Abstract
Glycoprotein B (gB), gC, gD, and gH:L of herpes simplex virus type 1 (HSV-1) are implicated in virus adsorption and penetration. gB, gD, and gH:L are essential for these processes, and their expression is necessary and sufficient to induce cell fusion. The current view is that these molecules act in concert as a functional complex, and cross-linking studies support this view (C. G. Handler, R. J. Eisenberg, and G. H. Cohen, J. Virol. 70:6067–6075, 1996). We examined the glycoprotein composition, with respect to gB, gC, gD, and gH, of mutant virions lacking individual glycoproteins and the sedimentation characteristics of glycoproteins extracted from these virions. The amounts of gB, gC, gD, or gH detected in virions did not alter when any one of these molecules was absent, and it therefore appears that they are incorporated into the virion independently of each other. The sedimentation characteristics of gB and gD from mutant virions were not different from those of wild-type virions. We confirmed that gB, gC, and gD could be cross-linked to each other on the virion surface but found that the absence of one glycoprotein did not alter the outcome of cross-linking reactions between the remaining molecules. The incorporation and arrangement of these glycoproteins in the virion envelope therefore appear to be independent of the individual molecular species. This is difficult to reconcile with the concept that gB, gC, gD, and gH:L are incorporated as a functional complex into the virion envelope.
The envelopes of all herpesviruses contain multiple integral membrane proteins. Alphaherpesvirus particles contain more than 10 transmembrane glycoproteins, but our knowledge of the organization of these molecules in the virus envelope and of the interactions between them is superficial. Several of these molecules, gC, gB, and gD, are known to be present as multimers (10, 17, 24, 31), and interactions between gH and gL (20), between gE and gI (21), and between gM and gN (22) have been demonstrated, but there is only limited evidence for higher-order interactions and organization.
There are, nevertheless, strong reasons for supposing that interactions between these molecules must occur. First, it is difficult to imagine a mechanism for the incorporation of transmembrane proteins into the virion envelope, in appropriate amounts, in the absence of interactions between these molecules or between them and the underlying tegument proteins. The site at which herpesviruses acquire their final envelope is uncertain, though the weight of evidence favors the Golgi or a Golgi-derived vesicle (13, 35), but regardless of the site it seems intuitively unlikely that the multiple virion envelope proteins accumulate independently at that compartment. Indeed, this possibility appears to be excluded by the fact that different virion envelope proteins exhibit different trafficking characteristics when expressed alone (1). Interaction between different virion glycoproteins therefore appears to be a prerequisite for assembly of the mature enveloped particle.
The function of the virion membrane proteins also implies complex interactions. Glycoproteins B, D, and H:L (gB, gD, and gH:L) are absolutely required for herpes simplex virus infectivity (9, 15, 25, 30), and this combination of proteins is necessary and sufficient to induce cell-cell fusion in a transient-transfection assay (33). It seems very unlikely that these molecules function independently, and this view is supported by the observation that they cannot cooperate in trans; all three molecules must be expressed on the same membrane (11). In the context of the virion these molecules could be organized into a functional complex via interactions with tegument proteins. However, since these three molecules alone are sufficient to induce cell-cell fusion, they can function independently of tegument proteins and do not require tegument components in order to form a functional unit.
Direct evidence for the existence of high-order complexes formed by the membrane proteins of herpes simplex virus type 1 (HSV-1) comes from chemical cross-linking studies. Zhu and Courtney (36), using cross-linking reagents capable of penetrating the virion membrane, observed the formation of very high molecular weight complexes which included virus-specific glycoproteins and major tegument proteins. Handler et al. (17), using nonpermeabilizing reagents, observed complexes which, on the basis of serological characteristics, contained gC, gB, gD, and gH:L, and since these molecules are all implicated in binding and entry of the virion, these authors argued that they interacted to form a functional complex. This argument was strengthened by the observation that the cross-linking characteristics were altered during virus entry into the cell, implying a conformational change in the complex during the process of membrane fusion (18). While this is a satisfying conclusion, it does conflict with immunoelectron microscopic observations which suggest that gC, gB, and gD form discrete morphological structures (32). Furthermore, since gC is dispensable for virion infectivity and for membrane fusion, whereas gB, gD, and gH:L are not, the interpretation of Handler et al. (17, 18) implies that the absence of gC from the complex does not affect its functional integrity.
Because of these inconsistencies we decided to reexamine the issue of complex formation between HSV-1 glycoproteins involved in virus entry. We reasoned that if these molecules interact to form a functional unit, then the composition and organization of the unit would be influenced by the absence of individual components. We therefore prepared virions lacking individual glycoproteins and examined these virions with respect to their glycoprotein composition, the sedimentation behavior of detergent-released glycoproteins, and the cross-linking behavior of the glycoprotein molecules.
MATERIALS AND METHODS
Cells.
