Abstract
Auditory processing is widely understood to occur differently in autism, though the patterns of brain activity underlying these differences are not well understood. The diversity of autism also means brain-wide networks may change in various ways to produce similar behavioral outputs. We used larval zebrafish to investigate auditory habituation in four genetic lines relevant to autism: fmr1, mecp2, scn1lab and cntnap2. In free-swimming behavioral tests, we found each line had a unique profile of auditory hypersensitivity and/or delayed habituation. Combining the optical transparency of larval zebrafish with genetically encoded calcium indicators and light-sheet microscopy, we then observed brain-wide activity at cellular resolution during auditory habituation. As with behavior, each line showed unique alterations in brain-wide spontaneous activity, auditory processing, and adaptation in response to repetitive acoustic stimuli. We also observed commonalities in activity across our genetic lines that indicate shared circuit changes underlying certain aspects of their behavioral phenotypes. These were predominantly in regions involved in sensory integration and sensorimotor gating rather than primary auditory areas. Overlapping phenotypes include differences in the activity and functional connectivity of the telencephalon, thalamus, dopaminergic regions, and the locus coeruleus, and excitatory/inhibitory imbalance in the cerebellum. Unique phenotypes include loss of activity in the habenula in scn1lab, increased activity in auditory regions in fmr1, and differences in network activity over time in mecp2 and cntnap2. Comparing these distinct but overlapping brain-wide auditory networks furthers our understanding of how diverse genetic factors can produce similar behavioral effects through a range of circuit- and network-scale mechanisms.
Introduction
Sensory processing in autism
Differences in sensory experience are common in autism, but the differences in neural circuitry underlying these traits is not well understood1–5. Human studies provide conflicting results about various measures of auditory processing in autism, and these discrepancies may arise from the etiological complexity of autism 6–8. More consistent results can be found in studies of syndromic forms of autism, such as fragile X syndrome (FXS) or Rett syndrome, but this approach risks poor representation of autism as a whole 7,9,10. There is evidence for reduced habituation to auditory stimuli in some autistic people and reduced adaptation of auditory cortex activity in FXS 8,11. There are several hypotheses explaining differences in brain activity in autism, including excitatory/inhibitory (E/I) imbalance, dopaminergic dysfunction, and altered cerebellar and brainstem function 12–15. It is not clear which of these link to changes in auditory habituation, or indeed whether different mechanisms are relevant to different etiologies. Animal models enable more incisive studies into brain function, but auditory habituation is under-studied in rodent models of autism compared to other measures of auditory function, despite its simplicity and potential to affect conclusions about differences in other auditory tests 8,16,17. While most animal models of autism only manipulate single genes, comparing across models can provide insights into the diversity of mechanisms underlying shared behavioral changes 16,18.
Zebrafish genetic lines for investigating brain-wide function
There is growing interest in using zebrafish to investigate differences in brain development in autism due to their genetic tractability and capacity for brain-wide calcium imaging 19–21. They enable investigations of responses to a range of different sensory stimuli including visual, acoustic, vestibular, olfactory, and water flow22–30. Indeed, differences in sensory processing in fmr1−/− fish have illustrated the advantages of capturing cellular resolution activity throughout the brain to describe phenotypes, with phenotypes characterized by differences in functional connectivity rather than in gross activity level 31,32. Furthermore, the relative efficiency of zebrafish research supports the shift in autism research towards comparing phenotypes across several different animal lines to better capture the complexity of autism etiology16,17,21,33,34.
Auditory habituation is well established in larval zebrafish, and pharmacological and optogenetic manipulations have found roles for dopamine, serotonin, glycine, and NMDA receptors in this process 35–40. Increased dopamine signaling increases the degree of habituation, while decreased dopamine signaling reduces the rate and degree of habituation 38,39. Serotonin has the opposite role to dopamine: increased serotonin reduces the degree of habituation while reducing serotonergic activity, particularly from the superior raphe, increases habituation38. In one study, glycine receptor blocker strychnine entirely eliminated habituation39. Blocking NMDA receptors also reduces the rate and/or degree of habituation35,36,39,40. Of note, reduced NMDAR activity has been linked to auditory phenotypes in rodent models of autism41,42.
Distinct but overlapping mechanisms for altered habituation
The reduction in response during habituation is generally viewed as a learned association to the innocuous nature of a stimulus. However, a superficially similar phenomenon of ‘induced passivity’ represents a different process: learning that behavioral responses are futile for escaping the stimulus. The networks underlying these two processes may or may not overlap, but both can be disrupted with NMDAR antagonist ketamine 36,43. Interpreting this induced passivity as behavioral adaptation fits with the newer narrative that habituation involves shifting response strategy, and is more complex than simply ‘learning to ignore’ stimuli 44. A putative passivity circuit in larval zebrafish has been described in the context of electric shock stimuli, where passivity was linked to increased activity in the ventral habenula and decreased activity in the dorsal thalamus and superior raphe43. These neurotransmitters and brain regions present diverse potential mechanisms for auditory habituation phenotypes in neurological conditions such as autism.
Here, we set out to study auditory habituation in four genetic lines associated with autism: fmr1, scn1lab, mecp2, and cntnap2, using both behavioral screening and imaging of whole-brain activity at single-neuron resolution. Each of these genes is associated with autism as well as its own syndrome: fmr1 with FXS, mecp2 with Rett syndrome, scn1lab with Dravet syndrome, and cntnap2 with Pitt-Hopkins like syndrome45. We find that each line has a unique behavioral phenotype in response to repetitive acoustic stimuli, and that they show distinct but overlapping changes in their brain-wide auditory networks during habituation. The results give a glimpse of the behavioral and functional complexity of autism-associated genes and identify network-scale alterations that could contribute to sensory changes in specific syndromic forms of autism.
Methods
Animals
All experiments were conducted on 6 days post fertilization zebrafish larvae on a Tüpfel-Longfin background. For imaging of fluorescent calcium transients, mitfa−/− fish transgenic for HuC:H2B-GCaMP6s were used46. Larvae were raised in embryo media (distilled water with 10% Hanks solution, consisting of 137mM NaCl, 5.4mM KCl, 0.25mM Na2HPO4, 0.44mM KH2PO4, 1.3mM CaCl2, 1.0mM 654 MgSO4 and 4.2mM NaHCO3 at pH 7.2) in an incubator at 28°C with a 14/10 hour light/dark cycle. The experimental room was maintained at approximately 26°C. Experimental animals were bred from parents heterozygous for the relevant autism-associated gene mutation to provide sibling wild-type controls for each dataset. Separate datasets were collected for each of the four lines: fmr1hu2787, mecp2fh232, scn1labΔ44 21,47, and cntnap2aya2188cntnap2bya2043 48. As cntnap2 is duplicated in zebrafish, parents of the experimental animals for the cntnap2 dataset were heterozygotes for both cntnap2a and cntnap2b. Following experiments, larvae were euthanized with ice and digested in 100 µL TE buffer with 1 µL ProK (New England Biolabs). They were then genotyped by PCR and Sanger sequencing. Primer sequences for each PCR can be found in Supplementary table 1. Larvae were genotyped after the experiment to maintain experimental blindness.
Free-swimming auditory habituation
Experimental set-up
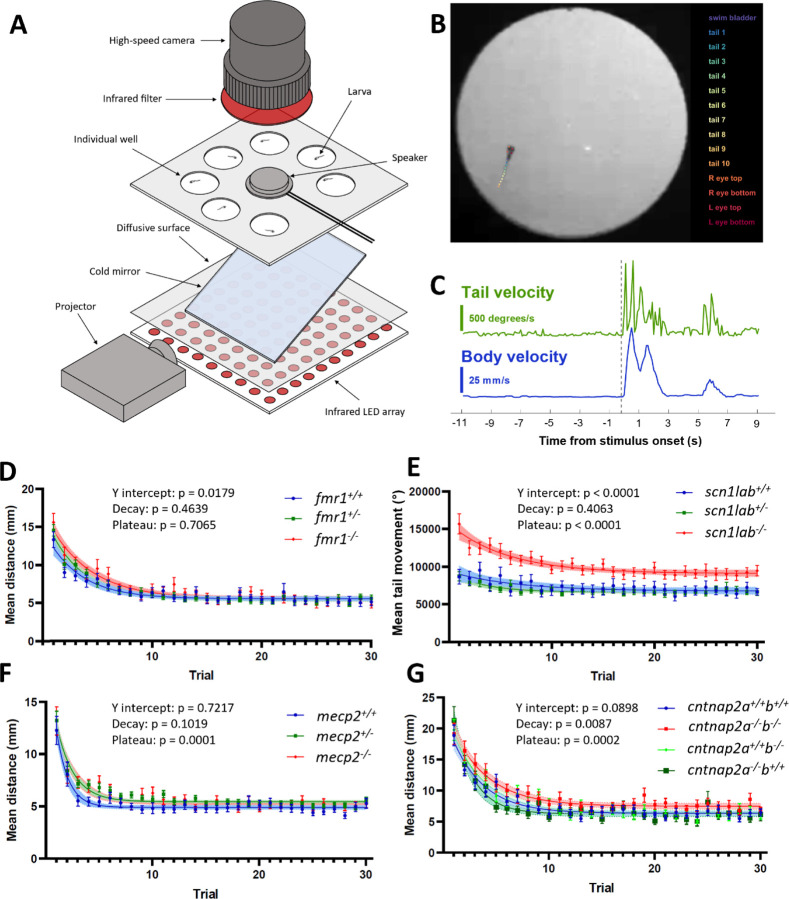
Initial behavioral phenotyping was conducted as part of a larger sensory phenotype screening procedure which also involved visual stimuli (data not shown). Free-swimming behavioral experiments were conducted on a custom-built behavioral rig (Figure 1A). Due to lack of swim bladder inflation in scn1lab−/− fish, for this mutant line all fish were partially embedded upright in 2% low melting point agarose, and behavioral responses assessed based on tail deflections. Fish were placed in seven individual circular wells of diameter 20 mm, arranged around a central speaker glued to the underside of the well plate. The speaker was driven by an amplifier (Dayton Audio DA30 2 × 15W Class D Bridgeable Mini Amplifier), which received input directly from the MATLAB code driving the experiment. The wells were illuminated from below by an array of infrared LEDs (840 nm). A projector delivered visual stimuli to an angled cold mirror to provide constant medium grey background light (500 lx) to the fish, and to deliver visual stimuli. Videos were recorded with a high-speed camera (Ximea xiB-64 model CB019MG-LX-X8G3), with an infrared filter, and with an exposure time of 1 ms and a framerate of 100 fps.
Figure 1:
Behavioral auditory habituation phenotypes in four genetic lines. A) Experimental set-up for recording behavior of seven larvae simultaneously. B) Tracking with DeepLabCut43. Automated identification of points in the swim bladder, eyes, and along the tail enables kinematic analysis of behavioral responses. C) Example outputs of tail velocity (green) and whole-body velocity (blue) in response to an auditory stimulus, indicated by the dotted line. D-G) Behavioral responses during habituation for fmr1, scn1lab, mecp2, and cntnap2, respectively. Responses are calculated for a 1-second window after the stimulus. For each group, the p-value is indicated for y-intercept (Y0), slope, and plateau, comparing fish carrying the mutation to wild-type siblings.
