Abstract
BACKGROUND:
Recent research indicates that there is a high prevalence of heart failure with preserved ejection fraction in patients with peripheral artery disease. We hypothesized that endovascular treatment (EVT) of flow-limiting peripheral stenosis improves left ventricular (LV) diastolic function.
METHODS:
Thirty patients with symptomatic peripheral artery disease and heart failure with preserved ejection fraction according to Heart Failure Association−preserved ejection fraction score who were scheduled for EVT or angiography were investigated at baseline, the day after EVT (n=25) or angiography (control, n=5), and at 4 months follow-up. Peripheral hemodynamics were determined by the total peripheral resistance, common femoral artery flow, and ankle brachial index. Aortic function was measured by arterial compliance, augmentation index, and pulse wave velocity. Aortic pulsatile load was estimated as the characteristic impedance of the proximal aorta and the magnitude of wave reflection (reflection coefficient). LV mass index, LV mean wall thickness, and systolic and diastolic function were assessed using echocardiography. Patient-centered outcomes were treadmill walking distance and New York Heart Association class.
RESULTS:
After EVT, peripheral hemodynamics changed significantly with a decrease in total peripheral resistance and an increase in common femoral artery flow and ankle brachial index. Aortic function improved after EVT, with significantly reduced augmentation index and pulse wave velocity and increased compliance immediately and at follow-up, resulting in a reduction in aortic pulsatile load (characteristic impedance of the proximal aorta and reflection coefficient). Concurrently, LV diastolic function improved after EVT compared with control, acutely and at follow-up, with increased septal and lateral e´ velocities and decreased E/e´ and left atrial volume index. The LV mass index and LV mean wall thickness decreased at follow-up. The New York Heart Association class and treadmill walking distance improved post-EVT at follow-up. Augmentation index, pulse wave velocity, and arterial compliance were identified as independent contributors to E/e´.
CONCLUSIONS:
Endovascular treatment of flow-limiting iliofemoral stenosis reduces aortic pulsatile load and concurrently lowers total peripheral resistance. This beneficial effect is associated with an acute and sustained improvement of left ventricular diastolic function.
REGISTRATION:
URL: http://www.clinicaltrials.gov; Unique identifier: NCT02728479.
Keywords: aorta, heart failure, peripheral arterial disease, pulse wave analysis, stroke volume
WHAT IS NEW?
Peripheral artery disease is associated with left ventricular diastolic function and heart failure with preserved ejection fraction.
The impact of endovascular treatment of flow-limiting iliofemoral stenosis on left ventricular diastolic function, aortic function, and peripheral hemodynamics in patients with peripheral artery disease and heart failure with preserved ejection fraction has not been studied.
Our data demonstrate that endovascular treatment of flow-limiting stenosis reduces aortic pulsatile load and concurrently lowers total peripheral resistance. This beneficial effect is associated with an acute and sustained improvement of left ventricular diastolic function in ameliorating heart failure.
WHAT ARE THE CLINICAL IMPLICATIONS?
Screening for peripheral artery disease in heart failure with preserved ejection fraction and vice versa is important to optimize treatment strategies in these high-risk patients.
See Editorial by Goudot and Gerhard-Herman
Peripheral artery disease (PAD) is associated with increased cardiovascular mortality and an increased risk for incident heart failure (HF).1 Furthermore, PAD is associated with arterial stiffness and an elevated aortic augmentation index (AIx). It is now appreciated that wave reflection and arterial stiffness are important determinants of age-related isolated systolic hypertension.2,3 As the aorta stiffens, its pressure-buffering function is impaired, resulting in an increase in aortic pulsatile load and aortic blood pressure (aBP). Isolated systolic hypertension occurs in up to 90% of patients with PAD.4 In the context of PAD, arterial stenoses and occlusions may cause local reflection of the pulse wave, increasing aortic augmentation and contributing to the development of isolated systolic hypertension, therefore increasing the left ventricular (LV) afterload, which ultimately leads to HF.5,6
Approximately 10% of patients with heart failure with preserved ejection fraction (HFpEF) have concomitant PAD.7 Functional limitations in walking capacity due to HF are likely to mask symptoms of PAD or vice versa, causing underestimation of the number of patients with both conditions. Patients with hypertension and HFpEF have increased vascular stiffness; therefore, this may play a role in coupling vascular stiffening, increased afterload, and impaired left ventricular diastolic function (LVDF).8–10
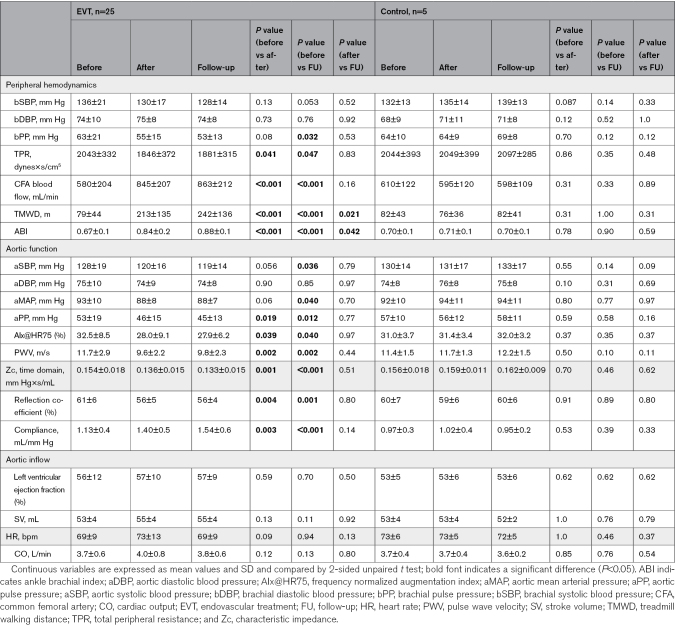
We and others have previously shown that peripheral EVT of flow-limiting stenosis acutely lowers aBP and brachial blood pressure (BP), accompanied by a lowering of the AIx.11,12 In this proof-of-concept study, we established a 3-element model to simultaneously and independently analyze (1) peripheral hemodynamics determined by the components of total peripheral resistance (TPR) in patients with PAD and HFpEF; (2) aortic function encompassing measures of the physicomechanic properties of the aortic wall, blood flow, and aortic pulsatile load; and (3) aortic inflow defined by LV systolic function. Simultaneous analysis of aortic inflow, aortic function, and peripheral hemodynamics before and after EVT enabled us to selectively investigate the impact of EVT on LVDF. We hypothesized that EVT of flow-limiting iliofemoral stenosis improves LVDF and patient-centered outcomes such as the New York Heart Association (NYHA) class and walking capacity by restoring peripheral perfusion and lowering aortic pulsatile and resistive load.