BHK cells were grown in Glasgow modified Eagle's medium supplemented with 10% newborn bovine serum and 10% tryptose phosphate broth. Vero cells, VD60 cells, CR1 cells, and D6 cells were grown in Glasgow modified Eagle's medium supplemented with 10% fetal bovine serum. VD60 cells, CR1 cells, and D6 cells are derived from Vero cells and are helper cell lines which supply HSV-1 gD, gH, and gB, respectively (4, 9, 25).
Viruses.
HSV-1 strain HFEM was used throughout. HFEM mutants lacking gB or gC have been described previously and are named HFEMΔUL27-Z and HFEMΔUL44-Z, respectively (3, 16). Mutants in which gD or gH coding sequences are replaced with a lacZ expression cassette were constructed using the methods described by Davis-Poynter et al. (11) and Browne et al. (7) and were named HFEMdelUS6-Z and HFEMdelUL22-Z, respectively. HSV-1 strains HFEM and HFEMΔUL44-Z were propagated in BHK cells. The gD-, gH-, and gB-negative mutants were grown in VD60 cells, CR1 cells, and D6 cells, respectively. All stocks were prepared using a multiplicity of infection (MOI) of 0.01.
Wild-type or gC-negative virions were prepared by infection of BHK cells at an MOI of 0.1, and the medium was collected after 48 h. In order to obtain virions lacking gB, gD, or gH, the relevant mutant was used to infect BHK cells at an MOI of 5, and after adsorption for 1 h the inoculum was removed and residual inoculum was inactivated by washing the cells with 40 mM citrate–135 mM NaCl–10 mM KCl, pH 3.0. Fresh medium was added, the cells were incubated, and the medium was harvested after 24 h.
Virus purification.
Tissue culture medium from infected cells was clarified by centrifugation at 2,000 × g for 10 min, and virus particles were then pelleted from the supernatant by centrifugation at 18,000 rpm for 2 h in a Beckman type 19 rotor at 4°C. The pellets were resuspended in a small volume of phosphate-buffered saline (PBS) and sonicated before being layered on 30-ml 15-to-30% Ficoll gradients in PBS. The gradients were centrifuged at 12,500 rpm for 90 min in a Beckman SW28 rotor at 4°C, and the visible band at the center of the gradient was harvested, diluted with PBS, and pelleted by centrifugation at 21,000 rpm in an SW28 rotor. The final pellet was resuspended in PBS, and the protein concentration was determined. Aliquots were then stored at −70°C at 1 to 2 mg/ml. Virus particle numbers were estimated by comparison with latex particles of known concentrations using negatively stained preparations as described by Watson et al. (34). All purified preparations contained at least 3 × 1011 enveloped virions per mg of protein.
Antibodies.
HSV-1 antigens were detected by immunoblotting or by immune precipitation using the following antibodies. gB was precipitated using monoclonal antibodies B/2153 and B/2182, both gifts from Anne Cross, Institute of Virology, Glasgow, United Kingdom. gC was precipitated with monoclonal antibody C/1001, also a gift from Anne Cross. gD was precipitated with monoclonal antibodies LP14 and LP2 (27), and gH was precipitated with monoclonal antibody LP11 (8). gB and gC were detected by immunoblotting using rabbit sera R69 and R47, respectively, both gifts from G. Cohen and R. Eisenberg, University of Pennsylvania, Philadelphia. gD and gH were detected by immunoblotting using monoclonal antibody LP14 or rabbit antiserum against a gH fusion protein expressed in Escherichia coli (12), respectively. The major tegument protein, VP16, was detected by immunoblotting using monoclonal antibody LP1 (26). Immunoprecipitation, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and immunoblotting were performed as described previously (7).
Cross-linking.
Bifunctional cross-linking reagents were obtained from Pierce and Warriner (Chester, United Kingdom). Sulfo-DST (disulfosuccinimydyl tartrate), DTSSP (3,3′-dithiobis[sulfosuccinimydal propionate]), and sulfo-EGS (ethylene glycol bis[sulfosuccinimydalsuccinate]) have spacer arm lengths of 6.4, 12, and 16 Å, respectively, and are hydrophilic reagents which do not permeate membranes. DSP (dithiobis[succinimidyl propionate]) is a 12-Å cross-linker which permeates the membrane. Preparations of purified virions at 1 mg/ml in PBS were treated with the appropriate reagent at a range of concentrations from 0.5 to 5 mM for 15 min on ice. The reaction was quenched by the addition of Tris-glycine, pH 7.5, to a final concentration of 50 mM. Control reactions were performed in the absence of cross-linker or were “prequenched” with Tris glycine before the addition of cross-linker. The reaction products were then subjected to SDS electrophoresis or were solubilized in 0.1% Triton X-100–0.1% sodium deoxycholate–0.01% SDS–15 mM NaCl–10 mM Tris-Cl, pH 7.5, for 30 min on ice prior to immune precipitation.
Sedimentation analysis.
