Abstract
Background and Objectives
If the proportion of calcium intake over a whole day is related to the risk of cognitive impairment in adults is still largely unknown. This research aimed to examine the relation of dietary calcium intake at dinner versus breakfast with the risk of cognitive impairment by using data from the China Health and Nutrition Survey (CHNS).
Methods and Study Design
A total of 2,099 participants (including 668 cognitive impairment) in the CHNS (1997–2006) were included. The participants were categorized into 5 groups in accordance with the ratio of dietary calcium intake at dinner and breakfast (Δ = dinner/breakfast). After adjustment was conducted for a series of confounding factors, Cox hazard regression modelling was performed to discuss the relation of Δ with cognitive impairment. Dietary substitution models were used to explore the changes in cognitive impairment risk when a 5% dietary calcium intake at dinner was replaced with dietary calcium intake at breakfast.
Results
Participants in the highest distribution of Δ showed a greater susceptibility to cognitive impairment than those in the lowest quintile, with an adjusted hazard ratio of cognitive impairment of 1.38 (95% CI: 1.08–1.76). When maintaining total calcium intake, substituting 5% of dietary calcium intake at dinner with calcium intake at breakfast was related to an 8% decrease in the risk of cognitive impairment.
Conclusions
Higher dietary calcium intake at dinner was associated with an increased risk of cognitive impairment, emphasizing the importance of appropriately distributing dietary calcium intake between breakfast and dinner.
Key Words: dietary intake, calcium, cognitive impairment, China Health and Nutrition Survey, dietary substitution model
Introduction
Cognitive impairment is a deficit in one or more key brain functions, such as memory, learning, concentration, and decision making.1, 2, 3 It can progress to dementia, mainly in the form of Alzheimer's disease.4 The global prevalence of dementia increased from 46.8 million in 2015 to 131.5 million in 2050,5 very consequential for human health in general. To meet the challenge of cognitive impairment, innovative prevention strategies are needed.
Diet ought to be a key modifiable factor in the prevention and management of cognitive impairment.6 Calcium is a functionally extensive essential macro-element, not only for bone and cardiovascular health, but also potentially in cognitive function linked to hormone secretion.7, 8, 9 Clock genes are genes with 24-hour autonomous cycles, driven by transcription–translation feedback loops, which control daily activities and maintain organ functions in many physiological processes.10 A great number of re-search evidence suggested that circadian rhythm disturbances may be related to cognitive impairment.11, 12 Calcium is involved in the synchronization of brain's circadian pacemaker role to do with regulation of molecular rhythms by clock genes.13, 14 Thus, the prevention and management of cognitive impairment might be pursued by improving circadian rhythms.15 Currently, some scholars found that the circadian clock system could interact with nutrients to affect biological functions.16, 17 Conceivably, this might be responsive to rectification of an apparent link to total dietary calcium intake of cognitive impairment.18 Hypothetically, the distribution of calcium intake across the whole day may affect the risk of cognitive impairment in adults.
In this study, the relationship of dietary calcium intake at dinner versus that at breakfast is explored as a putative risk factor for cognitive impairment in the China Health and Nutrition Survey (CHNS).
Methods
Study population
CHNS is an ongoing open cohort, and its purpose is to clarify how social, economic changes in China impact health behavior during whole life cycle. Detailed information on the CHNS has been provided previously.19
A total of 20,454 participants in the CHNS from 1997 to 2006 were enrolled in this study. Excluded participants included those under 55 years old, with missing essential information (n = 15,480), who participated in only one survey (n = 1,721), with missing essential information on dietary calcium intake at dinner, and with total energy intake < 500 or > 4,500 kcal/day (n = 1,154). After such cases were excluded, the total number of participants was 2,099 (1,058 men and 1,041 women).
This study was approved by the ethical committee of Qiqihar Medical University (ref: [2023] 28). This study adheres to the Helsinki declaration. All participants provided informed consent.
Dietary assessment
In the CHNS, detailed in-person interviews were administered with the use of a questionnaire to collect information on dietary information. The information about dietary intake was investigated for every member of family. Individual dietary calcium intake was equal to the sum of individual and household survey part. Mealtimes were divided as follow: breakfast, morning snack, lunch, afternoon snack, dinner, and evening snack. The food intake was listed in accordance with the mealtimes. Dietary calcium was calculated by 3 versions of Chinese Food Composition Table. The 1991 version was applied in 1997 and 2000, and the 2002/2004 (two books combined) versions were applied in 2004 and 2006.
Main exposure and outcome measure
The exposure variable was Δ. Cognitive function test was administered based on cognitive function screening test, containing four tasks: immediate memory (10 scores), delayed recall of a 10-word list (10 scores), counting backwards from 20 (two scores), and serial 7 subtraction (five scores).20 The total cognitive score ranged between 0 and 27, and higher scores indicated better cognitive function. In this research, the first quintile of the test score was selected to represent cognitive impairment.
