Abstract
Most patients infected with hepatitis C virus (HCV) become chronic carriers. Viruses that efficiently establish persistent infections must have effective ways of evading host defenses. In the case of HCV, little is known about how chronic infections are established or maintained. Besides hepatocytes, several reports suggest that HCV can infect T and B lymphocytes. Since T cells are essential for viral clearance, direct or indirect effects of HCV on T-cell function could influence the outcome of infection. Given that T-cell growth and differentiation require the cytokine interleukin 2 (IL-2), we asked whether HCV might modulate synthesis of IL-2. Portions of the HCV polyprotein were expressed in Jurkat cells under a variety of conditions. We found that the highly conserved HCV core protein, in combination with other stimuli, was able to dramatically activate transcription from the IL-2 promoter. The carboxy-terminal hydrophobic portion of the core protein was required for this activity. Activation was dependent on nuclear factor of activated T cells (NFAT), occurred in cells deficient in the tyrosine kinase p56lck, and could be blocked by addition of cyclosporin A and by depletion of calcium. These results suggest that the HCV core protein can activate transcription of the IL-2 promoter through the NFAT pathway. This novel activity may have consequences for T-cell development and establishment of persistent infections.
Hepatitis C virus (HCV) is the major cause of nonparenteral non-A, non-B hepatitis, affecting an estimated 170 million people worldwide (1, 77). HCV is an enveloped, single-stranded positive-sense RNA virus belonging to the family Flaviviridae (60). The 9.6-kb HCV genome consists of conserved terminal RNA elements flanking a single long open reading frame. The 5′ nontranslated region functions as an internal ribosome entry site to initiate translation of the viral polyprotein (41) that is cleaved by host and viral proteases to produce three structural and at least six nonstructural (NS) proteins (58).
A remarkable feature of HCV is its ability to establish chronic infections in a majority of those infected, regardless of immune status. Smoldering persistent replication of HCV is associated with the more severe sequelae of this disease, including chronic hepatitis, cirrhosis, and liver cancer (30). Although the high mutation rate of HCV is likely to play a role in maintaining persistent infection in the face of virus-specific immune responses, other mechanisms are undoubtedly also involved. For instance, two of the viral proteins, E2 (72) and NS5A (24, 25), appear to modulate interferon (IFN) resistance by interacting with the IFN-inducible, double-stranded RNA-stimulated protein kinase PKR. In addition, the HCV core (C) protein has recently been shown to bind to certain members of the tumor necrosis factor (TNF) receptor superfamily and modulate sensitivity to TNF-α in some cell types (12, 44, 55, 80).
Recently, several studies have begun to uncover immune response correlates of HCV clearance versus persistence. Overall, these data suggest that clearance is associated with Th responses directed against viral antigens such as NS3 (20) and strong early CD8+ cytotoxic T-cell (CTL) responses against multiple HCV epitopes (15). In contrast, robust humoral responses do not generally correlate with clearance, and in fact, HCV-specific antibodies in acute resolvers are often transient and disappear (2, 3). These trends suggest that a Th1 rather than a Th2-type response may be beneficial for controlling and clearing HCV (which is perhaps not surprising, given that CTLs play an important role in clearing most viral infections). What is not clear is why most individuals fail to clear HCV. The balance between humoral and cell-mediated immune response is regulated by multiple cytokines. Interleukin (IL)-2 is a key T-cell-specific mitogen and differentiation factor that is produced by helper T cells (18). The release of IL-2 is regulated at the level of transcription as well as by stabilization of mRNA (21). Other cytokines modulating T-cell differentiation include IL-4, IL-10, IL-12, and IFN-γ (18). Some viruses encode products that act to inhibit viral clearance by affecting the ratio of cytokines promoting cell- or antibody-mediated immunity (63). For instance, Epstein-Barr virus influences T-cell development by encoding BCRF1, and IL-10 homologue (31). Lymphotropic retroviruses like human T-cell lymphotropic virus type 1 (HTLV-1) and human immunodeficiency virus type 1 modulate IL-2 synthesis through expression of Tax and Tat, respectively (69, 76).
In vitro (17, 45, 68) and some (4, 5, 7) but not all (39) in vivo studies indicate that HCV may replicate in B and T lymphocytes. Given that several previous studies implicated the C protein in transcriptional regulation of host and viral promoters (54, 56, 57, 67), these observations prompted us to examine the impact of HCV C protein expression on the activity of the IL-2 promoter. The C protein is highly conserved among viral isolates and the first translation product in the HCV polyprotein. Signal peptidase mediates the cleavage after residue 191, separating the C from the downstream E1 glycoprotein (32). The C protein appears to be further processed at a site near residue 172 by a microsome-associated activity (60). These forms of HCV C are localized in the perinuclear reticular network in a pattern characteristic of the endoplasmic reticulum (46, 61, 79). C-terminally truncated forms of HCV C have also been observed (43), and deletion of the C-terminal hydrophobic portion unmasks functional nuclear localization signals found in the basic N-terminal domain (11, 52, 71). Such nucleus-localized forms of C have been implicated in transcription regulation (53, 54, 57, 67). Here we report that full-length but not truncated C protein activated transcription from the IL-2 promoter in Jurkat cells. This novel activity of HCV C may have consequences for T-cell development and viral pathogenesis.
MATERIALS AND METHODS
Chemical and immunological reagents.
All chemicals used were of analytical grade. Restriction endonucleases, T4 DNA polymerase, and T4 DNA ligase were obtained from New England Biolabs (Beverly, Mass.). DNase I, RNase T1, and Trizol were from Life Technologies (Rockville, Md.). Actinomycin D, cycloheximide, forskolin, ionomycin, and 12-O-tetradecanoylphorbol 13-acetate (TPA) were from Sigma Chemical Co. (St. Louis, Mo.). The T-cell receptor (TCR)-specific antibody C305 (9) was a gift from A. C. Chan (Washington University, St. Louis, Mo.), and the CD28-specific antibody 9.3 was provided by Bristol-Myers Squibb Pharmaceutical Research Institute (Seattle, Wash.). Horseradish peroxidase-linked anti-immunoglobulins were from Dako A/S (Glostrup, Denmark). Cyclosporin A was from Sandoz Pharmaceutical Corp. (East Hanover, N.J.). Beetle luciferin and Transfast were purchased from Promega Corp. (Madison, Wis.). The monoclonal antibody C7-50 (47) recognizing HCV C was a gift from J. R. Wands (Harvard Medical School, Boston, Mass.).
Cells, cell culture, and viral genomes.
Human Jurkat T cells and the derivative line J.CaM.1 were obtained from A. C. Chan. Both cell lines were cultured in RPMI 1640 medium (Life Technologies) supplemented with 10% fetal bovine serum (Sigma). HCV gene products were propagated in bacteria as recombinant plasmids. Complementary DNA from the H77 strain encoding the first 194 amino acids (aa) of the HCV polyprotein was cloned into plasmid pOPCMV (E. Agapov and C. M. Rice, unpublished). The resulting plasmid expresses its transcript from the cytomegalovirus (CMV) promoter and was designated pOP/HCV1-194. The HCV sequence was identical to the corresponding parts in infectious clones of the H77 strain (37, 78). The vector pOPCMV/ct (E. Agapov and C. M. Rice, unpublished) is a derivative of pOP13CAT (Clontech, Palo Alto, Calif.) with the original promoter from Rous sarcoma virus replaced by the CMV immediate-early promoter. Plasmid pOPCMV was constructed by removal of the gene encoding chloramphenicol acetyl transferase from pOPCMV/ct. To generate a truncated core protein, the KpnI-AftII fragment of pOP/HCV1-194 was replaced by a fragment encoding a stop codon after aa 152, resulting in plasmid pOP/HCV1-152. Plasmids pT7-luc202(−) and pT7-luc202(+) were designed for generation of a sequence-specific hybridization probe and a positive control, respectively, by cloning the 202-nucleotide (nt) proximal part of the luciferase gene in both orientations into pBluescript KS(−) (Stratagene, La Jolla, Calif.). Computer-readable sequences of these plasmids can be obtained via the Internet (http://www.ki.se/mtc/groups/mgm/anders_bergqvist/suppl.htm). In luciferase assays, the following reporter plasmids directing transcription of a luciferase reporter gene were used. Plasmids IL2-LUC (71a) and IL-2Luc (48a) contain nt −2060 to +40 and −326 to +47 of the murine and human IL-2 enhancers/promoters, respectively. NFAT-luc contains three copies of the nuclear factor of activated T cells (NFAT) site (−286 to −257 of the human IL-2 gene) linked to the human IL-2 promoter (−72 to +47) (48a). 2×AP1-luc contains two copies of the TPA response element in the rodent α chorionic gonadotropin gene linked to a minimal rat prolactin promoter (−36 to +37) (29). The plasmid NFκB-luc (gift from K. M. Murphy, Washington University, St. Louis, Mo.) was derived from Ig-κB-CAT (73) and contains two copies of the κB element from the immunoglobulin κ light-chain enhancer linked to the IFN-β gene TATA box (−53 to −11) driving luciferase expression.
