Abstract
The vaccinia virus (VV) E3L gene is responsible for providing interferon (IFN) resistance and a broad host range to VV in cell culture. The E3L gene product contains two distinct domains. A conserved carboxy-terminal domain, which is required for the IFN resistance and broad host range of the virus, has been shown to bind double-stranded RNA (dsRNA) and inhibit the antiviral dsRNA-dependent protein kinase, PKR. The amino-terminal domain, while conserved among orthopoxviruses, is dispensable in cell culture. To study the role of E3L in whole-animal infections, WR strain VV recombinants either lacking E3L (VVΔE3L) or expressing an amino-terminal (VVE3LΔ83N) or carboxy-terminal (VVE3LΔ26C) truncation of E3L were constructed. Whereas wild-type VV had a 50% lethal dose of approximately 104 PFU after intranasal infection, and elicited severe weight loss and morbidity, VVΔE3L was apathogenic, leading to no death, weight loss, or morbidity. VVΔE3L was also apathogenic after intracranial injection. Although the amino-terminal domain of E3L is dispensable for infection of cells in culture, both the amino- and carboxy-terminal domains of E3L were required for full pathogenesis in intranasal infections. These results demonstrate that the entire E3L gene is required for pathogenesis in the mouse model.
Vaccinia virus (VV) is the prototypical large double-stranded DNA virus, encoding approximately 190 genes. VV has been extensively studied as a safe alternative for a vaccine or gene therapy delivery vector (28, 29, 32, 43). One advantage is the ability to easily construct VV recombinants that express multiple foreign genes (9, 47). VV infection has been widely characterized in human subjects due to its widespread use as the smallpox vaccine (18). One important element of VV characterization is to study genes involved in pathogenesis, and those that influence the host range phenotype of the virus are logical candidates. Many VV genes initially considered nonessential have now been shown to be involved in host defense evasion (17, 41) or the well-documented poxvirus inhibition of the immune response (4, 25, 31) in whole-animal models. Some examples include the myxomavirus tumor necrosis factor alpha and gamma interferon (IFN-γ) receptors (26, 45), VV C4b-binding protein homologue (20), VV-encoded serpins (40), and a VV-encoded CC chemokine-binding protein (39).
E3L, one of the key IFN resistance genes encoded by VV, is required for VV replication in a wide range of host cells (2). However, until the present study, its in vivo importance had not been investigated. The E3L gene encodes a 190-amino-acid protein with a highly conserved carboxy-terminal double-stranded RNA-binding domain (dsRBD). E3L is a member of a large family of double-stranded RNA (dsRNA)-binding proteins which function, in vitro, to specifically bind dsRNA (but not single-stranded RNA or DNA) in a sequence-independent manner (1, 6). Other family members containing this conserved domain include both viral and cellular proteins. Several such members have been identified and extensively studied in vitro: the dsRNA-dependent protein kinase, PKR (8, 12, 24, 27, 30), group C rotavirus p8 (16, 21), Drosophila Staufen (5, 42), and Xenopus laevis RNA-binding protein A (36, 42).
The dsRBD of VV E3L has been shown to be required for both the IFN-resistant properties and the broad-host-range phenotype of VV (7). Many viruses synthesize dsRNA during replication or, in the case of VV, likely during convergent transcription at late times postinfection. dsRNA is a potent activator of two cellular IFN-inducible antiviral enzymes: PKR and 2′-5′ oligoadenylate (2′-5′A) synthetase. PKR becomes activated upon interaction with dsRNA and is able to phosphorylate the eukaryotic protein synthesis initiation factor 2 (eIF-2) on its small (α) subunit, initiating an inhibition of protein synthesis within the infected cell (37, 48). 2′-5′A synthetase also becomes activated by dsRNA, which in turn activates a latent endoribonuclease (RNase L), which then targets and cleaves cellular rRNA (15) and likely mRNA, halting protein synthesis within the cell. E3L inhibits the activation of PKR (8) and 2′-5′A synthetase (34), restoring function to the translational apparatus, thereby facilitating virus replication within the infected cell. This inhibition is dependent on the ability of E3L to bind dsRNA (7).
The amino terminus of E3L, however, is not required for PKR inhibition in vitro (7) or for IFN resistance or for a broad host range in cell culture (38). An amino-terminal deletion mutant of E3L (E3LΔ83N) that encodes a stable protein that retains its ability to bind dsRNA has been shown to functionally replace E3L in a VV infection. Virus expressing E3LΔ83N is IFN resistant and has broad-host-range characteristics in both single-cycle and multicycle (plaque formation) assays (7, 38). Since the amino terminus of E3L is not essential for the cell culture viability of VV, but the protein sequence is highly conserved among E3L genes in distantly related poxviruses (13), we hypothesized that this domain may represent a nonessential region of the E3L gene that may, in fact, be required for viral pathogenesis in an animal model. The aims of this study included determination of whether E3L is indeed important for VV infection in a mouse model and, more specifically, to determine whether both the amino- and carboxy-terminal domains are required.
MATERIALS AND METHODS
Plasmid construction.
Plasmids pMP-E3L and pMPE3LΔgpt are described in reference 19. Chang and Jacobs made the Δ83N mutant as follows. The AatII (blunt-ended)-SalI fragment of E3L (positions 84 to 190) was subcloned into pGEM3-5T vector (6), and the gene was subsequently cloned into the pMPE3LΔGPT recombination plasmid. The resultant plasmid, pMPΔ83N, contains sequences homologous to the flanking regions of the VV E3L gene as well as encoding the E. coli gpt selection gene, which allows a virus that has taken up the plasmid to replicate in the presence of mycophenolic acid. pMPΔ26C was made using whole-plasmid PCR of pMPE3L using divergent primers to delete amino acids known to be required for dsRNA binding.
Cell culture.
