Abstract
Background
Knowing the prevalence of myopia at school age is essential to implement preventive measures and appropriate interventions, ensure access to vision care, promote a healthier educational environment and improve academic performance. The purpose of this study was to determine the prevalence of myopia and its associated sociodemographic risk factors, as well as to estimate the coverage of myopia correction among adolescents in center of Portugal.
Methods
This cross-sectional study evaluated 1115 adolescents from the 5th to the 9th year of school, with an average of 12.9 years (SD = 1.5) ranging from 10.0 to 18.0 years. Optometric evaluations were carried out in a school environment and consisted of the evaluation of distance visual acuity, assessed using a logarithmic visual acuity chart (ETDRS charts 1 and 2) at 4 m, and measured by refractive error with a pediatric autorefractometer (Plusoptix), by non-cycloplegic. Myopia was defined as spherical equivalent (SE ≤ -0.50 diopter (D)) and uncorrected visual acuity (UVA ≤ 95VAR). Adjusted logistic regression analysis was applied to investigate risk factors.
Results
We found a myopia rate of 21.5% and a high myopia rate of 1.4%. Higher school level and attendance at urban schools were associated with myopia, but no association was found with age or sex. Only 34.6% of myopic adolescents use the best optical correction and 26.4% do not use any type of optical correction.
Conclusions
Data on the prevalence of refractive problems in Portugal are scarce and heterogeneous. This study, although regional, provides a valuable contribution with a clear and reproducible methodology, following international guidelines and filling gaps in the existing literature. The results show that the rate of myopia in this age group is similar to reports from other European studies. The high rate of adolescents with uncorrected or under-corrected myopia in Portugal is a problem that deserves attention.
Keywords: Adolescence, Myopia, Sociodemographic factors, Visual acuity, Myopia correction coverage, Urban-suburban disparity
Background
Myopia is a refractive condition that tends to develop in pre-adolescence, worsening during puberty and progressing into early adulthood [1]. The greater the degree of myopia, the greater the risk of ocular complications that can lead to vision loss that is not recoverable [2].
The definition of myopia, the methods used to measure ocular refraction and the inconsistent use of cycloplegics, influence the quantifications of myopia prevalence. In most epidemiological studies, myopia is defined by SE≤-0.50D and high myopia by SE≤-5.00D, with cycloplegic refraction [3]. However, the literature often uses non-cycloplegic refractive techniques and considers the same myopia definition [4–6]. Large-scale myopia studies rarely use cycloplegics, so there is a tendency to overestimate the rate of myopia [5].
The prevalence rates of myopia, when assessed using refractive techniques with cycloplegia, are higher in Asia than in compared to Europe [7]. Studies reporting non-cycloplegic refractive measurements show a similar pattern of differences but at even higher rates [4, 8]. Although cycloplegic refraction is considered the most appropriate technique for myopia studies, the use of cycloplegic means it takes a long time to measure refraction and can cause temporary side effects, such as blurred near vision and photophobia, which reduces adherence. [9].
Autorefractometers (AR) are instruments frequently used to obtain ocular refraction in epidemiological studies, but closed-field AR’s induce an overestimation of myopia. The use of open-field AR allows us to obtain refractive measurements close to cycloplegic refractive methods since it eliminates the stimulation of accommodation caused by instrument proximity [5]. It has also been recommended to measure non-cicloplegic autorefraction and visual acuity (VA) without correction, for higher accuracy in detecting myopia [9, 10]. The World Health Organization recommends measuring distance VA in vision screenings [11]. Employing a pinhole test in these screenings can reveal unmet refractive needs, as an improvement in VA with pinhole suggests the presence of correctable refractive errors [2, 11].
Although the magnitude of this problem presents geographic differences, an increase in the prevalence, incidence and progression rates has been observed globally. In Europe, population prevalence rates are estimated at around 40.0% and in certain parts of East Asia, rates exceed 60.0%, and there is strong evidence that these rates vary greatly with age [7]. This vision eye condition has become a growing concern in eye health, especially among school-age children and adolescents. Current trends show that children and adolescents are becoming myopic at an earlier age and that the degree of myopia continues to progress as these children age [2, 12]. The scientific literature reports that the prevalence of myopia tends to increase from the age of 6 years [7]. East Asia exhibits the highest rates of myopia, while Africa and South America have lower reported rates [13].
Health promotion and screening interventions are essential to prevent myopia and other refractive errors by identifying vision problems early. In addition, these actions can change behaviors by educating about the importance of spectacles and addressing common reasons for non-adherence to their use, such as discomfort or social stigma, thus improving acceptance and appropriate management of vision eye conditions. In Portugal, there is little data allowing to know the real extent of myopia. The National programme for eye health estimates that around 20.0% of children and around 50.0% of the adult population suffer from refractive errors in general, including myopia and other refractive conditions [14]. A study carried out with Portuguese university students recorded an increase in the prevalence of myopia from 23.4 to 41.3% between 2002 and 2014 [15]. Another study, based on the analysis of prescription and sales of ophthalmic lenses, estimated an increase in myopia from 40.0% in 2010 to more than 50.0% in 2020 [16].
The prevalence of refractive problems in Portugal is a topic where available data is relatively scarce and presents significant heterogeneity. Furthermore, these studies often present methodological descriptions that can be considered insufficiently detailed. This work aims to estimate the prevalence of myopia in adolescents who attend school from the 5th to the 9th year in the central region of Portugal. We also intend to understand the association of myopia with some sociodemographic parameters in these adolescents, and to estimate the coverage of myopia correction among this population.
