Abstract
Packaging of DNA into preformed capsids is a fundamental early event in the assembly of herpes simplex virus type 1 (HSV-1) virions. Replicated viral DNA genomes, in the form of complex branched concatemers, and unstable spherical precursor capsids termed procapsids are thought to be the substrates for the DNA-packaging reaction. In addition, seven viral proteins are required for packaging, although their individual functions are undefined. By analogy to well-characterized bacteriophage systems, the association of these proteins with various forms of capsids, including procapsids, might be expected to clarify their roles in the packaging process. While the HSV-1 UL6, UL15, UL25, and UL28 packaging proteins are known to associate with different forms of stable capsids, their association with procapsids has not been tested. Therefore, we isolated HSV-1 procapsids from infected cells and used Western blotting to identify the packaging proteins present. Procapsids contained UL15 and UL28 proteins; the levels of both proteins are diminished in more mature DNA-containing C-capsids. In contrast, UL6 protein levels were approximately the same in procapsids, B-capsids, and C-capsids. The amount of UL25 protein was reduced in procapsids relative to that in more mature B-capsids. Moreover, C-capsids contained the highest level of UL25 protein, 15-fold higher than that in procapsids. Our results support current hypotheses on HSV DNA packaging: (i) transient association of UL15 and UL28 proteins with maturing capsids is consistent with their proposed involvement in site-specific cleavage of the viral DNA (terminase activity); (ii) the UL6 protein may be an integral component of the capsid shell; and (iii) the UL25 protein may associate with capsids after scaffold loss and DNA packaging, sealing the DNA within capsids.
Formation of the infectious virion is the culmination of the lytic herpes simplex virus type 1 (HSV-1) life cycle. The overall process begins with replication of the viral double-stranded DNA genome to generate end-to-end, branched concatamers (reviewed in reference 68). At approximately the same time, capsid assembly initiates with the association of capsid shell and scaffold proteins, resulting in immature spherical procapsids containing an internal scaffold protein core (36, 37). Maturation of the procapsid involves the concurrent proteolytic processing of the scaffold by its associated protease, release of the scaffold from the capsid, cleavage of genomic DNA to unit length, and packaging of DNA into capsids. The above events are accompanied by angularization of the capsid to its final, stable icosahedral configuration. After DNA packaging, capsids acquire an additional layer of viral proteins known as the tegument and, ultimately, a lipid envelope containing viral glycoproteins (20).
Isolation of immature capsids from infected cells has provided a wealth of information about the complex process of DNA encapsidation. Three types of stable, angular capsids (A-, B-, and C-capsids) can be isolated from infected cells by sucrose gradient sedimentation (19, 44; reviewed in reference 20). C-capsids contain the viral genome and can mature to become infectious virions (44). B-capsids do not contain viral DNA but instead contain the proteolytically processed forms of the internal scaffold (35, 50). A-capsids lack both scaffold proteins and viral DNA and are believed to be by-products of DNA packaging incapable of maturation into infectious virions (59).
In contrast to the stable capsids described above, the unstable, spherical procapsid was initially identified as a transient precursor to the mature capsid in in vitro capsid assembly reactions (36, 37, 64) and has recently been isolated from HSV-1-infected cells (39). Procapsids are more rounded and porous than angular A-, B-, and C-capsids (64), contain unprocessed internal scaffold, and are unstable at low temperatures (36, 39, 52). The transition from procapsid to mature, angular capsid (8, 18, 47, 48, 52, 53) and the cleavage and packaging of viral DNA appear linked to the activity of the scaffold-associated protease since mutations in the protease block both processes (8, 18, 53). Furthermore, experiments using a temperature-sensitive virus with a reversible mutation in the protease demonstrate that the kinetics of scaffold cleavage, DNA cleavage, and DNA packaging are indistinguishable (8), lending further support to the idea that these processes occur in concert.
In addition to intact capsids (14, 45) and the activity of the scaffold-associated protease, cleavage of concatameric DNA to unit length and stable DNA encapsidation require the products of seven viral genes (2, 10, 26, 27, 32, 42, 49, 55, 63, 70). Viruses with mutations in UL6, UL15, UL17, UL28, UL32, and UL33 fail to cleave and package viral DNA into capsids, resulting in an accumulation of B-capsids containing processed scaffold proteins. In contrast, a null mutation in UL25 results in the cleavage of DNA without its stable encapsidation and in an increased accumulation of empty A-capsids (32).
The individual functions of the packaging proteins in the process of DNA cleavage and encapsidation are unclear. Several of the proteins are known to associate with capsids or with mature virions, and their differing patterns of association provide some insight into their unique functions in the packaging process. The UL6 and UL25 proteins are associated with A-, B-, and C-capsids as well as virions (1, 32, 41, 72). In contrast, different forms of UL15 are found on B- and C-capsids (54, 56, 72) and the UL28 protein is found preferentially associated with B- but not C-capsids (61, 72). Thus, the packaging of viral DNA into C-capsids is accompanied by dramatic changes in the capsid association of some packaging proteins.
To understand more about the individual roles of DNA cleavage and packaging proteins, we sought to determine if these proteins are associated with the procapsid, a structure that represents a stage prior to activity of the scaffold-associated protease, DNA packaging, and capsid angularization. To this end, we have isolated procapsids from HSV-1-infected cells and used Western blotting to examine the procapsid association of DNA-packaging proteins. For comparison, similar experiments were carried out with B- and C-capsids. We found that procapsids contained a complement of DNA-packaging proteins different from those of B- and C-capsids. Our results, combined with observations from other studies and approaches, support current models for HSV-1 DNA packaging including putative assignment of roles for several of the HSV-1 proteins involved in the packaging process.
MATERIALS AND METHODS
Cells and viruses.
Previously described procedures were used for growth of BHK cells (39), Vero cells (58), and the stably transformed Vero cell line BMS-MG22 (18). The propagation of HSV-1 (strain KOS) in Vero cells (58), the m100 virus in BMS-MG22 cells (18), and the UL15 deletion mutant virus hr81-1 in C-2 cells (70) was carried out as described previously. The tsProt.A (18) and ts1178 (57, 69) viruses were propagated in Vero cells at 34°C.
Expression of HSV-1 proteins in recombinant baculovirus-infected insect cells.
The UL28 gene was subcloned as a HindIII-BamHI fragment from the pECH82 plasmid (63) (kindly provided by Nels Pederson) into the HindIII and BglII sites of baculovirus transfer vector pVL1393 (Pharmingen). A similar construct expressing the UL15 cDNA was made by ligation of a fragment containing the 5′ end of the UL15 gene as an ApoI-AflII fragment [from the HindIII J fragment of HSV-1(KOS) DNA] to a second fragment containing the 3′ end of the UL15 gene as an AflII-NotI fragment (subcloned from the pT7ApoUL15C plasmid [70]). By three-way ligation, the UL15 fragments were inserted into the EcoRI and NotI sites of pVL1393. Recombinant baculoviruses were isolated using the BaculoGold system (Pharmingen) as specified by the manufacturer. For preparation of infected cell lysates, SF21 cells were infected at a multiplicity of infection of 0.1, incubated for 4 days at 27°C, and washed once in Dulbecco's phosphate-buffered saline (D-PBS) (8 g of NaCl per liter, 2.16 g of Na2HPO4 · 7H2O per liter, 0.2 g of KCl per liter, 0.2 g of KH2PO4 per liter), and the cell pellet was resuspended in loading buffer (50 mM Tris [pH 6.8], 100 mM dithiothreitol, 2% sodium dodecyl sulfate [SDS], 10% glycerol) for SDS-polyacrylamide gel electrophoresis (PAGE).
Capsid isolation.
B- and C-capsids were isolated from infected cells by sucrose gradient sedimentation, as previously described (58). For procapsid isolation, BHK cells were infected at a multiplicity of infection of 10 for 15 to 18 h. Infections with m100 virus were carried out at 37°C; infections with the tsProt.A and ts1178 viruses were carried out at 39°C. Cells were lysed and procapsids were isolated at room temperature essentially as described previously (39). Briefly, cells from five to six 150-cm2 flasks were pelleted and resuspended in 1 ml of lysis buffer (PBS, 1 mM EDTA, protease inhibitors) per flask. Lysates were then probe sonicated and precleared by centrifugation at 16,000 × g for 6 min. Where indicated in the text, lysates were further precleared by the addition of 100 μl of a monoclonal antibody (MAb) specific for bovine serum albumin per ml of lysate, incubation at room temperature for 30 min, and centrifugation at 16,000 × g for 5 min. A 100-μl sample of the VP5-specific MAb 6F10 (36, 60) was then added per ml of the precleared lysate, the mixture was incubated for an additional 15 min, and MAb-capsid complexes were collected by centrifugation at 16,000 × g for 2 min. The resulting pellet was resuspended in 200 μl of lysis buffer, and the sample was subjected to two additional rounds of MAb 6F10 precipitation. Samples were frozen at −80°C prior to SDS-PAGE.
SDS-PAGE and Western blots.
