Abstract
Objective
To explore the high-risk factors affecting the prognosis of pT1 − 2N1M0 patients after mastectomy, establish a nomogram prediction model, and screen the radiotherapy benefit population.
Method
The clinical data of 936 patients with pT1 − 2N1M0 who underwent mastectomy in the fourth hospital of Hebei Medical University from 2010 to 2016 were retrospectively analyzed. There were 583 patients received postmastectomy radiotherapy(PMRT), and 325 patients without PMRT. Group imbalances were mitigated using the propensity score matching (PSM) method, and the log-rank test was employed to compare overall survival (OS) and disease-free survival (DFS) between the cohorts. The efficacy of PMRT across various risk groups was evaluated using a nomogram model.
Result
The median follow-up period was 98 months, Patients who received PMRT demonstrated significantly improved 5-year and 8-year OS and DFS compared to those who did not (P < 0.001). Multivariate analysis revealed that age, primary tumor site, positive lymph node, stage, and Ki-67 level independently influenced OS, while age, primary tumor site, and stage independently affected DFS. PMRT drastically enhanced OS in the high-risk group (P = 0.001), but did not confer benefits in the low-risk and intermediate risk groups (P = 0.057, P = 0.099). PMRT led to a significant improvement in disease-free survival (DFS) among patients in the intermediate and high-risk groups (P = 0.036, P = 0.001), whereas the low-risk group did not experience a significant benefit (P = 0.475).
Conclusion
Age ≤ 40 years, tumor located in the inner quadrant or central area, T2 stage, 2–3 lymph nodes metastasis, and Ki67 > 30% were the high-risk factors affecting the prognosis of this cohort of patients. In OS nomogram, patients with a risk score of 149 or higher who received PMRT exhibited improved OS. Similarly, in DFS nomogram, patients with a risk score of 123 or higher who received PMRT demonstrated enhanced DFS.
Keywords: Breast cancer, Mastectomy, pN1, Prognosis, Nomogram model, Radiotherapy
Introduction
Breast cancer has become one of the significant diseases affecting women’s health. Postmastectomy radiotherapy (PMRT) was an important means of local treatment of breast cancer, which reduced the 10-year local recurrence rate (LRR) by 16.7%, and improved the long-term survival of patients [1, 2]. Treatment guidelines recommend that patients with tumor size greater than 5 cm or with 4 or more axillary positive lymph nodes (stage T3 − 4 or N2 − 3), - infiltration of the skin, and/or the pectoral muscle, inflammatory carcinoma and positive margins should receive PMRT. However, for patients with pT1 − 2N1M0, the value of PMRT remains controversial. According to the clinical guidelines for breast cancer of the National Comprehensive Cancer Network(NCCN), these patients underwent a transition from the option of considering radiotherapy to strongly considering it, and eventually to regional lymph node radiotherapy [3]. In the early years, the European Society for Medical Oncology(ESMO) clinical practice guidelines universally recommend PMRT for all patients in this cohort, but rather for patients with high-risk factors of recurrence, such as young age, presence of vascular invasion (LVI), and a lower number of removed nodes (NRN), etc [4]. However, recent ESMO guidelines recommend that PMRT should be considered in this patient cohort, even in the absence of high-risk factors [5]. Depending on the guidelines of the Chinese Society of Clinical Oncology [6], there is either a lack of high-level evidence supporting PMRT or a lack of evidence against it.
The necessity of radiotherapy for N1 patients remains unclear due to the limited clinical research evidence. According to the 2021 breast cancer radiotherapy guidelines of the Chinese Medical Association, considerable heterogeneity exists within in this cohort of patients [7].The question of whether patients with T1 − 2N1M0 breast cancer should receive PMRT remains controversial, and there is a lack of direct evidence supporting the use of PMRT in T1 − 2N1M0 patients. It is imperative to thoroughly weigh the benefits and risks of PMRT.
In this study, The high-risk factors of this cohort of patients after mastectomy were discussed. we tried to develop a nomogram prediction model to estimate the prognosis of pT1 − 2N1M0 breast cancer, identify the population benefiting from PMRT, and offer clinical guidance for the precise treatment of breast cancer.
Methods and materials
Eligibility criteria
We gathered the clinical data of patients with previously untreated breast cancer with T1 − 2N1M0 who were treated with mastectomy in the Fourth Hospital of Hebei Medical University from 2010 to 2016. This study was designed in accordance with the requirements of medical ethics and adheres to the principles of the Helsinki Declaration. The study protocol was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University.
The inclusion criteria were: (1) Pathology confirmed invasive breast cancer; (2) Absence of supraclavicular or internal mammary lymph node metastases, or distant metastases at initial diagnosis(Distant metastasis is defined as metastasis to distant sites outside the ipsilateral breast and regional lymph nodes, as assessed by imaging before surgery); (3) Receive breast mastectomy and the pathological stage was T1 − 2N1M0(American Joint Committee on Cancer staging system version 8); (4) Availability of complete clinical data. The exclusion criteria were: (1) Bilateral or occult breast cancer; (2) Received neoadjuvant therapy; (3) Absence of axillary lymph node dissection; (4) Male breast cancer; and 4) Merge with other malignant tumors.
A tolal of 936 patients with pT1 − 2N1M0 after mastectomy were retrospectively reviewed. 908 patients had complete follow-up data. Physical examination and imaging examination like mammography, ultrasound and MRI were usually used at initial diagnosis. Clinicopathological data were collected, including age, clinical stage, tumor morphology, histological grade, nodal status, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER-2) status and Ki-67.
Treatment methods
In the radiotherapy (RT) group, 579 patients received chemotherapy, representing 65.7% of the patients who received chemotherapy. 302 patients received chemotherapy, accounting for 34.3%, in non-radiotherapy(NRT) group. The chemotherapy scheme included CMF (Cyclophosphamide, methotrexate, fluorouracil), TAC (paclitaxel, anthracycline, Cyclophosphamide), Capecitabine, etc. In the RT group, 440 patients received endocrine therapy, representing 64.8% of those undergoing endocrine therapy. 239 patients received endocrine therapy, accounting for 35.2%, in the NRT group.