RK13 cells were cultured in minimal essential medium (MEM; Gibco, BRL) containing 5% fetal bovine serum (FBS), 50 μg of gentamicin per ml, and 0.1 mM nonessential amino acid solution (MEM5%; Gibco, BRL). Cells were incubated at 37°C with 5% CO2. BHK-21 cells were cultured in MEM (Gibco, BRL) containing 10% FBS and 50 μg of gentamicin per ml and incubated at 37°C with 5% CO2. HeLa S3 cells (American Type Culture Collection) were cultured in Dulbecco modified Eagle medium (Gibco, BRL) with 5% FBS and incubated at 37°C with 5% CO2.
Virus amplification.
The WR strain of VV was used for these studies. Infections of cell monolayers were performed after removing culture media in 100-μl volumes and incubating cells at 37°C and 5% CO2 for 1 h while rocking intermittently. Culture medium was replaced on the monolayer following infection. VV and VVE3LΔ83N were amplified in RK13 cell monolayers, and VVΔE3L and VVE3LΔ26C were amplified in BHK-21 cell monolayers in order to achieve maximum virus titer. Plaque formation was not visible in BHK cell monolayers without X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining. For titer determination, virus stocks were serially diluted in 1 mM Tris (pH 8.8), and plaque assays for all viruses were performed on RK13 cell monolayers; 24 to 30 h postinfection (hpi), the monolayers were stained with crystal violet (0.5% in 20% ethanol).
IVRs.
VVΔE3L was used as the parent virus for insertion of E3L mutants. In this virus, the E3L gene was removed from the WR strain of VV and replaced with the lacZ gene. In vivo recombinations (IVRs) were performed essentially as described elsewhere (19). Briefly, subconfluent RK13 cell monolayers were infected with VVΔE3L at a multiplicity of infection of 5 and simultaneously transfected with 1 μg of the plasmid containing an E3L mutant using Lipofectace (Gibco, BRL). At 30 hpi, the cells were harvested and the resultant recombinant virus was subjected to mycophenolic acid selection. Plaques were identified by a blue color when stained with X-Gal substrate. The IVR and the selection process were performed in BHK-21 cells for the Δ26C mutant due to the increased permissivity for viral replication in BHK-21 cells. The final resolution was performed in RK13 cells. Viruses were then amplified in RK13 cells for future infections and viral DNA sequencing. VVΔE3L and VVE3LΔ26C were amplified in BHK-21 cells due to the ability to achieve higher virus titers. Wild-type E3L revertant viruses were made by in vivo recombination of plasmid pMPE3L with each of VVΔE3L, VVE3LΔ83N, and VVE3LΔ26C.
Sequencing of virus mutants.
DNA was extracted from virally infected cells following three rounds of freezing (−80°C) and thawing (37°C), followed by 30-s sonication. Cell debris was removed by centrifugation at 700 × g for 10 min. Nucleic acid was obtained by phenol-chloroform-isoamyl alcohol extraction of the supernatant followed by chloroform-isoamyl alcohol extraction, ethanol precipitation, and solubilization in 10 mM Tris (pH 8.0)–0.1 mM EDTA. PCR was performed using convergent primers matching E3L flanking sequences. The PCR product was then gel purified, and the DNA was extracted from the band of interest and subjected to nucleotide sequencing.
Mice and in vivo infections.
C57BL/6 breeders were obtained from Charles River, and pathogen-free colonies were maintained at the Arizona State University Animal Resource Center. Both male and female animals between the ages of 4 and 6 weeks were used for experiments. Each cage contained seven to nine mice with approximately equal average age and weight and equal numbers of each sex, all between the ages of 4 and 6 weeks. A separate cage was used for each experimental condition (dose of each virus). An anesthetic cocktail containing xylazine (7.5 mg/ml; Phoenix Pharmaceuticals, St. Joseph, Mo.), acepromazine maleate (2.5 mg/ml), and ketamine (37.5 mg/ml; Fort Dodge Laboratories, Fort Dodge, Iowa) was prepared. Approximately 1 μl of cocktail was injected intramuscularly per g of body weight. Following anesthesia, virus was administered intranasally in 10-μl doses with a Rainin pipetman loaded with a gel loading tip. Mice were observed daily for mortality to assess the 50% lethal dose (LD50) (33). Intracranial injections on anesthetized animals were performed with 10 μl of virus, using a 27-gauge hypodermic needle and 1-ml syringe.
Weight loss.
Weight loss was determined by weighing each mouse every day or on alternate days postinfection. The percent weight gain or loss over time was determined by averaging the weights per cage at each time point divided by the initial average weight. Standard error was calculated for each dose to determine dose dependence.
Tissue distribution.
Animals were infected intranasally as described above with either 4 × 105 PFU of wild-type VV or 8 × 106 PFU of VVΔE3L. On alternate days beginning with 2 days postinfection, two animals were sacrificed by halothane overdose and then immediately dissected. The organs removed (liver, spleen, blood, lungs, brain, and nasal cavity) were immediately frozen in liquid nitrogen and stored at −80°C until the end of the time course. A 10% homogenate was prepared for each weighed organ by adding MEM5% with 2× gentamicin in a cleaned Dounce homogenizer. The nasal samples required the addition of approximately 0.1 g of sterilized sand (La Jolla Shores, Calif.) to thoroughly homogenize tissue. All homogenates were subjected to two rounds of freezing (−80°C)-thawing for 30 min on ice and then quick-thawing (37°C). Following the final thaw, samples were sonicated for 30 s and then subjected to a 10-min spin at 700 × g at 4°C to remove cell debris. Controls for determining the limit of detection in each tissue were performed simultaneously by adding known amounts of wild-type VV to tissue homogenates prior to the freeze-thaw steps. After spinning, supernatants were retained and dilutions were performed for all samples and then used for plaque assays on RK13 cells.
