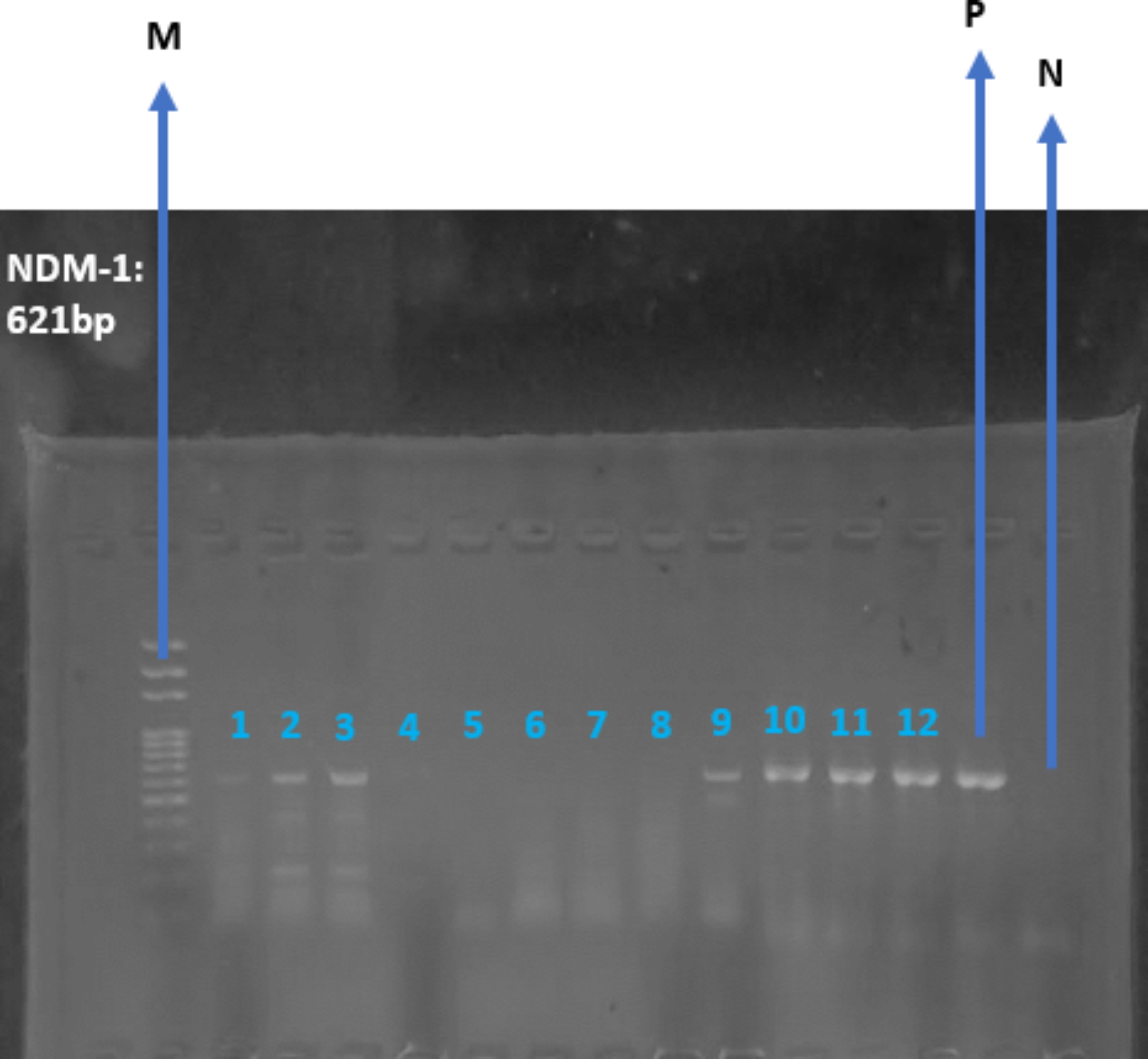
PCR amplification of Klebsiella pneumoniae for the detection of NDM-1 gene on gel electrophoresis with a generated amplicon of 621 bp.Uniplex. Lane M: DNA Ladder; (100 bp). Lane P: Positive control. Lane N: Negative control. Lanes 1, 2, 3, 9, 10, 11 and 12 were positive for NDM-1 gene. Lanes 4, 5, 6, 7 and 8 were negative for NDM-1 gene
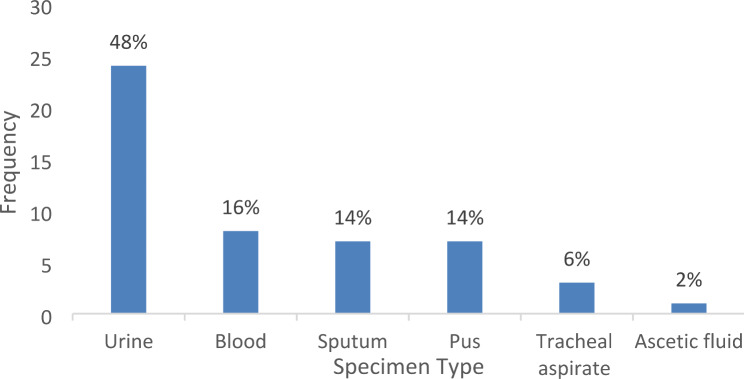
The second step involves the samples and the specimen types. The results of this phase are presented in a chart representing the frequency of the specimen types used. It was observed that Klebsiella pneumoniae was most frequently isolated from urine specimens, accounting for 24 (48%) of the cases, followed by blood, sputum, pus, tracheal aspirate, and ascitic fluid, which accounted for 8 (16%), 7 (14%), 7 (14%), 3 (6%), and 1 (2%) of the cases, respectively (Fig. 3). A total of 50 Klebsiella pneumoniae clinical isolates were sub-cultured and re-identified using biochemical tests. These isolates were obtained from different infection sites including 24 urine samples (48%), 8 blood samples (16%), 7 sputum samples (14%), 7 pus samples (14%), 3 tracheal aspirates (6%) and 1 Ascitic fluid sample (2%) (Table 3).
Fig. 3.
Bar chart showing the number of different specimen types in the clinical isolates of Klebsiella pneumoniae
Table 3.