For analysis of total infected-cell proteins, the cells were pelleted by centrifugation at 1,000 × g for 5 min, washed once in D-PBS, resuspended in loading buffer (58), probe sonicated, and boiled for 3 min prior to SDS-PAGE. Procapsid and B-, and C-capsid samples were precipitated by addition of trichloroacetic acid to 10% (final volume), incubation on ice for 10 min, and centrifugation at 12,000 × g for 20 min. The pellets were resuspended in alkaline loading buffer (200 mM Tris [pH 8.8], 100 mM dithiothreitol, 2% SDS, 10% glycerol), boiled for 3 min, and separated by electrophoresis in 4 to 20% polyacrylamide gradient gels (Bio-Rad) for analysis by Coomassie staining. For Western blotting, Tris-glycine gels with differing concentrations of acrylamide were run, as follows: 12% polyacrylamide SDS-PAGE gels for analysis of UL15 and UL25; 4 to 20% polyacrylamide SDS-PAGE gradient gels for analysis of VP5, VP23, VP24, VP21, VP22a, and UL6; and 7.5% polyacrylamide SDS-PAGE gels for analysis of UL28. The gels were transferred by electrophoresis to nitrocellulose blots and blocked as previously described (58). Antibodies were added to the blots for 2 h at the following dilutions: MAb 13-183 against VP5 (Advanced Biotechnologies Inc.) at 1:1,000, polyclonal antibody (PAb) NC2 (9) against VP19c at 1:10,000, PAb NC1 (9) against VP5 at 1:10,000, MAb 1D2 against VP23 (39) at 1:2,000, MAb MCA406 (Serotec Inc.) against VP21/VP22a at 1:10,000, MAb 9-2 (58), against VP24 at 1:1,000, PAb CL9 (27) against UL6 at 1:1,000, PAb ID2 (24) against UL25 at 1:1,000, and PAb AS14 (72) against UL28 at 1:1,000. The PAb against UL15, kindly provided by Bernard Roizman (3), was used at 1:1,000, while the AS9 antibody against UL15 (70) was used at 1:500 (AS9). Alkaline phosphatase-conjugated goat anti-mouse or anti-rabbit immunoglobulin G secondary antibodies (Bio-Rad) were added to blots for 2 h at a 1:4,000 dilution in blocking buffer (58) plus 0.1% Tween 20. Secondary antibodies were detected using the Immunstar chemiluminescent detection kit (Bio-Rad) as directed by the manufacturer and exposure of blots to Kodak XAR-5 film. Proteins were quantitated by densitometry of the resulting films using the Molecular Dynamics Personal Densitomer SI and ImageQuant software (version 4.0). To ensure that exposures were within the linear range of detection, serial dilutions of each sample were quantitated.
RESULTS
Isolation of procapsids from protease mutant virus-infected cells.
Procapsids are expected to be short-lived intermediates in wild-type HSV-1 infection. For procapsid purification, we made use of the observation that infection with viruses containing mutations in the scaffold-associated protease results in procapsid accumulation. The scaffold is the product of the 3′-coterminal UL26 and UL26.5 transcripts (31, 50), and it is the scaffold proteins that control the assembly of the capsid shell. The shared C-terminal sequences of UL26 and UL26.5 encode identical oligomerization (15, 43) and major capsid protein-binding (21, 40) domains. The unique N terminus of UL26 encodes the serine protease domain (17, 28–30), whose function is required for the transition of procapsids to A-, B-, and C-capsids (18). Autocleavage of the UL26 protease (16) generates the capsid proteins VP24 (containing the N-terminal protease domain) and VP21 (containing the C-terminal oligomerization domain), and cleavage of the UL26.5 protein generates the capsid protein VP22a (also containing the oligomerization domain) (13, 46, 67).
To isolate procapsids, we used the protease mutant viruses m100 and tsProt.A (viruses used or described in this study are listed in Table 1). The m100 virus (18) contains a frameshift mutation at amino acid 100 of UL26, resulting in translation of a truncated, partially missense form of the UL26 protease. The m100 virus expresses wild-type levels of the UL26.5 protein, allowing procapsid assembly to proceed (18). However, the absence of active protease (VP24) from capsids prevents scaffold processing and DNA packaging (18). In contrast to the m100 virus, the tsProt.A virus expresses a full-length UL26 protein (Table 1), which is temperature sensitive in its proteolytic activity (18). Two mutations within the N terminus of the tsProt.A UL26 protein inhibit protease activity at the nonpermissive temperature (Table 1) (18, 47). The UL26 mutations were discovered and identified in the protease mutant virus ts1201 by Preston et al. (47) and transferred into the strain KOS background by Gao et al. (18) to construct tsProt.A. Infection by either m100 or tsProt.A results in the synthesis of only procapsids under the restrictive conditions (18).
TABLE 1.
Mutant viruses described in these studies
| Virus | Genotype | Reference(s) |
|---|---|---|
| HSV-1(KOS) | Wild type | |
| m100 | ΔUL26 (frameshift at amino acid 100 of UL26, producing truncated/missense VP24) | 18 |
| ts1201 | ts UL26 (strain 17) (UL26 Y30F and A48V mutations) | 47 |
| tsProt.A | ts UL26 (ts1201 mutations in strain KOS background) | 18 |
| ts1178 (tsG8) | ts UL19 (VP5) | 57, 69 |
| hr81-1 | ΔUL15 (ICP6::lacZ insertion in exon 1) | 70 |
Previous attempts to isolate procapsids from cells have been unsuccessful due to their instability to sucrose gradient sedimentation (18, 52). Therefore we used a technique developed by Newcomb et al. for the isolation of in vitro-assembled procapsids (36) and more recently used to isolate procapsids from herpesvirus-infected cells (39). A conformation-specific MAb, 6F10, which recognizes the major capsid protein (60, 64), is bound to procapsids, forming large complexes that are separated from other proteins by centrifugation.
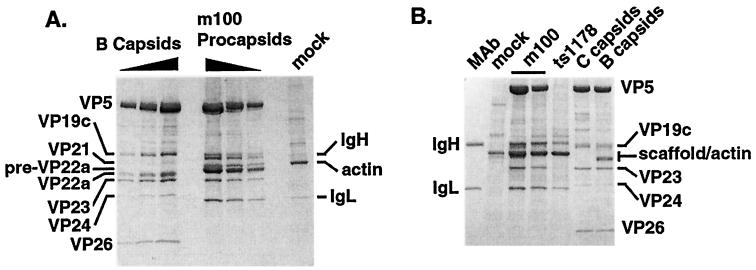
Figure 1A shows a Coomassie blue-stained SDS-PAGE gel of isolated m100 procapsids compared to sucrose-gradient purified B-capsids. We standardized the amounts of the various capsid forms by using the levels of the invariant capsid shell proteins VP5, VP19c, and VP23 (39). The 20 sides and 12 vertices of the capsid shell are composed of VP5 (virion protein 5, the major capsid protein 38, 65) organized into both six-membered rings (hexons) and five-membered rings (pentons). Tripartite complexes, or triplexes, composed of two copies of VP23 and one copy of VP19c, connect the capsid hexons and pentons (13, 38, 51). A fourth capsid shell protein, VP26, forms six-membered rings that decorate the external surface of capsid hexons but not pentons (7, 13, 73). While mature capsids but not procapsids contain VP26, the copy numbers of VP5, VP19c, and VP23 are invariant in all capsid forms (39).
FIG. 1.
Isolation of protease mutant procapsids by precipitation with a VP5-specific MAb. (A) Coomassie blue-stained SDS-PAGE gel comparing the compositions of sucrose gradient-purified B-capsids with m100 procapsids isolated by precipitation with MAb 6F10. Results for twofold dilutions of B-capsids and procapsids are shown. The positions of antibody heavy (IgH) and light (IgL) chains, capsid proteins, and cellular actin are indicated. A mock-infected cell lysate, treated identically to the 6F10 MAb, is shown as a control. The amount of sample loaded in the “mock” lane is equivalent to the largest amount of m100 procapsids loaded on the gel. (B) Coomassie blue-stained SDS-PAGE gel demonstrating the MAb precipitation technique does not isolate capsid proteins from a ts1178 (capsid-minus) extract. Two different antibody-isolated preparations of m100 procapsids are shown. Note also the comparison of the protein composition of sucrose-gradient purified HSV-1(KOS) B- and C-capsids with that of procapsids isolated from m100 infected cells.
Although the amounts of the capsid shell proteins VP5, VP19c, and VP23 were invariant in m100 procapsids and B-capsids, m100 procapsids contained, as expected, the unprocessed form of the UL26.5 scaffold protein, pre-VP22a, while B-capsids contained the processed form, VP22a. The UL26 proteolysis products, VP24 and VP21, were present in B-capsids but not in m100 procapsids (Fig. 1A). Although the truncated form of VP24 was detected in m100-infected cell lysates (see Fig. 2), it was not detected by Coomassie blue staining (Fig. 1) or Western blotting (data not shown) of m100 procapsids. m100 procapsids contained the expected MAb 6F10 heavy-chain (IgH) and light-chain (IgL) bands (Fig. 1). Few cellular proteins were precipitated from a similarly treated mock-infected cell lysate (Fig. 1A), with the most abundant protein identified as actin in Western blotting experiments (using MAb C4 [ICN Pharmaceuticals]) (data not shown). Actin was also found in m100 procapsid preparations and migrated slightly above the pre-VP22a protein in SDS-PAGE gels (Fig. 1A) (39).
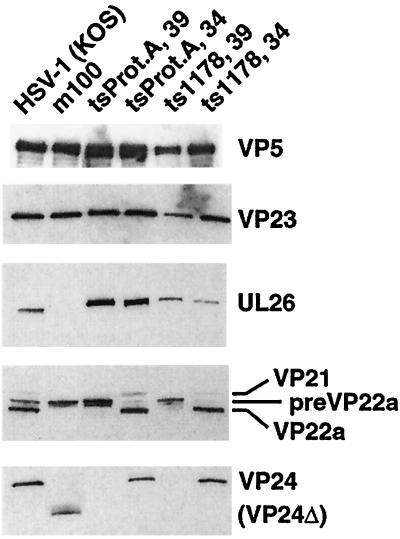
FIG. 2.
