Abstract
The in vivo persistence of gene-modified cells can be limited by host immune responses to transgene-encoded proteins. In this study we evaluated in a nonhuman primate model whether the administration of a nonmyeloablative regimen consisting of low-dose total-body irradiation with 200 cGy followed by immunosuppression with mycophenolate mofetil and cyclosporin A for 28 and 35 days, respectively, could be used to facilitate persistence of autologous gene-modified T cells when a transgene-specific immune response had already been established or to induce long-lasting tolerance in unprimed recipients. Two macaques (Macaca nemestrina) received infusions of T cells transduced to express either the enhanced green fluorescent protein and neomycin phosphotransferase genes or the hygromycin phosphotransferase and herpes simplex virus thymidine kinase genes. In the absence of immunosuppression, both macaques developed potent class I major histocompatibility complex-restricted CD8+ cytotoxic T-lymphocyte (CTL) responses that rapidly eliminated the gene-modified T cells and that persisted long term as memory CTL. Treatment with the nonmyeloablative regimen failed to abrogate preexisting memory CTL responses but interfered with the induction of transgene-specific CTL and facilitated in vivo persistence of gene-modified cells in an unprimed host. However, sustained tolerance to gene-modified T cells was not achieved with this regimen, indicating that further modifications will be required to permit sustained persistence of gene-modified T cells.
Genetically modified cells are being evaluated in studies for the correction of genetic diseases and to treat acquired diseases such as cancer and AIDS (2, 10, 25, 30). A potential obstacle to the in vivo persistence of these cells is the development of host immune responses to novel transgene-encoded proteins (38). Clinical trials and studies in immunocompetent animals demonstrated that cells modified with retroviral vectors to express a therapeutic gene and/or a selectable marker transgene such as the neomycin phosphotransferase (neo), hygromycin phosphotransferase (Hy), herpes simplex virus thymidine kinase (TK), or enhanced green fluorescent protein (EGFP) gene can elicit potent host immune responses (5, 28, 38, 47, 49). The immune mechanisms responsible for eliminating genetically altered cells included both CD8+ cytotoxic T-lymphocyte (CTL) responses specific for peptide fragments derived from intracellular proteins presented in association with class I major histocompatibility complex (MHC) molecules and/or antibody responses to transgene products expressed at the cell surface.
Several strategies have been investigated to overcome the obstacle of immune recognition. The coexpression of viral immune evasion genes, which have been identified to selectively interfere with class I MHC presentation of target antigens, can prevent CTL-mediated lysis of cells constitutively expressing transgene proteins in vitro (3, 36, 42). However, this strategy is restricted to intracellularly expressed proteins and might be limited in vivo by recognition of modified cells by natural killer cells, which preferentially eliminate cells lacking class I MHC molecules on the cell surface (19, 26).
Immunosuppressive regimens commonly applied following solid organ transplantation or hematopoietic stem cell transplantation (HSCT) have also been explored to circumvent host immune responses to vector- or transgene-encoded proteins. Long-term administration of cyclosporine A (CSP) alone or with methotrexate failed to abrogate host immune responses (11, 15, 28). Although a combination of CSP and cyclophosphamide prolonged in vivo gene expression after adenovirus-mediated gene transfer, secondary gene delivery was not tested and long-term immunosuppression with this approach is limited by the risk of infectious complications and other toxicities (11).
Recent studies by Storb et al. demonstrated that a low (200-cGy) dose of total-body irradiation (TBI) followed by transient postgrafting immunosuppression with mycophenolate mofetil (MMF) and CSP for 28 and 35 days, respectively, can induce stable mixed chimerism following allogeneic HSCT in a canine model and in humans (45, 46). This regimen is nonmyeloablative and causes only transient reduction in neutrophil and platelet counts. Moreover, recent studies using a nonmyeloablative regimen in the setting of autologous HSCT demonstrated sustained in vivo persistence of stem cells modified to express a marker gene, suggesting that this approach may circumvent the immunogenicity of transgene-encoded proteins (18, 41). In many settings, differentiated somatic cells rather than stem cells are used as vehicles for gene delivery. T cells can be efficiently modified by gene transfer, and studies in which a normal adenosine deaminase gene was introduced into autologous T cells demonstrated that these cells can be long-lived in vivo following transfer in immunodeficient hosts (4, 6). Unfortunately, the use of gene-modified T cells in immunocompetent hosts has been complicated by immune responses to transgene products (28, 38, 47). Therefore, we sought to determine in a nonhuman primate model (Macaca nemestrina) whether nonmyeloablative conditioning with low-dose TBI (200 cGy), MMF, and CSP could facilitate the persistence of gene-modified T cells in settings in which a transgene-specific memory T-cell response was already established or in which the transgene-encoded protein represented a neoantigen. The regimen failed to overcome an established transgene-specific memory CTL response but did interfere with the induction of a primary transgene-specific CD8+ CTL response and markedly prolonged the in vivo persistence of gene-modified T cells in an unprimed host.
MATERIALS AND METHODS
Retroviral vectors.
The retroviral vector pLGSN was constructed by ligating the EcoRI-NotI (Klenow filled) 767-bp fragment of pEGFP-1 into EcoRI- and HpaI-digested pLXSN (32). Vector stocks were made by using PG13 retrovirus-producing packaging cells (31). The LN (PG13) vector carrying the neo gene alone has been described elsewhere (32). The retroviral vector MSCVEGFP (PG13), carrying the EGFP gene, was provided by R. G. Hawley (University of Toronto, Toronto, Ontario, Canada). The retroviral vector HyTK (PA317), carrying the Hy and the herpes simplex virus TK genes as a single fusion gene, was provided by Targeted Genetics (Seattle, Wash.) (27). The PG13- and PA317 packaging cell lines were grown in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, Utah).
Transduction and expansion of M. nemestrina lymphocytes.
Peripheral blood mononuclear cells (PBMC) were separated by Ficoll-Hypaque 1077 density gradient centrifugation and then cultured in RPMI 1640 medium supplemented with 25 mM HEPES, 11% human AB serum (Gemini Bio-Products, Calabasas, Calif.), 25 μM 2-mercaptoethanol (Sigma Chemical Co., St. Louis, Mo.), 4 mM l-glutamine (GIBCO-BRL, Gaithersburg, Md.), and 2% purified human interleukin-2 (IL-2; Advanced Biotechnologies Incorporation, Columbia, Md.). Prior to transduction, cells were stimulated for 36 to 48 h with the immobilized anti-CD3 (SP34; 10 to 20 ng/ml; PharMingen, San Diego, Calif.), and anti-CD28 (9.3; 1 μg/ml; P. Martin, Fred Hutchinson Cancer Research Center, Seattle, Wash.) monoclonal antibodies (MAbs) and then placed into six-well plates that had been seeded 12 h earlier with 106 γ-irradiated (2,500 rad) packaging cells producing either the LGSN, LN, or HyTK retrovirus vector. After 48 h of coculture in the presence of protamine sulfate (8 μg/ml), nonadherent cells were collected and replated in fresh medium. For selection of transduced cells, either Geneticin (G418 sulfate; 1.0 mg/ml; GIBCO-BRL) or hygromycin B (0.2 mg/ml; Boehringer Mannheim, Indianapolis, Ind.) was added after 24 h. T cells were then expanded using anti-CD3 (10 ng/ml) and anti-CD28 (1 μg/ml) MAbs, in the presence of γ-irradiated human PBMC and herpesvirus papio-transformed macaque B-lymphoblastoid cell lines (B-LCL), and IL-2 as described previously (39). On day 13, cells from these cultures were cryopreserved in aliquots which could be thawed subsequently for further expansion and reinfusion.