Confounding measurements
Dietary confounders included total energy (kcal/day), protein, fat, carbohydrate, and dietary fiber (all with unit of g/day). Non-dietary confounding covariates included age (years), gender (men/women), urban index, smoking status (never smoked, current smoker, and former smoker), drinking (drinks/week), physical activity (MET-h/week), hypertension status (yes/no), type 2 diabetes mellitus status (T2DM, yes/no), and body mass index (BMI, kg/m2). The definitions of smoking status and physical activity were adopted from a previous study.21 The amount of alcohol consumed was detected based on the number of drinks, wherein a standard drink was any drink that contained 0.6 fluid ounces. T2DM was defined as HbA1c level ≥ 6.5%, or fasting plasma glucose level ≥ 7.0 mmol/L. Hypertension was defined as ≥ 140 mmHg of persistent systolic blood pressure measurements and/or ≥ 90 mmHg of diastolic blood pressure. BMI was calculated as weight in kilograms divided by the square of height in meters.
Statistical analysis
Statistical analyses were performed by R 3.5.3. Δ was categorized into quintiles. General linear models and chi-square (χ2) tests were applied to compare baseline characteristics by quintiles. Continuous variables were expressed as mean ± standard deviation (SD), classified variables as percentages. A p < 0.05 was regarded as significant criteria. Missing covariables < 5% were filled based on interpolation.
Cox proportional hazard regression models
Cox regression models were applied to evaluate Δ and the risk of cognitive impairment in different models. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were assessed by three different Cox hazard regression models, with the lowest quintile of Δ as the reference category. Model 1 was adjusted for age, gender, smoking status, drinking, urban index, and physical activity. On the basis of the model 1 covariates, model 2 was adjusted for total energy, carbohydrate, protein, fat, and dietary fiber intake. Finally, model 3 was adjusted for hypertension status, T2DM status, and BMI.
Dietary substitution models
Dietary substitution model is a new statistical method that holds total dietary calcium intake constant to assess the changes in cognitive impairment risk with a theoretical shift of the dietary calcium intake. The method of substitution analysis is used to study the substitution of dietary calcium at dinner with dietary calcium at breakfast under the premise of equal calcium intake, and then observed changes in epidemiological indicators. We established an equivalent dietary calcium substitution model to evaluate the change in cognitive impairment risk caused by switching dietary calcium intake from a single time period to another single time period. In present research, a series of dietary substitution models was performed to assess changes in the risk of cognitive impairment when a 5% dietary calcium intake at dinner was substituted with a 5% dietary calcium intake at breakfast in accordance with the different definition modes of breakfast and dinner.
Sensitivity analysis
The timing of breakfast and dinner intake was defined differently in different studies. Therefore, four sets of sensitivity analyses were performed to verify the stability of relationship between Δ and the risk of cognitive impairment. First, breakfast and a morning snack were considered breakfast, and the study data were reanalyzed. Second, dinner and an evening snack were considered dinner. Third, breakfast and a morning snack were considered breakfast, and dinner and evening snack were considered dinner. At last, the study data were analyzed again when the dietary calcium at breakfast and dinner was adjusted by total energy intake.
Results
Baseline characteristics
Up to 2,099 samples was used in this study, including 668 cognitive impairment cases. The characteristics of the baseline population of Δ in quintiles are shown in Table 1. Total protein, fat, carbohydrate, dietary fiber intake, dietary calcium intake, energy at breakfast and dinner, physical activity, urban index, hypertension, and T2DM status were significantly different across quintiles 1–5 (p < 0.05). No significant difference exists in age, gender, smoking, drinking, total energy intake, and BMI among the quintiles (p > 0.05).
Table 1.