METHODS
Data Sharing
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Cohort and Patients
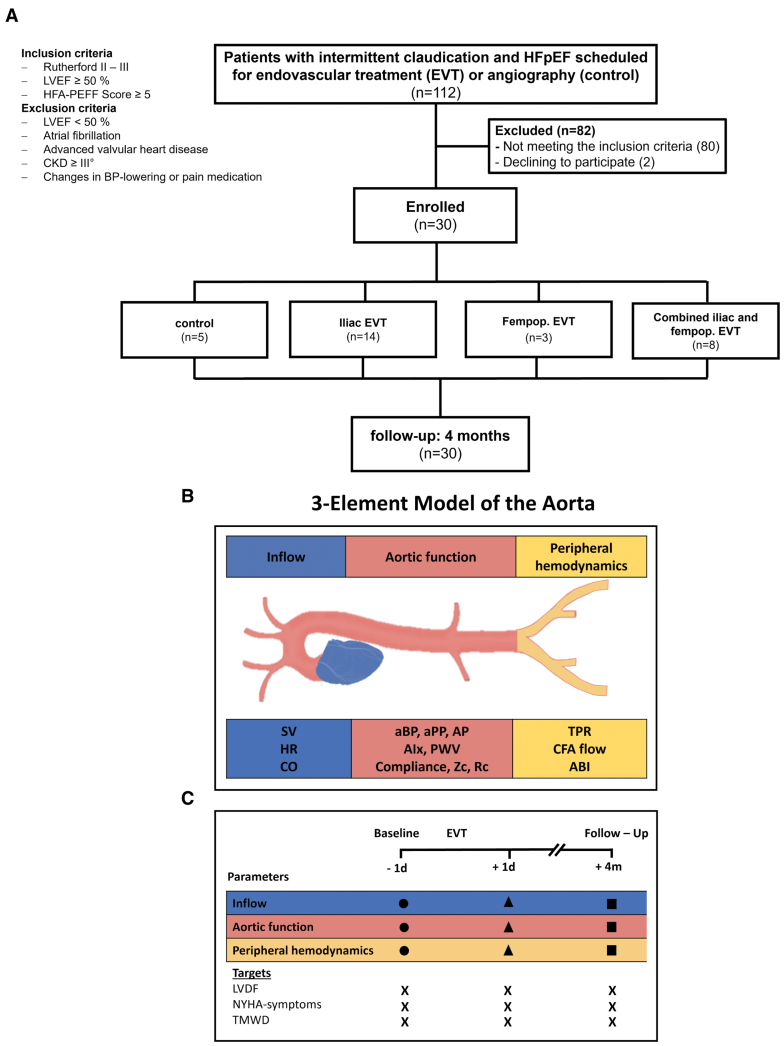
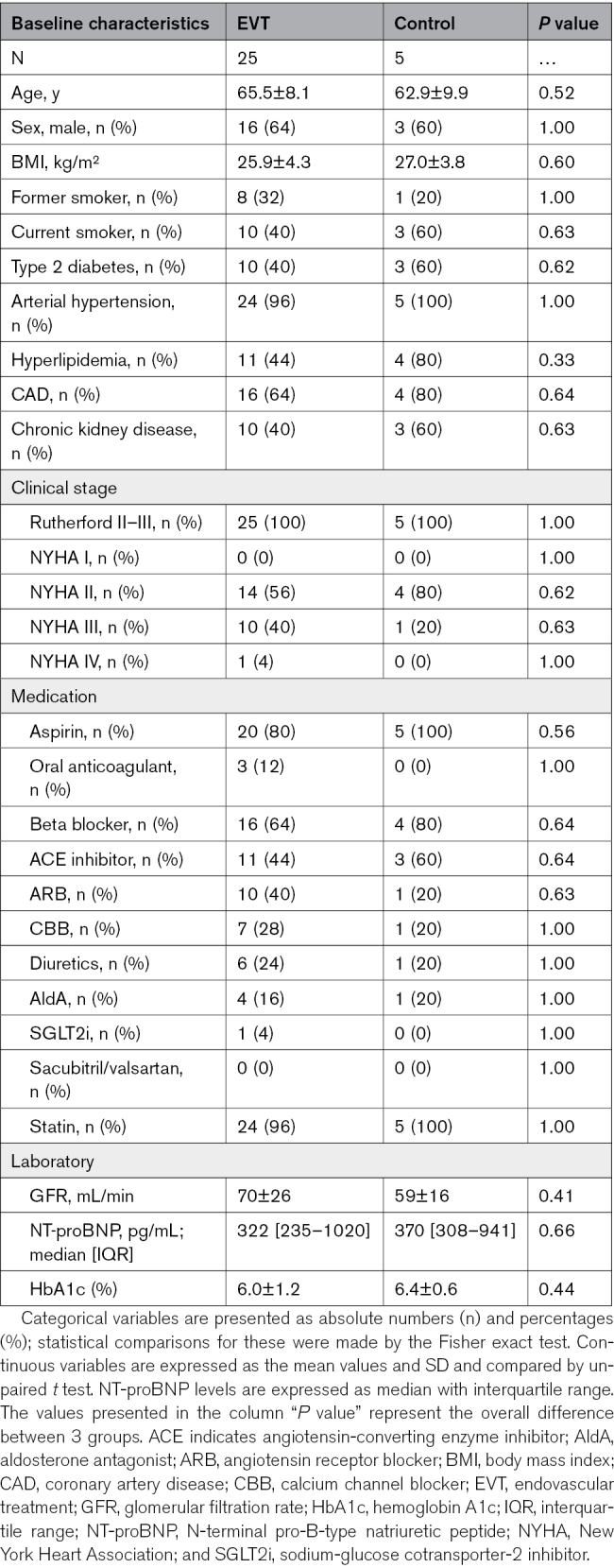
Patients with symptomatic Rutherford II or III PAD who were scheduled for EVT or angiography within the Duesseldorf PTA Registry (Clinical Trials identifier: NCT02728479) were eligible to participate if they had a LV ejection fraction ≥50% and an ESC Heart Failure Association−preserved ejection fraction score ≥5, implying “a high probability of HFpEF” according to the recent guideline recommendations.13 Major exclusion criteria included a prior documented reduction in the LV ejection fraction to <50%, documented atrial fibrillation, advanced valvular heart disease, chronic kidney disease of stage III or higher, and new or increased doses of BP-lowering or pain medication during follow-up. We prospectively investigated eligible patients at baseline 1 day before, 1 day after, and 4±1.3 months after EVT (n=25). Patients with diagnostic angiography served as a control (n=5). Patient-centered outcome measures were assessed by NYHA class and treadmill walking distance (TMWD; see the CONSORT [Consolidated Standards of Reporting Trials] diagram; Figure 1). The study was conducted after approval from the local ethics committee (study number: 2019-382-KFogU) and in accordance with the Declaration of Helsinki. All patients gave written informed consent before the procedure.
