Abstract
Objective
The persistent symptoms arising from COVID-19 infection pose a substantial threat to patients’ health, carrying significant implications. Amidst the evolving COVID-19 control strategies in China, healthcare workers (HCWs) endure considerable stress. This study aims to evaluate the prevalence of long COVID infections and their influencing factors among primary HCWs after epidemic control policy adjustment in Jiangsu.
Methods
A self-designed questionnaire was administered through on-site surveys among primary HCWs in five counties and districts within Jiangsu Province from July 4 to July 20, 2023. Logistic regression analysis was employed to identify factors associated with long COVID.
Results
The prevalence of long COVID among primary HCWs stood at 12.61%, with a 95% confidence interval (CI) of 11.67-13.55%. Among those affected, the most common long COVID symptoms were hypomnesia (4.90%, 95%CI: 4.29-5.51%), sleep difficulties (2.73%, 95%CI: 2.27-3.19%), fatigue (2.35%, 95%CI: 1.92-2.78%), disturbances in the reproductive system (1.93%, 95%CI: 1.54-2.32%), hair loss (1.85%, 95%CI: 1.47-2.23%), and myalgia/arthralgia (1.51%, 95%CI: 1.16-1.86%). Multivariate logistic regression revealed that older age groups (30–45 years (adjusted odds ratio (aOR) = 1.93, 95%CI: 1.44–2.58), 45–60 years (aOR = 2.82, 95%CI: 2.07–3.84)), females (aOR = 1.26, 95%CI: 1.03–1.55), and higher work stress (high stress (aOR = 1.52, 95%CI: 1.24–1.86), extremely high stress (aOR = 1.37, 95%CI: 1.03–1.82)) were more prone to long COVID. Conversely, individuals with educational attainment below the bachelor’s degree (aOR = 0.67, 95%CI: 0.55–0.82) and those who received four or more doses of the COVID-19 vaccine (aOR = 0.55, 95%CI: 0.33–0.92) were at a reduced risk.
Conclusion
This study investigates the prevalence of long COVID among primary HCWs and identifies key influencing factors. These findings are crucial for assisting in the early identification of COVID-19 patients at risk for long-term complications, developing targeted interventions aimed at optimizing healthcare resource allocation and enhancing the work conditions and quality of life of HCWs. To mitigate the prevalence of long COVID, healthcare providers and local authorities should implement effective measures, such as optimizing work-rest schedules and actively advocating for vaccination.
Keywords: Long COVID, Healthcare workers, Prevalence, Influencing factors, China
Introduction
Coronavirus disease 2019 (COVID-19) has posed a major threat to the health and socioeconomics of people worldwide since emerging from Wuhan in December 2019 [1]. As of Beijing Time, October 5, 2023, there have been 771,151,224 confirmed cases of COVID-19, including 6,960,783 deaths reported to the WHO worldwide [2]. With the continued prevalence of COVID-19 and the increasing number of recovered cases, there is substantial evidence to suggest that a higher proportion of individuals may experience long-term effects on multiple organs and systems even after the nucleic acid test has returned to a negative result. This condition is commonly referred to as ‘long COVID’.
Long COVID is a post-COVID-19 condition occurring in individuals with a history of probable or confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, usually 3 months from the onset, with symptoms lasting for at least 2 months and not attributable to an alternative diagnosis, as defined by the WHO [3]. The National Institute for Health and Clinical Excellence (NICE) in the UK distinguishes between symptoms that occur between 4 and 12 weeks after infection with COVID-19 (referred to as ‘Ongoing symptomatic COVID-19’, which is considered a subacute phase of the infection), and symptoms that persist for more than 12 weeks (termed ‘Post-COVID-19 syndrome’ or ‘chronic disease’) [4]. As the number of infections increased, the number of people experiencing long-term effects of COVID-19 also rose rapidly. As of March 5, 2023, about 1.9 million people in UK households (2.9% of the population) reported experiencing long COVID, with symptoms lasting over four weeks after their first confirmed or suspected COVID-19 infection, and not due to other causes [5]. Evidence indicates that a minimum of 65 million individuals worldwide are estimated to struggle with long COVID [6], with projections indicating its potential impact on over a billion individuals globally in the forthcoming years [7]. The most common symptoms include, but are not limited to, fatigue, shortness of breath, and cognitive dysfunction, which generally have an impact on everyday functioning [8].
The prevalence of long COVID varies depending on viral strains and duration of follow-up. Notably, the Omicron variant has been associated with lower rates compared to other variants. Research indicates prevalence rates of long COVID for historical variants at 48.1% (95% CI: 39.9–56.2%), Alpha variant at 35.9% (95% CI: 30.5–41.6%), and a combination of Delta and Omicron variants at 16.5% (95% CI: 12.4–21.4%) [9]. The prevalence of long COVID tends to decrease with longer follow-up periods. A study conducted during the 2021 Delta variant surge in India showed rates of 29.2% at 4 weeks and 9.3% at 6 months [10]. A follow-up study after the outbreak of the Omicron BA.2 variant in Shanghai in March 2022 reported that 8.89% of COVID-19 patients experienced WHO-defined long COVID, with a significant decrease in its incidence observed after one year [11]. Overall, an estimated 10–20% of cases currently experience long COVID, potentially affecting individuals of all age groups, including children [6]. Its ramifications cause widespread health, welfare, and livelihood damages globally, resulting in significant economic losses.
There is a considerable body of literature exploring the influencing factors of long COVID. Romero-Rodríguez et al. identified hospitalization, ICU admission, history of pneumonia, and vaccination as predictive factors for long COVID symptoms, with vaccination being the only negative predictor for all significant symptoms [12]. A multicenter survey across four major cities in China revealed that female gender, smoking, having ≥ 3 chronic diseases, and prolonged medication usage were risk factors for long COVID, while prior administration of ≥ 2 vaccine doses acted as a protective factor [13]. A retrospective cohort study following the Omicron surge in eastern India highlighted previous COVID-19 infection and having ≥ 2 symptoms during the acute phase as risk factors for developing long COVID [14]. Wang et al. identified through a prospective cohort study that individuals experiencing depression, anxiety, perceived stress, and greater concern about COVID-19 were more prone to develop long COVID related symptoms [15].