Status of capsid shell and scaffold proteins in mutant virus-infected cells. Shown are replicate Western blots of lysates from cells infected with the indicated viruses probed with antisera against VP5, VP23, and UL26 and UL26.5 scaffold proteins, and VP24. Temperature-sensitive mutant viruses tsProt.A and ts1178 were propagated at the permissive temperature (34°C) or the nonpermissive temperature (39°C), as noted. The positions of the relevant proteins are indicated to the right of the panels. ΔVP24 denotes the truncated/missense UL26 protein expressed by m100 (see Table 1).
To ensure that the technique specifically precipitated only proteins associated with procapsids, we used control lysates prepared from cells infected with a virus that fails to form capsids, ts1178 (also known as tsG8) (57, 69) (Table 1). ts1178 carries a temperature-sensitive mutation in the gene for the major capsid protein, VP5. To verify that capsid and scaffold proteins were properly expressed in ts1178-infected cells, lysates were compared to those from cells infected with the wild-type strain, HSV-1(KOS), and both protease mutant viruses, tsProt.A and m100. Blots were probed with antibodies against the capsid shell proteins VP5 and VP23, as well as the scaffold proteins encoded by the UL26 and UL26.5 genes. Compared to strain KOS-infected cell lysates, m100 lysates contained the expected pattern of UL26 and UL26.5 proteins (Fig. 2). The m100 lysate contained only the truncated form of VP24 (labeled VP24Δ; predicted molecular mass, 19 kDa), and not VP24 or VP21, and only the unprocessed form of the UL26.5 protein, pre-VP22a, and not the processed form, VP22a. ts1178-infected cells also displayed a defect in proteolytic cleavage at the nonpermissive temperature, producing full-length UL26 and the unprocessed form of UL26.5 protein, pre-VP22a. The pattern of protease processing in the ts1178-infected cells at the nonpermissive temperature resembled that seen in cells infected with the protease ts mutant virus, tsProt.A. Protease processing in both the ts1178- and tsProt.A-infected cells returned to wild-type levels on incubation at the permissive temperature. The temperature-sensitive defect in proteolytic processing in ts1178-infected cells has not been reported and is unexpected in light of the observation that a VP5 deletion mutant virus is not defective in proteolytic cleavage (14). At the nonpermissive temperature, the capsid shell proteins VP5, VP19c, and VP23 were present, albeit at slightly reduced levels, in ts1178-infected cell lysates (Fig. 2 and data not shown). Based on these results, we concluded that lysates from ts1178-infected cells (incubated at the nonpermissive temperature) provided a source of unassembled capsid shell proteins and unprocessed scaffold proteins for use as a control lysate in procapsid isolation experiments.
The MAb precipitation technique specifically isolated intact capsids, since capsid proteins VP5 and VP23 were not precipitated from ts1178-infected cell lysates (Fig. 1B, lane ts1178). The antibody heavy chain (labeled IgH) comigrates with the VP19c protein in this SDS-PAGE gel; however, Western blotting confirmed that VP19c was also absent from precipitates of capsid-minus samples (data not shown). Although the experiment in Fig. 1B failed to resolve the scaffold protein from cellular actin, Western blotting confirmed that the scaffold was also specifically isolated only in the presence of intact capsids (data not shown). In contrast to the HSV capsid proteins, some infected-cell proteins, including cellular actin, were also precipitated from ts1178-infected cells.
Association of DNA cleavage and packaging proteins with procapsids.
Four of the proteins essential for the process of DNA cleavage and packaging, the UL6, UL15, UL25, and UL28 proteins, are found associated with angular A-, B-, or C-capsids. Since relatively minor amounts of these proteins are associated with capsids, we used Western blotting with specific antisera to test for their association with procapsids. Unfortunately, in contrast to capsid shell and scaffold proteins, the isolation method used above proved to be unacceptable for analysis of the DNA-packaging proteins, since our controls indicated that several of the DNA-packaging proteins were nonspecifically isolated even in the absence of intact capsids (Fig. 3A). Although the UL6 protein (predicted molecular mass, 74 kDa) was specifically isolated only in the presence of intact capsids, this was not the case for the UL25 protein. The UL25 protein (predicted molecular mass, 63 kDa) was clearly detected in the capsid-minus ts1178 precipitate, indicating that its isolation was not dependent on the presence of intact capsids. Similarly, the UL15 and UL28 proteins were present in the capsid-minus ts1178 precipitates (data not shown). As a result, an improvement in the procapsid isolation protocol was required to remove the background of proteins not associated with intact capsids.
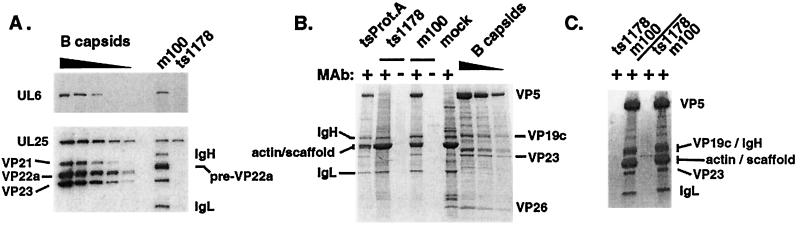
FIG. 3.
Refinement of the procapsid isolation technique. (A) Replicate Western blots probed with antibodies against the UL6 protein (upper panel) and the UL25, VP23, and scaffold proteins (lower panel). Twofold dilutions of HSV-1(KOS) B-capsids were run in parallel with procapsids isolated from m100-infected cells by using the basic MAb precipitation technique. An identically treated lysate from ts1178 (capsid-minus)-infected cells treated with MAb 6F10 is shown as a control. Note that UL25 is isolated from ts1178-infected cells in the absence of intact capsids. (B) Coomassie blue-stained gel of MAb 6F10-isolated tsProt.A and m100 procapsids isolated using the antibody precipitation technique without an antibody-preclearing step. Identically treated mock- and ts1178-infected cell samples are also shown. Twofold dilutions of sucrose gradient-purified HSV-1(KOS) B-capsids are included for comparison. Samples were treated in the absence (−) or presence (+) of the 6F10 MAb by using the procapsid isolation method described in Materials and Methods. (C) m100 procapsids and an identically treated ts1178 (capsid-minus) lysate after the addition of a preclearing step to the procapsid isolation method. Note the bands isolated from ts1178 (capsid-minus) extracts. The first two lanes show antibody-precleared, MAb 6F10-precipitated ts1178 and m100 samples collected using a brief (1-min) centrifugation under the conditions described in Materials and Methods. The next two lanes show similar samples collected after a longer (5-min) centrifugation step.
To improve the antibody-dependent isolation technique, we explored the factors affecting nonspecific precipitation of proteins in the absence of capsid structures. In the absence of the 6F10 antibody, no detectable proteins were isolated from ts1178 (capsid-minus) and m100 cell lysates (Fig. 3B). This result indicated that the majority of the contaminants were not simply insoluble proteins, since their isolation required the addition of the 6F10 antibody. We reasoned that some proteins might nonspecifically bind to the 6F10 antibody molecule and therefore added an antibody-preclearing step to the procapsid isolation process. Prior to addition of the 6F10 MAb, lysates were incubated with an antibody specific for bovine serum albumin and centrifuged to remove non-capsid-associated, antibody-reactive protein complexes (as described in Materials and Methods). This preclearing step resulted in a dramatic improvement in the specificity of the technique. The Coomassie blue-stained gel in Fig. 3C shows that procapsid proteins were present in the m100 sample but that a similarly treated capsid-minus sample (ts1178) was free of the majority of non-capsid-associated bands seen in Fig. 3A and B. The ratios of capsid shell proteins, VP5, VP19c, and VP23, were unchanged. Antibody-precleared mock-, ts1178 (capsid-minus)-, and m100-infected cell lysates contained fewer cellular proteins and undetectable or barely detectable levels of cellular actin (data not shown). Furthermore, m100 mutant procapsids isolated in this manner did not contain the truncated form of UL26 or the VP26 protein, and the procapsid preparations appeared nearly as clean as gradient-purified B-capsids. These observations suggest that the procapsid precipitation technique does not nonspecifically trap proteins in procapsid-antibody complexes.
Addition of the antibody-preclearing step allowed analysis of procapsids for the presence of DNA cleavage and packaging proteins. Figure 4 shows Western blots probed for the capsid shell proteins, VP5, VP23, and VP19c, as well as the UL6 and UL25 DNA packaging proteins. None of the proteins were detected in similarly treated capsid-minus samples (ts1178) (Fig. 4). The preclearing step also removed the background of non-capsid-associated UL15 and UL28 proteins (see Fig. 5 and 6).
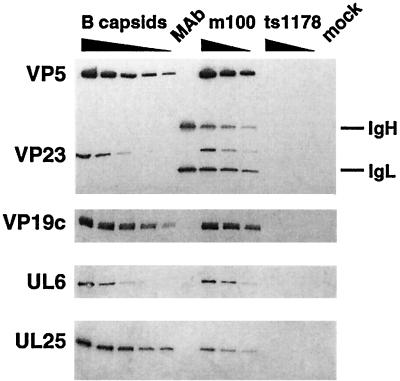
FIG. 4.
Procapsids contain decreased levels of UL25 but not UL6 protein. Twofold dilutions of sucrose gradient-purified HSV-1(KOS) B-capsids and antibody-precleared, MAb 6F10-isolated m100 procapsids are shown. MAb 6F10-treated ts1178 (capsid-minus) and mock-infected samples and a sample containing only the 6F10 MAb (MAb) are shown. Proteins were analyzed by Western blotting with antisera against VP5 and VP23 (top panel), VP19c, the UL6 protein, and the UL25 protein (bottom panel). The positions of antibody heavy (IgH) and light (IgL) chains are indicated.