Distribution of clinical isolates of Klebsiella pneumoniae
| Clinical specimen | Frequency (N) | Percentage (%) |
|---|---|---|
| Urine | 24 | 48 |
| Blood | 8 | 16 |
| Sputum | 7 | 14 |
| Pus | 7 | 14 |
| Tracheal aspirate | 3 | 6 |
| Ascitic fluid | 1 | 2 |
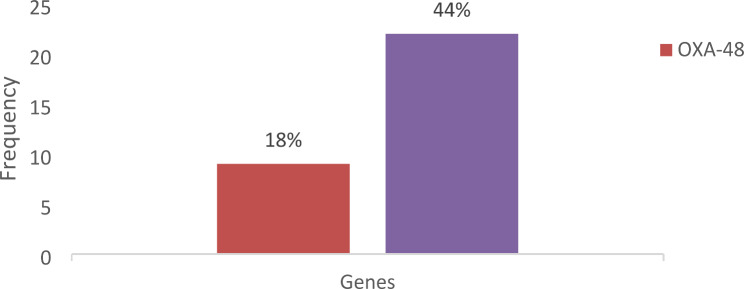
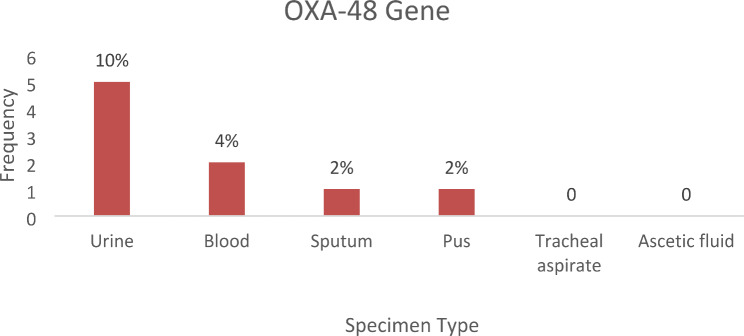
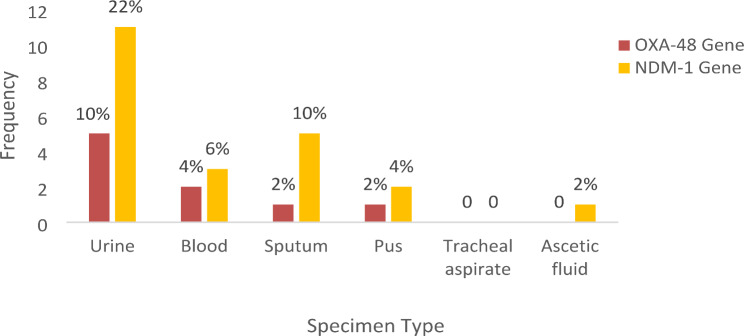
The results showed that the uniplex PCR assay for the detection of the OXA-48 gene was positive for 9 samples (18%) and 22 samples (44%) were positive for the NDM-1 gene (Fig. 4). OXA-48 gene was positive in 9 (18%) clinical isolates of Klebsiella pneumoniae in different specimen types. The urine specimen had the highest number of positive cases, with 5 (10%), followed by blood, sputum, and pus with 2 (4%), 1 (2%), and 1 (2%) positive cases, respectively. The OXA-48 gene was not detected in Klebsiella pneumoniae clinical isolates from tracheal aspirate and ascitic fluid specimens (Fig. 5).
Fig. 4.
Bar chart showing the comparison of the number of the number of positive samples for each gene
Fig. 5.
Bar chart showing the number of positive OXA-48 carbapenemase genes of Klebsiella pneumoniae present in each cultured specimen type
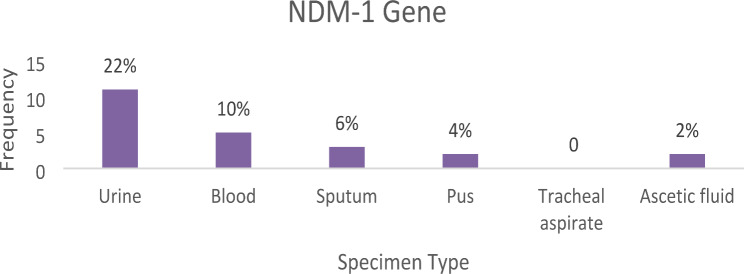
NDM-1 gene was positive in 22 (44%) clinical isolates of Klebsiella pneumoniae in different specimen types. The urine specimen had the highest number which was 11 (22%) followed by sputum, blood, pus and Ascitic fluid which was 5 (10%), 3 (6%), 2 (4%) and 1 (2%) respectively. The NDM-1 gene was not detected in Klebsiella pneumoniae clinical isolates from tracheal aspirate specimens (Fig. 6).
Fig. 6.
Bar chart showing the number of positive NDM-1 carbapenemase genes of Klebsiella pneumoniae present in each cultured specimen type
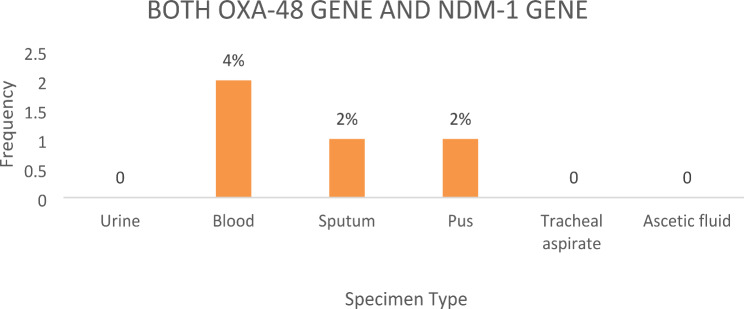
There was significant association between the carbapenemase genes among clinical isolates of Klebsiella pneumoniae and the sites of infection (Fig. 7). Both OXA-48 gene and NDM-1 gene were detected in 4 (8%) clinical isolates of Klebsiella pneumoniae in different specimen types. Blood specimen had the highest number of isolates with both genes, with 2 (4%), followed by sputum and pus with 1 (2%) each. Neither urine specimens, tracheal aspirates, nor ascitic fluid specimens contained both genes (Fig. 8).
Fig. 7.
Bar chart showing the number of specimen types that were positive for each gene
Fig. 8.
Bar chart showing the number of specimen types that were positive for both OXA-48 and NDM-1 genes
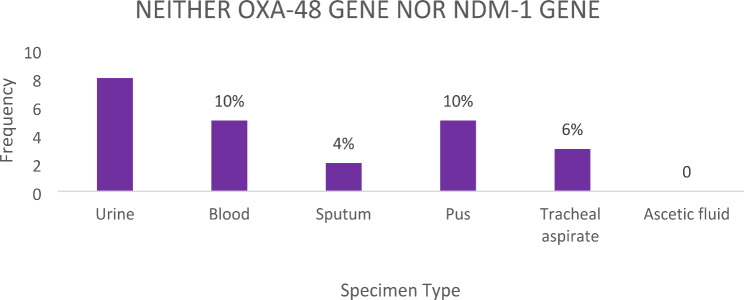
Not all clinical isolates of Klebsiella pneumoniae possessed either the OXA-48 or NDM-1 genes. A total of 23 (46%) clinical isolates of Klebsiella pneumoniae from different specimen types possessed neither the OXA-48 gene nor the NDM-1 gene. The urine specimen had the highest number of such cases, with 8 (16%), followed by blood, sputum, pus, and tracheal aspirates, with 5 (10%), 2 (4%), 5 (10%), and 3 (6%) cases, respectively (Fig. 9).
Fig. 9.
Bar chart showing the number of specimen types that had neither OXA-48 gene nor NDM-1 gene
The results of the Antibiotic Susceptibility Testing (AST) performed on the clinical isolates of Klebsiella pneumoniae showed that all the 50 (100%) clinical isolates of Klebsiella pneumoniae were resistant to meropenem. 9 (18%) clinical isolates of Klebsiella pneumoniae were resistant to azithromycin. 3 (6%) clinical isolates of Klebsiella pneumoniae were resistant to ciprofloxacin. 1 (2%) clinical isolate of Klebsiella pneumoniae was resistant to amoxicillin. 1 (2%) clinical isolate of Klebsiella pneumoniae was resistant to ceftriaxone. 1 (2%) clinical isolate of Klebsiella pneumoniae was resistant to levofloxacin. 1 (2%) clinical isolate of Klebsiella pneumoniae was resistant to cefepime. There was no resistance observed to ceftazidime, cefuroxime, gentamicin, piperacillin-tazobactam or amikacin (Table 4).