Analysis of gene expression.
Jurkat and J.CaM.1 cells were transfected by the DEAE-dextran procedure. Approximately 8 × 106 cells were transfected with 5 μg of DNA in the presence of 0.5 mg of DEAE-dextran per ml in 1.6 ml of Tris-buffered saline (25 mM Tris-Cl [pH 7.4], 140 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4, 0.9 mM CaCl2, 0.5 mM MgCl2). Equal amounts of effector and reporter plasmid DNA were used. The cells were aliquoted at 41 h posttransfection and stimulated with either 80 nM TPA, 2 μM ionomycin, 10 μM forskolin, or mouse ascites containing monoclonal antibodies recognizing the TCR (C305) or CD28 (9.3), both used at a dilution of 1:5,000. At 48 h posttransfection, the cells were washed in phosphate-buffered saline and lysed in a buffer containing 25 mM Tris-phosphate (pH 7.8), 2 mM dithiothreitol, 10% glycerol, and 1% Triton X-100. Luciferase activity was determined in a luminometer (EG & G Berthold, Wellesley, Mass., or Labsystems, Helsinki, Finland) using beetle luciferin reagent according to the manufacturer's instructions. All data shown were in the linear range of detection and obtained from representative experiments that had been repeated at least three times. Luciferase-specific RNA was detected by an RNase protection assay. Total RNA was isolated from transfected cells with Trizol followed by treatment with DNase I. Labeled probe RNA (305 nt) obtained by in vitro transcription of linearized pT7-luc202(−) was mixed with cellular RNA in annealing buffer (40 mM 1,4-piperazinediethanesulfonic acid [pH 6.4], 400 mM NaCl, 1 mM EDTA, 80% formamide) at 95°C for 60 s, followed by slow cooling to 45°C. Digestion of single-stranded RNA was performed at 30°C by addition of 360 U of RNase T1 in 12 volumes of digestion buffer (10 mM Tris-Cl [pH 7.5], 300 mM NaCl, 5 mM EDTA), and the reaction mixtures were then extracted with phenol-chloroform. Protected RNA fragments were separated on a 5% denaturing polyacrylamide gel and visualized by autoradiography.
Immunoblot analysis.
Jurkat cells (106) were transfected with 1.5 μg of DNA in the presence of 9 μl of Transfast. Forty hours posttransfection, the cells were washed with Tris-buffered saline (TBS) and solubilized by boiling in sodium dodecyl sulfate (SDS) sample buffer. Proteins were resolved on an SDS–15% polyacrylamide gel and transferred to a nitrocellulose membrane (Schleicher & Schuell Gmbh, Dassel, Germany) by using a semidry blotting apparatus. As the primary antibody, mouse ascites containing the HCV C-specific monoclonal antibody C7-50 was used at a final concentration of 1:2,000. Bound secondary horseradish peroxidase-linked antibody was then detected by enhanced chemiluminescence (62).
RESULTS
Increased activity of an IL-2 gene reporter in the presence of HCV C protein.
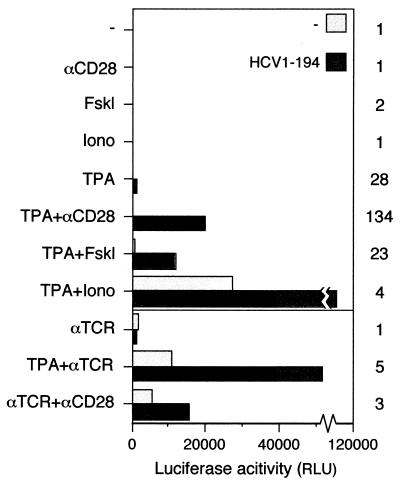
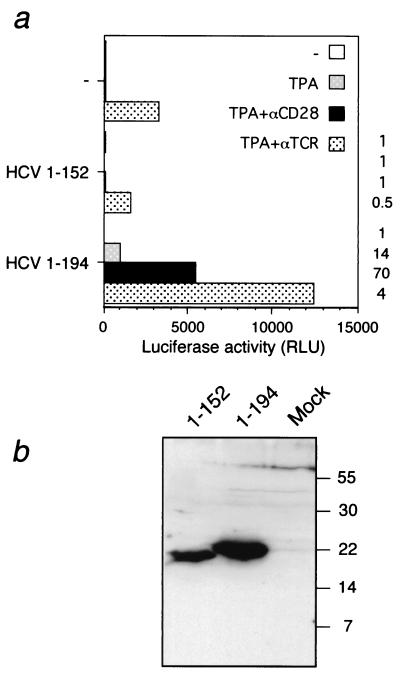
To investigate whether gene products of HCV can affect transcription of the IL-2 gene promoter, transient transfections were used to express viral protein in T cells. Since only a minority of the cells are susceptible to transfection, transcription from the IL-2 promoter was estimated indirectly in an enzymatic reporter assay. The possibility that the observed effects on enzyme activities were merely a result of posttranscriptional events rather than transcription per se was addressed in separate experiments (see below). Since the C protein has been shown to affect transcription from different promoters, we focused initially on this polypeptide. Jurkat cells were cotransfected with an effector plasmid, pOP/HCV1-194, and a reporter plasmid consisting of the murine IL-2 promoter adjacent to the luciferase gene. pOP/HCV1-194 expresses the proximal part of the viral genome encoding aa 1 to 194 and is processed to a 21-kDa form that is indistinguishable from C produced in the context of the entire HCV polyprotein. At 41 h posttransfection, cells were stimulated with different agents. Seven hours later, the cells were lysed and the luciferase activity was determined (Fig. 1). Without the C protein, transcription was observed when cells were stimulated with TPA in combination with either ionomycin or cross-linking of the TCR. Activity was also observed when antibodies against the TCR and the surface antigen CD28 were used simultaneously. Expression of the C protein alone in Jurkat cells was not sufficient to induce transcription from the IL-2 promoter. However, when cells transfected with pOP/HCV1-194 were stimulated with different inducers, a significant increase in luciferase activity was obtained. The level of activation was dependent upon the inducers used. The most dramatic effect of HCV C (20- to 130-fold increase) was observed when cells were stimulated with TPA alone or in combination with either anti-CD28 or forskolin. Much less (3- to 5-fold) activation by the C protein was observed with anti-TCR or ionomycin. In the absence of TPA or anti-TCR, no activity was detected. The same effects were obtained when a reporter construct containing the human IL-2 promoter was used (data not shown).
FIG. 1.
