Abstract
Alphaherpesviruses spread rapidly through dermal tissues and within synaptically connected neuronal circuitry. Spread of virus particles in epithelial tissues involves movement across cell junctions. Herpes simplex virus (HSV), varicella-zoster virus (VZV), and pseudorabies virus (PRV) all utilize a complex of two glycoproteins, gE and gI, to move from cell to cell. HSV gE/gI appears to function primarily, if not exclusively, in polarized cells such as epithelial cells and neurons and not in nonpolarized cells or cells that form less extensive cell junctions. Here, we show that HSV particles are specifically sorted to cell junctions and few virions reach the apical surfaces of polarized epithelial cells. gE/gI participates in this sorting. Mutant HSV virions lacking gE or just the cytoplasmic domain of gE were rarely found at cell junctions; instead, they were found on apical surfaces and in cell culture fluids and accumulated in the cytoplasm. A component of the AP-1 clathrin adapter complexes, μ1B, that is involved in sorting of proteins to basolateral surfaces was involved in targeting of PRV particles to lateral surfaces. These results are related to recent observations that (i) HSV gE/gI localizes specifically to the trans-Golgi network (TGN) during early phases of infection but moves out to cell junctions at intermediate to late times (T. McMillan and D. C. Johnson, J. Virol., in press) and (ii) PRV gE/gI participates in envelopment of nucleocapsids into cytoplasmic membrane vesicles (A. R. Brack, B. G. Klupp, H. Granzow, R. Tirabassi, L. W. Enquist, and T. C. Mettenleiter, J. Virol. 74:4004–4016, 2000). Therefore, interactions between the cytoplasmic domains of gE/gI and the AP-1 cellular sorting machinery cause glycoprotein accumulation and envelopment into specific TGN compartments that are sorted to lateral cell surfaces. Delivery of virus particles to cell junctions would be expected to enhance virus spread and enable viruses to avoid host immune defenses.
In patients suffering from recurrent facial or genital herpes simplex virus (HSV) infection or from shingles caused by varicella-zoster virus (VZV) infection in the skin, virus reactivation from latently infected sensory neurons is followed by rapid spread of infection through epidermal tissues. These alphaherpesviruses are extremely adept at moving from infected to uninfected epithelial cells and between neurons and other cells in the nervous system. Rapid and efficient progression of virus infection through tissues is particularly important, especially immediately following reactivation, when alphaherpesviruses race to produce progeny and spread to other hosts in the face of robust and fully primed host immunity. Cell-to-cell spread in epithelial tissues involves movement of virus particles across cell junctions, in a space that is resistant to the effects of virus-neutralizing antibodies. This process probably explains, in part, observations that the levels of neutralizing antibodies do not predict the severity of HSV lesions or the time to recrudescence (11).
HSV, VZV, and pseudorabies virus (PRV) express a heterodimer of two membrane glycoproteins, gE and gI, that functions to mediate cell-to-cell spread in epithelial and neuronal tissues (4, 9, 10, 12, 14, 18–20, 23–25, 28, 32, 33, 40, 42, 45). HSV and PRV gE/gI complexes are required for efficient spread of viruses between certain cultured epithelial cells, neurons, and other polarized cells with extensive cell junctions but are not needed for spread between highly transformed, nonpolarized cells, which do not form cell junctions (12, 13, 27, 42, 47, 51). For example, plaques formed by a gE-negative HSV mutant on monolayers of a keratinocyte cell line included eightfold fewer cells than plaques produced by wild-type HSV-1, yet there was no difference in cell-to-cell spread in monolayers of HeLa cells (47). Moreover, PRV and HSV gE/gI complexes are required for spread within synaptically connected neuronal circuitry in the peripheral and central nervous systems (3, 13, 32, 40, 42, 45). gE and gI are extensively complexed in virus-infected cells (19, 20), and it is the gE/gI complex that functions in cell-to-cell spread (12, 13, 19, 20, 35, 42, 52). In contrast to their effects on cell-to-cell spread, HSV and PRV gE/gI complexes do not appear to be required for entry of cell-free virus, i.e., virus particles applied to the apical surfaces of cells (12, 27). Given this observation and the specialized effects of gE/gI in polarized cells or cells that form extensive cell junctions, we hypothesized that gE/gI functions specifically in the movement of virus to and across cell junctions (14, 47). Consistent with this hypothesis, gE/gI can accumulate extensively at cell junctions after infection with HSV (14, 26a, 47).
The cytoplasmic domains of HSV and PRV gE/gI are essential for the process of cell-to-cell spread (26a, 39, 42, 47). These cytoplasmic domains, and those of VZV gE/gI, contain tyrosine (YXXØ, where Ø is a bulky hydrophobic amino acid) and dileucine motifs, as well as clusters of acidic amino acids that are phosphorylated (1, 2, 15, 16, 36, 39, 42, 47, 50). Motifs similar to these are known to promote endocytosis of cellular proteins and accumulation into trans-Golgi network (TGN) compartments by directed recycling from endosomal compartments (reviewed in references 5, 29, and 30). This occurs through interactions between the cytosolic domains of membrane proteins and AP-1 clathrin adapter complexes so that the proteins are incorporated into clathrin-coated vesicles and sorted to specialized endosomal or TGN compartments. However, in polarized cells, cells in which gE/gI plays an important role in cell-to-cell spread, tyrosine motifs and other cytoplasmic signals can cause selective sorting of cellular proteins into vesicles that are directed specifically to the basolateral domains of the plasma membrane, rather than to apical domains (17, 26, 30).
The molecular mechanisms by which alphaherpesviruses spread effectively from cell to cell in solid tissues are poorly understood. Since gE/gI functions selectively in this process, and not in entry of extracellular virus particles, gE/gI provides an important molecular tool for studying cell-to-cell spread. We previously proposed that gE/gI acts as a receptor-binding glycoprotein to mediate entry of virions specifically at cell junctions (14, 47; M. Huber, T. McMillan, T. Wisner, and D. C. Johnson, Abstr. 25th Int. Herpesvirus Workshop, Abstr. 7.06, 2000.). This hypothesis was based on our observation that gE/gI accumulated at cell junctions, late after HSV infection, consistent with selective retention there. Moreover, PRV gE/gI can mediate cell-to-cell spread in the absence of gD, a glycoprotein known to act as a receptor-binding protein during entry of extracellular virions (31, 37, 38), consistent with the hypothesis that gE/gI binds cellular ligands mediating entry in this setting. However, the observations that HSV and PRV gE/gI requires the cytoplasmic domain of gE to function in cell-to-cell spread, coupled with the extensive accumulation of gE/gI in the TGN during early phases of HSV infection or after expression by transfection or using adenovirus vectors (2, 26a), suggested that gE/gI does something other than act as a receptor-binding protein. Here, we show that gE/gI acts to sort nascent virions to the lateral surfaces of epithelial cells. This appears to involve the cytoplasmic domain of gE and AP-1 clathrin adapter complexes. Without gE, virions accumulated in the cytoplasm or were delivered to apical surfaces of cells.
MATERIALS AND METHODS
Cells and viruses.
HEC-1A human epithelial cells and MDBK, Vero, and HEp-2 cells, all from the American Type Culture Collection (ATCC), were grown as described previously (47). LLC-PK1 pig epithelial cells (ATCC) were maintained in alpha minimal essential medium containing 10% fetal bovine serum (FBS), and LLC-PK1 cells expressing μ1B (clone 4/1) (17) were grown in this medium containing 600 to 750 μg of G418/ml. Wild-type HSV type 1 (HSV-1) strain F and mutants derived from this strain, F-gEβ (13) and F-gEΔCT (47), were grown and titers were determined on Vero cells. PRV strain Becker was grown and titers were determined on PK-15 cells (ATCC).
Infection protocols.