Table 4.
Antibiotic susceptibility pattern of clinical isolates of Klebsiella pneumoniae
| Antibiotics | Number of isolates tested N (%) | Sensitive N (%) | Intermediate N (%) | Resistant N (%) |
|---|---|---|---|---|
| Ceftazidime | 50 (100) | 38 (76) | 12 (24) | - |
| Amoxicillin | 50 (100) | 37 (74) | 12 (24) | 1 (2) |
| Ceftriaxone | 50 (100) | 38 (76) | 11 (22) | 1 (2) |
| Cefuroxime | 50 (100) | 40 (80) | 10 (20) | - |
| Gentamicin | 50 (100) | 41 (82) | 9 (18) | - |
| Ciprofloxacin | 50 (100) | 32 (64) | 15 (30) | 3 (6) |
| Piperacillin-Tazobactam | 50 (100) | 49 (98) | 1 (2) | - |
| Meropenem | 50 (100) | - | - | 50 (100) |
| Amikacin | 50 (100) | 43 (86) | 7 (14) | - |
| Levofloxacin | 50 (100) | 35 (70) | 14 (28) | 1 (2) |
| Cefepime | 50 (100) | 32 (64) | 17 (34) | 1 (2) |
| Azithromycin | 50 (100) | 29 (58) | 12 (24) | 9 (18) |
Discussion
Carbapenem-resistant Enterobacteriaceae (CRE) frequently cause infections in immunocompromised patients, leading to prolonged hospital stays and increased mortality [3, 15, 24]. Despite the introduction of new antibiotics for CRE infections, combating antibiotic resistance remains challenging due to the limited availability of effective treatments and the lack of comprehensive clinical data from large randomized controlled trials. Diagnosing, treating, and preventing CRE infections are significant challenges. Notably, carbapenem-resistant Klebsiella pneumoniae (CRKP) is identified as a critical-priority pathogen among CREs [39]. The principal resistance mechanism of CRKP involves the production of carbapenemases, such as Klebsiella pneumoniae carbapenemases (KPC), New Delhi metallo-β-lactamase (NDM), imipenemase metallo-β-lactamase (IMP), Verona integron-encoded metallo-β-lactamase (VIM), and oxacillinase-48-type carbapenemases (OXA-48) [15, 28, 30, 40, 41].
This study aimed to detect OXA-48 and NDM-1 carbapenemase genes in clinical isolates of Klebsiella pneumoniae from patients attending a private tertiary hospital in southwestern Nigeria. Our results show an OXA-48 positivity rate of 18% and an NDM-1 positivity rate of 44% among Klebsiella pneumoniae isolates. This contrasts with findings from Odewale et al. [44], who reported a higher prevalence of OXA-48 at 28.9%, suggesting regional variations in prevalence. Our detection rate for NDM-1 is consistent with Olalekan et al. [19], where 62.5% of isolates were positive for blaNDM, although the presence of NDM-1 in our study was lower. This discrepancy might be attributed to differences in local epidemiological factors and healthcare practices. We found that 8% of the isolates possessed both OXA-48 and NDM-1 genes, predominantly in blood, sputum, and pus samples. This contrasts with Olowo-okere et al. [45], who reported a rare co-existence of carbapenemase genes. This variation could indicate a potential for gene co-expression that might be specific to our study region.
Furthermore, our study identified the highest prevalence of both carbapenemase genes in urine specimens, which aligns with the findings of Nkup et al. [16], who also reported a high prevalence of Klebsiella pneumoniae in urine samples. This observation underscores the importance of urine as a significant source of resistant isolates in Nigeria. Our study observed that 46% of the isolates were negative for both genes, which is consistent with findings from Doko et al. [18] and Oduyebo et al. [46]. This suggests that while carbapenem resistance is prevalent, not all resistant strains carry the tested carbapenemase genes, highlighting the presence of other resistance mechanisms or undetected genes. The absence of OXA-48 and NDM-1 in some isolates, as seen in Doko et al. [18], indicates that resistance might be mediated by other mechanisms or genes not covered in our study. Additionally, the lower detection of OXA-48 in our study compared to others might reflect differences in molecular techniques or the specific strains circulating in our region.
Beyond the borders of Nigeria, the 44% prevalence of the NDM-1 gene observed in this study was higher than the 17.6% reported in South India by Remya et al. [47] but lower than the 66% reported in Iran by Ghanbarinasab et al. [48]. These discrepancies could stem from differences in sample size or methodology. Conversely, the 18% prevalence of the OXA-48 gene found in this study was lower than the 55% reported in Croatia by Šuto et al. [49], the 66.7% in South India by Remya et al. [47], and the 52% and 100% in Egypt and Iran, respectively, reported by El-Domany et al. [50] and Ghanbarinasab et al. [48]. These variations may be due to differences in sample size, study population, or geographical location. Furthermore, both genes co-occurred in 8% of the isolates, unlike the 41.3% reported by Al-Zahrani et al. [27], possibly due to sample size differences.
ElFeky et al. [51] found a high dissemination of both NDM-1 and OXA-48 co-producing Enterobacterales in Egypt, indicating similar resistance patterns. These findings are consistent with our study, underscoring the predominance of these resistance mechanisms in the region. Studies in Bosnia and Herzegovina and India also reported similar resistance trends, highlighting the need for global surveillance and control measures [52–54]. Our observation of 100% meropenem resistance among all isolates reflects the broader challenge of multidrug resistance observed in various regions. This resistance rate is slightly higher than the 93.2% reported by Wang et al. [6] in China and significantly higher than the 29% reported by Šuto et al. [49] in Croatia. The lower resistance rates in these studies may be due to differences in the presence of resistance genes.
In this study, Klebsiella pneumoniae demonstrated high susceptibility to third- and fourth-generation cephalosporins: 76% to ceftazidime, 76% to ceftriaxone, and 64% to cefepime. These susceptibility rates are higher than those reported by Šuto et al. [49] in Croatia and Wang et al. [6] in China, where susceptibility rates were significantly lower. The discrepancies might be due to the presence of additional resistance genes in the isolates studied.
Aminoglycoside antibiotics showed high efficacy, with 82% and 86% susceptibility to gentamicin and amikacin, respectively. These rates were higher than those reported by An et al. [55] in Eastern China and Ghanbarinasab et al. [48] in Iran. The higher susceptibility rates in our study could be attributed to the absence of other resistance genes. Piperacillin-tazobactam showed 98% susceptibility, higher than the 80.27% reported by Remya et al. [47] in South India and the 7.5% reported by Wang et al. [6] in China. The high resistance reported by Hosseinzadeh et al. [56] in Iran and Alhazmi et al. [2] in Jeddah contrasts sharply with our findings. These differences could be due to variations in the presence of other resistance genes.