In the RT group, 58 patients (accounting for 23.7% of patients positive for HER-2 received targeted therapy, while in the NRT group, 9 patients (constituting 3.6%) received similar therapy. Targeted drug therapy primarily consisted of Trastuzumab alone or in combination with Pertuzumab.
There were 583(64.2%) cases received PMRT, while 325 cases (35.8%) did not. The decision to administer postmastectomy radiotherapy was primarily based on the results of multidisciplinary consultations, including specialists from breast surgery, radiology, and pathology departments. To some extent, this decision also considered the presence of high-risk factors such as high histological grade, ER/PR negativity, HER-2 positivity, presence of vascular invasion and neural invasion, and high Ki-67 expression. Radiation targets for 571 patients (90%) included the chest wall with infraclavicular (axillary level III) and supraclavicular fields, while for 8 patients (1.4%), the target encompassed the chest wall, infraclavicular (axillary level III) and supraclavicular regions, as well as the internal mammary lymph nodes (IMLNs). For 4 patients (0.7%), the target area extended to the chest wall, infraclavicular (axillary level III) and supraclavicular regions, as well as the internal mammary lymph nodes (IMLNs) and axillary lymph nodes (ALNs).
The median dose was 50 Gy (range, 46–50.4 Gy; dose per fraction, 1.8–2.0 Gy). Internal mammary nodes were irradiated when the tumor was located in the inner and central regions with concurrent axillary lymph node metastasis, irradiation of the internal mammary lymph nodes was considered after multidisciplinary consultation, including specialists from breast surgery, radiology, and pathology departments.
Follow up and endpoints
The median follow-up period was 98 (11–139) months. 109 (12.0%) patients died, including 96 patients of breast cancer and 13 patients of other causes; A total of 53(5.8%) and 127(14.0%) patients showed local recurrence and distant metastasis, respectively. There were 28 cases of loss to follow-up, resulting in a follow-up rate of 97%. Overall survival (OS) was calculated from the date of mastectomy to the time of death from any cause. Disease-free survival (DFS) was calculated from the time of disease recurrence, distant metastasis, death, or last follow-up.
Statistical analysis
SPSS 26.0, RStudio 4.1, X-tile 3.6 were used for statistical analysis. Patients’ clinical baseline characteristics were analyzed using the chi-square test. Group imbalances were mitigated using the propensity score matching with a caliper value set at 0.2. Univariate analysis was performed using the Kaplan-Meier (K-M) method, and survival curves between groups were compared using the log-rank test. Multivariate analysis was conducted using the Cox proportional hazards model. Nomograms were constructed using variables with a significance level of P < 0.05. Internal validation was performed using 1000 bootstrap samples to assess the accuracy of the consistency index. A calibration curve was generated to correct the predicted and observed probabilities. X-tile 3.6 was utilized to determine the optimal cutoff value for risk stratification, with a significance level of P < 0.05 considered statistically significant.
Result
Clinical characteristics
936 patients were enrolled, of whom 908 had complete follow-up data, with 583 in the RT group and 325 in the NRT group. The extracted variables included age, tumor location, histological grade, LVI, NRN, Lymph node metastasis(LNM), ER/PR status, HER-2 status, and stage. The groups were matched in a 1:1 ratio using propensity score matching (PSM), with a caliper value set at 0.2. There were 298 cases each in the RT and NRT groups after matching (Table 1).