For electron microscopy, cells were plated at 1.5 × 104 to 3.0 × 104 cells/cm2 on Thermanox plastic coverslips (Nunc) or on 0.4-μm-pore-size, 24-mm-diameter Transwell-COL polytetrafluorethylene filters coated with collagen (Costar) and then incubated for 5 to 7 days, with changes of medium every 2 days, until the cells became fully confluent and appeared tightly packed together in a cobblestone morphology. All the experiments whose results are reported here involved Thermanox coverslips because it was necessary to remove the supporting material (Thermanox or polytetrafluorethylene) once samples were embedded in epoxy, and cells often remained adhered tightly to the collagen-coated polytetrafluorethylene supports. Cells were infected with HSV-1 or PRV using 10 or 20 PFU/cell in medium containing 1 to 2% FBS for 2 h; then the virus inoculum was removed, and the cells were incubated for a further 15 to 18 h before the medium was removed and the cells were immediately fixed (without washing) with 2% glutaraldehyde (Sigma) for 15 to 20 min at room temperature. Cells growing on plastic dishes were infected with HSV-1 and then, after 20 h, were scraped from the plastic, pelleted in Eppendorf tubes, and fixed with 2% glutaraldehyde (see Table 3).
TABLE 3.
Distribution of virus particles in polarized MDBK and nonpolarized Vero cellsa
| Cells | Intracellular location | Virion structure | No. of particles/cellb
|
|
|---|---|---|---|---|
| Wild-type HSV-1 | F-gEβ | |||
| MDBK | Nucleus | Nonenveloped | 27 ± 3.9 | 19 ± 5.2 |
| Cytoplasm | Enveloped | 23 ± 3.0 | 111 ± 7.7 | |
| Nonenveloped | 3.2 ± 2.6 | 7.1 ± 4.1 | ||
| Cell surface | Enveloped | 88 ± 9.7 | 20 ± 3.8 | |
| Vero | Nucleus | Nonenveloped | 35 ± 2.6 | 37 ± 3.4 |
| Perinuclear space | Enveloped | 18 ± 4.1 | 12 ± 4.2 | |
| Cytoplasm | Enveloped | 31 ± 4.2 | 35 ± 2.1 | |
| Nonenveloped | 5.2 ± 2.4 | 6.4 ± 3.2 | ||
| Cell surface | Enveloped | 102 ± 6.7 | 89 ± 8.6 | |
MDBK or Vero cells growing in plastic dishes were infected with wild-type HSV-1 strain F or F-gEβ for 20 h. Cells were then fixed with glutaraldehyde, scraped from the plastic, and processed for electron microscopy. Approximately 2,200 wild-type virus particles and 2,200 F-gEβ particles were counted in the MDBK cell samples.
Data are averages with standard deviations.
Single-step growth curves.
HEC-1A or MDBK cells growing in 12-well (3.83-cm2) dishes were infected with wild-type HSV-1 or F-gEβ; then the cells were overlaid with 1.0 ml of medium containing 2% FBS. At various times, the medium was removed and centrifuged at 1,000 × g for 10 min, and additional medium containing 2% FBS was added to the cells, which were then scraped from the plastic. The cells were sonicated 3 times each for 40 s. The cells and cell culture supernatants were frozen at −70°C before the infectious-virus titers were determined on Vero cell monolayers.
Electron microscopy.
Cells were postfixed, prestained, and dehydrated as described previously (21). Coverslips or filters were embedded in epoxy resin, and the plastic or filter material was peeled away and replaced with resin. The samples were sectioned, collected on 300-mesh grids, and viewed with a Philips 300 transmission electron microscope. When the distribution of HSV or PRV particles was determined, only cells that appeared fully polarized, i.e., those with extensive cell junctions on both sides of the section, were considered.
RESULTS
gE/gI directs transport of HSV-1 particles to cell junctions in HEC-1A cells.
In order to determine the distribution of HSV-1 particles on various surfaces of polarized epithelial cells by electron microscopy, it was necessary to be able to orient the cells with respect to the growth substrate. HEC-1A human endometrial epithelial cells were grown on Thermanox plastic coverslips or on 0.4-μm-pore-size Transwell collagen-coated polytetrafluorethylene filters until the cells acquired a cobblestone morphology. Cells were infected with HSV for 16 to 18 h; then the medium was removed, and the cells were immediately fixed with glutaraldehyde without washing. Fixed cells were processed for electron microscopy while still attached to the plastic. However, it was difficult to view cells affixed to collagen-coated polytetrafluorethylene filters and Thermanox plastic because these materials were not stable in the electron beam. Thus, it was necessary to peel the plastic support from the cells and replace this material with epoxy. This was more difficult with the collagen-coated polytetrafluorethylene filters, as the cells adhered more tightly to material. Therefore, for all the experiments reported here, Thermanox coverslips were used. However, the morphology of cells growing on collagen-coated polytetrafluorethylene filters and the distribution of virions in these cells were not obviously different in more limited analyses.