FIG. 5.
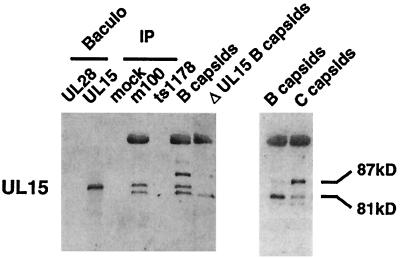
A single form of the UL15 protein is associated with procapsids. A Western blot obtained with antiserum against the C-terminal portion of the UL15 protein encoded within the second exon of the UL15 transcript is shown. The first two lanes contain control extracts from recombinant baculovirus-infected insect cells expressing the UL28 and UL15 proteins, respectively. Samples obtained by treatment of antibody-precleared mock-, m100-, and ts1178-infected cell lysates by the procapsid isolation method are shown under the heading IP. HSV-1(KOS) B-capsids and ΔUL15 B-capsids (hr81-2 mutant capsids) are included as controls. The right panel shows a second preparation of HSV-1(KOS) B- and C-capsids for comparison. A strong cross-reaction with the VP5 protein is present near the top of the Western blot. The positions of the 87- and 81-kDa UL15 immunoreactive proteins are indicated.
FIG. 6.
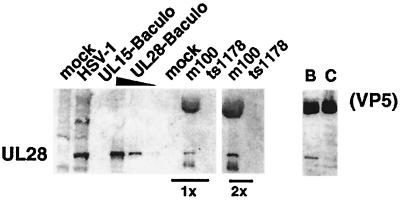
The UL28 protein is associated with procapsids. A Western blot obtained using antiserum against the UL28 protein is shown. Mock- and HSV-1(KOS)-infected cell lysates were analyzed in parallel with recombinant baculovirus-infected insect cell lysates expressing the UL15 protein (UL15-Baculo) and the UL28 protein (UL28-Baculo; three fivefold dilutions are shown). Two concentrations (labeled 1× and 2×) of mock-, m100-, and ts1178-infected precleared 6F10 MAb precipitates are shown. The right panel shows HSV-1(KOS) B- and C-capsids probed with UL28 antiserum.
The UL6 and UL25 proteins were clearly apparent in m100 procapsids (Fig. 4). Samples from similarly treated, mock-infected cell lysates did not contain cross-reacting proteins. Western blotting for the capsid shell proteins VP5, VP19c, and VP23 allowed a comparison of the relative amounts of UL6 and UL25 proteins in m100 procapsids and wild-type B-capsids. Twofold dilutions of m100 procapsids were compared to sucrose gradient-purified, wild-type B-capsids to enable a more precise comparison of protein amounts in these samples. UL6 protein levels were found to be similar in m100 procapsids and B-capsids, indicating that UL6 association with capsids occurs prior to protease processing of the scaffold proteins.
In contrast to the UL6 protein, the UL25 protein was reduced in m100 procapsids compared to B-capsids (Fig. 4). In the experiment shown, nearly sixfold less UL25 protein was found associated with m100 procapsids than with B-capsids. In multiple experiments, the amount of UL25 protein associated with procapsids was reproducibly reduced relative to that found in B-capsids (see Table 2).
TABLE 2.
UL25 protein levels are lowest in procapsids and highest in C-capsids
| Capsid type | % of UL25 protein relative to B-capsidsa
|
||||
|---|---|---|---|---|---|
| Expt. 1 | Expt. 2 | Expt. 3 | Expt. 4 | Mean | |
| Procapsidb | 19 | 17 | 25 | ND | 20 |
| B-capsid | 100 | 100 | 100 | 100 | 100 |
| C-capsid | 297 | NDc | 258 | 387 | 314 |
Protein levels were determined by densitometric scanning of film exposures of Western blots, as described in Materials and Methods. The results are presented as the percentage of UL25 protein relative to HSV-1(KOS) B-capsids, examined in parallel. Comparisons between samples and experiments were performed by setting levels for the VP5 protein to 100%.
Procapsids were isolated from m100-infected cells (experiments 1 and 2) or tsProt.A-infected cells (experiment 3). B- and C-capsids were isolated from HSV-1(KOS)-infected cells (experiments 1 to 4).
ND, not done.
The UL15 protein is associated with procapsids.
Studies have suggested that association of the UL15 (predicted molecular mass, 81 kDa) and UL28 (predicted molecular mass, 86 kDa) proteins with the capsid is dynamic since they are found on some but not all forms of capsids (54, 56, 61, 72). We found that the 81-kDa UL15 protein and the UL28 protein are associated predominantly with B- but not C-capsids (72). Figure 5 shows Western blots of different preparations of capsids and infected cell lysates probed with antiserum against the UL15 protein (AS9 antiserum) (70). Since several bands are detected by the UL15 antiserum, analysis is aided by comparison of these samples to capsids from a UL15 deletion mutant virus (hr81-2 virus) (70) (Fig. 5, lane ΔUL15 B-capsids). Four unique immunoreactive bands were detected in the B- and C-capsid preparations. An 81-kDa band found in B-capsids comigrates in SDS-PAGE gels with UL15 protein expressed in recombinant baculovirus-infected insect cells (Fig. 5, lane UL15 Baculo) at the predicted molecular mass for UL15. The 81-kDa form also comigrates with the most abundant form of the UL15 protein expressed in HSV-1-infected cells (data not shown). The level of the 81-kDa UL15 was diminished in C-capsids in the experiment in Fig. 5, in agreement with previous observations (72). Furthermore, quantitative analysis of four different preparations of wild-type capsids revealed that the 81-kDa form of UL15 was more abundant in B-capsids than in C-capsids (data not shown). Baines and coworkers reported different size species of UL15 (83, 80, and 79 kDa) that we did not detect with our antiserum and gel conditions (3, 54, 56). However, we found that their antiserum (kindly provided by Bernard Roizman 3) reacted almost exclusively with the 81-kDa form of UL15 protein expressed in recombinant baculovirus, in HSV-1-infected cells, and in B-capsids (data not shown), using our gel conditions.
Three additional unique bands were also detected with the AS9 antiserum. Control Western blots with antiserum against VP5 demonstrated that the largest AS9-reactive band in Fig. 5 represents a strong cross-reaction of the AS9 antiserum with the VP5 protein. In addition, a band smaller in apparent molecular mass than the 81-kDa UL15 protein was detected with the AS9 antiserum. These larger and smaller proteins were also present in the ΔUL15 B-capsids (Fig. 5), confirming that they do not represent additional forms of the UL15 protein. A fourth unique band (labeled 87 kDa in Fig. 5) is recognized by the AS9 antiserum in wild-type capsids but not in the ΔUL15 B-capsids. Although the relative amounts of the 87-kDa protein were somewhat variable (two different preparations of B-capsids are shown in Fig. 5), the 87-kDa protein was reproducibly more abundant in C-capsids than in B-capsids. This pattern of bands agrees with those previously observed (72) using the AS9 antiserum. However, the antiserum described by Baines and Roizman (3) failed to detect this 87-kDa band (data not shown), despite the fact that both antisera were raised against identically expressed recombinant UL15 fusion proteins. Therefore, the nature of the 87-kDa species detected by the UL15 antiserum is unknown, although its addition to capsids correlates well with maturation of the capsid and encapsidation of DNA. We speculate that the 87-kDa protein may represent a strong cross-reaction of the AS9 antibody with a tegument protein, although the possibility that the 87-kDa protein is another viral or host-encoded protein, or a modified form of UL15, has not been ruled out.
We found that m100 procapsids contained the 81-kDa form of UL15 by using our AS9 antiserum (Fig. 5) and that of Baines et al. (reference 3 and data not shown) but did not contain detectable amounts of the 87-kDa protein found in C-capsids (Fig. 5). The larger and smaller bands seen in m100 procapsids are not forms of UL15, since they are also observed in hr81-2 (ΔUL15) mutant B-capsids. UL15 proteins were not detected in capsid-minus (ts1178) and mock-infected precipitates, confirming that the UL15 protein is associated with procapsids.
The UL28 protein is associated with both procapsids and B-capsids.
We next examined procapsids for the presence of the UL28 protein (Fig. 6). Previous reports have demonstrated that the UL28 protein is associated predominantly with B- but not C-capsids (61, 72). The UL28 protein migrated as a single immunoreactive band in HSV-1-infected cell lysates and in recombinant baculovirus-infected insect cell extracts (Fig. 6). In agreement with previous studies, we found the UL28 protein predominantly associated with B- but not C-capsids (61, 72). The slower-migrating protein present at the top of the Western blots represents a cross-reaction of the UL28 antiserum with VP5. We found that m100 procapsids contained the UL28 protein (Fig. 6). The antiserum against UL28 also detected a band of higher mobility than UL28 in procapsids. This faster-migrating band may be a cross-reacting (non-UL28) protein or a breakdown product of UL28. The finding that both the UL15 and UL28 proteins are associated with procapsids demonstrates that, in this respect, procapsids are more similar to scaffold-containing B-capsids than to DNA-containing C-capsids.
Association of packaging proteins with tsProt.A procapsids.