Our study demonstrated a 74% susceptibility rate to amoxicillin, significantly higher than the rates reported by Wang et al. [6] in China and Argente et al. [57] in Catalonia, who found much lower susceptibility rates. While the high sensitivity rate to amoxicillin observed in this study is unconventional, it highlights the potential for unique resistance profiles in specific bacterial populations. It is possible that the Klebsiella pneumoniae isolates in our study harbor genetic mutations or variations that could impact their typical resistance profiles. Such variations might include the loss or alteration of the genes encoding penicillinases or other resistance mechanisms, leading to unexpected susceptibility to amoxicillin. While Klebsiella pneumoniae is known to possess chromosomal SHV-type beta-lactamases, it is possible that some of the isolates in our study acquired plasmid-borne beta-lactamase genes. These plasmid-encoded beta-lactamases may contribute to resistance against beta-lactam antibiotics. However, the observed susceptibility to amoxicillin in a minority of the isolates could be due to other factors, such as variations in gene expression or the presence of alternative resistance mechanisms that do not completely inhibit the effectiveness of the antibiotic.
Klebsiella pneumoniae is a highly adaptable organism with significant genetic diversity. Mutations or deletions in the genes encoding the SHV-type beta-lactamases could lead to variations in resistance patterns. Such genetic variability among clinical isolates could explain the unexpected susceptibility to amoxicillin observed in our study. Whole-genome sequencing of these isolates could further elucidate these genetic differences. The phenotypic expression of antibiotic resistance can vary among different isolates, even within the same species. Environmental factors, selective pressures, and genetic background can influence the expression of resistance genes. This phenotypic heterogeneity could account for the observed variation in amoxicillin susceptibility. The ecological niche and evolutionary pressures faced by the clinical isolates may have led to differential expression of resistance mechanisms. In a clinical setting, the selective pressure exerted by the use of various antibiotics can shape the resistance profile of bacterial populations. The isolates we tested might have been exposed to selective pressures that favored the retention or loss of certain resistance traits, including those against amoxicillin [58–60].
Our study was conducted in a specific geographical region (Southwestern Nigeria) where local epidemiological factors might influence the resistance profiles of bacterial populations. These factors could include differences in antibiotic usage patterns, infection control practices, or the presence of unique bacterial strains. We also observed susceptibility to quinolones: 64% to ciprofloxacin and 70% to levofloxacin, significantly higher than the rates reported by Wang et al. [6] in China and Argente et al. [57] in Catalonia. Again, these differences could be attributed to regional variations in the prevalence of resistance genes or local healthcare practices.
The susceptibility rate to carbapenems in our study was 20%, slightly higher than the rate reported by El-Domany et al. [50] in Egypt, who found a 13% susceptibility rate. These rates underscore the challenge of treating infections caused by carbapenem-resistant Klebsiella pneumoniae. Interestingly, our study showed that 42% of the isolates were susceptible to colistin, a stark contrast to the 0% reported by Olowo-okere et al. [45] in Nigeria. This difference might be due to variations in local colistin usage patterns or resistance mechanisms.
The high isolation rate of CRKP from urine samples in our study (46%) was similar to the 52% reported by Rojas et al. [61] in the United States and consistent with the findings of Satlin et al. [62] and El-Sayed et al. [63]. However, studies in Poland [6] and South Africa [64] reported higher isolation rates from sputum and bloodstream infections, respectively. This variation underscores the need for regional surveillance and tailored interventions. Overall, this study highlights the alarming prevalence of carbapenem-resistant Klebsiella pneumoniae due to OXA-48 and NDM-1 genes, reflecting global trends. Carbapenemase genes are often located on integrons with other cassette genes, contributing to multidrug resistance (MDR). The widespread nature of these resistance genes underscores the urgent need for comprehensive surveillance, robust infection control practices, and international cooperation to mitigate the spread of these formidable pathogens [65–68].
In the context of One Health, the global interconnectedness of ecosystems plays a crucial role in the dissemination of antibiotic-resistant bacteria and their genetic elements. Resistant strains and their genes can be transported across ecosystems through water, air, and soil. Contaminated water sources in one region can carry resistant strains to distant areas. Wildlife, livestock, and pets can also spread resistant bacteria and genes across borders. Animals traveling between regions or countries can introduce new resistant strains into different ecosystems. Human activities through global travel and trade facilitate the movement of resistant bacteria. Patients traveling for medical treatment, as well as international shipping and trade, can spread resistant strains across continents [65, 69].
Furthermore, antibiotic use in agriculture can select for resistant strains, which then spread through food, waste, and environmental contamination. Cross-border healthcare activities, including patient transfers and international medical collaborations, can contribute to the spread of resistant bacteria and genes. These interconnected pathways highlight the importance of global surveillance and cooperative strategies to address antimicrobial resistance [69, 70]. Understanding these dynamics is crucial for developing effective interventions and control measures to combat the spread of carbapenem-resistant strains like those detected in our study.
Limitations of this study
The limitations of this study include: (1) A small number of clinical isolates were used for the study due to the high cost associated with molecular detection of the resistance genes of interest in this research setting. (2) The study was conducted over just two months. This short duration may not fully capture the variability in antibiotic resistance patterns over time. (3) While the study detected the presence of carbapenemase genes, it did not investigate their actual expression levels. Gene expression profiles could provide insights into the functional relevance of these genes in the resistance of Klebsiella pneumoniae strains to carbapenems, and (4) The study focused on two specific carbapenemase genes (OXA-48 and NDM-1). Other resistance genes that contribute to Klebsiella pneumoniae resistance were not explored due to the high cost of investigation.
Recommendations
Based on the limitations identified in the study, the following recommendations are proposed to address these shortcomings and enhance the robustness and applicability of future research: (1) Increase the sample size to obtain a more representative sample of clinical isolates, enabling more accurate estimation of the prevalence and distribution of OXA-48 and NDM-1 genes among Klebsiella pneumoniae strains. Larger sample sizes can enhance the statistical power of analyses and facilitate subgroup analyses by patient demographics, infection sites and antimicrobial resistance profiles. (2) Future studies with longer durations are necessary to provide a more robust and comprehensive analysis of resistance trends. (3) Conduct longitudinal studies to elucidate temporal trends in the prevalence and genetic diversity of Klebsiella pneumoniae strains, allowing for the assessment of changes in resistance gene expression over time and their association with clinical outcomes. Longitudinal data can provide valuable insights into the dynamics of Klebsiella pneumoniae infections and inform targeted intervention strategies. (4) Expand the scope of molecular analysis to include other antimicrobial resistance determinants and genetic markers associated with Klebsiella pneumoniae resistance. Whole-genome sequencing and functional genomics approaches can provide a comprehensive understanding of the genetic basis of Klebsiella pneumoniae antimicrobial resistance, guiding the development of novel therapeutics and diagnostics. (5) Implement population-based surveillance programs to monitor the prevalence and epidemiology of Klebsiella pneumoniae infections at the community level, facilitating early detection of outbreaks and identification of emerging antimicrobial resistance patterns. (6) Collaboration between healthcare institutions, public health agencies, and academic research centers can enhance data sharing and facilitate collaborative research efforts to combat Klebsiella pneumoniae infections effectively. By implementing these recommendations, future research endeavors can address the limitations identified in this study and advance our understanding of Klebsiella pneumoniae antimicrobial resistance and clinical management strategies, ultimately improving patient outcomes and public health outcomes.