Virions were pelleted from tissue culture supernatants, and samples containing approximately 1010 virions in 400 μl of PBS were made 1% with respect to octylglucoside or digitonin and kept on ice for 30 min. The sample was then loaded onto a 15-ml 5-to-20% sucrose gradient in 10 mM triethanolamine–100 mM NaCl, pH 7.5, containing the detergent used to lyse the virus particles. Bovine serum albumin, alcohol dehydrogenase, and apoferritin were included in the sample as internal molecular weight markers with weights of 65,000 (65K), 150K, and 440K, respectively. Gradients were centrifuged at 35,000 rpm for 15.5 h in a Beckman SW40 Ti rotor at 6°C. The gradient was then harvested into 0.5-ml fractions, and each fraction was subjected to SDS electrophoresis and immunoblotting to locate the position of viral proteins in the gradient. Internal molecular weight markers were detected by staining with Coomassie blue.
RESULTS
Sedimentation analysis.
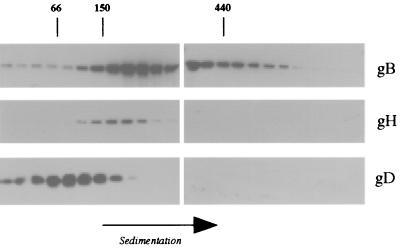
Wild-type virions were disrupted in digitonin or octylglucoside and subjected to sucrose density gradient centrifugation in the same detergents. Figure 1 shows the distribution of gB, gD, and gH:L in the gradient fractions after disruption of the virions in octylglucoside. gB is broadly distributed, with the majority sedimenting between the 150K marker and the 440K marker but a significant proportion sedimenting more rapidly. In contrast, the gH:L complex sediments as a discrete species with an Mr of about 170,000, consistent with the size of a gH:L heterodimer. The majority of gD sediments between the 66K (albumin) and 150K markers. Similar results were obtained when digitonin was used to disrupt the virions except that, in repeated experiments, gD appeared to sediment more rapidly in digitonin gradients, with the peak fractions coinciding with the 150K marker.
FIG. 1.
Sedimentation of detergent-released gB, gD, and gH. Wild-type virions were dissociated in octylglucoside, and the products were sedimented through a 5-to-20% sucrose gradient. The gradient was harvested into 24 fractions, and samples from each fraction were subjected to SDS-PAGE. gB, gD, and gH were detected by immunoblotting, and molecular weight markers included in the gradient were detected by staining.
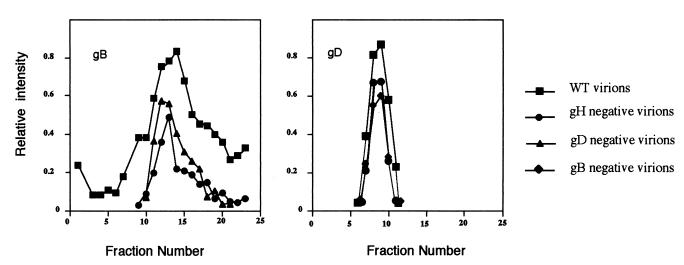
These results are broadly consistent with previous observations—namely, that gD and gB form dimers but higher-molecular-weight forms of gB are also observed (10, 17, 31). Data on gH:L are limited to studies of soluble secreted forms of the protein, which behave as a single heterodimer (29). The sedimentation profiles shown in Fig. 1 provide no evidence for interactions between gB, gD, and gH:L but do not exclude the possibility. We therefore repeated these experiments using preparations of virions lacking gB, gD, or gH to find whether the absence of individual glycoprotein species modified the sedimentation behavior of the remaining glycoproteins. Figure 2 shows the sedimentation of gB from wild-type, gD-negative, and gH-negative virions and the sedimentation of gD from wild-type, gB-negative, and gH-negative virions. It is apparent that the sedimentation of gD is entirely unaffected by the absence of gB or gH. The sedimentation of gB is broadly similar in wild-type, gH-negative, and gD-negative virions. There is some evidence of a reduction in the amounts of higher-molecular-weight gB species in gH-negative and gD-negative virions, though this is of doubtful significance given the semiquantitative nature of the data at low signal strengths.
FIG. 2.
Sedimentation of glycoproteins from mutant virions. Wild-type virions or virions lacking gB, gD, or gH were dissociated with digitonin, and gB and gD were sedimented and detected by immunoblotting as described in the legend to Fig. 1. The amount of bound secondary antibody was estimated directly by chemiluminescent imaging. Sedimentation is from left to right.
Glycoprotein composition of viruses.