Since the issuance of the ‘Notice on Further Optimizing and Implementing COVID-19 Prevention and Control Measures’ by the Joint Prevention and Control Mechanism of the State Council on December 7, 2022 [16], virus transmission has rapidly escalated, resulting in a sharp rise in infection cases in China. Healthcare workers (HCWs), being at the forefront in combating COVID-19, face heightened risks and might be more susceptible to long COVID. Foreign studies have documented the occurrence of long COVID among HCWs. An observational multicenter study conducted in India, covering the period from July to October 2021 among HCWs infected with COVID-19, reported an overall prevalence rate of 30.34% for long COVID [17]. To our knowledge, there is no similar research conducted in China. Therefore, it seems urgent to conduct relevant studies to understand the prevalence of long COVID after epidemic control policy adjustment.
Therefore, in July 2023, we conducted a follow-up study among primary HCWs in Jiangsu Province who had contracted COVID-19, aiming to comprehensively understand the current status of long COVID in this population, accurately assess associated risk factors, and early identify high-risk groups for long COVID. This study intends to provide support and research evidence for the revision of fundamental healthcare policies.
Materials and methods
Study subjects
Previously, we conducted a study on COVID-19 infections and the prevalence, characteristics, and predictors of ongoing symptoms (lasting > 28 days) of COVID-19 one and a half months after adjustments the epidemic prevention and control policy [18]. This study employed the same methodology but changed the questionnaire completion method from online to on-site. From July 4th to July 20th, 2023, a follow-up survey was carried out to assess long COVID symptoms among 5,754 primary HCWs in five counties and districts of Jiangsu Province. All participants had been involved in the initial survey and were primary HCWs who may have been directly involved in the diagnosis, care, and treatment of COVID-19. The questionnaire was developed according to the survey’s purpose and references, and subsequently underwent refinement during an expert workshop. The finalized questionnaire was distributed through the Questionnaire Star platform (https://www.wjx.cn/). At the follow-up site, investigators initially provided an explanation of the questionnaire, and participants subsequently used their smartphones to scan a QR-code for response submission. Participants could consult the investigators at any time if they encountered any difficulties during the completion process. Investigators conducted on-site proofreading and review of the collected questionnaires to ensure accurate completion. The survey encompassed demographic information and information regarding symptoms associated with long COVID.
The study protocol was approved by the Ethics Committee of the Jiangsu Provincial Centre for Disease Control and Prevention (No. JSJK2023-B010-01). Additionally, all participants voluntarily joined this study and signed an informed consent form before completing the questionnaire.
Definition
In our study, long COVID is defined as the presence of signs and symptoms consistent with COVID-19 during or after infection, lasting more than 12 weeks and not explained by other diagnoses [4]. Individuals would be diagnosed with ‘long COVID’ if they experienced symptoms persisting for more than 90 days, and if they presented one or more of the following symptoms: fatigue, cough, breathing difficulties, nasal congestion/runny nose, cardiac issues, anaemia, headache/dizziness, sleep difficulties, anxiety, depression, brain fog, hypomnesia, hair loss, smell/taste disorder, nausea/vomiting, diarrhea/constipation, myalgia/arthralgia, limb numbness, skin rash, or disturbance of reproductive system [19–21].
Since January 8, 2023, China has implemented “Class B control” for COVID-19 infections, and the nucleic acid strategy has been adjusted to “test at will“ [22]. The number of people undergoing nucleic acid testing has sharply declined. This study concentrates on individuals who have either been infected or likely to be infected. In this study, infected persons were defined as those who tested positive for COVID-19 nucleic acid, those who tested positive for COVID-19 antigen, those who tested positive for both COVID-19 nucleic acid and antigen, as well as those who showed symptoms related to COVID-19 but were not tested. Based on the literature, the strain of infection involved in this investigation is most likely to be the Omicron strain [23].
The body mass index (BMI) was calculated as weight in kilograms divided by the square of height in metres. The BMI classification criteria for Chinese adults are outlined as follows [24]: underweight is defined as BMI < 18.5 kg/m2, normal weight is defined as BMI 18.5–23.9 kg/m2, overweight is defined as BMI 24.0–27.9 kg/m2, and obesity was defined as BMI ≥ 28.0 kg/m2.
Sample size and statistical analysis
According to our analysis of data collected from primary HCWs in Jiangsu Province who were infected from December 2022 to January 2023, the prevalence rate of ongoing symptoms was 14.83%. Additionally, a follow-up survey conducted after the Omicron BA.2 outbreak in Shanghai from March to June 2022 found that 8.89% COVID-19 patients self-reported the presence of long COVID symptoms [11]. In our study, assuming a prevalence rate of 8.89% for long COVID, alongside a two-tailed alpha level set at 0.05, and a two-sided 95% Clopper-Pearson CI width of 0.01778 (with a relative error of 10%), the minimum sample size required was computed using PASS 15.0 software, resulting in 4,489 participants, factoring in a permissible non-response rate of up to 10%.
The final data were exported to Microsoft Excel and subsequently analyzed using the statistical software R 4.2.3. Continuous variables were reported as means (standard deviation, SD), while categorical variables were presented as frequencies and percentages. The Nightingale rose diagram was utilized to illustrate the distribution of persistent symptoms following COVID-19 infection. Researchers conducted both univariate and multivariate logistic regression analyses to identify factors associated with the development of long COVID symptoms among primary HCWs. In the univariate analyses, variables associated with infection (P < 0.2) were subsequently included in multivariate logistic stepwise regression analyses. Odds ratio (OR) and 95%CI or adjusted odds ratio (aOR) and 95%CI, quantified the risk associated with long COVID symptoms. Multivariate outcomes will be graphically depicted using forest plots. A two-sided P-value of < 0.05 was considered as indicative of statistical significance.
Results
Participants
In July 2023, we conducted an investigation into the long COVID situations among participants of the initial survey by randomly selecting five counties/districts from the locations where the 34,090 respondents of the first survey were situated. These selected areas comprised the Ganyu District in Lianyungang City, Funing County, and Yandu District in Yancheng City, Kunshan and Changshu, which are county-level cities in Suzhou. A total of 5,754 primary HCWs participated in the survey. After excluding duplicate questionnaires, the data from the initial baseline survey were cross-referenced with the data from this follow-up survey by matching citizen identification number. Subsequently, participants with inconsistent responses to at least one of the four questions (citizen identification number, COVID-19 infection between 1 December 2022 and 20 January 2023, education level, and history of COVID-19 vaccination between the two surveys) were excluded. This process led to the identification of 5,541 participants, of whom 4,757 were infected.