Methods
Study design and participants
This is an epidemiological, cross-sectional and observational study. Participants were children and adolescents attending the 2nd cycle of basic education (5th and 6th grades) and the 3rd cycle of basic education (7th, 8th and 9th grades) in Covilhã, a city in the central area of Portugal.
All schools in the urban area of the municipality where the study was conducted were included, covering 2 schools from the second cycle and 4 schools from the third cycle of basic education. Due to the small number of students in suburban schools and their significant geographic dispersion, 2 from each educational cycle in suburban area were selected based on having the highest number of enrolled students. All children enrolled in the participating schools were invited to join the study, with those receiving authorization from their legal guardians included, without participant randomization.
The inclusion criteria were being a child /adolescent attending the 2nd or 3rd cycle of basic education, aged between 10 and 18 years old, having the authorization from their legal tutor and providing verbal consent on the day of the screening. Incomplete screening records or those with poor cooperation were excluded from the data analysis. Students undergoing treatment with orthokeratology or atropine were also excluded, as this treatment can temporarily influence visual acuity and myopia measurement.
Procedures
The study protocol consisted of the acquisition of refractive measurements in eye screening actions in schools. The study was approved from the Ethics Committee of the National School of Public Health (CEENSP nº 29/2023) and was previously authorized by the Ministry of Education (nº 1307100001). Data were collected between November 2023 and February 2024. The examination and vision testing was performed by AN and MC.
Socio-demographic data were collected, such as age, sex, school level, school location (urban or suburban area), place of birth, and special educational needs.
All study volunteers underwent monocular distance visual acuity measurement and ocular refraction assessement using an autorefractometer Additionally, for participants who wore spectacles on the screening day, the prescription value of the spectacles was also recorded.
Visual acuity
VA was measured with ETDRS (Original Series Chart 1 and Chart 2; Good-Lite; USA) at 4 m under photopic lighting conditions. The lighting in the room was measured with a digital luxmeter (Luxmeter PCE-L335; PCE instruments; Tobarra, Spain) and values equal to or greater than 400 lx were considered acceptable [17]. The ETDRS charts are considered reliable, repeatable and easy to use in screening actions [18]. All VA were recorded on the Visual Acuity Rating scale (VAR), which is a more intuitive system for using a logarithmic charts and allows scoring letter by letter instead of line by line [18, 19]. In this rating system, each letter has a score of 1VAR; each line has 5VAR and the decimal VA = 1.0 is equivalent to 100VAR, and decimal VA = 0.8 is equivalent to 95VAR.
The protocol recommended by the WHO was followed to calculate the effective refractive correction coverage rate [2]. To determine UVA, all children were assessed monocularly and without any refractive correction. Visual acuity with usual correction (VAUC) was assessed in all children who wore glasses or contact lenses with their usual correction. In cases where the presented visual acuity (PVA) - defined as UVA for those not wearing corrective lenses or VAUC for those who did - was less than 95VAR, pinhole visual acuity (phVA) was also assessed. The diametre of pinhole was 1.5 mm. The same procedure was applied to record all visual acuity measurements. The patient started at the 80VAR line on the chart (equivalente 0,4 logMAR) and continued reading downwards until reaching a line where they could no longer correctly identify at least three letters. If the patient couldn´t read the 80VAR line, they started at the top of the cgart. The final score was based on the number of letters correctly identified. A different card was used for each eye to avoid learning effects.
Autorefraction
AR was performed under non-cycloplegic conditions, using the PlusOptix, model A09 (PlusOptix; Nuremberg, Germany). The PlusOptix is a device that measures ocular refraction at a distance of 1 m from the eyes, reducing the effects of instrumental myopia compared to closed-field AR. The refraction obtained with the PlusOptix A09 has shown agreement with the refraction of cycloplegic retinoscopy and is indicated as a screening method in myopic children [20, 21]. The ocular refraction of each participant was measured three times and the mean value of the SE of the three measurements was calculated. The SE was obtained by adding the spherical component to half the cylindrical component of the ocular refraction measured with the AR. When PlusOptix reported that the participant’s ocular refraction exceeded its measurement capacity, the refraction of the student’s usual spectacles was considered.
Definition of myopia
In screening activities, some authors recommend the combined use of refraction and VA, recognizing that this combination maximizes the sensitivity of screening in signaling myopia [10, 11, 22]. For children over 6 years of age, some authors recommend a decimal VA ≥ 1.0, equivalent to 0.0logMAR or 100VAR [23, 24], other authors recommend a decimal VA ≥ 0.8, equivalent to 0.1logMAR or 95VAR [9, 24].
In this study, the criteria of UAV < 95VAR and SE≤-0.50D were used to define myopia. To facilitate comparison with other studies, only the SE≤-0.50D criterion was also used. To characterize severity, we considered high myopia SE≤-6.00D, moderate myopia − 6.00D < SE≤-3.00D and mild myopia − 3.00D < SE≤-0.50D.
Statistical analysis
The data were analyzed using SPSS version 28 (IBM SPSS Statistics; New York, USA). Continuous variables were expressed as mean (SD) and categorical variables were presented as counts or proportions. The study of differences between the eyes for the continuous variables was carried out using the paired samples t-test. Chi-square test was used to compare categorical variables between groups. A multivariate logistic regression analysis was carried out using a stepwise backward method to explore the sociodemographic factors associated with myopia. The results of the logistic regression were reported as odds ratios (OR). For all analyses, a two-sided p-value < 0.05 was considered statistically significant. Confidence intervals (CI) were calculated at 95%.