Table 1.
Clinical data characteristics of 908 patients after mastectomy before and after PSM
| Variable | Pre PSM | After PSM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N=908 | RT N=583(%) |
NRT N=325(%) |
χ2 value | P value | N=596 | RT N=298(%) |
NRT N=298(%) |
χ2 value | P value | ||
| Age, y | 13.025 | <0.001 | 0.883 | 0.347 | |||||||
| ≤40 | 119 | 94(79.0) | 25(21.0) | 44 | 19(43.2) | 25(56.8) | |||||
| >40 | 789 | 489(62.0) | 300(38.0) | 552 | 279(50.5) | 273(49.5) | |||||
| Tumor location | 0.099 | 0.753 | 0.275 | 0.600 | |||||||
| Outer | 615 | 397(64.6) | 218(35.4) | 402 | 204(50.7) | 198(49.3) | |||||
| Inner /central | 293 | 186(63.5) | 107(36.5) | 194 | 94(48.5) | 100(51.5) | |||||
| Histological grade | 10.830 | 0.013 | 1.367 | 0713 | |||||||
| I | 18 | 11(61.1) | 7(38.9) | 13 | 6(46.2) | 7(53.8) | |||||
| II | 447 | 273(61.1) | 174(38.9) | 300 | 150(50.0) | 150(50.0) | |||||
| III | 276 | 199(72.1) | 77(27.9) | 164 | 87(53.0) | 77(47.0) | |||||
| Unknown | 167 | 100(59.9) | 67(40.1) | 119 | 55(46.2) | 64(53.8) | |||||
| LVI | 11.226 | 0.001 | 0.065 | 0.798 | |||||||
| No | 537 | 321(59.8) | 216(40.2) | 381 | 192(50.4) | 189(49.6) | |||||
| Yes | 371 | 262(70.6) | 109(29.4) | 215 | 106(49.3) | 109(50.7) | |||||
| Ki-67,% | 5.262 | 0.022 | <0.001 | 1.000 | |||||||
| ≤30 | 465 | 282(60.6) | 183(39.4) | 312 | 156(50.0) | 156(50.0) | |||||
| >30 | 443 | 301(67.9) | 142(32.1) | 284 | 142(50.0) | 142(50.0) | |||||
| NRN | 2.028 | 0.154 | 1.170 | 0.279 | |||||||
| <10 | 23 | 18(78.3) | 5(21.7) | 14 | 9(64.3) | 5(35.7) | |||||
| ≥10 | 885 | 565(63.8) | 320(36.2) | 582 | 289(49.7) | 293(50.3) | |||||
| LNM | 65.787 | <0.001 | 0.910 | 0.634 | |||||||
| 1 | 427 | 219(51.3) | 208(48.7) | 351 | 170(48.4) | 181(51.6) | |||||
| 2 | 297 | 211(71.0) | 86(29.0) | 182 | 96(52.7) | 86(47.3) | |||||
| 3 | 184 | 153(83.2) | 31(16.8) | 63 | 32(50.8) | 31(49.2) | |||||
| ER/PR status | 4.409 | 0.036 | 0.885 | 0.347 | |||||||
| Positive | 726 | 454(62.5) | 272(37.5) | 483 | 237(49.1) | 246(50.9) | |||||
| Negative | 182 | 129(70.9) | 53(29.1) | 113 | 61(54.0) | 52(46.0) | |||||
|
HER-2 status |
13.878 | 0.001 | 3.216 | 0.200 | |||||||
| Negative | 558 | 350(62.7) | 208(37.3) | 373 | 179(48.0) | 194(52.0) | |||||
| Positive | 244 | 177(72.5) | 67(27.5) | 151 | 85(56.3) | 66(43.7) | |||||
| Unknown | 106 | 56(52.8) | 50(47.2) | 72 | 34(47.2) | 38(52.8) | |||||
| stagea | 0.079 | 0.778 | 0.007 | 0.935 | |||||||
| T1 | 419 | 267(63.7) | 152(36.3) | 275 | 137(49.8) | 138(50.2) | |||||
| T2 | 489 | 316(64.6) | 173(35.4) | 321 | 161(50.2) | 160(49.8) | |||||
Abbreviations aOnly including T stage; PSM: propensity score matching; RT: radiotherapy; NRT: non-radiotherapy; LVI: lymphovascular invasion; NRN: number of removed nodes; LNM: lymph node metastasis; ER/PR status: Estrogen Receptor/Progesterone Receptor; HER-2
Status: Human epidermal growth factor receptor 2
Survival analysis
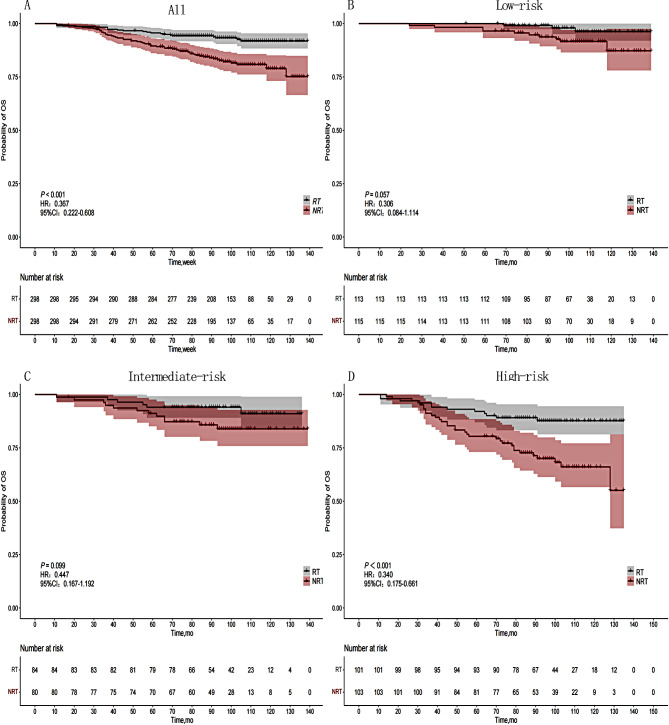
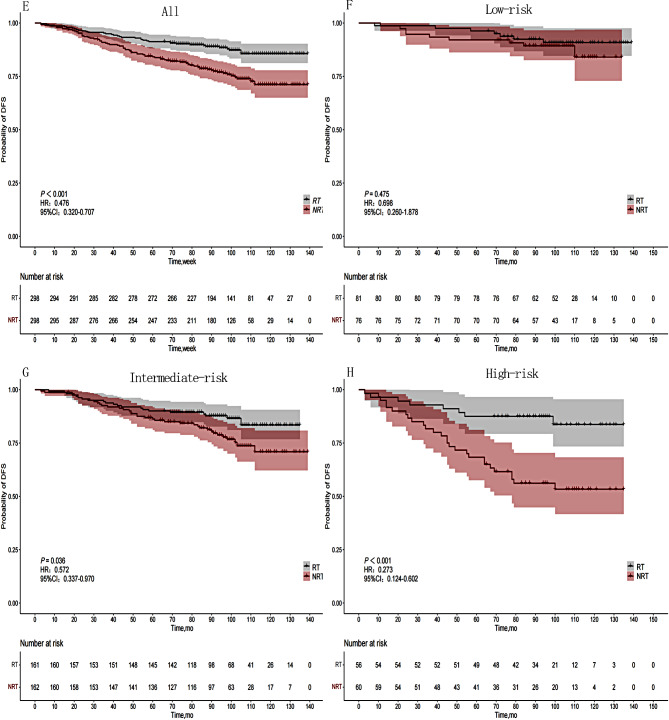
Univariate analysis revealed statistically significant differences in OS among age, tumor location, Ki-67, LNM, and stage. The 5-year OS for the RT group and NRT group were 95.6% and 91.8%, respectively, while the 8-year OS were 89.5% and 78.9%, respectively (P < 0.001). Statistically significant differences were observed in age, tumor location, and stage with respect to DFS. The 5-year DFS for the RT group and NRT group were 91.3% and 85.6%, respectively, while the 8-year DFS were 84.1% and 71.1%, respectively (P < 0.001). (Table 2) (Figs. 1-A and 2-E).
Table 2.
