Abstract
Background
Bromadiolone is a wide-use long-acting anticoagulant rodenticide known to cause severe coagulation dysfunction. At present, there have been no detailed reports of acute kidney injury (AKI) resulting from bromadiolone poisoning.
Case presentation
A 27-year-old woman was admitted to the hospital due to severe coagulopathy and severe AKI. Coagulation test revealed a prothrombin time exceeding 120 s and an international normalized ratio (INR) greater than 10. Further examination for coagulation factors showed significantly reduced level of factors II, VII, IX and X, indicating a vitamin K deficiency. The AKI was non-oliguric and characterized by gross dysmorphic hematuria. Following the onset of the disease, the patient’s serum creatinine rose from 0.86 to 6.96 mg/dL. Suspecting anticoagulant rodenticide poisoning, plasma bromadiolone was identified at a concentration of 117 ng/mL via gas chromatography/mass spectrometry. All other potential causes of AKI were excluded, except for the presence of a horseshoe kidney. The patient’s kidney function fully recovered after the coagulopathy was corrected with high doses of vitamin K and plasma transfusion. At a follow-up 160 days post-discharge, the coagulation function had normalized, and the serum creatinine had returned to 0.51 mg/dL.
Conclusion
Bromadiolone can induce AKI through a severe and prolonged coagulation disorder. Kidney function can be restored within days following treatment with high-dose vitamin K1.
Keywords: Acute kidney injury, Coagulopathy, Anticoagulant rodenticide, Bromadiolone, Poisoned, Vitamin K antagonists
Background
Poison control centers across the United States receive about 2.3 million reports of human poisonings annually, of which rodenticides account for about 0.5% [1]. Rodenticides include long-acting “superwarfarin” anticoagulants, warfarin, and phosphide, with anticoagulant rodenticides being the most common. Bromadiolone, a second-generation biscoumarin rodenticide and a member of the “superwarfarin” class, is known for its high fat solubility, long half-life in vivo, and significantly stronger anticoagulant effect compared to warfarin [2]. Due to its chemical structure’s similarity to vitamin Kl, bromadiolone will competitively inhibit vitamin K1 when it enters the body, inhibit vitamin K1 epoxide reductase [2], affecting the synthesis of coagulation factors II, VII, IX and X in the liver. This disruption, in turn, impairs the production of thrombin and fibrin, leading to coagulation dysfunction and a prolonged coagulation time. Additionally, bromadiolone causes sustained damage to capillaries, increasing the permeability of blood vessel walls and heightening the risk of bleeding [3].
Clinically, the primary manifestations of bromadiolone-related poisoning are mainly bleeding complications in various organs and tissues related to coagulation dysfunction, such as gastrointestinal tract, urogenital tract and intracranial hemorrhage, with symptoms often beginning 24 to 48 h post-exposure [4]. Some patients may be complicated with rare symptoms such as acute toxic encephalopathy [5], spontaneous abortion [6], pericardial and mediastinal hemorrhages [7], hematoperitoneum [8]. Notably, 16% transient acute kidney injury (AKI) was record in 42 hospitalization due to brodifacoum (another superwarfarin) poisoning [9], but here have been no detailed information of AKI related to superwarfarin poisoning in humans. In this report, we present a case of severe AKI caused by bromadiolone poisoning and highlight the potential pathogenesis and rapid recovery of renal function following the correction of severe coagulation dysfunction.
Case presentation
A 27-year-old female patient was admitted with complaints of hematuria and black stools for over 11 days. Eleven days before admission, the patient reported multiple blood blisters in the right buccal mucos after eating out and subsequently developed gross hematuria with foamy urine later that evening. After seven days of persistent hematuria, she sought medical attention at a local hospital. Initial laboratory results indicated normal hemoglobin (126 g/L), an elevated platelet count (420 × 109/L), impaired renal function (creatinine: 0.86 mg/dL), hematuria (urine erythrocyte 3+) and proteinuria (urine protein 3+), and abnormal coagulation function, including a prothrombin time (PT) > 180.0 s, activated partial thromboplastin time (APTT) > 180 s,, international normalized ratio (INR) > 10 and hyperfibrinogenemia (7.65 g/L). Despite the administration of antibiotics and plasma transfusions, her condition deteriorated, evidenced by a notable reduction in hemoglobin levels (90 g/L) and exacerbated kidney dysfunction (serum creatinine 4.71 mg/dL). She was then transferred to our hospital. Further lab tests showed a decline in hemoglobin (51 g/L), elevated urea (25.55 mmol/L) and creatinine (6.95 mg/dL) levels, along with gross hematuria (urine red blood cells 3298/µl), leukocyturia, and abnormal coagulation parameters (Table 1). A chest and abdominal CT scan ruled out thoracic and abdominal hemorrhage. In the emergency room, the patient was treated with anti-infection measures (levofloxacin 0.4 g per day), acid suppression, hemostasis and blood transfusion before being transferred. to our department.