Results
A total of 1115 students from urban and suburban schools took part in the study. The average age was 12.9 (SD = 1.5) years, ranging from 10.0 to 18.0 years. The male sex represented 50.9% of the total sample, and 67.4% of the students attended urban schools. There was also a rate of 11.7% of adolescents flagged in school files as having special educational needs (SEN) and 15.6% of participants were from other countries. The majority of migrant students originated from America (n = 99, with 92 from Brazil) and Africa (n = 49, with 43 from Angola). There were 19 adolescents from other European countries and 7 from Asia. The origin of 2 migrant students was not documented. The characteristics of the sample according to various factors are presented in Table 1. The results of the study of the differences between the groups, as well as the prevalence of myopia according to each of the factors analyzed, are also included.
Table 1.
General characteristics of the sample
| Characteristics | Size [N (%)] |
Age [years] (Average ± SD) |
UVA [< 95VAR] N(%) |
Myopia | ||||
|---|---|---|---|---|---|---|---|---|
| SE≤-0.50D | SE≤-0.50D and UVA < 95VAR | |||||||
| N(%) | p-value (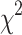 ) ) |
N(%) | p-value (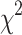 ) ) |
|||||
| Total sample | 1115(100) | 12.7 ± 1.5 | 516(46.3) | 262(23.5) | -- | 240(21.5) | -- | |
| Sex | Male | 568(51.0) | 12.7 ± 1.5 | 245(43.1) | 133(23.4) | 0.957 | 121(21.3) | 0.857 |
| Female | 547(49.0) | 12.7 ± 1.5 | 271(49.5) | 129(23.6) | 119(21.8) | |||
| Nature | Portuguese | 941(84.4) | 12.6 ± 1.5 | 438(46.5) | 221(23.5) | 0.982 | 201(21.4) | 0.756 |
| Migrants | 174(15.6) | 12.8 ± 1.5 | 78(44.9) | 41(23.6) | 39(22.4) | |||
| School level | 2nd cycle | 437(39.2) | 11.2 ± 0.7 | 190(43.5) | 77(17.8) | < 0.001** | 74(16.9) | 0.003** |
| 3rd cycle | 678(60.8) | 13.6 ± 1.0 | 326(48.1) | 185(27.3) | 166(24.5) | |||
| SEN | Positive | 131(11.7) | 13.0 ± 1.4 | 74(56.5) | 29(21.1) | 0.686 | 25(19.1) | 0.469 |
| Negative | 984(88.3) | 12,6 ± 1.5 | 442(44.9) | 233(23.7) | 215(21.8) | |||
| School location | Urban | 751(67.4) | 12.8 ± 1.5 | 360(47.9) | 195(26) | 0.005** | 176(23.4) | 0.026* |
| Suburban | 364(32.6) | 12.5 ± 1.5 | 156(42.9) | 67(18.4) | 64(17.6) | |||
N - counts; % - proportions; SD – standard deviation - UVA – uncorrected visual acuity; VAR – visual acuity rating scale; SE – spherical equivalent; SEN – special educational needs
*Significant at 0.05 level; ** significant at 0.001 level
Prevalence of myopia and risk factors
The mean values for UVA were 90.6 ± 17VAR and 89.4 ± 17VAR for the right and left eyes respectively, and this difference was statistically significant (t = 5.656, p < 0.001). The visual acuity of the worst eye was used to classify myopia. An UVA worse than 95VAR in at least one eye occurred in 516 participants (46.3%; 95% CI: 42.4–50.4%) (Table 1).
For the SE≤-0.50D criterion, a prevalence of myopia was found to be 23.4% (95% CI: 21.0–26.0%), and for the SE≤-0.50D and UAV < 95VAR criteria, it was 21.5% (95% CI: 18.9–24.4%). The average value of the SE of the myopic population (n = 262) was − 2.70D (SD = 1.86), in a range between − 0.50D and − 10.37D. Considering SE≤-6.00D, we account for 16 cases, that is a rate of 1.4% (95% CI: 0.9–2.3%) was found for high myopia. The average value of the SE in high myopia was − 7.52 (SD = 1.32).
The proportion of myopic participants was not significantly different between girls and boys, between Portuguese and migrant students or between participants with and without SEN. However, it was significantly different between the school level, with a higher proportion of adolescents with myopia in the 3rd cycle; as well as between schools in urban and rural areas, with a higher proportion found in schools in the urban areas. These results was observed for both myopia classification criteria.
The association between the presence of myopia and age, sex, geographical location of the school and school level was studied using the odds ratio (OR) (Table 2).
Table 2.
Myopia risk factors
| Factor | OR crude (95% CI) | p-value | OR Adjusted (95% CI) | p-value |
|---|---|---|---|---|
| Age (numeric) | 1.097 (0.996–1.208) | 0.061 | 0.924 (0.786–1.085) | 0.336 |
|
Sex [male vs. female] |
1.027 (0.772–1.367) | 0.854 | 1.008 (0.756–1.344) | 0.958 |
| School location [suburban vs. urban] | 1.435 (1.044–1.973) | 0.026* | 1.409 (1.022–1.941) | 0.036* |
|
School level [2nd cycle vs. 3rd cycle] |
1.590 (1.172–2.158) | 0.003** | 1.889 (1.152–3.097) | 0.012* |
*Significant at 0.05 level; ** significant at 0.001 level
The crude OR revealed an association between myopia and the school location, as well as between myopia and the school level. The adjusted OR showed that adolescents from urban schools were 1.4 times more likely to have myopia than those from rural schools, after adjusting for age, sex and cycle of studies. Adolescents in the 3rd cycle of studies were also 1.9 times more likely to have myopia than adolescents in the 2nd cycle, after adjusting for age, sex and school location.