Plaque assays for tissue distribution studies were performed using 100 μl of the prepared dilutions (neat, 1:10, 1:100, and 1:1,000) to infect 70% confluent RK13 cells in 24-well tissue culture plates (BD). Twenty-four hours postinfection, all monolayers were stained with crystal violet to determine titer (PFU per gram of tissue).
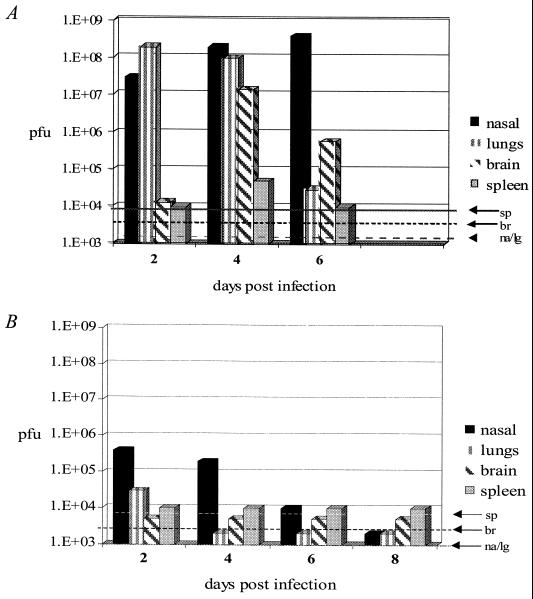
Detection limits were determined for each tissue by addition of 10, 100, 1,000, or 10,000 PFU of wild-type virus to uninfected control tissue homogenates. Controls were performed in triplicate, and limits of detection were assessed (brain, 5,000 PFU; nasal turbinate, 2,000 PFU; lung, 2,000 PFU; and spleen, 104 PFU). Limits of detection were based on the fold decrease in plaques observed compared to the known input virus (brain, 50-fold; nasal turbinate, 20-fold; lung, 20-fold; and spleen, 100-fold). Reported viral titers (Fig. 4) take into account the limit of detection; thus, plaque counts were multiplied by the absolute value of the fold decrease observed in controls.
FIG. 4.
Viral spread. Nasal turbinates (na), lungs (lg), brain (br), and spleen (sp) were harvested from infected pairs of mice on alternate days after intranasal infection (4 × 105 PFU of wild-type VV [A] or 4 × 106 PFU of VVΔE3L [B]). Organs were homogenized, and then plaque assays were performed to determine titers of detectable viable virus expressed as PFU per gram of tissue. Average titers are plotted. Limits of detection are indicated for each tissue (dashed lines). Most mice infected with wild-type VV did not survive to day 8; thus, tissues were not sampled on that day.
RESULTS
E3L is a virulence gene.
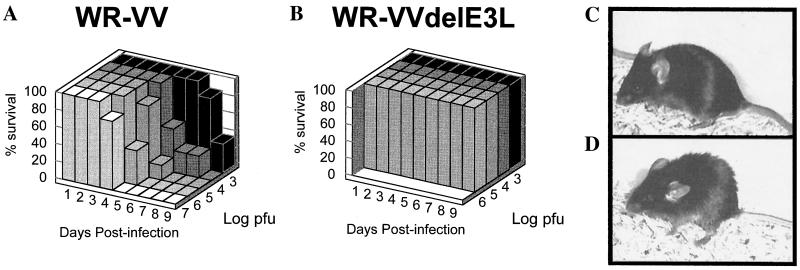
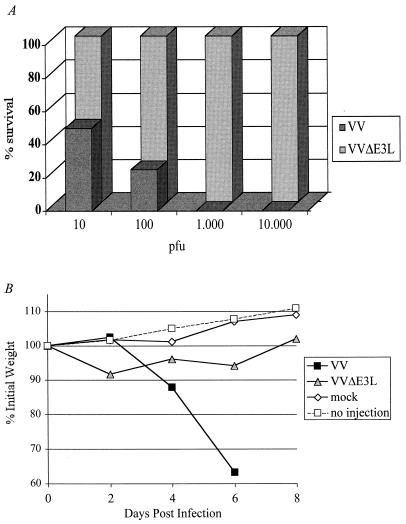
The WR strain of VV was originally derived from serial passage in mouse brain and thus is a highly virulent strain with which to perform pathogenesis studies (18). To study the role of the E3L gene in virulence, wild-type WR strain VV was compared with a WR strain from which E3L was deleted and replaced with a gene encoding β-galactosidase (VVΔE3L) (Fig. 1). WR strain VVΔE3L was phenotypically indistinguishable in cells in culture from the previously characterized Copenhagen strain VVΔE3L, in that both viruses were IFN sensitive and failed to replicate in HeLa cells (data not shown). Intranasal infections of C57BL/6 mice were performed with 10 μl of wild-type VV or VVΔE3L at various dilutions as described in Materials and Methods. Animals were observed for 14 days postinfection, at which time it was clear that the surviving animals were thriving and no longer appeared sick. Mortality was noted for each dilution of each virus. Percent survival for each virus is shown in Fig. 2A. Animals infected with wild-type VV showed 100% mortality at doses of ≥104 PFU; the LD50 is less than 103 PFU (33). We routinely obtained an LD50 of approximately 104 PFU after intranasal infection (data not shown). The wild-type VV-infected mice showed distinct signs of illness, including ruffled fur (Fig. 2D) and lack of activity. On the other hand, VVΔE3L-infected mice were indistinguishable from uninfected animals (Fig. 2B and C). No morbidity or mortality was observed at the highest attainable dose of VVΔE3L, 106 PFU. In other experiments, no animals infected with up to 4 × 106 PFU of VVΔE3L have died (data not shown). Clearly, the E3L gene is required for VV pathogenesis in this animal model.