Characteristics of the participants according to the ratio of dietary calcium intake in CHNS (1997-2006)
| Variable | Q1 | Q2 | Q3 | Q4 | Q5 | p value |
|---|---|---|---|---|---|---|
| < 0.96 | 0.96-1.44 | 1.45-2.53 | 2.54-5.78 | ≥ 5.79 | ||
| Case/N | 129/420 | 131/419 | 112/421 | 130/419 | 166/420 | |
| Age (years) | 61.9 (6.7) | 60.8 (6.4) | 61.2 (7.0) | 61.0 (6.3) | 61.1 (6.4) | 0.199 |
| Gender (Women) | 204 (48.6) | 197 (47.0) | 212 (50.4) | 212 (50.6) | 216 (51.4) | 0.714 |
| Current smoking [n, (%)] | 122 (29.0) | 138 (32.9) | 131 (31.1) | 122 (29.1) | 128 (30.5) | 0.744 |
| Drinking (drinks/week) | 4.3 (13.9) | 5.3 (15.1) | 4.9 (14.5) | 5.6 (14.6) | 5.7 (15.3) | 0.166 |
| Physical activity (MET-h/week) | 47.9 (84.5) | 40.5 (75.3) | 46.7 (78.7) | 51.5 (84.1) | 51.0 (85.3) | <0.001 |
| Urban index | 59.0 (21.6) | 52.8 (20.4) | 61.1 (19.8) | 64.3 (17.4) | 68.0 (14.4) | <0.001 |
| Total energy intake (kcal/day) | 2167 (599) | 2217 (642) | 2126 (638) | 2212 (615) | 2198 (625) | 0.553 |
| Total protein intake (g/day) | 66.4 (23.3) | 64.7 (23.8) | 63.6 (21.0) | 66.8 (23.6) | 69.7 (22.9) | 0.012 |
| Total fat intake (g/day) | 69.2 (37.2) | 65.7 (35.8) | 71.9 (39.3) | 77.0 (36.4) | 79.5 (41.5) | <0.001 |
| Total carbohydrate intake (g/day) | 325 (114) | 347 (119) | 311 (109) | 317 (108) | 304 (101) | <0.001 |
| Total dietary fiber intake (g/day) | 11.7 (6.5) | 12.8 (9.9) | 10.6 (6.1) | 10.1 (8.0) | 10.3 (8.3) | <0.001 |
| Calcium intake at breakfast (mg/day) | 172 (131) | 110 (72.7) | 77.9 (50.9) | 44.2 (27.3) | 21.0 (14.7) | <0.001 |
| Calcium intake at dinner (mg/day) | 98.6 (56.8) | 128 (81.4) | 147 (94.7) | 165 (103) | 247 (229) | <0.001 |
| Energy at breakfast (kcal/day) | 667 (247) | 653 (254) | 559 (224) | 510 (220) | 447 (168) | <0.001 |
| Energy at dinner (kcal/day) | 722 (240) | 785 (270) | 780 (263) | 852 (278) | 887 (297) | <0.001 |
| Hypertension [(n, (%)] | 192 (45.7) | 161 (38.4) | 168 (39.9) | 144 (34.3) | 172 (41.0) | 0.018 |
| T2DM [(n, (%)] | 79 (18.8) | 71 (16.9) | 71 (16.9) | 71 (16.9) | 81 (19.3) | 0.048 |
| BMI (kg/m2) | 23.2 (3.8) | 23.0 (3.5) | 23.5 (3.5) | 22.8 (3.3) | 23.4 (3.6) | 0.821 |
Q, quintile. T2DM, type 2 diabetes mellitus. BMI, body mass index
Continuous variables were described as mean (SD) whereas categorical variables were described as n (%)
The significant difference were checked by Generalized linear models and χ2 test
Cox proportional hazard regression models
The Cox regression model of the relationship between Δ and cognitive impairment were exhibited in Table 2. The results showed that the highest quintile of Δ was more likely to increase the risk of cognitive impairment than the lowest quintile. The adjusted HR of cognitive impairment was 1.38 (95% CI: 1.08–1.76) after adjusting for age, gender, smoking status, drinking, urban index, physical activity, total energy, carbohydrate, protein, fat, dietary fiber intake, hypertension status, T2DM status, and BMI.
Table 2.
The association between the risk of cognitive impairment and the ratio of dietary calcium intake by Cox proportional hazard regression model (n = 2,099)
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | |
|---|---|---|---|---|---|---|
| Δ | < 0.96 | 0.96-1.44 | 1.45-2.54 | 2.55-5.78 | ≥ 5.79 | |
| Case/N | 129/420 | 131/419 | 112/421 | 130/419 | 166/420 | |
| Model 1 | 1 (ref.) | 1.00 (0.78,1.28) | 0.96 (0.74,1.24) | 1.02 (0.79,1.31) | 1.29 (1.02,1.64) | 0.053 |
| Model 2 | 1 (ref.) | 1.02 (0.80,1.30) | 0.94 (0.73,1.21) | 1.01 (0.79,1.30) | 1.37 (1.07,1.74) | 0.024 |
| Model 3 | 1 (ref.) | 1.02 (0.80,1.30) | 0.96 (0.74,1.24) | 1.00 (0.78,1.29) | 1.38 (1.08,1.76) | 0.022 |
Model 1 was adjusted for age, gender, smoking status, drinking, urban index, and physical activity; Model 2 was further adjusted for total energy, carbohydrate, protein, fat, and dietary fiber intake; Model 3 was further adjusted for hypertension, T2DM status, and BMI.