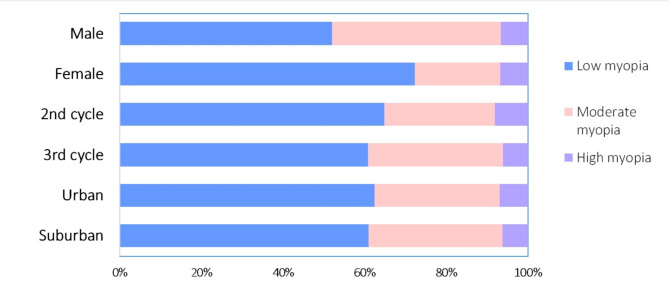
Figure 1 shows the distribution of myopia severity, according to sociodemographic characteristics. Low myopia is more common in all subgrups, but there were sex differences (χ2 = 11.868, p = 0.003). Low myopia is more common in both boys and girls, but of the universe of myopic boys (121), 52.0% have low myopia and 41.3% have moderate myopia, while of the universe of myopic girls (119), 72.3% have a low degree of myopia and 21.0% have moderate myopia. In the studied sample, boys have the highest proportion of moderate myopia. The distribution of myopia severity did not reveal differences between adolescents at different school levels (χ2 = 1.077, p = 0.584) ou between school location (χ2 = 0.109, p = 0.947).
Fig. 1.
Myopia distribution by severity. Legend (Low myopia, Moderate myopia, High myopia). The number in the bars corresponds to the number of adolescents with the condition
Covarage of myopia correction
We found that 35.8% of the screened population reported wearing spectacles or contact lenses (n = 400). There were significant differences between sex in the use of spectacles, with a higher proportion of girls (218 girls, 54,5% and 182 boys, 45.5%) reporting the use of these devices (χ2 = 6.409, p = 0.011). However, no significant differences were found between urban and suburban areas, nor among different levels of education. Among the adolescents who reported using some optical correction, 13.0% (95% CI: 9.7–16.3%) did not show up with their usual correction on the screening day (n = 53). Among the adolescents who attended with their usual optical correction (n = 347), the majority (n = 212) used a myopic prescription, with SE ≤-0.50D. However, 36 of the students who use myopia correction do not meet the myopia criterion (UVA < 95VAR AND AR SE >-0.50D). Hence, of the 240 students with myopia that have been identified, 176 use optical correction. In summary, we found a myopia rate of 21.5% (95% CI: 18.9–24.4%), of which 73.3% (95% CI: 67.8–78.9%) already use some optical correction. Moreover 3.2% (95% CI: 0.8–5.6%) of the sample use prescriptions for myopia while they not need it. It was also noted that the majority use monofocal lenses, with only 12 reported cases using myopia control lenses. There were no records of orthokeratology or atropine usage.
Table 3 shows the counts and proportions of adolescents who habitually use optical correction, according to presenting VA (UVA for those who do not use any correction, or VAUC for those who have spectacles or contact lenses). It also shows the number of cases in which VA improved when measured with the pinhole. It can be observed that only 34.6% (95% CI: 28.6–40.6%) of the myopic population is optically well corrected. Of the myopic teenagers who already use optical correction, a large percentage use insufficient correction to achieve a good vision. It was observed that 38.7% (95% CI: 32.5–44.9%) of the myopic population uses partial correction and 26.7% (95% CI: 21.1–32.3%) does not use any type of correction. The assessment of VA with pinhole in uncorrected or partially corrected myopic adolescents (n = 157) revealed that in 80.3% (95% CI: 74.1–86.5%) of cases it is possible to improve vision with adequate optical correction.
Table 3.
Counts and proportions of myopic adolescents who already use some optical correction, according to the limits of uncorrected visual acuity (UVA) and corrected visual acuity (VAUC). SE – spherical equivalent; PhVA – pinhole visual acuity
| Criteria | N | % |
|---|---|---|
| SE≤ ( -0.50D) and UVA < 95VAR | 240 | 100 |
| VAUC ≥ 95VAR [already wear spectacles or Contact lenses] | 83 | 34.6 |
| VAUC < 95VAR [already wear spectacles or Contact lenses] | 93 | 38.7 |
| UVA < 95VAR [do not wear spectacles or Contact lenses] | 64 | 26.7 |
| PhVA (N= (93 + 64)) [improved] | 126 | 80.3% |
Discussion
This study evaluated the prevalence of myopia in adolescents attending school from the 5th to the 9th year. For the SE≤-0.50D and UVA < 95VAR criteria, there was a prevalence of myopia of 21.5% (95%CI:18.9–24.4%) and for high myopia there was a prevalence of 1.4% (95%CI:0.9–2.3%). Attending the 3rd cycle of studies and attending schools in urban areas were factors associated with a higher prevalence of myopia, while age and sex were not associated with increased odds of myopia. We also observed that only 34.6% (95% CI: 28.6–40.6%) of myopic students were well-corrected and 26.7% (95% CI: 21.1–32.3%) did not use any optical refraction.
Myopia is notably more prevalent in Asia, with scientific literature indicating that children and adolescents in East Asia experience exceptionally high rates of myopia. In some regions, the prevalence has been reported to exceed 80.0% [25]. Given the limited information on myopia prevalence among adolescents in Portugal, it is more practical to analyze and compare myopia trends within the European context, where data are more robust. While extensive research exists in regions such as China, utilizing data from European countries provides a more relevant comparison to Portugal’s situation and enables a more immediate and applicable analysis of local trends and predictors.
Studies on the prevalence of myopia in European children and adolescents are few, and those we found that had been published in the last 5 years report rates ranging from 10% in Sweden to 24.8% in Austria [26, 27]. When cycloplegic refraction is used, rates are lower [26, 28, 29] than when cycloplegia is not used [27, 30]. It should also be noted that most studies use SE≤-0.50D as the definition of myopia [22, 26, 28–30] but some studies use a more myopic cutoff point [31] and the joint assessment of autorefraction and visual acuity [32].