Figure 1.
Overview of the study cohort, 3-element model of the aorta, and study protocol. A, CONSORT diagram. B, Three-element model of the aorta depicting parameters of inflow, aortic function, and peripheral hemodynamics. C, The study protocol. ABI indicates ankle brachial index; aBP, aortic blood pressure; AIx, augmentation index; AP, augmentation pressure; aPP, aortic pulse pressure; BP, blood pressure; CFA, common femoral artery; CKD, chronic kidney disease; CO, cardiac output; CONSORT, Consolidated Standards of Reporting Trials; HFpEF, heart failure with preserved ejection fraction; HR, heart rate; LVDF, left ventricular diastolic function; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PWV, pulse wave velocity; RC, reflection coefficient; SV, stroke volume; TMWD, treadmill walking distance; TPR, total peripheral resistance; and Zc, characteristic impedance of the proximal aorta.
Three-Element Model of Aortic Function and Its Impact on LVDF
To assess the impact of endovascular treatment of flow-limiting stenosis in PAD on LVDF, we defined a 3-element model encompassing measures of (1) peripheral hemodynamics, (2) aortic function, and (3) aortic inflow. Independent of that, multiple echocardiographic indexes of LVDF were measured and correlated to changes in physical exertion capacity.
Peripheral Hemodynamics
The outflow of the aorta is critically determined by its afterload. To analyze changes in peripheral hemodynamics before, during, and after EVT, we measured the brachial BP, ankle brachial index (ABI), common femoral artery (CFA) flow, and TPR determined by the ratio of cardiac output (CO) to aortic mean arterial pressure (aMAP).
Aortic Function
The major function of the aorta is to distribute blood flow adequately to peripheral tissues and to adapt the pulsatile CO. To monitor the major determinants of aortic function, we analyzed the physicomechanic indices of the aortic wall (compliance and stiffness), aBP, and pulsatile characteristics of the aortic flow (aortic pulse pressure). The characteristic impedance of the proximal aorta (Zc) and the magnitude of wave reflection were calculated to assess the metrics of aortic pulsatile load.
Aortic Inflow
To assess the impact of EVT on LVDF, it is important to ensure that the systolic LV function determining the amount of aortic inflow remains constant before, during, and after EVT. For this purpose, we measured the heart rate, stroke volume (SV), and CO.
LVDF and Indexes of Physical Exercise Capacity
EVT-related changes in the aforementioned 3 components may affect LVDF, left atrial function, secondary symptoms of HF, and exercise capacity. Therefore, we analyzed the echocardiographic functional and morphological indexes of LVDF and the left atrium together with symptoms according to the NYHA classification and TMWD.
Endovascular Treatment
Iliac lesions were treated with angioplasty (Passeo 35, Biotronic), followed by the implantation of balloon-expandable stents (Dynamic, Biotronic). Femoropopliteal arteries were treated with angioplasty, followed by drug-coated balloon treatment (Passeo-Lux 18, Biotronic), and if necessary, with self-expandable nitinol stents (Innova, Boston Scientific). To further stratify lesion characteristics, the stented segment was calculated as a cylindrical volume (V) as follows: Vstent=π×r²×l.
Measures of Peripheral Hemodynamics
Office measurements were performed, including a standardized vascular ultrasound assessment (10 MHz transducer; Vivid I, GE) and measurement of the ABI. Brachial BP was measured using an automated clinical digital sphygmomanometer (Dynamap Vital Signs Monitor, Dinamap, General Electric Health Care, Solingen, Germany). CFA blood flow was calculated as volume flow [mL/min]=π×r2[cm]×Vmean[cm/s]×60. TPR was estimated as aMAP divided by CO.