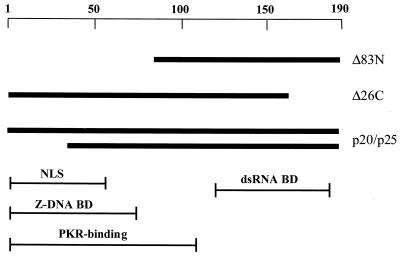
FIG. 1.
Virus constructs. Wild-type VV encodes the E3L gene products, p20 and p25. The functional domains of E3L are shown: the conserved dsRBD (dsRNA-BD); sequences homologous to a known Z-DNA-binding domain (Z-DNA BD); a putative nuclear localization signal (NLS); and sequences reported to be involved in interaction with PKR. For these experiments the E3L gene was deleted from the WR strain of VV, and the lacZ gene encoding β-galactosidase was inserted (VVΔE3L). Two viruses, E3LΔ26C and E3LΔ83N, were constructed from VVΔE3L by IVR with truncated versions of E3L. Revertants encoding wild-type E3L were reconstructed for all mutants.
FIG. 2.
E3L is a virulence gene. Four- to six-week-old C57BL/6 mice were infected intranasally with the indicated dose of either wild-type VV (A) or VVΔE3L (B), and percent survival was determined. Mice were infected intranasally with 4 × 106 PFU of VVΔE3L (C) or wild-type VV (D) and photographed on day 4 postinfection. Ruffled fur is observed in the wild-type VV-infected animal, while the VVΔE3L-infected animal has smooth fur like that of an uninfected animal (not shown).
Weight loss is dose dependent.
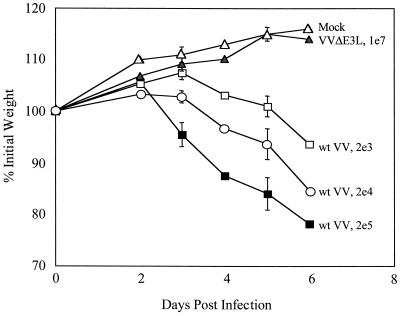
Individual mice were weighed on alternate days following intranasal infection in order to monitor the degree of sickness. Weight loss has been consistently used to measure pathogenesis and directly correlates with fever in poxvirus infections in animals (3). Weight loss was determined for mice infected with each dilution of wild-type VV (Fig. 3). Weight loss after intranasal infections with the WR strain of VV exhibited dose dependence. Weight gain rather than loss was seen for uninfected animals as well as VVΔE3L-infected animals, indicating that the virus is unable to cause detectable pathogenesis in the absence of the E3L gene (Fig. 3). These results are consistent with the absence of morbidity and mortality (Fig. 2) observed in VVΔE3L- compared to wild-type-infected animals.
FIG. 3.
VVΔE3L does not induce weight loss. Animals were infected as described for Fig. 1 and weighed at the indicated times. Average percentage of initial weight for four to six animals infected with each dose of virus is plotted versus time (days postinfection). wt, wild type.
Viral spread.
To determine the mechanism of pathogenesis, we determined virus spread and replication in various tissues. Mice were infected intranasally with wild-type VV or VVΔE3L. Two animals were sacrificed in each group on alternate days postinfection, and the spleen, lungs, brain, and nasal cavities were removed and immediately snap-frozen. Samples were later homogenized as described in Materials and Methods, and virus titers in each tissue were determined by plaque assay. High titers of virus in the lungs and nasal turbinates were observed in wild-type VV-infected mice by 2 days postinfection (Fig. 4A). Virus could be detected in the spleens and brains of infected animals by 4 days postinfection. All animals died by 8 days postinfection. Low levels of virus could be detected in the nose and lungs of animals infected with VVΔE3L at 2 days postinfection. The infection appeared to be completely resolved by 6 days postinfection (Fig. 4B). At no time was virus detected in the spleens or brains of animals infected with VVΔE3L. These results suggest that only wild-type virus exhibited evidence of a productive systemic infection.
Neurovirulence.
To assess neurovirulence directly, intracraial infections were performed with wild-type VV and VVΔE3L (Fig. 5A). Increasing doses of each virus in a total volume of 10 μl were administered to the brains of 4- to 6-week-old mice. The intracranial LD50 for wild-type VV was found to be 10 to 100 PFU, consistent with previous findings by Turner (44). As Fig. 5A demonstrates, the intracranial LD50 for VVΔE3L is greater than 104 PFU, as no animals died even at this dose; moreover, animals infected intracranially with up to 107 PFU of VVΔE3L have all survived (data not shown). Weight loss following injection with 103 PFU of each virus was assessed. Animals infected with wild-type VV lost weight following infection, whereas only a transient, marginal weight loss was observed in animals infected with VVΔE3L (Fig. 5B). Taken together, these results demonstrate that VVΔE3L does not cause disease even when injected directly into the central nervous system.
FIG. 5.
Neurovirulence. Intracranial injections were performed with 10 μl of increasing doses of wild-type VV and VVΔE3L. (A) Percent survival plotted against increasing doses of virus; (B) weights on alternate days postinfection. Too few VV-infected mice were alive after day 6 postinfection for weights to be sampled at this dose.
Full-length E3L is required for pathogenesis.