The myopia rate found in the present study is similar to that reported in other studies from European countries. A comparison of our results with reports from other studies that used more conservative criteria to define myopia (e.g., SE≤-0.50 and UVA ≤ 95VAR) reveals that myopia is slightly more prevalent among adolescents in Portugal (21.5%) than in Bulgaria (19.0%) [26], and very similar to the prevalence reported in Germany (21.5%), where the definition of myopia used a cutoff point SE≤-0.75D [31]. For a broader comparison with the SE≤-0.50D criterion, we found a prevalence rate of 23.4%. This value is very close to that reported by other studies with children and adolescents in Europe, which used the same definition of myopia. In Austria, a rate of 24.8% was found between the ages of 15 and 18, and in Spain, a rate of 20.1% was reported in children aged 6 to 7 [22, 30].
The prevalence of myopia and associated risk factors among children has not yet been determined. It is known that genetic and environmental factors play a role in its etiology. Risk factors for myopia may include a combination of genetic, environmental and lifestyle factors, with the most obvious being genetics, time outdoors, near work and sex [33]. The literature also reports that the prevalence of myopia increases with age, is more frequent in girls and in the urban areas [22, 34]. In the present study, there was no association between myopia and age, but an association was found with school level, with a higher prevalence of mypia in the 3rd cycle. Although a higher school level necessarily requires an older age, the age-adjusted multivariate analysis revealed that age has no association and that the probability of myopia is 1.9 times greater in adolescents in the 3rd cycle. We believe that this association is influenced by other factors that also contribute to myopia, such as the intensity of close work and excessive use of digital screens [34]. Adolescents in the 3rd cycle of studies have a greater academic workload, which requires them to dedicate more time to tasks with near vision. Furthermore, the excessive use of digital screens, both for academic support and leisure, tends to be greater among older adolescents [35].
Regarding sex, there is no consensus in the literature, with older studies reporting that men have a higher prevalence of myopia, while more recent studies report that women show higher prevelances [34]. Other authors also report finding no association between sex and myopia [36], in line with the results from our study. The urban environment is also described as a factor associated with myopia and urban-rural differences tend to be stronger where there is a greater disparity in living conditions [37, 38]. This study also found this association, with adolescents attending an urban school being 1.4 times more likely to have myopia than those attending a suburban school. In a study carried out in India, where the location of the school was also taken into account, it was observed that the rate of myopia was 1.3 times higher in urban schools than in suburban schools [39].
Multi-ethnic population-based studies suggest that the prevalence of myopia varies according to ethnicity. The scientific literature reports that the prevalence of myopia is highest in Asian populations (above 50.0%), and lowest in African regions (around 15.0%) and shows values between 20.0 and 40.0% in Europe and America [3, 13]. In our study, no significant differences were found in myopia rates between Portuguese and migrant adolescents. For the most conservative criterion, SE≤-0.50D and UVA < 95VAR, the prevalence of myopia was 21.4% for the Portuguese and 22.4% for the migrants’ adolescents. The migrant population in this study was mostly from Brazil and African countries, with a low rate of students from Asia. We believe that the low representation of Asian adolescents is the main reason why the migrant population had a prevalence rate similar to that of adolescents born in Portugal.
Scientific literature reports that children with special educational needs have a higher prevalence of vision dysfunction when compared to population samples, and one of the main causes of this disability is refractive errors [40]. In our study, there were no significant differences in the proportion of myopic adolescents between those with (vs. without) SEN. Since adolescents with low levels of autonomy and low capacity for collaboration in the acquisition of measurements have been excluded from the study, adolescents from the SEN group with greater potential for vision impairment may have been left out of our sample. On the other hand, this analysis is limited to myopia, and refractive errors such as hyperopia or astigmatism in individuals with SEN may be more frequent [41].
Another finding from our study that deserves reflection concerns the use of optical correction. Other authors report that the use of corrective spectacles improves the cognitive and educational well-being, psychological well-being, mental health, and quality of life of school-age children and adolescents [42]. Several authors have reported high rates of uncorrected myopia in school-age children [24, 43]. Our study found that only 34.6% of adolescents with myopia were well-corrected, with 38.7% being under-corrected, and 26.7% not using any correction. According to WHO recommendations, in screening activities, an improvement in visual acuity with a pinhole means that the problem of vision impairment can be solved with the use of suitable spectacles [11]. In the present study, when evaluating visual acuity with the pinhole in uncorrected or undercorrected myopic participants, an improvement was obtained in 80.3% of cases, which means that these adolescents can see their vision improved with a simple pair of appropriately prescribed spectacles. We also found that there is a significant percentage of teenagers who report having spectacles, but who do not use them regularly (13.0%). Several studies have explored compliance to spectacle use in impairement vision due to refractive errors, and a systematic review reveals that non-adherence rates in children are hiegh, even when glasses are freely provided. The reasons for non-adherence are varief, including factors such as broken glasses, forgetfulness, parental perceptions, and peer pressure [44, 45]. The design of the present study did not allow us to explore the reasons for this behavior, but it reinforces the message that teenagers’ refusal to wear prescribed spectacles puts their eye health and their professional and academic future at risk [42]. Health professionals and the educational community must come together to raise awareness of the risks of non-compliance with spectacles, promote educational campaigns, and debunk myths and beliefs.