Table 1.
Laboratory examination
| Reference Range, This Hospital |
4 days before this admission | 2 days before this admission | Emergency room/ on this admission | 1 day after admission | 3 days after admission (discharge) | |
|---|---|---|---|---|---|---|
| Prothrombin time (s) | 8–14 | — | > 180.0 | 17.1 | 44.7 | 29.9 |
| International normalized ratio | 0.9–1.1 | — | > 10 | 1.50 | 4.05 | 2.68 |
| Activated partial thromboplastin time (s) | 25.0–31.3 | — | > 180 | 31.8 | 42.3 | 39.6 |
| Thrombin time (s) | 15–21 | — | 14.9 | 14.7 | 15.3 | 15.6 |
| Fibrinogen (g/L) | 2.0–4.0 | — | 7.65 | 6.04 | 6.61 | 4.09 |
| Antithrombin (%) | 80–120 | — | — | — | 100 | — |
| Hemoglobin(g/L) | 115–150 | 126 | 90 | 51→66 | 80 | 90 |
| Platelet count(*10^9/L) | 125–350 | 420 | 516 | 245 | 298 | 366 |
| Leukocyte(*10^9/L) | 3.5–9.5 | 16.03 | 24.43 | 12.87 | NA | 10.8 |
| Neutrophilspercentage | 40–75 | 81 | 85 | 82 | 93 | 72 |
| Lymphocytes percentage | 20–50 | 11 | 7 | 10 | 5 | 0.18 |
| Urea (mmol/L) | 2.6–7.5 | — | — | 26 | 14 | 10 |
| Creatinine (mg/dL) | 0.56–0.83 | 0.85 | 4.70 | 6.96 | 1.59 | 1.05 |
| Corrected calcium (mmol/L) | 2.10–2.54 | 1.75 | 1.98 | 2.33 | 2.32 | |
| Urine color | Clear to yellow | red | red | red | yellow | yellow |
| Urine erythrocyte (/ul) | 0–17 | 1860 | — | 3298 | — | 40 |
| Urinary white blood cell (/ul) | 0–28 | 20/HP | — | 1006 | — | 33 |
| Urine volume (ml) | 800–2500 | — | — | 4130 | 1700 | 2750 |
| Urinary protein | negative | 1+ | 2+ | +- | – | +- |
| Urine albumin (mg/L) | < 30 | — | — | 76.7 | — | — |
| Urine protein (mg/L) | < 100 | — | — | 1867 | — | — |
| Urinary albumin to creatinine ratio (mg/g) | 0–30 | — | — | 241 | — | — |
| Urinary protein to creatinine ratio (mg/g) | 0–150 | — | — | 5866 | — | — |
| Urinary N-acetyl-beta-D-glucosaminidase (U/L) | 0–10.6 | — | — | 4.7 | — | — |
| Urine α-1 microglobulin (mg/L) | 0–14 | — | — | 5.5 | — | — |
| Urine sediment | — | — | Gross dysmorphic RBC | — | Microscopic dysmorphic RBC |
RBC: red blood cell
Upon admission, the physical examination revealed normal body temperature, an elevated pulse rate, and normal blood pressure. The patient displayed ecchymosis on various body parts, including the chest wall, left elbow, left groin, right middle thigh, and left ankle. Obvious moist rales were heard in both lower lungs, while the heart and abdominal examination were normal. There was no lower limb edema. The patient’s medical history did not indicate any pre-existing hematological or coagulation disorders. Random urinary chemistry showed the fractional excretion of sodium of 5.8% (suggesting little possibility of pre-renal AKI), normal urinary N-acetyl-beta-D-glucosaminidase and urinary α-1 microglobulin concentration, and an obvious discrepancy between urinary protein to creatinine ratio (5866 mg/g) and urinary albumin to creatinine ratio (241 mg/g). Random urine sediment indicated gross dysmorphic red blood cells (Table 1). The kidney ultrasound indicated a horseshoe kidney without kidney stones or hydronephrosis.