Stimuli
The auditory habituation stimulus train was preceded by stimuli for screening of other audiovisual phenotypes, including sounds at different volumes and looming stimuli. Sound stimuli for the auditory habituation test were 500 ms white noise bursts at 108 dBSPL, with 2 ms on and off ramps. The interstimulus interval was 5 seconds, and stimuli were divided into two blocks. The first block consisted of 30 stimuli, followed by a 90 second break, then the recovery block consisted of 15 stimuli.
Analysis
Videos were segmented using python (version 3.7.6), and the activity of fish was tracked using DeepLabCut49, with a model trained on in-house data for larval zebrafish (Figure 1B-C). Responses were analyzed with custom MATLAB (2022) scripts. In the scn1lab dataset where the fish were partially immobilized, only the tail activity was analyzed. Responses were summed within a 1-second window after the stimulus onset.
Response intensities were compared between genotypes with a non-linear regression to a one-phase decay in GraphPad Prism (v9.4.1). This enabled comparison of whether each dataset can be explained by curves with the same coefficients in the equation . These values represent the y-intercept (a, initial response intensity), slope (b, rate of habituation), and plateau (c, extent of habituation).
Calcium imaging of brain activity during auditory habituation
Data acquisition
Imaging of calcium fluorescence was conducted with a custom-built light-sheet microscope 31,50,51. Fish were held in a 3D-printed plastic chamber with glass coverslip walls, filled with embryo media (Supplementary Figure 1A). Fish were fully immobilized in 2% low melting point agarose, except for the mecp2 dataset, where the tail was cut free to allow a range of motion of 90 degrees to each side. This enabled imaging of tail activity using a camera below the experimental chamber, for confirmation that movements otherwise interpreted from motion correction of brain imaging indeed correlated to real tail movements (data not shown).
The brain was imaged by scanning two perpendicular sheets of light through 50 z-planes at a step size of 5 µm, to cover a total volume of 250 µm with a frame rate 100 fps and binning of 4, resulting in volumetric acquisition of 2 Hz (Supplementary Figure 1B). The imaging column was as previously described50, consisting of a 20x water immersion objective, a filter to exclude the 488 nm wavelength light from the excitation laser, an electrically tunable lens, and a high-speed camera (PCO edge 5.5). Acquisition and delivery of acoustic stimuli were controlled with Micro Manager software (version 1.4) 52, and a custom written GUI in MATLAB (2022).
Stimuli
Acoustic stimuli were delivered via a speaker (Dayton Audio DAEX-9–4SM Skinny Mini Exciter Audio, Haptic Item Number 295–256) affixed to the back wall of the experimental chamber, so that sounds were delivered directly into the embryo media filling the chamber27. The stimulus train was again part of a wider screening protocol: firstly, a separate recording of 10 minutes of spontaneous activity (data not shown), then a second recording of sounds at different volumes (data not shown), followed by the auditory habituation paradigm. The auditory habituation stimulus train was composed of 100 ms white noise bursts with 2 ms on and off ramps, set to a volume equivalent to 96 dBSPL in air. Due to the requirement for smaller speakers to fit on the experimental chamber, it was not possible to deliver sounds precisely matching those in the free-swimming set-up. The interstimulus interval was 3 seconds, and again the stimuli were broken into two blocks, except for the scn1lab dataset, which did not have a recovery block. The first block consisted of 20 stimuli, followed by 10 stimuli in the second block after a 1-minute break.
Analysis of neuronal traces
Regions of interest (ROIs) representing individual neurons, and their fluorescent traces over time were extracted using Suite2p (Supplementary Figure 1B)53. The mean stack of images was then warped, first to a template brain averaged from 10 wild-type larvae, then to the Zbrain reference brain space, using the ANTs warping algorithm54,55. A mask of all the brain regions in this reference atlas was then used to exclude any extraneous ROIs identified outside the brain or in the eyes. The extraction and warping steps were performed using the high-performance computing cluster at the University of Melbourne. The remaining analysis was conducted in MATLAB (version 2022). The ΔF/F of the fluorescent traces was calculated using a sliding window of 201 timepoints, and a smoothing kernel of 7 timepoints.
Correlation to auditory stimuli and motion were calculated by linear regression to theoretical calcium transients at stimulus timings and timings of motion correction as outputted by Suite2p (Supplementary Figure 1C-D). Comparisons of metrics without data for each neuron were performed with Wilcoxon ranked-sum tests. For voxel-wise spatial comparison of various measures, neurons were averaged in 3-dimensional cubes of edge length 10µm. Mean activity traces of neurons within these cubes were fitted to a curve described by the function to obtain three curve fit parameters.
We performed graph theory analysis with the brain connectivity toolbox for MATLAB56. For each fish, a correlation matrix across all neurons was produced based on correlations in ΔF/F activity within the period of interest, and autocorrelations were removed. The matrix was binarized using one of two methods: the top 10% highest correlations, or correlation coefficients above a threshold of 0.3. The degree of each neuron was then calculated as the proportion of the maximum possible number of edges.
We compared a range of metrics between genotypes within anatomical regions, using linear mixed effects models. The equation used was ‘Y ~ genotype + (1|genotype:fishID)’, where Y is a vector of some response metric for each cell within a region. The fixed effect is the genotype, and the random effect is the individual fish 57. The region list came from the Zbrain reference atlas, with custom added masks for the octavolateralis nucleus and the granule cells of the cerebellum. We set inclusion criteria for brain regions that at least 60% of wild-type fish from each of the 4 datasets must have at least 5 neurons identified in that region. We further excluded regions in which we would not expect to find cell nuclei, and small regions defined by expression of certain markers. A full list of p-values from all comparisons can be found in the Supplementary Information.
We calculated the ratio between the degree in the gad1b and vglut2 parts of the cerebellum for the fmr1 and scn1lab datasets, excluding any ROIs which overlapped between the two, using the average degree per fish for each time period and a repeated measures ANOVA, with a Dunn-Sidak test for multiple comparisons.
Total motor activity, genotype, and correlation threshold for the top 10% of edges in the cntnap2 dataset were compared with a linear mixed-effects model with the equation ‘Y ~ motor + (1|genotype)’, where Y is the correlation threshold, the fixed effect is motor activity, and the random effect is the genotype. Each data point is one fish in this analysis.
Results
Free-swimming auditory habituation phenotypes
As expected, the behavioral responses to auditory stimuli were well modelled by an exponential decay curve. Within each dataset, we compared fit metrics of the y-intercept (the calculated y value of the curve at the initial stimulus), decay rate (slope), and final plateau value. For the fmr1 dataset, the y-intercept was higher for the fmr1−/− (14.50, n = 38) and fmr1+/− (13.79, n = 77) fish than the fmr1+/+ controls (12.27, n = 41, p = 0.0179, Figure 1D). However, the slope was not different between genotypes (fmr1+/+ 0.319, fmr1+/− 0.320, fmr1−/− 0.263, p = 0.4639), and neither was the plateau (fmr1+/+ 5.57, fmr1+/− 5.43, fmr1−/− 5.55, p = 0.7065). The fmr1 mutation therefore produces an initial sensitivity phenotype, but the rate of habituation is the same as wild types, and habituation eventually reaches the same plateau as in wild-type siblings. Therefore, if the degree of habituation is measured as relative to the initial response, the habituation strength could be considered increased in the fmr1−/− fish.
As scn1lab−/− larvae do not consistently swim upright, we embedded their heads in agarose and measured their responses based on tail movements rather than distance travelled (Figure 1E). Very striking differences in the mutants were apparent in the y-intercept (scn1lab+/+ 9002, n = 17, scn1lab+/− 8674, n =47, scn1lab−/− 14639, n = 31, p < 0.0001) and the plateau (scn1lab+/+ 6769, scn1lab+/− 6639, scn1lab−/− 9092, p < 0.0001), but not in the decay rate of the curve (scn1lab+/+ 0.158, scn1lab+/− 0.317, scn1lab−/− 0.177, p = 0.4063). The scn1lab homozygous mutants therefore have a very strong hypersensitivity phenotype, with a reduced extent of habituation in absolute measures.
In the mecp2 line (Figure 1F), the y-intercept was not different between genotypes (mecp2+/+ 12.28, n = 40, mecp2+/− 12.76, n = 107, mecp2−/− 12.47, n = 50, p = 0.7217), nor was the decay rate (mecp2+/+ 0.907, mecp2+/− 0.618, mecp2−/− 0.5714, p = 0.4639). However, the plateau was significantly higher in the mecp2−/− fish (5.334) and mecp2+/− fish (5.442) than the mecp2+/+ fish (4.900, p = 0.0001). The mecp2 phenotype is therefore reduced extent of habituation.
For the cntnap2 line, both the decay rate (cntnap2a+/+b+/+ 0.337, n = 31, cntnap2a+/+b−/− 0.320, n = 25, cntnap2a−/−b+/+ 0.519, n = 21, cntnap2a−/−b−/− 0.293, n = 30, p = 0.0087) and the plateau level (cntnap2a+/+b+/+ 6.355, cntnap2a+/+b−/− 6.443, cntnap2a−/−b+/+ 6.256, cntnap2a−/−b−/− 7.480, p = 0.0002) were significantly different between genotypes (Figure 1G). For the plateau, this difference is driven by the cntnap2a−/−b−/− fish having a higher plateau response rate than the other genotypes. The cntnap2a−/− b−/− fish have a slower decay rate than the wild types and cntnap2a+/+b−/− fish, but cntnap2a−/−b+/+ fish diverge in the other direction, with a faster habituation rate. The y-intercept did not reach the significance threshold (cntnap2a+/+b+/+ 17.88, cntnap2a+/+b−/− 18.89, cntnap2a−/−b+/+ 21.00, cntnap2a−/−b−/− 19.37, p = 0.0898), but the strongest difference is between the wild type initial response, and the highest initial response of the cntnap2a−/−b+/+ fish. Overall, the homozygous mutant of both paralogs habituates both more slowly and to a lesser extent than wild types.
Brain-wide imaging of auditory habituation phenotypes
Brain-wide auditory phenotypes at different scales
Whole-brain measures
To understand the brain activity underlying the behavioral auditory habituation phenotypes for each of the four genetic lines, we performed calcium imaging using light-sheet microscopy and the genetically encoded calcium indicator GCaMP6s, expressed in the nuclei of all neurons. This enabled detection of activity at cellular resolution across the full volume of the brain. To quantify activity across the brain-wide network, we used Suite2p53 to identify regions of interest (ROIs) generally corresponding to individual neurons31,58, and then extracted fluorescence across the experiment for each ROI. The number of ROIs segmented across the whole brain was not different between mutant and wild-type larvae for any of our genetic lines (Supplementary Figure 1E). We next measured correlation to motor activity using the motion correction output from Suite2p (Supplementary Figure 1D). Most movements detected were strong and likely stimulus-evoked rather than spontaneous, as expected in restrained fish without visual feedback 59. The amount of motor activity was not different to wild types in fmr1−/−, mecp2−/− or cntap2a−/−b−/− fish, but was significantly higher in scn1lab−/− fish, recapitulating the strong behavioral hypersensitivity (Supplementary Figure 1F). We also measured correlation to auditory stimuli, either only to the auditory habituation train or to all sounds (Supplementary Figure 1C, G-H), since the inclusion of quieter sounds aids in distinguishing auditory-specific from stimulus-evoked motor responses. There were no significant brain-wide differences in auditory correlation in any genetic line, indicating that phenotypes arise at the level of sub-regions within the brain.