Activation of transcription from the IL-2 promoter by HCV C protein. Jurkat cells were cotransfected with pOP/HCV1-194 (HCV1-194), expressing the entire HCV C protein, together with the reporter plasmid IL2-LUC. As a negative control, plasmid pOPCMV/ct(−) or the empty vector pOPCMV (data not shown) was used. Cells were stimulated with TPA, forskolin (Fskl), ionomycin (Iono), or with antibodies to the TCR (αTCR) or CD28 (αCD28) as indicated. Luciferase activity was determined in a luminometer and is presented as relative light units (RLU). The activation factor is defined as the ratio of the activity obtained in the presence versus that in the absence of HCV C protein.
Gene reporter activation by HCV C is specific for NFAT elements.
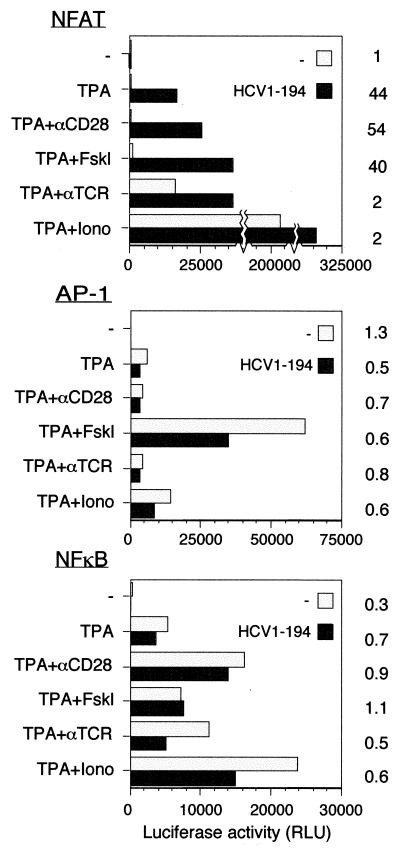
The upstream regulatory region of the IL-2 promoter is complex and consists of multiple binding sites for several different transcription factors (see Fig. 8) (64). To investigate whether activation by the C protein is a general effect on transcription or an effect specific for some of these upstream elements, a comparison between different promoters was performed. The effect of C on transcription was analyzed by using reporter plasmids containing multimers of binding sites for different transcription factors upstream of the luciferase gene. NFAT-luc contains a trimer of the target element for the transcription factor NFAT. 2×AP1-luc and NFκB-luc consist of dimers of the TPA-responsive element and the NFκB site, respectively. While transcription from 2×AP1-luc and NFκB-luc could be induced by different agents, no further activation was observed in the presence of HCV C (Fig. 2). In contrast, when NFAT-luc was used, an increased level of activity was observed in the presence of the C protein. When stimulated with TPA, alone or in combination with anti-CD28 or forskolin, HCV C activated transcription by 40- to 50-fold. When anti-TCR or ionomycin was used as a coinducer with TPA, high reporter activity was observed in the absence of the C protein. Under these conditions, the presence of HCV C only had an enhancing effect.
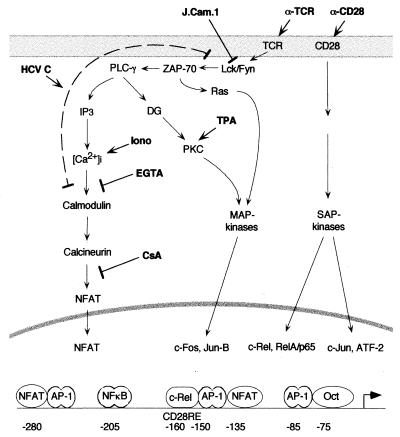
FIG. 8.
Simplified scheme of the structure of the IL-2 promoter and of signaling pathways involved in its regulation (adapted from reference 64). The targets for artificial inducers and inhibitors used in this study as well as putative targets for HCV C are indicated. See text for further details. PLC-γ, phospholipase C-γ; DG, diacylglycerol; Iono, ionomycin; MAP, mitogen-activated protein; SAP, stress-activated protein.
FIG. 2.
Transcriptional activation by HCV C protein can be mediated by NFAT response elements. Jurkat cells were cotransfected with an effector plasmid, pOP/HCV1-194 (HCV1-194), together with different reporter plasmids. As reporter plasmids, NFAT-luc (NFAT), 2×AP1-luc (AP-1), and NFκB-luc (NFκB) were used. As a negative control, the plasmid pOPCMV/ct(−) was used. Cells were stimulated as described in the legend to Fig. 1. Activation factors are shown to the right of the graph.
Transcriptional activation of an NFAT-controlled gene reporter by HCV C.
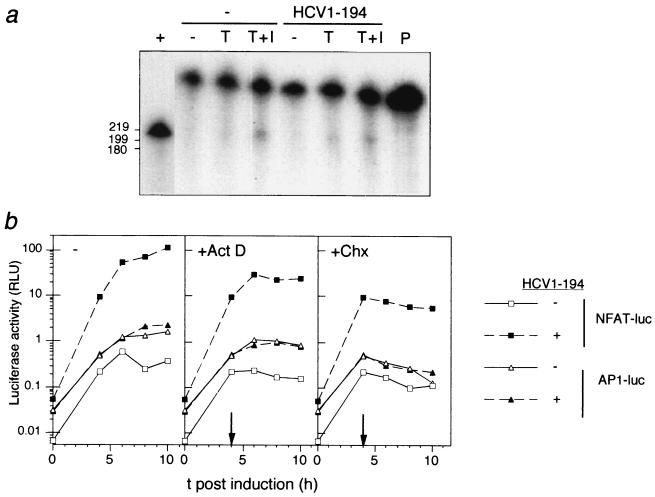
Reporter assays are indirect methods for estimation of transcriptional activity that depend on stability of the reporter enzyme and processing, translation, and degradation of RNA. In the analysis of different promoter elements shown in Fig. 2, both the entire luciferase coding region and most of the transcribed noncoding regions in the reporter plasmids were identical. Hence, it is likely that the specific effect of HCV C on luciferase activity, obtained with the reporter gene controlled by NFAT motifs, was due to increased transcription. Nevertheless, to directly address whether C affects the steady-state levels of luciferase RNA, an RNase protection assay was performed. Total RNA from Jurkat cells transfected with NFAT-luc together with either pOP/HCV1-194 or a negative control was analyzed. Cells stimulated with both TPA and ionomycin contained detectable levels of luciferase-specific RNA (Fig. 3a). Expression of HCV C in cells stimulated with TPA alone increased the steady-state level of specific RNA moderately, suggesting that the data obtained in the luciferase assays did not exclusively reflect an altered translation rate or protein stability. However, the increased steady-state levels of luciferase-specific RNA could be a result of altered processing or stability of RNA. To rule out this possibility, the kinetics of luciferase accumulation in transfected cells were characterized by using selective inhibitors that block synthesis of macromolecules. Since the reporter protein activities obtained with NFAT-luc in the absence of HCV were too small to give reproducible kinetics, 2×AP1-luc was included as a control. This reporter plasmid was not affected by the C protein (Fig. 2). At 4 h postinduction, transcription or translation was blocked with actinomycin D or cycloheximide, respectively. In the presence of cycloheximide, luciferase activity decayed exponentially with similar rates in all transfected cells, suggesting that luciferase degradation was slow, with an average protein half-life of 7 h (Fig. 3b). After blocking transcription by addition of actinomycin D, the kinetics of luciferase accumulation showed a similar pattern in all sets of cultures, suggesting that the translational efficiency was not significantly affected by the C protein. Furthermore, the accumulation of luciferase activity from 4 to 6 h postinduction was approximately 60% in the presence of actinomycin D compared to control cells without inhibitor, suggesting that the half-life of the mRNA was less than 2 h and was not dependent on either reporter plasmid or expression of HCV C.
FIG. 3.