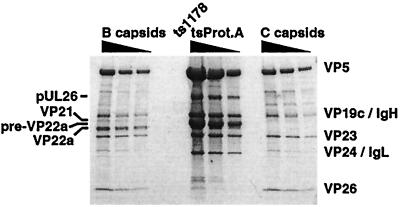
Unlike m100 procapsids, which lack the UL26 protease and are unable to package viral DNA, tsProt.A procapsids contain full-length UL26 protein and can be reversed in vivo to form DNA-containing capsids (8). To investigate the role of the full-length UL26 protein in recruitment of the DNA-packaging machinery to the capsid, we tested tsProt.A procapsids for the presence of the UL6, UL15, UL25, and UL28 proteins. tsProt.A procapsids, purified by the refined MAb 6F10 isolation technique, are shown in the Coomassie blue-stained SDS-PAGE gel in Fig. 7. In contrast to m100 procapsids, which entirely lack the UL26 proteins, tsProt.A procapsids contained the full-length UL26 protein in an unprocessed form (labeled pUL26, Fig. 7). tsProt.A procapsids also contained primarily the unprocessed form of the UL26.5 protein, pre-VP22a, although an extremely small amount of processed VP22a was detected. When similar amounts of B- and C-capsids were compared to tsProt.A procapsids, the VP26 protein was not detected in procapsids (Fig. 7, compare the first dilution of B- and C-capsids to the third dilution of tsProt.A procapsids), confirming our published findings (39).
FIG. 7.
Analysis of tsProt.A procapsids. Shown is a Coomassie blue-stained SDS-PAGE gel comparing twofold dilutions of sucrose gradient-purified HSV-1(KOS) B- and C-capsids and tsProt.A procapsids isolated using the precleared MAb 6F10 precipitation technique. The relative positions of the capsid shell proteins and the scaffold proteins are indicated. Note the presence of the full-length, unprocessed UL26 scaffold protein (labeled pUL26) in tsProt.A procapsids.
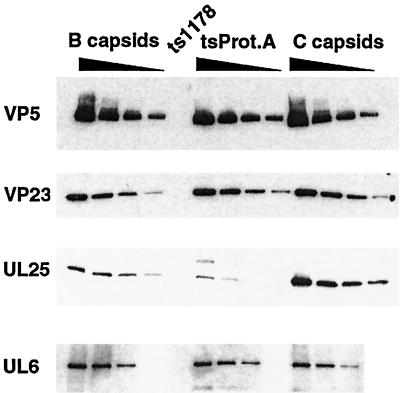
We examined tsProt.A procapsids to determine whether the presence of the UL26 protein affected the association of DNA cleavage and packaging proteins. Figure 8 shows replicate Western blots of tsProt.A procapsids probed with antibodies against the VP5 and VP23 capsid shell proteins and the UL6 and UL25 DNA-packaging proteins. The amount of UL6 protein associated with tsProt.A capsids was similar to that in wild-type B-capsids. We also found that the UL15 and UL28 proteins were present in tsProt.A procapsids in amounts and species that resembled those found in m100 procapsids (data not shown). These results indicate that the presence of the unprocessed UL26 protein did not affect the association of the UL6, UL15, and UL28 proteins with procapsids.
FIG. 8.
DNA-packaging proteins associated with tsProt.A procapsids. Replicate Western blots of twofold dilutions of sucrose gradient-purified HSV-1(KOS) B- and C-capsids and precleared MAb 6F10-isolated tsProt.A procapsids were probed with antibodies against the VP5, VP23, UL25, and UL6 proteins. The larger protein detected by the UL25 antiserum in the tsProt.A samples represents a cross-reaction with the UL26 protein since it also reacts with antisera to the UL26 protein (data not shown).
In agreement with the results obtained with m100 procapsids, examination of tsProt.A procapsids with UL25 antiserum revealed a decreased amount of procapsid-associated UL25 protein relative to that in wild-type B-capsids (Fig. 8). In the experiment in Fig. 8, the amount of UL25 was reduced approximately fourfold from that in wild-type B-capsids (see Table 2). An additional band, with higher molecular mass than the UL25 protein, was also recognized by the UL25 antiserum in tsProt.A procapsids. This probably represents a cross-reaction with the full-length UL26 protein, since antibody specific for VP24 also recognizes a band with the same molecular mass (data not shown).
Because association of the UL25 protein with capsids seemed to change in relation to the proteolytic processing of the internal scaffold proteins, we investigated whether the amount of UL25 protein also changes in response to scaffold release and DNA packaging. Figure 8 shows the results of a representative Western blotting experiment comparing the relative amounts of UL25 protein associated with wild-type B- and C-capsids. Comparison of twofold dilutions of B- and C-capsids shows that similar amounts of capsids were compared (based on the VP5 and VP23 signals). While the amounts of the UL6 protein present in B- and C-capsids were comparable, an increased amount of UL25 protein was associated with C-capsids. In the experiment shown, nearly threefold more UL25 protein was detected in C-capsids than in B-capsids.
Quantitative comparison of the data from multiple experiments (Table 2) shows that the amount of UL25 protein was consistently four- to sixfold lower in procapsids than in B-capsids and approximately three- to fourfold higher in C-capsids than in B-capsids. When similar quantitations were performed for the UL6 protein, no measurable differences were detected in the levels of UL6 associated with procapsids or B- or C-capsids (data not shown). The values shown in Table 2 translate into an average 15.7-fold-higher level of UL25 protein associated with C-capsids than with procapsids (Table 2).
DISCUSSION
The process of HSV-1 DNA packaging bears long-recognized similarities to the packaging of DNA by double-stranded DNA bacteriophages. The products of the UL6, UL15, UL17, UL25, UL28, UL32, and UL33 genes are required for stable packaging of DNA into HSV-1 capsids. By analogy to well-characterized bacteriophage systems, the HSV-1 DNA-packaging proteins are expected to encompass a number of functions. HSV-1 DNA-packaging proteins might be expected to include a terminase complex, which recognizes and cleaves nascent viral DNA to unit length and inserts the DNA into capsids; a “portal” protein, which forms a pore in the capsid through which DNA is packaged; and “cork” proteins, which appear to seal the capsid after DNA is inserted, stabilizing the packaged DNA within capsids (reviewed in references 4, 6, and 33). Like their bacteriophage counterparts, these HSV-1 proteins would be expected to be present at distinct sites and at different copy numbers within capsids. It is currently unclear how many copies of each HSV-1 DNA-packaging protein are present per capsid since the proteins are not visible by Coomassie blue staining or in capsid structural determinations. Therefore, copy number determination will require quantitative Western blotting of capsids against purified protein standards. Unfortunately, the insolubility of these proteins has so far prevented their purification to homogeneity, and so quantitation has not been possible.
Several lines of evidence have been presented (described below) that support the hypotheses that the products of UL15 and UL28 may be involved in terminase activity and that the UL25 protein may seal capsids after DNA packaging. Furthermore, the association of similar levels of UL6 with all forms of capsids is not inconsistent with a role as a portal protein which forms an integral part of the capsid shell. To complete the analogy to the bacteriophage counterparts, these proteins are expected to be present in various maturational stages of capsids. While their presence in some capsid types has been documented, the most immature capsid, the procapsid, has not previously been examined due to technical difficulties in its isolation. We have used a novel technique to isolate procapsids (36, 39), and in this work we have refined the technique to enable an examination of the DNA packaging proteins. The results of this work lend further support to the above hypothetical functions for individual HSV-1 cleavage and packaging proteins.
Several lines of evidence point to the involvement of the UL15 and UL28 gene products in the cleavage and packaging of viral DNA into capsids, perhaps even as components of the terminase itself. In bacteriophage systems, the terminase is generally a two-subunit enzyme complex which is transiently associated with procapsids during packaging but is absent from the mature DNA-containing capsid (5, 34). Previously, the HSV-1 81-kDa UL15 protein (72) and the UL28 protein (61, 72) were shown to be predominantly associated with B-capsids but not DNA-containing C-capsids. The observation that the HSV-1 UL15 and UL28 proteins are transiently associated with intermediate B-capsids, combined with the published observations described below, prompted Yu and Weller (72) to hypothesize that these proteins might form the herpesvirus terminase complex. This proposal is consistent with the observation that the UL15 protein has homology to the ATP-binding motif within the large subunit of the bacteriophage T4 terminase protein (12). Most bacteriophage terminases bind and hydrolyze ATP, which is believed to provide energy for the translocation of DNA into capsids (6). In the HSV-1 system, the UL15 ATP-binding motif is required for DNA cleavage and packaging (71) and the packaging of DNA is blocked on depletion of ATP (11). Additionally, several lines of evidence point toward an interaction between the UL15 and UL28 proteins. The capsid localization of the UL15 protein requires the presence of the UL28 protein, suggesting an interaction of these proteins on capsids (72). Further suggestion of a physical interaction between the two proteins comes from the observation that the UL15 and UL28 proteins copurify from infected cells (23) and may interact to localize to the nucleus (23, 24). In addition, on selection with agents that inhibit cleavage and packaging in the related herpesvirus human cytomegalovirus, resistance mutations can arise in either the UL15 or UL28 homologs (25, 66). Although definitive biochemical evidence is required to identify the HSV-1 terminase proteins, the existing data are consistent with a role for the UL15 and UL28 proteins in the DNA cleavage and packaging process.
In the present work we show that the 81-kDa UL15 protein and the UL28 protein associate with capsids at a stage earlier than the B-capsid, i.e., the procapsid. These results imply that the herpesvirus DNA-packaging machinery begins its interaction with capsids at the procapsid stage, prior to protease cleavage of the internal scaffold proteins and the coincident structural changes in the capsid shell. Also, an 87-kDa protein detected with the UL15 antiserum, previously shown to be found primarily in C-capsids rather than B-capsids (72), was absent from procapsids. Further analysis of the 87-kDa protein may be enlightening, since its high-level addition to the capsid seems to correlate with DNA packaging. Since procapsids and B-capsids have in common the presence of the internal scaffold proteins, our results suggest that loss of scaffolding protein and packaging of viral DNA correlate with loss of the 81-kDa UL15 and UL28 proteins from the capsid and addition of the 87-kDa protein identified by the UL15 antiserum. We feel that these changes in capsid interaction may be triggered by DNA packaging rather than scaffold loss, since we recently reported that mutant B-capsids lacking the bulk of the scaffold have B-capsid levels of both of these proteins (58). The apparent correlation between DNA packaging and loss of the 81-kDa UL15 and UL28 proteins from the capsid is consistent with the proposal that they are directly involved in the packaging process.