Demographic characteristics of infected primary HCWs
In this study, all results are analyzed based on the participation of 4,757 infected primary HCWs. The mean age of the participants was 39.55 ± 10.61 years, and 31.20% were male. BMI calculations using self-reported height and weight revealed that 51.69% of the participants were classified as normal weight, while 12.47% were categorized as obesity. More than half of the participants (58.10%) held a bachelor’s degree and above. Among the participants, the most common profession was doctor, accounting for 2,118 (44.52%), followed by nurses with 1,339 (28.15%). The majority were employees, with only 16.46% in leader and middle management. For a comprehensive overview of the demographic profile of the participants, please refer to Table 1.
Table 1.
Demographic characteristics of infected primary HCWs in Jiangsu Province
| Variable | Total, N (%) | |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 39.55 (10.61) | |
| Gender | ||
| Male | 1,484 (31.20) | |
| Female | 3,273 (68.80) | |
| BMI | ||
| Under weight | 260 (5.47) | |
| Normal weight | 2,459 (51.69) | |
| Over weight | 1,445 (30.38) | |
| Obesity | 593 (12.47) | |
| Educational level | ||
| Below the bachelor’s degree | 1,993 (41.90) | |
| Bachelor’s degree and above | 2,764 (58.10) | |
| Profession | ||
| Doctor | 2,118 (44.52) | |
| Nurse | 1,339 (28.15) | |
| Pharmacist | 327 (6.87) | |
| Medical technician | 559 (11.75) | |
| Other position | 414 (8.70) | |
| Position | ||
| Employee | 3,974 (83.54) | |
| Middle management | 627 (13.18) | |
| Leader | 156 (3.28) |
Prevalence, major symptoms of long COVID and comparison of major symptoms between the two surveys
The symptoms number observed in long COVID patients are presented in Table 2. Out of the 4,757 infected individuals, 600 (12.61%, 95%CI: 11.67-13.55%) self-reported experiencing long COVID symptoms, while 264 (5.55%, 95%CI: 4.90-6.20%) reported two or more symptoms, accounting for 44.00% of patients with long COVID.
Table 2.
The symptoms number of long COVID among primary HCWs
| Number of symptoms | Frequency | Proportion (%) |
|---|---|---|
| 0 | 4,157 | 87.39 |
| 1 | 336 | 7.06 |
| ≥ 2 | 264 | 5.55 |
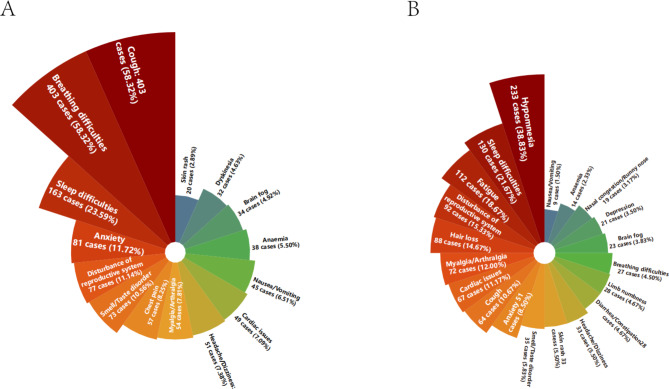
The most common symptoms of long COVID were hypomnesia (233/4,757, 4.90%, 95%CI: 4.29-5.51%), sleep difficulties (130/4,757, 2.73%, 95%CI: 2.27-3.19%), fatigue (112/4,757, 2.35%, 95%CI: 1.92-2.78%), disturbances in the reproductive system (92/4,757, 1.93%, 95%CI: 1.54-2.32%), hair loss (88/4,757, 1.85%, 95%CI: 1.47-2.23%), and myalgia/arthralgia (72/4,757, 1.51%, 95%CI: 1.16-1.86%). Comparing the ongoing symptoms collected in the initial baseline survey with the symptoms concurrently reported in the current follow-up survey of long COVID symptoms, a notable decrease in the instances of breathing difficulties (58.32% vs. 4.50%) and cough (58.32% vs. 10.67%) became apparent. However, there was a slight increase in the prevalence of myalgia/arthralgia (7.81% vs. 12.00%), cardiac issues (7.09% vs. 11.17%), disturbance of reproductive system (11.14% vs. 15.33%), and skin rash (2.89% vs. 5.50%). The detailed distribution of the two surveys is depicted in Fig. 1.
Fig. 1.
Nightingale rose diagram: illustration of > 28 and ≥ 91 days symptoms in patients with long COVID. The area of each colored bar indicates the number of cases. (A) signifies the percentage of patients experiencing ongoing symptoms lasting > 28 days among the 691 patients surveyed at baseline; (B) signifies the percentage of patients with long COVID symptoms lasting ≥ 91 days among the 600 patients in the follow-up survey
Logistic regression on factors influencing of long COVID
The comprehensive examination of factors contributing to long COVID among primary HCWs is elucidated in Table 3. Significant disparities (p < 0.05) were observed between individuals with and without long COVID across multiple parameters: age, education level, position, medical history, work in a fever clinic, COVID-19 vaccination doses, normal rest on weekend or holidays, and work stress. Prevalence rates for long COVID were 13.50% among the 30–45 age group, 15.45% among the 45–60 age group, and 6.67% for those under 30. Those holding a bachelor’s degree and above displayed a prevalence of 14.22%, while those with education below the bachelor’s degree showed a prevalence of 10.39%. Remarkably, middle management exhibited a significantly higher prevalence of long COVID at 15.15% compared to ordinary employees and unit leaders. Individuals with medical history (15.23%) and those working in fever clinics (14.09%) had higher long COVID prevalence compared to their counterparts. Regarding COVID-19 vaccine doses, prevalence rates were 18.69% for 0–1 doses, 14.07% for 2–3 doses, and 10.60% for 4 or more doses. Participants who normally rested during weekends or holidays had a notably lower prevalence of 7.61% compared to occasional normal resters.
Table 3.