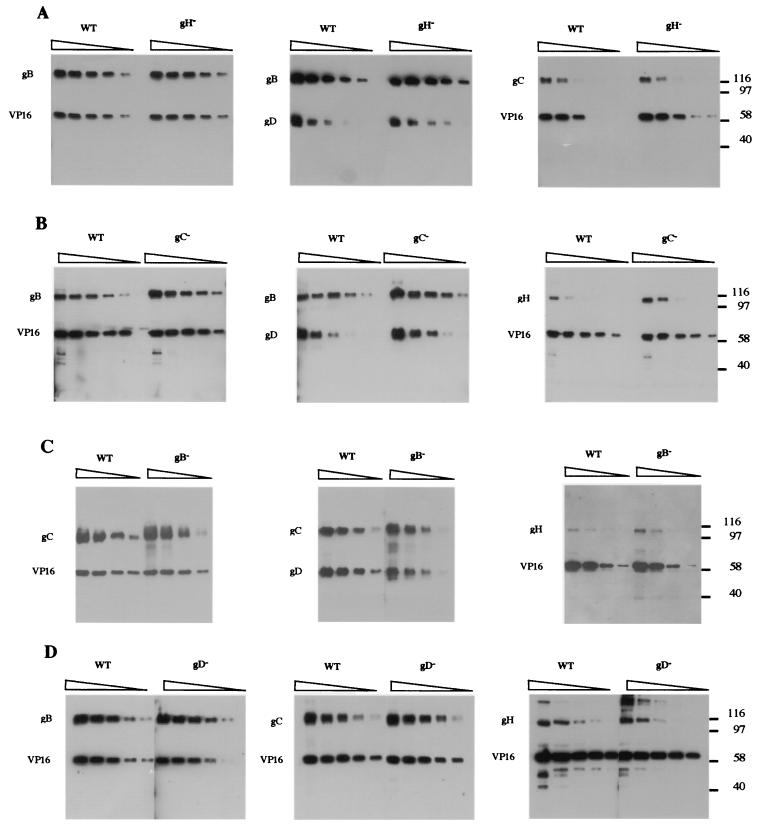
Given the complexity of the glycoprotein composition of HSV virions, it seems unlikely that the different glycoproteins are assembled independently into the virion envelope. If the glycoproteins involved in attachment and entry assemble into a functional complex, then it is reasonable to suppose that the formation of this complex precedes envelopment, and this view is supported by the fact that gB, gD, and gH:L mediate cell-cell fusion in the absence of other virion components. It follows that the absence of one of these proteins might prevent the formation of the complex and affect the incorporation of other components of the complex into the virion. We therefore examined the glycoprotein composition of virions lacking either gB, gC, gD, or gH:L. Equal numbers of wild-type and mutant virions were denatured in SDS under reducing conditions, and twofold serial dilutions were subjected to SDS-PAGE in parallel. The electrophoresis products were then subjected to immunoblotting to detect gB, gC, gD, or gH. The tegument protein, VP16, was simultaneously detected to provide an internal control for equal loading of wild-type and mutant virions. This control was not included when gD was detected because of the similar migration rates of gD and VP16. Representative immunoblots are shown in Fig. 3. Given the combined errors of particle counting, transfer to nitrocellulose, and antigen detection, these results must be interpreted with caution, but it is apparent that the composition of virions with respect to gB, gC, gD, and gH is largely unaffected by the absence of any one of these glycoprotein species. We were unable to detect any gross difference between mutant and wild-type virions, and we conclude that these proteins must be incorporated independently of each other into the virion envelope.
FIG. 3.
Glycoprotein composition of mutant virions. Serial twofold dilutions of wild-type virions or virions lacking gB, gC, gD, or gH were subjected to SDS-PAGE, the electrophoresis products were transferred to nitrocellulose, and the blots were probed for virion glycoproteins. Mutant and wild-type virions were compared on individual gels, and in each instance the undiluted sample (left lane) contained approximately 5 μg of protein. VP16 was simultaneously detected as an internal control for virion load. Where gD was detected, gB or gC was simultaneously detected as a loading control. Thus, in series A, the left panel shows that the VP16 loads for wild-type and gH-negative virions are similar and that the gB content is also similar. In the center panel, which compares the gD content, gB is therefore used as an internal control for loading. Series A, comparison of wild-type and gH-negative virions; series B, comparison of wild-type and gC-negative virions; series C, comparison of wild-type and gB-negative virions; series D, comparison of wild-type and gD-negative virions.
Cross-linking studies.
A number of studies using bifunctional cross-linking reagents have established that the luminal domains of HSV-1 envelope glycoproteins can be cross-linked to tegument proteins and that different glycoprotein species can be cross-linked to each other via their external domains. Handler et al. (17) found that intermolecular cross-links could be established between gC, gB, gD, and gH using a 12-Å cross-linker that was incapable of penetrating the envelope, and they interpreted their data as indicating that these molecules formed a functional complex that is required for virion attachment and penetration. If this is so, then it is reasonable to suppose that the formation and structure of such a complex would be dependent on the presence of each of its components. In the absence of one component we would therefore expect the cross-linking pattern of the remaining components to be altered. We therefore examined the cross-linking characteristics of glycoproteins in wild-type virions and mutant virions lacking individual glycoproteins. In a preliminary series of experiments wild-type virions were treated with non-membrane-permeative cross-linkers 6.4, 12, and 16 Å in length, and the reaction products were subjected to SDS-PAGE and immunoblotting.