Conclusion
In conclusion, Carbapenemase genes (OXA-48 and NDM-1) were present in varying degree (18% and 44% respectively) in the clinical isolates of Klebsiella pneumoniae recovered from various sites of infections of patients attending a private tertiary hospital in southwestern Nigeria. The detection of different carbapenemase genes of Klebsiella pneumoniae isolates suggests that they are associated with resistance accompanied by serious implications for patients and healthcare workers. The outcome of this study underscores the results of previous studies and contributes significantly to the understanding of resistance of Klebsiella pneumoniae, offering insights into gene distribution and the potential for targeted interventions in the clinical setting. The combination of molecular methods and clinical data provides a holistic view of Klebsiella pneumoniae infections in the studied population. The findings offer valuable insights into the diversity of gene distribution among specimen types and the potential of molecular techniques in Medical Microbiology. This study serves as a foundation for future research and underscores the importance of continued vigilance and proactive measures in managing Klebsiella pneumoniae infections in healthcare settings.
Abbreviations
- AST
Antibiotic Susceptibility Test
- BLAST
Basic Local Alignment Search Tool
- BUHREC
Babcock University Health Research Ethics Committee
- BUTH
Babcock University Teaching Hospital
- CLSI
Clinical and Laboratory Standard Institute
- CRE
Carbapenem-resistant Enterobacteriaceae
- CRKP
Carbapenem-resistant Klebsiella pneumoniae
- CSF
Cerebrospinal Fluid
- DNA
Deoxyribonucleic Acid
- EDTA
Ethylenediaminetetraacetic Acid
- ESBL
Extended-spectrum beta-lactamase
- GES
Guiana extended-spectrum
- IMP
Imipenemasemetallo-β-lactamase
- KPC
Klebsiella pneumoniaecarbapenemase
- NCBI
National Center for Biotechnology Information
- NDM
New Delhi metallo-β-lactamase
- OXA
48-Oxacillinase-48-type carbapenemases
- PCR
Polymerase Chain Reaction
- TBE
Tris-Boric EDTA
- UV
Ultraviolet
- VIM
Verona integron-encoded metallo-β-lactamase
Author contributions
Study concept and design: CBO & SSE; Literature search: CBO, SSE, OAK, ACI, AAA, MUI, GEI, AOO, POA & MFK; Acquisition of data: CBO, SSE, OAK, ACI & AAA; Analysis and interpretation of data: CBO, SSE, MUI & GEI; Statistical analysis: CBO, SSE & OAK; Study supervision: SSE & OAK; Drafting of the manuscript: CBO, SSE, OAK, ACI, & AAA; Critical revision of the manuscript for important intellectual content: CBO, SSE, OAK % ACI; Final approval of the manuscript: CBO, SSE, OAK, ACI, AAA, MUI, GEI, AOO, POA & MFK.
Funding
None.
Data availability
No new data were generated. The primer sequences and optimization protocols utilized in this study for the detection of OXA-48 and NDM-1 genes in Klebsiella pneumoniae isolates were adapted from a previously published work available online at: https://doi.org/10.1016/j.diagmicrobio.2010.12.002.
Declarations
Ethical approval
Ethical approval for this study was granted by the Babcock University Health Research Ethics Committee (BUHREC) with ethical approval registration number: BUHREC 914/23. BUHREC is the Institutional Review Board (IRB) of the university.
Informed consent
Informed consent was obtained from each participant. The purpose and nature of the study, as well as the method of sample collection was explained to them properly. Afterwards, participants was requested to voluntarily complete the consent form in their own handwriting and endorsed by their signatures as proof of willingness to provide samples for the test. They were assured of the confidentiality.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yu W, Luo Q, Shen P, Chen Y, Xu H, Xiao Y, &Qiu Y. New options for bloodstream infections caused by colistin- or ceftazidime/avibactam-resistant Klebsiella pneumoniae. Int J Antimicrob Agents. 2021;58(6):106458. 10.1016/j.ijantimicag.2021.106458. 10.1016/j.ijantimicag.2021.106458 [DOI] [PubMed] [Google Scholar]
- 2.Alhazmi W, Al-Jabri A, Al-Zahrani I. The Molecular Characterization of Nosocomial Carbapenem-Resistant Klebsiella pneumoniae Co-Harboring bla NDM and bla OXA-48 in Jeddah. Microbiology Research. 2022;13(4):753 – 64. 10.3390/microbiolres13040054
- 3.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. ClinMicrobiol Rev. 2012;25(4):682–707. 10.1128/CMR.05035-11 [DOI] [PMC free article] [PubMed]
- 4.Nordmann P. Carbapenemase-producing Enterobacteriaceae: overview of a major public health challenge. Medicine and Infectious Diseases. 2014;44(2):51–6. 10.1016/j.medmal.2013.11.007 [DOI] [PubMed]
- 5.Sharma R, Garcia E, Diep JK, Lee VH, Minhaj F, Jermain B, Ellis-Grosse EJ, Abboud CS, Rao GG. Pharmacodynamic and immunomodulatory effects of polymyxin B in combination with fosfomycin against KPC-2-producing Klebsiella pneumoniae. Int J Antimicrob Agents. 2022;59(4):106566. 10.1016/j.ijantimicag.2022.106566. 10.1016/j.ijantimicag.2022.106566 [DOI] [PubMed] [Google Scholar]
- 6.Wang N, Zhan M, Liu J, Wang Y, Hou Y, Li C et al. Prevalence of carbapenem-resistant Klebsiella pneumoniae infection in a Northern province in China: Clinical characteristics, drug resistance and geographical distribution. Infect Drug Resist. 2022;15:569–579. 10.2147/IDR.S347343 [DOI] [PMC free article] [PubMed]
- 7.Hu Y, Liu C, Shen Z, Zhou H, Cao J, Chen S et al. Prevalence, risk factors and molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae in patients from Zhejiang, China, 2008–2018. Emerging Microbes and Infections. 2020;9(1):1771–9. 10.1080/22221751.2020.1799721 [DOI] [PMC free article] [PubMed]
- 8.Mouktaroudi M, Kotsaki A, Giamarellos-Bourboulis EJ. Meropenem-vaborbactam: a critical positioning for the management of infections by Carbapenem-resistant Enterobacteriaceae. Expert Rev Anti-Infective Therapy. 2022;20(6):809–18. 10.1080/14787210.2022.2030219. 10.1080/14787210.2022.2030219 [DOI] [PubMed] [Google Scholar]