Further examination of coagulation factors revealed vitamin K deficiency, with significantly reduced levels of factors II (26.9↓%), VII (16.9↓%), IX (28.2↓%), and X (26.2↓%). The levels of factor V (76.1%), factor XI (94.8%) were normal and factor VII (208.9↑%) was elevated. Given this patient’s severe coagulopathy, characterized by low activity of vitamin K1-dependent clotting factor, prolonged PT and INR, and normal liver function, we considered the possibility of exposure to a coumarin-based anticoagulant rodenticide. Therefore, a toxicological examination was performed on the first day of admission, which revealed a plasma bromadiolone concentration of 117 ng/ml by gas chromatography/mass spectrometry.
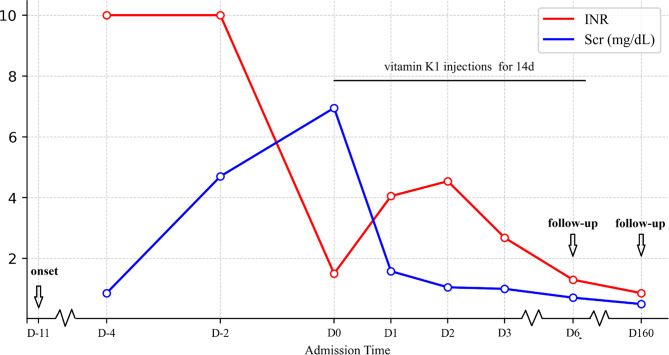
The patient received treatment, including vitamin K1 injections (two 10 mg dose intramuscularly before the confirmation of bromadiolone intoxication, and 20 mg twice daily intramuscularly after the confirmation), 1.5 units of red blood cell transfusion to correct anemia, and other symptomatic therapies. On the first day after admission, renal function tests indicated the improved levels of urea (14 mmol/L) and creatinine (1.59 mg/dL). Coagulation function remained abnormal, with prolonged PT and APTT, while the fibrinogen and platelet counts were within or above the normal range (Table 1). After three days of treatment, creatinine levels decreased further to 1.05 mg/dL (Fig. 1), INR dropped to 2.68, and urine color returned to normal. Due to bromadiolone’s long half-life, repeat measurement of its levels was not performed.
Fig. 1.
Relationship between INR and serum creatinine in this patient
During a telephone follow-up three days after discharge, the patient exhibited normal renal function (serum creatinine of 0.71 mg/dL), with coagulation function and blood routine returning to normal. Vitamin K treatment was discontinued under the recommendation of the local physician 11 days after discharge. Six months later, the patient was re-examined in the local hospital, where routine blood and coagulation function were within normal ranges, and renal function was stable (serum creatinine of 0.51 mg/dL). Urine test showed 1 + urine glucose, 1 + urine occult blood, 2 + urine leukocyte esterase. The patient is advised to continue close monitoring kidney function, urine examination, and kidney ultrasound during follow-ups. Consent for publication of this case was obtained and provided to the journal in accordance with BMC policy.
Discussion
Severe coagulation disorders can potentially result in the development of AKI. Anticoagulant-related nephropathy (ARN) is characterized by AKI when other causes are excluded and an INR above 3.0 following anticoagulant therapy. ARN typically presents with a distinct pattern of glomerular hemorrhage on kidney biopsy, most commonly associated with anticoagulants like warfarin and other vitamin K antagonists (such as fluindione and acenocoumarol), and direct oral anticoagulants. ARN is more likely to occur in individuals with preexisting glomerular disease, with renal biopsies often revealing IgA nephropathy as the most common type [10]. Overanticoagulation can lead to the disruption of the glomerular filtration barrier and diffuse glomerular hemorrhage, which manifests on renal biopsy as numerous renal tubules and Bowman space are filled with red cells and red cell casts, causing obstruction, ischemia, and eventual obliteration [11, 12]. In most ARN cases, the severity of renal failure, red cell tubular casting, and hematuria is disproportionately severe when compared to the observed changes in glomerular morphology [10]. Prior research suggests that anticoagulation may exacerbate glomerular disease rather than directly affecting the glomerular filtration barrier [10].