To expand T cells for adoptive transfer, aliquots of cells transduced to express either the EGFP and neo genes or the HyTK gene were stimulated in 25-cm2 flasks. On day 13, T cells were harvested, washed three times with Dulbecco's phosphate-buffered saline, and resuspended in 50 ml of isotonic saline solution containing 2% autologous serum for infusion. Prior to infusion, cells were tested by flow cytometry for expression of EGFP, CD3, CD4, and CD8 and by PCR for the presence of the transgene.
Generation and transduction of B-LCL.
B-LCL were established by infecting PBMC with fresh supernatant from S394-1X1055 cells containing herpesvirus papio as described previously (20). B-LCL were transduced with the LGSN, LN, MSVEGFP, or HyTK retroviral vector by cocultivation as described above and either selected with G418 (1 mg/ml) or hygromycin B (0.2 mg/ml) or sorted for expression of EGFP on a Ventage instrument (Becton Dickinson, Mountain View, Calif.).
Animals and experimental design.
Healthy adult pig-tailed macaques (M. nemestrina) were housed at the University of Washington Regional Primate Research Center under American Association for Accreditation of Laboratory Animal Care-approved conditions. Protocols were approved by the Institutional Review Board and Animal Care and Use Committee. All procedures and blood draws were performed as described elsewhere (24, 33).
A schematic diagram of the experimental design is shown in Fig. 1. Previous clinical trials indicated that a gene-modified T-cell dose of 109/m2 resulted in an easily detectable frequency of transferred cells in peripheral blood (8, 38). Thus, this cell dose was chosen for each infusion in this study. For immunosuppression, a single dose of TBI (200 cGy) was given from opposing 60Co sources (7 cGy/min) prior to the T-cell infusion. CSP (1.5 mg/kg intravenously [i.v.] twice a day [b.i.d.]; Sandoz Pharmaceuticals Corporation, East Hanover, N.J.) was begun 3 days before TBI was administered to ensure a sufficient drug level, and MMF (10 mg/kg i.v. b.i.d.; donated by Roche Laboratories Inc., Nutley, N.J.) was started on the day T cells were transferred. MMF and CSP were continued for 4 and 5 weeks, respectively (46). Drug levels were monitored, and doses were adjusted as needed. Standard supportive care was provided (24). A lymph node biopsy was performed 3 days after the last cell dose.
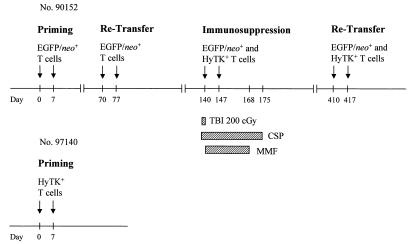
FIG. 1.
Schematic diagram of the experimental design. Each arrow indicates an infusion of 109 autologous gene-modified T cells/m2. For immunosuppression, macaque 90152 was given a nonmyeloablative dose (200 cGy) of TBI prior to the infusion of both EGFP/neo- and HyTK-modified T cells (day 140). CSP (1.5 mg/kg b.i.d. i.v.) was administered on days 137 to 175, and MMF (10 to 15 mg/kg b.i.d. i.v.) was given on days 140 to 168.
Flow cytometric analysis and cell sorting.
PBMC and T cells were analyzed for expression of CD3 by indirect immunofluorescence using the murine immunoglobulin (Ig) G3 MAb SP34 (PharMingen) as primary antibody followed by fluorescein isothiocyanate-conjugated or phycoerythrin-conjugated goat-anti-mouse IgG MAb (Tago Immunologicals, Camarillo, Calif.) as secondary antibody. Fluorescein isothiocyanate- or phycoerythrin-conjugated antibodies used for immunophenotyping included anti-CD4 (Leu 3a), anti-CD8 (Leu 2a), anti-CD16 (Leu 11a), and anti-CD20 (Leu 16) MAbs and isotype-matched irrelevant control antibodies (Becton Dickinson). Transferred EGFP-expressing T cells were enumerated in PBMC by analyzing >100,000 gated events for each analysis. PBMC from control animals were used to define negative fluorescence gates, and dead cells were excluded based on staining with propidium iodide (1 μg/ml; Sigma). Fluorescence analysis was performed on a FACScan flow cytometer (Becton Dickinson), and data were analyzed using CellQuest software.
Lymphoproliferative assay.
PBMC (105/well) were plated in triplicate and stimulated with concavalin A (ConA; 10 μg/ml; Sigma) or anti-CD3 and anti-CD28 MAbs as described above and incubated for 96 h at 37°C. For mixed lymphocyte reactions, an equal number of γ-irradiated (3,500 rad) allogeneic PBMC was added. Cells were pulsed with [3H]thymidine (2.5 μCi/well; NEN Products, Boston, Mass.) 16 h before harvest. In some experiments, cells were incubated in the presence of CSP (0.01 to 10 μg/ml) or mycophenolic acid (0.01 to 10 μM; Sigma), the pharmacologically active compound of the prodrug form MMF. The stimulation index was calculated as described elsewhere (8).
Cytotoxicity assays.
Cytotoxic responses specific to EGFP, neo, or HyTK were assessed by methods described previously (3, 38). Briefly, PBMC obtained from the animals before and after infusion were stimulated at a responder-to-stimulator ratio of 2:1 twice 1 week apart with γ-irradiated autologous T cells expressing either the EGFP/neo, neo, or HyTK gene. Cells from these cultures were assayed in a chromium release assay at various effector-to-target (E/T) ratios for specific recognition of autologous 51Cr-labeled B-LCL or T cells, either parental or transduced to express EGFP, EGFP/neo, neo, or HyTK. In some experiments, CD4+ or CD8+ T-cell subpopulations were isolated before the assay by using MACS MS+/RS+ separation columns (Miltenyi Biotec, Auburn, Calif.) according to the manufacturer's directions. To evaluate class I MHC-restricted cytolytic responses, target cells were preincubated (60 min, 4°C) with either the class I MHC MAb W6/32 (25 μg/ml; D. Geraghty, Fred Hutchinson Cancer Research Center) or the anti-HLA-DR MAb L243 (HB55; American Type Culture Collection, Manassas, Va.) (dilution of 1:10 [ascites fluid]; V. Groh, Fred Hutchinson Cancer Research Center). Both W6/32 and L243 cross-react with macaque MHC (34, 35).
LDA.
CTL precursors (CTLp) specific for EGFP or HyTK were quantified by limiting dilution analysis (LDA) of PBMC obtained before and after transfer of T cells (38). Twenty-four replicates of PBMC were plated in 96-well round-bottomed plates at concentrations of 375 to 100,000 cells per well with 2 × 103 autologous γ-irradiated (3,000 rad) EGFP- or HyTK-transduced T cells per well as stimulators and 5 × 104 autologous γ-irradiated PBMC per well as feeder cells. IL-2 (2%) was added on day 3, 6, 9, and 12. Wells were assayed on day 14 in a chromium release assay for specific recognition of autologous B-LCL, either parental or expressing EGFP, neo, or HyTK. CTL frequencies and confidence intervals were determined by maximum likelihood analysis as described elsewhere (12, 21).
Fluorescent probe PCR assay (TaqMan).