Dietary substitution analysis
Four sets of dietary substitution analyses are shown in Figure 1, in which the changes in cognitive impairment risk were demonstrated. The results showed that when 5% of dietary calcium consumed at dinner was replaced by 5% of dietary calcium consumed at breakfast, the cognitive impairment risk reduced by 8% (HR: 0.92, 95% CI: 0.87–0.98). Under condition of breakfast and a morning snack were considered as breakfast, or dinner and an evening snack were considered as dinner, the cognitive impairment risk also decreased by 9% and 9% (HR: 0.91, 95% CI: 0.86–0.97; HR: 0.91, 95% CI: 0.86–0.98). When breakfast and a morning snack were considered as breakfast, and dinner and an evening snack were considered as dinner, the cognitive impairment risk decreased by 9% (HR: 0.91, 95% CI: 0.86–0.96).
Figure 1.
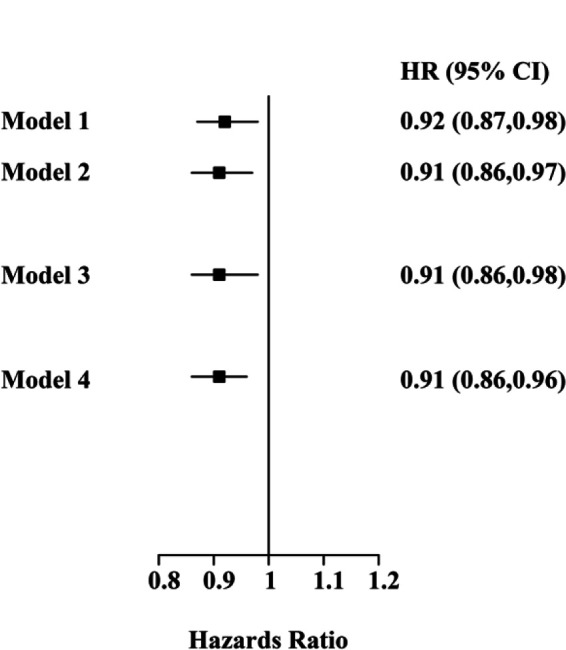
Adjusted HRs for cognitive impairment risk: dietary substitution of calcium intake from dinner to breakfast. Adjustments included age, gender, smoking status, drinking, urban index, physical activity, total energy, carbohydrate, protein, fat, dietary fiber, hypertension status, T2DM status, and BMI. Model 1, replacing 5% dietary calcium intake at dinner with dietary calcium intake at breakfast; Model 2, replacing 5% dietary calcium intake at dinner with dietary calcium intake at breakfast, when breakfast and a morning snack were considered as breakfast; Model 3, replacing 5% dietary calcium intake at dinner with dietary calcium intake at breakfast, when dinner and an evening snack were considered as dinner; Model 4, replacing 5% dietary calcium intake at dinner with dietary calcium intake at breakfast, when breakfast and a morning snack were considered as breakfast, and dinner and an evening snack were considered as dinner
Sensitivity analysis
In accordance with different definitions of breakfast and dinner, four sets of sensitivity analyses were conducted, and found that the results were in accordance with the previous research conclusion in Table 3. When breakfast and a morning snack were consumed as breakfast, or dinner and an evening snack were consumed as dinner, the highest quintile of Δ indicated a significant increasing risk of cognitive impairment compared with the lowest quintile, and the adjusted HRs were 1.34 and 1.32. When dinner and an evening snack were consumed as dinner, and breakfast and a morning snack was consumed as breakfast, the adjusted HR was 1.37 (95% CI: 1.07–1.76). When dietary calcium at breakfast and dinner was adjusted by total energy intake, the adjusted HR of cognitive impairment in the highest quintile of Δ was 1.59 (95% CI: 1.25–2.03) after controlling for a series of confounding factors.
Table 3.