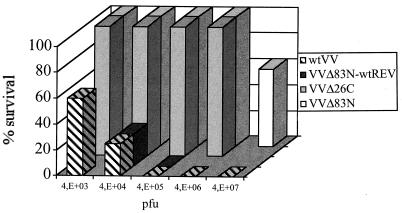
Intranasal infections were performed with various dilutions of wild-type VV, VVΔE3L, VVE3LΔ26C, and VVE3LΔ83N. Pathogenesis for VVE3LΔ26C (Fig. 6) was indistinguishable from that for VVΔE3L (Fig. 2), in that infection with up to 4 × 106 PFU did not kill infected animals. This was not unexpected, given the dominant role of the dsRBD of E3L in viral replication and IFN resistance in cell culture infections (7, 19). Surprisingly, VVE3LΔ83N was much less pathogenic than wild-type VV: only mice infected with very high doses (4 × 107 PFU) of VVE3LΔ83N died (Fig. 6). Mice infected with lower doses (as low as to 4 × 105 PFU) exhibited symptoms of pathogenesis (unlike VVΔE3L or VVE3LΔ26C), such as ruffled fur, weight loss, and lack of activity; however, the mice recovered by the end of the experiment. The calculated intranasal LD50 for VVE3LΔ83N was approximately 4 × 107 PFU. This finding demonstrates that wild-type VV is greater than 1,000-fold more lethal to mice than the amino-terminal deletion mutant VVE3LΔ83N.
FIG. 6.
Both carboxy- and amino-terminal domains of E3L are required for pathogenesis. Four- to six-week-old c57BL/6 mice were infected intranasally with 10 μl of virus with increasing doses of wild-type VV (wtVV), VVE3LΔ26C, VVE3LΔ83N, or a wild-type revertant of VVE3LΔ83N (VVE3LΔ83N-wtREV), and percent survival was determined.
To ensure that the loss of pathogenesis detected with our engineered mutants of E3L was not due to spurious mutations inadvertently introduced into the virus during mutant construction, revertant viruses were constructed by replacing the mutant E3L gene with a wild-type allele (Fig. 6). All wild-type revertants were able to fully restore wild-type virulence as assessed by mortality following intranasal infections.
DISCUSSION
In this report we have demonstrated the importance of the E3L gene of VV to pathogenesis in a mouse model. When administered intranasally, wild-type VV is at least 10,000-fold more virulent than VVΔE3L. In fact, no morbidity or mortality was detected at the highest attainable dose of the virus. The WR strain is a highly neurovirulent strain of VV, and replication in the central nervous system is likely the cause of death (18). Wild-type VV spread systemically, ultimately leading to infection of the brain, whereas VVΔE3L was found only in the respiratory tract, indicating that virus deleted for E3L does not induce a systemic infection in mice. To directly assess neurovirulence, VVΔE3L was administered directly to the brain via intracranial injections. These experiments demonstrated that E3L is required for viral pathogenesis in the brain. To determine whether both the amino- and carboxy-terminal domains of E3L were required for pathogenesis, an amino-terminal deletion mutant (VVE3LΔ83N) as well as a carboxy-terminal deletion mutant (VVE3LΔ26C) were constructed and used for intranasal infections. The results of these experiments demonstrate that while the amino terminus is dispensable for supporting replication in cells in culture, both the carboxy-terminal dsRBD and the amino terminus of E3L are required for full viral pathogenesis.
Wild-type VV (WR) infections of C57BL/6 mice by the intranasal route had an LD50 of approximately 104 PFU. This is consistent with the results found by Turner (44) as well as the results of Williamson et al. (49) for BALB/c mice. VVΔE3L did not result in mortality, and thus the LD50 is greater than 107 PFU. In fact, the animals appeared active and healthy and showed no visible signs of illness. These results clearly demonstrate that the E3L gene product is a virulence factor in vivo. This was not unexpected given the dramatic difference between wild-type VV and VVΔE3L in IFN resistance and host range in cells in culture. It remains unclear whether it is the inability of VVΔE3L to replicate in some cells in a mouse in the absence of IFN or the sensitivity of this virus to IFN that is responsible for the lack of pathogenesis in the mouse model.
The reduction in virulence is observed not only in lethality of the virus but also in weight loss. Weight loss has been correlated directly with fever and is a reliable method of determining relative pathogenesis (3). Our studies have demonstrated dose-dependent weight loss with wild-type VV. On the contrary, VVΔE3L-infected animals did not lose weight (Fig. 3). Viral spread was dramatically different between wild-type VV and VVΔE3L. VVΔE3L-infected animals cleared the virus by 6 days postinfection, and it did not appear to spread beyond a respiratory infection. In wild-type VV-infected animals, high titers of replication competent virus were isolated from the nose and lungs throughout the course of infection. Virus was isolated consistently from the brains of wild-type VV-infected mice but never from those of VVΔE3L-infected animals (Fig. 4). The direct question was then raised: Is the E3L gene product required for neurovirulence? Intracranial infection with wild-type VV resulted in an LD50 of between 10 and 100 PFU, comparable to the results of Turner (44). VVΔE3L, however, was attenuated at least 40,000-fold by the intracranial route of inoculation. In these studies, pathogenesis was not detected in VVΔE3L at any dose tested, up to 4 × 106 PFU.
Our working model, based on results from cells in culture, to describe the mechanism by which E3L provides IFN resistance to VV is an intracellular one. E3L is made early during VV infection and is available to bind viral dsRNA that results from the absence of precise late transcription termination. In this regard, E3L inhibits the dsRNA-dependent activation of the IFN-induced antiviral enzymes PKR and 2′-5′A synthetase, allowing protein synthesis in the cell to proceed normally and ultimately providing a means for productive viral replication (10, 34).
Clearly this model is at least partially inconsistent with the results reported here. Although the carboxy-terminal dsRBD is required for viral pathogenesis, these in vivo experiments have also demonstrated an important role for the amino terminus of E3L, which is not necessary to satisfy the above model. The amino terminus of E3L is not dispensable for in vivo infections. In fact, there is at least a 1,000-fold decrease in virulence in a virus expressing the amino-terminal deletion of E3L. This is likely not due to a second-site mutation in the virus, since we were able to reconstitute wild-type E3L from VVE3LΔ83N via a second recombination event and restore full virulence to the virus (Fig. 6).