- 9.Fuster B, Tormo N, Salvador C, Gimeno C. Detection of two simultaneous outbreaks of Klebsiella pneumoniae coproducing OXA-48 and NDM-1 carbapenemases in a tertiary-care hospital in Valencia, Spain. New Microbes and New Infections. 2020;34:100660. 10.1016/j.nmni.2020.100660 [DOI] [PMC free article] [PubMed]
- 10.Paul M, Carrara E, Retamar P, Tängdén T, Bitterman R, Bonomo RA, de Waele J, Daikos GL, Akova M, Harbarth S, Pulcini C, Garnacho-Montero J, Seme K, Tumbarello M, Lindemann PC, Gandra S, Yu Y, Bassetti M, Moutin JW, Tacconelli E, Rodriguez-Bano J. European Society of Clinical Microbiology and Infectious diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli. Clin Microbiol Infect. 2022;28(4):521–47. 10.1016/j.cmi.2021.11.025. 10.1016/j.cmi.2021.11.025 [DOI] [PubMed] [Google Scholar]
- 11.Karlowsky JA, Lob SH, Khan A, Chen WT, Woo PCY, Seto WH, Ip M, Leung S, Wong QWL, Chau RWY, DeRyke AC, Young K, Motyl MR, Sahm DF. Activity of ceftolozane/tazobactam against Gram-negative isolates among different infections in Hong Kong: SMART 2017–2019. J Med Microbiol. 2022;71(4):1487. Available from:. 10.1099/jmm.0.001487 [DOI] [PubMed] [Google Scholar]
- 12.Gaibani P, Lombardo D, Bussini L, Bovo F, Munari B, Giannella M et al. Epidemiology of Meropenem/Vaborbactam Resistance in KPC-Producing Klebsiella pneumoniae Causing Bloodstream Infections in Northern Italy, 2018. Antibiotics, 2021; 10(5), 536. 10.3390/antibiotics10050536 [DOI] [PMC free article] [PubMed]
- 13.Umair M, Walsh TR, Mohsin M. A systematic review and meta-analysis of carbapenem resistance and its possible treatment options with focus on clinical Enterobacteriaceae: thirty years of development in Pakistan. Heliyon. 2024;10(7):e28052. 10.1016/j.heliyon.2024.e28052. 10.1016/j.heliyon.2024.e28052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007;20(3):440–58. 10.1128/CMR.00001-07. 10.1128/CMR.00001-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitout JD, Nordmann P, Poirel L. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother. 2015;59(1):5873–5884. 10.1128/AAC.01019-15 [DOI] [PMC free article] [PubMed]
- 16.Barguigua A, Zerouali K, Katfy K, El Otmani F, Timinouni M, Elmdaghri N. Occurrence of OXA-48 and DM-1 carbapenemase-producing Klebsiella pneumoniae in a Moroccan university hospital in Casablanca, Morocco. Infection, Genetics and Evolution. 2015;31:142-8. 10.1016/j.meegid.2015.01.010 [DOI] [PubMed]
- 17.Solgi H, Badmasti F, Giske CG, Aghamohammad S, Shahcheraghi F. Molecular epidemiology of NDM-1-and OXA-48-producing Klebsiella pneumoniae in an Iranian hospital: clonal dissemination of ST11 and ST893. Journal of Antimicrobial Chemotherapy. 2018;73(6):1517-24. 10.1093/jac/dky081 [DOI] [PubMed]
- 18.Bush K, Bradford PA. Interplay between β-lactamases and new β-lactamase inhibitors. Nature Reviews Microbiology, 2019; 17(5), 295–306. 10.1038/s41579-019-0159-8 [DOI] [PubMed]
- 19.Al-Zahrani IA, Aljabri A, Alhazmi WA, Yasir M, Abujamel T, Alghamdi AK, Azhar EI. Genomic analysis of extensively drug resistant (XDR) Klebsiella pneumoniae high-risk clone ST14 co-harboring blaNDM and blaOXA-48 recovered from Saudi Arabia. Journal of Infection and Public Health. 2024;17(4):669 – 75. 10.1016/j.jiph.2024.02.011 [DOI] [PubMed]
- 20.Bougouizi A, Chekroud Z, Rahab H, Boumegoura A, Touati A. Prevalence and characterization of Carbapenem-Resistant Enterobacterales among inpatients and outpatients in Skikda, Algeria. The Journal of Infection in Developing Countries. 2024;18(03):383–390. 10.3855/jidc.18263 [DOI] [PubMed]
- 21.Takei S, Tabe Y, Miida T, Hishinuma T, Khasawneh A, Kirikae T, Sherchand JB, Tada T. Multidrug-resistant Klebsiella pneumoniae clinical isolates producing NDM-and OXA-type carbapenemase in Nepal. Journal of Global Antimicrobial Resistance. 2024; 37, 233–243. 10.1016/j.jgar.2024.04.008 [DOI] [PubMed]
- 22.Moghadampour M, Rezaei A, Faghri J. The emergence of bla OXA-48 and bla NDM among ESBL-producing Klebsiella pneumoniae in clinical isolates of a tertiary hospital in Iran. ActaMicrobiologicaetImmunologicaHungarica. 2018;65(3):335 – 44. 10.1556/030.65.2018.034 [DOI] [PubMed]
- 23.Nagaraj G, Shamanna V, Govindan V, Rose S, Sravani D, Akshata KP et al. High-resolution genomic profiling of carbapenem-resistant Klebsiella pneumoniae isolates: a multicentric retrospective indian study. Clin Infect Dis. 2021;73(4):300–7. 10.1093/cid/ciab767 [DOI] [PMC free article] [PubMed]
- 24.El Naggar NM, Shawky RM, Serry FM, Emara M. The Increased Prevalence of rmpA Gene in Klebsiella pneumoniae Isolates Coharboringbla NDM and bla OXA-48-like Genes. Microbial Drug Resistance. 2024 May 21. 10.1089/mdr.2023.0296 [DOI] [PubMed]
- 25.Abbasi E, Ghaznavi-Rad E. High frequency of NDM-1 and OXA-48 carbapenemase genes among Klebsiella pneumoniae isolates in central Iran. BMC microbiology. 2023;23(1):98. 10.1186/s12866-023-02840-x [DOI] [PMC free article] [PubMed]
- 26.Bošnjak Z, Hasman H, Hansen F, Hammerum AM, Roer L, Jurić I, Budimir A. Co-occurrence of triple carbapenemase genes, bla VIM-2, bla NDM-1, and bla OXA-48 in Enterobacter hormaechei clinical isolates–first report from Croatia. Journal of Chemotherapy. 2024 May 9:1–5. 10.1080/1120009X.2024.2354107 [DOI] [PubMed]
- 27.Veeraraghavan B, Shankar C, Karunasree S, Kumari S, Ravi R, Ralph R. Carbapenem-resistant Klebsiella pneumoniae isolated from bloodstream infection: Indian experience. Pathog Glob Health. 2017;111(5):240–246. 10.1080/20477724.2017.1340128 [DOI] [PMC free article] [PubMed]
- 28.Doi Y, O’Hara JA, Lando JF, Querry AM, Townsend BM, Pasculle AW et al. Co-production of NDM-1 and OXA-232 by Klebsiella pneumoniae. Emerging Infectious Diseases. 2014;20(1):163–5. 10.3201/eid2001.130904 [DOI] [PMC free article] [PubMed]