Four sensitivity analysis results for the association between the risk of cognitive impairment and the ratio of dietary calcium intake at dinner versus breakfast according to different definitions of breakfast and dinner (n = 36,164) and calcium intake adjusted by energy by cox hazards regression model (n = 2,099)
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | |
|---|---|---|---|---|---|---|
| Sensitivity analysis 1 | ||||||
| Δ | <0.62 | 0.62-0.97 | 0.98-1.51 | 1.52-3.02 | ≥3.03 | |
| Case/N | 118/420 | 131/420 | 133/419 | 119/420 | 167/420 | |
| Model 1 | 1 (ref.) | 1.08 (0.84,1.39) | 1.19 (0.92,1.52) | 1.06 (0.82,1.37) | 1.32 (1.03,1.68) | 0.047 |
| Model 2 | 1 (ref.) | 1.06 (0.83,1.37) | 1.14 (0.89,1.47) | 1.04 (0.80,1.35) | 1.35 (1.06,1.73) | 0.03 |
| Model 3 | 1 (ref.) | 1.03 (0.80,1.32) | 1.14 (0.88,1.46) | 1.02 (0.79,1.33) | 1.34 (1.05,1.72) | 0.028 |
| Sensitivity analysis 2 | ||||||
| Δ | <1.66 | 1.66-1.83 | 1.84-3.21 | 3.22-7.26 | ≥7.27 | |
| Case/N | 125/420 | 120/420 | 113/419 | 144/420 | 166/420 | |
| Model 1 | 1 (ref.) | 0.91 (0.71,1.17) | 0.84 (0.65,1.08) | 1.14 (0.89,1.45) | 1.22 (0.96,1.55) | 0.026 |
| Model 2 | 1 (ref.) | 0.93 (0.72,1.20) | 0.85 (0.65,1.09) | 1.15 (0.90,1.47) | 1.30 (1.02,1.65) | 0.009 |
| Model 3 | 1 (ref.) | 0.92 (0.72,1.19) | 0.85 (0.66,1.11) | 1.14 (0.89,1.46) | 1.32 (1.03,1.68) | 0.007 |
| Sensitivity analysis 3 | ||||||
| Δ | <0.79 | 0.79-1.20 | 1.21-1.91 | 1.92-3.58 | ≥3.59 | |
| Case/N | 108/420 | 138/420 | 115/419 | 142/420 | 165/420 | |
| Model 1 | 1 (ref.) | 1.13 (0.88,1.46) | 1.14 (0.87,1.48) | 1.24 (0.96,1.60) | 1.33 (1.04,1.70) | 0.022 |
| Model 2 | 1 (ref.) | 1.13 (0.88,1.46) | 1.11 (0.85,1.44) | 1.22 (0.94,1.57) | 1.36 (1.06,1.75) | 0.016 |
| Model 3 | 1 (ref.) | 1.11 (0.86,1.44) | 1.11 (0.85,1.44) | 1.21 (0.94,1.57) | 1.37 (1.07,1.76) | 0.012 |
| Sensitivity analysis 4 | ||||||
| Δ | <0.78 | 0.78-1.09 | 1.10-1.70 | 1.71-3.35 | ≥3.36 | |
| Case/N | 117/420 | 133/420 | 115/419 | 124/420 | 179/420 | |
| Model 1 | 1 (ref.) | 0.99 (0.77,1.27) | 0.90 (0.69,1.16) | 1.04 (0.81,1.35) | 1.54 (1.21,1.95) | <0.001 |
| Model 2 | 1 (ref.) | 0.98 (0.77,1.27) | 0.88 (0.68,1.14) | 1.01 (0.78,1.31) | 1.57 (1.23,1.99) | <0.001 |
| Model 3 | 1 (ref.) | 0.99 (0.77,1.28) | 0.89 (0.69,1.15) | 1.03 (0.80,1.33) | 1.59 (1.25,2.03) | <0.001 |
Model 1 was adjusted for age, gender, smoking status, drinking, urban index, and physical activity; Model 2 was further adjusted for total energy, carbohydrate, protein, fat, and dietary fiber; Model 3 was further adjusted for hypertension status, T2DM status, and BMI.
Discussion
To the authors' knowledge, this research was the first to discuss the relation between Δ and the incidence of cognitive impairment based on national representative sample from China. The results demonstrated that, higher dietary calcium intake at dinner increased the risk of cognitive impairment than at breakfast, highlighting the importance of allocating calcium to breakfast and dinner throughout the day. Meanwhile, replacing 5% calcium intake at dinner with calcium intake at breakfast reduced the risk of cognitive impairment by 8%, which further supported the health benefits of allocating calcium to breakfast and dinner. Increasing calcium intake at breakfast and reducing calcium-rich foods in proportion at dinner are necessary to reduce the risk of cognitive impairment.
Obesity is a key risk factor for cognitive impairment,22 and its reasons may include the following: First, obesity can cause inflammation and increase the level of factors mediating inflammation, especially IL-6 level.23 A growing body of research suggested that IL-6 could disrupt neural circuits responsible for cognitive pairs. Second, obesity may disrupt the blood–brain barrier and activate neuroinflammation, which could lead to cognitive impairment.24 A study has shown that eating breakfast every day showed increased daily calcium intake,25 and a statistically significant negative relation was found between calcium intake and BMI.26, 27 Skipping breakfast or reducing the percentage of daily calcium intake from breakfast may increase the risk of obesity. Therefore, insufficient dietary calcium intake at breakfast could lead to obesity and increase the risk of cognitive impairment.