Univariate survival analysis of OS and DFS in 596 patients after mastectomy
| Variable | N | OS(%) | P value | DFS(%) | P value | ||
|---|---|---|---|---|---|---|---|
| 5-y | 8-y | 5-y | 8-y | ||||
| Age, y | 0.007 | 0.007 | |||||
| ≤ 40 | 44 | 84.0 | 73.8 | 72.6 | 67.5 | ||
| >40 | 552 | 93.3 | 91.6 | 88.9 | 83.6 | ||
| Tumor location | 0.003 | 0.021 | |||||
| Outer | 402 | 93.5 | 90.2 | 88.5 | 84.7 | ||
| Inner/central | 194 | 90.7 | 82.2 | 86.0 | 77.5 | ||
| Histological grade | 0.418 | 0.630 | |||||
| I | 13 | 100 | 100 | 100 | 100 | ||
| II | 300 | 93.0 | 87.7 | 89.3 | 83.1 | ||
| III | 164 | 90.2 | 86.3 | 81.7 | 79.5 | ||
| Unknown | 119 | 94.1 | 88.0 | 90.7 | 82.7 | ||
| ER/PR status | 0.167 | 0.806 | |||||
| Positive | 483 | 93.4 | 88.7 | 88.8 | 82.8 | ||
| Negative | 113 | 89.3 | 83.4 | 83.1 | 80.9 | ||
| HER-2 status | 0.439 | 0.329 | |||||
| Negative | 373 | 94.1 | 88.5 | 89.2 | 83.4 | ||
| Positive | 151 | 89.3 | 87.2 | 86.7 | 84.3 | ||
| Unknown | 72 | 91.7 | 84.7 | 81.9 | 73.7 | ||
| Ki-67,% | 0.011 | 0.172 | |||||
| ≤ 30 | 312 | 95.5 | 90.9 | 91.3 | 84.4 | ||
| >30 | 284 | 89.4 | 84.3 | 83.8 | 81.8 | ||
| NRN | 0.241 | 0.258 | |||||
| <10 | 14 | 78.6 | 78.6 | 78.6 | 70.7 | ||
| ≥ 10 | 582 | 92.9 | 87.9 | 87.9 | 82.7 | ||
| LNM | 0.029 | 0.089 | |||||
| 1 | 351 | 95.1 | 90.8 | 89.7 | 84.8 | ||
| 2 | 182 | 90.0 | 83.5 | 85.6 | 78.4 | ||
| 3 | 63 | 85.7 | 82.1 | 82.5 | 80.7 | ||
| Stagea | 0.004 | 0.005 | |||||
| T1 | 275 | 95.6 | 91.0 | 92.7 | 86.4 | ||
| T2 | 321 | 90.0 | 84.8 | 83.4 | 79.0 | ||
| LVI | 0.054 | 0.417 | |||||
| No | 381 | 93.9 | 89.9 | 89.5 | 83.5 | ||
| Yes | 215 | 90.2 | 83.9 | 84.7 | 80.5 | ||
Abbreviations LVI: lymphovascular invasion; NRN: number of removed nodes; LNM: lymph node metastasis; ER/PR: Estrogen Receptor/Progesterone Receptor; HER-2: Human epidermal growth factor receptor 2; OS: Overall survival; DFS: Disease-free survival
aOnly including T stage
Fig. 1.
OS curves of 596 patients in different risk groups based on the RT status. (A) Entire cohort, (B) Low-risk group, (C) Intermediate-risk group, (D) High-risk group
Fig. 2.
DFS curves of 596 patients in different risk groups based on the RT status. (E) Entire cohort, (F) Low-risk group, (G) Intermediate-risk group, (H) High-risk group
Age (P = 0.005), tumor location (P < 0.001), LNM (P = 0.015), stage (P = 0.006), Ki-67 (P = 0.021) were found to be independent prognostic factors for OS. Age (P = 0.001), tumor location (P = 0.010), and stage (P = 0.002) were independent prognostic factors for DFS (Table 3).
Table 3.