To explore whether such small subregions had altered activity in our mutants, we used a voxel-wise subsampling approach, creating 10μm cubes throughout the brain and looking for differences across genotypes for a range of metrics (Figures 2–5, B-C). For each of our genetic lines, this approach revealed a unique profile of regions where activity in mutants diverged from wild types (detailed below).
Figure 2:
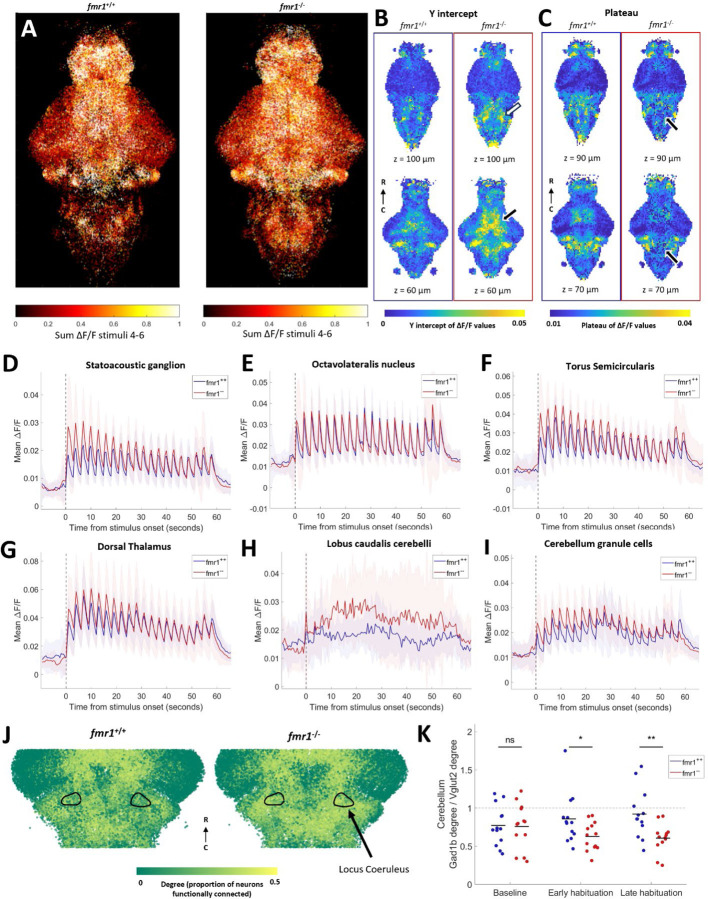
Auditory habituation phenotypes in fmr1. A) All segmented neurons from all fish, colored by sum of activity between stimuli 4 and 6. B-C) Comparison within 10 µm cubes of curve fit values during habituation period. Two different z-depths for each measure are shown for fmr1+/+ (left, n = 12) and fmr1−/− (right, n = 13). B) Y-intercept values are higher in the fmr1−/− fish, notably in the thalamus (white arrow) and hindbrain (black arrow). C) Plateau values are lower in fmr1−/− fish than wild types in several parts of the hindbrain (black arrows). D-I) Mean activity of all neurons in the SAG (D), ON (E), TS (F) dorsal thalamus (G) lobus caudalis cerebelli (H), and the granule cell region of the cerebellum (I). D-I: Shading indicates SD. J) A subset of ROIs, colored by degree, identified using the top 10% of edges from correlation during the whole habituation period. Black outlines indicate the locus coeruleus. K) Ratio between the degree of all neurons in gad1b and vglut2 regions of the cerebellum at different periods during habituation. Degree is based on the top 10% of edges. Each dot represents one fish, and black lines indicate the means. Significant effect of genotype (p = 0.0289) and interaction between genotype and time (p = 0.0401), but no significant effect of time alone (p = 0.9167, repeated measures ANOVA). No difference between genotypes at baseline (p = 0.9063), but significantly lower gad1b:vglut2 ratio in fmr1−/− in the early (p = 0.0380) and late (p = 0.0050) habituation periods (Dunn-Sidak test).
Figure 5:
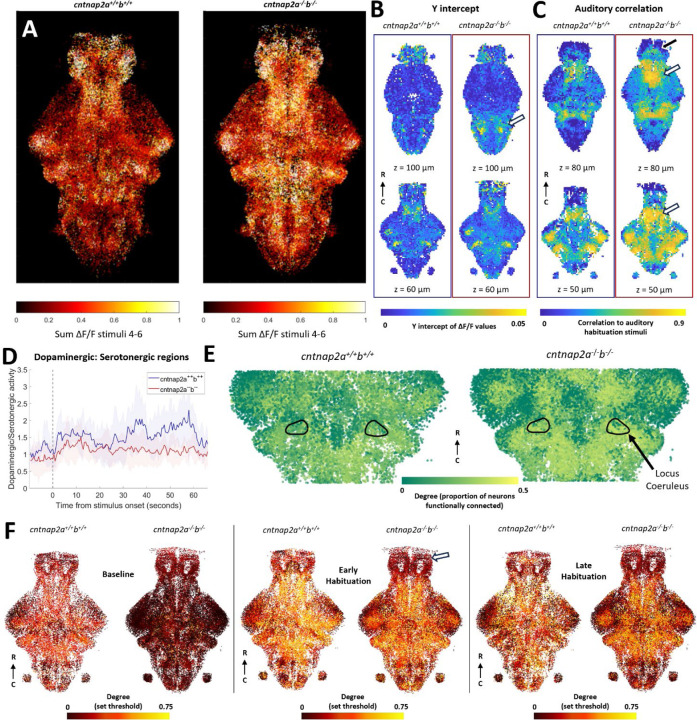
Auditory habituation phenotype in cntnap2. A) All segmented neurons from all fish, colored by the sum of fluorescent activity between stimuli 4 and 6. B) Voxel-based representations of curve fits during habituation period. Two different z-depths for each measure are shown for cntnap2a+/+b+/+ (left, n = 5) and cntnap2a−/−b−/− (right, n = 7). Y-intercepts are higher in cntnap2a−/−b−/− fish than wild types in the pineal and most of the rhombencephalon (white arrow). C) Voxel-based auditory correlation values during the habituation period. Auditory correlation is not different in most of the brain in cntnap2a−/−b−/− fish, except for the subpallium (black arrow), preoptic area (white arrows), and pineal. D) Mean ratio of activity in dopaminergic regions versus serotonergic regions. Shading indicates SD. E) Sub-selection of neurons, colored by degree, as determined using the top 10% of edges from correlation during the whole habituation period. Black outlines indicate the locus coeruleus. F) All neurons from all fish in a section of interest between 50–75 µm depth in the z-dimension. Each neuron is colored by its degree as defined by a set correlation threshold during the baseline, early habituation, and late habituation periods. At baseline, neurons have lower degree throughout the brain in the cntnap2a−/−b−/− fish compared to wild types. In the early habituation period, only the telencephalon has lower degree cntnap2a−/−b−/− fish compared to wild types (white arrow).
Cellular-resolution analyses within anatomical regions
Similarly, we performed analyses within specific brain regions, as defined in the Zbrain atlas55. Within these sub-regions we compared several different metrics using cellular-level data, but with a linear mixed effects model that allow us to control for fish of origin57. The significance of the effect of genotype across brain regions and metrics are presented in Supplementary Figure 2. These metrics include the auditory and motion correlation as described above, and the three parameters from exponential curve fits to auditory responses. We also compared the number of neurons segmented, and the sum of ΔF/F values in different time periods during the experiment. Lastly, we incorporated the degree measure of functional connectivity, calculated using correlations between all neurons, allowing us to detect differences in network dynamics that would only be evident with cellular resolution. These analyses were intended to characterize changes in brain activity that may contribute to auditory habituation phenotypes.
The summary grids in Supplementary Figure 2 illustrate the distribution of phenotypes throughout the brains of each genotype, with full p-values reported in the Supplementary Material. The scn1lab−/− fish clearly have many more differences across metrics and brain regions, which is unsurprising given their dramatic behavioral phenotype. None of the phenotypes were common across three or all four genotypes, but there were several instances in which two mutants showed similar effects (Supplementary Figure 2).
Brain wide phenotypes in four genetic autism lines
Auditory structures are hyperresponsive in fmr1
The behavioral phenotype in fmr1−/− animals was specifically in the y-intercept, representing higher initial responses to acoustic stimuli (Figure 1). Consistent with the behavioral phenotype, there is broadly stronger activity in individual neurons across the brains of fmr1−/− animals (Figure 2A). When these data are represented using the voxel-based approach, the y-intercept of neuronal activity is also higher in several brain regions, including broad regions across the diencephalon and mesencephalon (Figure 2B, Supplementary Figure 2A). There is also a portion of the hindbrain that has a lower plateau value compared to wild types (Figure 2C), consistent with the proportionally deeper behavioral habituation observed in mutants.
When single-neuron data are partitioned into defined brain regions, elevated responses are seen throughout the core auditory pathway early in the stimulus train (Figure 2D-G). In the statoacoustic ganglion (SAG), homologous to the cochlear and vestibular ganglia in mammals 60–62, the initial response strength is elevated (p = 0.0232, Figure 2D), closely mirroring the behavioral hypersensitivity (Figure 1D), before dropping to wild type levels later in the stimulus train. In the octavolateralis nucleus (ON), homologous to the cochlear nucleus60, this effect is not seen across all neurons (p = 0.3454, Figure 2E), but upon closer inspection, strongly responding auditory neurons in the ON have elevated responses early in the stimulus train (Supplementary Figure 3). These results suggest that, while these responses are masked by the large and diverse population of neurons in the ON, there are elevated auditory signals in this structure. Similar elevations in initial response strength are present in the torus semicircularis (TS, p = 0.0352, Figure 2F), homologous to the inferior colliculus63, and the dorsal thalamus (p = 0.0459, Figure 2G), the auditory region within the thalamus62.
Beyond the defined auditory processing pathway, cellular-resolution data reveal stronger responses in fmr1−/− animals for the lobus caudalis (the vestibular region64) of the cerebellum (Figure 2H) and a region corresponding to cerebellar granule cells (Figure 2I). The increase in the lobus caudalis does not attenuate like the auditory pathway (Figure 2D-G), showing some degree of elevation throughout the stimulus train. Other observations of cellular-resolution data include increased activity at baseline in the telencephalon of fmr1−/− fish (p = 0.0434), and an increased number of neurons detected in the pineal (p = 0.0070, Supplementary Figure 2A).