The alteration in circadian rhythm may be another mechanism to explain the observations in this study. Circadian rhythm disruption could lead to cognitive impairment and psychiatric illness, such as depression. Diet is a vital external factor influencing the synchronization of endogenous clocks. Nutrient intake and chronotype may play an important role in circadian mechanisms. A study has demonstrated that calcium and its signalling pathway could adjust the molecular mechanism of periodicity of circadian behaviour.28 Eating too much calcium-rich food at dinner disrupts the level of clock genes, leading to circadian dysregulation.29
Epidemiological studies have shown that circadian disruption has an effect on the key markers of cardiovascular function and risk, thereby increasing cardiovascular risk factors, include hypertension and diabetes.30 Hypertension is a key risk factor for cognitive impairment.31, 32 Studies have shown that hypertension induces vascular hypertrophy, changes cerebrovascular structure, and damages brain microcirculation, thus promoting the occurrence of cognitive impairment.22
Studies have found that circadian rhythm disturbance is an important risk factor for intestinal microflora disorder.33 Recent research showed that the metabolites produced by intestinal microbes in the process of dietary metabolism, such as trimethylamine, trimethylamine oxide, short-chain fatty acids, and secondary bile acids, were associated with cognitive impairment. Intestinal microbiome imbalance may be related to cognitive impairment. The gut microbiome produces large amounts of amyloid, which is different from that in the central nervous system, and their release is closely related to immune system condition. The amyloid peptides and lipopolysaccharides synthesized by visceral microorganisms based on activation of inflammatory signals could lead to pathophysiological changes in cognitive impairment.34 Therefore, too much calcium at dinner may cause cognitive impairment by causing dysregulation of the intestinal flora.
Immune response and lipid metabolism are major pathways in cognitive impairment.35 An animal study showed that high calcium intake at dinner significantly increased serum triglyceride and high-density lipoprotein levels compared with high calcium intake at breakfast, leading to lipid metabolism disorders. The possible reason is that the mRNA level of circadian rhythm genes per1 and clock is inhibited,36 which could lead to circadian disruption.
The significance of present research as follow: First, the CHNS, a high quality database of Chinese in diet survey. Second, this research highlighted the importance of timing of calcium intake. Increasing calcium intake at breakfast and reducing calcium intake at dinner could decrease the risk of cognitive impairment. Third, a series of sensitivity analyses and dietary substitution models was performed to conform the stability of research results from different angles, and all results emphasized the importance of proper distribution of dietary calcium throughout the day. However, this study also has several limitations. First, in the CHNS, the intake of various nutrients was obtained through a dietary questionnaire, and measurement errors were unavoidable and may have biased the results. Second, the participants in this study were recruited from 1996 to 2007; their dietary structure may have changed over the 9 years. Moreover, dietary calcium intake data at first entry into the cohort were taken as baseline data, which could have biased the results of the Cox proportional hazard regression models. Third, some confounding factors that were not detected may impact the results.
Conclusion
Participants in the highest distribution of Δ showed a greater susceptibility to cognitive impairment compared to those in the lowest quintile, with an adjusted hazard ratio of cognitive impairment of 1.38 (95% CI: 1.08–1.76). Furthermore, when maintaining total calcium intake, substituting 5% of dietary calcium intake at dinner with dietary calcium intake at breakfast was related to an 8% decrease in the risk of cognitive impairment. In conclusion, higher dietary calcium intake at dinner was associated with an increased risk of cognitive impairment, emphasizing the importance of appropriately distributing dietary calcium intake between breakfast and dinner.
Acknowledgements
We thank the National Institute for Nutrition and Health, China Center for Disease Control and Prevention, Carolina Population Center (P2C-HD-050924, T32-HD-007168); the University of North Carolina, Chapel Hill; the National Institutes of Health (R01-HD-30880, DK-056350, R24-HD-050924, R01-HD-38700); and the National Institutes of Health Fogarty International Center (D43-TW-009077, D43-TW-007709) for financial support for the CHNS data collection.
Author Disclosures
The authors declare no conflict of interest.
This study was funded by the Department of Education, Heilongjiang Province [Grant number: 2022-KYYWF-0810].
Funding Statement
This study was funded by the Department of Education, Heilongjiang Province [Grant number: 2022-KYYWF-0810].