Multivariate analysis for OS and DFS in 596 patients after Mastectomy
| Variable | OS | DFS | ||||
|---|---|---|---|---|---|---|
| HR(95%CI) | Coef | P | HR(95%CI) | Coef | P | |
| Age, y | ||||||
| > 40 | Reference | Reference | ||||
| ≤ 40 | 2.57(1.340–4.94) | 0.945 | 0.005 | 2.53(1.43–4.47) | 0.929 | 0.001 |
| Tumor location | ||||||
| Outer | Reference | Reference | ||||
| Inner/central | 2.24(1.416–3.54) | 0.806 | <0.001 | 1.66(1.13–2.43) | 0.505 | 0.010 |
| LNM | ||||||
| 1 | Reference | – | – | – | ||
| 2 | 1.84(1.124–3.01) | 0.610 | 0.015 | |||
| 3 | 2.00(0.995–4.02) | 0.694 | 0.052 | |||
| Stagea | ||||||
| T1 | Reference | Reference | ||||
| T2 | 2.02(1.225–3.34) | 0.704 | 0.006 | 1.90(1.28–2.82) | 0.641 | 0.002 |
| Ki-67 | – | – | – | |||
| ≤ 30% | Reference | |||||
| >30% | 1.74(1.089–2.80) | 0.557 | 0.021 | |||
Abbreviations HR: Hazard ratio; CI: confidence interval; LNM: lymph node metastasis
aOnly including T stage
Establishment and validation of nomogram model
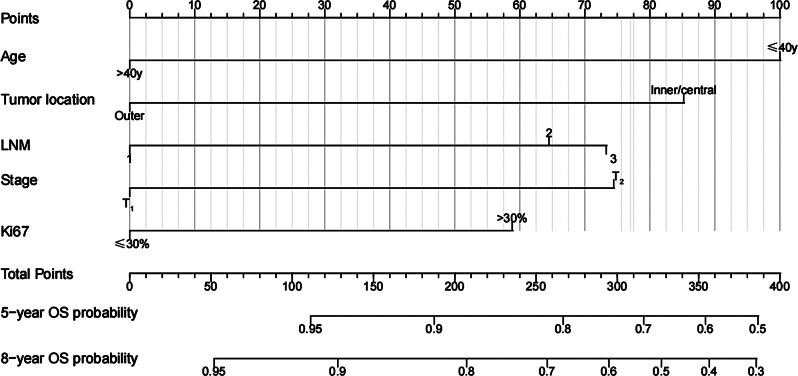
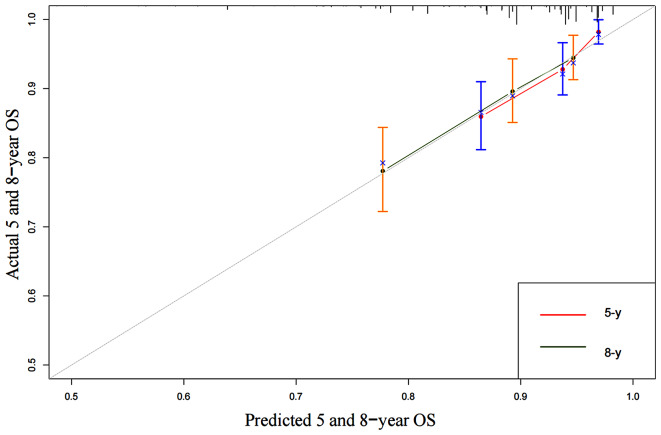
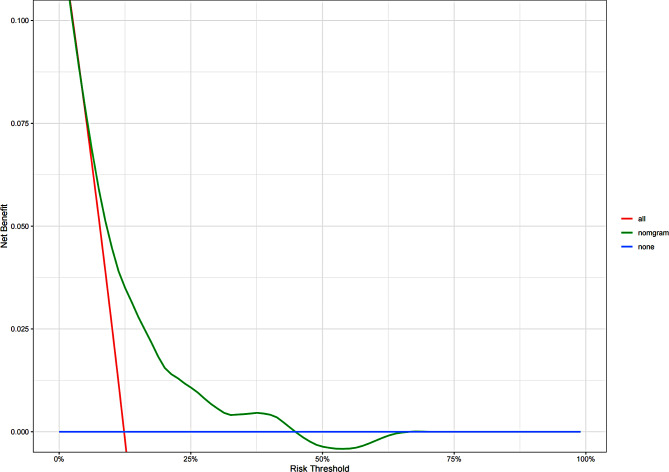
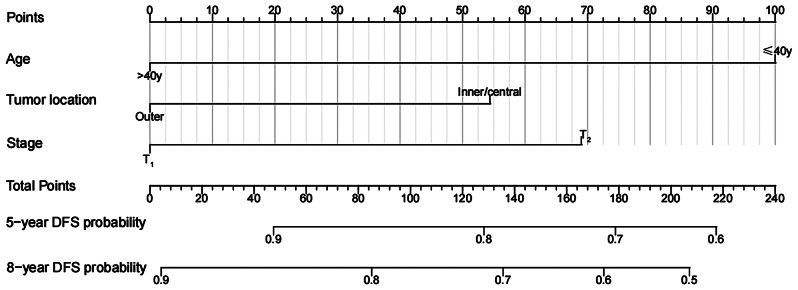
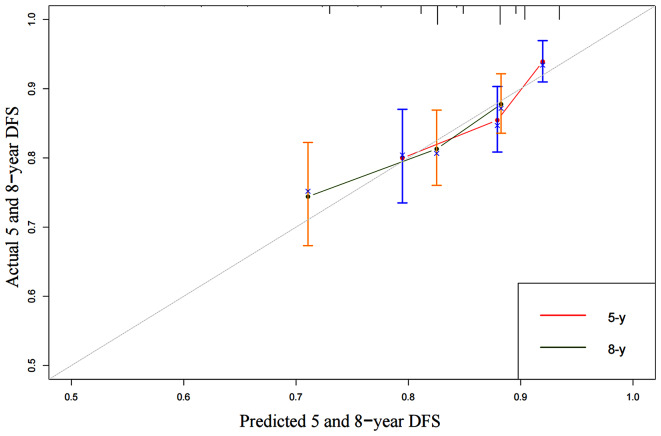
We developed an OS nomogram based on independent prognostic factors affecting OS (Fig. 3). The consistency index of this model was 0.707 (95% CI: 0.649–0.765), indicating good consistency with the calibration curves (Fig. 4). The DCA demonstrated that when the threshold probability ranged from 6.3 to 32.3%, the net benefit of applying the nomogram was significantly higher than the “no intervention” and “full intervention” strategies, suggesting that the nomogram has good clinical applicability (Fig. 5). Patients with a total score of ≤ 100 points were classified as the low-risk group, those with a total score of ≥ 149 points were classified as the high-risk group, and others were classified as the Intermediate-risk group. The 5-year and 8-year OS of those who received PMRT in the low-risk group were 100% and 97.9%, respectively, while those who did not were 96.5% and 91.6% (P = 0.057); In the intermediate-risk group, patients who received PMRT were 94.0% and 91.6%, respectively, while those who did not were 91.2% and 83.9% (P = 0.099); In the high-risk group, patients who received PMRT were 92.1% and 87.7%, respectively, while those who did not were 80.4% and 70.0% (P < 0.001) (Table 4; Fig. 1B-D).
Fig. 3.
OS nomogram of 596 patients after mastectomy
Fig. 4.
5-year and 8-year calibration curves for OS nomogram of 596 patients after mastectomy
Fig. 5.
Decision curve analysis for the OS nomgram
Table 4.