In applying graph theory to data from individual neurons, we found higher functional connectivity (as measured by degree) in the locus coeruleus of fmr1−/− fish throughout the experiment (Figure 2J, p = 0.0002). In the cerebellum, there are divergent degree differences in the vglut2- versus the gad1b-enriched areas as habituation proceeds (Supplementary Figure 2). This divergence is of particular interest given their opposing effects on cerebellar output64,65. We therefore compared the mean degree of cells within these regions as a ratio over the course of the habituation period (Figure 2K). During the baseline the mean degree is not different between wil types and fmr1−/− fish, but in the habituation period the vglut2-enriched area has higher functional connectivity than the gad1b-enriched area in fmr1−/− fish. This observation suggests that activity in the excitatory eurydendroid cells is more tightly coupled to brain-wide activity than the inhibitory Purkinje cells in the fmr1−/− fish.
Widespread hyper-excitation and loss of habenular activity in scn1lab
Qualitatively, scn1lab−/− animals show broader and stronger activity across the brain in response to auditory stimuli, which is less specifically located in auditory regions such as the thalamus, TS, and cerebellum compared to wild types (Figure 3A). Our voxel-based approach showed that the scn1lab−/− fish also had clear differences in the number of neurons detected in specific brain regions.
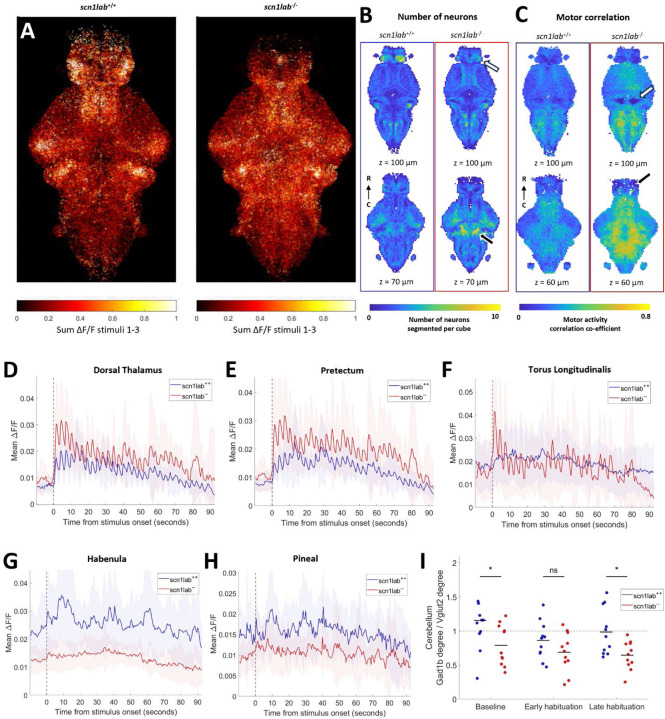
Figure 3:
Auditory habituation phenotype in scn1lab. A) All segmented neurons from all fish, colored by sum of activity between stimuli 1 and 3. B) Comparison within 10 µm cubes of number of neurons segmented. Two different z-depths for each measure are shown for scn1lab+/+ (left, n = 11) and scn1lab−/− (right, n = 11). Fewer neurons were segmented in the habenula (white arrow), and more in the cerebellum (black arrow) in scn1lab−/− fish than wild types. C) Comparison within 10 µm cubes of motor correlation values. Motor correlation is higher in most of the brain in scn1lab−/− fish, except for the granule cells of the cerebellum (white arrow) and the telencephalon (black arrow). D-H) Mean activity of all neurons in the dorsal thalamus (D), pretectum (E), torus longitudinalis (F), habenula (G) and pineal (H). D-H: Shading indicates SD. I) Ratio between the degree of all neurons in the gad1b region of the cerebellum and the vglut2 region of the cerebellum at different periods during habituation. Degree is based on the top 10% of edges design. Each dot represents one fish, black lines indicate the means. Significant effect of genotype (p = 0.0078), non-significant effect of time (p = 0.0567) and interaction between genotype and time (p = 0.4682, repeated measures ANOVA). The ratio is significantly lower in scn1lab−/− fish at baseline (p = 0.0437) and in the late habituation period (p = 0.0102), but not different in the early habituation period (p = 0.1629, Dunn-Sidak test).
While the overall number of neurons detected brain-wide was not different (Supplementary Figure 1E), more neurons are detected throughout the mesencephalon (p = 0.0488) and rhombencephalon (p = 0.0031, including the cerebellum p = 0.0255), but fewer in the telencephalon (p = 0.0417), and notably the habenula (p = 0.0006, Figure 3B). These observations are consistent with increased proliferative cells and reduced forebrain volume of scn1lab−/− fish21. We also found strikingly increased motor correlations throughout most brain regions in the mutants (Figure 3C) and increased auditory correlation during the habituation period in most of these regions (Supplementary Figure 2B). The granule cells of the cerebellum deviate from this general trend, having lower correlation to both motor activity (p < 0.0001, Figure 3C) and auditory stimuli (p = 0.0006).
Analyses of cellular-resolution data in particular brain regions also reveal profound differences in the scn1lab−/− brain. Stimulus-evoked activity within the diencephalon diverges drastically from wild types, in opposing directions in different sub-regions. The scn1lab−/− fish show increased overall activity in the thalamus, particularly the dorsal thalamus, and also in the pretectum (Figure 3D and E, Supplementary Figure 2B). The y-intercept (p = 0.0219 ) and auditory correlation (p < 0.0001) are increased in the primarily glutamatergic66 torus longitudinalis, with clear auditory responses in scn1lab−/− but not wild types (Figure 3F). The habenula (Figure 3G) and pineal (Figure 3H), on the other hand, have drastically reduced activity in scn1lab−/− fish. In the case of the habenula this represents an almost total loss of activity. Activity is also generally decreased in the telencephalon, especially in late habituation (p = 0.0010), and decreased at baseline in the vagal ganglia (p = 0.0075) and vagal motor neuron cluster (p = 0.0205, Supplementary Figure 2B).
Similarly to fmr1, in scn1lab−/− fish there is diverging functional connectivity of neurons in the gad1b and vglut2-enriched areas of the cerebellum (Figure 3I), indicating imbalance between the role of excitatory and inhibitory populations.
Reduced dopamine activity and changes in functional connectivity in mecp2
Unsurprisingly given its mild behavioral phenotype, mecp2 shows only subtle changes in auditory processing and motor correlations. Indeed, a qualitative mapping of activity strength across the brain early in the stimulus train (when the behavioral phenotype is strongest) shows similar patterns across mecp2−/− larvae and their wild-type siblings (Figure 4A). There are no widespread increases in the plateau or late habituation period ΔF/F in the brain activity of mecp2−/− fish that could explain the behavioral phenotype, but there are differences in other more specific measurements that could contribute to behavior, including increases in the plateau of activity in the statoacoustic ganglion and the lobus caudalis cerebelli (Supplementary Figure 2C).
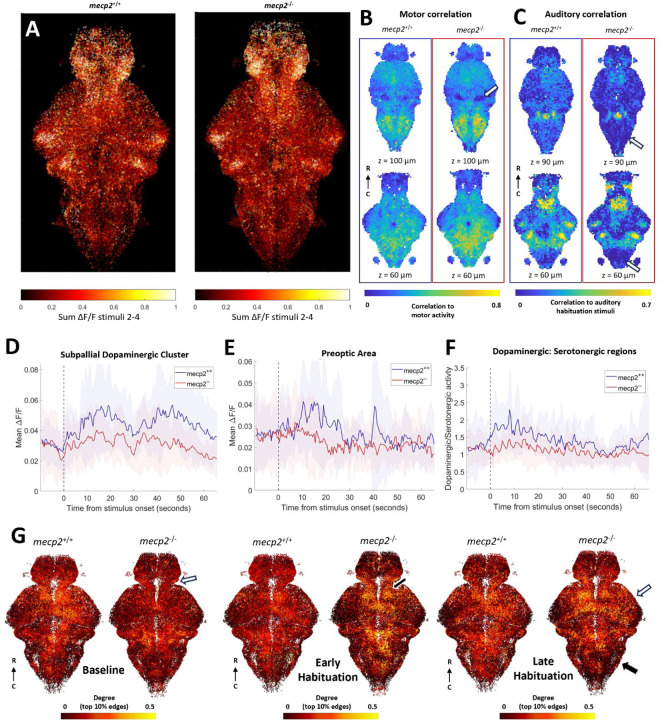
Figure 4:
Auditory habituation phenotype in mecp2. A) All segmented neurons from all fish, colored by the sum of fluorescent activity between stimuli 2 and 4. B) Comparison within 10 µm cubes of motor correlation values. Two different z-depths for each measure are shown for mecp2+/+ (left, n = 10) and mecp2−/− (right, n = 13). Motor correlations are not different in most of the brain in mecp2−/− fish, except for the granule cells of the cerebellum (white arrow). C) Auditory correlation during the habituation period is decreased in mecp2−/− fish in the inferior olive and the posterior hindbrain (white arrows). Mean activity of all neurons in the subpallial dopaminergic cluster (D)and the preoptic area (E). F) Mean ratio between activity in dopaminergic regions and serotonergic regions. D-F: Shading indicates SD. G) All neurons from all fish in a region of interest in the z-dimension. Each neuron colored by its degree as defined by the top 10% of edges during the baseline, early habituation, and late habituation periods. Differences in degree between mecp2−/− and wild-type fish are indicated in the diencephalon at baseline (white arrow), the dorsal thalamus (black arrow) during early habituation, and in the rhombencephalon (black arrow) and the mesencephalon (white arrow) during late habituation.
Looking at the motor correlations across voxels, we observe a decrease in the region of the cerebellar granule cells (p = 0.0112, Figure 4B), similar to what we observed for scn1lab. The correlation to auditory stimuli in the habituation period is lower in parts of the rhombencephalon, the inferior olive (p = 0.0282) and the preoptic area (p =0.0481, Figure 4C). The only difference in the sum of activity at baseline is an increase in the inferior olive in mecp2−/− fish compared to wild types (p = 0.0199, Supplementary Figure 2C).
The mecp2−/− fish have decreased activity during late habituation for neurons in dopaminergic regions such as the subpallial dopaminergic cluster (p < 0.0001 Figure 4D) and the preoptic area (p = 0.0309, Figure 4E), and a decreased number of ROIs detected in the ventral thalamus (p = 0.0169, Supplementary Figure 2C). To address the opposing effects of dopamine and serotonin on habituation, we calculated the ratio of mean activity in dopaminergic regions (preoptic area, subpallial dopaminergic cluster, pretectal dopaminergic cluster and dopaminergic cluster of the ventral thalamus) with the mean activity in serotonergic regions (superior raphe, inferior raphe and pineal). While there is more dopaminergic than serotonergic activity in wild types, particularly at the beginning of the auditory habituation block, the mean ratio is close to 1 throughout the period for mecp2−/− fish, and this difference between genotypes is most pronounced during critical habituation period (Figure 4F).