Measures of Aortic Function
The systolic aBP, diastolic aBP, aMAP, aortic pulse pressure, and AIx@HR75 were determined by applanation tonometry measured at the radial artery using the SphygmoCor system with transfer function (AtCor Medical, Sydney, Australia). The average values of 3 repeated measurements were noted. The SphygmoCor system was used for the measurement of the carotid-to-femoral PWV (cfPWV) according to current guidelines.14 The femoral artery on the noninterventional side was chosen as the femoral derivation point for the measurement of cfPWV. For bilateral procedures, the limb with the higher ABI was chosen as the femoral derivation point. Arterial compliance was estimated by the ratio of SV to aortic pulse pressure.15 The Zc was calculated according to Mitchell et al16 as follows: the aortic pressure curve and the LV outflow tract flow were digitized using a graph plotting program (WebPlotDigitizer), then we calculated the slope of the 2 superimposed graphs to estimate the Zc. With this superimposed graph, the magnitude of wave reflection, defined as the reflection coefficient, was calculated as described by Chirinos et al.17
Measures of Aortic Inflow
Transthoracic and Doppler echocardiography were performed by our echocardiography laboratory, which was blinded to the clinical information and treatment assignment, with commercially available ultrasound systems (GE Healthcare, Chicago, IL). The Simpson method was used to calculate the LV ejection fraction. SV was determined as the product of the LV outflow tract area and the velocity time integral; CO was derived as the product of heart rate and SV.
Left Ventricular Diastolic Function
Transmitral flow, containing the E/A ratio, was assessed by pulsed wave Doppler at the top of mitral valve leaflets. The mitral annulus velocity was obtained from tissue Doppler imaging mode at septal and lateral annulus with pulsed wave Doppler. The E wave of transmitral flow was divided by the septal and lateral mitral annulus velocities and averaged as the E/e´mean ratio. LV mass index and LV wall thickness were assessed for each patient. Left atrial parameters, including the left atrial area, left atrial volume, and left atrial volume index, were assessed in 4- and 2-chamber views. Continuous wave Doppler was used to obtain the peak tricuspid regurgitant velocity as a measure of systolic pulmonary artery pressure.
Patient-Centered Outcomes: TMWD and NYHA Class
PAD-related clinical evaluations included staging by the Rutherford classification and TMWD if possible. The NYHA class was assessed at baseline 1 day before and at follow-up after EVT or diagnostic angiography.
Statistical Analyses
Categorical variables are presented as absolute numbers (n) and percentages (%); statistical comparisons for these variables were made by Fisher exact test. Continuous variables are expressed as the mean values and standard deviations and were compared by unpaired t test. Changes in parameters (delta) were calculated as postangioplasty values minus baseline (pre angioplasty) values and expressed as the means with 95% CIs. Within-subject changes with single comparisons in hemodynamics and echocardiographic parameters were analyzed using a paired Student t test. Linear relationships between continuous variables were expressed as Pearson’s r. Statistical significance was assumed at P≤0.05. Linear univariate and multivariate regression analyses were performed to test the relationships between independent variables of peripheral hemodynamics, aortic function, aortic inflow (CO), and the dependent variable E/e´. All individual baseline, post-EVT, and follow-up observations of patients participating in the study were entered into the models, thus including repeated measures. The model was tested for collinearity between measures of aortic inflow, function, and peripheral hemodynamics. Potential confounders were entered into stepwise linear regression models, including standard modifiable risk factors for cardiovascular disease (diabetes, hyperlipidemia, hypertension, and smoking), age, BMI, CKD, sex, and CAD.
Data were analyzed using GraphPad Prism version 6.00 (La Jolla, California, IL) and IBM SPSS software version 25.0 (Armonk, NY).
RESULTS
Study Population and Procedures
A total of 30 patients with symptomatic PAD and concomitant HFpEF (see Tables S1 and S2 for the Heart Failure Association−preserved ejection fraction scores and Figure S1) were included. Baseline characteristics between these 2 groups were not statistically different (Table 1). Exposure to HF medications was similar between the 2 groups at baseline (Table 1). The detailed HF medication burden at baseline and follow-up is summarized in Table S3. Detailed lesion and procedural characteristics are shown in Tables S4 and S5. The control group was managed conservatively or scheduled for surgery based on the lesion characteristics after diagnostic angiography (Table S5). No major adverse cardiac and cerebrovascular events or major adverse limb events occurred during follow-up (Table S6).
Table 1.
Baseline Clinical and Demographic Characteristics of PAD Patients
Endovascular Treatment Leads to a Sustained Decrease in TPR and an Increase in ABI and CFA Blood Flow
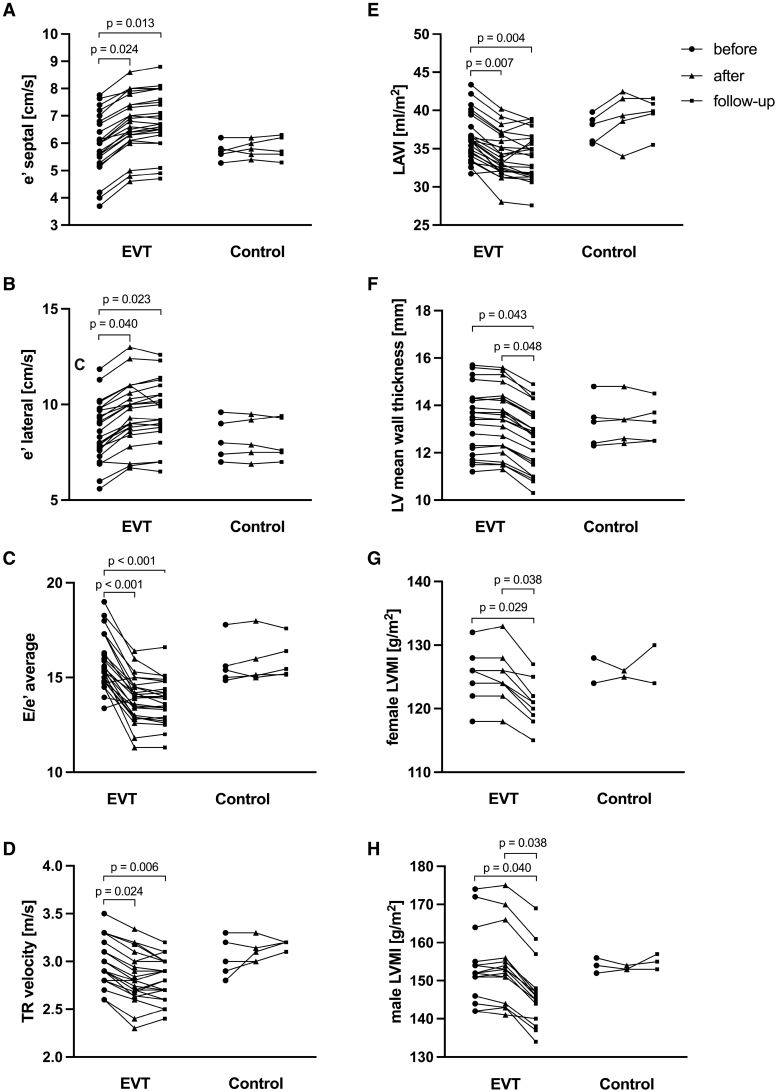
The ABI of the treated leg did not differ between groups (Table S3) at baseline and significantly increased from 0.67±0.14 to 0.84±0.17 and 0.88±0.14 at follow-up after EVT (Table 2). There was no significant change in the ABI in the control group. TPR decreased significantly (P=0.041) after successful EVT with a sustained effect at follow-up (Table 2), whereas no significant change was noted in the control group.