PCR amplifications and analysis of the neo or HyTK gene were performed by using a quantitative real-time PCR assay (TaqMan) (16). DNA (0.3 to 1 μg) was amplified in duplicate or triplicate with neo-specific primers (5′-GGA TTG CAC GCA GGT TCT C-3′ and 5′-AGA GCA GCC GAT TGT CTG TT-3′) and a fluorescence-tagged probe (5′-FAM-TGC CCA GTC ATA GCC GAA TAG CCT CTC CAT-TAMRA-3′; Synthegen, Houston, Tex.) (9). For HyTK, the specific primers 5′-TAC ACA AAT CGC CCG CAG A-3′ and 5′-AGC CTG GTC GAA CGC AGA C-3′ were used with the probe 5′-FAM-CGA CTT CTA CAC AGC CAT CGG TCC AGA-TAMRA-3′. These primers were designed using Primer Express software (Perkin-Elmer Applied Biosystems, Foster City, Calif.). Standards consisted of dilutions of DNA extracted from a human T-cell clone transduced with a single copy of the neo or HyTK vector (8, 38). The limit of detection of the real-time PCR assay was ∼10 copies per 106 cells. Negative controls consisted of DNA extracted from PBMC obtained preinfusion or from control animals or water. Samples were subjected to 50°C for 2 min and 95°C for 10 min, followed by 42 cycles of amplification at 95°C for 25 s and 60°C for 1 min, using an ABI PRISM 7700 sequence detection system (Perkin-Elmer).
RESULTS
In vivo persistence of autologous gene-modified T cells in macaques.
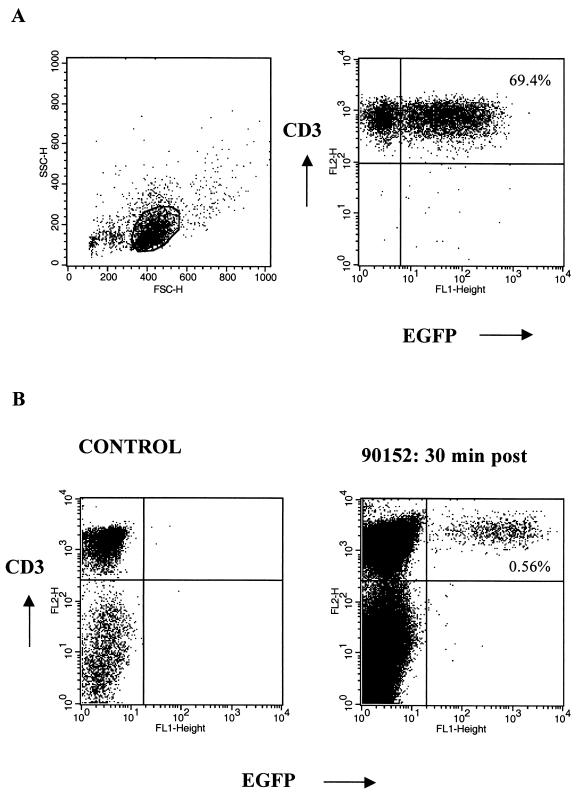
Autologous T cells were transduced to express either both the EGFP and neo genes or the HyTK gene, expanded in vitro, and adoptively transferred twice 1 week apart to different macaques (Fig. 1). The EGFP/neo retroviral vector was produced in LGSN PG13 packaging cells and transduced 69.4% of the T cells (Fig. 2A). This expression remained stable with subsequent cell expansion, making drug selection unnecessary. A lower gene transfer efficiency of approximately 11% was achieved following transduction of T cells with the amphotropic PA317 HyTK packaging cells. Thus, T cells from these cultures were selected in hygromycin B prior to expansion to increase the fraction of cells expressing the transgene. Efficient expansion of gene-modified macaque T cells to cell numbers of >109 was achieved in vitro by using anti-CD3 and anti-CD28 stimulation. Phenotypic analysis of the EGFP/neo-marked T cells prior to infusion revealed that a mean of 76.2% (range, 70.0 to 83.6%) were CD3+ CD8+ and 26.4% (range, 18.3 to 29.4%) were CD3+ CD4+. A mean of 95.6% (range, 91.8 to 97.7%) of the HyTK+ T cells were CD3+ CD8+ T cells, and 5.1% (range, 2.2 to 10.5%) were CD3+ CD4+.
FIG. 2.
(A) Successful gene transfer of M. nemestrina T lymphocytes, demonstrated by representative flow cytometry analysis of EGFP expression in T cells after transduction with the LGSN (PG13) retroviral vector. Gene transfer was accomplished using an optimized transduction protocol including anti-CD3 and anti-CD28 stimulation of T cells. Viable cells were stained with anti-CD3 MAb and analyzed by flow cytometry. Percentages of cells positive for both EGFP and CD3 are as indicated. (B) Transferred EGFP-expressing T cells are detectable by flow cytometry in vivo. PBMC from a control animal (left) and PBMC collected from macaque 90152 at 30 min postinfusion (day 410) (right) were analyzed for expression of both EGFP and CD3. Percentages of cells positive for both EGFP and CD3 are as indicated.
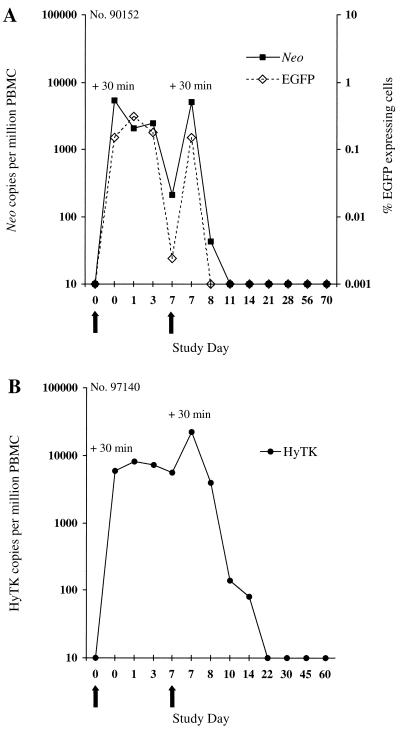
The persistence of transferred T cells in peripheral blood was examined by flow cytometry for the presence of EGFP-expressing cells (Fig. 2B) and by PCR for neo or HyTK sequences. In the macaque receiving EGFP/neo-marked T cells, 5,427 neo copies per 106 cells were detected 30 min after the first infusion, and these cells were slightly reduced in frequency by days 1 and 3 (Fig. 3A). However, only 211 neo copies per 106 cells were detected by day 7, and the frequency of neo+ cells declined rapidly over 24 h following the second cell dose to undetectable levels by day 3 postinfusion. Analysis of PBMC samples by flow cytometry for expression of EGFP confirmed the pattern of persistence of EGFP/neo-marked T cells (Fig. 3A). Transferred EGFP-expressing cells were present for 3 days after the first infusion in 0.15 to 0.31% of PBMC (∼1,454 to 3,142 per 106 cells). However, this number declined to low levels on day 7, and the cells were not detectable 24 h after the second cell dose.
FIG. 3.
Analysis of PBMC for the in vivo persistence of gene-modified T lymphocytes following in vivo priming. (A) Frequency of EGFP/neo-modified T cells transferred to macaque 90152 in peripheral blood. PBMC collected before and at the indicated days after infusion were analyzed by the quantitative real-time PCR assay for the presence of the neo sequence or by flow cytometry for EGFP-expressing cells. For enumeration of transferred EGFP-expressing T cells in PBMC, >100,000 viable cells were analyzed for each assay. Negative fluorescence gates were defined using PBMC from control animals. (B) Detection of HyTK-modified T cells transferred to macaque 97140 in circulating PBMC by the real-time PCR assay. Arrows indicate the days of T-cell infusions.