With graph theory using the top 10% method, we observe differences in functional connectivity at the gross level of brain regions (Figure 4G). Degree is lower in mecp2−/− fish compared to wild types in the baseline period in the diencephalon (p =0.0380), and elevated in the dorsal thalamus in mecp2−/− fish in the early habituation period (p = 0.0221, Supplementary Figure 2C). In the late habituation period, high degree neurons are reduced in the rhombencephalon (p = 0.0156) and increased in the mesencephalon (p =0.0326) in the mecp2−/− fish compared to wild types.
Increased activity and disrupted functional connectivity in cntnap2.
The cntnap2a−/−b−/− double knockouts have an (insignificantly) elevated y-intercept, slower habituation, and a higher plateau compared to wild-type siblings (Figure 2G). When these combined effects are strongest, during early habituation, neurons across the brain are more responsive to auditory stimuli (Figure 5A). The cntnap2a−/−b−/− larvae have several brain regions with increased y-intercepts, including most of the hindbrain, but fewer that have differences in decay rate or plateau (Supplementary Figure 2D, Figure 5B). There is also increased correlation to auditory stimuli during the habituation period in the preoptic area, subpallium, and eminentia thalami (thalamic eminence) (Figure 5C).
We observed both decreased activity in dopaminergic regions (preoptic area, thalamic dopaminergic cluster, tegmentum and subpallial dopaminergic cluster) and increased activity in serotonergic regions (superior raphe and pineal, Supplementary Figure 2D). While the ratio between dopaminergic activity and serotonergic activity in the mutants (as calculated for mecp2) shows a similar increase at the beginning of the stimulus train to that of the wild types, it remains below the mean value for wild types until the end of the auditory stimuli (Figure 5D). This suggests that the temporal dynamics of this ratio are preserved at the onset of the sound, but not in the later part of the train, when the behavioral phenotype is strongest.
According to graph theory measurements with the top 10% thresholding method, functional connectivity in early habituation in cntnap2a−/−b−/− fish is decreased across several parts of the forebrain, and consequently increased in the rhombencephalon (p = 0.0197). The locus coeruleus also has higher functional connectivity during the whole period (p = 0.0001, Figure 5E). With the hard threshold method, functional connectivity is broadly reduced at baseline (Figure 5F). This effect is not due to differences in motor activity at baseline: both motor activity (p = 0.0029) and genotype (p = 3.08 ×10–6) have significant effects on the threshold required to attain the top 10% of correlations (linear mixed-effect model). The decreased functional connectivity persists in the telencephalon (p = 0.0008) and parts of diencephalon into early but not late habituation (Figure 5F). The few regions without lower degree during the baseline include monoaminergic regions such as the subpallium, preoptic area, locus coeruleus, and superior raphe (Supplementary Figure 2D). Fish mutant for only cntnap2a or cntnap2b generally resemble the double mutants, but the mutant phenotype diverges between the single mutants in the functional connectivity of the locus coeruleus and the set threshold functional connectivity over time (Supplementary Figure 4).
Discussion
Unique phenotypic fingerprints for each gene
In this study, we have used auditory habituation as a paradigm to characterize the behavioral and brain-wide phenotypes for four genetic lines with relevance to autism. Each gene showed a different combination of traits describing its behavioral phenotype and each had its own profile of activity changes across the brain. The points of overlap, but also the distinctions between the lines’ phenotypes, raise interesting questions about the various ways in which changes in brain activity could lead to altered perception and behavior. This approach is a first step toward understanding the diverse and multigenic ways in which sensation and behavior are altered across the autism spectrum in humans.
Initial hyperresponsiveness in fmr1
The behavioral phenotype of fmr1 was limited to an increase in the initial responsiveness (Figure 1D). Correspondingly, we found increased initial response amplitude in several auditory brain structures, including the SAG, torus semicircularis, and thalamus, and auditory neurons in the ON. We therefore postulate that the auditory hypersensitivity phenotype arises within the auditory pathway, which drives increased behavioral output.
Surprisingly, we also found some differences in the late habituation period, despite the lack of a behavioral phenotype in the plateau of responses. The plateau of several rhombomeres of the hindbrain is also decreased in fmr1−/− fish, suggesting lower activity in motor output regions. Indeed, there is also increased activity in the granule cells of the cerebellum in the early part of the habituation period (Figure 2), which may represent more inhibition of motor output67–70. The fmr1−/− larvae may therefore undergo stronger adaptation to reach the same behavioral plateau as the wild types, having started from a greater initial response.
While the hypersensitivity fits with previous zebrafish fmr1 studies32, the lack of a phenotype in the habituation to sounds here is at odds with what would be expected from studies of auditory habituation in FXS8. It is also different to the decreased auditory adaptation in the brain activity of Fmr1−/− mice, although different age or stimulus presentation rates may explain this discrepancy71.
Drastically increased responsiveness in scn1lab
The behavioral phenotype in scn1lab was the strongest of the four genes, with highly elevated initial response and plateau responses (Figure 1E). Unsurprisingly, we also found the most dramatic differences in brain activity in scn1lab mutants (Supplementary Figure 2B). Because there are differences in the volume and number of proliferative cells in the brains of scn1lab−/− fish21, we infer that neuronal proliferation, migration, differentiation and/or survival is altered in the brain. These changes likely lead to some neurons playing different roles within the network, such as those in the torus longitudinalis responding completely differently in scn1lab−/− animals than wild types (Figure 3F).
We observed both increases and reductions in activity in distinct parts of the brains of scn1lab−/− fish. Activity was increased in the thalamus, pretectum, tegmentum, and torus longitudinalis. Conversely, activity was reduced in the telencephalon, habenula, pineal, and vagal ganglia. A previous study using pERK/tERK staining to infer activity levels found decreased activity at baseline throughout the brains of scn1ab−/− fish, though most strikingly in the telencephalon and habenula21. Our results recapitulate this forebrain phenotype, but also uncover various changes in activity across the rest of the brain.
The habenula has a role in suppressing anxiety or fear responses in zebrafish 43,72–75. The putative ‘induced passivity’ network involves increased activity in the habenula and decreased activity in the dorsal thalamus 43. The loss of activity in the habenula and increased activity in the dorsal thalamus we observe in scn1lab−/− fish may therefore represent an ‘anti-passivity’ network, which leads to greatly increased behavioral output. However, our methods only measure activity of neurons, and glia have also been shown to be important for passivity59.
The higher correlation to motion throughout the brains of scn1lab−/− animals (Figure 3C) may be due to greater physical movement in scn1lab−/− fish, which recruit brain-wide activity more than the comparatively smaller movements of the wild types. An alternate explanation is functional hyperconnectivity across the mutant brain, which would tie to the role for scn1lab in epilepsy76. However, we do not observe gross differences in graph theory measures of network connectivity.
Higher plateau of responses in mecp2
The behavioral phenotype for mecp2 was specifically an increase in the plateau of the response rates, suggesting decreased habituation (Figure 1F). A recent study measured auditory habituation in mecp2−/− zebrafish larvae, and found no differences in the habituation as measured by the likelihood of startle77. It is not clear whether this study may have found an increase in the plateau of response rates using the distance travelled, as we have here, instead of startle probability. In our study, there were no differences in pure activity level in auditory regions to explain this phenotype. The only regions with a higher plateau of responses were the SAG and lobus caudalis cerebelli, which is generally regarded to be part of the vestibular network61. The vestibular and auditory systems are functionally intertwined in larval zebrafish, however, and these higher plateaus may therefore correspond to altered auditory, rather than vestibular processing78.
We also observe differences in the functional connectivity of the brain networks over time in the major divisions of the brain (Figure 4G). In the early part of habituation period, where there are no behavioral phenotypes, only the dorsal thalamus has higher functional connectivity in mecp2−/− fish. By the end of the habituation period, when the behavioral phenotype emerges, the balance of edges in the network is shifted toward the mesencephalon and away from the rhombencephalon in mecp2−/− fish. This matched timing with the behavioral phenotype suggests it may have functional consequences for the behavior of the animal. In contrast to fmr1 and scn1lab, where an initial hypersensitivity either is (fmr1) or is not (scn1lab) compensated for during habituation, mecp2 provides an example of a genetic line with a normal initial response that only exhibits a phenotype as habituation plays out. It will be interesting to explore whether the earlier divergence of functional connectivity in the dorsal thalamus may be linked to the ensuing shift in functional connectivity across the brain.
Slower habituation and higher plateau in cntnap2
In the behavior of cntnap2a−/−b−/− fish, we observed a slower decay rate and a higher plateau of responses to auditory stimuli (Figure 1G). Surprisingly, given that there was no significant behavioral difference in the initial response, we found far more differences in the y-intercept of brain activity than either the decay rate or plateau (Supplementary Figure 2D). These increases occurred throughout the rhombencephalon, as well as in the pineal and subpallial dopaminergic cluster. Our best explanation for this discrepancy is that, given the difficulty of getting a large experimental n for a duplicated gene such as cntnap2, we lacked statistical power to identify what is a real y-intercept phenotype, yielding only an insignificant trend (Figure 1G).
We also observed widespread decreased functional connectivity throughout the brain in cntnap2a−/−b−/− fish at baseline, independent of differences in motor activity (Figure 5F). In the early habituation period, these differences disappear except in the telencephalon and habenula, and by the late habituation period, the network connectivity is comparable to that in wild types. This initial lack of functional connectivity in the network, and late recruitment of higher-order integrative structures, may underlie the reduced ability of the network to adapt to auditory stimuli over time. Interestingly, each of the single mutants resembled one aspect of these functional connectivity phenotypes (Supplementary Figure 4H). Each mutation may therefore contribute differently to the cumulative phenotype of the double mutant.
Similarly to scn1lab−/− regions outside of the forebrain, we did not observe widespread changes in activity at baseline as reported in a previous paper that used pERK/tERK staining to infer brain activity21 Our baseline is measured over a fairly short period preceding stimuli, whereas the increased pERK/tERK activity may be related to freely swimming in the environment over a longer period. The observed decrease in habituation to auditory stimuli does, however, fit with previous studies in Cntnap2 knock-out rats79,80.
Overlapping phenotypes across genetic lines
There were not overlapping phenotypes between genes in the primary auditory regions, rather shared circuitry changes appear to be in the sensorimotor and modulatory regions.
The y-intercept of neurons in rhombomeres 3–5 is higher in scn1lab−/− and cntnap2a−/−b−/− fish than wild types, which likely relates to activity in motor regions driving the increased initial behavioral response in each of those lines. Conversely, the plateau of activity in this part of the hindbrain is decreased in fmr1−/− fish, which supports the idea that the network is more strongly adapted from a higher initial point to reach the same plateau as wild types.