The 12-Å reagent (DTSSP) gave the greatest yield of cross-linked products: the amounts of monomeric gD, gB, and gC were greatly reduced, and these proteins were detected as a heterogeneous population of molecules migrating more slowly than a 205K marker in 7% gels (data not shown). We observed no forms of gH other than the monomer and the gH:L heterodimer, but this assay detects gH with poor efficiency compared to the other glycoproteins, and our failure to find higher-molecular-weight forms could be due merely to a lack of sensitivity. The observed cross-linking of gB, gC, and gD into high-molecular-weight species was due to reactions external to the envelope because the electrophoretic migration of the tegument protein VP16 was entirely unaffected by these cross-linking reactions. In contrast, similar reactions using a membrane-permeative 12-Å cross-linker (DSP) yielded very high molecular weight forms of VP16 that barely entered a 7% acrylamide gel.
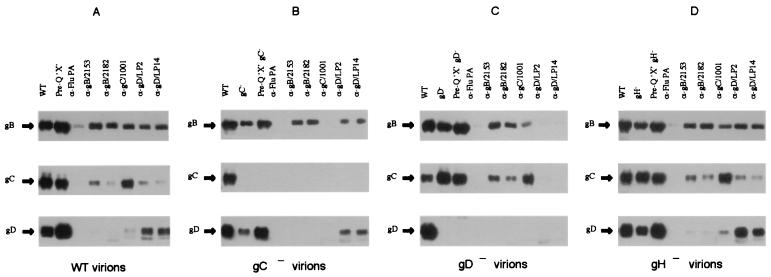
Cross-linking reactions using DTSSP were repeated with preparations of virions lacking gC, gD, or gH. The results were very similar to those obtained with wild-type virions, but because of the high molecular weight and heterogeneity of the cross-linked species these results were difficult to interpret with confidence. We therefore attempted to determine the approximate composition of the cross-linked species by subjecting virions to cross-linking with DTSSP, dissociating the virions with detergent, and immunoprecipitating the cross-linked complexes with monoclonal antibodies. The content of the immunoprecipitates was then analyzed by SDS-PAGE under reducing conditions followed by immunoblotting (DTSSP contains a disulfide group, and cross-linking can therefore be reversed by thiol reducing agents). The results of such an experiment using wild-type virions are shown in Fig. 4A and, in agreement with results reported by Handler et al. (17), provide solid evidence for the existence of complexes composed of different glycoproteins. Thus, monoclonal antibodies against gD or gC precipitate complexes containing gB, and antibodies against gD or gB precipitate complexes containing gC. The specificity of the immunoprecipitation reactions is demonstrated in the subsequent panels. Thus, anti-gC and anti-gD antibodies precipitate no detectable products from gC-negative (Fig. 4B) and gD-negative (Fig. 4C) virions, respectively. Although these data demonstrate the immune precipitation of multicomponent complexes, the results are not reciprocal. Figure 4A shows that two anti-gD antibodies efficiently precipitated complexes containing gB but neither of the gB antibodies precipitated gD. This is somewhat unsatisfactory but might be due to inaccessibility of the relevant gB epitopes in cross-linked molecules. The purpose of the experiments shown in Fig. 4 was to obtain evidence that the absence of one glycoprotein would modify the organization of others such that the pattern of cross-linking would change. We obtained no such evidence. Figure 4D shows the results of cross-linking and immune precipitation experiments using gH-negative virions. The results are identical to those obtained with wild-type virions (Fig. 4A). Similarly the absence of gC (Fig. 4B) has no effect on cross-linking reactions between gB and gD, and the absence of gD (Fig. 4C) has no effect on reactions between gB and gC. If we interpret the products of these cross-linking reactions as evidence for the existence of a specific functional complex, then it appears that when one component of the complex is absent the organization of the remaining components is unaltered.
FIG. 4.
Cross-linked complexes from wild-type and mutant virions. Wild-type virions or mutant virions lacking gC, gD, or gH were treated with 2.5 mM DTSSP, a 12-Å cross-linking reagent. The membrane proteins were released with detergent and immune precipitated with antibodies to gB, gC, or gD. Immune precipitates were heated in dithiothreitol to break cross-links, and the products were subjected to SDS-PAGE. gB, gC, and gD were then detected by immunoblotting. WT, cross-linked wild-type virions; Pre-Q, virions from reactions that were prequenched with Tris-glycine prior to addition of the cross-linking reagent. These lanes are included to indicate the amount of each glycoprotein species available for immune precipitation. Subsequent lanes show the products of immune precipitates formed by the antibodies indicated above each lane. Anti-Flu PA (a gift from Paul Digard, University of Cambridge) is an antibody raised against a subunit of the influenza virus polymerase, which was used as a negative control. Series A, wild-type virions; series B, gC-negative virions; series C, gD-negative virions; series D, gH-negative virions.