Table 2.
Three-Element Model of the Aorta Encompassing Measures of Peripheral Hemodynamics, Aortic Function, and Aortic Inflow
The total CFA blood flow significantly increased after successful EVT and at follow-up, whereas no significant change in the CFA blood flow was noted in the control group (Table 2).
Normalized Aortic Function Mediated Through Improved Peripheral Hemodynamics
Systolic aBP decreased after EVT. The mean difference 1 day after EVT was −8 mm Hg (P=0.056) and −9 mm Hg at follow-up (P=0.036). Diastolic aBP was not affected by EVT. AMAP decreased after EVT with a mean difference of −5 mm Hg 1 day after EVT and −5 mm Hg at follow-up, whereas no significant change was noted in the control group (Table 2). The mean aortic pulse pressure decrease was −7 mm Hg and −8 mm Hg at follow-up compared with baseline. There was no significant change in aBP in the control group (Table 2).
Arterial compliance significantly increased after successful EVT and at follow-up, whereas no significant change was noted in the control group (Table 2). PWV significantly decreased after successful EVT and at follow-up, whereas a modest increase was noted at follow-up in the control group (Table 2). When changes in arterial compliance and PWV were adjusted for aMAP, only arterial compliance increased significantly after successful EVT at follow-up (Table S7). AP and AIx@HR75 were significantly lower after EVT and at follow-up, while there was no significant change in the control group (Table 2).
Zc and reflection coefficient as metrics of aortic pulsatile load decreased after EVT and at follow-up (Figure S2; Table 2). Zc decreased significantly after EVT and at follow-up, whereas no significant change was noted in the control group (Figure S2; Table 2). Reflection coefficient decreased significantly after EVT and at follow-up, while there was no significant change in the control group (Figure S2; Table 2).
Constant Aortic Inflow After Endovascular Treatment
As shown in Table S8, there were no significant differences in baseline measures of aortic inflow between the groups. In both groups, LV ejection fraction, SV, and CO remained unchanged 1 day after the procedure and at follow-up. Table 2 summarizes measures of aortic inflow.
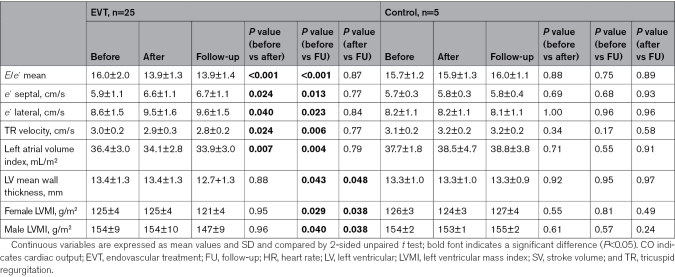
Immediate and Chronic Improvement of Markers for LVDF After Endovascular Treatment
As shown in Table S8, there were no significant differences in the baseline echocardiographic parameter LVDF between the groups. Markers of LVDF acutely improved after successful angioplasty with a sustained effect at follow-up, with an increase in lateral and septal e´ velocities and decreases in E/e´, tricuspid regurgitant, and left atrial volume index. LV mass index and mean LV wall thickness decreased at follow-up in successfully treated patients (Figure 2; Table 3). A summary of the changes in LVDF between EVT and control at different time points is shown in Table S9 and summarized in Figure 2. In a subgroup analysis, the effect of bilateral EVT (n=8) on E/e´ and left atrial volume index was greater than that of unilateral EVT (n=17; Figure S3). Improvements in TMWD, ABI, and CFA flow are associated with a reduction in E/e´ (Figure S4). When stent volume was calculated as a cylinder, the effect of larger stent volume had a greater effect on E/e´ than smaller volumes (Figure S5).
Figure 2.
Peripheral endovascular treatment (EVT) improves left ventricular diastolic function (LVDF). Functional and morphological changes in LVDF after EVT. Septal e´ (A), lateral e´ (B), E/e´ average (C), tricuspid regurgitation (TR; D), left atrial volume index (LAVI; E), LV mean wall thickness (F), and LV mass index in female (LVMI; G) and male (H) patients before (circle), after EVT (triangle), and at the follow-up (square) after elective EVT (n=25) or diagnostic angiography alone as a control (n=5). Unpaired t test was used to calculate P values.
Table 3.
Parameters of Left Ventricular Diastolic Function Before, After, and at Follow-Up After Endovascular Treatment or Diagnostic Angiography as a Control
Improved TMWD and NYHA Class at Follow-Up
TMWD improved significantly after EVT, whereas no significant change was observed after angiography. The mean TMWD improved from 79±44 m at baseline to 213±135 m and 242±136 in treated patients at follow-up (Table 2).