The reduced correlation to motor activity in the granule cells of the cerebellum is a striking phenotype in scn1lab−/− and mecp2−/− fish (Figure 6), particularly because it is scaled to the intensity of the behavioral phenotype of each gene. Previous studies in larval zebrafish suggest suppression of granule cell activity is required for stimulus-evoked behavioral responses and preventing immobility 67–70. Reduced granule cell activity in scn1lab and mecp2 mutants at the time of stimulus-evoked movements may therefore contribute to sustained higher behavioral responses in both these lines.
Figure 6:
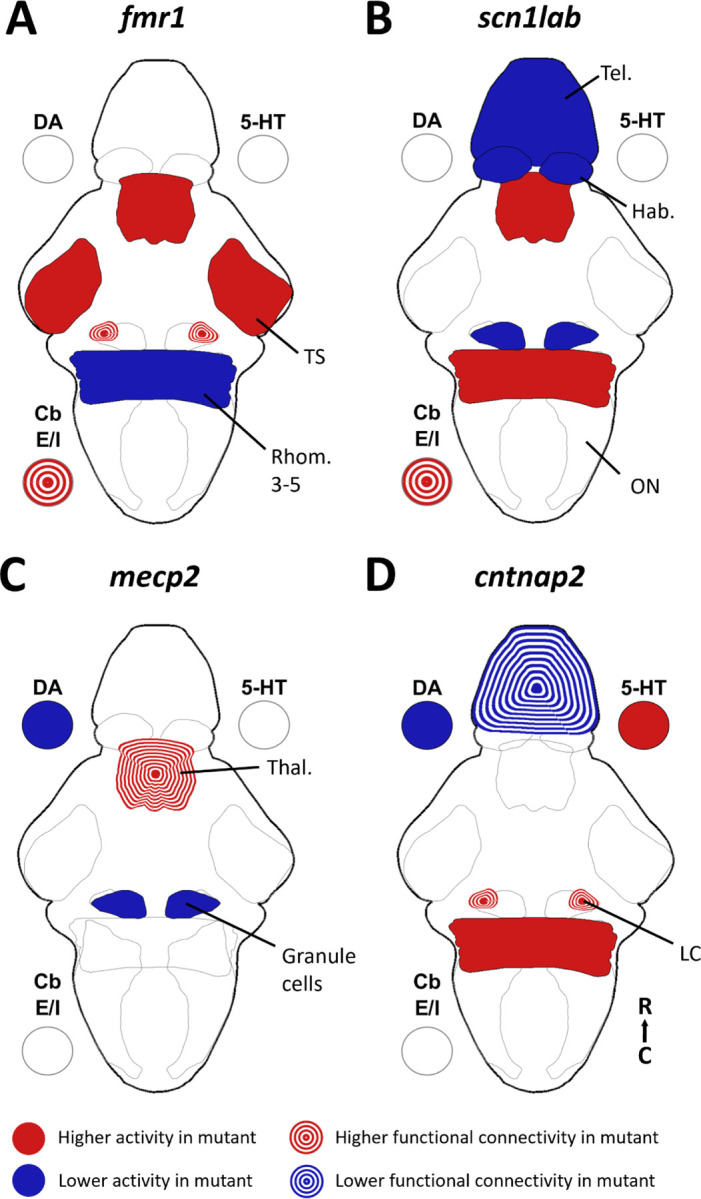
Summary of brain activity underlying auditory habituation phenotypes. A summary of the brain-wide phenotypes for activity and functional connectivity found in fmr1 (A), scn1lab (B), mecp2 (C), and cntnap2 (D). 5-HT = serotonin, DA =dopamine, Cb E/I = cerebellum excitatory/inhibitory regions, Hab = habenula, LC = locus coeruleus, ON = octavolateralis nucleus, Rhomb. = rhombomere, Tel. = telencephalon, Thal = thalamus, TS = torus semicircularis.
We found that the functional connectivity of the gad1b-enriched and vglut2-enriched parts of the cerebellum (as measured by the degree) diverged similarly in both fmr1 and scn1lab mutants (Supplementary Figure 2,Figure 6). While there is some heterogeneity of cell types within these regions, we take these populations to be representative of the Purkinje cells (gad1b) and eurydendroid cells (vglut2). The functional connectivity of the eurydendroid cells is higher than that of the Purkinje cells in both the fmr1 and scn1lab mutants, particularly in the late habituation period. This divergence in connectivity of excitatory and inhibitory regions may relate to the theory of E/I imbalance in autism12. Considering the crucial role of the cerebellum in sensorimotor processing, this divergence in connectivity seems likely to affect behavioral output. It is particularly interesting for fmr1 that this connectivity phenotype is strongest in the late habituation period when there is not a behavioral phenotype in the plateau of responses, indicating that while the behavioral plateau reached resembles that of the wild types, the underlying brain activity is not the same.
The cntnap2a−/−b−/− and mecp2−/− fish both have differences in monoaminergic activity (Figure 6), which fit with what we may expect based on pharmacological studies 38,39. Indeed, pharmacological manipulations of dopamine and serotonin receptors affected baseline activity in a different cntnap2a−/− b−/− line48. The combination of dopaminergic and serotonergic phenotypes, and the earlier onset of these differences in cntnap2, may explain why the behavioral output of these fish diverges from wild types earlier in the stimulus train than in mecp2 fish, which have only dopaminergic differences. The difference in ratio of dopaminergic to serotonergic activity was recapitulated in both of the single gene cntnap2 mutants (Supplementary Figure 4E). There are also some reductions in activity in dopaminergic areas of forebrain in scn1lab−/− fish, but these are less likely to be dopamine-specific given the reduced number of dopaminergic forebrain neurons in scn1lab−/− fish21 and the overall reduction in telencephalic activity (Supplementary Figure 2). Interestingly, the dopaminergic effects are specific to the subpallium and preoptic area, and the serotonergic differences in cntnap2 are stronger in the pineal than the superior raphe, the serotonergic region typically associated with habituation38.
Functional connectivity over the full habituation period is increased in the locus coeruleus in both fmr1 and cntnap2 fish (Figure 6). The locus coeruleus is a noradrenergic center, whose activity is associated with alertness81. This increased recruitment of the locus coeruleus within the brain network may indicate a generally more alert state in these fish, leading to hyperresponsiveness to auditory stimuli. We also observe an increased plateau of activity in the locus coeruleus in scn1lab−/− fish (Supplementary Figure 2), which may represent more persistent alertness late in the habituation period, consistent with the observed behavior.
We observe differences in the activity or functional connectivity of parts of or the whole telencephalon in all three of the genes that have higher plateau of responses: mecp2, scn1lab and cntnap2 (Figure 6). The telencephalon is the seat of higher-order processing in the zebrafish brain82,83, so it is unsurprising that it would have involvement in adaptation to stimuli. It is notable that fmr1, the only gene not to have a behavioral phenotype in the plateau of responses, does not have significant changes to the activity of the telencephalon.
Future directions
We have identified a range of differences in brain activity in these four autism-relevant genetic lines. In each, we have addressed brain-wide function in a fundamental way, observing activity across the entire brain at cellular resolution. Doing so has permitted several separate and complementary approaches for assessing brain-wide activity and the ways in which networks may change differently in different autism-relevant mutant lines.
This approach, in its current form, comes with important limitations, especially because we cannot assess the synaptic relationships between the neurons that we observe and cannot conclusively determine the neurons’ neurotransmitter subtypes. As such, while these results are comprehensive from one perspective, they represent merely a departure point for better understanding the mechanisms by which information flow changes in the brains of these fish. Future studies could shed greater light on these changes by performing calcium imaging while co-labelling neurons with transgenic markers for neurotransmitter subtypes, such as the GABAergic, glutamatergic, dopaminergic, and serotonergic populations that our study implicate. Further, optogenetic techniques could be employed to manipulate parts of the circuit in wild types that we have implicated in our mutants, directly testing the hypothesized impacts on activity elsewhere in the brain and on behavior. More in-depth behavioral analysis may also provide more insight into brain activity underlying changing response strategies.
Additionally, it would be interesting to add more genes to this collection. Here we have presented four different autism-associated genes, but this only scratches the surface of autism’s genetic complexity and phenotypic diversity. Adding more lines with different combinations of behavioral and neural phenotypes would enable a greater understanding of which combinations of brain activity phenotypes link to which behaviors. Ultimately, this will lead to a fuller appreciation of the relationship among genetics, perception, and behavior.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the University of Queensland’s Biological Resources aquatics team and the Danio rerio University of Melbourne facility (DrUM, Melbourne, Australia) for maintenance of zebrafish lines, the Queensland Brain Institute workshop staff for 3D-printing of imaging chambers, and Dr Summer Thyme for her feedback on the manuscript.
Funding
Support was provided by a Simons Foundation Research Award (625793), two ARC Discovery Project Grants (DP220103812 and DP230102614), and an NHMRC Investigator Grant (2027072) to EKS. The research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number R01NS118406 to EKS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Support was also provided by an ARC DECRA award (DE220100691 & DE230100972) to CL and IAF, an NHMRC Ideas Grant (2012140) to IAF and a Simons Foundation Research Award (573508) to EJH.
MW is supported by a University of Queensland RTP scholarship.
Funding Statement
Support was provided by a Simons Foundation Research Award (625793), two ARC Discovery Project Grants (DP220103812 and DP230102614), and an NHMRC Investigator Grant (2027072) to EKS. The research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number R01NS118406 to EKS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Support was also provided by an ARC DECRA award (DE220100691 & DE230100972) to CL and IAF, an NHMRC Ideas Grant (2012140) to IAF and a Simons Foundation Research Award (573508) to EJH.
MW is supported by a University of Queensland RTP scholarship.
Footnotes
Ethics approval
All work was performed in accordance with research application SBS/341/19 and breeding application IMB/271/19/BREED, which was approved by the Anatomical Biosciences Animal Ethics Committee at the University of Queensland, or research application 2022-24987-35220-5, which was approved by the SLA-2 Animal Ethics Committee at the University of Melbourne.