Univariate logistic regression for factors associated with long COVID
| Variable | Non-long COVID (n = 4,157) | Long COVID (n = 600) | OR (95%CI) | p-value | |
|---|---|---|---|---|---|
| Gender | Male | 1,311 (88.34%) | 173 (11.66%) | ||
| Female | 2,846 (86.95%) | 427 (13.05%) | 1.14 (0.94–1.37) | 0.182 | |
| Age | Under 30 years old | 896 (93.33%) | 64 (6.67%) | ||
| 30 ~ 45 years old | 1,800 (86.50%) | 281 (13.50%) | 2.19 (1.65–2.90) | < 0.001 | |
| 45 ~ 60 years old | 1,345 (84.54%) | 246 (15.46%) | 2.56 (1.92–3.41) | < 0.001 | |
| More than 60 years old | 116 (92.80%) | 9 (7.20%) | 1.09 (0.53–2.24) | 0.823 | |
| BMI | Normal weight | 2,147 (87.31%) | 312 (12.69%) | ||
| Obesity | 517 (87.18%) | 76 (12.82%) | 1.01 (0.77–1.32) | 0.933 | |
| Over weight | 1,266 (87.61%) | 179 (12.39%) | 0.97 (0.80–1.18) | 0.784 | |
| Under weight | 227 (87.31%) | 33 (12.69%) | 1.00 (0.68–1.47) | 0.998 | |
| Education level | Bachelor’s degree and above | 2,371 (85.78%) | 393 (14.22%) | ||
| Below the bachelor’s degree | 1,786 (89.61%) | 207 (10.39%) | 0.70 (0.58–0.84) | < 0.001 | |
| Profession | Other position | 360 (86.96%) | 54 (13.04%) | ||
| Doctor | 1,849 (87.30%) | 269 (12.70%) | 0.97 (0.71–1.33) | 0.848 | |
| Nurse | 1,186 (88.57%) | 153 (11.43%) | 0.86 (0.62–1.20) | 0.373 | |
| Pharmacist | 273 (83.49%) | 54 (16.51%) | 1.32 (0.88–1.98) | 0.185 | |
| Medical technician | 489 (87.48%) | 70 (12.52%) | 0.95 (0.65–1.40) | 0.810 | |
| Position | Employee | 3,488 (87.77%) | 486 (12.23%) | ||
| Middle management | 532 (84.85%) | 95 (15.15%) | 1.28 (1.01–1.63) | 0.041 | |
| Leader | 137 (87.82%) | 19 (12.18%) | 1.00 (0.61–1.62) | 0.985 | |
| Smoking status | Never smoking | 3,693 (87.45%) | 530 (12.55%) | ||
| Used to smoke | 111 (85.38%) | 19 (14.62%) | 1.19 (0.73–1.96) | 0.485 | |
| Smoking | 353 (87.38%) | 51 (12.62%) | 1.01 (0.74–1.37) | 0.966 | |
| Drinking status | Never drinking | 3,265 (87.30%) | 475 (12.70%) | ||
| Used to drink | 245 (88.13%) | 33 (11.87%) | 0.93 (0.64–1.35) | 0.688 | |
| Drinking | 647 (87.55%) | 92 (12.45%) | 0.98 (0.77–1.24) | 0.851 | |
| Dietary structure | Balanced diet | 3,027 (87.79%) | 421 (12.21%) | ||
| Meat-based diet | 555 (88.38%) | 73 (11.62%) | 0.95 (0.73–1.23) | 0.679 | |
| Vegetable-based diet | 575 (84.43%) | 106 (15.57%) | 1.33 (1.05–1.67) | 0.017 | |
| Weekly frequency of exercise | 0 times | 1,146 (87.02%) | 171 (12.98%) | ||
| 1–3 times | 2,224 (87.94%) | 305 (12.06%) | 0.92 (0.75–1.12) | 0.409 | |
| 4–6 times | 515 (87.14%) | 76 (12.86%) | 0.99 (0.74–1.32) | 0.940 | |
| 7 and more times | 272 (85.00%) | 48 (15.00%) | 1.18 (0.84–1.67) | 0.342 | |
| Medical history | No | 3,300 (88.09%) | 446 (11.91%) | ||
| Yes | 857 (84.77%) | 154 (15.23%) | 1.33 (1.09–1.62) | 0.005 | |
| Work in a fever clinic | No | 2,138 (88.82%) | 269 (11.18%) | ||
| Yes | 2,019 (85.91%) | 331 (14.09%) | 1.30 (1.10–1.55) | 0.003 | |
| COVID-19 vaccine doses | 0 ~ 1 doses | 87 (81.31%) | 20 (18.69%) | ||
| 2 ~ 3 doses | 2,155 (85.93%) | 353 (14.07%) | 0.71 (0.43–1.17) | 0.183 | |
| 4 and more doses | 1,915 (89.40%) | 227 (10.60%) | 0.52 (0.31–0.85) | 0.010 | |
| Normal rest on weekends or holidays | Always | 413 (92.39%) | 34 (7.61%) | ||
| Often | 1269 (89.62%) | 147 (10.38%) | 1.41 (0.95–2.08) | 0.085 | |
| Occasionally | 2,475 (85.52%) | 419 (14.48%) | 2.06 (1.43–2.96) | < 0.001 | |
| Daily working hours | > 8 h | 1,477 (86.17%) | 237 (13.83%) | ||
| ≤ 8 h | 2,680 (88.07%) | 363 (11.93%) | 0.84(0.71–1.01) | 0.058 | |
| Weekly working days | > 5 days | 3,464 (87.06%) | 515 (12.94%) | ||
| ≤ 5 days | 693 (89.07%) | 85 (10.93%) | 0.83(0.65–1.05) | 0.122 | |
| Work stress | Moderate | 2,076 (89.95%) | 232 (10.05%) | ||
| Low | 222 (93.28%) | 16 (6.72%) | 0.64 (0.38–1.09) | 0.102 | |
| High | 1,376 (83.95%) | 263 (16.05%) | 1.71 (1.42–2.07) | < 0.001 | |
| Extremely high | 483 (84.44%) | 89 (15.56%) | 1.65 (1.27–2.15) | < 0.001 |
No statistically significant differences were observed between the long COVID group and the non-long COVID group in terms of gender, BMI, profession, smoking status, drinking status, dietary structure, weekly frequency of exercise, daily working hours, and weekly working days. The individual characteristics of the long COVID and non-long COVID groups can be found in Table 3.