- 29.Kumarasamy K, Kalyanasundaram A. Emergence of Klebsiella pneumoniaeisolate co-producing NDM-1 with KPC-2 from India. Journal of Antimicrobial Chemotherapy. 2012;67(1):243–4. 10.1093/jac/dkr431 [DOI] [PubMed]
- 30.Pourakbari B, Mamishi S, Poormohammadi S et al. High prevalence of carbapenem resistance and clonal expansion of blaNDM gene in Klebsiella pneumoniae isolates in an Iranian referral pediatric hospital. Gut Pathog 16, 17 (2024). 10.1186/s13099-024-00611-1 [DOI] [PMC free article] [PubMed]
- 31.Cuzon G, Ouanich J, Gondret R, Naas T, Nordmann P. Outbreak of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in France. Antimicrobial Agents and Chemotherapy. 2011;55(5):2420–3. 10.1128/AAC.01452-10 [DOI] [PMC free article] [PubMed]
- 32.Barwa R, Shaaban M. Molecular characterization of Klebsiella pneumoniae clinical isolates with elevated resistance to carbapenems. The open microbiology journal. 2017;11:152. 10.2174/1874285801711010152 [DOI] [PMC free article] [PubMed]
- 33.Nkup J, Joseph S, Agabi Y, David V, Hashimu Z, Madubulum CC, David A, Otobo U, Goyil G, Cirfat N, Anejo-Okopi JA. Molecular detection of carbapenem resistant Klebsiella pneumoniae isolated from clinical specimens in Jos, Nigeria. Microbes Infect Dis. 2023;4(2):555–62. 10.21608/MID.2022.145897.1329. 10.21608/MID.2022.145897.1329 [DOI] [Google Scholar]
- 34.Maduakor UC, Eleazar CI, Mba CG, Obodochukwu CC, Eberechukwu CL, Ogu CO. Metallo-Beta-Lactamase Producing isolates of Escherichia coli and Klebsiella pneumoniae and their resistance profiles in Enugu, Nigeria: a threat to Public Health. J Adv Microbiol. 2024;24(2):11–9. 10.9734/jamb/2024/v24i2791. 10.9734/jamb/2024/v24i2791 [DOI] [Google Scholar]
- 35.Doko HI, Enejiyon SO, Wuna MM, Adedeji SA, Sa’adu M, Fasasi RA, Adabara NU. Molecular characterization OfCarbapenem resistant Klebsiella Pneumoniae from Wound surfaces of patients attending General Hospital Minna, Nigeria. Fudma J Sci. 2024;8(3):233–41. 10.33003/fjs-2024-0803-2494. 10.33003/fjs-2024-0803-2494 [DOI] [Google Scholar]
- 36.Olalekan A, Onwugamba F, Iwalokun B, Mellmann A, Becker K, Schaumburg F. High proportion of carbapenemase-producing Escherichia coli and Klebsiella pneumoniae among extended-spectrum β-lactamase-producers in Nigerian hospitals. J Global Antimicrob Resist. 2020;21:8–12. 10.1016/j.jgar.2019.09.007. 10.1016/j.jgar.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 37.Akinyemi KO, Abegunrin RO, Iwalokun BA, Fakorede CO, Makarewicz O, Neubauer H, Pletz MW, Wareth G. The emergence of Klebsiella pneumoniae with reduced susceptibility against Third Generation cephalosporins and carbapenems in Lagos hospitals, Nigeria. Antibiotics. 2021;10(2):142. 10.3390/antibiotics10020142. 10.3390/antibiotics10020142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ekpunobi NF, Mgbedo UG, Okoye LC, Agu KC. Prevalence of Esbl Genes in Klebsiella pneumoniae from Individuals with Community Acquired Urinary Tract Infections in Lagos State, Nigeria. Preprints 2024, 10.20944/preprints202403.1702.v1
- 39.Akinola O, Dahunsi O. Whole Genome Sequencing Revealing Antibiotics Resistance Pattern and Virulence Factors in KlebsiellaQuasipneumoniae Subsp. Similipneumoniae From Hospital Wastewater in South-West Nigeria. SimilipneumoniaeFrom Hospital Wastewater in South-West Nigeria. 10.2139/ssrn.4760762
- 40.Ibrahim Y, Sani Y, Saleh Q, Saleh A, Hakeem G. Phenotypic detection of extended spectrum beta lactamase and carbapenemase co-producing clinical isolates from two tertiary hospitals in Kano, North West Nigeria. Ethiop J Health Sci. 2017;27(1):3–10. 10.4314/ejhs.v27i1.2. 10.4314/ejhs.v27i1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muhammad BA. Survey for Carbapenem resistant Klebsiella Pneumoniae among patients attending Aminu Kano Teaching Hospital, Kano, Nigeria. UMYU J Microbiol Res (UJMR). 2023;8(2):181–9. 10.47430/ujmr.2382.021. 10.47430/ujmr.2382.021 [DOI] [Google Scholar]
- 42.Irekeola AA, Shueb RH, Abd Rahman EN, Afolabi HA, Wada Y, Elmi AH, Hakami MA, Alghzwani SM, Elnoubi OA, Alshehri AA. High prevalence of Carbapenem-resistant Enterobacterales (CRE) in human samples from Nigeria: a systematic review and Meta-analysis. Heliyon 2024 Jul 20. 10.1016/j.heliyon.2024.e34926 [DOI] [PMC free article] [PubMed]
- 43.Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes.Diagnostic microbiology and infectious disease. 2011;70(1):119–23. Available online at: 10.1016/j.diagmicrobio.2010.12.002 [DOI] [PubMed]
- 44.Odewale G, Jibola-Shittu MY, Ojurongbe O, Olowe RA, Olowe OA. Genotypic determination of Extended Spectrum β-Lactamases and carbapenemase production in clinical isolates of Klebsiella pneumoniae in Southwest Nigeria. Infect Disease Rep. 2023;15(3):339–53. 10.3390/idr15030034. 10.3390/idr15030034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olowo-okere A, Ibrahim YK, Olayinka BO, Ehinmidu JO, Mohammed Y, Nabti LZ, Rolain JM, Diene SM. Phenotypic and genotypic characterization of clinical carbapenem-resistant Enterobacteriaceae isolates from Sokoto, northwest Nigeria. New Microbes New Infections. 2020;37:100727. 10.1016/j.nmni.2020.100727. 10.1016/j.nmni.2020.100727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oduyebo OO, Falayi OM. 1,; Oshun, P; Ettu, A O. 2015. 10.4103/1117-1936.173973
- 47.Remya P, Shanthi M, Sekar U. Prevalence and clonal relatedness of NDM and OXA-48-producing Klebsiella pneumoniae in a tertiary care hospital in South India. J Lab Physicians. 2019;11(4):312–316. 10.4103/JLP.JLP_111_19 [DOI] [PMC free article] [PubMed]
- 48.Ghanbarinasab F, Haeili M, Ghanati SN, Moghimi M. High prevalence of OXA-48-like and NDM-1 carbapenemases among carbapenem-resistant Klebsiella pneumoniae of clinical origin from Iran. Iranian Journal of Microbiology. 2023;15(5):609–15. 10.18502/ijm.v15i5.13866 [DOI] [PMC free article] [PubMed]