Effect of PMRT on OS and DFS in different risk groups
| Group | RT | No. | 5-y and 8-y OS(%) | HR(95%CI) | P |
|---|---|---|---|---|---|
| All(N = 596) | Yes | 298 | 95.6/91.8 | 0.367(0.222–0.608) | <0.001 |
| No | 298 | 89.5/78.9 | |||
| Low-risk(N = 228) | Yes | 113 | 100.0/97.9 | 0.306(0.084–1.114) | 0.057 |
| No | 115 | 96.5/91.6 | |||
| Intermediate-risk(N = 164) | Yes | 84 | 94.0/91.6 | 0.447(0.167–1.192) | 0.099 |
| No | 80 | 91.2/83.9 | |||
| High-risk(N = 204) | Yes | 101 | 92.1/87.7 | 0.340(0.175–0.661) | <0.001 |
| No | 103 | 80.4/70.0 |
| Group | RT | No. | 5-y and 8-y DFS(%) | HR(95%CI) | P |
|---|---|---|---|---|---|
| All(N = 596) | Yes | 298 | 91.3/85.6 | 0.476(0.320–0.707) | <0.001 |
| No | 298 | 84.1/71.1 | |||
| Low-risk(N = 157) | Yes | 81 | 96.3/90.9 | 0.698(0.260–1.878) | 0.475 |
| No | 76 | 92.1/89.3 | |||
| Intermediate-risk(N = 323) | Yes | 161 | 90.1/87.7 | 0.572(0.337–0.970) | 0.036 |
| No | 162 | 86.3/77.8 | |||
| High-risk(N = 116) | Yes | 56 | 87.5/83.7 | 0.273(0.124–0.602) | <0.001 |
| No | 60 | 68.3/56.1 |
Among the three risk groups based on OS nomgram. Low risk indicates a risk score ≤ 100, intermediate risk indicates a risk score of 100 to 149, and high risk indicates a risk score ≥ 149. Among the three risk groups based on OS nomgram. Low risk indicates a risk score <54, intermediate risk indicates a risk score of 54 to 123, and high risk indicates a risk score ≥ 123
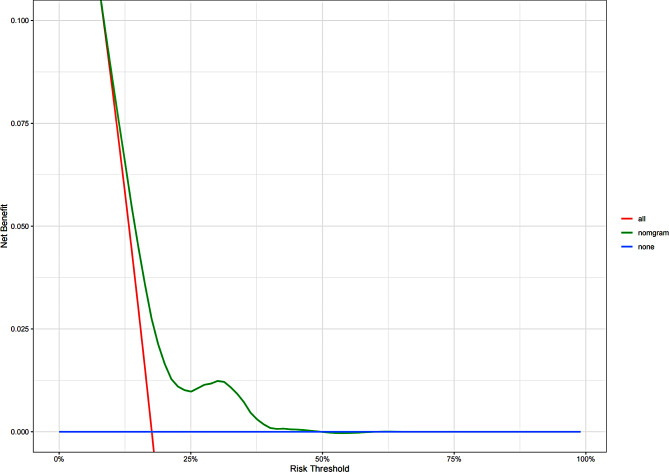
We developed an DFS nomogram based on independent prognostic factors affecting DFS (Fig. 6). The consistency index of this model was 0.623 (95% CI: 0.571–0.675), indicating good consistency with the calibration curves (Fig. 7). The DCA demonstrated that when the threshold probability ranged from 8.9 to 50%, the net benefit of applying the nomogram significantly higher than the “no intervention” and “full intervention” strategies, suggesting that the nomogram has good clinical applicability (Fig. 8). Patients with a total score of < 54 points was classified as low-risk group, a total score of ≥ 123 points was classified as high-risk group, and other patients were classified as intermediate-risk group. In the low-risk group, the 5-year and 8-year DFS among those who received PMRT were 96.3% and 90.9%, respectively, while those who did not were 92.1% and 89.3% (P = 0.475). In the moderate-risk group, the 5-year and 8-year DFS among those who received PMRT were 90.1% and 87.7%, respectively, while those who did not were 86.3% and 77.8% (P = 0.036). In the high-risk group, the 5-year and 8-year DFS among those who received PMRT were 87.5% and 83.7%, respectively, while those who did not were 68.3% and 56.1% (P < 0.001)(Table 4; Fig. 2F-H).
Fig. 6.
DFS nomogram of 596 patients after mastectomy
Fig. 7.
5-year and 8-year calibration curves for the DFS nomogram of 596 patients after mastectomy
Fig. 8.
Decision curve analysis for the DFS nomgram
Discussion
Several early clinical research studies have confirmed that PMRT can improve local control (LC) and long-term survival in breast cancer patients [8–10]. However, there has been no randomized controlled trial specifically focusing on patients with pT1 − 2N1M0 to ascertain the beneficial effects of PMRT. Overgaard M et al. [11] reported that PMRT reduced the 15-year loco-regional failure rate from 27 to 4% and improved OS from 48 to 57% for patients with 1–3 lymph node-positive breast cancer. The findings of Headon H’s meta-analysis indicated a modest enhancement in OS with PMRT [12]. A randomized controlled study conducted by Ragaz J et al. [13] demonstrated that PMRT enhanced LRFS, DFS, and BCSS in patients with pT1 − 2N1M0. However, some studies have suggested that while PMRT improved LC in this cohort, it did not confer a benefit in terms of OS [14–18]. Additionally, some scholars argued that PMRT did not provide any benefits [19]. This study utilized a large sample dataset and balanced intergroup differences through PSM, demonstrating that PMRT significantly improved patient DFS and OS. In comparison with other studies, its conclusions appear more reliable.
For this cohort, domestic and foreign guidelines unanimously recommend that PMRT may benefit people with clinical high-risk factors. Previous studies have identified several high-risk factors affecting the prognosis of this cohort, including age < 40 years, T2 stage, high histological grade, ER/PR negativity, LVI, inner quadrant tumor, and HER−2 overexpression [20–22]. In the present study, tumor quadrant, LNM, T stage, and Ki−67 were identified as independent prognostic factors for OS, whereas age, tumor quadrant, and T stage were independent prognostic factors for DFS, which was consistent with prior research.