(a) Steady-state levels of luciferase-specific RNA determined in an RNase protection assay. Jurkat cells were cotransfected with a reporter plasmid, NFAT-luc, together with either an effector plasmid, pOP/HCV1-194 (HCV1-194), or a control plasmid, pOPCMV/ct(−). Where indicated, cells were stimulated with TPA (T) and ionomycin (I) at 40 h posttransfection. At 7 h postinduction, cellular RNA was extracted, and luciferase-specific RNA was detected by RNase protection analysis. As a positive control (+), in vitro-transcribed RNA corresponding to the proximal part of the luciferase gene was used (shorter exposure in autoradiography). Undigested probe (P) was loaded to the right, and the positions of labeled RNA markers run in parallel are indicated to the left (in nt). (b) Kinetics of luciferase accumulation in transfected cells in the presence of transcriptional and translational inhibitors. Jurkat cells were cotransfected with pOP/HCV1-194 (HCV1-194) together with either NFAT-luc or 2×AP1-luc. As a negative control, plasmid pOPCMV/ct(−) was used. Forty hours posttransfection (zero hours in the graph), the cells were stimulated with TPA. Where indicated with arrows, transcription and translation were blocked by addition of actinomycin D (Act D) and cycloheximide (Chx) to final concentrations of 2 and 10 μg/ml, respectively. At different intervals, the cells were harvested and the luciferase activity was determined.
C-terminal part of HCV C protein is required for transcriptional activation.
Mutational analyses of HCV C have revealed that the carboxy-terminal domain of the protein is not required for either transcriptional modulation of the c-myc and hepatitis B virus (HBV) promoters (54, 67) or interaction with the lymphotoxin-β receptor (12, 44). To investigate whether the carboxy-terminal part of C is required for transcriptional activation of the IL-2 promoter, a deletion mutant encoding the first 152 aa was made (pOP/HCV1-152). Jurkat cells were transfected with this mutant and then stimulated with TPA, alone or in combination with antibodies to either CD28 or TCR. In contrast to full-length HCV C, no activation of transcription from the IL-2 promoter was observed with pOP/HCV1-152 when cells were stimulated with TPA and antibodies to CD28 (Fig. 4a). Expression of the predicted C protein forms from these plasmids was confirmed by Western blotting (Fig. 4b). Although the truncated form of HCV C was detected at slightly lower levels than pOP/HCV1-194, this difference was substantially less than the effect observed on transcription. In addition, the inability of pOP/HCV1-152 to stimulate transcription from the IL-2 promoter was reproduced with three different transfection procedures (data not shown). Additional C-terminal deletion mutants encoding C proteins terminating after aa 123, 80, or 61 were also unable to increase transcription from the IL-2 promoter (data not shown). However, these negative results are difficult to interpret since we were unable to verify the expression of these small and highly basic polypeptides by Western blotting.
FIG. 4.
(a) Effect of deletion of the C terminus of HCV C protein on transactivation. Jurkat cells were cotransfected with IL2-LUC together with either pOP/HCV1-194 (HCV1-194) or pOP/HCV1-152 (HCV1-152). As a negative control, plasmid pOPCMV/ct(−) was used. Cells were stimulated and luciferase activity was determined as described in the legend to Fig. 1. Activation factors, defined by the ratio of luciferase activity of HCV1-194 or HCV1-152 to the control plasmid, is shown to the right of the graph. (b) Detection of HCV C proteins by Western blotting. Jurkat cells were transfected with plasmids expressing either the full-length pOP/HCV1-194 (HCV1-194) or truncated pOP/HCV1-152 (HCV1-152) HCV C protein. Cellular extracts were subjected to electrophoresis on a 15% polyacrylamide gel. The proteins were transferred to a nitrocellulose membrane, and the C protein was detected using an HCV C-specific monoclonal antibody. The positions of molecular size markers are indicated at the right (in kilodaltons).
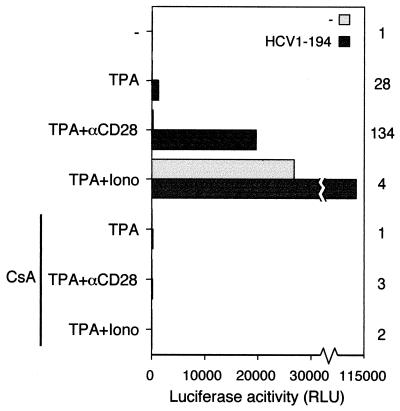
Activation of transcription from the IL-2 promoter by the HCV C protein is sensitive to cyclosporin A.
DNA binding and activation by the transcription factor NFAT is sensitive to the immune-suppressive drug cyclosporin A (22). This effect is mediated by inactivation of the phosphatase calcineurin, which becomes unable to dephosphorylate the cytoplasmic subunit of NFAT, preventing its translocation to the nucleus (6). To investigate whether transcriptional activation by HCV C is sensitive to inhibition of calcineurin, cyclosporin A was added prior to stimulation. In the presence of cyclosporin A, no induction of transcription was observed when different inducers were added to the cells (Fig. 5). Transcriptional activation of the IL-2 promoter by the HTLV-1 protein Tax is not sensitive to inhibition of calcineurin by cyclosporin A (69). Cotransfection experiments revealed that HCV C and HTLV-1 Tax could activate transcription from the IL-2 promoter synergistically (data not shown). When cyclosporin A was added prior to induction, the activity returned to the level obtained with HTLV-1 Tax alone.
FIG. 5.
Activation of transcription from the IL-2 promoter by HCV C protein is sensitive to cyclosporin A. Transient-transfection assays were performed as described in the legend to Fig. 1. Where indicated, cyclosporin A (CsA) at a concentration of 50 ng/ml was added to the cells 30 min prior to stimulation with TPA, anti-CD28, or ionomycin (Iono). Some of the data presented are also shown in Fig. 1. Activation factors are shown to the right of the graph.
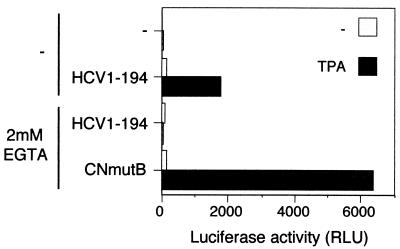
Activation by the HCV C protein is inhibited by depletion of extracellular calcium.
Engagement of the TCR in Jurkat cells causes a rapid increase in the concentration of intracellular calcium, which is a prerequisite for nuclear translocation of the transcription factor NFAT (14, 36). To investigate whether the effect on transcription by HCV C is dependent on the presence of calcium, the effect on removal of calcium was analyzed. Prior to induction, calcium was removed from the environment by replacing the original medium with calcium-deficient medium containing the chelator EGTA. Under these conditions, TPA stimulation of cells expressing the C protein had no effect on transcription (Fig. 6). To verify that the cells were indeed responsive to TPA during this treatment, a parallel set of cells was transfected with plasmid pBJ5-CNMUT2B. This plasmid encodes a mutant calcineurin that is constitutively active and therefore independent of stimulation by calcium (14). When transfected with pBJ5-CNMUT2B, transcription could be induced by TPA in cells depleted of calcium.
FIG. 6.
Transcriptional activation by the HCV C protein is abrogated by depletion of calcium. Jurkat cells were cotransfected with NFAT-luc and either pOP/HCV1-194 (HCV1-194), negative control pOPCMV/ct(−), or pBJ5-CNMUT2B (CNmutB). One hour before stimulation with TPA, one half of each set of cells was shifted to calcium-deficient medium containing 2 mM EGTA. At 7 h poststimulation, luciferase activity was determined.
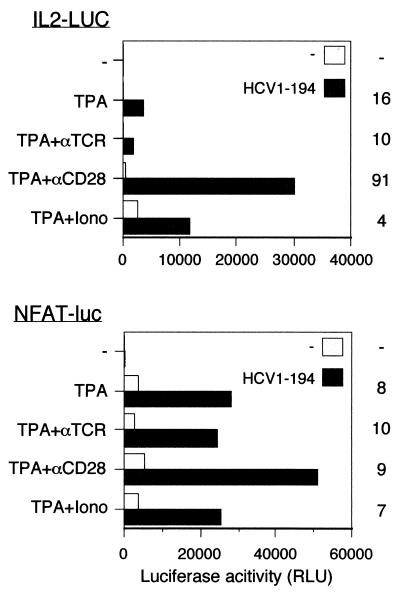
Transcriptional activation by the HCV C protein is independent of the protein tyrosine kinase p56lck.