A similar pattern of persistence was observed in the macaque receiving HyTK-modified T cells (Fig. 3B). Transferred cells persisted at high levels for 7 days after the first cell dose, but the frequency of HyTK copies declined >150-fold by 3 days after the second infusion, and HyTK-marked cells were no longer detectable after day 14.
CD8+ CTL specific for the transgene products are elicited after transfer of gene-modified T cells and persist as memory cells.
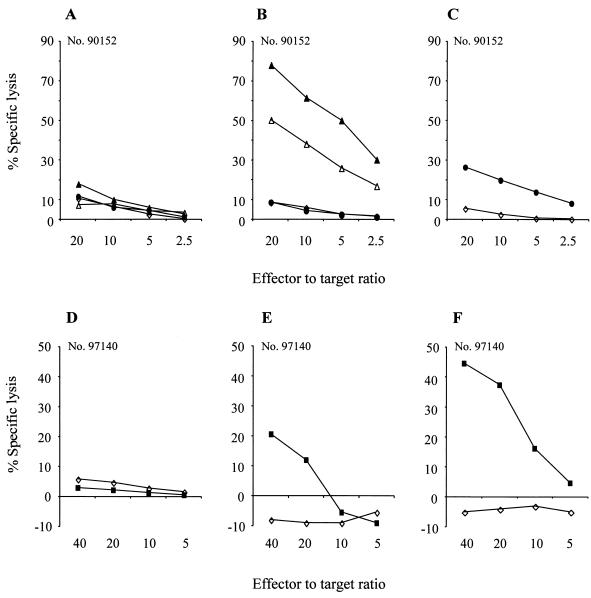
To determine if the short persistence of gene-modified T cells reflected the induction of a host immune response against transgene-encoded proteins, PBMC were obtained from the macaques before and at intervals after infusion and stimulated with γ-irradiated autologous T cells transduced to express either EGFP/neo, neo, or HyTK. No cytolytic activity was detected in cultures of preinfusion PBMC, indicating the absence of primary CTL responses to EGFP, neo, and HyTK (Fig. 4A and D). However, in the macaque receiving LGSN-modified T cells, both EGFP- and neo-specific CTL responses were first observed by 7 days after the first cell dose (Fig. 4B and C). In the animal receiving HyTK-expressing T cells, a HyTK-specific cytolytic response was first detectable on day 8 (Fig. 4E) and was stronger by day 10 (Fig. 4F).
FIG. 4.
CTL responses specific for the transgene products are elicited following transfer of gene-modified T cells. (A) Analysis of PBMC collected from macaque 90152 prior to the infusion of EGFP/neo-modified T cells for the presence of EGFP/neo-specific CTL. (B and C) Analysis of PBMC collected from macaque 90152 on day 7 following infusion of EGFP/neo-modified T cells for the presence of EGFP-specific CTL (B) and neo-specific CTL (C). Aliquots of PBMC were analyzed following stimulation with autologous T cells expressing EGFP and/or neo in a chromium release assay for recognition of target cells, either parental (◊) or transduced to express the EGFP (▵) and neo (●) genes alone and together (▴). (D to F) PBMC from macaque 97140 obtained before (D) and on days 8 (E) and 10 (F) following the first cell dose were cocultured with autologous HyTK-modified T cells and then assayed for HyTK-specific cytolytic activity against target cells, either parental (◊) or transduced to express the HyTK gene (■).
The lytic activity of these cultures could potentially reflect several populations of effector cells, including CD8+ class I MHC-restricted and/or CD4+ class II MHC-restricted T cells or non-MHC-restricted natural killer cells. Positively selected CD4+ T cells from these cultures revealed little EGFP/neo- and HyTK-specific cytolytic activity (Table 1). In contrast, enrichment of CD8+ T cells from these cultures augmented the cytolytic activity, suggesting that the lytic activity was mediated predominantly by this subset of T cells. This was confirmed by preincubating the targets with anti-class I and anti-class II MHC MAbs. Treatment of target cells with class I MAb significantly reduced the lysis of autologous EGFP/neo-expressing targets, whereas treatment with class II MAb had no effect (Table 2). These results indicated that transfer of gene-modified T cells elicited potent class I MHC-restricted CD8+ CTL responses specific for epitopes derived from transgene proteins.
TABLE 1.
Effects of selection of T-cell subsets on cytotoxicity of CTL cultures
| E/T | % Lysis
|
|||||
|---|---|---|---|---|---|---|
| EGFP-expressing target cell
|
HyTK-expressing target cell
|
|||||
| Unselected | CD4+ enriched | CD8+ enriched | Unselected | CD4+ enriched | CD8+ enriched | |
| Macaque 90152a | ||||||
| 1.25:1 | 40.2 | 15.5 | 67.3 | |||
| 0.625:1 | 38.4 | 5.7 | 51.8 | |||
| Macaque 97140b | ||||||
| 40:1 | 33.6 | −13.2 | 44.7 | |||
| 20:1 | 18.2 | −13.3 | 37.5 | |||
Lysis of parental target cells was <5% at all E/T ratios (data not shown).
Lysis of parental target cells was <−3.5% at all E/T ratios (data not shown).
TABLE 2.
Preincubation with anti-class I MAb blocks cytotoxic activity specific for transgene-encoded proteins
| E/T | % Lysis
|
|||||
|---|---|---|---|---|---|---|
| EGFP-expressing target cell
|
HyTK-expressing target cell
|
|||||
| Untreated | Anti-class I MAb | Anti-class II MAb | Untreated | Anti-class I MAb | Anti-class II MAb | |
| Macaque 90152a | ||||||
| 10:1 | 50.9 | 29.6 | 55.6 | |||
| 5:1 | 40.4 | 17.4 | 43.2 | |||
| Macaque 97140b | ||||||
| 40:1 | 32.2 | 13.7 | 43.7 | |||
| 10:1 | 21.0 | 7.7 | 25.7 | |||
Lysis of parental target cells was <5% at all E/T ratios (data not shown).
Lysis of parental target cells was <8.5% at all E/T ratios (data not shown).
We next determined whether the CTL responses persisted long term following in vivo priming. Analysis of PBMC collected on a biweekly basis following priming demonstrated that strong EGFP- and neo-specific cytolytic reactivity persisted during the follow-up and was present even 10 weeks following the first cell dose (Fig. 5A and B). Similarly, in the macaque receiving HyTK-expressing T cells, HyTK-specific cytolytic reactivity was detectable in samples of PBMC more than 12 weeks after the first cell dose (data not shown).
FIG. 5.