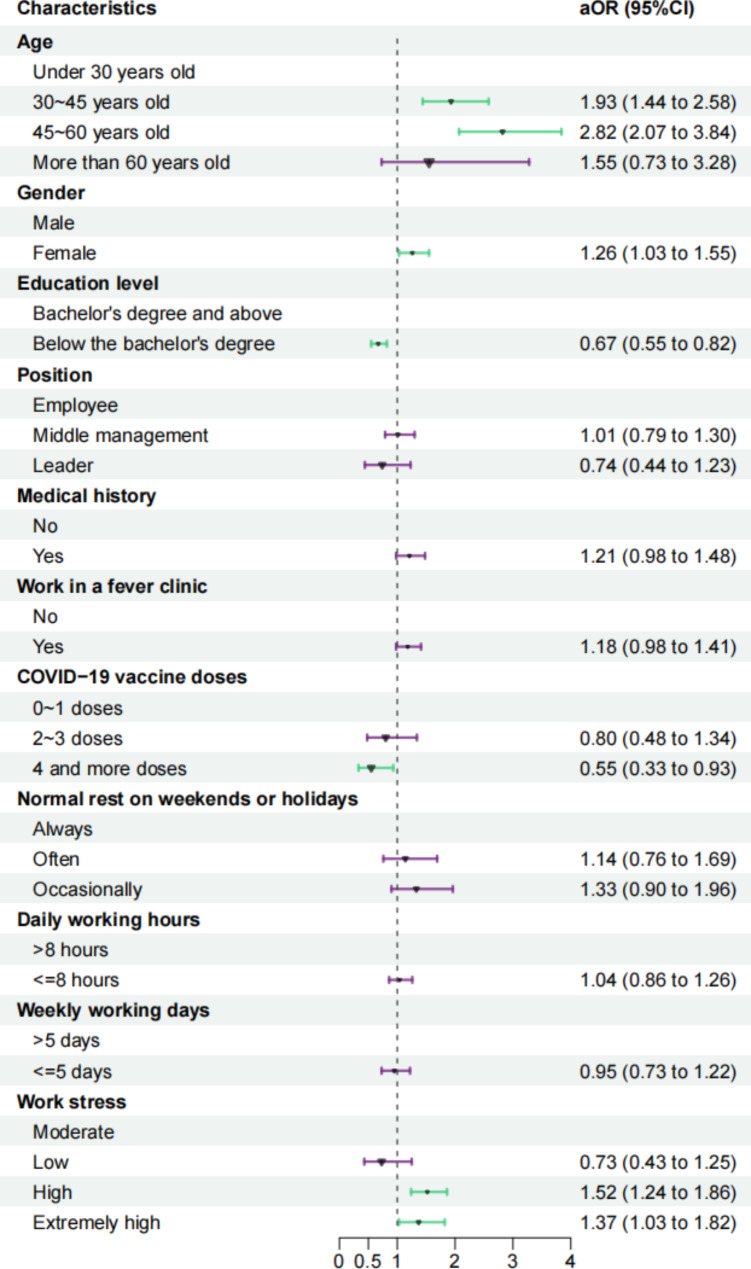
Variables with a p-value of 0.2 or lower in the univariate logistic regression analysis were included in the multivariate logistic regression. The findings, depicted in Fig. 2, revealed significant associations indicating that older age, female, and higher work stress were correlated with an increased risk of long COVID prevalence. Specifically, individuals aged 30–45 years (aOR = 1.93, 95%CI: 1.44–2.58) and 45–60 years (aOR = 2.82, 95%CI: 2.07–3.84) exhibited a higher likelihood of developing long COVID compared to those under 30 years old. Additionally, females have a higher risk of developing long COVID compared to males (aOR = 1.26, 95%CI: 1.03–1.55). Furthermore, individuals reporting high stress (aOR = 1.52, 95%CI: 1.24–1.86) and extremely high stress (aOR = 1.37, 95%CI: 1.03–1.82) were at a greater risk of experiencing long COVID. Conversely, individuals with education levels below a bachelor’s degree (aOR = 0.67, 95%CI: 0.55–0.82) and those who had received four doses of the COVID-19 vaccine (aOR = 0.55, 95%CI: 0.33–0.92) were associated with a reduced risk of developing the disease.
Fig. 2.
Forest plot of factors associated with long COVID in multivariate logistic regression. The OR are the exponentiated coefficients of the logistic model
Discussion
In our study, 600 individuals (12.61% of the total sample of 4,757) experienced long COVID symptoms within six months post-infection. Among those with long COVID, 44.00% exhibited two or more symptoms. The most prevalent long COVID symptoms included hypomnesia, sleep difficulties, fatigue, disturbances in the reproductive system, hair loss, and myalgia/arthralgia. In the multivariate logistic regression, risk factors associated with the development of long COVID included older age, female and higher work stress, while protective factors included educational attainment below a bachelor’s degree and receiving four or more vaccine doses.
While it is currently observed that new variants of COVID-19 lead to milder cases [25], they may have serious health consequences for specific populations such as pregnant individuals [26, 27]. There is an urgent need for in-depth research on the impact of these new variants on the long-term development of COVID-19, aiming to mitigate the public health crisis and formulate more effective management strategies. The primary objective of this study, conducted after adjustment of the epidemic control policy in China, is to estimate the proportion of individuals infected with SARS-CoV-2 who self-report long-term symptoms after six months. Furthermore, we seek to comprehend the primary symptoms and their influencing factors, with the aim of assessing the impact of the Omicron pandemic on individuals’ health status.
Long COVID has emerged as a significant global public health concern [28]. Recent global analyses have reported a cumulative prevalence of long COVID ranging from 9–63% [29]. A meta-analysis encompassing 51 cohort studies, involving 33,573 patients, revealed an overall long COVID prevalence of 65.8% (95%CI: 47.7-83.9%). Specifically, the Omicron variant exhibited a substantially lower long COVID at 28.4% (95%CI: 7.9-49.0%), while the wild-type and Alpha variants were 60.5% (95%CI: 40.4-80.6%) and 66.1% (95%CI: 42.2-89.9%) respectively [30]. Overall, the Omicron variant demonstrated a lower prevalence of long COVID post-infection compared to other variants. During our study, the prevailing strain identified was Omicron [31]. Therefore, the reported prevalence of long COVID associated with this strain (12.61%) aligns with the historical study range. However, our long COVID prevalence was higher compared to a prospective cohort study in Shanghai, China, which reported 8.89% [11]. This discrepancy may be attributed to their stringent definition, which required laboratory confirmation or clinical diagnosis for COVID-19 patients, whereas our criteria included individuals with COVID-19 like symptoms but without formal testing, potentially leading to an overestimation of long COVID prevalence. This also stands as a limitation of our study.