References
- 1.Marco E.J., Hinkley L.B.N., Hill S.S., and Nagarajan S.S. (2011). Sensory processing in autism: A review of neurophysiologic findings. Pediatric Research CS, 48R. 10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crane L., Goddard L., and Pring L. (2009). Sensory processing in adults with autism spectrum disorders. Autism 13, 215–228. 10.1177/1362361309103794. [DOI] [PubMed] [Google Scholar]
- 3.Simpson K., Adams D., Alston-Knox C., Heussler H.S., and Keen D. (2019). Exploring the Sensory Profiles of Children on the Autism Spectrum Using the Short Sensory Profile-2 (SSP-2). Journal of Autism and Developmental Disorders 4S, 2069–2079. 10.1007/s10803-019-03889-2. [DOI] [PubMed] [Google Scholar]
- 4.Tomchek S.D., and Dunn W. (2007). Sensory processing in children with and without autism: a comparative study using the short sensory profile. American Journal of Occupational Therapy C1, 190–200. 10.5014/ajot.61.2.190. [DOI] [PubMed] [Google Scholar]
- 5.Mansour Y., Burchell A., and Kulesza R.J. (2021). Central Auditory and Vestibular Dysfunction Are Key Features of Autism Spectrum Disorder. Frontiers in Integrative Neuroscience 15, 32. 10.3389/FNINT.2021.743561/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J., Barstein J., Ethridge L.E., Mosconi M.W., Takarae Y., and Sweeney J.A. (2013). Resting state EEG abnormalities in autism spectrum disorders. Journal of Neurodevelopmental Disorders 5. 10.1186/1866-1955-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sztainberg Y., and Zoghbi H.Y. (2016). Lessons learned from studying syndromic autism spectrum disorders. Nature Neuroscience 1S, 1408–1418. 10.1038/nn.4420. [DOI] [PubMed] [Google Scholar]
- 8.Sinclair D., Oranje B., Razak K.A., Siegel S.J., and Schmid S. (2017). Sensory processing in autism spectrum disorders and Fragile X syndrome-From the clinic to animal models. Neuroscience and biobehavioral reviews 7C, 235–253. 10.1016/j.neubiorev.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stamou M., Streifel K.M., Goines P.E., and Lein P.J. (2013). Neuronal connectivity as a convergent target of gene × environment interactions that confer risk for Autism Spectrum Disorders. Neurotoxicology and Teratology 3C, 3–16. 10.1016/j.ntt.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon S.H., Choi J., Lee W.J., and Do J.T. (2020). Genetic and Epigenetic Etiology Underlying Autism Spectrum Disorder. Journal of Clinical Medicine S, 966. 10.3390/jcm9040966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merchie A., and Gomot M. (2023). Habituation, Adaptation and Prediction Processes in Neurodevelopmental Disorders: A Comprehensive Review. Brain Sciences 13, 1110. 10.3390/brainsci13071110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parrella N.-F., Hill A.T., Dipnall L.M., Loke Y.J., Enticott P.G., and Ford T.C. (2024). Inhibitory dysfunction and social processing difficulties in autism: A comprehensive narrative review. Journal of Psychiatric Research 1CS, 113–125. 10.1016/j.jpsychires.2023.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Pavăl D. (2017). A Dopamine Hypothesis of Autism Spectrum Disorder. Dev Neurosci 3S, 355–360. 10.1159/000478725. [DOI] [PubMed] [Google Scholar]
- 14.Su L.-D., Xu F.-X., Wang X.-T., Cai X.-Y., and Shen Y. (2021). Cerebellar Dysfunction, Cerebro-cerebellar Connectivity and Autism Spectrum Disorders. Neuroscience 4C2, 320–327. 10.1016/j.neuroscience.2020.05.028. [DOI] [PubMed] [Google Scholar]
- 15.Seif A., Shea C., Schmid S., and Stevenson R.A. (2021). A Systematic Review of Brainstem Contributions to Autism Spectrum Disorder. Frontiers in Integrative Neuroscience 15, 39. 10.3389/FNINT.2021.760116/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilde M., Constantin L., Thorne P.R., Montgomery J.M., Scott E.K., and Cheyne J.E. (2022). Auditory processing in rodent models of autism: a systematic review. Journal of Neurodevelopmental Disorders 14, 48. 10.1186/s11689-022-09458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Degroote S., Hunting D., and Takser L. (2017). Improved assessment of sensorimotor gating in animal models relevant to ASD: A data modelling approach to quantify PrePulse Inhibition of the acoustic startle reflex. Journal of neuroscience methods 27C, 13–22. 10.1016/j.jneumeth.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Scott K.E., Schulz S.E., Moehrle D., Allman B.L., Cardy J.E.O., Stevenson R.A., and Schmid S. (2021). Closing the species gap: Translational approaches to studying sensory processing differences relevant for autism spectrum disorder. Autism Research 14, 1322–1331. 10.1002/AUR.2533. [DOI] [PubMed] [Google Scholar]
- 19.Washbourne P. (2023). Can we model autism using zebrafish? Dev Growth Differ. 10.1111/dgd.12888. [DOI] [PubMed] [Google Scholar]
- 20.Rea V., and Van Raay T.J. (2020). Using Zebrafish to Model Autism Spectrum Disorder: A Comparison of ASD Risk Genes Between Zebrafish and Their Mammalian Counterparts. Frontiers in Molecular Neuroscience 13, 207. 10.3389/fnmol.2020.575575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinschutz Mendes H., Neelakantan U., Liu Y., Fitzpatrick S.E., Chen T., Wu W., Pruitt A., Jin D.S., Jamadagni P., Carlson M., et al. (2023). High-throughput functional analysis of autism genes in zebrafish identifies convergence in dopaminergic and neuroimmune pathways. Cell Rep 42, 112243. 10.1016/j.celrep.2023.112243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanwalleghem G., Schuster K., Taylor M.A., Favre-Bulle I.A., and Scott E.K. (2020). Brain-Wide Mapping of Water Flow Perception in Zebrafish. Journal of Neuroscience 40, 4130–4144. 10.1523/JNEUROSCI.0049-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Favre-Bulle I.A., Stilgoe A.B., Rubinsztein-Dunlop H., and Scott E.K. (2017). Optical trapping of otoliths drives vestibular behaviours in larval zebrafish. Nature Communications 8. https://doi.org/ARTN 630 10.1038/s41467-017-00713-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jetti S.K., Vendrell-Llopis N., and Yaksi E. (2014). Spontaneous activity governs olfactory representations in spatially organized habenular microcircuits. Curr Biol 24, 434–439. 10.1016/j.cub.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Baier H., and Scott E.K. (2024). The Visual Systems of Zebrafish. 10.1146/annurevneuro-111020-104854. [DOI] [PubMed] [Google Scholar]
- 26.Fero K., Yokogawa T., and Burgess H.A. (2011). The behavioral repertoire of larval zebrafish. Neuromethods 52, 249–291. 10.1007/978-1-60761-922-2_12. [DOI] [Google Scholar]
- 27.Poulsen R.E., Scholz L.A., Constantin L., Favre-Bulle I., Vanwalleghem G.C., and Scott E.K. (2021). Broad frequency sensitivity and complex neural coding in the larval zebrafish auditory system. Current Biology 31, 1977–1987.e4. 10.1016/j.cub.2021.01.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Privat M., Romano S.A., Pietri T., Jouary A., Boulanger-Weill J., Elbaz N., Duchemin A., Soares D., and Sumbre G. (2019). Sensorimotor Transformations in the Zebrafish Auditory System. Current Biology 2S, 4010–4023.e4. 10.1016/J.CUB.2019.10.020/SENSORIMOTOR_TRANSFORMATIONS_IN_THE_ZEBRAFISH_AUDITORY_SYSTEM.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migault G., van der Plas T.L., Trentesaux H., Panier T., Candelier R., Proville R., Englitz B., Debrégeas G., and Bormuth V. (2018). Whole-Brain Calcium Imaging during Physiological Vestibular Stimulation in Larval Zebrafish. Current Biology 28, 3723–3735.e6. 10.1016/J.CUB.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunn T.W., Gebhardt C., Naumann E.A., Riegler C., Ahrens M.B., Engert F., and Del Bene F. (2016). Neural Circuits Underlying Visually Evoked Escapes in Larval Zebrafish. Neuron 8S, 613– 628. 10.1016/j.neuron.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marquez-Legorreta E., Constantin L., Piber M., Favre-Bulle I.A., Taylor M.A., Blevins A.S., Giacomotto J., Bassett D.S., Vanwalleghem G.C., and Scott E.K. (2022). Brain-wide visual habituation networks in wild type and fmr1 zebrafish. Nat Commun 13, 895. 10.1038/s41467-022-28299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Constantin L., Poulsen R.E., Scholz L.A., Favre-Bulle I.A., Taylor M.A., Sun B., Goodhill G.J., Vanwalleghem G.C., and Scott E.K. (2020). Altered brain-wide auditory networks in a zebrafish model of fragile X syndrome. BMC Biol 18, 125. 10.1186/s12915-020-00857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zerbi V., Pagani M., Markicevic M., Matteoli M., Pozzi D., Fagiolini M., Bozzi Y., Galbusera A., Scattoni M.L., Provenzano G., et al. (2021). Brain mapping across 16 autism mouse models reveals a spectrum of functional connectivity subtypes. Mol Psychiatry 2C, 7610–7620. 10.1038/s41380-021-01245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellegood J., Anagnostou E., Babineau B.A., Crawley J.N., Lin L., Genestine M., DiCicco-Bloom E., Lai J.K.Y., Foster J.A., Peñagarikano O., et al. (2015). Clustering autism: using neuroanatomical differences in 26 mouse models to gain insight into the heterogeneity. Mol Psychiatry 20, 118–125. 10.1038/mp.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts A.C., Reichl J., Song M.Y., Dearinger A.D., Moridzadeh N., Lu E.D., Pearce K., Esdin J., and Glanzman D.L. (2011). Habituation of the C-Start Response in Larval Zebrafish Exhibits Several Distinct Phases and Sensitivity to NMDA Receptor Blockade. Plos One C. https://doi.org/ARTN e29132 10.1371/journal.pone.0029132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolman M.A., Jain R.A., Liss L., and Granato M. (2011). Chemical modulation of memory formation in larval zebrafish. Proceedings of the National Academy of Sciences of the United States of America 108, 15468–15473. 10.1073/pnas.1107156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts A.C., Bill B.R., and Glanzman D.L. (2013). Learning and memory in zebrafish larvae. Frontiers in Neural Circuits 7. https://doi.org/Artn 126 10.3389/Fncir.2013.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pantoja C., Hoagland A., Carroll E.C., Karalis V., Conner A., and Isacoff E.Y. (2016). Neuromodulatory Regulation of Behavioral Individuality in Zebrafish. Neuron S1, 587–601. 10.1016/j.neuron.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson J.C., Shoenhard H., and Granato M. (2023). Integration of cooperative and opposing molecular programs drives learning-associated behavioral plasticity. PLOS Genetics 1S, e1010650. 10.1371/journal.pgen.1010650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Png W.Y., Tang P.Y., Ogawa S., Parhar I., and Mok S.Y. (2021). Startle Habituation: A Tool for Assessing Information Processing Deficits in Zebrafish Model of Schizophrenia. Sains Malaysiana 50, 201–206. 10.17576/JSM-2021-5001-20. [DOI] [Google Scholar]