In contrast to the other DNA-packaging proteins, we find that the level of UL6 protein associated with procapsids, B-capsids, and C-capsids is invariant. In this respect, UL6 resembles the structural proteins that form the capsid, perhaps indicating that it forms an integral, minor component of the capsid shell. In support of this hypothesis, the association of UL6 with capsids is independent of other packaging proteins (32, 72). Moreover, UL6 is required for efficient capsid association of the UL15 protein, suggesting that capsid-associated UL6 may serve as a docking site for the cleavage and packaging machinery (72). These UL6 traits are reminiscent of the portal protein of bacteriophage capsids. The bacteriophage portal protein forms a unique vertex of the capsid through which DNA is packaged and subsequently injected into the host cell on infection (reviewed in references 4 and 6). It is certainly possible that UL6 might be present at evenly distributed sites within the capsid. However, since UL6 is the only essential DNA-packaging protein known to associate in similar amounts with all forms of capsids, it is intriguing to speculate that UL6 may possibly form the portal of DNA entry into herpesvirus capsids.
At present, it is unclear how herpesvirus DNA enters the capsid. Unlike bacteriophage capsids, no unique vertex is readily apparent in electron micrographs of HSV-1 capsids. Although high-resolution three-dimensional reconstructions of the capsid have been obtained, icosahedral averaging of these structures would obscure such a unique structure if it exists. The above observations suggest that the HSV portal may be small and difficult to visualize. Alternatively, if all of the vertices of the HSV capsid are indeed initially equivalent, perhaps only a single vertex is activated to package DNA by contact with or docking of the DNA cleavage and packaging machinery. A requirement for portal protein activation has been suggested for bacteriophage systems (reviewed in reference 6), where such activation may provide a mechanism to prevent premature attempts at packaging during early stages of capsid assembly. In the bacteriophage lambda system, it has been postulated that proteolysis of a subset of portal protein molecules may make the portal competent to package viral DNA (6). Further analysis of the HSV-1 procapsid could reveal the mechanism of DNA entry, and the isolation of tsProt.A procapsids may facilitate these studies. tsProt.A procapsids can be reversed to package DNA in vivo (8) and therefore may provide a unique opportunity to evaluate the events of DNA cleavage and packaging in greater detail in a defined in vitro system.
Unlike the UL6 protein, the association of the UL25 protein with capsids seems to change as a result of protease cleavage, scaffold loss, and DNA packaging. Both m100 and tsProt.A procapsids contain less UL25 than do B-capsids. B-capsids, in turn, contain less UL25 than do DNA-containing C-capsids. Data presented by others for HSV-1 (Fig. 9 of reference 32) and pseudorabies virus (Fig. 2 of reference 22) agree with our findings in that the level of UL25 protein shown associated with C-capsids appears greater than that associated with B-capsids. We propose that the level of UL25 protein within capsids is inversely regulated by the amount of scaffold proteins found within capsids since we recently observed that mutant B-capsids lacking the bulk of the internal scaffold proteins contain a level of UL25 protein similar to that in wild-type DNA-containing C-capsids (58). In agreement with this idea, the total amount of scaffold protein found in procapsids is somewhat larger than that found in B-capsids (39). The level of UL25 protein is reduced so dramatically in procapsids from that in C-capsids (>15-fold) that it is tempting to speculate that UL25 may in fact be absent from the procapsid. Perhaps the low level of UL25 seen in our procapsid blots represents leakiness of the mutant phenotypes or partial maturation of the procapsid during its isolation. Although treatment with guanidine hydrochloride is often used to judge the strength of protein association with angular capsids (35), we were unable to use this method on procapsids since guanidine hydrochloride treatment entirely disrupts the structure of the procapsid (W. Newcomb and J. Brown, unpublished data). Nevertheless, the simplest explanation for our findings is that UL25 is absent from the procapsid in vivo and is fully incorporated into the capsid only after DNA packaging is completed.
A model in which the majority of the UL25 protein is added after scaffold loss and DNA packaging correlates well with the phenotype of a UL25-null mutant virus (32). While mutations in the other genes that are essential for HSV-1 DNA packaging result in the accumulation of only B-capsids, infection with the UL25 deletion mutant virus results in an accumulation of both A- and B-capsids. DNA-containing C-capsids are not produced, although viral DNA is properly cleaved to unit length. Since A-capsids are thought to be produced as a consequence of abortive DNA cleavage and packaging, this observation led to the hypothesis that UL25 may be required to seal the capsids after DNA is cleaved and packaged (32). If this is indeed the case, then the DNase-sensitive, unit-length genomes and empty A-capsids might be produced by transient packaging and release of packaged genomes, as suggested by McNab et al. (32). This model is in agreement with the idea that addition of UL25 protein upon DNA packaging may stabilize the packaged DNA within the capsid. In further support of the notion that UL25 is added late in the process of DNA cleavage and packaging, the association of other cleavage and packaging proteins with capsids is independent of the presence of UL25 (72).
The ability of the capsid to change its overall shape from a spherical, porous procapsid to a more sealed, angular capsid presumably involves many changes in protein tertiary structures, including both inter- and intramolecular effects. However, the changes in packaging proteins associated with the various maturational stages of capsids cannot be simply explained by different affinities for angular versus spherical capsid conformations. Indeed, there are examples of proteins that bind to all capsid types (UL6, VP24, and MAb 6F10), those that are lost on capsid angularization (scaffold proteins), those that are gained on angularization (VP26 and UL25), and those that are lost (81-kDa UL15 and UL28) or gained (UL15-reactive 87-kDa protein and UL25) on DNA packaging.
Further experiments must be performed to test for other proteins, both host and viral, that may be associated with procapsids and involved in capsid maturation. We have examined procapsids for the association of UL6, UL15, UL25, and UL28; however, three additional proteins are known to be essential for the DNA encapsidation, the UL17, UL32, and UL33 proteins (10, 26, 49, 55). In the absence of the UL17 and UL32 proteins, capsids and viral DNA do not properly colocalize in infected cells (26, 62). Although these proteins have not been unequivocally identified as capsid components, it is entirely possible that they interact transiently with the procapsid during its maturation to facilitate the interaction of capsids with viral DNA. In fact, both host and phage proteins have been found to facilitate the interaction of capsids with DNA and to enhance the cleavage of DNA in the bacteriophage lambda system (6).
Numerous intriguing questions about HSV-1 DNA cleavage and packaging remain. The process of DNA packaging is obviously complex, requiring not only the interaction of replicated viral DNA and preformed capsids but also the proper timing of capsid maturational events for the stable encapsidation of viral DNA. The signals that transmit the events of capsid maturation, presumably involving the capsid scaffold (and protease), the capsid shell, viral DNA, and the cleavage and packaging proteins, are as yet undefined. It is clear that capsids are required to trigger DNA cleavage, since cleavage is inhibited in the absence of intact capsids (14, 45). However, it is unclear how the associations and dissociations of DNA-packaging proteins are timed to allow the packaging of DNA into capsids. For example, the signals that trigger the loss of the 81-kDa UL15 and UL28 proteins from the capsid on DNA packaging are unknown, as are those that trigger the addition of UL25 to the capsid. Determining the precise capsid-binding sites of the UL6, UL15, UL25, and UL28 proteins may provide some insight into these questions.
ACKNOWLEDGMENTS
We are grateful to Gary Cohen and Roselyn Eisenberg for providing the NC1 and NC2 antibodies, to Bernard Roizman for providing antiserum to the UL15 protein, to Priscilla Schaffer for permission to use the ts1178 virus, and to Nels Pederson for providing the pECH82 plasmid. We appreciate scientific discussions concerning the UL15 protein with Joel Baines. A.K.S., M.G., and D.J.T. thank Richard Colonno for supporting these studies.
W.W.N. and J.C.B. were supported by NIH grant AI41644 and NSF award MCB9904879. D.Y. and S.K.W. were supported by NIH grant AI37549.