DISCUSSION
gB, gD, and the gH:L complex are essential for virion infectivity and are necessary and sufficient to induce cell-cell fusion in a short-term transfection assay. gC is also implicated in the infection process and is required for efficient binding of virions to the cell surface, though conflicting evidence for this function has been obtained with different virus strains (16, 19). It is reasonable to suppose that the processes of receptor-binding and membrane fusion are mediated by a functional complex of these molecules rather than by their independent action, and this is consistent with the observation that cell fusion is observed only when gB, gD, and gH:L are present on the same membrane; these molecules do not appear to cooperate in trans (11). Handler et al. (17), using cross-linking reagents that could not penetrate the virion envelope, observed cross-linking reactions between gB, gC, gD, and gH:L on the virion surface and concluded that these proteins must interact to form a functional complex. If such a functional complex exists, then it must be capable of forming in the absence of other virion components, because expression of gB, gD, and gH:L alone is sufficient to induce fusion. It follows that this hypothetical complex forms prior to virion envelopment and that it is the complex that is incorporated into the envelope during budding. These arguments led us to investigate the properties of these molecules in virions that lacked individual glycoproteins. Our expectation was that the absence of one glycoprotein would alter the physical characteristics of other molecules of the complex and thus provide further evidence for the existence of a complex and of the interactions which influence its formation. In fact, we obtained no such evidence.
gD was detergent extracted from wild-type, gB-negative, or gH-negative virions and exhibited identical sedimentation characteristics. gB extracted from wild-type, gD-negative, or gH-negative virions also showed similar sedimentation characteristics. These are negative results, and they cannot be interpreted unambiguously because we cannot be sure that intermolecular interactions are stable to detergent extraction. Nevertheless, these results provide no evidence for interactions between gD and gH or gB or for interaction between gB and gH. An analysis of the glycoprotein content of mutant virions lacking gB, gC, gD, or gH revealed that the absence of any one of these proteins seemed to have no effect on the composition of virions with respect to the remaining three. These results, also, must be interpreted with caution because differences as great as twofold might not be detected. Nevertheless, we conclude that these four glycoproteins assemble into the virion independently of each other, a conclusion which is difficult to reconcile with the idea that they form a functional complex prior to virion formation. While these results throw no light on the possible interaction between these proteins, they are reassuring in other respects. Much of our current understanding of the functions of gC, gD, gB, and gH:L comes from studies of deletion mutants lacking these proteins, and the conclusions drawn from these studies are predicated on the assumption that the absence of one protein does not affect virion composition with respect to the others. The results reported here show that this assumption is correct.
The results of our cross-linking studies are, perhaps, the most difficult to reconcile with the idea that gB, gC, gD, and gH:L form a functional complex. Our results are entirely consistent with those of Handler et al. (17) in that we, also, observed cross-linking between gB and gC, gB and gD, and gD and gC. However, in the absence of gH, gD, or gC the remaining available interactions appear to be unaltered. The concept that four proteins are organized into a functional complex such that the absence of one member of the complex has no effect on the spatial arrangement of the others seems untenable. Instead we are forced to conclude that these molecules are arranged independently in the virion but are sufficiently closely packed that cross-linking between them can occur. This is consistent with the observation that cross-linking is reduced during virus entry (18), because the virion glycoproteins would become spatially diluted during membrane fusion.
If, as we suspect, gB, gC, gD, and gH are incorporated independently into the virion envelope and are arranged independently within it, then we are obliged to ask what interactions and signals are involved in their assembly into the envelope. An obvious possibility is that each of these proteins reacts, via its cytoplasmic tail or transmembrane region, with tegument components. This appears to be excluded by the observation that the cytoplasmic tail of gD can be deleted without substantial loss of viability (14, 28) and by the finding that an alternative transmembrane region and cytoplasmic tail can be substituted in gD without altering the specific infectivity or gD content of recombinant virions (35). An alternative view is that other virion membrane proteins are involved in targeting the envelopment process, but this seems unlikely because all the envelope proteins apart from gB, gD, and gH:L are dispensable for the production of infectious virions. Our view of “dispensability” is, however, questioned by recent data obtained using pseudorabies virus (PRV). Mutants of this virus lacking gE or gM are viable, but mutants lacking both proteins fail to produce enveloped virions, and this defect is compensated by cell lines which provide either protein in trans (5). It appears that in PRV, gE and gM play a role in the envelopment process but that each protein can compensate, at least partially, for the absence of the other. The function of PRV gE and gM in envelopment is obscure, though the cytoplasmic tail of gE appears to be the key component of this molecule which compensates for the absence of gM (6), and it remains to be seen whether similar results will be obtained with other alphaherpesviruses.
It is also possible that the incorporation of alphaherpesvirus glycoproteins into the virion envelope involves no specific signals or interactions: these proteins may accumulate to sufficient levels in cytoplasmic membranes that their incorporation is an inevitable consequence of budding. This is a somewhat unsatisfying concept, but a number of unrelated transmembrane proteins have been found in alphaherpesvirus virions, and this has been interpreted as indicating that incorporation of membrane proteins into the envelope is essentially a passive process (23). These data are, however, difficult to interpret because the efficiency of incorporation is not easy to assess. Anderson et al. (2) found that vesicular stomatitis virus G protein was incorporated into HSV-1 virions but that the efficiency of incorporation was increased if the transmembrane domain was replaced by that of HSV-1 gD. This result strongly implies that the gD transmembrane sequence contains signals which direct efficient incorporation into the virion envelope, but this interpretation is confounded by the observation that the transmembrane sequence of gD can be replaced with the corresponding domain of the enzyme 2-sialyl transferase with no detectable decrease in gD incorporation or virion infectivity (35). Taken together, these data argue against the incorporation of herpes simplex virus envelope proteins by a purely random process but give no clues as to the specificities involved. The results reported in this paper suggest interactions between the essential membrane proteins gB, gD, and gH:L are of no importance in incorporating these molecules into the virion and argue that these molecules do not form a functional complex.