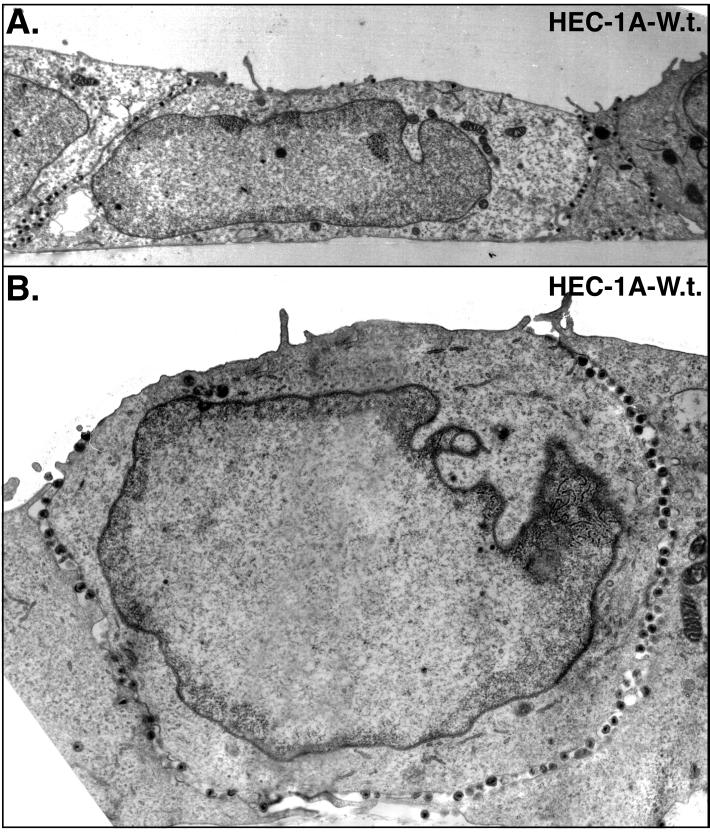
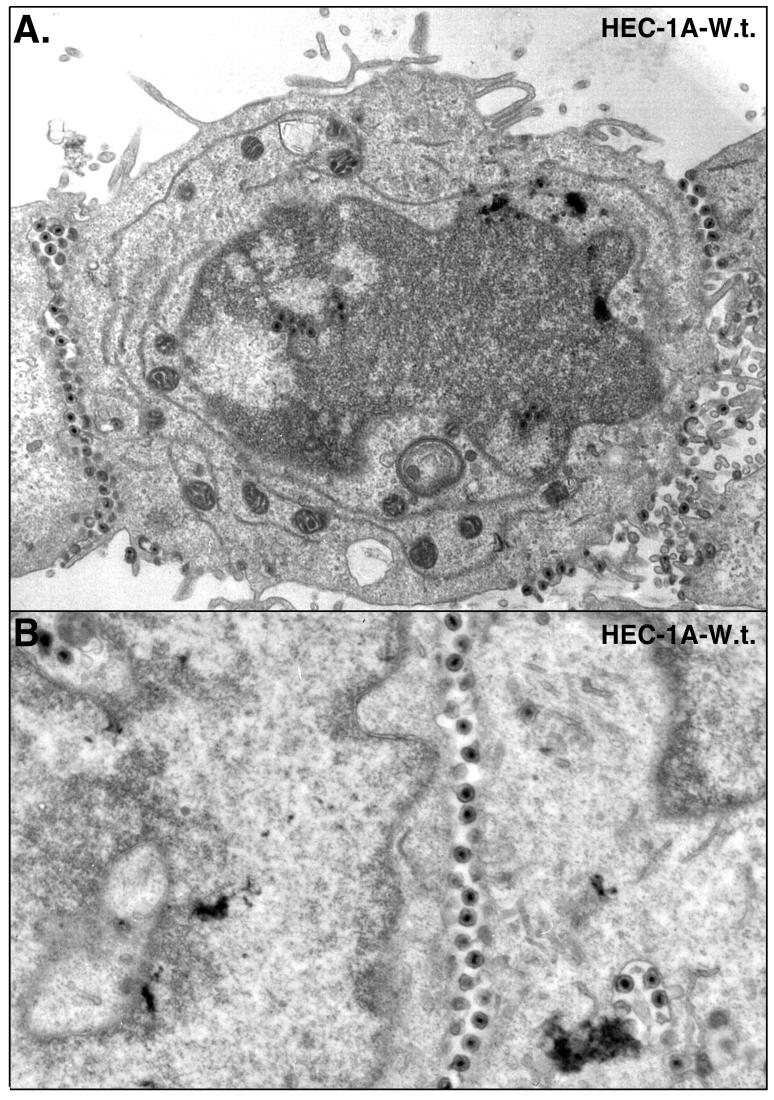
Epithelial cells infected with wild-type HSV-1 displayed large numbers of virions that accumulated at cell junctions (Fig. 1 and 2). In these sections, the majority of virus particles were seen along the entire lateral surfaces of the cells, in the narrow spaces between the cells. Virions were seen adjacent to sites of adherens junctions and desmosomes, which were not grossly altered until later times. For the most part, cells remained adhered one to another until after 20 h of infection. As virus particles began to build up in the space between cells at later times, the distance between cells in some cases increased (Fig. 2), and virions were observed on apical surfaces adjacent to the borders with cell junctions (Fig. 1A). Along lateral surfaces, virions were frequently attached to both cells (Fig. 2). By contrast, HEC-1A cells that did not form extensive contact with other cells, in less confluent areas of the monolayers, displayed a more random distribution of virus particles (not shown). With these cells, it was difficult to specify whether virions were on apical versus lateral surfaces because in the absence of cell junctions, these surfaces were not distinct. The results in Fig. 1 and 2 show that polarized HEC-1A cells displayed extensive accumulation of virions along lateral surfaces at cell junctions, and fewer virions were observed on the basal and apical surfaces of these cells.
FIG. 1.
Electron micrographs of HEC-1A cells infected with wild-type HSV-1. HEC-1A human epithelial cells were grown to confluence on plastic coverslips and then infected with wild-type HSV-1. After 16 to 18 h, the cells were fixed and processed for electron microscopy. The basal surface is along the bottom of each image.
FIG. 2.
Electron micrographs of HEC-1A cells infected with wild-type HSV-1 as described in the legend to Fig. 1.
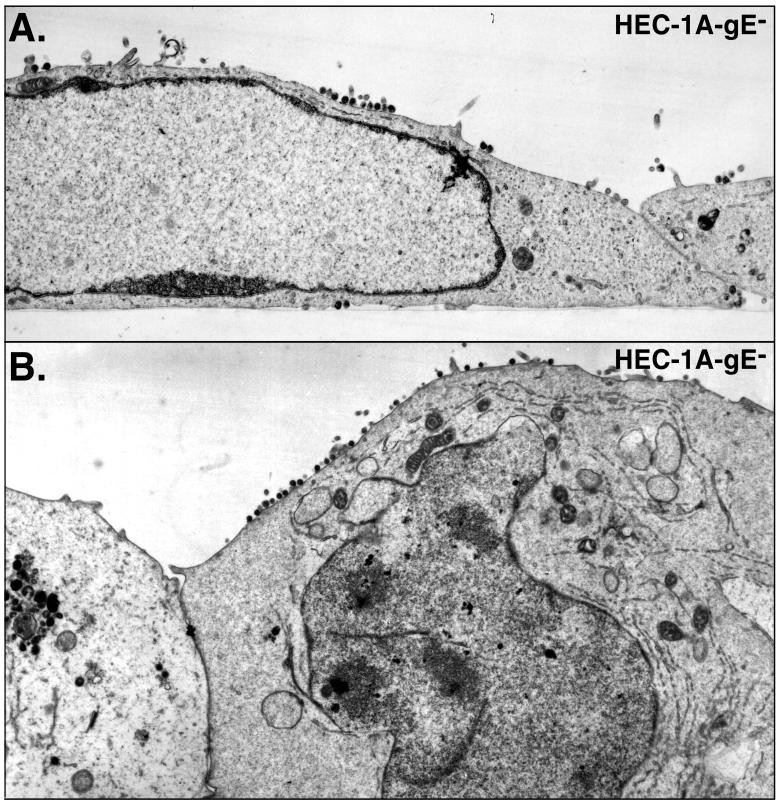
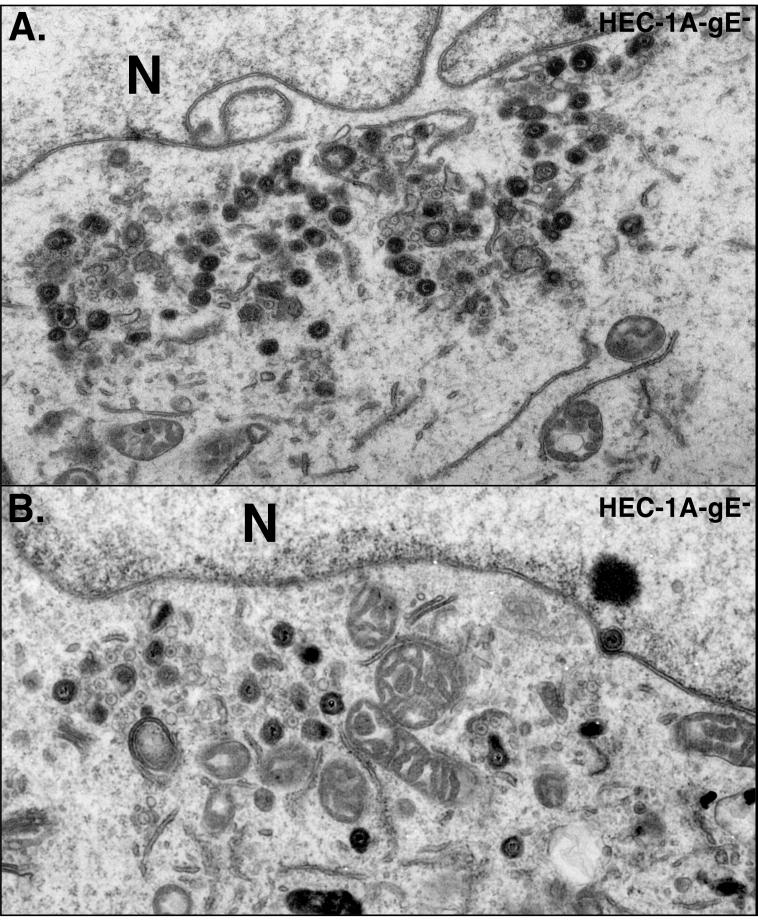
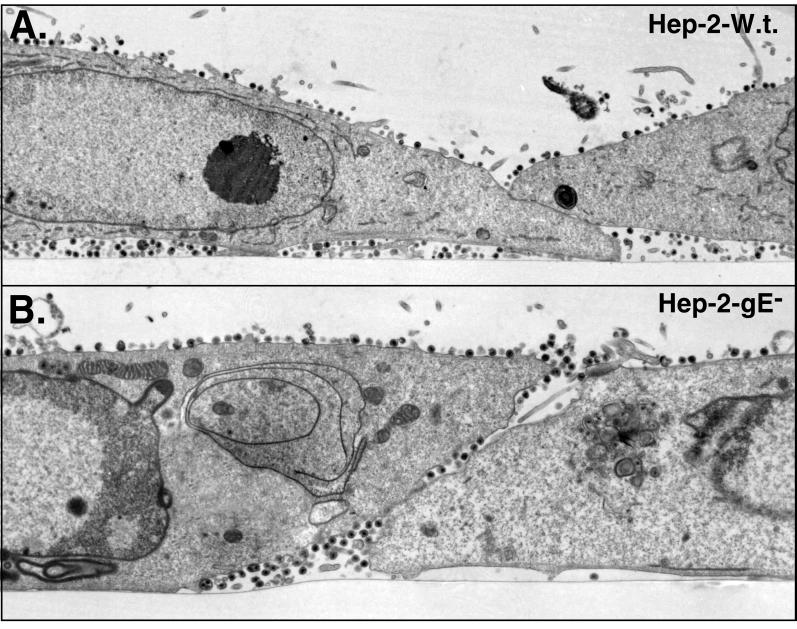
When HEC-1A cells were infected with HSV-1 mutant F-gEβ, which does not express gE (13), many fewer virions were observed along the lateral surfaces of cells, at cell junctions (Fig. 3). Instead, F-gEβ particles accumulated more frequently on apical surfaces. In addition, there was accumulation of enveloped and, in some cases, nonenveloped virions in the cytoplasm of F-gEβ-infected cells (Fig. 4). The distribution of virus particles on the different surfaces of cells (basal, lateral, and apical) was quantified by counting virus particles in 65 to 80 HEC-1A cells infected with either the wild type or F-gEβ (Table 1). Approximately 15-fold fewer virions were found at the junctions of cells infected by F-gEβ compared with wild-type-infected cells. Cells infected with F-gEβ exhibited 10-fold more virus particles on the apical surfaces than those infected with wild-type HSV. Moreover, there were twofold fewer virions found on all plasma membrane surfaces (apical plus basal plus lateral) in F-gEβ-infected than in wild-type-HSV-infected HEC-1A cells. This decrease in the total cell surface virions appeared to relate to an accumulation of virions in cytoplasmic vesicles. However, there were too few intracellular virions in these sections to allow an accurate comparison. A rescued version of F-gEβ, F-gEβR (14), behaved similarly to wild-type HSV (data not shown). In a similar analysis of nonpolarized HEp-2 cells, both wild-type and F-gEβ particles were found uniformly distributed over most of the cell surfaces (apical, lateral, and basal surfaces) (Fig. 5; Table 1). This is consistent with earlier observations that the effects of gE/gI in mediating cell-to-cell spread were restricted to cells that form extensive cell junctions, such as epithelial cells and neurons, and were not observed with other, highly transformed cells (13).