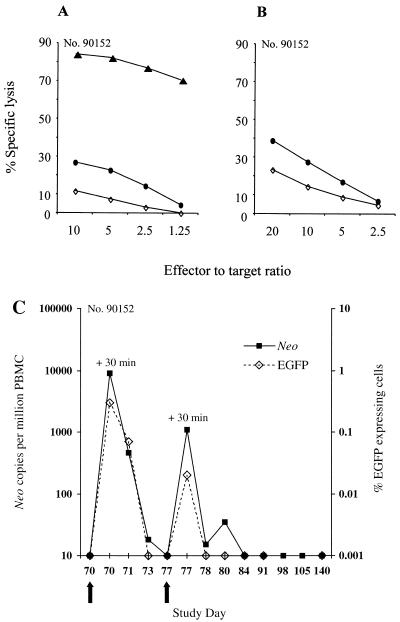
Transferred gene-modified T cells induce potent memory T-cell responses specific for the transgene products. (A and B) Aliquots of PBMC from macaque 90152 were collected on day 70 prior to the readministration of EGFP/neo-modified T cells and cocultured with autologous T cells either expressing both EGFP and neo (A) or neo alone (B). Cells from these cultures were then assayed in a chromium release assay for recognition of target cells, either parental (◊) or expressing both the EGFP and neo genes (▴) or the neo (●) gene alone. (C) Frequency of EGFP/neo-modified T cells readministered to macaque 90152 in peripheral blood. PBMC collected before and at the indicated days postinfusion were analyzed by real-time PCR for the presence of the neo sequence as described in the text or by flow cytometry to enumerate EGFP-expressing cells. Arrows indicate the days of T-cell infusions.
To evaluate whether these transgene-specific memory CTL mediated immune elimination of gene-modified cells even after a prolonged period, EGFP/neo-modified T cells were readministered on days 70 and 77 to the previously sensitized macaque 90152. The transferred cells were rapidly eliminated, consistent with the establishment of a potent memory T-cell response to the transgene products (Fig. 5C). Moreover, LDA of samples of PBMC collected prior and after retransfer of T cells revealed a substantial boost of EGFP-specific CTLp in PBMC following reexposure to transgene-expressing cells from a frequency of 1 per 10,076 (95% confidence interval, 1 per 7,658 to 1 per 13,258) to 1 per 710 (95% confidence interval, 1 per 510 to 1 per 989) 6 weeks postinfusion (Table 3).
TABLE 3.
LDA of EGFP-specific CTLp frequenciesa
| Time point | Inverse frequency | 95% Confidence interval
|
|
|---|---|---|---|
| Lower | Upper | ||
| Predose | 1/3,394,664 | 1/325,043 | 1/35,452,985 |
| Study day 70 postdose | 1/10,076 | 1/7,658 | 1/13,258 |
| Study day 111 postdose | 1/710 | 1/510 | 1/989 |
Frequencies of CTLp were estimated by LDA of PBMC obtained from macaque 90152 before (predose) and 70 days after the first cell dose and 41 days after readministration of EGFP/neo-marked cells (study day 111). Inverse frequency (inverse of the calculated CTLp frequency for autologous EGFP/neo-expressing target cells) and confidence interval were determined by maximum likelihood analysis. Inverse frequencies of CTLp for autologous target cells transduced to express the neo gene were <1/1,000,000 (data not shown).
In vivo persistence of gene-modified T cells given with transient immunosuppression.
To evaluate if administration of a transient immunosuppressive regimen could facilitate persistence of gene-modified T cells despite a memory CD8+ CTL response to the transgene product or induce tolerance against a novel transgene-encoded protein, macaque 90152, previously sensitized against EGFP and neo but not against HyTK, received both EGFP/neo- and HyTK-modified T cells on days 140 and 147. The T cells were infused after a single 200-cGy dose of TBI and initiation of 28- and 35-day courses of MMF and CSP, respectively (Fig. 1).
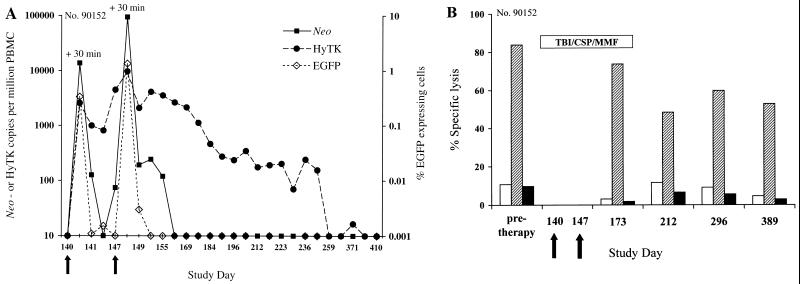
The EGFP/neo-marked T cells disappeared rapidly after the infusion with kinetics similar to those observed with the infusions on days 70 and 77 when EGFP/neo-marked T cells were given without immunosuppression (Fig. 6A). Thus, the immunosuppressive regimen failed to eliminate or inhibit the function of memory T-cell responses to EGFP or neo. In contrast, the transferred HyTK-modified T cells persisted in the peripheral blood at high levels (>250 HyTK copies per 106 cells) for more than 60 days postinfusion (Fig. 6A). The frequency of HyTK-modified cells then declined gradually, in part reflecting repopulation of the peripheral lymphoid compartment after withdrawal of the immunosuppression. However, more than 100 copies of the HyTK gene per 106 cells were still present >100 days postinfusion. The HyTK-marked cells continued to decline to low levels and were sporadically detectable until day 371. The HyTK gene could still be detected if samples of PBMC collected on day 371 were cultured in the presence of hygromycin B (data not shown). These results suggest that the transient immunosuppressive regimen efficiently interfered with the induction of HyTK-specific CTL and permitted the prolonged persistence of HyTK-modified T cells in vivo.
FIG. 6.
(A) Analysis of PBMC for the presence of both EGFP/neo- and HyTK-modified T cells transferred with a transient nonmyeloablative regimen to macaque 90152. The frequency of neo or HyTK sequences was evaluated in samples of DNA derived from PBMC collected before and on the indicated days after infusion, using the real-time PCR assay. The frequency of transferred EGFP-expressing T cells in the same samples of PBMC was assessed by flow cytometry as described in the text. Arrows indicate the days of T-cell infusions. (B) EGFP/neo- and HyTK-specific CTL responses before and after immunosuppressive treatment. Aliquots of PBMC obtained from macaque 90152 at the days indicated on the horizontal axis were stimulated twice with autologous T cells expressing either both the EGFP and neo genes or the HyTK gene and then assayed in a chromium release assay at various E/T ratios for recognition of target cells, either parental (white bars) or transduced to express either both the EGFP and neo genes (hatched bars) or the HyTK gene (black bars). The percent specific lysis of the target cells indicated on the vertical axis is shown for an E/T ratio of 20:1. The presence of EGFP-specific CTL was evaluated in samples of PBMC collected on day 371 instead of day 389. The duration of the treatment is indicated by the horizontal bar, and arrows indicate the days of T-cell infusions.
Evaluation of CTL responses during and following immunosuppression.
To evaluate the impact of the immunosuppressive regimen on the preexisting CTL responses to EGFP and neo, and the development of a primary CTL response to HyTK, samples of PBMC were examined before and after therapy for the presence of cytolytic activity against either EGFP, neo, or HyTK. EGFP- and neo-specific CTL were detectable in cultures of PBMC collected during and/or following immunosuppressive treatment (Fig. 6B). The persistence of these cytolytic effector cells was not likely due to inadequate dosing of CSP or MMF in macaques since in vitro exposure to levels of both CSP and mycophenolic acid comparable to those present in vivo inhibited lymphoproliferative responses of macaque T cells to ConA or allogeneic lymphocytes by 71.7 to 84.5% or 94.4 to 99.0%, respectively (data not shown).
In contrast, no HyTK-specific cytolytic reactivity was detected in any of the cultures of PBMC collected until day 389 (Fig. 6B). Lymphocyte counts of >400/μl and 800 to 1,000/μl were first detectable after periods of 48 and 75 days, respectively, and lymphoproliferative responses to ConA, anti-CD3 and anti-CD28 MAbs, and allogeneic lymphocytes recovered after day 163 posttherapy (data not shown). Thus, our results indicated that the immunosuppressive regimen blocked the development of HyTK-specific CTL.