We consider that there exists a causal relationship between coagulation dysfunction due to bromadiolone intoxication and AKI in this patient. The supporting evidence is as follows: Firstly, previous reports on ARN indicate that any agent or condition causing severe coagulopathy may lead to ARN [10], thus laying a theoretical foundation for our hypothesis. Secondly, the patient’s serum creatinine level increased over several days following an abnormal rise in INR and rapidly improved after correcting coagulation dysfunction, suggesting that AKI was induced by coagulation dysfunction. Thirdly, we have ruled out other potential causes of AKI, encompassing hypotension, obstructive nephropathy, intrinsic renal factors, lupus nephropathy, ANCA-associated vasculitis, anti-GBM nephropathy, and so on. Fourthly, the patient’s urine examination results indicated pleomorphic gross hematuria, suggesting the leakage of red blood cells through the glomerular basement membrane, which aligns with the pathological characteristics of anticoagulant-related nephropathy.
In this case, the recovery of kidney function surpassed what has been reported in cases of ARN. Previously documented cases of classic ARN have often demonstrated limited or negligible recovery of kidney function (Table 2). This discrepancy in outcomes might be attributed to the following two factors: First, a significant number of patients may have had pre-existing kidney diseases or decreased glomerular filtration rate (GFR) prior to the onset of ARN. These underlying renal disorders may render the kidney more susceptible to ARN and restrict the kidney’s inherent capacity for self-repair. The cornerstone of treatment for ARN is ensuring an adequate urine flow to expel the obstructive elements, such as red blood cells and damaged tubules. Most patients with ARN may have no enough urine flow from decreased GFR or additional fluid limited by heart function, which potentially contributing to the unfavorable prognosis. In contrast, this patient had adequate urine output and received unlimited fluid therapy that contributing rapid recovery of kidney function. Secondly, many patients requiring long-term anticoagulant therapy often have cardiovascular conditions such as atrial fibrillation, necessitating the use of medications like warfarin to prevent thrombosis. These drugs demand regular monitoring of clotting function to ensure that the INR falls within the therapeutic range. For example, the utilization of warfarin for anticoagulation in patients with atrial fibrillation mandates precise control of INR within the range of 2.0 to 3.0. Maintaining INR within this range may not completely arrest kidney bleeding and can consequently impede the recovery of renal function. In contrast, the rapid decrease of INR from above 10 to the normal range may effectively correct coagulation disorder and halter renal bleeding in this case.
Table 2.
Main results of renal recovery in patients with classic anticoagulant-related nephropathy
| Year | Author | Age/Sex | Anticoagulant | Baseline SCr (mg/dL) | Peak SCr (mg/dL) | Peak INR | SCr at discharge/Follow-up (mg/dL) | Follow up time |
|---|---|---|---|---|---|---|---|---|
| 2017 | Góis [13] | 84/M | acenocoumarol | 1.0 | 4.68 | 6.96 | 3.45/1.7 | 11 m |
| 2018 | Golla [14] | 50/F | acenocoumarol | 0.9 | 7.6 | 4.70 | 1.1/NA | 2 w |
| 2020 | Chamberlain [15] | 85/F | warfarin | 2.26 | 5.20 | 3.0 | NA/3.39 | 1 m |
| 2020 | Tennekoon [16] | 61/M | warfarin | 2.38 | 6.45 | 3.5 | 3.39/NA | 4 m |
| 2021 | Nonaka [17] | 73/M | warfarin | 1.1 | 9.0 | 3.9 | NA/1.6 | 1 y |
| 2022 | Bhandari [18] | 64/M | warfarin | NA | 8.6 | 6.36 | NA/5.49 | 3 m |
| 2023 | Brittanee [19] | 78/M | warfarin | 0.96 | 12.88 | 6.9 | 0.9/NA | NA |
| 2023 | This study | 27/F | bromadiolone | 0.51 | 6.95 | > 10 | 0.9/0.51 | 160d |
Note: the literatures without serum creatinine value at discharge or during follow-up were deleted; NA: not available
In cases of bromadiolone or other superwarfarin poisoning, the primary treatment strategy involves administering high doses of vitamin K1 and transfusion [4]. The recommended initial dose of vitamin K1 typically ranges from 10 to 50 mg, administered intravenously, depending on the severity of the coagulopathy. In some cases, higher doses, up to 100 mg, may be required [4, 20]. Treatment often needs to be continued for an extended period, with twice-daily dosing, due to the short half-life of vitamin K1. Pervious research showed elimination of bromadiolone followed a two-compartment model, with an estimated half-life of 3.5 day from ingestion to day 8, and estimated half-life of 24 days from day 8 to day 107 [21]. Therefore, this patient may require a high-dose of vitamin K1 for 14 days and could discontinue therapy thereafter. Regular monitoring of coagulation parameters, such as PT and INR, is essential to adjust the dosage and duration of vitamin K1 therapy.