FIG. 3.
Electron micrographs of HEC-1A cells infected with F-gEβ. HEC-1A cells were grown as described for Fig. 1 and then infected with F-gEβ, a gE− HSV-1 mutant, for 16 to 18 h before the cells were fixed and processed for electron microscopy.
FIG. 4.
Electron micrographs of HEC-1A cells infected with F-gEβ, as described for Fig. 3. N, nucleus.
TABLE 1.
Cell surface distribution of virions produced by wild-type and gE mutant HSV-1 on the surfaces of HEC-1A and HEp-2 cellsa
| Cells | Virus | No. of virus particles per cellb
|
|||
|---|---|---|---|---|---|
| Apical | Basal | Cell junctions | Total | ||
| HEC-1A | Wild-type HSV | 81 (1.2) | 265 (3.9) | 1,525 (22.4) | 1,871 (27.5) |
| F-gEβ | 600 (7.7) | 211 (2.7) | 98 (1.3) | 909 (11.7) | |
| HEp-2 | Wild-type HSV | 786 (21.8) | 363 (10.1) | 101 (2.8) | 1,250 (34.7) |
| F-gEβ | 880 (23.8) | 518 (14.0) | 81 (2.2) | 1,479 (40.0) | |
Confluent monolayers of human HEC-1A or HEp-2 nonpolarized kidney cells growing on Thermanox plastic coverslips were infected with wild-type HSV-1 strain F, F-gEβ, or F-gEΔCT for 16 to 18 h. The cells were then fixed and sectioned perpendicular to the plane of the cell monolayer and plastic. Electron microscopy was used to quantify the numbers of particles on apical, basal, or lateral (cell junctions) surfaces.
The data are total numbers of virions counted, and the numbers in parentheses are the average numbers of particles per cell.
FIG. 5.
Electron micrographs of HEp-2 cells infected with wild type (W.t.) or F-gEβ. HEp-2 (nonpolarized human cells) were grown to confluence on plastic coverslips, infected with either wild-type HSV-1 (A) or F-gEβ (B), and processed for electron microscopy after 17 h.
It should be noted that these studies were more technically difficult than standard protocols in which cells are scraped from the dish and pelleted and sections through the pellets are analyzed. Here, analyses involved monolayers of cells laid out in a strip. Fewer cells, especially fully polarized cells with numerous cell surface virions, were available for counting. There were often substantial differences in the numbers of particles present on the surfaces of individual cells. The aim of this analysis was not to compare the variation between individual cells infected with a given virus but to compare the overall particle distribution with different viruses. Thus, Table 1 includes the total number of virions counted and particles per cell without standard deviations. However, the data convincingly show that wild-type HSV accumulates extensively at cell junctions and F-gEβ accumulates primarily on apical surfaces.
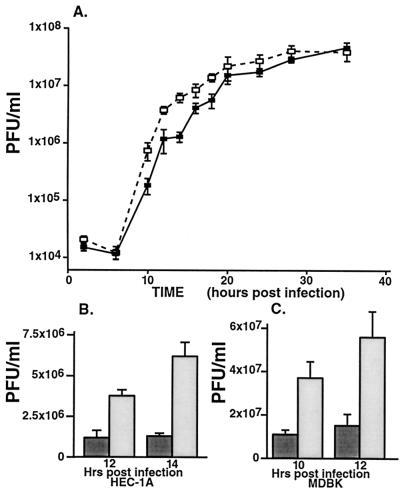
In order to examine transport of virions to the apical surfaces of epithelial cells and the associated shedding of virus into the cell culture supernatant, medium was harvested throughout a single round of infection. The titers of the infectious viruses in these culture fluids were determined by using plaque assays. At intermediate times of infection (12 to 18 h), F-gEβ-infected HEC-1A cells released four- to fivefold more infectious virus into the medium than wild-type HSV-1 (Fig. 6A and B). However, at later time points (24 to 35 h), the amounts of mutant and wild-type virus in the medium were similar, consistent with leakage of virus into the apical compartment as tight junctions were compromised and cells began to round. The total amounts of infectious virus produced by mutant and wild-type HSV-1 (cells and medium combined) at the intermediate times were similar; e.g., at 14 h there were 5.9 × 106 PFU of wild-type HSV-1 and 8.2 × 106 PFU of F-gEβ/ml. Thus, the kinetics of virus production was not different. Increased amounts of infectious F-gEβ in the medium at intermediate times correlated with the 10-fold increase in virus particles on the apical surfaces of HEC-1A cells observed by electron microscopy (Table 1). Together, these results in part explain the reduced levels of virus particles observed at cell junctions. Previously, the quantities of infectious wild-type HSV and F-gEβ in cell culture supernatants of nonpolarized Vero cells were not found to differ (13).