The E3L gene product of VV has two distinct domains: a conserved carboxy-terminal dsRBD and a conserved amino-terminal domain. Previous studies have shown that the carboxy-terminal domain of E3L is required for replication in HeLa cells and is also required for replication in the presence of IFN (6, 7, 38, 46). This work also demonstrated that the amino terminus of E3L, despite its conservation among distantly related poxviruses, is nonessential for replication in HeLa cells and nonessential for viral growth in the presence of IFN. Thus, the work described here is the first to suggest a role for the amino terminus of the E3L gene during virus infection.
The amino terminus of the E3L gene has also been shown to be necessary for counteracting the effects of mammalian PKR expression in the heterologous yeast system (35). Expression of PKR in yeast leads to eIF-2α phosphorylation and a slow-growth phenotype. Expression of E3L can reverse the slow-growth phenotype induced by PKR expression. In yeast, both the amino-terminal and carboxy-terminal domains are required for rescue of eIF-2α phosphorylation and the slow-growth phenotype mediated by PKR.
While the amino terminus is not required to support replication in animal cells in culture, it is required to fully suppress PKR in VV-infected HeLa cells (J. O. Langland and B. L. Jacobs, unpublished data). Infection of HeLa cells with VVΔE3L leads to PKR activation and eIF-2α phosphorylation by 3 to 6 hpi, with a concomitant inhibition of both viral and host-protein synthesis. Infection with amino-terminal mutants of E3L suppresses eIF-2α phosphorylation at early but not late times postinfection (9 to 12 hpi). Despite this late phosphorylation of eIF-2α, protein synthesis continues unabated, and virus replicates normally in these cells. It is at present unclear whether eIF-2α phosphorylation in some cells is responsible for the inhibition of pathogenesis described in this report for mice infected with VVE3LΔ83N.
The amino terminus of E3L shares sequence similarity with two known cellular proteins, an RNA-specific adenosine deaminase, ADAR (22), and the murine tumor stroma and activated macrophage protein, DLM-1 (11). Both cellular proteins can be induced by treatment with IFN. The E3L homologous domain on ADAR (Zα domain) has been shown to bind to Z-DNA (23). The amino-terminal domain of E3L can also bind Z-DNA (A. Herbert and A. Rich, personal communication).
Several other biochemical characteristics have been mapped to the amino-terminal domain of E3L. Genetic screens have suggested that the amino terminus of E3L interacts directly with PKR (35). A mutation that prevented interaction in a yeast two-hybrid assay also failed to rescue yeast from the slow-growth phenotype mediated by PKR. The amino terminus has also been shown to be necessary for nuclear localization of E3L (7, 50). Wild-type E3L-encoded proteins can be found in both the nucleus and cytoplasm of infected cells. In fact, the E3L-encoded proteins are the only VV proteins known to localize to the nucleus (50). Amino-terminal mutants of E3L do not migrate to the nucleus but are present predominantly in a perinuclear location in infected cells (8). Finally, the amino terminus has been shown to be necessary for formation of oligomeric complexes larger than dimers (14). It is at present unclear which of the four biochemical characteristics that map to the amino terminus, Z-DNA binding, nuclear localization, PKR interaction, and higher-order oligomer formation, are important for pathogenesis.
The study of the pathogenesis determinants of VV is important if this virus is to be used for vaccine or gene delivery purposes. The E3L gene can be considered a virulence gene, since it is not absolutely required for replication in cells in culture (2) but is required for pathogenesis in mice. This is especially true of the amino terminus of E3L. Thus, E3L can be added to the list of genes, including the genes for thymidine kinase (17), the virokines (41), and complement control factor (39), which might be mutated to alter pathogenesis of vaccine or gene delivery vectors.
ACKNOWLEDGMENTS
We thank David Bloom, without whose assistance initiating this work would not have been possible.
This work was supported by grant CA 48654 from the National Institutes of Health and contract 20002 from the Arizona Disease Control Research Commission.
REFERENCES
- 1.Bass B L, Hurst S R, Singer J D. Binding properties of newly identified Xenopus proteins containing dsRNA-binding motifs. Curr Biol. 1994;4:301–314. doi: 10.1016/s0960-9822(00)00069-5. [DOI] [PubMed] [Google Scholar]
- 2.Beattie E, Kauffman E B, Martinez H, Perkus M E, Jacobs B L, Paoletti E, Tartaglia J. Host-range restriction of vaccinia virus E3L-specific deletion mutants. Virus Genes. 1996;12:89–94. doi: 10.1007/BF00370005. [DOI] [PubMed] [Google Scholar]
- 3.Bloom D C, Edwards K M, Hager C, Moyer R W. Identification and characterization of two nonessential regions of the rabbitpox virus genome involved in virulence. J Virol. 1991;65:1530–1542. doi: 10.1128/jvi.65.3.1530-1542.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buller R M, Palumbo G J. Poxvirus pathogenesis. Microbiol Rev. 1991;55:80–122. doi: 10.1128/mr.55.1.80-122.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bycroft M, Grunert S, Murzin A G, Proctor M, St. Johnston D. NMR solution structure of a dsRNA binding domain from Drosophila staufen protein reveals homology to the N-terminal domain of ribosomal protein S5. EMBO J. 1995;14:3563–3571. doi: 10.1002/j.1460-2075.1995.tb07362.x. . (Erratum, 14:4385.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang H W, Jacobs B L. Identification of a conserved motif that is necessary for binding of the vaccinia virus E3L gene products to double-stranded RNA. Virology. 1993;194:537–547. doi: 10.1006/viro.1993.1292. [DOI] [PubMed] [Google Scholar]