- 49.Šuto S, Bedenic B, Likic S. Diffusion of OXA-48 carbapenemase among urinary isolates of Klebsiella pneumoniae in non-hospitalized elderly patients. BMC Microbiol. 2022;22(30). 10.1186/s12866-022-02443-y [DOI] [PMC free article] [PubMed]
- 50.El-Domany RA, El-Banna T, Sonbol F, Abu-Sayedahmed SH. Co-existence of NDM-1 and OXA-48 genes in Carbapenem Resistant Klebsiella pneumoniae clinical isolates in Kafrelsheikh, Egypt. African health sciences. 2021;21(2):489 – 96. 10.4314/ahs.v21i2.2 [DOI] [PMC free article] [PubMed]
- 51.ElFeky DS, Awad AR, Mowafy HL, Baddour MM, Hamed RM. Dissemination of NDM-1 and OXA-48 co-producing carbapenem-resistant Enterobacterales at two tertiary hospitals in Egypt. Egyptian Journal of Medical Microbiology. 2024;33(2):163 – 74. 10.21608/ejmm.2024.288181.1247
- 52.Ljubović AD, Granov Đ, Zahirović E, Čamdžić A, Muhić A, Bešić IS. Predominance of OXA-48 carbapenemase-producing Klebsiella pneumoniae strains in tertiary hospital in Sarajevo, Bosnia and Herzegovina. Biomolecules and Biomedicine. 2024 May 2. 10.17305/bb.2024.10406
- 53.Srivastava D, Bajpai S, Singh S, Singh MR. Molecular Characterization of Blaoxa-48 And Blandm-1 Resistant Genes In Carbapenem Resistance Klebsiella pneumoniae Clinical Isolates: A Cross Sectional Study. Biochemical & Cellular Archives. 2024;24(1). 10.51470/BCA.2024.24.1.107
- 54.TaşkınKafa AH, Aslan R, DurnaDaştan S, Çeli̇k C, Hasbek M, Emi̇noğlu A. Molecular diversity of Klebsiella pneumoniae clinical isolates: antimicrobial resistance, virulence, and biofilm formation. Nucleosides, Nucleotides & Nucleic Acids. 2024 Apr 19:1–7. 10.1080/15257770.2024.2344741 [DOI] [PubMed]
- 55.An X, Xie W, Zheng X, Zhao L, Wu Y, Qu Y. Detection and analysis of carbapenem-resistant Klebsiella pneumoniae resistance genes in five hospitals in Qingdao City. Chinese. 2020;32(2):145–9. 10.3760/cma.j.cn121430-20200110-00027 [DOI] [PubMed]
- 56.Hosseinzadeh Z, Ebrahim-Saraie HS, Sarvari J, Mardaneh J, Dehghani B, Rokni-Hosseini SM, Motamedifar M. Emerge of bla NDM-1 and bla OXA-48-like harboring carbapenem-resistant Klebsiella pneumoniae isolates from hospitalized patients in southwestern Iran. Journal of the Chinese Medical Association. 2018;81(6):536 – 40. 10.1016/j.jcma.2017.08.015 [DOI] [PubMed]
- 57.Argente M, Miró E, Martí C, Vilamala A, Alonso-Tarrés C, Ballester F et al. Molecular characterization of OXA-48 carbapenemase-producing Klebsiella pneumoniae strains after a carbapenem resistance increase in Catalonia. EnfermedadesInfecciosasMicrobiologíaClínica. 2019;37(2):82–8. 10.1016/j.eimc.2018.02.003 [DOI] [PubMed]
- 58.Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae: Here is the storm! Trends in Molecular Medicine. 2012;18(5):263–72. 10.1016/j.molmed.2012.03.003 [DOI] [PubMed]
- 59.Nordmann P, Poirel L, Dortet L. Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2012;18(9):1503–7. 10.3201/eid1809.120355 [DOI] [PMC free article] [PubMed]
- 60.Karaman E, Çiçek AÇ, Şemen V, ŞabanBeriş F. Characterization of resistance genes and replicon typing in Carbapenem-resistant Klebsiella pneumoniae strains. Annals of Clinical Microbiology and antimicrobials. 2024;23(1):19. 10.1186/s12941-024-00672-9 [DOI] [PMC free article] [PubMed]
- 61.Rojas LJ, Salim M, Cober E, Richter SS, Perez F, Salata RA et al. Colistin resistance in carbapenem-resistant Klebsiella pneumoniae: laboratory detection and impact on mortality. Clin Infect Dis. 2017;64(6):711–8. 10.1093/cid/ciw805 [DOI] [PMC free article] [PubMed]
- 62.Satlin MJ, Kubin CJ, Blumenthal JS, Cohen AB, Furuya EY, Wilson SJ et al. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother. 2011;55(12):5893–9. 10.1128/AAC.00387-11 [DOI] [PMC free article] [PubMed]
- 63.El-Sayed AK, Abou-Dobara MI, Saleh HH, Ahmad YF. Detection of blaOXA-48 gene in carbapenem-resistant Klebsiella pneumoniae and Escherichia coli from urine samples. Scientific Journal for Damietta Faculty of Science. 2024;14(1):119 – 28. 10.21608/SJDFS.2024.270443.1160
- 64.Malinga NZZ, Shobo CO, Molechan C, Amoako DG, Zishiri OT, Bester LA. Molecular surveillance and dissemination of Klebsiella pneumoniae on frequently encountered surfaces in South African Public Hospitals. Microbial Drug Resistance. 2021;28(3). 10.1089/mdr.2020.0546 [DOI] [PubMed]
- 65.Durmuş MA, Aydin MD. Detection of Carbapenem Resistance Using the Genotypic and Phenotypic Methods in Klebsiella pneumoniae. Duzce Medical Journal. 2024;26(1):15–20. 10.18678/dtfd.1383748
- 66.Mawla NN, Nessa M, Nandi P, Rahman MM, Faruk BS. Molecular Detection of Carbapenemase Genes among Carbapenem Resistant Gram-Negative Bacilli in Tertiary Care Hospital, Bangladesh. 10.21275/SR24502173236
- 67.Addis E, Unali I, Bertoncelli A, Ventura A, Cecchetto R, Mazzariol A. Different OXA-Carbapenemases in Genetically Unrelated Klebsiella pneumoniae Strains Isolated in a North Italian Hospital During Multidrug Resistance Screening. Microbial Drug Resistance. 2024 Jan 2. 10.1089/mdr.2023.0134 [DOI] [PubMed]
- 68.El Naggar NM, Shawky RM, Serry FME et al. Investigating the relationship between carbapenemase production and biofilm formation in Klebsiella pneumoniae clinical isolates. BMC Res Notes 17, 49 (2024). 10.1186/s13104-024-06708-9 [DOI] [PMC free article] [PubMed]
- 69.Diallo OO, Baron SA, Abat C, Colson P, Chaudet H, Rolain JM. Antibiotic resistance surveillance systems: a review. Journal of Global Antimicrobial Resistance. 2020;23:430–8. 10.1016/j.jgar.2020.10.009 [DOI] [PubMed]
- 70.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–27. 10.1016/S1473-3099(17)30753-3 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated. The primer sequences and optimization protocols utilized in this study for the detection of OXA-48 and NDM-1 genes in Klebsiella pneumoniae isolates were adapted from a previously published work available online at: https://doi.org/10.1016/j.diagmicrobio.2010.12.002.