FIG. 6.
Infectious HSV found in cell culture supernatants of epithelial cells. HEC-1A cells (A and B) and MDBK cells (C) were infected with wild-type HSV-1 (filled symbols and bars) or with F-gEβ (open symbols and bars). After various times, the cell culture supernatants (and cells [data not shown]) were harvested, and titers of infectious virus were determined on Vero cell monolayers. The value at each time point is the mean for five independent dishes, and standard deviations are shown. Panel A is a logarithmic scale, whereas panels B and C are linear scales.
The cytoplasmic tail of gE is involved in directed transport of HSV to MDBK cell junctions.
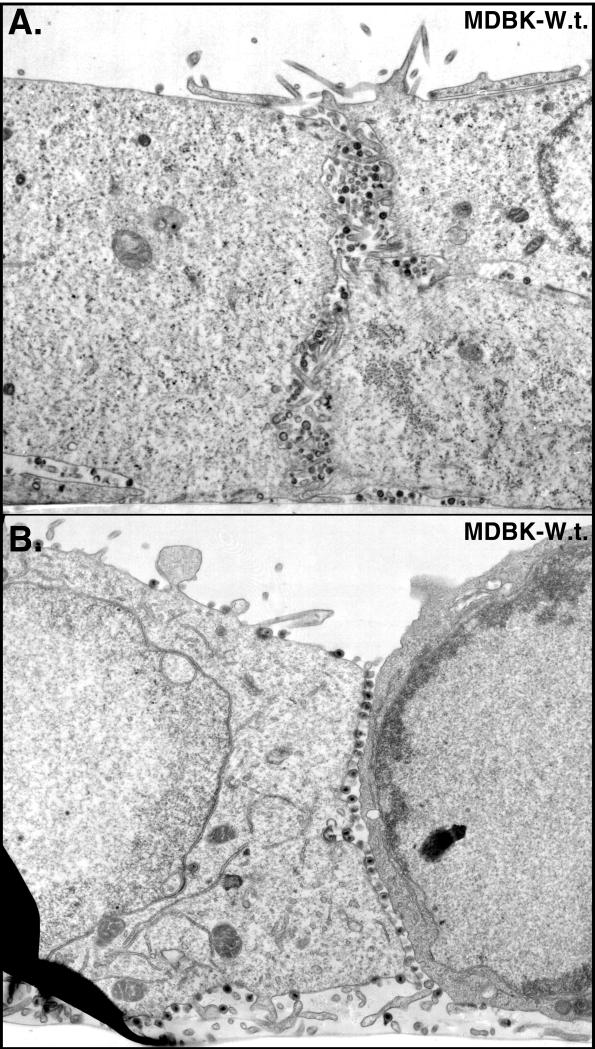
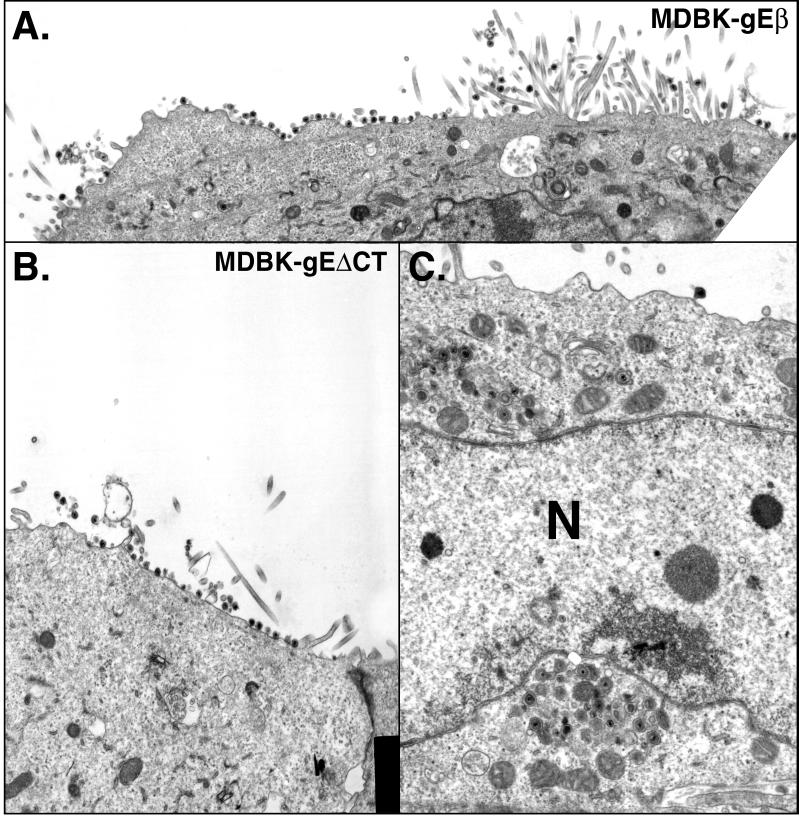
These results were extended to a second epithelial cell line, MDBK cells, and to a second mutant, F-gEΔCT, lacking only the cytoplasmic domain of gE (47). The cytoplasmic domain of gE contains sequences, including tyrosine sorting motifs and clusters of acidic residues, that are essential for cell-to-cell spread and for localization to the TGN and cell junctions (26a, 47). Again, the majority of wild-type HSV-1 particles accumulated along the lateral surfaces of MDBK cells, at cell junctions (Fig. 7 and Table 2). By contrast, there were 15- to 20-fold fewer F-gEβ particles at cell junctions and 3- to 4-fold more F-gEβ particles on the apical surfaces of MDBK cells (Fig. 8A and Table 2). Moreover, three- to fourfold more infectious F-gEβ was released into the cell culture supernatants of MDBK cells than into supernatants of wild-type-HSV-infected MDBK cells at intermediate times after infection (Fig. 6C). The mutant F-gEΔCT behaved similarly to F-gEβ: there were 20- to 30-fold fewer virions at cell junctions and increased numbers of virions on the apical surface (Fig. 8B and C; Table 2). Therefore, the cytoplasmic domain of gE is required for accumulation of virions at epithelial cell junctions.
FIG. 7.
Electron micrographs of MDBK cells infected with wild-type (W.t.) HSV. Cells were fixed and processed for electron microscopy after 17 h.
TABLE 2.
Cell surface distribution of enveloped HSV-1 on the surfaces of MDBK cellsa
| Virus | No. of virus particles per cellb
|
|||
|---|---|---|---|---|
| Apical | Basal | Cell junctions | Total | |
| Wild type | 168 (3.3) | 653 (12.8) | 2,101 (41.2) | 2,922 (57.3) |
| F-gEβ | 611 (10.0) | 334 (5.5) | 116 (1.9) | 1,061 (17.4) |
| F-gEΔCT | 653 (11.9) | 158 (2.9) | 66 (1.2) | 877 (15.9) |
MDBK cells growing on Thermanox plastic coverslips were infected with wild-type HSV-1 strain F, F-gEβ, or F-gEΔCT for 16 h. The cells were then fixed and processed for electrom microscopy as described for Table 1.
The total numbers of particles on apical or basal surfaces or at cell junctions are shown, and the numbers in parentheses are the average numbers of particles per cell.
FIG. 8.
Electron micrographs of MDBK cells infected with F-gEβ (A) or F-gEΔCT (B and C). Cells were fixed and processed for electron microscopy after 17 h. N, nucleus.