According to prior literature, the most commonly reported symptoms of long COVID encompass fatigue, breathlessness, olfactory dysfunction, myalgia, cough, memory impairment, attention disorders, headache, hair loss, brain fog, anxiety, and depression [32–35]. In our study, we assessed 20 prevalent long COVID symptoms, with hypomnesia (4.89%) emerging as the most frequent. This contrasts slightly with other studies where fatigue was identified as the predominant long COVID symptom, potentially attributed to the heightened cognitive demands experienced by healthcare professionals in comparison to the general populace [34, 36]. Additionally, our investigation highlighted disturbances in the reproductive system (1.94%) as one of the most frequently occurring symptoms of long COVID. Previous studies suggest a reasonable hypothesis that SARS-CoV-2 infection may impact the reproductive organs in both females and males, potentially influencing human fertility as well as aspects of pregnancy processes and outcomes to some degree [37]. Furthermore, a retrospective matched cohort study utilizing the UK primary care database unveiled a noteworthy association between reduced libido (OR = 2.36, 95%CI: 1.61–3.47) and long COVID [38].
A study involving 4,182 COVID-19 cases from the UK, USA, and Sweden discovered that the likelihood of developing long COVID increased with age, corroborating our own findings [39]. Similarly, a systematic review comprising 25 observational studies also pinpointed advanced age as a common risk factor for long COVID [40]. A single-center prospective cohort study conducted at San Paolo Hospital in Milan, Italy, unveiled a significant correlation between older age and the risk of long COVID [41]. Individuals within the 30–60 age bracket exhibit a higher likelihood of developing long COVID compared to those under 30 years old. This tendency might be attributed to the higher prevalence of pre-existing conditions like diabetes, high blood pressure, and cardiovascular disease among older individuals, coupled with the relatively weaker immune system observed in this age group [42].
At the outset, we initially noted no substantial variations between genders. However, subsequent multivariate analyses revealed a significant association between female and the risk of long COVID. Consistently across most studies, women exhibited a higher risk compared to men [38, 41]. This discrepancy may stem from differences in hormone levels between genders and potentially due to women’s heightened attentiveness to bodily changes.
Our study reveals that higher levels of work stress are associated with an increased risk of developing long COVID. Previous research has already indicated that elevated stress levels during the acute phase of COVID-19 infection are closely linked to symptom onset. A study conducted among healthcare professionals caring for COVID-19 patients in five prominent hospitals in Singapore and India demonstrated a strong correlation between physical symptoms like sore throat, nausea, vomiting, insomnia, loss of appetite, and heightened levels of anxiety, stress, and depression [43]. Regarding the relationship between stress and long COVID, a cross-sectional online survey conducted on social media platforms (Twitter and Facebook) showed that stress is one of the common triggers for the recurrence or exacerbation of long COVID symptoms [44].
Based on our research, individuals with education levels below a bachelor’s degree may have a reduced likelihood of developing long COVID compared to those with a bachelor’s degree and above. This could be attributed to healthcare professionals with lower educational attainment being more inclined to work in general medical practices or primary healthcare settings, which typically have fewer encounters with severe or potentially high-risk COVID-19 cases. Conversely, HCWs with higher educational levels are more likely to be stationed in specialized medical institutions or emergency centers, where they may have more frequent encounters with severe cases. Additionally, a population-based prospective cohort study has also shown that lower educational levels (adjusted hazard ratio (aHR) = 0.77, 95%CI: 0.64–0.93) act as a protective factor for the prolongation of persistent symptoms [45]. From this, it can be inferred that lower educational levels may correlate with shorter durations of persistent symptoms, thereby reducing the likelihood of experiencing long COVID symptoms.
Vaccination against COVID-19 offers protective benefits against the development of long COVID symptoms. A multicenter population survey in China demonstrated that receiving two or more doses of the COVID-19 vaccine in the past was associated with protection [13]. This finding is consistent with our study. However, our results suggest that receiving four or more doses of the COVID-19 vaccine in the past provides even greater protection. The subtle variation in outcomes between the two studies may be attributed to China’s active promotion of inhalable vaccines from December 2022 to January 2023. In our research, receiving two to three doses of the COVID-19 vaccine did not emerge as a protective factor against long COVID. This leads us to infer that the protective effect of the vaccine against long COVID may be time-sensitive. Simultaneously, it emphasizes the significance of enhancing primary healthcare workers’ understanding of preventive measures and promoting self-protection awareness. Nevertheless, further research is warranted to furnish compelling evidence.
The study exhibited several strengths: the overwhelming majority of respondents possessed knowledge in preventive or clinical medicine, heightening the accuracy of their self-reported findings. Additionally, all respondents were engaged in primary healthcare institutions, contributing to a high response rate. Moreover, on-site counseling, proofreading, and auditing further bolstered response accuracy, thus augmenting the reliability of the study’s results. However, our study has several acknowledged limitations. Firstly, the absence of mandatory nucleic acid testing might have resulted in undetected asymptomatic COVID-19 cases, potentially introducing variability in the overall self-reported rate of long COVID among the study subjects. Furthermore, the lack of testing could result in both underestimation and overestimation of the infection prevalence, as this study classified individuals who showed symptoms related to COVID-19 but were not tested as infected persons. Secondly, our study lacks a control or comparator group, potentially leading to an overestimation of the prevalence due to the broad and non-specific symptoms of long COVID. Additionally, recall bias may have resulted from the large gap between the period of infection (1 December 2022 to 20 January 2023) and the time of the survey (July 2023). Lastly, our analyses were confined to primary HCWs in Jiangsu Province, which might restrict the applicability of our findings to China as a whole or other regions.
In response to these limitations, future studies should consider designing cohort studies in more diverse regions and populations, incorporating nucleic acid or antigen testing to ensure accurate case identification and to further explore the incidence of long COVID and its influencing factors.