The main strength of this work lies in its analysis of data on myopia from a large sample of adolescents in the central region of Portugal, providing valuable insights into the prevalence of myopia in Portugal. However, there are also some limitations. One of the main limitations of this study is the fact that cycloplegic refraction was not used. Nevertheless, we sought a methodological design that would minimize this aspect, looking for a reliable alternative. An open-field autorefractometer was used, an instrument that is described as the closest technique to cycloplegic refraction [21, 37]. Another important measure was to combine the spherical equivalent measurement with uncorrected visual acuity, as proposed by others authors [9, 10], enabling to confer more confidence to the myopia prevalence values found in the present study. The definition of a refractive threshold and a visual acuity threshold as a cut-off point for myopia is therefore an added value and strengthens the findings of this study. The selection of the eye with poorer visual acuity may have contributed to some overestimation of myopia prevalence compared to studies that consider only one eye. However, this approach has also been adopted in similar studies [28, 32]. The association between myopia prevalence and the presence of modifiable environmental risk factors (e.g., shorter distance and longer time spent for near work) was not addressed in this study, representing an opportunity for future work. Studying modifiable environemental risk factors is fundamental for understanding which habits and behaviors of adolescents are associated with the development of myopia, providing relevant evidence for the development of recommendations for its prevention and management.
Conclusions
This paper is a cross-sectional study of myopia in adolescents at a center in Portugal. It shows that myopia in adolescence is comparable to that reported by other European countries, being at the upper end of reported rates (above 20.0%). Moreover, it showed that mypia was higher among higher school levels and among students of urban schools.
The high prevalence of uncorrected or under-corrected myopia is a worrying aspect. Another pertinent aspect concerns non- compliance with spectacles, as a considerable number of students who reported having spectacles were not wearing them at the time of the assessment. Adolescents’ refusal to wear their usual spectacles puts their ocular health and their school and professional future at risk.
The epidemiological burden of myopia among schoolchildren necessitates a cross-sectoral approach, involving both health and education sectors, to ensure systematic screening, effective refractive error services, optical correction, and ongoing follow-up for affected children. Our results also highlight the critical need for public education on eye care and the development of an effective and sustainable school-age vision screening program to prevent vision impairment and blindness. By integrating public education with practical screening initiatives, we can ensure early detection and treatment, ultimately safeguarding children’s vision health.
Acknowledgements
We thank the Clinical and Experimental Center for Vision Sciences and UBImedical for their support with the necessary materials and assistance in data collection.
Abbreviations
- SE
Spherical equivalent
- AR
Autorefroctometer
- VA
Visual acuity
- ETDRS
Early Treatment of Diabetic Retinopathy Study
- UVA
Uncorrected visual acuity
- VAUC
Visual acuity with usual correction
- PhVA
Pinhole visual acuity
- VAR
Visual Acuity Rating
- OR
Odds ratio
- CI
Confidence interval
- SEN
Special educational needs
Author contributions
AFN, MCBS and CAG contributed to the concept of the study. AFN and MC acquired and analyzed the data. AFN and CAG helped with the interpretation of the data. AFN and MC drafted the manuscript. MCBS and CAG supervised the study. All authors read and approved the final manuscript.
Funding
No funding was received for this research.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study conformed to the principles of the Declaration of Helsinki, and informed consent was signed by the participants’ parents. The Ethics Committee of the National School of Public Health, approved this study (approval number CEENSP nº 29/2023).
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ducloux A, Marillet S, Ingrand P, Bullimore MA, Bourne RA, Leveziel N. Progression of myopia in teenagers and adults: a nationwide longitudinal study of a prevalent cohort. Br J Nurs. 2021;0:1–6. 10.1136/bjophthalmol-2021-319568. 10.1136/bjophthalmol-2021-319568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keel S, Müller A, Block S, Bourne R, Burton MJ, Chatterji S, et al. Keeping an eye on eye care: monitoring progress towards effective coverage. Lancet Glob Heal. 2021;9(10):e1460–4. 10.1016/S2214-109X(21)00212-6. 10.1016/S2214-109X(21)00212-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–42. 10.1016/j.ophtha.2016.01.006. 10.1016/j.ophtha.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 4.Grzybowski A, Kanclerz P, Tsubota K, Lanca C, Saw SM. A review on the epidemiology of myopia in school children worldwide. BMC Ophthalmol. 2020;20:1–11. 10.1186/s12886-019-1220-0. 10.1186/s12886-019-1220-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berke A. Prevalence of myopia in children and adults in Europe and North America. Optometry Contact Lenses. 2021;1(2):48–55. 10.54352/dozv.YCPT5231. 10.54352/dozv.YCPT5231 [DOI] [Google Scholar]
- 6.Singh H, Singh H, Latief U, Tung GK, Shahtaghi NR, Sahajpal NS, et al. Myopia, its prevalence, current therapeutic strategy and recent developments: a review. Indian J Ophthalmol. 2022;70(8):2788–99. 10.4103/ijo.IJO_2415_21. 10.4103/ijo.IJO_2415_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Németh J, Tapasztó B, Aclimandos WA, Kestelyn P, Jonas JB, De Faber JH, et al. Update and guidance on management of myopia. European Society of Ophthalmology in cooperation with International Myopia Institute. Eur J Ophthalmol. 2021;31(3):853–83. 10.1177/1120672121998960. 10.1177/1120672121998960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flitcroft DI, He M, Jonas JB, Jong M, Naidoo K, Ohno-Matsui K, et al. IMI–defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Investig Ophthalmol Vis Sci. 2019;60(3):M20–30. 10.1167/iovs.18-25957. 10.1167/iovs.18-25957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Ying GS, Fu X, Zhang R, Meng J, Gu F, et al. Prevalence of myopia and vision impairment in school students in Eastern China. BMC Ophthalmol. 2020;20:1–10. 10.1186/s12886-019-1281-0. 10.1186/s12886-019-1281-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu H, Shao Y, Yuan H, Yan B. Age-determined referral criteria of myopia for large-scale vision screening. Eye Sci. 2015;30(4):151–5. 10.3978/j.issn.1000-4432.2015.11.03. 10.3978/j.issn.1000-4432.2015.11.03 [DOI] [PubMed] [Google Scholar]
- 11.WHO - World Health Organization. Vision and eye screening implementation handbook. Licence: CC BY-NC-SA 3.0 IGO. 2023. ISBN:978-92-4-008245-8.