As with HEC-1A cells, fewer enveloped particles were observed on all the combined plasma membrane surfaces of F-gEβ-infected MDBK cells than of wild type-infected cells (Table 2). In order to determine whether virus particles accumulated in the cytoplasm, MDBK cells were fixed with glutaraldehyde, scraped from the plastic, and processed for electron microscopy as a cell pellet rather than affixed to plastic as in previous experiments. This allowed analysis of large numbers of virus particles, especially for F-gEβ-infected cells, because cell pellets contained larger numbers of cells. As with previous analyses there were decreased numbers of virus particles on the surfaces (all surfaces) of cells infected with F-gEβ compared with wild type-infected MDBK cells (Table 3). There were also 4.8-fold more enveloped virions, present in cytoplasmic vesicles, in F-gEβ-infected MDBK cells than in wild-type-HSV-infected cells. In some sections, we observed what appeared to be accumulation of cytosolic nucleocapsids in F-gEβ-infected cells (Fig. 4 and 8C), but there was not a consistent difference in their numbers. Comparable numbers of nucleocapsids were observed in nuclei, suggesting that early stages of replication were not different. The distribution of wild-type HSV-1 and F-gEβ particles in nonpolarized Vero cells did not differ (Table 3). Therefore, the loss of gE leads to accumulation of enveloped virions in cytoplasmic membrane vesicles of polarized epithelial cells, in addition to an apical distribution of particles.
Cell-to-cell spread of PRV is enhanced by μ1B, a component of AP-1 clathrin adapters involved in basolateral sorting.
Targeting of membrane proteins to the basolateral surfaces of epithelial cells can involve AP-1 clathrin adapter complexes (17, 30). Recently, AP-1 complexes containing an epithelial cell-specific component, μ1B, were found to sort proteins to basolateral surfaces (17). LLC-PK1, a pig epithelial cell line that lacks μ1B, was transfected with μ1B and there was increased sorting of proteins to the basolateral surfaces. We sought to determine whether μ1B could affect sorting of HSV particles; however, LLC-PK1 cells were inefficiently infected by HSV and produced few HSV progeny. Moreover, there were no available human epithelial cells lacking μ1B. The related pig alphaherpesvirus, PRV, produced relatively large numbers of progeny in LLC-PK1 cells. HSV gE/gI and PRV gE/gI are closely related glycoproteins and function in a highly similar manner: both heterodimers are crucial for cell-to-cell spread between epithelial cells, in epithelial tissues, and between connected neurons in vitro. Moreover, mutations in the cytosolic domains of these glycoproteins affect sorting of the glycoproteins and cell-to-cell spread in a similar or identical fashion (reviewed in references 2, 26a, 39, 42, and 47). Thus, we tested whether μ1B could alter the distribution of PRV particles in LLC-PK1 epithelial cells. Parental LLC-PK1 cells or cells stably transfected with μ1B (17) were infected with PRV, and virus particles were counted in electron micrographs. There were 4-fold fewer PRV particles on the apical surfaces of μ1B-transfected cells than on parental LLC-PK1 cells and 2- to 2.5-fold more viruses on both the basal surfaces and at cell junctions (Table 4). Moreover, there was two- to threefold more virus present in the cell culture supernatant of parental LLC-PK1 cells than of μ1B-transfected cells (data not shown). These results were not as substantial as those obtained with HEC-1 or MDBK cells, probably because LLC-PK1 cells did not form as extensive cell junctions. However, these results showed clearly that AP-1 complexes containing μ1B play an important role in sorting PRV to the basolateral surfaces and cell junctions of epithelial cells. This is the first instance, that we are aware of, of a definition of the cellular sorting machinery that directs virions to specific plasma membrane domains.
TABLE 4.
Cell surface distribution of enveloped PRV on the surfaces of LLC-PK1 cellsa
| Cells | No. of virus particles per cell
|
|||
|---|---|---|---|---|
| Apical | Basal | Cell junctions | Total | |
| Parental | 406 (13.1) | 242 (7.8) | 140 (4.5) | 788 (25.4) |
| μ1B transfectant | 106 (3.3) | 490 (15.3) | 320 (10.0) | 916 (28.6) |
Parental LLC-PK-1 cells or stably transfected cells expressing μ1B were grown on plastic coverslips and infected with wild-type PRV for 16 h Cells were then fixed and processed for electron microscopy as described for Table 1.
DISCUSSION
There is now considerable biochemical and genetic evidence that alphaherpesviruses acquire their final envelope from cytoplasmic membranes (6, 7, 43, 46). As a result, enveloped virions reside, for a time, within cytoplasmic membrane vesicles before being transported to cell surfaces. Here, we have demonstrated that in polarized epithelial cells, the majority of HSV virions move from cytosolic vesicles specifically to the lateral surfaces. The lateral surfaces of these epithelial cells include extensive cell junctions: adherens junctions, desmosomes, and gap junctions, as well as tight junctions near the apical surfaces. HSV particles accumulated along the entire lateral surfaces of these cells, in the narrow spaces between the cells, with fewer particles found on the basal surfaces and only rare particles observed on the apical surfaces. Fewer HSV particles were observed on the lateral surfaces of cells not in contact with another cell, hence our conclusion that particles are targeted to cell junctions. Without gE, there were 15- to 30-fold fewer virions at cell junctions, 4- to 10-fold more virus on apical surfaces, 4- to 5-fold more virus shed into the cell culture fluids, and accumulation of 4- to 5-fold more virus in cytoplasmic vesicles. Therefore, the gE/gI complex functions to selectively sort HSV particles to lateral surfaces and specifically to cell junctions. The cytoplasmic domain of gE is essential for this targeting. A similar picture was seen with PRV, although the cells used in these experiments (LLC-PK1 cells) did not form as extensive junctions with one another, and so the distribution of PRV particles was more variable.
How does gE/gI sort the entire HSV particle to cell junctions? Cellular membrane proteins are sorted to basolateral surfaces and separated from apical proteins in the TGN by recognition of sorting motifs (tyrosine, dileucine, and acidic domains) that interact with AP-1 clathrin adapter complexes (reviewed in references 5, 26, 29, 30, and 48). One example of this is the interaction between tyrosine motifs in the cytosolic domains of cellular proteins and μ1B-substituted AP-1 clathrin adapters that causes specific sorting of cellular membrane proteins to basolateral domains of LLC-PK1 epithelial cells (17). Similar or identical motifs have been implicated in the endocytosis of membrane proteins and recycling of proteins back to the TGN, but in polarized cells these signals can interact with sorting machinery, e.g., μ1B-substituted AP-1, to direct transport of clathrin-coated vesicles from the TGN specifically to the basolateral membranes. VZV, PRV, and HSV gE/gI complexes all localize to the TGN, either after transfection or during the early stages of virus infection (1, 2, 26a, 44, 49), and these glycoprotein complexes are endowed with a plethora of cytoplasmic sorting motifs that appear to be involved in TGN localization. Accumulation of gE/gI in the TGN, along with other viral glycoproteins, probably promotes envelopment of cytoplasmic nucleocapsids into these compartments (26a, 49, 50). Evidence has recently been presented that PRV gE/gI, in collaboration with a second glycoprotein, gM, drives envelopment of capsids into cytosolic vesicles (6). PRV mutants lacking gE (or its cytoplasmic domain) as well as a second glycoprotein, gM, accumulated large numbers of nucleocapsids in the cytoplasm (6). We propose that gE/gI is sorted to specific TGN compartments, vesicles that are in the process of being sorted to basolateral surfaces, in part through interactions between gE/gI cytoplasmic domain motifs and μ1B-substituted AP-1 clathrin adapters. Once localized to these TGN vesicles, gE/gI promotes envelopment of virus nucleocapsids so that virions bud into the vesicles. The vesicles are targeted specifically to lateral surfaces of these epithelial cells and virions accumulate at cell junctions. Without gE/gI or the gE cytoplasmic domain, envelopment occurs more randomly in the TGN or into other cytoplasmic membranes, there is accumulation of enveloped virions in cytoplasmic vesicles, and nascent virions are frequently transported to apical surfaces. This model is discussed in more detail in a forthcoming paper (26a).