REFERENCES
- 1.Ali M A, Forghani B, Cantin E M. Characterization of an essential HSV-1 protein encoded by the UL25 gene reported to be involved in virus penetration and capsid assembly. Virology. 1996;216:278–283. doi: 10.1006/viro.1996.0061. [DOI] [PubMed] [Google Scholar]
- 2.Baines J D, Cunningham C, Nalwanga D, Davison A. The U(L)15 gene of herpes simplex virus type 1 contains within its second exon a novel open reading frame that is translated in frame with the U(L)15 gene product. J Virol. 1997;71:2666–2673. doi: 10.1128/jvi.71.4.2666-2673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baines J D, Poon A P, Rovnak J, Roizman B. The herpes simplex virus 1 UL15 gene encodes two proteins and is required for cleavage of genomic viral DNA. J Virol. 1994;68:8118–8124. doi: 10.1128/jvi.68.12.8118-8124.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazinet C, King J. The DNA translocating vertex of dsDNA bacteriophage. Annu Rev Microbiol. 1985;39:109–129. doi: 10.1146/annurev.mi.39.100185.000545. [DOI] [PubMed] [Google Scholar]
- 5.Becker A, Marko M, Gold M. Early events in the in vitro packaging of bacteriophage lambda DNA. Virology. 1977;78:291–305. doi: 10.1016/0042-6822(77)90100-3. [DOI] [PubMed] [Google Scholar]
- 6.Black L W. DNA packaging in dsDNA bacteriophages. In: Calendar R, editor. The bacteriophages. Vol. 2. New York, N.Y: Plenum Press; 1988. pp. 321–373. [Google Scholar]
- 7.Booy F P, Trus B L, Newcomb W W, Brown J C, Conway J F, Steven A C. Finding a needle in a haystack: detection of a small protein (the 12-kDa VP26) in a large complex (the 200-MDa capsid of herpes simplex virus) Proc Natl Acad Sci USA. 1994;91:5652–5656. doi: 10.1073/pnas.91.12.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Church G A, Wilson D W. Study of herpes simplex virus maturation during a synchronous wave of assembly. J Virol. 1997;71:3603–3612. doi: 10.1128/jvi.71.5.3603-3612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen G H, Ponce de Leon M, Diggelmann H, Lawrence W C, Vernon S K, Eisenberg R J. Structural analysis of the capsid polypeptides of herpes simplex virus types 1 and 2. J Virol. 1980;34:521–531. doi: 10.1128/jvi.34.2.521-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham C, Davison A J. A cosmid-based system for constructing mutants of herpes simplex virus type 1. Virology. 1993;197:116–124. doi: 10.1006/viro.1993.1572. [DOI] [PubMed] [Google Scholar]
- 11.Dasgupta A, Wilson D W. ATP depletion blocks herpes simplex virus DNA packaging and capsid maturation. J Virol. 1999;73:2006–2015. doi: 10.1128/jvi.73.3.2006-2015.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davison A J. Channel catfish virus: a new type of herpesvirus. Virology. 1992;186:9–14. doi: 10.1016/0042-6822(92)90056-u. [DOI] [PubMed] [Google Scholar]
- 13.Davison M D, Rixon F J, Davison A J. Identification of genes encoding two capsid proteins (VP24 and VP26) of herpes simplex virus type 1. J Gen Virol. 1992;73:2709–2713. doi: 10.1099/0022-1317-73-10-2709. [DOI] [PubMed] [Google Scholar]
- 14.Desai P, DeLuca N A, Glorioso J C, Person S. Mutations in herpes simplex virus type 1 genes encoding VP5 and VP23 abrogate capsid formation and cleavage of replicated DNA. J Virol. 1993;67:1357–1364. doi: 10.1128/jvi.67.3.1357-1364.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desai P, Person S. Molecular interactions between the HSV-1 capsid proteins as measured by the yeast two-hybrid system. Virology. 1996;220:516–521. doi: 10.1006/viro.1996.0341. [DOI] [PubMed] [Google Scholar]
- 16.DiIanni C L, Drier D A, Deckman I C, McCann P J, Liu F, Roizman B, Colonno R J, Cordingley M G. Identification of the herpes simplex virus-1 protease cleavage sites by direct sequence analysis of autoproteolytic cleavage products. J Biol Chem. 1993;268:2048–2051. [PubMed] [Google Scholar]
- 17.DiIanni C L, Stevens J T, Bolgar M, O'Boyle D R, Weinheimer S P, Colonno R J. Identification of the serine residue at the active site of the herpes simplex virus type 1 protease. J Biol Chem. 1994;269:12672–12676. [PubMed] [Google Scholar]
- 18.Gao M, Matusick-Kumar L, Hurlburt W, DiTusa S F, Newcomb W W, Brown J C, McCann P J, Deckman I, Colonno R J. The protease of herpes simplex virus type 1 is essential for functional capsid formation and viral growth. J Virol. 1994;68:3702–3712. doi: 10.1128/jvi.68.6.3702-3712.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson W, Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972;10:1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Homa F L, Brown J C. Capsid assembly and DNA packaging in herpes simplex virus. Rev Med Virol. 1997;7:107–122. doi: 10.1002/(sici)1099-1654(199707)7:2<107::aid-rmv191>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Hong Z, Beaudet-Miller M, Durkin J, Zhang R, Kwong A D. Identification of a minimal hydrophobic domain in the herpes simplex virus type 1 scaffolding protein which is required for interaction with the major capsid protein. J Virol. 1996;70:533–540. doi: 10.1128/jvi.70.1.533-540.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaelin K, Dezelee S, Masse M J, Bras F, Flamand A. The UL25 protein of pseudorabies virus associates with capsids and localizes to the nucleus and to microtubules. J Virol. 2000;74:474–482. doi: 10.1128/jvi.74.1.474-482.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koslowski K M, Shaver P R, Casey II J T, Wilson T, Yamanaka G, Sheaffer A K, Tenney D J, Pederson N E. Physical and functional interactions between the herpes simplex virus UL15 and UL28 DNA cleavage and packaging proteins. J Virol. 1999;73:1704–1707. doi: 10.1128/jvi.73.2.1704-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koslowski K M, Shaver P R, Wang X Y, Tenney D J, Pederson N E. The pseudorabies virus UL28 protein enters the nucleus after coexpression with the herpes simplex virus UL15 protein. J Virol. 1997;71:9118–9123. doi: 10.1128/jvi.71.12.9118-9123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krosky P M, Underwood M R, Turk S R, Feng K W, Jain R K, Ptak R G, Westerman A C, Biron K K, Townsend L B, Drach J C. Resistance of human cytomegalovirus to benzimidazole ribonucleosides maps to two open reading frames: UL89 and UL56. J Virol. 1998;72:4721–4728. doi: 10.1128/jvi.72.6.4721-4728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamberti C, Weller S K. The herpes simplex virus type 1 cleavage/packaging protein, UL32, is involved in efficient localization of capsids to replication compartments. J Virol. 1998;72:2463–2473. doi: 10.1128/jvi.72.3.2463-2473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamberti C, Weller S K. The herpes simplex virus type 1 UL6 protein is essential for cleavage and packaging but not for genomic inversion. Virology. 1996;226:403–407. doi: 10.1006/viro.1996.0668. [DOI] [PubMed] [Google Scholar]
- 28.Liu F, Roizman B. Characterization of the protease and other products of amino-terminus-proximal cleavage of the herpes simplex virus 1 UL26 protein. J Virol. 1993;67:1300–1309. doi: 10.1128/jvi.67.3.1300-1309.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu F, Roizman B. Differentiation of multiple domains in the herpes simplex virus 1 protease encoded by the UL26 gene. Proc Natl Acad Sci USA. 1992;89:2076–2080. doi: 10.1073/pnas.89.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu F Y, Roizman B. The herpes simplex virus 1 gene encoding a protease also contains within its coding domain the gene encoding the more abundant substrate. J Virol. 1991;65:5149–5156. doi: 10.1128/jvi.65.10.5149-5156.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu F Y, Roizman B. The promoter, transcriptional unit, and coding sequence of herpes simplex virus 1 family 35 proteins are contained within and in frame with the UL26 open reading frame. J Virol. 1991;65:206–212. doi: 10.1128/jvi.65.1.206-212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNab A R, Desai P, Person S, Roof L L, Thomsen D R, Newcomb W W, Brown J C, Homa F L. The product of the herpes simplex virus type 1 UL25 gene is required for encapsidation but not for cleavage of replicated viral DNA. J Virol. 1998;72:1060–1070. doi: 10.1128/jvi.72.2.1060-1070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murialdo H, Becker A. Head morphogenesis of complex double-stranded deoxyribonucleic acid bacteriophages. Microbiol Rev. 1978;42:529–576. doi: 10.1128/mr.42.3.529-576.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murialdo H, Siminovitch L. The morphogenesis of bacteriophage lambda. IV. Identification of gene products and control of the expression of the morphogenetic information. Virology. 1972;48:785–823. doi: 10.1016/0042-6822(72)90162-6. [DOI] [PubMed] [Google Scholar]
- 35.Newcomb W W, Brown J C. Structure of the herpes simplex virus capsid: effects of extraction with guanidine hydrochloride and partial reconstitution of extracted capsids. J Virol. 1991;65:613–20. doi: 10.1128/jvi.65.2.613-620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newcomb W W, Homa F L, Thomsen D R, Booy F P, Trus B L, Steven A C, Spencer J V, Brown J C. Assembly of the herpes simplex virus capsid: characterization of intermediates observed during cell-free capsid formation. J Mol Biol. 1996;263:432–446. doi: 10.1006/jmbi.1996.0587. [DOI] [PubMed] [Google Scholar]
- 37.Newcomb W W, Homa F L, Thomsen D R, Trus B L, Cheng N, Steven A, Booy F, Brown J C. Assembly of the herpes simplex virus procapsid from purified components and identification of small complexes containing the major capsid and scaffolding proteins. J Virol. 1999;73:4239–4250. doi: 10.1128/jvi.73.5.4239-4250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newcomb W W, Trus B L, Booy F P, Steven A C, Wall J S, Brown J C. Structure of the herpes simplex virus capsid. Molecular composition of the pentons and the triplexes. J Mol Biol. 1993;232:499–511. doi: 10.1006/jmbi.1993.1406. [DOI] [PubMed] [Google Scholar]