- 12.Burton MJ, Faal HB, Ramke J, Ravilla T, Holland P, Wang N et al. (2019) Announcing The Lancet Global Health Commission on Global Eye Health. Lancet Glob Heal. 2019;7(12):e1612–3. 10.1016/S2214-109X(19)30450-4 [DOI] [PubMed]
- 13.Baird PN, Saw SM, Lança C, Guggenheim JA, Smith IE, Zhou X, et al. Myopia Nat Rev Dis Prim. 2020;6(1):99. 10.1038s41572-020-00231-4. 10.1038/s41572-020-00231-4 [DOI] [PubMed] [Google Scholar]
- 14.SNS - Direção Geral Saúde. Programa Nacional para a Saúde Da Visão - revisão e extensão a 2020. DGS; 2016.
- 15.Jorge J, Braga A. Changes in Myopia Prevalence among First-Year University students in 12 year. Optom Vis Sci. 2016;93(10):1262–7. 10.1097/OPX.0000000000000926. 10.1097/OPX.0000000000000926 [DOI] [PubMed] [Google Scholar]
- 16.Carneiro VA, González-Méijome JM. Prevalence of refractive error in Portugal estimated from ophthalmic lens manufacturing data: ten-years analysis. PLoS ONE. 2023;18(4):e0284703. 10.1371/journal.pone.0284703. 10.1371/journal.pone.0284703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tidbury LP, Czanner G, Newsham D. Fiat lux: the effect of illuminance on acuity testing. Graefe’s Archive Clin Experimental Ophthalmol. 2016;254(6):1091–7. 10.1007/s00417-016-3329-7. 10.1007/s00417-016-3329-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott DB. The good (logMAR), the bad (Snellen) and the ugly (BCVA, number of letters read) of visual acuity measurement. Ophthalmic Physiol Opt. 2016;36(4):355–8. 10.1111/opo.12310. 10.1111/opo.12310 [DOI] [PubMed] [Google Scholar]
- 19.Bailey IL, Lovie-Kitchin JE. Visual acuity testing. From the laboratory to the clinic. Vision Res. 2013;90:2–9. 10.1016/j.visres.2013.05.004. 10.1016/j.visres.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 20.Payerols A, Eliaou C, Trezeguet V, Villain M, Daien V. Accuracy of PlusOptix A09 distance refraction in pediatric myopia and hyperopia. BMC Ophthalmol. 2016;6:1–7. 10.1186/s12886-016-0247-8. 10.1186/s12886-016-0247-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yilmaz I, Ozkaya A, Alkin Z, Ozbengi S, Yazici AT, Demirok A. Comparison of the Plusoptix A09 and Retinomax K-Plus 3 with retinoscopy in children. J Pediatr Ophthalmol Strabismus. 2015;52(1):37–42. 10.3928/01913913-20141230-06. 10.3928/01913913-20141230-06 [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Xie H, Morgan I, Chen J, Yao C, Zhu J, et al. How to conduct school myopia screening: comparison among myopia screening tests and determination of associated cutoffs. Asia-Pacific J Ophthalmol. 2022;11(1):12–8. 10.1097/APO.0000000000000487. 10.1097/APO.0000000000000487 [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Liu J, Ma, Zhang Q, Li R, He X, et al. Prevalence of myopia in 3-14-year-old Chinese children: a school-based cross-sectional study in Chengdu. BMC Ophthalmol. 2021;21(1):318. 10.1186/s12886-021-02071-6. 10.1186/s12886-021-02071-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Li Y, Qiu K, Zhang R, Lu X, Luo L, et al., et al. Prevalence of myopia and uncorrected myopia among 721 032 schoolchildren in a city-wide vision screening in southern China: the Shantou Myopia Study. Br J Ophthalmol. 2023;107(12):1798–805. 10.1136/bjo-2021-320940. 10.1136/bjo-2021-320940 [DOI] [PubMed] [Google Scholar]
- 25.We XX, Yu LL, Majid AZA, Xu Y. Study on the prevalence of myopia and its associated factors in China: a systemic review. Eur Rev Med Pharmacol Sci. 2023;27(17). 10.26355/eurrev_202309_33559. [DOI] [PubMed]
- 26.Demir P, Baskaran K, Theagarayan B, Gierow P, Sankaridurg P, Macedo AF. Refractive error, axial length, environmental and hereditary factors associated with myopia in Swedish children. Clin Experimental Optometry. 2021;104(5):595–601. 10.1080/08164622.2021.1878833. 10.1080/08164622.2021.1878833 [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Vass C, Smith L, Juan A, Waldhör T. Thirty-five-year trend in the prevalence of refractive error in Austrian conscripts based on 1.5 million participants. Br J Ophthalmol. 2020;104(10):1338–44. 0.1136/bjophthalmol-2019-315024. 10.1136/bjophthalmol-2019-315024 [DOI] [PubMed] [Google Scholar]
- 28.Harrington SC, Stack J, O’Dwyer V. Risk factors associated with myopia in schoolchildren in Ireland. Br J Ophthalmol. 2019;103(12):1803–9. 10.1136/bjophthalmol-2018-313325. 10.1136/bjophthalmol-2018-313325 [DOI] [PubMed] [Google Scholar]
- 29.Guillon-Rolf R, Grammatico-Guillon L, Leveziel N, Pelen F, Durbant E, Chammas J, Khanna RK. Refractive errors in a large dataset of French children: the ANJO study. Sci Rep. 2022;12(1):4069. 10.1038/s41598-022-08149-5. 10.1038/s41598-022-08149-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez-Peregrina C, Martinez-Perez C, Villa-Collar C, González-Pérez M, González-Abad A, Sánchez-Tena MA. (2021) The prevalence of myopia in children in Spain: an updated study in 2020. International Journal of Environmental Research and Public Health. 2021; 18(23): 12375. 10.3390/ijerph182312375 [DOI] [PMC free article] [PubMed]