References
- 1.Drew DA, Weiner DE, Sarnak MJ. Cognitive Impairment in CKD: Pathophysiology, Management, and Prevention. Am J Kidney Dis. 2019;74:782–790. doi: 10.1053/j.ajkd.2019.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li T, Zhuang D, Cai S, Ding F, Tian F, Huang M, Li L, Chen W, Li K, Sheng J. Low serum calcium is a novel predictor of unfavorable prognosis after traumatic brain injury. Heliyon. 2023;9:e18475–e18475. doi: 10.1016/j.heliyon.2023.e18475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estrella ML, Durazo-Arvizu RA, Gallo LC, Isasi CR, Perreira KM, Vu TT, et al. Associations between perceived neighborhood environment and cognitive function among middle-aged and older women and men: Hispanic Community Health Study/Study of Latinos Sociocultural Ancillary Study. Soc Psychiatry Psychiatr Epidemiol. 2020;55:685–696. doi: 10.1007/s00127-019-01829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.2022 Alzheimer's disease facts and figures Alzheimer's & dementia: the journal of the Alzheimer's Association. 2022;18:700–789. doi: 10.1002/alz.12638. [DOI] [PubMed] [Google Scholar]
- 5.2021 Alzheimer's disease facts and figures Alzheimer's & dementia: the journal of the Alzheimer's Association. 2021;17:327–406. doi: 10.1002/alz.12328. [DOI] [PubMed] [Google Scholar]
- 6.Vlachos GS, Scarmeas N. Dietary interventions in mild cognitive impairment and dementia. Dialogues Clin Neurosci. 2019;21:69–82. doi: 10.31887/DCNS.2019.21.1/nscarmeas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian DY, Tian J, Shi CH, Song B, Wu J, Ji Y, Wang RH, Mao CY, Sun SL, Xu YM. Calcium intake and the risk of stroke: an up-dated meta-analysis of prospective studies. Asia Pac J Clin Nutr. 2015;24:245–252. doi: 10.6133/apjcn.2015.24.2.22. [DOI] [PubMed] [Google Scholar]
- 8.Jiao D, Jiang C. Nutritional therapy of older osteoporotic people with supplemental calcium and vitamin D: side effects, fracture rates, and survival - an internationalised meta-analysis. Asia Pac J Clin Nutr. 2024;33:1–10. doi: 10.6133/apjcn.202403_33(1).0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomaschitz A, Pilz S. Interplay between sodium and calcium regulatory hormones: a clinically relevant research field. Hypertension. 2014;63:212–214. doi: 10.1161/hypertensionaha.113.02253. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi T, Leise TL, Kingsbury NJ, Diemer T, Wang LL, Henson MA, Welsh DK. Calcium Circadian Rhythmicity in the Suprachiasmatic Nucleus: Cell Autonomy and Network Modulation. eNeuro. 2017;4 doi: 10.1523/eneuro.0160-17.2017. ENEURO.0160-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmet P, Alberti KGMM, Stern N, Bilu C, El-Osta A, Einat H, Kronfeld-Schor N. The Circadian Syndrome: is the Metabolic Syndrome and much more! J Intern Med. 2019;286:181–191. doi: 10.1111/joim.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng D, Yang M, Hu L, Liu T, Zhang H, Sun X, Wang X, Chen Y, Jin Y, Liu R. Rifaximin protects against circadian rhythm disruption-induced cognitive impairment through preventing gut barrier damage and neuroinflammation. J Neurochem. 2022;163:406–418. doi: 10.1111/jnc.15701. [DOI] [PubMed] [Google Scholar]
- 13.Horigane SI, Ozawa Y, Yamada H, Takemoto-Kimura S. Calcium signalling: a key regulator of neuronal migration. J Biochem. 2019;165:401–409. doi: 10.1093/jb/mvz012. [DOI] [PubMed] [Google Scholar]
- 14.Jubiz W, Canterbury JM, Reiss E, Tyler FH. Circadian rhythm in serum parathyroid hormone concentration in human subjects: correlation with serum calcium, phosphate, albumin, and growth hormone levels. J Clin Invest. 1972;51:2040–2046. doi: 10.1172/jci107010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maiese K. Cognitive Impairment and Dementia: Gaining Insight through Circadian Clock Gene Pathways Biomolecules. 2021;11:1002–1002. doi: 10.3390/biom11071002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnston JD. Physiological responses to food intake throughout the day. Nutr Res Rev. 2014;27:107–118. doi: 10.1017/s0954422414000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oike H, Oishi K, Kobori M. Nutrients, Clock Genes, and Chrononutrition. Curr Nutr Rep. 2014;3:204–212. doi: 10.1007/s13668-014-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reicks M, Degeneffe D, Ghosh K, Bruhn C, Goodell LS, Gunther C, et al. Parent calcium-rich-food practices/perceptions are associated with calcium intake among parents and their early adolescent children. Public Health Nutr. 2012;15:331–340. doi: 10.1017/s1368980011001133. [DOI] [PubMed] [Google Scholar]