- 39.Newcomb W W, Trus B L, Cheng N, Steven A C, Sheaffer A K, Tenney D J, Weller S K, Brown J C. Isolation of herpes simplex virus procapsids from cells infected with a protease-deficient mutant virus. J Virol. 2000;74:1663–1673. doi: 10.1128/jvi.74.4.1663-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oien N L, Thomsen D R, Wathen M W, Newcomb W W, Brown J C, Homa F L. Assembly of herpes simplex virus capsids using the human cytomegalovirus scaffold protein: critical role of the C terminus. J Virol. 1997;71:1281–1291. doi: 10.1128/jvi.71.2.1281-1291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel A H, MacLean J B. The product of the UL6 gene of herpes simplex virus type 1 is associated with virus capsids. Virology. 1995;206:465–478. doi: 10.1016/s0042-6822(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 42.Patel A H, Rixon F J, Cunningham C, Davison A J. Isolation and characterization of herpes simplex virus type 1 mutants defective in the UL6 gene. Virology. 1996;217:111–123. doi: 10.1006/viro.1996.0098. [DOI] [PubMed] [Google Scholar]
- 43.Pelletier A, Dô F, Brisebois J J, Lagacé L, Cordingley M G. Self-association of herpes simplex virus type 1 ICP35 is via coiled-coil interactions and promotes stable interaction with the major capsid protein. J Virol. 1997;71:5197–5208. doi: 10.1128/jvi.71.7.5197-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perdue J L, Cohen J C, Randall C C, O'Callaghan D J. Biochemical studies of the maturation of herpesvirus nucleocapsid species. Virology. 1976;74:194–208. [PubMed] [Google Scholar]
- 45.Person S, Desai P. Capsids are formed in a mutant virus blocked at the maturation site of the UL26 and UL26.5 open reading frames of herpes simplex virus type 1 but are not formed in a null mutant of UL38 (VP19C) Virology. 1998;242:193–203. doi: 10.1006/viro.1997.9005. [DOI] [PubMed] [Google Scholar]
- 46.Person S, Laquerre S, Desai P, Hempel J. Herpes simplex virus type 1 capsid protein, VP21, originates within the UL26 open reading frame. J Gen Virol. 1993;74:2269–2273. doi: 10.1099/0022-1317-74-10-2269. [DOI] [PubMed] [Google Scholar]
- 47.Preston V G, Coates J A, Rixon F J. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J Virol. 1983;45:1056–1064. doi: 10.1128/jvi.45.3.1056-1064.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Register R B, Shafer J A. Alterations in catalytic activity and virus maturation produced by mutation of the conserved histidine residues of herpes simplex virus type 1 protease. J Virol. 1997;71:8572–8581. doi: 10.1128/jvi.71.11.8572-8581.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reynolds A E, Fan Y, Baines J D. Characterization of the U(L)33 gene product of herpes simplex virus 1. Virology. 2000;266:310–318. doi: 10.1006/viro.1999.0090. [DOI] [PubMed] [Google Scholar]
- 50.Rixon F J, Cross A M, Addison C, Preston V G. The products of herpes simplex virus type 1 gene UL26 which are involved in DNA packaging are strongly associated with empty but not with full capsids. J Gen Virol. 1988;69:2879–2891. doi: 10.1099/0022-1317-69-11-2879. [DOI] [PubMed] [Google Scholar]
- 51.Rixon F J, Davison M D, Davison A J. Identification of the genes encoding two capsid proteins of herpes simplex virus type 1 by direct amino acid sequencing. J Gen Virol. 1990;71:1211–1214. doi: 10.1099/0022-1317-71-5-1211. [DOI] [PubMed] [Google Scholar]
- 52.Rixon F J, McNab D. Packaging-competent capsids of a herpes simplex virus temperature-sensitive mutant have properties similar to those of in vitro-assembled procapsids. J Virol. 1999;73:5714–5721. doi: 10.1128/jvi.73.7.5714-5721.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robertson B J, McCann P J, Matusick-Kumar L, Newcomb W W, Brown J C, Colonno R J, Gao M. Separate functional domains of the herpes simplex virus type 1 protease: evidence for cleavage inside capsids. J Virol. 1996;70:4317–4328. doi: 10.1128/jvi.70.7.4317-4328.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salmon B, Baines J D. Herpes simplex virus DNA cleavage and packaging: association of multiple forms of U(L)15-encoded proteins with B capsids requires at least the U(L)6, U(L)17, and U(L)28 genes. J Virol. 1998;72:3045–3050. doi: 10.1128/jvi.72.4.3045-3050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salmon B, Cunningham C, Davison A J, Harris W J, Baines J D. The herpes simplex virus type 1 U(L)17 gene encodes virion tegument proteins that are required for cleavage and packaging of viral DNA. J Virol. 1998;72:3779–3788. doi: 10.1128/jvi.72.5.3779-3788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salmon B, Nalwanga D, Fan Y, Baines J D. Proteolytic cleavage of the amino terminus of the U(L)15 gene product of herpes simplex virus type 1 is coupled with maturation of viral DNA into unit-length genomes. J Virol. 1999;73:8338–8348. doi: 10.1128/jvi.73.10.8338-8348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schaffer P A, Aron G M, Biswal N, Benyesh-Melnick M. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology. 1973;52:57–71. doi: 10.1016/0042-6822(73)90398-x. [DOI] [PubMed] [Google Scholar]
- 58.Sheaffer A K, Newcomb W W, Brown J C, Gao M, Weller S K, Tenney D J. Evidence for the controlled incorporation of UL26, the herpes simplex virus type-1 protease, into capsids. J Virol. 2000;74:6838–6848. doi: 10.1128/jvi.74.15.6838-6848.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sherman G, Bachenheimer S. Characterization of intranuclear capsids made by ts morphogenic mutants of HSV-1. Virology. 1988;163:471–480. doi: 10.1016/0042-6822(88)90288-7. [DOI] [PubMed] [Google Scholar]
- 60.Spencer J V, Trus B L, Booy F P, Steven A C, Newcomb W W, Brown J C. Structure of the herpes simplex virus capsid: peptide A862–H880 of the major capsid protein is displayed on the rim of the capsomer protrusions. Virology. 1997;228:229–235. doi: 10.1006/viro.1996.8392. [DOI] [PubMed] [Google Scholar]
- 61.Taus N S, Baines J D. Herpes simplex virus 1 DNA cleavage/packaging: the UL28 gene encodes a minor component of B capsids. Virology. 1998;252:443–449. doi: 10.1006/viro.1998.9475. [DOI] [PubMed] [Google Scholar]
- 62.Taus N S, Salmon B, Baines J D. The herpes simplex virus 1 UL 17 gene is required for localization of capsids and major and minor capsid proteins to intranuclear sites where viral DNA is cleaved and packaged. Virology. 1998;252:115–125. doi: 10.1006/viro.1998.9439. [DOI] [PubMed] [Google Scholar]
- 63.Tengelsen L A, Pederson N E, Shaver P R, Wathen M W, Homa F L. Herpes simplex virus type 1 DNA cleavage and encapsidation require the product of the UL28 gene: isolation and characterization of two UL28 deletion mutants. J Virol. 1993;67:3470–3480. doi: 10.1128/jvi.67.6.3470-3480.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trus B L, Booy F P, Newcomb W W, Brown J C, Homa F L, Thomsen D R, Steven A C. The herpes simplex virus procapsid: structure, conformational changes upon maturation, and roles of the triplex proteins VP19c and VP23 in assembly. J Mol Biol. 1996;263:447–462. doi: 10.1016/s0022-2836(96)80018-0. [DOI] [PubMed] [Google Scholar]
- 65.Trus B L, Newcomb W W, Booy F P, Brown J C, Steven A C. Distinct monoclonal antibodies separately label the hexons or the pentons of herpes simplex virus capsid. Proc Natl Acad Sci USA. 1992;89:11508–11512. doi: 10.1073/pnas.89.23.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Underwood M R, Harvey R J, Stanat S C, Hemphill M L, Miller T, Drach J C, Townsend L B, Biron K K. Inhibition of human cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is mediated through the UL89 gene product. J Virol. 1998;72:717–725. doi: 10.1128/jvi.72.1.717-725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weinheimer S P, McCann P J, O'Boyle D R, Stevens J T, Boyd B A, Drier D A, Yamanaka G A, DiIanni C L, Deckman I C, Cordingley M G. Autoproteolysis of herpes simplex virus type 1 protease releases an active catalytic domain found in intermediate capsid particles. J Virol. 1993;67:5813–5822. doi: 10.1128/jvi.67.10.5813-5822.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weller S K. Herpes simplex virus DNA replication and genome maturation. In: Cooper G M, Temin R, Sugden B, editors. Implications of the DNA provirus: Howard Temin's scientific legacy. Washington, D.C.: ASM Press; 1995. pp. 189–213. [Google Scholar]
- 69.Weller S K, Carmichael E P, Aschman D P, Goldstein D J, Schaffer P A. Genetic and phenotypic characterization of mutants in four essential genes that map to the left half of HSV-1 UL DNA. Virology. 1987;161:198–210. doi: 10.1016/0042-6822(87)90186-3. [DOI] [PubMed] [Google Scholar]
- 70.Yu D, Sheaffer A K, Tenney D J, Weller S K. Characterization of ICP6::lacZ insertion mutants of the UL15 gene of herpes simplex virus type 1 reveals the translation of two proteins. J Virol. 1997;71:2656–2665. doi: 10.1128/jvi.71.4.2656-2665.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu D, Weller S K. Genetic analysis of the UL 15 gene locus for the putative terminase of herpes simplex virus type 1. Virology. 1998;243:32–44. doi: 10.1006/viro.1998.9041. [DOI] [PubMed] [Google Scholar]
- 72.Yu D, Weller S K. Herpes simplex virus type 1 cleavage and packaging proteins UL15 and UL28 are associated with B but not C capsids during packaging. J Virol. 1998;72:7428–7439. doi: 10.1128/jvi.72.9.7428-7439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou Z H, Prasad B V, Jakana J, Rixon F J, Chiu W. Protein subunit structures in the herpes simplex virus A-capsid determined from 400 kV spot-scan electron cryomicroscopy. J Mol Biol. 1994;242:456–469. doi: 10.1006/jmbi.1994.1594. [DOI] [PubMed] [Google Scholar]