ACKNOWLEDGMENTS
We thank Helena Browne for her advice and critical discussion.
This work was supported by the Wellcome Trust, United Kingdom (grant no. 036076) and by a Cooperative Group Grant from the Medical Research Council, United Kingdom.
REFERENCES
- 1.Alconada A, Bauer U, Sodeik B, Hoflack B. Cellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J Virol. 1999;73:377–387. doi: 10.1128/jvi.73.1.377-387.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson D B, Laquerre S, Goins W F, Cohen J B, Glorioso J C. Pseudotyping of glycoprotein D-deficient herpes simplex virus type 1 with vesicular stomatitis virus glycoprotein G enables mutant virus attachment and entry. J Virol. 2000;74:2481–2487. doi: 10.1128/jvi.74.5.2481-2487.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babic N, Rodger G, Arthur J, Minson A C. A study of primary neuronal infection by mutants of herpes simplex virus type 1 lacking dispensable and non-dispensable glycoproteins. J Gen Virol. 1999;80:2403–2409. doi: 10.1099/0022-1317-80-9-2403. [DOI] [PubMed] [Google Scholar]
- 4.Boursnell E M, Entwisle C, Blakeley D, Roberts C, Duncan I A, Chisholm S E, Martin G M, Jennings R, Ni C D, Sobek I, Inglis S C, McLean C S. A genetically inactivated herpes simplex virus type 2 (HSV-2) vaccine provides effective protection against primary and recurrent HSV-2 disease. J Infect Dis. 1997;175:16–25. doi: 10.1093/infdis/175.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brack A R, Dijkstra J M, Granzow H, Klupp B G, Mettenleiter T C. Inhibition of virion maturation by simultaneous deletion of glycoproteins E, I, and M of pseudorabies virus. J Virol. 1999;73:5364–5372. doi: 10.1128/jvi.73.7.5364-5372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brack A R, Klupp B G, Granzow H, Tirabassi R, Enquist L W, Mettenleiter T C. Role of the cytoplasmic tail of pseudorabies virus glycoprotein E in virion formation. J Virol. 2000;74:4004–4016. doi: 10.1128/jvi.74.9.4004-4016.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browne H, Bell S, Minson T, Wilson D W. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J Virol. 1996;70:4311–4316. doi: 10.1128/jvi.70.7.4311-4316.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckmaster E A, Gompels U, Minson A C. Characterisation and physical mapping of an HSV1 glycoprotein of approximately 115 × 103 molecular weight. Virology. 1984;139:408–413. doi: 10.1016/0042-6822(84)90387-8. [DOI] [PubMed] [Google Scholar]
- 9.Cai W Z, Person S, Warner S C, Zhou J H, DeLuca N A. Linker-insertion nonsense and restriction-site deletion mutations of the gB glycoprotein gene of herpes simplex virus type 1. J Virol. 1987;61:714–721. doi: 10.1128/jvi.61.3.714-721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claesson-Welsh L, Spear P G. Oligomerization of herpes simplex virus glycoprotein B. J Virol. 1986;60:803–806. doi: 10.1128/jvi.60.2.803-806.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis-Poynter N, Bell S, Minson T, Browne H. Analysis of the contributions of herpes simplex virus type 1 membrane proteins to the induction of cell-cell fusion. J Virol. 1994;68:7586–7590. doi: 10.1128/jvi.68.11.7586-7590.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai P J, Schaffer P A, Minson A C. Excretion of non-infectious virus particles lacking glycoprotein H by a temperature-sensitive mutant of herpes simplex virus type 1: evidence that gH is essential for virion infectivity. J Gen Virol. 1988;69:1147–1156. doi: 10.1099/0022-1317-69-6-1147. [DOI] [PubMed] [Google Scholar]
- 13.Enquist L W, Husak P J, Banfield B W, Smith G A. Infection and spread of alphaherpesviruses in the nervous system. Adv Virus Res. 1999;51:237–247. doi: 10.1016/s0065-3527(08)60787-3. [DOI] [PubMed] [Google Scholar]
- 14.Feenstra V, Hodaie M, Johnson D C. Deletions in herpes simplex virus glycoprotein D define nonessential and essential domains. J Virol. 1990;64:2096–2102. doi: 10.1128/jvi.64.5.2096-2102.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forrester A, Farrell H, Wilkinson G, Kaye J, Davis P N, Minson T. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol. 1992;66:341–348. doi: 10.1128/jvi.66.1.341-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffiths A, Renfrey S, Minson T. Glycoprotein-C deficient mutants of two strains of herpes simplex virus type 1 exhibit unaltered adsorption characteristics on polarised or non-polarised cells. J Gen Virol. 1998;79:807–812. doi: 10.1099/0022-1317-79-4-807. [DOI] [PubMed] [Google Scholar]