Conclusion
In summary, this study revealed that the prevalence of long COVID among primary HCWs in five districts/counties of Jiangsu Province, China, is approximately 12.61% (95%CI: 11.67-13.55%). Hypomnesia, sleep difficulties, fatigue, disturbances in the reproductive system, hair loss, and myalgia/arthralgia were the most common long COVID symptoms. The research found that older age, female and higher work stress are more likely to lead to long COVID, while having an educational level below a bachelor’s degree and receiving four or more doses of the vaccine offer protective effects. Our study results hold the potential to assist in early identification of COVID-19 patients at risk for long-term complications and in the development of tailored rehabilitation plans. To mitigate the prevalence of long COVID, healthcare providers and local authorities should implement effective measures, such as optimizing work-rest schedules and actively advocating for vaccination.
Acknowledgements
Not applicable.
Abbreviations
- COVID-19
Coronavirus disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- NICE
The National Institute for Health and Clinical Excellence
- CI
Confidence interval
- HCWs
Healthcare workers
- BMI
Body mass index
- SD
Standard deviation
- OR
Odds ratio
- aOR
adjusted odds ratio
- aHR
adjusted hazard ratio
Author contributions
H.C. and Y.Q.: study design, data curation, statistical analysis, and writing of the draft. B.L., R.M., P.M., M.F. and Y.S.: study design, data curation, and review of the draft. H.G., B.X., Z.S. and Y.L.: study design and review of the draft. B.C., J.X. and Y.Z.: study design, conceptualization and critical review of the draft.
Funding
This research was supported by Health Commission of Nanjing, China (ZKX22019) and the 2022 annual open project of Jiangsu Provincial Primary Health Development and General Practice Medical Education Research Center (No. 2022A02).
Data availability
The primary data used in this study is available upon request from the Corresponding author.
Declarations
Consent for publication
Not applicable.
Conflict of interest
The authors declared they had no conflicts of interest.
Ethics approval and consent to participate
This research was approved by the Ethics Committee of the Jiangsu Provincial Center for Disease Prevention and Control (Reference number: JSJK2023-B010-01). Informed consent was obtained from all participants in this study. Participants were made aware of the purpose and procedures of the study, and their freedom to withdraw from the study at any time without negative consequences.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hualing Chen and Yongkang Qian contributed equally to this work.
Contributor Information
Yongjie Zhang, Email: 371578804@qq.com.
Jinshui Xu, Email: 353112354@qq.com.
Bingwei Chen, Email: drchenbw@126.com.
References
- 1.Dhama K, Nainu F, Frediansyah A, et al. Global emerging Omicron variant of SARS-CoV-2: impacts, challenges and strategies. J Infect Public Health. 2023;16(1):4–14. 10.1016/j.jiph.2022.11.024. 10.1016/j.jiph.2022.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus (COVID-19) Dashboard. Accessed October 9. 2023. https://covid19.who.int
- 3.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV, WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22(4):e102–7. 10.1016/S1473-3099(21)00703-9. 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19. National Institute for Health and Care Excellence (NICE). 2020. Accessed October 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK567261/ [PubMed]
- 5.Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK - Office for National Statistics. Accessed October 9. 2023. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/30march2023
- 6.The Lancet, Long COVID. 3 years in. Lancet. 2023;401(10379):795. 10.1016/S0140-6736(23)00493-2. 10.1016/S0140-6736(23)00493-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang L. Americans still facing COVID risk. Accessed October 9, 2023. https://www.chinadaily.com.cn/a/202205/12/WS627c7610a310fd2b29e5c3a5.html
- 8.Yelin D, Moschopoulos CD, Margalit I, et al. ESCMID rapid guidelines for assessment and management of long COVID. Clin Microbiol Infect. 2022;28(7):955–72. 10.1016/j.cmi.2022.02.018. 10.1016/j.cmi.2022.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azzolini E, Levi R, Sarti R, et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in Health Care workers. JAMA. 2022;328(7):676–8. 10.1001/jama.2022.11691. 10.1001/jama.2022.11691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arjun MC, Singh AK, Pal D, et al. Characteristics and predictors of long COVID among diagnosed cases of COVID-19. PLoS ONE. 2022;17(12):e0278825. 10.1371/journal.pone.0278825. 10.1371/journal.pone.0278825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai J, Lin K, Zhang H, et al. A one-year follow-up study of systematic impact of long COVID symptoms among patients post SARS-CoV-2 omicron variants infection in Shanghai, China. Emerg Microbes Infect. 2023;12(2):2220578. 10.1080/22221751.2023.2220578. 10.1080/22221751.2023.2220578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero-Rodríguez E, Pérula-de Torres LÁ, Castro-Jiménez R, et al. Hospital admission and vaccination as predictive factors of long COVID-19 symptoms. Front Med (Lausanne). 2022;9:1016013. 10.3389/fmed.2022.1016013. 10.3389/fmed.2022.1016013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong MCS, Huang J, Wong YY, et al. Epidemiology, Symptomatology, and risk factors for long COVID symptoms: Population-Based, Multicenter Study. JMIR Public Health Surveill. 2023;9:e42315. 10.2196/42315. 10.2196/42315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arjun MC, Singh AK, Roy P, et al. Long COVID following Omicron wave in Eastern India-A retrospective cohort study. J Med Virol. 2023;95(1):e28214. 10.1002/jmv.28214. 10.1002/jmv.28214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S, Quan L, Chavarro JE, et al. Associations of Depression, anxiety, worry, perceived stress, and loneliness prior to infection with risk of Post–COVID-19 conditions. JAMA Psychiatry. 2022;79(11):1081–91. 10.1001/jamapsychiatry.2022.2640. 10.1001/jamapsychiatry.2022.2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The State Council Joint Prevention and Control Mechanism announces the. Notice on Further Optimizing the Implementation of COVID-19 Prevention and Control Measures. Accessed October 9, 2023. https://www.gov.cn/xinwen/2022-12/07/content_5730475.htm