The Src-related protein tyrosine kinase p56lck is required for induction of IL-2 synthesis induced by stimulation of the TCR (70). The J.CaM.1 cell line is a p56lck-deficient derivative of Jurkat cells (70). J.CaM.1 cells were cotransfected with pOP/HCV1-194 together with a luciferase reporter plasmid to investigate whether p56lck is required for transcriptional activation by HCV C. The entire IL-2 promoter and the NFAT motif linked to the luciferase gene were used as reporter plasmids. When J.CaM.1 cells were stimulated with TPA, an 8- to 16-fold increase in transcription was observed in the presence of HCV C with the entire IL-2 promoter as well as with NFAT-luc (Fig. 7). Less effect on transcription was obtained by the C protein when J.CaM.1 cells were stimulated with TPA in combination with ionomycin.
FIG. 7.
Activation of transcription by the HCV C protein in cells deficient in the p56lck kinase. J.CaM.1 cells were cotransfected with pOP/HCV1-194 (HCV1-194) and either IL2-LUC or NFAT-luc. As a negative control, plasmid pOPCMV/ct(−) was used. Activation factors are shown to the right of the graph.
DISCUSSION
In this report, we show that expression of full-length but not truncated HCV C protein can activate NFAT-mediated transcription of the IL-2 promoter in Jurkat cells. IL-2 synthesis in T cells is tightly regulated at the transcriptional level; we conducted several studies to define the step at which the HCV C protein might act. Transcription of the IL-2 gene is induced by engagement of cell surface receptors, which results in triggering of signal transduction cascades and, subsequently, binding of different transcription factors to specific elements in the IL-2 promoter (Fig. 8) (21, 64). Cross-linking TCRs initiates signals that involve tyrosine kinase-dependent activation of phospholipase C-γ and generation of diacylglycerol and inositol-1,4,5-trisphosphate (IP3) (16, 33). Downstream signaling includes activation of both p21ras mitogen-activated protein kinase- (35) and calcium-dependent pathways (34). Under normal circumstances, stimulation of multiple Jurkat cell surface receptors is required to induce IL-2 transcription (42). The requirements for induction can be bypassed in vitro by activation of protein kinase C with phorbol esters and by triggering intracellular calcium release with ionophores (21). We found that HCV C was able to substitute for some of these stimuli. In the presence of HCV C, induction of transcription from the IL-2 promoter was observed in cells stimulated with TPA alone. When the cells were costimulated with anti-CD28 or forskolin, a significant increase in luciferase activity was observed in the presence of the C protein. In contrast, HCV C was much less potent in activating transcription when cells were costimulated with ionomycin or anti-TCR, both of which induce calcium release into the cytoplasm. Released calcium binds to calmodulin, which then activates the phosphatase calcineurin (14). Upon dephosphorylation by calcineurin, the cytoplasmic component of NFAT translocates to the nucleus, where it cooperates with members of the Fos and Jun families to bind DNA (36).
HCV C did not have a general effect on transcription, since activation was only observed when NFAT binding sites were present in the reporter plasmid (Fig. 2), suggesting that NFAT elements were sufficient for activation of the IL-2 promoter by the C protein. The requirement for NFAT activation was further supported by the observation that HCV C substitutes for inducers that trigger release of calcium. In contrast to their effect on the entire IL-2 promoter, forskolin and anti-CD28 were inactive with the trimeric NFAT motif repeats. This is consistent with studies that had mapped the elements responsive to these agents outside of the NFAT motifs (13, 23). The AP-1 and NFκB motifs in the IL-2 promoter are nonconsensus motifs that differ slightly from the elements present in the reporter constructs used in this study (64). Since these differences may result in altered affinity for transcription factors, an auxiliary role for elements other than the NFAT motifs cannot be excluded.
Activation of the IL-2 promoter by HCV C was abrogated by addition of cyclosporin A as well as by depletion of extracellular calcium by addition of EGTA. Cyclosporin A inhibits NFAT-dependent transcription by preventing calcineurin-dependent dephosphorylation of NFAT (22). The cyclosporin A results suggest that the effect of HCV C was mediated by the transcription factor NFAT and that active calcineurin was required for this activity. The sensitivity to calcium depletion suggests that release of calcium into the cytoplasm is required for transcriptional activation by the C protein. Hence, HCV C must be acting upstream of both these events. The T-cell-specific tyrosine kinase p56lck is required for induction of IL-2 synthesis upon stimulation of the TCR (70). Hence, TCR cross-linking induces neither calcium release nor IL-2 synthesis in the p56lck-deficient cell line J.CaM.1. In these cells, HCV C was able to induce transcription from the entire IL-2 promoter as well as from multiple NFAT elements. These results showed that p56lck was dispensable for transcriptional activation by HCV C, suggesting that the C protein must act downstream of p56lck in the signal transduction cascade.
To induce high-level transcription from the entire IL-2 promoter in the presence of HCV C and TPA, costimulation by either anti-CD28 or forskolin was required. Cross-linking CD28 includes signals that involve phosphorylation of c-Jun and IκB-a by stress-activated protein kinases (75). Transcriptional activation has been reported to be mediated by binding of c-Rel (26, 65) or RelA/p65 (38) to CD28RE, a κB-like site in the IL-2 promoter (23). The adenylate cyclase activator forskolin has been widely used as an inducer of cyclic AMP-dependent kinase (protein kinase A [PKA]). PKA has several potential targets in the cell and probably exerts several effects. HCV C can be phosphorylated in vitro by both PKA and PKC (66). However, it is unlikely that the effect of forskolin was due to phosphorylation of the C protein alone, since the result was dependent on promoter type (Fig. 1 and 2). In stimulated Jurkat cells, activation of PKA by forskolin causes impaired nuclear translocation and DNA binding of RelA/p65, resulting in decreased transcription of the IL-2 gene (48). However, under these conditions both the expression and DNA binding of c-Rel were increased. When analyzing the effects of HCV C on transcriptional activation, we observed similar effects by costimulation of either PKA or CD28. Therefore, it is conceivable that a single factor was triggered by two independent pathways. One potential factor is c-Rel, which can be induced by both forskolin and anti-CD28 (26, 48, 65).
Effects on transcription of different promoters by HCV C have been described before (54, 67). However, our data for the IL-2 promoter differ from previously published results. Whereas the first 122 aa of HCV C were sufficient for modulating the c-myc and HBV promoters, the C-terminal portion was required for activation of the IL-2 promoter since a truncated derivative consisting of residues 1 to 152 was inactive (Fig. 2). The presence of the carboxy-terminal part of HCV C has been shown to be critical for its localization to the perinuclear endoplasmic reticulum (ER) membrane (52, 61, 71). This suggests that the subcellular localization of the C protein or some other function of the C-terminal hydrophobic residues may be of importance for its ability to stimulate transcription from the IL-2 promoter. Release of calcium from intracellular stores upon TCR stimulation is mediated via activation of the IP3 receptor, which is located in the ER membrane (49, 50). One conceivable model is that ER-localized HCV C stimulates calcium signaling, either by increasing the basal concentration of calcium in the ER, resulting in a more vigorous response upon stimulation, or by facilitating calcium release.