- 31.Philipp D, Vogel M, Brandt M, Rauscher FG, Hiemisch A, Wahl S, et al. The relationship between myopia and near work, time outdoors and socioeconomic status in children and adolescents. BMC Public Health. 2022;22(1):2058. 10.1186/s12889-022-14377-1. 10.1186/s12889-022-14377-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dragomirova M, Antonova A, Stoykova S, Mihova G, Grigorova D. Myopia in Bulgarian school children: prevalence, risk factors, and health care coverage. BMC Ophthalmol. 2022;22(1):248. 10.1186/s12886-022-02471-2. 10.1186/s12886-022-02471-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ying ZQ, Li DL, Zheng XY, Zhang XF, Pan CW. (2024) Risk factors for myopia among children and adolescents: an umbrella review of published meta-analyses and systematic reviews. British Journal of Ophthalmology. 2024;108(2):167–174. 10.1136/bjo-2022-322773 [DOI] [PubMed]
- 34.Morgan IG, Wu PC, Ostrin LA, Tideman JL, Yam JC, Lan W, et al. IMI risk factors for myopia. Invest Ophthalmol Vis Sci. 2021;62(5):3. 10.1167/iovs.62.5.3. 10.1167/iovs.62.5.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lissak G. Adverse physiological and psychological effects of screen time on children and adolescents: literature review and case study. Environ Res. 2018;164:149–57. 10.1016/j.envres.2018.01.015. 10.1016/j.envres.2018.01.015 [DOI] [PubMed] [Google Scholar]
- 36.Hansen MH, Hvid-Hansen A, Jacobsen N, Kessel L. Myopia prevalence in Denmark–a review of 140 years of myopia research. Acta Ophthalmol. 2021;99(2):118–27. 10.1111/aos.14562. 10.1111/aos.14562 [DOI] [PubMed] [Google Scholar]
- 37.Rudnicka AR, Kapetanakis VV, Wathern AK, Logan NS, Gilmartin B, Whincup PH, et al. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: implications for aetiology and early prevention. Br J Ophthalmol. 2016;100(7):882–90. 0.1136/bjophthalmol-2015-307724. [DOI] [PMC free article] [PubMed]
- 38.Wang Y, Liu L, Zhang L. Rural-urban differences in prevalence of and risk factors for refractive errors among school children and adolescents aged 6–18 years in Dalian, China. Front Public Health. 2022;10:917781. 10.3389/fpubh.2022.917781. 10.3389/fpubh.2022.917781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gopalakrishnan A, Hussaindeen JR, Sivaraman V, Swaminathan M, Wong YL, Armitage JA, et al. Myopia and its association with near work, outdoor time, and housing type among schoolchildren in south India. Optom Vis Sci. 2023;100(1):105–10. 10.1097/OPX.0000000000001975. 10.1097/OPX.0000000000001975 [DOI] [PubMed] [Google Scholar]
- 40.Choi KY, Wong HY, Cheung HN, Tseng JK, Chen CC, Wu CL, et al. Impact of visual impairment on balance and visual processing functions in students with special educational needs. PLoS ONE. 2022;17(4):e0249052. 10.1101/2020.09.28.20202879. 10.1101/2020.09.28.20202879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielsen LS, Skov L, Jensen H. (2007). Visual dysfunctions and ocular disorders in children with developmental delay. II. Aspects of refractive errors, strabismus and contrast sensitivity. Acta Ophthalmologica Scandinavica. 2007;85(4):419–426. 10.1111/j.1600-0420.2007.00881.x [DOI] [PubMed]
- 42.Pirindhavellie GP, Yong AC, Mashige KP, Naidoo KS, Chan VF. The impact of spectacle correction on the well-being of children with vision impairment due to uncorrected refractive error: a systematic review. BMC Public Health. 2023;23(1):1575. 10.1186/s12889-023-16484-z. 10.1186/s12889-023-16484-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang M, Luensmann D, Fonn D, Woods J, Jones D, Gordon K, Jones L. Myopia prevalence in Canadian school children: a pilot study. Eye. 2018;32(6):1042–7. 0.1038/s41433-018-0015-5. 10.1038/s41433-018-0015-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhirar N, Dudeja S, Duggal M, Gupta PC, Jaiswal N, Singh M, et al. Compliance to spectacle use in children with refractive errors-a systematic review and meta-analysis. BMC Ophthalmol. 2020;20(1):71. 10.1186/s12886-020-01345-9. 10.1186/s12886-020-01345-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu L, Feng J, Zhang M. Implementing interventions to promote spectacle wearing among children with refractive errors: a systematic review and meta-analysis. Front Public Health. 2023;11:1053206. 10.3389/fpubh.2023.1053206. 10.3389/fpubh.2023.1053206 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.