- 17.Handler C G, Eisenberg R J, Cohen G H. Oligomeric structure of glycoproteins in herpes simplex virus type 1. J Virol. 1996;70:6067–6075. doi: 10.1128/jvi.70.9.6067-6070.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Handler C G, Cohen G H, Eisenberg R J. Cross-linking of glycoprotein oligomers during herpes simplex virus type 1 entry. J Virol. 1996;70:6076–6082. doi: 10.1128/jvi.70.9.6076-6082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herold B C, WuDunn D, Soltys N, Spear P G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchinson L, Browne H, Wargent V, Davis-Poynter N, Primorac S, Goldsmith K, Minson A C, Johnson D C. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J Virol. 1992;66:2240–2250. doi: 10.1128/jvi.66.4.2240-2250.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson D C, Feenstra V. Identification of a novel herpes simplex virus type 1 induced glycoprotein which complexes with gE and binds immunoglobulin. J Virol. 1987;61:2208–2216. doi: 10.1128/jvi.61.7.2208-2216.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jöns A, Dijkstra J M, Mettenleiter T C. Glycoproteins M and N of pseudorabies virus form a disulfide-linked complex. J Virol. 1998;72:550–557. doi: 10.1128/jvi.72.1.550-557.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keil G M. Fusion of the green fluorescent protein to amino acids 1 to 71 of bovine respiratory syncytial virus glycoprotein G directs the hybrid polypeptide as a class II membrane protein into the envelope of recombinant bovine herpesvirus-1. J Gen Virol. 2000;81:1051–1055. doi: 10.1099/0022-1317-81-4-1051. [DOI] [PubMed] [Google Scholar]
- 24.Kikuchi G E, Glorioso J C, Nairn R. Cross-linking studies show that herpes simplex virus type 1 glycoprotein C molecules are clustered in the membrane of infected cells. J Gen Virol. 1990;71:455–458. doi: 10.1099/0022-1317-71-2-455. [DOI] [PubMed] [Google Scholar]
- 25.Ligas W M, Johnson D C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988;62:1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLean C, Buckmaster A, Hancock D, Buchan A, Fuller A, Minson A. Monoclonal antibodies to three non-glycosylated antigens of herpes simplex virus. J Gen Virol. 1982;63:297–305. doi: 10.1099/0022-1317-63-2-297. [DOI] [PubMed] [Google Scholar]
- 27.Minson A C, Hodgman T C, Digard P, Hancock D C, Bell S E, Buckmaster E A. An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralisation. J Gen Virol. 1986;67:1001–1013. doi: 10.1099/0022-1317-67-6-1001. [DOI] [PubMed] [Google Scholar]
- 28.Muggeridge M I, Wilcox W C, Cohen G H, Eisenberg R J. Identification of a site on herpes simplex virus type 1 glycoprotein D that is essential for infectivity. J Virol. 1990;64:3617–3626. doi: 10.1128/jvi.64.8.3617-3626.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng T, Ponce de Leon M, Novotny M J, Jiang H, Lambris J D, Dubin G, Spear P G, Cohen G H, Eisenberg R J. Structural and antigenic analysis of a truncated form of the herpes simplex virus gH-gL complex. J Virol. 1998;72:6092–6103. doi: 10.1128/jvi.72.7.6092-6103.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roop C, Hutchinson L, Johnson D C. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol. 1993;67:2285–2297. doi: 10.1128/jvi.67.4.2285-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarmiento M, Spear P G. Membrane proteins specified by herpes simplex viruses. IV. Conformation of the virion glycoprotein designated VP7(B2) J Virol. 1979;29:1159–1167. doi: 10.1128/jvi.29.3.1159-1167.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stannard L M, Fuller A O, Spear P G. Herpes simplex virus glycoproteins associated with different morphological entities projecting from the virion envelope. J Gen Virol. 1987;68:715–725. doi: 10.1099/0022-1317-68-3-715. [DOI] [PubMed] [Google Scholar]
- 33.Turner A, Bruun B, Minson T, Browne H. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J Virol. 1998;72:873–875. doi: 10.1128/jvi.72.1.873-875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watson D H, Russell W C, Wildy P. Electron microscopy particle counts on herpes virus using the phosphotungstate negative staining technique. Virology. 1963;19:250–260. doi: 10.1016/0042-6822(63)90062-x. [DOI] [PubMed] [Google Scholar]
- 35.Whiteley A, Bruun B, Minson T, Browne H. Effects of targeting herpes simplex virus type 1 gD to the endoplasmic reticulum and trans-Golgi network. J Virol. 1999;73:9515–9520. doi: 10.1128/jvi.73.11.9515-9520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Q, Courtney R J. Chemical cross-linking of virion envelope and tegument proteins of herpes simplex virus type 1. Virology. 1994;204:590–599. doi: 10.1006/viro.1994.1573. [DOI] [PubMed] [Google Scholar]