Although neutralizing antibodies provide protective immunity, most viral infections are cleared by CTL-mediated immune response. In HBV infections, viral replication is dramatically downregulated by the cytokines TNF-α and IFN-γ without major cytopathology (27, 28). It has been suggested that, besides lysis by CTLs, antiviral cytokines might also be of importance for clearance in HCV infections (10). Antiviral cytokines are associated with a type 1 immune response. The significance of the cytokine profile in HCV-infected patients has been addressed in clinical studies. In one study, secretion of the type 1 cytokines IFN-γ and IL-2 in the acute phase of the infection was associated with recovery (74). In another report, type 1 cytokines were found in healthy anti-HCV-positive donors, while a type 2 cytokine profile characterized chronic HCV carriers (76a). Therapy with IFN-α has been shown to reduce the viral load in chronic HCV patients (19). Treatment with IFN-α is accompanied by decreased secretion of the type 2 cytokines IL-4 and IL-10 (8), but not by stimulation of CTL activity (59). Since IL-2 is a T-cell differentiation factor, it is conceivable that altered inducibility of IL-2 by the C protein might affect the T-cell phenotype and the cytokine profile. Interestingly, expression of HCV C by recombinant vaccinia virus in mice was recently found by Large et al. (40) to be associated with an immune suppression characterized by a decreased CTL response and decreased expression of the cytokines IFN-γ and, to lesser extent, IL-2. We do not know whether the reason for the different effects on IL-2 expression in the in vivo study compared to our study are due to species-specific factors or other differences in the experimental systems. Finally, it should be noted that, besides IL-2, other genes—e.g., the genes for the cytokines IL-4, TNF-α, IFN-γ, and granulocyte-macrophage colony-stimulating factor—have binding sites for NFAT in their promoter regions (51). It is thus conceivable that their synthesis is also influenced by HCV C. If so, some of these effects on other genes might be even more relevant for HCV pathogenesis than the effect on the IL-2 promoter.
In summary, a form of HCV C that is present during natural infection in humans was found to promote transcription from the IL-2 promoter in Jurkat cells in the presence of other stimuli. Activation was mediated by the transcription factor NFAT. Although the biological relevance of this novel C protein activity in the context of HCV infection remains to be established, further studies to examine this pathway in other human T-cell lines to confirm these results or uncover cell type-specific differences are warranted and may lead to a better understanding of the mechanisms involved in HCV persistence and pathogenesis.
ACKNOWLEDGMENTS
We thank the following colleagues for fruitful discussions and for providing antibodies, plasmids, and cell lines: Andrew C. Chan, Kenneth M. Murphy, Talal Shatila, Thomas H. Steinberg, and Jack R. Wands. We are also grateful to Arash Grakoui, Göran Magnusson, Stefan Schwartz, and Catharina Svensson for critical reading of the manuscript.
This work was supported by Public Health Service grant CA57973 (C.M.R.), the Swedish Cancer Society (A.B.), and the Swedish Foundation for Strategic Research (A.B.). A.B. was supported by fellowships from the Swedish Society for Medical Research, the Wenner-Gren Center Foundation for Scientific Research, the Swedish Institute, and the Swedish Council for Medical Research.
REFERENCES
- 1.Alter M J. Epidemiology of hepatitis C. Hepatology. 1997;26:62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 2.Bassett S E, Brasky K M, Lanford R E. Analysis of hepatitis C virus-inoculated chimpanzees reveals unexpected clinical profiles. J Virol. 1998;72:2589–2599. doi: 10.1128/jvi.72.4.2589-2599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassett S E, Thomas D L, Brasky K M, Lanford R E. Viral persistence, antibody to E1 and E2, and hypervariable region 1 sequence stability in hepatitis C virus-inoculated chimpanzees. J Virol. 1999;73:1118–1126. doi: 10.1128/jvi.73.2.1118-1126.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blight K J, Lesniewski R R, LaBrooy J T, Gowans E J. Detection and distribution of hepatitis C-specific antigens in naturally infected liver. Hepatology. 1994;20:553–557. [PubMed] [Google Scholar]
- 5.Bouffard P, Hayashi P H, Acevedo R, Levy N, Zeldis J B. Hepatitis C virus is detected in a monocyte/macrophage subpopulation of peripheral blood mononuclear cells of infected patients. J Infect Dis. 1992;166:1276–1280. doi: 10.1093/infdis/166.6.1276. [DOI] [PubMed] [Google Scholar]
- 6.Bram R J, Hung D T, Martin P K, Schreiber S L, Crabtree G R. Identification of the immunophilins capable of mediating inhibition of signal transduction by cyclosporin A and FK506: roles of calcineurin binding and cellular location. Mol Cell Biol. 1993;13:4760–4769. doi: 10.1128/mcb.13.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bronowicki J P, Loriot M A, Thiers V, Grignon Y, Zignego A L, Brechot C. Hepatitis C virus persistence in human hematopoietic cells injected into SCID mice. Hepatology. 1998;28:211–218. doi: 10.1002/hep.510280127. [DOI] [PubMed] [Google Scholar]
- 8.Cacciarelli T V, Martinez O M, Gish R G, Villanueva J C, Krams S M. Immunoregulatory cytokines in chronic hepatitis C virus infection: pre- and posttreatment with interferon alfa. Hepatology. 1996;24:6–9. doi: 10.1002/hep.510240102. [DOI] [PubMed] [Google Scholar]
- 9.Chan A C, Irving B A, Fraser J D, Weiss A. The ζ chain is associated with a tyrosine kinase and upon T-cell antigen receptor stimulation associates with ZAP-70, a 70-kDa tyrosine phosphoprotein. Proc Natl Acad Sci USA. 1991;88:9166–9170. doi: 10.1073/pnas.88.20.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang K M, Rehermann B, Chisari F V. Immunopathology of hepatitis C. Springer Semin Immunopathol. 1997;19:57–68. doi: 10.1007/BF00945025. [DOI] [PubMed] [Google Scholar]
- 11.Chang S C, Yen J H, Kang H Y, Jang M H, Chang M F. Nuclear localization signals in the core protein of hepatitis C virus. Biochem Biophys Res Commun. 1994;205:1284–1290. doi: 10.1006/bbrc.1994.2804. [DOI] [PubMed] [Google Scholar]
- 12.Chen C M, You L R, Hwang L H, Lee Y H. Direct interaction of hepatitis C virus core protein with the cellular lymphotoxin-beta receptor modulates the signal pathway of the lymphotoxin-beta receptor. J Virol. 1997;71:9417–9426. doi: 10.1128/jvi.71.12.9417-9426.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen D, Rothenberg E V. Interleukin 2 transcription factors as molecular targets of cAMP inhibition: delayed inhibition kinetics and combinatorial transcription roles. J Exp Med. 1994;179:931–942. doi: 10.1084/jem.179.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clipstone N A, Crabtree G R. Identification of calcineurin as a key signaling enzyme in T-lymphocyte activation. Nature (London) 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 15.Cooper S, Erickson A L, Adams E J, Kansopon J, Weiner A J, Chien D Y, Houghton M, Parham P, Walker C M. Analysis of a successful immuner response against hepatitis C virus. Immunity. 1999;10:439–449. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- 16.Crabtree G R, Clipstone N A. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem. 1994;63:1045–1083. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- 17.Cribier B, Schmitt C, Bingen A, Kirn A, Keller F. In vitro infection of peripheral blood mononuclear cells by hepatitis C virus. J Gen Virol. 1995;76:2485–2491. doi: 10.1099/0022-1317-76-10-2485. [DOI] [PubMed] [Google Scholar]