To our acknowledge, there have been no detailed reports of AKI in adult patients caused by bromadiolone poisoning at present. This case firstly presents a rare instance of bromadiolone-induced AKI accompanied by pleomorphic gross hematuria. The kidney function could restore within several days after treatment with large dose of vitamin K1. This clinical case could extend clinicians’ current knowledge of AKI caused by ingestion of bromadiolone. Our findings suggest that AKI can be caused by severe coagulopathy, and that correcting severe coagulopathy may help treat AKI. However, further research is needed to confirm this relationship.
There are several limitations of this case report. Firstly, a kidney biopsy was not conducted to confirm ARN in the patient due to the coagulation disorder and the presence of a horseshoe kidney [22]. Typically, renal biopsy is not initially recommended in patients receiving anticoagulant therapy due to the heightened risk of bleeding. Secondly, our observations lacked similar cases for comparative analysis, emphasizing the necessity for further validation of our findings.
Conclusion
This case suggested that bromadiolone can cause AKI due to a prolonged and severe coagulation disorder. Unlike other cases of anticoagulant-related nephropathy, kidney function may recover within days after treatment with a large dose of vitamin K1. The findings are crucial for clinicians managing coagulopathy in critical care, as recognizing the potential link between severe coagulopathy and AKI could lead to earlier identification, monitoring, and intervention, thereby improving patient outcomes. Routine renal function monitoring in cases of severe coagulopathy, along with early correction of coagulation abnormalities, should be integral in managing patients at risk for AKI.
Acknowledgements
We want to thank the involving nurses for their help.
Abbreviations
- AKI
Acute kidney injury
- INR
International normalized ratio
- PT
Prothrombin time
- APTT
Activated partial thromboplastin time
- NA
Not Available
- ARN
Anticoagulant-related nephropathy
- GFR
Glomerular filtration rate
Author contributions
Material preparation, data collection, and literature search were performed by WH. WB analyzed and interpreted the patient’s data. The first draft of the manuscript was written by WH and all authors commented on previous versions of the manuscript. WB provided diagnoses and treatments for the patient in this case. XC and MH supervised the manuscript drafting. All authors read and approved the final manuscript.
Funding
This report was supported by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions (CN), General Project of the National Natural Science Foundation of China (81970639, 82151320), Jiangsu Provincial Medical Key Discipline (Laboratory) Cultivation Unit (JSDW202206). The funders had no role in the design of the study or in the collection analysis or interpretation of the data.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University. The ethic committee waived the need for informed consent for this case report and the data were analyzed anonymously.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gummin DD, Mowry JB, Beuhler MC, Spyker DA, Brooks DE, Dibert KW, Rivers LJ, Pham NPT, Ryan ML. 2019 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 37th Annual Report. Clin Toxicol (Phila) 2020;58(12):1360–1541. [DOI] [PubMed]
- 2.Sharma P, Bentley P. Of rats and men: superwarfarin toxicity. Lancet. 2005;365(9459):552–3. 10.1016/S0140-6736(05)70773-X [DOI] [PubMed] [Google Scholar]
- 3.Park BK, Leck JB. A comparison of vitamin K antagonism by warfarin, difenacoum and brodifacoum in the rabbit. Biochem Pharmacol. 1982;31(22):3635–9. 10.1016/0006-2952(82)90587-1 [DOI] [PubMed] [Google Scholar]
- 4.King N, Tran MH. Long-acting anticoagulant rodenticide (superwarfarin) poisoning: a review of its historical development, epidemiology, and Clinical Management. Transfus Med Rev. 2015;29(4):250–8. 10.1016/j.tmrv.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 5.Li Q, Yu W, Qu Y, Wang JQ, Mao N, Kang H. Acute toxic encephalopathy following bromadiolone intoxication: a case report. BMC Neurol. 2021;21(1):8. 10.1186/s12883-020-02034-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipton RA, Klass EM. Human ingestion of a ‘superwarfarin’ rodenticide resulting in a prolonged anticoagulant effect. JAMA. 1984;252(21):3004–5. 10.1001/jama.1984.03350210052030 [DOI] [PubMed] [Google Scholar]