In some of these epithelial cells, especially human HEC-1A cells, there was preferential movement of HSV to lateral surfaces, and specifically to cell junctions, rather than to basal domains. Thus, it appears that there is some level of specificity beyond targeting of virions broadly to basolateral surfaces. In monolayers of epithelial cells, cell junctions contain proteins not observed in the basal domains of the plasma membrane, e.g., cell adhesion molecules that are retained there specifically. Moreover, the basal domains contain integrins that do not accumulate in lateral domains. Therefore, there are either additional sorting or specific retention mechanisms that can produce asymmetry, even at early stages of differentiation of these epithelial cells (48).
The accumulation of large numbers of HSV virions at cell junctions may be related to the use of monolayers of epithelial cells that were uniformly infected. Virus progeny produced by one cell might be unable to enter an adjacent cell which was also infected by HSV. It is well known that HSV receptors can be blocked by overexpression of gD, a receptor-binding protein (8, 22). Thus, it is likely that virions produced by an HSV-infected epithelial cell would encounter blocked receptors, causing accumulation of virions at cell junctions. In tissues, the situation would be different: virions would move rapidly across cell junctions to adjacent uninfected cells without accumulation. However, the accumulation of virus particles at cell junctions observed here serves to indicate the direction of virion transport. One could argue that accumulation at junctions occurs by preferential “trapping” of virions and that this trapping does not occur as extensively on apical surfaces. However, there were abundant quantities of F-gEβ virions on the apical surfaces of F-gEβ-infected epithelial cells and on nonpolarized HEp-2 cells infected with wild-type HSV. In vivo, the architecture of cells in tissues may be quite different from that in epithelial cell monolayers, and virus spread may also differ from that seen here. For example, spread of HSV from infected neurons into the basal layers of the cornea involves virus movement to apical surfaces of the stromal cells, but subsequent movement through the epithelial cell layer of the cornea involves lateral spread (34).
Our results illustrate a novel strategy for directed cell-to-cell spread of an animal virus. By taking advantage of cellular sorting machinery designed to deliver cellular proteins to specific plasma membrane domains, gE/gI causes nascent virions to be incorporated into TGN compartments that are subsequently sorted to the lateral surfaces of polarized cells. Directed movement of alphaherpesvirus particles to lateral surfaces, those involved in cell junctions, would be expected to increase the rate or extent of virus spread to neighboring uninfected cells. In solid tissues, a similar form of directed spread between mucosal or ocular epithelial cells would be expected to hasten the spread of virus. This might be especially important following reactivation from latency, when alphaherpesviruses must replicate and spread rapidly to outrun fully primed host immunity. By directing nascent virus particles away from the epithelial apical surface, herpesviruses would avoid contact with components of the immune system, e.g., antibodies. In the nervous system, a similar process probably occurs, so that gE/gI promotes sorting to synaptic or adherens junctions, promoting movement from neuron to neuron. Therefore, reduced pathogenesis and neuropathogenesis of gE− or gI− mutants would appear to relate, at least in part, to the inability of virions to move to specific surfaces in polarized cells. In neurons, this process probably involves gE/gI but may also involve another glycoprotein, US9, encoded by an adjacent gene (6a).
ACKNOWLEDGMENTS
We thank Ira Mellman for the gift of μ1B-transfected LLC-PK1 cells. We are indebted to Irene Smith, Mary Huber, Tom McMillan, Jay Nelson, and Gary Thomas for critical review of the manuscript and helpful discussions. D.C.J. especially thanks Mary Huber for her optimistic support while the paper was being prepared and submitted for publication.
We acknowledge support from NIH grant CA 73996.
REFERENCES
- 1.Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- 2.Alconada A, Bauer U, Sodeik B, Hoflack B. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J Virol. 1999;73:377–387. doi: 10.1128/jvi.73.1.377-387.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babic N, Klupp B, Brack A, Mettenleiter T C, Ugolini G, Flamand A. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology. 1996;219:279–284. doi: 10.1006/viro.1996.0247. [DOI] [PubMed] [Google Scholar]
- 4.Balan P, Davis-Poynter N, Bell S, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J Gen Virol. 1994;75:1245–1258. doi: 10.1099/0022-1317-75-6-1245. [DOI] [PubMed] [Google Scholar]
- 5.Bonifacino J S, Dell'Angelica E C. Molecular bases for recognition of tyrosine-based sorting signals. J Cell Biol. 1999;145:923–926. doi: 10.1083/jcb.145.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brack A R, Klupp B G, Granzow H, Tirabassi R, Enquist L W, Mettenleiter T C. Role of the cytoplasmic tail of pseudorabies virus glycoprotein E in virion formation. J Virol. 2000;74:4004–4016. doi: 10.1128/jvi.74.9.4004-4016.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Brideau A D, Eldridge M G, Enquist L W. Directional transneuronal infection by pseudorabies virus is dependent on an acidic internalization motif in the Us9 cytoplasmic tail. J Virol. 2000;74:4549–4561. doi: 10.1128/jvi.74.10.4549-4561.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browne H, Bell S, Minson T, Wilson D W. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J Virol. 1996;70:4311–4316. doi: 10.1128/jvi.70.7.4311-4316.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campadelli-Fiume G, Arsenakis M, Farabegoli F, Roizman B. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J Virol. 1988;62:159–167. doi: 10.1128/jvi.62.1.159-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Card J P, Whealy M E, Robbins A K, Enquist L W. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J Virol. 1992;66:3032–3041. doi: 10.1128/jvi.66.5.3032-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen J I, Nguyen H. Varicella-zoster virus glycoprotein I is essential for growth of virus in Vero cells. J Virol. 1997;71:6913–6920. doi: 10.1128/jvi.71.9.6913-6920.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corey L, Spear P G. Infections with herpes simplex viruses (1) N Engl J Med. 1986;314:686–691. doi: 10.1056/NEJM198603133141105. [DOI] [PubMed] [Google Scholar]
- 12.Dingwell K S, Brunetti C R, Hendricks R L, Tang Q, Tang M, Rainbow A J, Johnson D C. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J Virol. 1994;68:834–845. doi: 10.1128/jvi.68.2.834-845.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dingwell K S, Doering L C, Johnson D C. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J Virol. 1995;69:7087–7098. doi: 10.1128/jvi.69.11.7087-7098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dingwell K S, Johnson D C. Herpes simplex virus gE/gI facilitates cell-to-cell spread and binds to components of cell junctions. J Virol. 1998;72:8933–8942. doi: 10.1128/jvi.72.11.8933-8942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edson C M. Phosphorylation of neurotropic alphaherpesvirus envelope glycoproteins: herpes simplex virus type 2 gE2 and pseudorabies virus gI. Virology. 1993;195:268–270. doi: 10.1006/viro.1993.1372. [DOI] [PubMed] [Google Scholar]
- 16.Edson C M, Hosler B A, Waters D J. Varicella-zoster virus gpI and herpes simplex virus gE: phosphorylation and Fc binding. Virology. 1987;161:599–602. doi: 10.1016/0042-6822(87)90157-7. [DOI] [PubMed] [Google Scholar]
- 17.Folsch H, Ohno H, Bonifacino J S, Mellman I. A novel clathrin adapter complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs L, Mulder J T, Van Oirshcot J T, Gielkens A L, Kimman T G. Deleting two amino acids in glycoprotein gI of pseudorabies virus decreases virulence and neurotropism for pigs, but does not affect immunogenicity. J Gen Virol. 1993;74:2201–2206. doi: 10.1099/0022-1317-74-10-2201. [DOI] [PubMed] [Google Scholar]