Grouping model of risk factors have been established by some scholars to explore which risk factors combine to yield the optimal benefit from PMRT [23–26]. However, these studies solely established prognostic groups based on the number of risk factors, which may overlook the decisive influence of key factors and diminish the feasibility of grouping. In the present study, a nomogram model for OS and DFS was constructed to elucidate the contribution of different factors to outcomes and identify those with the greatest impact on cohort prognosis. Patients were stratified into low, intermediate-risk, and high-risk groups based on the model scores, and the efficacy of radiotherapy across these groups was assessed. According to our findings, PMRT did not confer significant benefits to patients in the low-risk group, in terms of either DFS or OS. However, for the medium and high-risk groups, PMRT markedly improved DFS, with particularly noteworthy improvements observed in the high-risk group for OS.
In reviewing pertinent literature, it is evident that some scholars have also devised nomogram models to identify populations that may benefit from PMRT [27, 28]. The model established by Tang Y et al. [27] concluded that PMRT significantly improved he OS of patients in the intermediate-risk and high-risk groups, but not in the low-risk group. The study by Hou N et al. [28] concluded that PMRT only improved OS in the high-risk group. In the present study, a more rigorous selection was employed for identifying high-risk factors to be included in our model, which differed from the aforementioned models. While variations in the risk factors considered across different clinical studies may influence results to some extent, these models nonetheless offer valuable guidance for clinical practice.
Admittedly, the current study had limitations. First, being a retrospective study conducted at a single center, it was prone to recall bias and selection bias. due to the late implementation of neoadjuvant chemotherapy and genetic testing at our center, these factors were not included in the study analysis. Second, Since 546 patients reached the 5-year follow-up, accounting for 91.6%, and 335 patients reached the 8-year follow-up, accounting for 56.2%, the estimate of 8-year survival has minimal bias. Finally, the nomogram model developed in this study has not undergone validation using external datasets, and its reliability awaits confirmation through validation in independent datasets.
The current study had two main strengths. Firstly, employing post-randomization significantly improved intergroup balance and yielded conclusions similar to previous studies. Secondly, the selection of variables included in the nomogram was more stringent, and internal validation was conducted using a more rigorous scientific Bootstrap method.
In conclusion, among breast cancer patients with pT1 − 2N1M0 who have undergone mastectomy, age ≤ 40 years, tumor located in the inner quadrant or central region, LVI, T2 stage, 2–3 LNMs, and Ki−67 > 30% were identified as high-risk factors influencing patient prognosis. In OS nomogram, patients with a risk score of 149 or higher who received PMRT exhibited improved OS. Similarly, in DFS nomogram, patients with a risk score of 123 or higher who received PMRT demonstrated enhanced DFS.
Acknowledgements
Not available.
Author contributions
Author 1: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Writing—original draft, Visualization. Author 2: Conceptualization, Methodology, Investigation, Validation, Writing—original draft, Supervision. Author 3: Methodology, Investigation, Validation, Data curation, Visualization. Author 4: Validation, Investigation, Data curation, Visualization. Author 5: Validation, Data curation, Visualization. Author 6: Validation, Data curation, Visualization, Author 7: Validation, Writing—review and editing, Visualization. Author 8: Validation, Writing—review and editing, Supervision. Author 9: Conceptualization, Methodology, Formal analysis, Writing—original draft, Writing—review and editing, Funding acquisition.
Funding
Heibei Province medical science research project (20240090). Heibei Province medical science research project (20240018). Clinical Medicine Outstanding Talent Training Project Funded by Hebei Provincial Government (ZF2024123).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This retrospective study was carried out using the opt-out method for the case series of our hospital. The study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University. and was conducted in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was waived by our Institutional Review Board because of the retrospective nature of our study.
Consent for publication
The authors have no ethical, legal, or financial conflicts of interest related to this article. All the authors have read and approved the manuscript for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Clarke M, Collins R, Darby S, et al. Early breast Cancer trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–106. 10.1016/S0140-6736(05)67887-7. 10.1016/S0140-6736(05)67887-7 [DOI] [PubMed] [Google Scholar]
- 2.EBCTCG, McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–35. 10.1016/S0140-6736(14)60488-8. 10.1016/S0140-6736(14)60488-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gradishar WJ, Moran MS, Abraham J, et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(6):691–722. 10.6004/jnccn.2022.0030. 10.6004/jnccn.2022.0030 [DOI] [PubMed] [Google Scholar]
- 4.Aebi S, Davidson T, Gruber G, et al. Primary breast can-cer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up[J]. Ann Oncol. 2011;24(3):106–14. 10.1093/annonc/mdr371. 10.1093/annonc/mdr371 [DOI] [PubMed] [Google Scholar]
- 5.Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–220. 10.1093/annonc/mdz189. 10.1093/annonc/mdz189 [DOI] [PubMed] [Google Scholar]
- 6.Breast cancer Professional Committee of China Anti Cancer Association Guidelines and Specifications for breast cancer Diagnosis and Treatment of China Anti Cancer Association. (2021) Chinese Journal of Cancer, 2021; 31 (10): 954–1040. 10.19401/j.cnki.1007-3639.2021.10.013