The animal treated with this regimen experienced an unexpectedly prolonged period of myelosuppression. On day 22 of the regimen, absolute neutrophil counts declined below 500/μl and remained at this level until day 91, and platelet counts remained below 20,000/μl until day 119 (study day 259). However, the animal continued to eat and maintained its weight, and there was no evidence of underlying diseases or of infectious or hemostatic complications.
HyTK-specific CTL are elicited after secondary administration of gene-modified T cells.
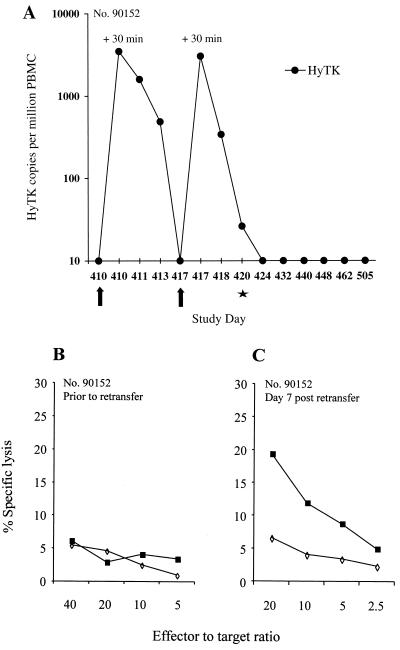
To determine if immune tolerance to the HyTK transgene product had been induced, HyTK-modified T cells were readministered to the animal 9 months after the immunosuppressive treatment. At this time, lymphocyte subsets had recovered to normal levels, and lymphoproliferative responses to mitogens such as ConA or anti-CD3 and anti-CD28 MAbs, as well as mixed lymphocyte responses, were in the normal range. Analysis of PBMC samples by PCR demonstrated that 3,316 copies of the HyTK gene per 106 cells were detectable 30 min after the first infusion on study day 410 and were present in a reduced frequency of 1,449 or 411 HyTK copies per 106 cells on days 1 and 3 postinfusion, respectively, but declined to undetectable levels by day 7 (Fig. 7A). However, after the second cell dose, the frequency of HyTK copies decreased 10-fold during 24 h and continued to rapidly decline to <30 copies per 106 cells on day 3. Cells became undetectable by day 7. To evaluate whether the marked cells were sequestered in lymphoid tissues, a lymph node biopsy was performed. PCR analysis of cell suspensions indicated the absence of transferred cells.
FIG. 7.
HyTK-specific CTL are elicited following readministration of HyTK-modified T cells to macaque 90152. (A) In vivo persistence of HyTK-modified T cells. Samples of PBMC collected before and on the indicated days after infusion were evaluated by real-time PCR for the frequency of HyTK sequences. Arrows indicate the days of T-cell infusions, and the star denotes an inguinal lymph node biopsy. (B and C) CTL responses specific for target antigens derived from HyTK in samples of PBMC collected before (study day 389) (B) and 7 days after (study day 417) (C) infusion. PBMC were cocultured with autologous γ-irradiated HyTK-expressing T cells and then assayed for HyTK-specific cytolytic activity against target cells, either parental (◊) or transduced to express the HyTK gene (■).
No HyTK-specific CTL response was detectable prior to readministration of cells (Fig. 7B), but HyTK-specific cytolytic effector T cells were detectable on day 7 postinfusion (Fig. 7C) and persisted during the follow-up (data not shown). Thus, although the transient immunosuppressive treatment delayed the initiation of a primary immune response to HyTK, it failed to induce permanent immune tolerance.
DISCUSSION
A major obstacle to the in vivo persistence of gene-modified cells is the development of a host immune response to transgene-encoded proteins (28, 38). The design of immunomodulatory approaches to circumvent immune recognition of these cells in immunocompetent hosts has been difficult (3, 11, 15). Myeloablative treatment regimens could potentially be used, since host immune responses against novel transgene-encoded proteins expressed in gene-modified cells have not been found under these conditions in large animal models and in patients with malignant diseases (1, 7, 13, 17, 23). However, the acute and long-term toxicities of complete myeloablative conditioning with high-dose TBI and/or chemotherapy are undesirable in patients with nonmalignant disorders. Recent studies by Storb et al. demonstrated that a nonmyeloablative TBI dose of 200 cGy followed by transient immunosuppression with MMF and CSP is capable of inducing persistent immune tolerance across MHC barriers of allogeneic HSCT in a canine model and in humans. Therefore, we evaluated this approach as a potential strategy to overcome immune recognition of gene-modified T cells (45, 46).
Our results demonstrated that the transfer of autologous T cells transduced to express either the EGFP and neo genes or the HyTK gene elicited rapid and potent class I MHC-restricted CD8+ CTL responses specific to these transgene products. This in vivo priming resulted in strong and persistent transgene-specific memory T-cell responses, which were maintained during and following a nonmyeloablative immunosuppressive regimen and rapidly eliminated gene-modified cells readministered several months later. These results have implications for the treatment of inherited protein-null genetic deficiency disorders, since in this circumstance, cells expressing the therapeutic gene product may be similarly immunogenic. Our observations demonstrate that once a transgene-specific CD8+ CTL response is established, its modulation might be very difficult and immune rejection may limit subsequent efforts to correct the defect by administering gene-modified cells. Thus, the prevention of primary host immune responses against transgene-encoded proteins will be essential for successful gene therapy.
In this study, the nonmyeloablative immunosuppressive regimen was effective in preventing the induction of CTL specific for a novel transgene product and permitted the prolonged (for >7 months) persistence of gene-modified T cells in vivo. However, in contrast to the nonmyeloablative allogeneic HSCT setting, in which permanent tolerance to allogeneic hematopoietic stem cells is frequently achieved, persistent immune tolerance to the transgene product was not observed and reinfusion of gene-modified T cells 9 months following the regimen led to the induction of CTL specific for the transgene product.
Several factors may explain the inability to achieve persistent tolerance to the transgene product. First, there is evidence that the sustained presence of the antigen is required for persistent immune tolerance, and the shorter life span of T cells than of permanently engrafted and self-renewing hematopoietic stem cells may have resulted in the eventual loss of the antigen (14, 37, 40). Studies of adenosine deaminase-deficient patients have shown that transferred genetically corrected T cells can survive in vivo for more than 4 years (4, 6). However, in this setting of congenital immunodeficiency, the gene-modified T cells had a survival advantage compared with nontransduced cells. Gene-marked Epstein-Barr virus-specific CTL lines administered to immunocompromised patients undergoing T-cell-depleted bone marrow transplantation were detectable >18 months postinfusion (17). However, after 4 to 5 months the detectable frequencies of the transferred neo-marked T cells were substantially lower than observed early after the infusion, and the presence of the neo gene could be demonstrated by PCR only after culturing samples of PBMC with autologous Epstein-Barr virus-transformed B-LCL in vitro. Similarly, in our study transferred HyTK-marked T cells declined to undetectable levels more than 7 months postinfusion in the absence of a measurable HyTK-specific CTL response. This decline may in part reflect dilution due to repopulation of the lymphoid compartment following the immunosuppressive therapy as well as a limited life span of the mature gene-modified T cells. Thus, a decrease of the HyTK-marked T cells below a threshold needed to maintain tolerance might explain the failure to observe permanent tolerance with this approach.