- 17.Shukla AK, Atal S, Banerjee A, et al. An observational multi-centric COVID-19 sequelae study among health care workers. Lancet Reg Health Southeast Asia. 2022;10:100129. 10.1016/j.lansea.2022.100129. 10.1016/j.lansea.2022.100129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu B, Ma R, Xu J, et al. Primary healthcare workers’ COVID-19 infection status following implementation of adjusted epidemic prevention and control strategies: a cross-sectional study in Jiangsu, China. Front Public Health. 2023;11. 10.3389/fpubh.2023.1297770. [DOI] [PMC free article] [PubMed]
- 19.Davis HE, McCorkell L, Vogel JM, Topol EJ, Long COVID. Major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133–46. 10.1038/s41579-022-00846-2. 10.1038/s41579-022-00846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. 10.1038/s41598-021-95565-8. 10.1038/s41598-021-95565-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.da Costa E, Silva GR, Moura WÉA, Dos Santos KC, et al. Long-term symptoms after mild coronavirus disease in healthy Healthcare professionals: a 12-Month prospective cohort study. Int J Environ Res Public Health. 2023;20(2):1483. 10.3390/ijerph20021483. 10.3390/ijerph20021483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Notice on Issuing the Overall Plan for Implementing Class B Management of New Coronavirus Infections. Accessed October 11. 2023. https://www.gov.cn/xinwen/2022-12/27/content_5733739.htm
- 23.Pan Y, Wang L, Feng Z, et al. Characterisation of SARS-CoV-2 variants in Beijing during 2022: an epidemiological and phylogenetic analysis. Lancet. 2023;401(10377):664–72. 10.1016/S0140-6736(23)00129-0. 10.1016/S0140-6736(23)00129-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou BF, Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96. [PubMed] [Google Scholar]
- 25.Antonelli M, Pujol JC, Spector TD, Ourselin S, Steves CJ. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet. 2022;399(10343):2263–4. 10.1016/S0140-6736(22)00941-2. 10.1016/S0140-6736(22)00941-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adjei S, Hong K, Molinari NAM, et al. Mortality risk among patients hospitalized primarily for COVID-19 during the omicron and Delta variant pandemic periods - United States, April 2020-June 2022. MMWR Morb Mortal Wkly Rep. 2022;71(37):1182–9. 10.15585/mmwr.mm7137a4. 10.15585/mmwr.mm7137a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villar J, Soto Conti CP, Gunier RB, et al. Pregnancy outcomes and vaccine effectiveness during the period of omicron as the variant of concern, INTERCOVID-2022: a multinational, observational study. Lancet. 2023;401(10375):447–57. 10.1016/S0140-6736(22)02467-9. 10.1016/S0140-6736(22)02467-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prashar J. Long covid: conceptualizing the challenges for public health. J Public Health (Oxf). 2023;45(3):771–9. 10.1093/pubmed/fdac153. 10.1093/pubmed/fdac153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lippi G, Sanchis-Gomar F, Henry BM. COVID-19 and its long-term sequelae: what do we know in 2023? Pol Arch Intern Med. 2023;133(4):16402. 10.20452/pamw.16402. 10.20452/pamw.16402 [DOI] [PubMed] [Google Scholar]
- 30.Du M, Ma Y, Deng J, Liu M, Liu J. Comparison of long COVID-19 caused by different SARS-CoV-2 strains: a systematic review and Meta-analysis. Int J Environ Res Public Health. 2022;19(23):16010. 10.3390/ijerph192316010. 10.3390/ijerph192316010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ioannidis JPA, Zonta F, Levitt M. Estimates of COVID-19 deaths in Mainland China after abandoning zero COVID policy. medRxiv. Published online January 13, 2023:2022.12.29.22284048. 10.1101/2022.12.29.22284048 [DOI] [PMC free article] [PubMed]
- 32.Healey Q, Sheikh A, Daines L, Vasileiou E. Symptoms and signs of long COVID: a rapid review and meta-analysis. J Glob Health. 2022;12:05014. 10.7189/jogh.12.05014. 10.7189/jogh.12.05014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marjenberg Z, Leng S, Tascini C, et al. Risk of long COVID main symptoms after SARS-CoV-2 infection: a systematic review and meta-analysis. Sci Rep. 2023;13:15332. 10.1038/s41598-023-42321-9. 10.1038/s41598-023-42321-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. medRxiv Published Online January. 2021;30. 2021.01.27.21250617. [DOI] [PMC free article] [PubMed]
- 35.Premraj L, Kannapadi NV, Briggs J, et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci. 2022;434:120162. 10.1016/j.jns.2022.120162. 10.1016/j.jns.2022.120162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Natarajan A, Shetty A, Delanerolle G, et al. A systematic review and meta-analysis of long COVID symptoms. Syst Rev. 2023;12:88. 10.1186/s13643-023-02250-0. 10.1186/s13643-023-02250-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markiewicz-Gospodarek A, Wdowiak P, Czeczelewski M, et al. The impact of SARS-CoV-2 infection on fertility and female and male Reproductive systems. J Clin Med. 2021;10(19):4520. 10.3390/jcm10194520. 10.3390/jcm10194520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706–14. 10.1038/s41591-022-01909-w. 10.1038/s41591-022-01909-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–31. 10.1038/s41591-021-01292-y. 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cabrera Martimbianco AL, Pacheco RL, Bagattini ÂM, Riera R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: a systematic review. Int J Clin Pract. 2021;75(10):e14357. 10.1111/ijcp.14357. 10.1111/ijcp.14357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bai F, Tomasoni D, Falcinella C, et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect. 2022;28(4):611e. 9-611.e16. 10.1016/j.cmi.2021.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mansell V, Hall Dykgraaf S, Kidd M, Goodyear-Smith F. Long COVID and older people. Lancet Healthy Longev. 2022;3(12):e849–54. 10.1016/S2666-7568(22)00245-8. 10.1016/S2666-7568(22)00245-8 [DOI] [PubMed] [Google Scholar]
- 43.Chew NWS, Lee GKH, Tan BYQ, et al. A multinational, multicentre study on the psychological outcomes and associated physical symptoms amongst healthcare workers during COVID-19 outbreak. Brain Behav Immun. 2020;88:559–65. 10.1016/j.bbi.2020.04.049. 10.1016/j.bbi.2020.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ziauddeen N, Gurdasani D, O’Hara ME, et al. Characteristics and impact of Long Covid: findings from an online survey. PLoS ONE. 2022;17(3):e0264331. 10.1371/journal.pone.0264331. 10.1371/journal.pone.0264331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi Y, Strobl R, Apfelbacher C et al. Persistent symptoms and risk factors predicting prolonged time to symptom-free after SARS–CoV–2 infection: an analysis of the baseline examination of the German COVIDOM/NAPKON-POP cohort. Infection. Published online May 25, 2023:1–16. doi:10/gr9xgm. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The primary data used in this study is available upon request from the Corresponding author.