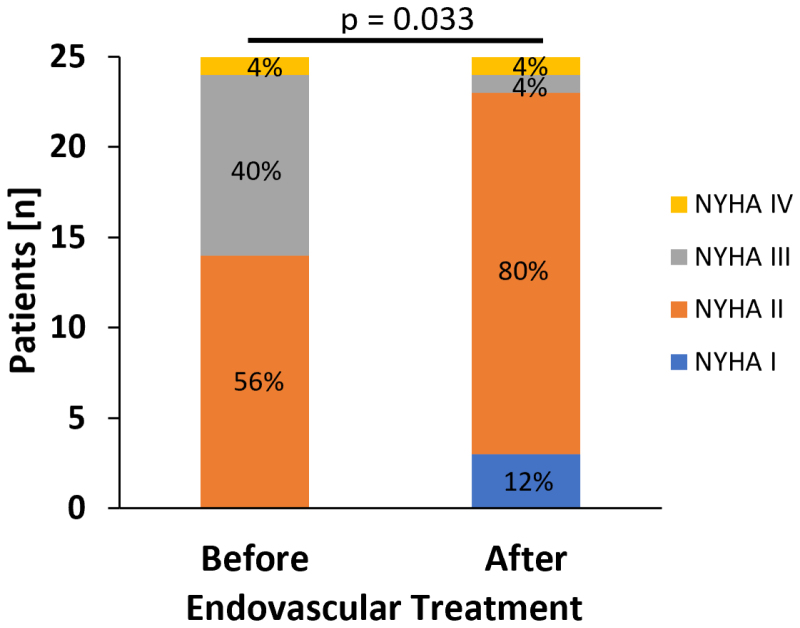
In addition to a limitation in the walking distance, most patients were affected by coexisting dyspnea (Table 1). As shown in Figure 3, the NYHA class significantly decreased at follow-up after successful EVT (P=0.033), whereas the NYHA class remained unchanged in the control group.
Figure 3.
Reduction in NYHA class after endovascular treatment of flow-limiting iliofemoral stenosis. NYHA class (blue indicates NYHA I, orange indicates NYHA II, gray indicates NYHA III, and yellow indicates NYHA IV) at baseline before angioplasty (n=25) of flow-limiting stenosis and at follow-up. Wilcoxon signed-rank test was used to calculate P value. NYHA indicates New York Heart Association.
Independent Contributors to E/e´
Among hemodynamic and nonhemodynamic factors, components of physicomechanical properties at the level of aortic function (PWV, AIx, and arterial compliance) independently contributed to E/e´ in multivariate analysis, but systolic aBP and aMAP did not (Table S10). Measures of the level of aortic outflow were not independently associated with E/e´.
To determine whether adjustment for comorbidities weakened the association between indexes of aortic function and E/e´, stepwise linear regression models were repeated to include comorbidities. The model remained largely unchanged, with parameters of aortic function (arterial compliance, AIx, and PWV) that were significant in all models, even after Bonferroni adjustment for multiple testing (Table S11).
DISCUSSION
This study shows for the first time that endovascular treatment of flow-limiting stenosis in PAD patients with HFpEF reduces aortic pulsatile load and concurrently lowers TPR. This beneficial effect is associated with acute and sustained improvement of LVDF that ameliorates HF, independent of standard modifiable cardiovascular risk factors. Physicomechanical properties of the aorta (AIx, PWV, and arterial compliance) were identified as independent contributors to E/e´.
PAD and HFpEF
This study was conducted in a unique cohort of patients with severe symptomatic PAD of the iliofemoral segment who were scheduled for endovascular treatment and concomitant HFpEF. PAD and HFpEF share similar cardiovascular risk factors, such as hypertension, diabetes, dyslipidemia, and advanced age, and this condition is present in ≈15% of patients with HFpEF.7 The actual prevalence of HF in patients with PAD might be underestimated and vice versa, since symptoms can be masked by coexisting claudication or dyspnea, depending on the severity of the disease burden. Claudication may be masked by reduced exercise capacity due to fluid overload as a result of high LV filling pressures in HFpEF, leading to underdiagnosis of PAD in these patients. Recent data suggest that the presence of PAD in patients with HFpEF is associated with adverse cardiovascular events.18 Therefore, screening for PAD in HFpEF may be useful to optimize treatment strategies in this high-risk cohort. In this trial, all patients experienced claudication and dyspnea (≥NYHA II), but most patients’ quality of life was predominantly limited by claudication, complicating the NYHA assessment. Approximately 35% of patients with HFpEF presented dyspnea on exertion, which might have been masked in patients with the HFpEF and PAD phenotypes.19
Endovascular Treatment of Flow-Limiting Stenosis Increases Regional Blood Flow, Consecutively Decreasing TPR
All patients in this study were affected by symptomatic flow-limiting iliofemoral stenosis with a markedly reduced ABI and TMWD at baseline. Endovascular treatment of flow-limiting iliofemoral stenosis led to a significant increase in CFA blood flow of the target leg with a concomitant increase in arterial perfusion pressure, as indicated by an elevated ABI and improved functionality with an increased TMWD. This regional hemodynamic effect translated into a decrease in TPR lasting 4 months after endovascular treatment. Endovascular treatment of flow-limiting stenosis achieved a remarkable systolic aBP lowering of ≈8 to 9 mm Hg and ≈7 systolic brachial BP 1 day after angioplasty and at the 4-month mean follow-up.