A second explanation is suggested by recent studies which showed that transferred allogeneic hematopoietic stem cells induced central tolerance to donor antigen by negative selection (22). The presence of systemic mixed donor/recipient hematopoietic microchimerism in solid organ recipients with stable long-term graft survival has led to the hypothesis that the establishment of mixed microchimerism may induce sustained tolerance (29, 43, 44, 48). However, it has still not been determined whether the presence of donor hematopoietic cells is simply a marker of tolerance or is a prerequisite for the development of tolerance.
In conclusion, we have shown that gene-modified T cells can induce memory CD8+ CTL responses which persisted during and following a potent immunosuppressive treatment consisting of low-dose TBI (200 cGy), MMF, and CSP. However, this regimen prevented the induction of a primary immune response against transgene-encoded proteins and markedly prolonged the in vivo persistence of gene-modified T cells. Sustained tolerance was not achieved, possibly due to the requirement for the continued presence of the antigen. This problem might be overcome by repeat administration of gene-modified T cells at intervals to ensure that the antigen persists or by introducing the gene into hematopoietic stem cells (18, 41).
ACKNOWLEDGMENTS
We thank Gary Millen, Robert G. Andrews, and the staff of the University of Washington Regional Primate Research Center for assistance.
C.B. is supported by the Deutsche Krebshilfe, Dr. Mildred-Scheel-Stiftung fuer Krebsforschung. H.-P.K. is a Markey Molecular Medicine Investigator. This work was supported by National Institutes of Health grants DK47754 (H.-P.K.), DK56465 (H.-P.K.), AI43650 (S.R.R.), AI41754 (S.R.R.), CA18029 (S.R.R.), and NCVDG AI26503 (P.D.G.).
REFERENCES
- 1.An D S, Wersto R P, Agricola B A, Metzger M E, Lu S, Amado R G, Chen I S Y, Donahue R E. Marking and gene expression by a lentivirus vector in transplanted human and nonhuman primate CD34+ cells. J Virol. 2000;74:1286–1295. doi: 10.1128/jvi.74.3.1286-1295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson W F. Human gene therapy. Nature. 1998;392(Suppl.):25–30. doi: 10.1038/32058. [DOI] [PubMed] [Google Scholar]
- 3.Berger C, Xuereb S, Johnson D C, Watanabe K S, Kiem H-P, Greenberg P D, Riddell S R. Expression of herpes simplex virus ICP47 and human cytomegalovirus US11 prevents recognition of transgene products by CD8+ cytotoxic T lymphocytes. J Virol. 2000;74:4465–4473. doi: 10.1128/jvi.74.10.4465-4473.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaese R M, Culver K W, Miller D A, Carter C S, Fleisher T, Clerici M, Shearer J, Chang L, Chiang Y, Tolstoshev P, Greenblatt J J, Rosenberg S A, Klein H, Berger M, Mullen C A, Ramsey W J, Muul L, Morgan R A, Anderson W F. T lymphocyte-directed gene therapy for ADA− SCID: initial trial results after 4 years. Science. 1995;270:475–480. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- 5.Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, Ponzoni M, Rossini S, Mavilio F, Traversari C, Bordignon C. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 6.Bordignon C, Notarangelo L D, Nobili N, Ferrari G, Casorati G, Panina P, Mazzolari E, Maggioni D, Rossi C, Servida P, Ugazio A G, Mavilio F. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA− immunodeficient patients. Science. 1995;270:470–475. doi: 10.1126/science.270.5235.470. [DOI] [PubMed] [Google Scholar]
- 7.Brenner M K, Rill D R, Holladay M S, Heslop H E, Moen R C, Buschle M, Krance R A, Santana V M, Anderson W F, Ihle J N. Gene marking to determine whether autologous marrow infusion restores long-term haemopoiesis in cancer patients. Lancet. 1993;342:1134–1137. doi: 10.1016/0140-6736(93)92122-a. [DOI] [PubMed] [Google Scholar]
- 8.Brodie S J, Lewinsohn D A, Patterson B K, Jiyamapa D, Krieger J, Corey L, Greenberg P D, Riddell S R. In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat Med. 1999;5:34–41. doi: 10.1038/4716. [DOI] [PubMed] [Google Scholar]
- 9.Brodie S J, Patterson B K, Lewinsohn D A, Diem K, Spach D, Greenberg P D, Riddell S R, Corey L. HIV-specific cytotoxic T lymphocytes traffic to lymph nodes and localize at sites of HIV replication and cell death. J Clin Investig. 2000;105:1407–1417. doi: 10.1172/JCI8707. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova J-L, Bousso P, Le Deist F, Fischer A. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 11.Dai Y, Schwarz E M, Gu D, Zhang W-W, Sarvetnick N, Verma I M. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de St. Groth S F. The evaluation of limiting dilution assays. J Immunol Methods. 1982;49:R11–R23. doi: 10.1016/0022-1759(82)90269-1. [DOI] [PubMed] [Google Scholar]
- 13.Dunbar C E, Cottler-Fox M, O'Shaughnessy J A, Doren S, Carter C, Berenson R, Brown S, Moen R C, Greenblatt J, Stewart F M, Leitman S F, Wilson W H, Cowan K, Young N S, Nienhuis A W. Retrovirally marked CD34-enriched peripheral blood and bone marrow cells contribute to long-term engraftment after autologous transplantation. Blood. 1995;85:3048–3057. [PubMed] [Google Scholar]
- 14.Ehl S, Aichele P, Ramseier H, Barchet W, Hombach J, Pircher H, Hengartner H, Zinkernagel R M. Antigen persistence and time of T-cell tolerization determine the efficacy of tolerization protocols for prevention of skin graft rejection. Nat Med. 1998;4:1015–1019. doi: 10.1038/2001. [DOI] [PubMed] [Google Scholar]
- 15.Engelhardt J F, Ye X, Doranz B, Wilson J M. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci USA. 1994;91:6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heid C A, Stevens J, Livak K J, Williams P M. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 17.Heslop H E, Ng C Y C, Li C, Smith C A, Loftin S K, Krance R A, Brenner M K, Rooney C M. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 18.Huhn R D, Tisdale J F, Agricola B, Metzger M E, Donahue R E, Dunbar C E. Retroviral marking and transplantation of rhesus hematopoietic cells by nonmyeloablative conditioning. Hum Gene Ther. 1999;10:1783–1790. doi: 10.1089/10430349950017464. [DOI] [PubMed] [Google Scholar]
- 19.Kärre K. How to recognize a foreign submarine. Immunol Rev. 1997;155:5–9. doi: 10.1111/j.1600-065x.1997.tb00935.x. [DOI] [PubMed] [Google Scholar]
- 20.Kent S J, Corey L, Agy M B, Morton W R, McElrath M J, Greenberg P D. Cytotoxic and proliferative T cell responses in HIV-1-infected Macaca nemestrina. J Clin Investig. 1995;95:248–256. doi: 10.1172/JCI117647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kent S J, Woodward A, Zhao A. Human immunodeficiency virus type 1 (HIV-1)-specific T cell responses correlate with control of acute HIV-1 infection in macaques. J Infect Dis. 1997;176:1188–1197. doi: 10.1086/514112. [DOI] [PubMed] [Google Scholar]