- 18.Curfs J H, Meis J F, Hoogkamp-Korstanje J A. A primer on cytokines: sources, receptors, effects, and inducers. Clin Microbiol Rev. 1997;10:742–780. doi: 10.1128/cmr.10.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Bisceglie A M. Hepatitis C. Lancet. 1998;351:351–355. doi: 10.1016/S0140-6736(97)07361-3. [DOI] [PubMed] [Google Scholar]
- 20.Diepolder H M, Zachoval R, Hoffmann R M, Wierenga E A, Santantonio T, Jung M C, Eichenlaub D, Pape G R. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346:1006–1007. doi: 10.1016/s0140-6736(95)91691-1. [DOI] [PubMed] [Google Scholar]
- 21.Favero J, Lafont V. Effector pathways regulating T cell activation. Biochem Pharmacol. 1998;56:1539–1547. doi: 10.1016/s0006-2952(98)00213-5. [DOI] [PubMed] [Google Scholar]
- 22.Flanagan W M, Corthesy B, Bram R J, Crabtree G R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991;352:803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- 23.Fraser J D, Irving B A, Crabtree G R, Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991;251:313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- 24.Gale M, Jr, Blakely C M, Kwieciszewski B, Tan S L, Dossett M, Tang N M, Korth M J, Polyak S J, Gretch D R, Katze M G. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol Cell Biol. 1998;18:5208–5218. doi: 10.1128/mcb.18.9.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gale M J, Jr, Korth M J, Tang N M, Tan S L, Hopkins D A, Dever T E, Polyak S J, Gretch D R, Katze M G. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology. 1997;230:217–227. doi: 10.1006/viro.1997.8493. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh P, Tan T H, Rice N R, Sica A, Young H A. The interleukin 2 CD28-responsive complex contains at least three members of the NF kappa B family: c-Rel, p50, and p65. Proc Natl Acad Sci USA. 1993;90:1696–1700. doi: 10.1073/pnas.90.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guidotti L G, Ando K, Hobbs M V, Ishikawa T, Runkel L, Schreiber R D, Chisari F V. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc Natl Acad Sci USA. 1994;91:3764–3768. doi: 10.1073/pnas.91.9.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 29.Hattori M, Tugores A, Westwick J, Veloz L, Leffert H L, Karin M, Brenner D A. Activation of activator protein 1 during hepatic acute phase response. Am J Physiol. 1993;264:G95–G103. doi: 10.1152/ajpgi.1993.264.1.G95. [DOI] [PubMed] [Google Scholar]
- 30.Hoofnagle J H. Hepatitis C: the clinical spectrum of disease. Hepatology. 1997;26:15S–20S. doi: 10.1002/hep.510260703. [DOI] [PubMed] [Google Scholar]
- 31.Hsu D H, de Waal Malefyt R, Fiorentino D F, Dang M N, Vieira P, de Vries J, Spits H, Mosmann T R, Moore K W. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 1990;250:830–832. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- 32.Hussy P, Langen H, Mous J, Jacobsen H. Hepatitis C virus core protein: carboxy-terminal boundaries of two processed species suggest cleavage by a signal peptide peptidase. Virology. 1996;224:93–104. doi: 10.1006/viro.1996.0510. [DOI] [PubMed] [Google Scholar]
- 33.Imboden J B, Stobo J D. Transmembrane signalling by the T cell antigen receptor: perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985;161:446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imboden J B, Weiss A, Stobo J D. The antigen receptor on a human T cell line initiates activation by increasing cytoplasmic free calcium. J Immunol. 1985;134:663–665. [PubMed] [Google Scholar]
- 35.Izquierdo M, Leevers S J, Marshall C J, Cantrell D. p21ras couples the T cell antigen receptor to extracellular signal-regulated kinase 2 in T lymphocytes. J Exp Med. 1993;178:1199–1208. doi: 10.1084/jem.178.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain J, McCaffrey P G, Miner Z, Kerppola T K, Lambert J N, Verdine G L, Curran T, Rao A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature (London) 1993;365:352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- 37.Kolykhalov A A, Agapov E V, Blight K J, Mihalik K, Feinstone S M, Rice C M. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science. 1997;277:570–574. doi: 10.1126/science.277.5325.570. [DOI] [PubMed] [Google Scholar]
- 38.Lai J H, Horvath G, Subleski J, Bruder J, Ghosh P, Tan T H. RelA is a potent transcriptional activator of the CD28 response element within the interleukin-2 promoter. Mol Cell Biol. 1995;15:4260–4671. doi: 10.1128/mcb.15.8.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lanford R E, Chavez D, Chisari F V, Sureau C. Lack of detection of negative-strand hepatitis C virus RNA in peripheral blood mononuclear cells and other extrahepatic tissues by the highly strand-specific rTth reverse transcriptase-PCR. J Virol. 1995;69:8079–8083. doi: 10.1128/jvi.69.12.8079-8083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Large M K, Kittlesen D J, Hahn Y S. Suppression of host immune response by the core protein of hepatitis C virus: possible implications for hepatitis C virus persistence. J Immunol. 1999;162:931–938. [PubMed] [Google Scholar]
- 41.Lemon S H, Honda M. Internal ribosome entry sites within the RNA genomes of hepatitis C virus and other flaviviruses. Semin Virol. 1997;8:274–288. [Google Scholar]
- 42.Linsley P S, Greene J L, Tan P, Bradshaw J, Ledbetter J A, Anasetti C, Damle N K. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992;176:1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lo S-Y, Masiarz F, Hwang S B, Lai M M C, Ou J-H. Differential subcellular localization of hepatitis C virus core gene products. Virology. 1995;213:455–461. doi: 10.1006/viro.1995.0018. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto M, Hsieh T-Y, Zhu N, van Arsdale T, Hwang S B, Jeng K-S, Gorbalenya A E, Lo S-Y, Ou J-H, Ware C F, Lai M M C. Hepatitis C virus core protein interacts with the cytoplasmic tail of lymphotoxin-B receptor. J Virol. 1997;71:1301–1309. doi: 10.1128/jvi.71.2.1301-1309.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizutani T, Kato N, Saito S, Ikeda M, Sugiyama K, Shimotohno K. Characterization of hepatitis C virus replication in cloned cells obtained from a human T-cell leukemia virus type 1-infected cell line, MT-2. J Virol. 1996;70:7219–7223. doi: 10.1128/jvi.70.10.7219-7223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moradpour D, Englert C, Wakita T, Wands J R. Characterization of cell lines allowing tightly regulated expression of hepatitis C virus core protein. Virology. 1996;222:51–63. doi: 10.1006/viro.1996.0397. [DOI] [PubMed] [Google Scholar]
- 47.Moradpour D, Wakita T, Tokushige K, Carlson R I, Krawczynski K, Wands J R. Characterization of three novel monoclonal antibodies against hepatitis C virus core protein. J Med Virol. 1996;48:234–241. doi: 10.1002/(SICI)1096-9071(199603)48:3<234::AID-JMV4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 48.Neumann M, Grieshammer T, Chuvpilo S, Kneitz B, Lohoff M, Schimpl A, Franza B R, Jr, Serfling E. RelA/p65 is a molecular target for the immunosuppressive action of protein kinase A. EMBO J. 1995;14:1991–2004. doi: 10.1002/j.1460-2075.1995.tb07191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48a.Northrop J P, Ullman K S, Crabtree G R. Characterization of the nuclear and cytoplasmic components of lymphoid specific nuclear factor of activated T cells (NF-AT) complex. J Biol Chem. 1993;268:2917–2923. [PubMed] [Google Scholar]
- 49.Putney J W., Jr Type 3 inositol 1,4,5-trisphosphate receptor and capacitative calcium entry. Cell Calcium. 1997;21:257–261. doi: 10.1016/s0143-4160(97)90050-6. [DOI] [PubMed] [Google Scholar]