- 7.Kruse JA, Carlson RW. Fatal rodenticide poisoning with brodifacoum. Ann Emerg Med. 1992;21(3):331–6. 10.1016/S0196-0644(05)80900-X [DOI] [PubMed] [Google Scholar]
- 8.Cox P, Ofili-Yebovi D. Managing life-threatening haematoperitoneum in a non-pregnant patient secondary to bromadiolone self-poisoning. Clin Med (Lond). 2017;17(Suppl 3):s3. 10.7861/clinmedicine.17-3-s3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelkar AH, Smith NA, Martial A, Moole H, Tarantino MD, Roberts JC. An outbreak of Synthetic Cannabinoid-Associated Coagulopathy in Illinois. N Engl J Med. 2018;379(13):1216–23. 10.1056/NEJMoa1807652 [DOI] [PubMed] [Google Scholar]
- 10.Brodsky SV, Satoskar A, Hemminger J, Rovin B, Hebert L, Ryan MS, Nadasdy T. Anticoagulant-related nephropathy in kidney biopsy: a single-Center Report of 41 cases. Kidney Med. 2019;1(2):51–6. 10.1016/j.xkme.2019.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodsky SV, Satoskar A, Chen J, Nadasdy G, Eagen JW, Hamirani M, Hebert L, Calomeni E, Nadasdy T. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. Am J Kidney Diseases: Official J Natl Kidney Foundation. 2009;54(6):1121–6. 10.1053/j.ajkd.2009.04.024 [DOI] [PubMed] [Google Scholar]
- 12.Brodsky S, Eikelboom J, Hebert LA. Anticoagulant-related nephropathy. J Am Soc Nephrol. 2018;29(12):2787–93. 10.1681/ASN.2018070741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Góis M, Azevedo A, Carvalho F, Nolasco F. Anticoagulant-related nephropathy in a patient with IgA nephropathy. BMJ Case Rep 2017, 2017. [DOI] [PMC free article] [PubMed]
- 14.Golla A, Goli R, Nagalla VK, Kiran BV, Raju DSB, Uppin MS. Warfarin-related Nephropathy. Indian J Nephrol. 2018;28(5):378–81. 10.4103/ijn.IJN_3_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamberlain AJ, Carasco K, Skene A, Lancefield T, Mount PF. Anticoagulant-related nephropathy: a common complication hiding in plain sight. Intern Med J. 2020;50(10):1295–6. 10.1111/imj.15030 [DOI] [PubMed] [Google Scholar]
- 16.Tennekoon HD, Kousios A, Gardiner R, Moran L, Goodall D, Galliford J, Taube D, Roufosse C. Anticoagulant-related nephropathy in a renal transplant recipient. Kidney Int Rep. 2020;5(11):2089–96. 10.1016/j.ekir.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nonaka E, Takashima T, Matsumoto K, Fukuda M, Rikitake S, Miyazono M. Warfarin-related nephropathy: a case report of a delayed renal function improvement. Clin Case Rep. 2021;9(5):e04105. 10.1002/ccr3.4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhandari G, Tiwari V, Gupta A, Gupta P, Bhargava V, Malik M, Gupta A, Bhalla AK, Rana DS. Double whammy: anticoagulant-related nephropathy with leukocytoclastic vasculitis due to warfarin. CEN Case Rep. 2022;11(1):154–8. 10.1007/s13730-021-00642-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brittanee S, Matthew D, Hannah S, Gregory G. Warfarin related kidney damage: a confusing case of Thrombophlebitis Masquerading as infection. J Prim Care Community Health. 2023;14:21501319231159978. 10.1177/21501319231159978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guodong L, Jieyi W, Xiaobo P, Xiaoxia L, Zhongying L, Zhiguo P, Zewu Q, Jianguang D. Retrospective analysis of clinical characteristics of and treatment strategies for patients with long-acting anticoagulant rodenticide poisoning. Basic Clin Pharmacol Toxicol. 2022;131(1):74–82. 10.1111/bcpt.13734 [DOI] [PubMed] [Google Scholar]
- 21.Lo VM, Ching CK, Chan AY, Mak TW. Bromadiolone toxicokinetics: diagnosis and treatment implications. Clin Toxicol (Phila). 2008;46(8):703–10. 10.1080/15563650701504366 [DOI] [PubMed] [Google Scholar]
- 22.Luciano RL, Moeckel GW. Update on the native kidney biopsy: core curriculum 2019. Am J Kidney Dis. 2019;73(3):404–15. 10.1053/j.ajkd.2018.10.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.