- 22.Khan A, Tomita Y, Sykes M. Thymic dependence of loss of tolerance in mixed allogeneic bone marrow chimeras after depletion of donor antigen. Transplantation. 1996;62:380–387. doi: 10.1097/00007890-199608150-00014. [DOI] [PubMed] [Google Scholar]
- 23.Kiem H-P, Andrews R G, Morris J, Peterson L, Heyward S, Allen J M, Rasko J E J, Potter J, Miller A D. Improved gene transfer into baboon marrow repopulating cells using recombinant human fibronectin fragment CH-296 in combination with interleukin-6, stem cell factor, FLT-3 ligand, and megacaryocyte growth and development factor. Blood. 1998;92:1878–1886. [PubMed] [Google Scholar]
- 24.Kiem H-P, Heyward S, Winkler A, Potter J, Allen J M, Miller D A, Andrews R G. Gene transfer into marrow repopulating cells: comparison between amphotropic and gibbon ape leukemia virus pseudotyped retroviral vectors in a competitive repopulation assay in baboons. Blood. 1997;90:4638–4645. [PubMed] [Google Scholar]
- 25.Kohn D B, Nolta J A, Crooks G M. Clinical trials of gene therapy using hematopoietic stem cells. In: Thomas E D, Blume K G, Forman S J, editors. Hematopoietic cell transplantation. Malden, Mass: Blackwell Science; 1999. pp. 97–102. [Google Scholar]
- 26.Lanier L L, Corliss B, Phillips J H. Arousal and inhibition of human NK cells. Immunol Rev. 1997;155:145–154. doi: 10.1111/j.1600-065x.1997.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 27.Lupton S D, Brunton L L, Kalberg V A, Overall R A. Dominant positive and negative selection using a hygromycin phosphotransferase-thymidine kinase fusion gene. Mol Cell Biol. 1991;11:3374–3378. doi: 10.1128/mcb.11.6.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutzko C, Kruth S, Abrams-Ogg A C G, Lau K, Li L, Clark B R, Ruedy C, Nanji S, Foster R, Kohn D, Shull R, Dubé I D. Genetically corrected autologous stem cells engraft, but host immune responses limit their utility in canine α-l-iduronidase deficiency. Blood. 1999;93:1895–1905. [PubMed] [Google Scholar]
- 29.Maeda T, Eto M, Nishimura Y, Nomoto K, Kong Y-Y, Nomoto K. Role of peripheral hemopoietic chimerism in achieving donor-specific tolerance in adult mice. J Immunol. 1993;150:753–762. [PubMed] [Google Scholar]
- 30.Miller A D. Human gene therapy comes of age. Nature. 1992;357:455–460. doi: 10.1038/357455a0. [DOI] [PubMed] [Google Scholar]
- 31.Miller A D, Garcia J V, von Suhr N, Lynch C N, Wilson C, von Eiden M. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol. 1991;65:2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller A D, Rosman G J. Improved retroviral vectors for gene transfer and expression. BioTechniques. 1989;7:980–988. [PMC free article] [PubMed] [Google Scholar]
- 33.Morton W R, Knitter G H, Smith P M, Susor T G, Schmitt K. Alternatives to chronic restraint of nonhuman primates. J Am Vet Med Assoc. 1987;191:1282–1286. [PubMed] [Google Scholar]
- 34.O'Doherty U, Ignatius R, Bhardwaj N, Pope M. Generation of monocyte-derived dendritic cells from precursors in rhesus macaque blood. J Immunol Methods. 1997;207:185–194. doi: 10.1016/s0022-1759(97)00119-1. [DOI] [PubMed] [Google Scholar]
- 35.Parham P, Sehgal P K, Brodsky F M. Anti-HLA-A,B,C monoclonal antibodies with no alloantigenic specificity in humans define polymorphisms in other primate species. Nature. 1979;279:639–641. doi: 10.1038/279639a0. [DOI] [PubMed] [Google Scholar]
- 36.Ploegh H L. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 37.Ramsdell F, Fowlkes B J. Maintenance of in vivo tolerance by persistence of antigen. Science. 1992;257:1130–1134. doi: 10.1126/science.257.5073.1130. [DOI] [PubMed] [Google Scholar]
- 38.Riddell S R, Elliott M, Lewinsohn D A, Gilbert M J, Wilson L, Manley S A, Lupton S D, Overell R W, Reynolds T C, Corey L, Greenberg P D. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat Med. 1996;2:216–223. doi: 10.1038/nm0296-216. [DOI] [PubMed] [Google Scholar]
- 39.Riddell S R, Greenberg P D. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128:189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 40.Rocha B, Tanchot C, von Boehmer H. Clonal anergy blocks in vivo growth of mature T cells and can be reversed in the absence of antigen. J Exp Med. 1993;177:1517–1521. doi: 10.1084/jem.177.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenzweig M, MacVittie T J, Harper D, Hempel D, Glickman R L, Johnson R P, Farese A M, Whiting-Theobald N, Linton G F, Yamasaki G, Jordan C T, Malech H L. Efficient and durable gene marking of hematopoietic progenitor cells in nonhuman primates after nonablative conditioning. Blood. 1999;94:2271–2286. [PubMed] [Google Scholar]
- 42.Schowalter D B, Tubb J C, Liu M, Wilson C B, Kay M A. Heterologous expression of adenovirus E3-gp19K in an E1a-deleted adenovirus vector inhibits MHC I expression in vitro, but does not prolong transgene expression in vivo. Gene Ther. 1997;4:351–360. doi: 10.1038/sj.gt.3300398. [DOI] [PubMed] [Google Scholar]
- 43.Shapiro R, Rao A S, Fontes P, Zeevi A, Jordan M, Scantlebury V P, Vivas C, Gritsch H A, Corry R J, Egidi F, Rugeles M T, Rilo H, Aitouche A, Demetris A J, Rosner G, Trucco M, Rybka W, Irish W, Fung J J, Starzl T E. Combined simultaneous kidney/bone marrow transplantation. Transplantation. 1995;60:1421–1425. doi: 10.1097/00007890-199560120-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Starzl T E, Murase N, Thomson A, Demetris A J. Liver transplants contribute to their own success. Nat Med. 1996;2:163–165. doi: 10.1038/nm0296-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Storb R, Yu C, McSweeney P. Mixed chimerism after transplantation of allogeneic hematopoietic cells. In: Thomas E D, Blume K G, Forman S J, editors. Hematopoietic cell transplantation. Malden, Mass: Blackwell Science; 1999. pp. 287–295. [Google Scholar]
- 46.Storb R, Yu C, Wagner J L, Deeg H J, Nash R A, Kiem H-P, Leisenring W, Shulman H. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- 47.Stripecke R, del Carmen Villacres M, Skelton D C, Satake N, Halene S, Kohn D B. Immune response to green fluorescent protein: implications for gene therapy. Gene Ther. 1999;6:1305–1312. doi: 10.1038/sj.gt.3300951. [DOI] [PubMed] [Google Scholar]
- 48.Taniguchi H, Toyoshima T, Fukao K, Nakauchi H. Presence of hematopoietic stem cells in the adult liver. Nat Med. 1996;2:198–203. doi: 10.1038/nm0296-198. [DOI] [PubMed] [Google Scholar]
- 49.Walker R E, Carter C S, Muul L, Natarajan V, Herpin B R, Leitman S F, Klein H G, Mullen C A, Metcalf J A, Baseler M, Falloon J, Davey Jr R T, Kovacs J A, Polis M A, Masur H, Blaese R M, Lane H C. Peripheral expansion of pre-existing mature T cells is an important means of CD4+ T-cell regeneration HIV-infected adults. Nat Med. 1998;4:852–856. doi: 10.1038/nm0798-852. [DOI] [PubMed] [Google Scholar]