- 50.Rao A. Signaling mechanisms in T cells. Crit Rev Immunol. 1991;10:495–519. [PubMed] [Google Scholar]
- 51.Rao A, Luo C, Hogan P G. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 52.Ravaggi A, Natoli G, Primi D, Albertini A, Levrero M, Cariani E. Intracellular localization of full-length and truncated hepatitis C virus core protein expressed in mammalian cells. J Hepatol. 1994;20:833–836. doi: 10.1016/s0168-8278(05)80157-6. [DOI] [PubMed] [Google Scholar]
- 53.Ray R B, Ghosh A K, Meyer K, Ray R. Functional analysis of a transrepressor domain in the hepatitis C virus core protein. Virus Res. 1999;59:211–217. doi: 10.1016/s0168-1702(98)00138-5. [DOI] [PubMed] [Google Scholar]
- 54.Ray R B, Lagging L M, Meyer K, Steele R, Ray R. Transcriptional regulation of cellular and viral promoters by the hepatitis C virus core protein. Virus Res. 1995;37:209–220. doi: 10.1016/0168-1702(95)00034-n. [DOI] [PubMed] [Google Scholar]
- 55.Ray R B, Meyer K, Steele R, Shrivastava A, Aggarwal B B, Ray R. Inhibition of tumor necrosis factor (TNF-alpha)-mediated apoptosis by hepatitis C virus core protein. J Biol Chem. 1998;273:2256–2259. doi: 10.1074/jbc.273.4.2256. [DOI] [PubMed] [Google Scholar]
- 56.Ray R B, Steele R, Meyer K, Ray R. Hepatitis C virus core protein represses p21WAF1/Cip1/Sid1 promoter activity. Gene. 1998;208:331–336. doi: 10.1016/s0378-1119(98)00030-4. [DOI] [PubMed] [Google Scholar]
- 57.Ray R B, Steele R, Meyer K, Ray R. Transcriptional repression of p53 promoter by hepatitis C virus core protein. J Biol Chem. 1997;272:10983–10986. doi: 10.1074/jbc.272.17.10983. [DOI] [PubMed] [Google Scholar]
- 58.Reed K E, Rice C M. Molecular characterization of hepatitis C virus. Curr Stud Hematol Blood Transfus. 1998;62:1–37. doi: 10.1159/000060472. [DOI] [PubMed] [Google Scholar]
- 59.Rehermann B, Chang K M, McHutchison J G, Kokka R, Houghton M, Chisari F V. Quantitative analysis of the peripheral blood cytotoxic T lymphocyte response in patients with chronic hepatitis C virus infection. J Clin Investig. 1996;98:1432–1440. doi: 10.1172/JCI118931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rice C M. Flaviviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 1. New York, N.Y: Lippincott-Raven Publishers; 1996. pp. 931–959. [Google Scholar]
- 61.Santolini E, Migliaccio G, La Monica N. Biosynthesis and biochemical properties of the hepatitis C virus core protein. J Virol. 1994;68:3631–3641. doi: 10.1128/jvi.68.6.3631-3641.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schneppenheim R, Budde U, Dahlmann N, Rautenberg P. Luminography—a new, highly sensitive visualization method for electrophoresis. Electrophoresis. 1991;12:367–372. doi: 10.1002/elps.1150120508. [DOI] [PubMed] [Google Scholar]
- 63.Seow H F. Pathogen interactions with cytokines and host defence: an overview. Vet Immunol Immunopathol. 1998;63:139–148. doi: 10.1016/s0165-2427(98)00090-7. [DOI] [PubMed] [Google Scholar]
- 64.Serfling E, Avots A, Neumann M. The architecture of the interleukin-2 promoter: a reflection of T lymphocyte activation. Biochim Biophys Acta. 1995;1263:181–200. doi: 10.1016/0167-4781(95)00112-t. [DOI] [PubMed] [Google Scholar]
- 65.Shapiro V S, Truitt K E, Imboden J B, Weiss A. CD28 mediates transcriptional upregulation of the interleukin-2 (IL-2) promoter through a composite element containing the CD28RE and NF-IL-2B AP-1 sites. Mol Cell Biol. 1997;17:4051–4058. doi: 10.1128/mcb.17.7.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shih C M, Chen C M, Chen S Y, Lee Y H. Modulation of the trans-suppression activity of hepatitis C virus core protein by phosphorylation. J Virol. 1995;69:1160–1171. doi: 10.1128/jvi.69.2.1160-1171.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shih C M, Lo S J, Miyamura T, Chen S Y, Lee Y H. Suppression of hepatitis B virus expression and replication by hepatitis C virus core protein in HuH-7 cells. J Virol. 1993;67:5823–5832. doi: 10.1128/jvi.67.10.5823-5832.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shimizu Y K, Iwamoto A, Hijikata M, Purcell R H, Yoshikura H. Evidence for in vitro replication of hepatitis C virus genome in a human T-cell line. Proc Natl Acad Sci USA. 1992;89:5477–5481. doi: 10.1073/pnas.89.12.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siekewitz M, Feinberg M B, Holbrook N J, Wong-Staal F, Greena W C. Activation of the interleukin-2 and interleukin-2 receptor (Tac) promoter expression by the transactivator (tat) gene product of human T cell leukemia virus type I. Proc Natl Acad Sci USA. 1987;84:5389–5393. doi: 10.1073/pnas.84.15.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Straus D, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki R, Matsuura Y, Susuki T, Ando A, Chiba J, Harada S, Saito I, Miyamura T. Nuclear localization of the truncated hepatitis C virus core protein with its hydrophobic C terminus deleted. J Gen Virol. 1995;76:53–61. doi: 10.1099/0022-1317-76-1-53. [DOI] [PubMed] [Google Scholar]
- 71a.Szabo S J, Gold J S, Murphy T L, Murphy K M. Identification of cis-acting regulatory elements controlling interleukin-4 gene expression in T cells: roles for NF-Y and NF-ATc. Mol Cell Biol. 1993;13:4793–4805. doi: 10.1128/mcb.13.8.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taylor D R, Shi S T, Romano P R, Barber G N, Lai M M. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science. 1999;285:107–110. doi: 10.1126/science.285.5424.107. [DOI] [PubMed] [Google Scholar]
- 73.Thanos D, Maniatis T. The high mobility group protein HMG I(Y) is required for NF-kappa B-dependent virus induction of the human IFN-beta gene. Cell. 1992;71:777–789. doi: 10.1016/0092-8674(92)90554-p. [DOI] [PubMed] [Google Scholar]
- 74.Tsai S L, Liaw Y F, Chen M H, Huang C Y, Kuo G C. Detection of type 2-like T-helper cells in hepatitis C virus infection: implications for hepatitis C virus chronicity. Hepatology. 1997;25:449–458. doi: 10.1002/hep.510250233. [DOI] [PubMed] [Google Scholar]
- 75.Ward S G. CD28: a signalling perspective. Biochem J. 1996;318:361–377. doi: 10.1042/bj3180361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Westendorp M O, Li W M, Frank R W, Krammer P H. Human immunodeficiency virus type 1 Tat upregulates interleukin-2 secretion in activated T cells. J Virol. 1994;68:4177–4185. doi: 10.1128/jvi.68.7.4177-4185.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76a.Woitas R P, Lechmann M, Jung G, Kaiser R, Sauerbruch T, Spengler U. CD30 induction and cytokine profiles in hepatitis C virus core-specific peripheral blood T lymphocytes. J Immunol. 1997;159:1012–1018. [PubMed] [Google Scholar]
- 77.World Health Organisation. Hepatitis C. Wkly Epidemiol Rec. 1997;72:65–69. [Google Scholar]
- 78.Yanagi M, Purcell R H, Emerson S U, Bukh J. Transcripts from a single full-length cDNA clone of hepatitis C virus are infectious when directly transfected into the liver of a chimpanzee. Proc Natl Acad Sci USA. 1997;94:8738–8743. doi: 10.1073/pnas.94.16.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yasui K, Wakita T, Tsukiyama-Kohara K, Funahashi S I, Ichikawa M, Kajita T, Moradpour D, Wands J R, Kohara M. The native form and maturation process of hepatitis C virus core protein. J Virol. 1998;72:6048–6055. doi: 10.1128/jvi.72.7.6048-6055.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu N, Khoshnan A, Schneider R, Matsumoto M, Dennert G, Ware C, Lai M M. Hepatitis C virus core protein binds to the cytoplasmic domain of tumor necrosis factor (TNF) receptor 1 and enhances TNF-induced apoptosis. J Virol. 1998;72:3691–3697. doi: 10.1128/jvi.72.5.3691-3697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]