- 19.Johnson D C, Feenstra V. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J Virol. 1987;61:2208–2216. doi: 10.1128/jvi.61.7.2208-2216.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson D C, Frame M C, Ligas M W, Cross A M, Stow N D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988;62:1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson D C, Spear P G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982;43:1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson R M, Spear P G. Herpes simplex virus glycoprotein D mediates interference with herpes simplex virus infection. J Virol. 1989;63:819–827. doi: 10.1128/jvi.63.2.819-827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura H, Straus S E, Williams R K. Varicella-zoster virus glycoproteins E and I expressed in insect cells form a heterodimer that requires the N-terminal domain of glycoprotein I. Virology. 1997;233:382–391. doi: 10.1006/viro.1997.8625. [DOI] [PubMed] [Google Scholar]
- 24.Kritas S K, Pensaert M B, Mettenleiter T C. Role of envelope glycoproteins gI, gp63 and gIII in the invasion and spread of Aujeszky's disease virus in the olfactory nervous pathway of the pig. J Gen Virol. 1994;75:2319–2327. doi: 10.1099/0022-1317-75-9-2319. [DOI] [PubMed] [Google Scholar]
- 25.Kudelova M, Kostal M, Cervenakova L, Rajcani J, Kaerner H C. Pathogenicity and latency competence for rabbits of the herpes simplex virus type 1 ANGpath gC and gE defective mutants. Acta Virol. 1991;35:438–449. [PubMed] [Google Scholar]
- 26.Matter K. Epithelial polarity: sorting out the sorters. Curr Biol. 2000;10:R39–R42. doi: 10.1016/s0960-9822(99)00256-0. [DOI] [PubMed] [Google Scholar]
- 26a.McMillan, T. N., and D. C. Johnson. Cytoplasmic domain of herpes simplex virus gE causes accumulation in the trans=Golgi network, a site of virus envelopment and sorting of virions to cell junctions. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 27.Mettenleiter T C, Schreurs C, Zuckermann F, Ben-Porat T. Role of pseudorabies virus glycoprotein gI in virus release from infected cells. J Virol. 1987;61:2764–2769. doi: 10.1128/jvi.61.9.2764-2769.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mettenleiter T C, Zsak L, Kaplan A S, Ben-Porat T, Lomniczi B. Role of a structural glycoprotein of pseudorabies in virus virulence. J Virol. 1987;61:4030–4032. doi: 10.1128/jvi.61.12.4030-4032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molloy S S, Anderson E D, Jean F, Thomas G. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 1999;9:28–35. doi: 10.1016/s0962-8924(98)01382-8. [DOI] [PubMed] [Google Scholar]
- 30.Mostov K, ter Beest M B A, Chapin S J. Catch the u1B train to the basolateral surface. Cell. 1999;99:121–122. doi: 10.1016/s0092-8674(00)81643-8. [DOI] [PubMed] [Google Scholar]
- 31.Mulder W, Pol J, Kimman T, Kok G, Priem J, Peeters B. Glycoprotein D-negative pseudorabies virus can spread transneuronally via direct neuron-to-neuron transmission in its natural host, the pig, but not after additional inactivation of gE or gI. J Virol. 1996;70:2191–2200. doi: 10.1128/jvi.70.4.2191-2200.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulder W A, Jacobs L, Priem J, Kok G L, Wagenaar F, Kimman T G, Pol J M. Glycoprotein gE-negative pseudorabies virus has a reduced capability to infect second- and third-order neurons of the olfactory and trigeminal routes in the porcine central nervous system. J Gen Virol. 1994;75:3095–3106. doi: 10.1099/0022-1317-75-11-3095. [DOI] [PubMed] [Google Scholar]
- 33.Neidhardt H, Schroder C H, Kaerner H C. Herpes simplex virus type 1 glycoprotein E is not indispensable for viral infectivity. J Virol. 1987;61:600–603. doi: 10.1128/jvi.61.2.600-603.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Hara P T, Chin M S, LaVail J H. The spread of herpes simplex virus type 1 from trigeminal neurons to the murine cornea: an immunoelectron microscopy study. J Virol. 2000;74:4776–4786. doi: 10.1128/jvi.74.10.4776-4786.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olson J K, Grose C. Complex formation facilitates endocytosis of the varicella-zoster virus gE:gI Fc receptor. J Virol. 1998;72:1542–1551. doi: 10.1128/jvi.72.2.1542-1551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson J K, Grose C. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J Virol. 1997;71:4042–4054. doi: 10.1128/jvi.71.5.4042-4054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peeters B, Pol J, Gielkens A, Moormann R. Envelope glycoprotein gp50 of pseudorabies virus is essential for virus entry but is not required for viral spread in mice. J Virol. 1993;67:170–177. doi: 10.1128/jvi.67.1.170-177.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rauh I, Mettenleiter T C. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J Virol. 1991;65:5348–5356. doi: 10.1128/jvi.65.10.5348-5356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tirabassi R S, Enquist L W. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J Virol. 1999;73:2717–2728. doi: 10.1128/jvi.73.4.2717-2728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tirabassi R S, Enquist L W. Role of the envelope protein gE in the pseudorabies virus life cycle. J Virol. 1998;72:4571–4579. doi: 10.1128/jvi.72.6.4571-4579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tirabassi R S, Enquist L W. Role of the pseudorabies virus gI cytoplasmic tail in neuroinvasion, virulence, and posttranslational N-linked glycosylation. J Virol. 2000;74:3505–3516. doi: 10.1128/jvi.74.8.3505-3516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tirabassi R S, Townley R A, Eldridge M G, Enquist L W. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J Virol. 1997;71:6455–6464. doi: 10.1128/jvi.71.9.6455-6464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Gendersen I R, Brandimarti M, Torrisi G, Campadelli-Fiume G, van Meer G. The phospholipid composition of extracellular herpes simplex virions differs from that of the host cell nuclei. Virology. 1994;200:831–836. doi: 10.1006/viro.1994.1252. [DOI] [PubMed] [Google Scholar]
- 44.Whealy E M, Card J P, Meade R P, Robbins A K, Enquist L W. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J Virol. 1991;65:1066–1081. doi: 10.1128/jvi.65.3.1066-1081.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whealy E M, Card J P, Robbins A K, Dubin J R, Rziha H J, Enquist L W. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J Virol. 1993;67:3786–3797. doi: 10.1128/jvi.67.7.3786-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whiteley A, Brunn B, Minson T, Browne H. Effects of targeting of herpes simplex virus type gD to the endoplasmic reticulum and trans-Golgi network. J Virol. 1999;73:9515–9520. doi: 10.1128/jvi.73.11.9515-9520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wisner T, Brunetti C, Dingwell K, Johnson D C. The extracellular domain of herpes simplex virus gE is sufficient for accumulation at cell junctions but not for cell-to-cell spread. J Virol. 2000;74:2278–2287. doi: 10.1128/jvi.74.5.2278-2287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeaman C, Grindstaff K K, Nelson W J. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- 49.Zhu Z, Gershon M D, Hao Y, Ambron R T, Gabel C A, Gershon A A. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J Virol. 1995;69:7951–7959. doi: 10.1128/jvi.69.12.7951-7959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu Z, Hao Y, Gershon M D, Ambron R T, Gershon A A. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J Virol. 1996;70:6563–6575. doi: 10.1128/jvi.70.10.6563-6575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zsak L, Zuckermann F, Sugg N, Ben-Porat T. Glycoprotein gI of pseudorabies virus promotes cell fusion and virus spread via direct cell-to-cell transmission. J Virol. 1992;66:2316–2325. doi: 10.1128/jvi.66.4.2316-2325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zuckermann F A, Mettenleiter T C, Schreurs C, Sugg N, Ben-Porat T. Complex between glycoproteins gI and gp63 of pseudorabies virus: its effect on virus replication. J Virol. 1988;62:4622–4626. doi: 10.1128/jvi.62.12.4622-4626.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]