Reduced Aortic Pulsatile Load Mediated by a Decrease in TPR After Endovascular Treatment
As noted above, a high-risk population of patients with a high burden of cardiovascular risk factors and severe symptomatic PAD characterized by a severely impaired peripheral perfusion and reduced TMWD was investigated. As previously described, we observed an acute lowering of the AIx through endovascular treatment of iliofemoral stenosis.12 Importantly, this acute effect persisted for 4 months, which corroborates previous studies by Jacomella et al.20 This suggests that in patients with peripheral arterial lesions, these may act as major pulse reflection sites, increasing the aortic systolic BP by aortic augmentation. Arterial lesions represent a point of impedance that enhances pulse wave reflection. Therefore, endovascular treatment of a flow-limiting stenosis reduces the local impedance, which reduces the magnitude of the backward wave, as indicated by a reduced reflection coefficient, allowing the pulse wave to travel further distally. Thus, the change in the AIx induced by endovascular treatment is explained by a primary decrease in the contribution of the pressure wave reflected at the lesion and thereby a secondary decrease in aortic BP, thus decreasing the AP.12 Moreover, we observed a pronounced acute and chronic lowering of the PWV in successfully treated patients. The PWV is strongly correlated with BP and, in particular, the MAP.21,22 According to the concept of pulse wave reflection and sites of impedance, we speculate that through a BP-dependent decrease in the PWV, the arrival of reflected waves to the proximal aorta occurs later, which leads to an additional decrease in the AIx. This results in improved ventricular-arterial coupling through a beneficial reduction in aortic pulsatile load, as evidenced by decreased Zc, decreased reflection coefficient, and increased arterial compliance.
Lowered Aortic Pulsatile Load Is Associated With Acute and Chronic Improvement of the LVDF
Our study demonstrates that peripheral endovascular treatment acutely improves peripheral hemodynamics and lowers the aortic pulsatile load, which is associated with favorable improvement in the LVDF. We found a rapid improvement in the LV filling pressure, as shown by a reduction in early mitral inflow velocity relative to early diastolic LV relaxation (E/e′) as a main measure of LVDF, an effect that was already significant 1 day after peripheral endovascular treatment and exhibited a sustained effect at follow-up. This functional effect was accompanied by a morphological decrease in the left atrial volume index, which was sustained until the end of the study. Moreover, we observed a regression of the LV mass index after 4 months, indicating favorable LV remodeling. Bilateral endovascular treatment of flow-limiting stenoses had a greater impact on LVDF than unilateral treatment. At the end of the study, <10% of patients were affected by NYHA class III or higher symptoms.
Multivariate analysis with different models consistently showed that the physicomechanical properties of aortic function (AIx, PWV, and arterial compliance) were identified as contributors to mean E/e′, whereas the nonpulsatile elements of aortic function (aMAP), systolic aBP, and aortic outflow (TPR, CFA flow, and ABI) did not affect E/e′ independently. Similar to the study of Borlaug et al,23 the present study shows that markers of the level of aortic function (arterial compliance), but not peripheral hemodynamics (TPR), are associated with E/e′.
These data support the hypothesis that wave reflections play an important role in abnormal ventricular-arterial interaction with premature arrival of the back wave at the proximal aorta in mid-to-late systole, resulting in systolic pressure augmentation as observed in patients with HFpEF.24 EVT of flow-limiting stenosis results in a favorable improvement in the characteristic impedance of the proximal aorta, reduced magnitude of wave reflections expressed by reduced AIx, systolic aBP, and post-EVT reflection coefficient with improved diastolic function.
Moreover, to avoid symptomatic claudication, patients with PAD might restrict their physical activity, potentially leading to deconditioning and deterioration of cardiovascular fitness. Therefore, by improving lower extremity blood flow, endovascular treatment of flow-limiting peripheral stenosis promotes patient mobility and quality of life in the short term.
Limitations
This single-center investigation had some limitations. First, in this proof-of-concept study, the number of patients was small; therefore, clinical outcomes should be evaluated in large-scale trials to support the generalizability of our results. The study was not randomized; therefore, in this cohort, significant and even nonsignificant characteristics and unmeasured confounders might have biased the results. However, the core laboratory assessing LVDF was blinded to EVT. We measured the changes in hemodynamics and echocardiographic parameters within a short time frame after EVT and the improvements in LVDF in the mid-term follow-up, indicating the sustainability of the acute beneficial effects of EVT on LVDF, but without assessing outcome data in rehospitalization due to HFpEF in the long-term period.
Conclusions
Our data demonstrated that successful endovascular treatment of flow-limiting stenosis in patients with definite HFpEF acutely led to improvement of diastolic function, with a sustained effect at the 4-month follow-up. This favorable effect was accompanied by a clinically relevant reduction in NYHA class. Physicomechanical properties of aortic function (AIx, PWV, and arterial compliance) were identified as independent contributors to E/e´. Whether improvement of HFpEF in patients with PAD through endovascular treatment of flow-limiting stenosis improves their overall prognosis has to be tested.
ARTICLE INFORMATION
Sources of Funding
This study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) CRC/TRR259, grant 397484323 to Dr Quast.
Disclosures
None.
Supplemental Material
Tables S1–S11
Figures S1–S5
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ABI
- ankle brachial index
- aBP
- aortic blood pressure
- AIx
- augmentation index
- aMAP
- aortic mean arterial pressure
- BP
- blood pressure
- CFA
- common femoral artery
- CO
- cardiac output
- HFpEF
- heart failure with preserved ejection fraction
- LV
- left ventricular
- LVDF
- left ventricular diastolic function
- NYHA
- New York Heart Association
- PAD
- peripheral artery disease
- PWV
- pulse wave velocity
- SV
- stroke volume
- TMWD
- treadmill walking distance
- TPR
- total peripheral resistance
- Zc
- characteristic impedance of the proximal aorta
S. Baasen and M. Stern contributed equally.
For Sources of Funding and Disclosures, see page 815.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCHEARTFAILURE.123.011258.
Contributor Information
Sven Baasen, Email: sven.baasen@med.uni-duesseldorf.de.
Manuel Stern, Email: manuel.stern@med.uni-duesseldorf.de.
Patricia Wischmann, Email: Patricia.Wischmann@med.uni-duesseldorf.de.
Johanna Schremmer, Email: johanna.schremmer@med.uni-duesseldorf.de.
Roberto Sansone, Email: Roberto.Sansone@med.uni-duesseldorf.de.
Maximilian Spieker, Email: maximilian.spieker@med.uni-duesseldorf.de.
Georg Wolff, Email: guwolff@hotmail.com.