- 19.Zhang B, Zhai FY, Du SF, Popkin BM. The China Health and Nutrition Survey, 1989-2011. Obes Rev. 2014;15:2–7. doi: 10.1111/obr.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Z, El-Obeid T, Riley M, Li M, Page A, Liu J. High Chili Intake and Cognitive Function among 4582 Adults: An Open Cohort Study over 15 Years. Nutrients. 2019;11:1183–1183. doi: 10.3390/nu11051183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren X, Yang X, Jiang H, Han T, Sun C. The association of energy and macronutrient intake at dinner vs breakfast with the incidence of type 2 diabetes mellitus in a cohort study: The China Health and Nutrition Survey, 1997-2011. J Diabetes. 2021;13:882–892. doi: 10.1111/1753-0407.13185. [DOI] [PubMed] [Google Scholar]
- 22.Buie JJ, Watson LS, Smith CJ, Sims-Robinson C. Obesity-related cognitive impairment: The role of endothelial dysfunction. Neurobiol Dis. 2019;132:104580–104580. doi: 10.1016/j.nbd.2019.104580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almuraikhy S, Kafienah W, Bashah M, Diboun I, Jaganjac M, Al-Khelaifi F, et al. Interleukin-6 induces impairment in human subcutaneous adipogenesis in obesity-associated insulin resistance. Diabetologia. 2016;59:2406–2416. doi: 10.1007/s00125-016-4031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallières L, Lacroix S, Rivest S. Influence of interleukin-6 on neural activity and transcription of the gene encoding corticotrophin-releasing factor in the rat brain: an effect depending upon the route of administration. Eur J Neurosci. 1997;9:1461–1472. doi: 10.1111/j.1460-9568.1997.tb01500.x. [DOI] [PubMed] [Google Scholar]
- 25.Pinho-Pompeu M, Paulino DSM, Surita FG. Influence of breakfast and meal frequency in calcium intake among pregnant adolescents. Matern Child Nutr. 2020;16:e13034–e13034. doi: 10.1111/mcn.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovejoy JC, Champagne CM, Smith SR, de Jonge L, Xie H. Ethnic differences in dietary intakes, physical activity, and energy expenditure in middle-aged, premenopausal women: the Healthy Transitions Study. Am J Clin Nutr. 2001;74:90–95. doi: 10.1093/ajcn/74.1.90. [DOI] [PubMed] [Google Scholar]
- 27.Pereira MA, Jacobs DR Jr, Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: the CARDIA Study. JAMA. 2002;287:2081–2089. doi: 10.1001/jama.287.16.2081. [DOI] [PubMed] [Google Scholar]
- 28.Cavieres-Lepe J, Ewer J. Reciprocal Relationship Between Calcium Signaling and Circadian Clocks: Implications for Calcium Homeostasis, Clock Function, and Therapeutics. Front Mol Neurosci. 2021;14:666673–666673. doi: 10.3389/fnmol.2021.666673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carruth BR, Skinner JD. The role of dietary calcium and other nutrients in moderating body fat in preschool children. Int J Obes Relat Metab Disord. 2001;25:559–566. doi: 10.1038/sj.ijo.0801562. [DOI] [PubMed] [Google Scholar]
- 30.Koszewicz M, Jaroch J, Brzecka A, Ejma M, Budrewicz S, Mikhaleva LM, et al. Dysbiosis is one of the risk factor for stroke and cognitive impairment and potential target for treatment. Pharmacol Res. 2021;164:105277–105277. doi: 10.1016/j.phrs.2020.105277. [DOI] [PubMed] [Google Scholar]
- 31.Dye L, Boyle NB, Champ C, Lawton C. The relationship between obesity and cognitive health and decline. Proc Nutr Soc. 2017;76:443–454. doi: 10.1017/s0029665117002014. [DOI] [PubMed] [Google Scholar]
- 32.Kendig MD, Morris MJ. Reviewing the effects of dietary salt on cognition: mechanisms and future directions. Asia Pac J Clin Nutr. 2019;28:6–14. doi: 10.6133/apjcn.201903_28(1).0002. 10.6133/apjcn. 201903_28(1).0002 . [DOI] [PubMed] [Google Scholar]
- 33.Cochrane A, Robertson IH, Coogan AN. Association between circadian rhythms, sleep and cognitive impairment in healthy older adults: an actigraphic study. J Neural Transm (Vienna) 2012;119:1233–1239. doi: 10.1007/s00702-012-0802-2. [DOI] [PubMed] [Google Scholar]
- 34.Gomes JM, Costa JA, Alfenas RC. Could the beneficial effects of dietary calcium on obesity and diabetes control be mediated by changes in intestinal microbiota and integrity? Br J Nutr. 2015;114:1756–1765. doi: 10.1017/s0007114515003608. [DOI] [PubMed] [Google Scholar]
- 35.Qiu S, Palavicini JP, Wang J, Gonzalez NS, He S, Dustin E, et al. Adult-onset CNS myelin sulfatide deficiency is sufficient to cause Alzheimer's disease-like neuroinflammation and cognitive impairment. Mol Neurodegener. 2021;16:64–64. doi: 10.1186/s13024-021-00488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao LM, Xie CY, Zhang TY, Wu X, Yin YL. Maternal supplementation with calcium varying with feeding time daily during late pregnancy affects lipid metabolism and transport of placenta in pigs. Biochem Biophys Res Commun. 2018;505:624–630. doi: 10.1016/j.bbrc.2018.09.143. [DOI] [PubMed] [Google Scholar]