- 7.Chang H W, Uribe L H, Jacobs B L. Rescue of vaccinia virus lacking the E3L gene by mutants of E3L. J Virol. 1995;69:6605–6608. doi: 10.1128/jvi.69.10.6605-6608.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang H W, Watson J C, Jacobs B L. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc Natl Acad Sci USA. 1992;89:4825–4829. doi: 10.1073/pnas.89.11.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coupar B E, Andrew M E, Boyle D B. A general method for the construction of recombinant vaccinia viruses expressing multiple foreign genes. Gene. 1988;68:1–10. doi: 10.1016/0378-1119(88)90593-8. [DOI] [PubMed] [Google Scholar]
- 10.Davies M V, Chang H W, Jacobs B L, Kaufman R J. The E3L and K3L vaccinia virus gene products stimulate translation through inhibition of the double-stranded RNA-dependent protein kinase by different mechanisms. J Virol. 1993;67:1688–1692. doi: 10.1128/jvi.67.3.1688-1692.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu Y, Comella N, Tognazzi K, Brown L F, Dvorak H F, Kocher O. Cloning of DLM-1, a novel gene that is up-regulated in activated macrophages, using RNA differential display. Gene. 1999;240:157–163. doi: 10.1016/s0378-1119(99)00419-9. [DOI] [PubMed] [Google Scholar]
- 12.Gunnery S, Mathews M B. RNA binding and modulation of PKR activity. Methods. 1998;15:189–198. doi: 10.1006/meth.1998.0623. [DOI] [PubMed] [Google Scholar]
- 13.Haig D M, Fleming S. Immunomodulation by virulence proteins of the parapoxvirus orf virus. Vet Immunol Immunopathol. 1999;72:81–86. doi: 10.1016/s0165-2427(99)00119-1. [DOI] [PubMed] [Google Scholar]
- 14.Ho C K, Shuman S. Physical and functional characterization of the double-stranded RNA binding protein encoded by the vaccinia virus E3 gene. Virology. 1996;217:272–284. doi: 10.1006/viro.1996.0114. [DOI] [PubMed] [Google Scholar]
- 15.Iordanov M S, Paranjape J M, Zhou A, Wong J, Williams B R, Meurs E F, Silverman R H, Magun B E. Activation of p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase by double-stranded RNA and encephalomyocarditis virus: involvement of RNase L, protein kinase R, and alternative pathways. Mol Cell Biol. 2000;20:617–627. doi: 10.1128/mcb.20.2.617-627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs B L, Langland J O, Brandt T. Characterization of viral double-stranded RNA-binding proteins. Methods. 1998;15:225–232. doi: 10.1006/meth.1998.0626. [DOI] [PubMed] [Google Scholar]
- 17.Johnson G P, Goebel S J, Paoletti E. An update on the vaccinia virus genome. Virology. 1993;196:381–401. doi: 10.1006/viro.1993.1494. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan C. Vaccinia virus: a suitable vehicle for recombinant vaccines? Arch Virol. 1989;106:127–139. doi: 10.1007/BF01311044. [DOI] [PubMed] [Google Scholar]
- 19.Kibler K V, Shors T, Perkins K B, Zeman C C, Banaszak M P, Biesterfeldt J, Langland J O, Jacobs B L. Double-stranded RNA is a trigger for apoptosis in vaccinia virus-infected cells. J Virol. 1997;71:1992–2003. doi: 10.1128/jvi.71.3.1992-2003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotwal G J, Hugin A W, Moss B. Mapping and insertional mutagenesis of a vaccinia virus gene encoding a 13,800-Da secreted protein. Virology. 1989;171:579–587. doi: 10.1016/0042-6822(89)90627-2. [DOI] [PubMed] [Google Scholar]
- 21.Langland J O, Pettiford S, Jiang B, Jacobs B L. Products of the porcine group C rotavirus NSP3 gene bind specifically to double-stranded RNA and inhibit activation of the interferon-induced protein kinase PKR. J Virol. 1994;68:3821–3829. doi: 10.1128/jvi.68.6.3821-3829.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, George C X, Patterson J B, Samuel C E. Functionally distinct double-stranded RNA-binding domains associated with alternative splice site variants of the interferon-inducible double-stranded RNA-specific adenosine deaminase. J Biol Chem. 1997;272:4419–4428. doi: 10.1074/jbc.272.7.4419. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Herbert A, Rich A, Samuel C E. Double-stranded RNA-specific adenosine deaminase: nucleic acid binding properties. Methods. 1998;15:199–205. doi: 10.1006/meth.1998.0624. [DOI] [PubMed] [Google Scholar]
- 24.McCormack S J, Samuel C E. Mechanism of interferon action: RNA-binding activity of full-length and R-domain forms of the RNA-dependent protein kinase PKR—determination of KD values for VAI and TAR RNAs. Virology. 1995;206:511–519. doi: 10.1016/s0042-6822(95)80067-0. [DOI] [PubMed] [Google Scholar]
- 25.McFadden G, Graham K, Barry M. New strategies of immune modulation by DNA viruses. Transplant Proc. 1996;28:2085–2088. [PubMed] [Google Scholar]
- 26.McFadden G, Graham K, Ellison K, Barry M, Macen J, Schreiber M, Mossman K, Nash P, Lalani A, Everett H. Interruption of cytokine networks by poxviruses: lessons from myxoma virus. J Leukoc Biol. 1995;57:731–738. doi: 10.1002/jlb.57.5.731. [DOI] [PubMed] [Google Scholar]