Florian Bönner, Email: florian.boenner@krankenhaus-dueren.de.
Christine Quast, Email: Christine.Quast@med.uni-duesseldorf.de.
Christian Heiss, Email: c.heiss@surrey.ac.uk.
REFERENCES
- 1.Ostergren J, Sleight P, Dagenais G, Danisa K, Bosch J, Qilong Y, Yusuf S; HOPE Study Investigators. Impact of ramipril in patients with evidence of clinical or subclinical peripheral arterial disease. Eur Heart J. 2004;25:17–24. doi: 10.1016/j.ehj.2003.10.033 [DOI] [PubMed] [Google Scholar]
- 2.Heiss C, Pitcher A, Belch JJF, De Carlo M, Reinecke H, Baumgartner I, Mazzolai L, Aboyans V. The year in cardiology: aorta and peripheral circulation. Eur Heart J. 2020;41:501–508b. doi: 10.1093/eurheartj/ehz939 [DOI] [PubMed] [Google Scholar]
- 3.Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:1237–1263. doi: 10.1016/j.jacc.2019.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Safar ME, Priollet P, Luizy F, Mourad JJ, Cacoub P, Levesque H, Benelbaz J, Michon P, Herrmann MA, Blacher J. Peripheral arterial disease and isolated systolic hypertension: the ATTEST study. J Hum Hypertens. 2009;23:182–187. doi: 10.1038/jhh.2008.121 [DOI] [PubMed] [Google Scholar]
- 5.O’Rourke MF, Safar ME, Dzau V. The cardiovascular continuum extended: aging effects on the aorta and microvasculature. Vasc Med. 2010;15:461–468. doi: 10.1177/1358863X10382946 [DOI] [PubMed] [Google Scholar]
- 6.Kahan T. The importance of myocardial fibrosis in hypertensive heart disease. J Hypertens. 2012;30:685–687. doi: 10.1097/HJH.0b013e328350e5db [DOI] [PubMed] [Google Scholar]
- 7.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530 [DOI] [PubMed] [Google Scholar]
- 8.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050 [DOI] [PubMed] [Google Scholar]
- 9.Kass DA. Ventricular arterial stiffening: integrating the pathophysiology. Hypertension. 2005;46:185–193. doi: 10.1161/01.HYP.0000168053.34306.d4 [DOI] [PubMed] [Google Scholar]
- 10.Chirinos JA, Kips JG, Jacobs DR, Jr, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis). J Am Coll Cardiol. 2012;60:2170–2177. doi: 10.1016/j.jacc.2012.07.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe K, Takahashi H, Watanabe T, Otaki Y, Kato S, Tamura H, Nishiyama S, Arimoto T, Shishido T, Watanabe M. Endovascular revascularization improves the central hemodynamics and augmentation index in patients with peripheral artery disease. Intern Med. 2020;59:37–44. doi: 10.2169/internalmedicine.3413-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busch L, Heinen Y, Stern M, Wolff G, Ozaslan G, Tzetou K, Sansone R, Heiss C, Kelm M. Angioplasty of flow-limiting stenosis reduces aortic and brachial blood pressure in patients with peripheral artery disease. J Am Heart Assoc. 2021;10:e019724. doi: 10.1161/JAHA.120.019724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pieske B, Tschope C, De Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the heart failure association (HFA) of the European society of cardiology (ESC). Eur Heart J. 2019;40:3297–3317. doi: 10.1093/eurheartj/ehz641 [DOI] [PubMed] [Google Scholar]
- 14.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–448. doi: 10.1097/HJH.0b013e32834fa8b0 [DOI] [PubMed] [Google Scholar]
- 15.Chemla D, Hebert JL, Coirault C, Zamani K, Suard I, Colin P, Lecarpentier Y. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol. 1998;274:H500–H505. doi: 10.1152/ajpheart.1998.274.2.H500 [DOI] [PubMed] [Google Scholar]
- 16.Mitchell GF, Tardif JC, Arnold JM, Marchiori G, O’Brien TX, Dunlap ME, Pfeffer MA. Pulsatile hemodynamics in congestive heart failure. Hypertension. 2001;38:1433–1439. doi: 10.1161/hy1201.098298 [DOI] [PubMed] [Google Scholar]
- 17.Chirinos JA. Ventricular-arterial coupling: Invasive and non-invasive assessment. Artery Res. 2013;7:2–14. doi: 10.1016/j.artres.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandesara PB, Hammadah M, Samman-Tahhan A, Kelli HM, O’Neal WT. Peripheral artery disease and risk of adverse outcomes in heart failure with preserved ejection fraction. Clin Cardiol. 2017;40:692–696. doi: 10.1002/clc.22716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omote K, Verbrugge FH, Sorimachi H, Omar M, Popovic D, Obokata M, Reddy YNV, Borlaug BA. Central haemodynamic abnormalities and outcome in patients with unexplained dyspnoea. Eur J Heart Fail. 2023;25:185–196. doi: 10.1002/ejhf.2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacomella V, Shenoy A, Mosimann K, Kohler MK, Amann-Vesti B, Husmann M. The impact of endovascular lower-limb revascularisation on the aortic augmentation index in patients with peripheral arterial disease. Eur J Vasc Endovasc Surg. 2013;45:497–501. doi: 10.1016/j.ejvs.2013.01.026 [DOI] [PubMed] [Google Scholar]
- 21.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham heart study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa [DOI] [PubMed] [Google Scholar]
- 22.Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham heart study. Circulation. 2010;122:1379–1386. doi: 10.1161/CIRCULATIONAHA.109.914507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, Kass DA. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol. 2007;50:1570–1577. doi: 10.1016/j.jacc.2007.07.032 [DOI] [PubMed] [Google Scholar]
- 24.Chirinos JA. Deep phenotyping of systemic arterial hemodynamics in HFpEF (part 1): physiologic and technical considerations. J Cardiovasc Transl Res. 2017;10:245–259. doi: 10.1007/s12265-017-9735-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.