- 7.Chinese Medical Doctor Association Radiation Tumor Therapy Doctor Branch Guidelines for radiotherapy of breast cancer. (Chinese Medical Doctor Association 2020) Chinese Journal of Radiation Oncology, 2021, 30 (04): 321–342. 10.3760/cma.j.cn113030-20210107-00010
- 8.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337(14):949–55. 10.1056/NEJM199710023371401. 10.1056/NEJM199710023371401 [DOI] [PubMed] [Google Scholar]
- 9.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353(9165):1641–8. 10.1016/S0140-6736(98)09201-0. 10.1016/S0140-6736(98)09201-0 [DOI] [PubMed] [Google Scholar]
- 10.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337(14):956–62. 10.1056/NEJM199710023371402. 10.1056/NEJM199710023371402 [DOI] [PubMed] [Google Scholar]
- 11.Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 2007;82(3):247–53. 10.1016/j.radonc.2007.02.001. 10.1016/j.radonc.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 12.Headon H, Kasem A, Almukbel R, et al. Improvement of survival with postmastectomy radiotherapy in patients with 1–3 positive axillary lymph nodes: a systematic review and meta-analysis of the current literature. Mol Clin Oncol. 2016;5(4):429–36. 10.3892/mco.2016.971. 10.3892/mco.2016.971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97(2):116–26. 10.1093/jnci/djh297. 10.1093/jnci/djh297 [DOI] [PubMed] [Google Scholar]
- 14.Huang CJ, Hou MF, Chuang HY, et al. Comparison of clinical outcome of breast cancer patients with T1-2 tumor and one to three positive nodes with or without postmastectomy radiation therapy. Jpn J Clin Oncol. 2012;42(8):711–20. 10.1093/jjco/hys080. 10.1093/jjco/hys080 [DOI] [PubMed] [Google Scholar]
- 15.Cosar R, Uzal C, Tokatli F, et al. Postmastectomy irradiation in breast in breast cancer patients with T1-2 and 1–3 positive axillary lymph nodes: is there a role for radiation therapy? Radiat Oncol. 2011;30(6):28. 10.1186/1748-717X-6-28. 10.1186/1748-717X-6-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He ZY, Wu SG, Zhou J. Postmastectomy radiotherapy improves disease-free survival of high risk of locoregional recurrence breast cancer patients with T1-2 and 1 to 3 positive nodes. PLoS ONE. 2015;10(3):e0119105. 10.1371/journal.pone.0145972. 10.1371/journal.pone.0145972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Moran MS, Huo Q, et al. Post-mastectomy radiotherapy for breast cancer patients with t1-t2 and 1–3 positive lymph nodes: a meta-analysis. PLoS ONE. 2013;8(12):e81765. 10.1371/journal.pone.0081765. 10.1371/journal.pone.0081765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WANG SL, YU ZH, LI YX, et al. The role of postmastectomy radiotherapy in breast cancer patients with T1-T2 and one to three posi-tive axillary nodes. Chin J Radiation Oncol. 2009;18(4):291–4. [Google Scholar]
- 19.Muhsen S, Moo TA, Patil S, et al. Most breast Cancer patients with T1-2 tumors and one to three positive lymph nodes do not need Postmastectomy Radiotherapy. Ann Surg Oncol. 2018;25(7):1912–20. 10.1245/s10434-018-6422-9. 10.1245/s10434-018-6422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang PS, Chen CM, Liu MC, et al. Radiotherapy can decrease locoregional recurrence and increase survival in mastectomy patients with T1 to T2 breast cancer and one to three positive nodes with negative estrogen receptor and positive lymphovascular invasion status. Int J Radiat Oncol Biol Phys. 2010;77(2):516–22. 10.1016/j.ijrobp.2009.05.016. 10.1016/j.ijrobp.2009.05.016 [DOI] [PubMed] [Google Scholar]
- 21.Gaffney DK, Tsodikov A, Wiggins CL. Diminished survival in patients with inner versus outer quadrant breast cancers. J Clin Oncol. 2003;21(3):467–72. 10.1200/JCO.2003.12.047. 10.1200/JCO.2003.12.047 [DOI] [PubMed] [Google Scholar]
- 22.Su YL, Li SH, Chen YY, et al. Post-mastectomy radiotherapy benefits subgroups of breast cancer patients with T1-2 tumor and 1–3 axillary lymph node(s) metastasis. Radiol Oncol. 2014;48(3):314–22. 10.2478/raon-2013-0085. 10.2478/raon-2013-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park HJ, Shin KH, Kim JH, et al. Incorporating risk factors to identify the indication of post-mastectomy radiotherapy in N1 breast cancer treated with optimal systemic therapy: a multicenter analysis in Korea (KROG 14–23). Cancer Res Treat. 2017;49(3):739–47. 10.4143/crt.2016.405. 10.4143/crt.2016.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo C, Zhong X, Deng L, et al. Nomogram predicting locoregional recurrence to assist decision-making of postmastectomy radiotherapy in patients with T1-2N1 breast cancer. Int J Radiat Oncol Biol Phys. 2019;103(4):905–12. 10.1016/j.ijrobp.2018.11.005. 10.1016/j.ijrobp.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Wen G, Tang Y, et al. Effectiveness of the AJCC 8th edition staging system for selecting patients with T1-2N1 breast cancer for post-mastectomy radiotherapy: a joint analysis of 1986 patients from two institutions. BMC Cancer. 2020;20(1):792. 10.1186/s12885-020-07267-5. 10.1186/s12885-020-07267-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Truong PT, Olivotto IA, Kader HA, et al. Berthelet E. selecting breast cancer patients with T1-T2 tumors and one to three positive axillary nodes at high postmastectomy locoregional recurrence risk for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61(5):1337–47. 10.1016/j.ijrobp.2004.08.009. 10.1016/j.ijrobp.2004.08.009 [DOI] [PubMed] [Google Scholar]
- 27.Tang Y, Zhang YJ, Zhang N, et al. Nomogram predicting survival as a selection criterion for postmastectomy radiotherapy in patients with T1 to T2 breast cancer with 1 to 3 positive lymph nodes. Cancer. 2020;126(16):3857–66. 10.1002/cncr.32963. 10.1002/cncr.32963 [DOI] [PubMed] [Google Scholar]
- 28.Hou N, Zhang J, Yang L, et al. A prognostic risk stratification model to identify potential Population benefiting from Postmastectomy Radiotherapy in T1-2 breast Cancer with 1–3 positive Axillary Lymph Nodes. Front Oncol. 2021;11:640268. 10.3389/fonc.2021.640268. 10.3389/fonc.2021.640268 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.