- 27.McMillan N A, Carpick B W, Hollis B, Toone W M, Zamanian-Daryoush M, Williams B R. Mutational analysis of the double-stranded RNA (dsRNA) binding domain of the dsRNA-activated protein kinase, PKR. J Biol Chem. 1995;270:2601–2606. doi: 10.1074/jbc.270.6.2601. [DOI] [PubMed] [Google Scholar]
- 28.Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc Natl Acad Sci USA. 1996;93:11341–11348. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moss B, Carroll M W, Wyatt L S, Bennink J R, Hirsch V M, Goldstein S, Elkins W R, Fuerst T R, Lifson J D, Piatak M, Restifo N P, Overwijk W, Chamberlain R, Rosenberg S A, Sutter G. Host range restricted, non-replicating vaccinia virus vectors as vaccine candidates. Adv Exp Med Biol. 1996;397:7–13. doi: 10.1007/978-1-4899-1382-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nanduri S, Carpick B W, Yang Y, Williams B R, Qin J. Structure of the double-stranded RNA-binding domain of the protein kinase PKR reveals the molecular basis of its dsRNA-mediated activation. EMBO J. 1998;17:5458–5465. doi: 10.1093/emboj/17.18.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palumbo G J, Buller R M, Glasgow W C. Multigenic evasion of inflammation by poxviruses. J Virol. 1994;68:1737–1749. doi: 10.1128/jvi.68.3.1737-1749.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paoletti E, Tartaglia J, Taylor J. Safe and effective poxvirus vectors—NYVAC and ALVAC. Dev Biol Stand. 1994;82:65–69. [PubMed] [Google Scholar]
- 33.Reed L J, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 34.Rivas C, Gil J, Melkova Z, Esteban M, Diaz-Guerra M. Vaccinia virus E3L protein is an inhibitor of the interferon (i.f.n.)-induced 2-5A synthetase enzyme. Virology. 1998;243:406–414. doi: 10.1006/viro.1998.9072. [DOI] [PubMed] [Google Scholar]
- 35.Romano P R, Zhang F, Tan S L, Garcia-Barrio M T, Katze M G, Dever T E, Hinnebusch A G. Inhibition of double-stranded RNA-dependent protein kinase PKR by vaccinia virus E3: role of complex formation and the E3 N-terminal domain. Mol Cell Biol. 1998;18:7304–7316. doi: 10.1128/mcb.18.12.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryter J M, Schultz S C. Molecular basis of double-stranded RNA-protein interactions: structure of a dsRNA-binding domain complexed with dsRNA. EMBO J. 1998;17:7505–7513. doi: 10.1093/emboj/17.24.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samuel C E, Kuhen K L, George C X, Ortega L G, Rende-Fournier R, Tanaka H. The PKR protein kinase—an interferon-inducible regulator of cell growth and differentiation. Int J Hematol. 1997;65:227–237. doi: 10.1016/s0925-5710(96)00544-0. [DOI] [PubMed] [Google Scholar]
- 38.Shors S T, Beattie E, Paoletti E, Tartaglia J, Jacobs B L. Role of the vaccinia virus E3L and K3L gene products in rescue of VSV and EMCV from the effects of IFN-alpha. J Interferon Cytokine Res. 1998;18:721–729. doi: 10.1089/jir.1998.18.721. [DOI] [PubMed] [Google Scholar]
- 39.Smith G L. Vaccinia virus immune evasion. Immunol Lett. 1999;65:55–62. doi: 10.1016/s0165-2478(98)00125-4. [DOI] [PubMed] [Google Scholar]
- 40.Smith G L, Howard S T, Chan Y S. Vaccinia virus encodes a family of genes with homology to serine proteinase inhibitors. J Gen Virol. 1989;70:2333–2343. doi: 10.1099/0022-1317-70-9-2333. [DOI] [PubMed] [Google Scholar]
- 41.Smith G L, Symons J A, Alcami A. Immune modulation by proteins secreted from cells infected by vaccinia virus. Arch Virol Suppl. 1999;15:111–129. doi: 10.1007/978-3-7091-6425-9_8. [DOI] [PubMed] [Google Scholar]
- 42.St. Johnston D, Brown N H, Gall J G, Jantsch M. A conserved double-stranded RNA-binding domain. Proc Natl Acad Sci USA. 1992;89:10979–10983. doi: 10.1073/pnas.89.22.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tartaglia J, Cox W I, Pincus S, Paoletti E. Safety and immunogenicity of recombinants based on the genetically-engineered vaccinia strain, NYVAC. Dev Biol Stand. 1994;82:125–129. [PubMed] [Google Scholar]
- 44.Turner G S. Respiratory infection of mice with vaccinia virus. J Gen Virol. 1967;1:399–402. doi: 10.1099/0022-1317-1-3-399. [DOI] [PubMed] [Google Scholar]
- 45.Upton C, Mossman K, McFadden G. Encoding of a homolog of the IFN-gamma receptor by myxoma virus. Science. 1992;258:1369–1372. doi: 10.1126/science.1455233. [DOI] [PubMed] [Google Scholar]
- 46.Watson J C, Chang H W, Jacobs B L. Characterization of a vaccinia virus-encoded double-stranded RNA-binding protein that may be involved in inhibition of the double-stranded RNA-dependent protein kinase. Virology. 1991;185:206–216. doi: 10.1016/0042-6822(91)90768-7. [DOI] [PubMed] [Google Scholar]
- 47.Whitton J L, Sheng N, Oldstone M B, McKee T A. A “string-of-beads” vaccine, comprising linked minigenes, confers protection from lethal-dose virus challenge. J Virol. 1993;67:348–352. doi: 10.1128/jvi.67.1.348-352.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams B R. PKR; a sentinel kinase for cellular stress. Oncogene. 1999;18:6112–6120. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- 49.Williamson J D, Reith R W, Jeffrey L J, Arrand J R, Mackett M. Biological characterization of recombinant vaccinia viruses in mice infected by the respiratory route. Gen Virol. 1990;71:2761–2767. doi: 10.1099/0022-1317-71-11-2761. . (Erratum, 72:474, 1991.) [DOI] [PubMed] [Google Scholar]
- 50.Yuwen H, Cox J H, Yewdell J W, Bennink J R, Moss B. Nuclear localization of a double-stranded RNA-binding protein encoded by the vaccinia virus E3L gene. Virology. 1993;195:732–744. doi: 10.1006/viro.1993.1424. [DOI] [PubMed] [Google Scholar]