- 41.Saunders J.A., Tatard-Leitman V.M., Suh J., Billingslea E.N., Roberts T.P., and Siegel S.J. (2013). Knockout of NMDA Receptors in Parvalbumin Interneurons Recreates Autism-Like Phenotypes. Autism Research C, 69–77. 10.1002/aur.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gandal M.J., Anderson R.L., Billingslea E.N., Carlson G.C., Roberts T.P.L., and Siegel S.J. (2012). Mice with reduced NMDA receptor expression: more consistent with autism than schizophrenia? Genes, Brain and Behavior 11, 740–750. 10.1111/j.1601-183X.2012.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andalman A.S., Burns V.M., Lovett-Barron M., Broxton M., Poole B., Yang S.J., Grosenick L., Lerner T.N., Chen R., Benster T., et al. (2019). Neuronal Dynamics Regulating Brain and Behavioral State Transitions. Cell 177, 970–985. 10.1016/j.cell.2019.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDiarmid T.A., Yu A.J., and Rankin C.H. (2019). Habituation Is More Than Learning to Ignore: Multiple Mechanisms Serve to Facilitate Shifts in Behavioral Strategy. BioEssays 41, 1900077. 10.1002/BIES.201900077. [DOI] [PubMed] [Google Scholar]
- 45.Zweier C., de Jong E.K., Zweier M., Orrico A., Ousager L.B., Collins A.L., Bijlsma E.K., Oortveld M.A.W., Ekici A.B., Reis A., et al. (2009). CNTNAP2 and NRXN1 are mutated in autosomal-recessive Pitt-Hopkins-like mental retardation and determine the level of a common synaptic protein in Drosophila. Am J Hum Genet 85, 655–666. 10.1016/j.ajhg.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen T.W., Wardill T.J., Sun Y., Pulver S.R., Renninger S.L., Baohan A., Schreiter E.R., Kerr R.A., Orger M.B., Jayaraman V., et al. (2013). Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 4SS, 295–300. 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kroll F., Powell G.T., Ghosh M., Gestri G., Antinucci P., Hearn T.J., Tunbak H., Lim S., Dennis H.W., Fernandez J.M., et al. (2021). A simple and effective F0 knockout method for rapid screening of behaviour and other complex phenotypes. Elife 10, e59683. 10.7554/eLife.59683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffman E.J., Turner K.J., Fernandez J.M., Cifuentes D., Ghosh M., Ijaz S., Jain R.A., Kubo F., Bill B.R., Baier H., et al. (2016). Estrogens Suppress a Behavioral Phenotype in Zebrafish Mutants of the Autism Risk Gene, CNTNAP2. Neuron 8S, 725–733. 10.1016/j.neuron.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathis A., Mamidanna P., Cury K.M., Abe T., Murthy V.N., Mathis M.W., and Bethge M. (2018). DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci 21, 1281–1289. 10.1038/s41593-018-0209-y. [DOI] [PubMed] [Google Scholar]
- 50.Taylor M.A., Vanwalleghem G.C., Favre-Bulle I.A., and Scott E.K. (2018). Diffuse light-sheet microscopy for stripe-free calcium imaging of neural populations. Journal of Biophotonics 11. 10.1002/jbio.201800088. [DOI] [PubMed] [Google Scholar]
- 51.Favre-Bulle I.A., Vanwalleghem G., Taylor M.A., Rubinsztein-Dunlop H., and Scott E.K. (2018). Cellular-Resolution Imaging of Vestibular Processing across the Larval Zebrafish Brain. Current Biology 28, 3711-+. 10.1016/j.cub.2018.09.060. [DOI] [PubMed] [Google Scholar]
- 52.Edelstein A., Amodaj N., Hoover K., Vale R., and Stuurman N. (2010). Computer control of microscopes using µManager. Curr Protoc Mol Biol Chapter 14, Unit14.20. 10.1002/0471142727.mb1420s92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pachitariu M., Stringer C., Dipoppa M., Schröder S., Rossi L.F., Dalgleish H., Carandini M., and Harris K. (2016). Suite2p: beyond 10,000 neurons with standard two-photon microscopy. bioRxiv, 061507. 10.1101/061507. [DOI] [Google Scholar]
- 54.Avants B.B., Epstein C.L., Grossman M., and Gee J.C. (2008). Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis 12, 26–41. 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Randlett O., Wee C.L., Naumann E.A., Nnaemeka O., Schoppik D., Fitzgerald J.E., Portugues R., Lacoste A.M.B., Riegler C., Engert F., et al. (2015). Whole-brain activity mapping onto a zebrafish brain atlas. Nature Methods 12, 1039–1046. 10.1038/Nmeth.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rubinov M., and Sporns O. (2010). Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 52, 1059–1069. 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Aarts E., Verhage M., Veenvliet J.V., Dolan C.V., and van der Sluis S. (2014). A solution to dependency: using multilevel analysis to accommodate nested data. Nat Neurosci 17, 491–496. 10.1038/nn.3648. [DOI] [PubMed] [Google Scholar]
- 58.Wilde M., Poulsen R., Qin W., Arnold J., Favre-Bulle I.A., Mattingley J.B., Scott E.K., and Stednitz S.J. (2024). Evidence for auditory stimulus-specific adaptation but not deviance detection in larval zebrafish brains. Preprint at bioRxiv, 10.1101/2024.06.14.597058. [DOI] [Google Scholar]
- 59.Mu Y., Bennett D.V., Rubinov M., Narayan S., Yang C.T., Tanimoto M., Mensh B.D., Looger L.L., and Ahrens M.B. (2019). Glia Accumulate Evidence that Actions Are Futile and Suppress Unsuccessful Behavior. Cell 178, 27–43.e19. 10.1016/J.CELL.2019.05.050. [DOI] [PubMed] [Google Scholar]
- 60.Whitfield T.T., Riley B.B., Chiang M.-Y., and Phillips B. (2002). Development of the zebrafish inner ear. Developmental Dynamics 223, 427–458. 10.1002/dvdy.10073. [DOI] [PubMed] [Google Scholar]
- 61.Baeza-Loya S., and Raible D.W. (2023). Vestibular physiology and function in zebrafish. Front. Cell Dev. Biol. 11. 10.3389/fcell.2023.1172933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fay R.R., and Edds-Walton P.L. (2008). Structures and Functions of the Auditory Nervous System ofFishes. In Fish Bioacoustics: With 81 Illustrations, Webb J. F., Fay R. R., and Popper A. N., eds. (Springer; ), pp. 49–97. 10.1007/978-0-387-73029-5_3. [DOI] [Google Scholar]
- 63.Vanwalleghem G., Heap L.A., and Scott E.K. (2017). A profile of auditory-responsive neurons in the larval zebrafish brain. Journal of Comparative Neurology 525, 3031–3043. 10.1002/CNE.24258. [DOI] [PubMed] [Google Scholar]
- 64.Pose-Méndez S., Schramm P., Valishetti K., and Köster R.W. (2023). Development, circuitry, and function of the zebrafish cerebellum. Cell Mol Life Sci 80, 227. 10.1007/s00018-023-04879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bae Y.-K., Kani S., Shimizu T., Tanabe K., Nojima H., Kimura Y., Higashijima S., and Hibi M. (2009). Anatomy of zebrafish cerebellum and screen for mutations affecting its development. Developmental Biology 330, 406–426. 10.1016/j.ydbio.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 66.Folgueira M., Riva-Mendoza S., Ferreño-Galmán N., Castro A., Bianco I.H., Anadón R., and Yáñez J. (2020). Anatomy and Connectivity of the Torus Longitudinalis of the Adult Zebrafish. Frontiers in Neural Circuits 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knogler L.D., Kist A.M., and Portugues R. (2019). Motor context dominates output from purkinje cell functional regions during reflexive visuomotor behaviours. eLife 8, e42138. 10.7554/eLife.42138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knogler L.D., Markov D.A., Dragomir E.I., Štih V., and Portugues R. (2017). Sensorimotor Representations in Cerebellar Granule Cells in Larval Zebrafish Are Dense, Spatially Organized, and Non-temporally Patterned. Current Biology 27, 1288–1302. 10.1016/j.cub.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 69.Matsuda K., Yoshida M., Kawakami K., Hibi M., and Shimizu T. (2017). Granule cells control recovery from classical conditioned fear responses in the zebrafish cerebellum. Sci Rep 7, 11865. 10.1038/s41598-017-10794-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhandiwad A.A., Chu N.C., Semenova S.A., Holmes G.A., and Burgess H.A. (2022). A cerebellar-prepontine circuit for tonic immobility triggered by an inescapable threat. Science Advances 8, eabo0549. 10.1126/sciadv.abo0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lovelace J.W., Wen T.H., Reinhard S., Hsu M.S., Sidhu H., Ethell I.M., Binder D.K., and Razak K.A. (2016). Matrix metalloproteinase-9 deletion rescues auditory evoked potential habituation deficit in a mouse model of Fragile X Syndrome. Neurobiology of Disease 8S, 126–135. 10.1016/j.nbd.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen F., Chen S., Liu S., Zhang C., and Peng G. (2015). Effects of lorazepam and WAY-200070 in larval zebrafish light/dark choice test. Neuropharmacology S5, 226–233. 10.1016/j.neuropharm.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 73.Agetsuma M., Aizawa H., Aoki T., Nakayama R., Takahoko M., Goto M., Sassa T., Amo R., Shiraki T., Kawakami K., et al. (2010). The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat Neurosci 13, 1354–1356. 10.1038/nn.2654. [DOI] [PubMed] [Google Scholar]
- 74.Duboué E.R., Hong E., Eldred K.C., and Halpern M.E. (2017). Left Habenular Activity Attenuates Fear Responses in Larval Zebrafish. Curr Biol 27, 2154–2162.e3. 10.1016/j.cub.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee A., Mathuru A.S., Teh C., Kibat C., Korzh V., Penney T.B., and Jesuthasan S. (2010). The Habenula Prevents Helpless Behavior in Larval Zebrafish. Current Biology 20, 2211–2216. 10.1016/j.cub.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 76.Gorman K.M., Peters C.H., Lynch B., Jones L., Bassett D.S., King M.D., Ruben P.C., and Rosch R.E. (2021). Persistent sodium currents in SCN1A developmental and degenerative epileptic dyskinetic encephalopathy. Brain Communications. 10.1093/BRAINCOMMS/FCAB235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Santistevan N.J., Ford C.T., Gilsdorf C.S., and Grinblat Y. Behavioral and transcriptomic analyses of mecp2 function in zebrafish. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics n/a, e32981. 10.1002/ajmg.b.32981. [DOI] [PubMed] [Google Scholar]
- 78.Favre-Bulle I.A., Taylor M.A., Marquez-Legorreta E., Vanwalleghem G., Poulsen R.E., Rubinsztein-Dunlop H., and Scott E.K. (2020). Sound generation in zebrafish with Bio-Opto-Acoustics (BOA). bioRxiv, 2020.06.09.143362. 10.1101/2020.06.09.143362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scott K.E., Schormans A.L., Pacoli K.Y., De Oliveira C., Allman B.L., and Schmid S. (2018). Altered auditory processing, filtering, and reactivity in the cntnap2 knock-out rat model for neurodevelopmental disorders. Journal of Neuroscience 38, 8588–8604. 10.1523/JNEUROSCI.0759-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Möhrle D., Wang W., Whitehead S.N., and Schmid S. (2021). GABAB Receptor Agonist R-Baclofen Reverses Altered Auditory Reactivity and Filtering in the Cntnap2 Knock-Out Rat. Frontiers in Integrative Neuroscience 0, 25. 10.3389/FNINT.2021.710593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lovett-Barron M., Andalman A.S., Allen W.E., Vesuna S., Kauvar I., Burns V.M., and Deisseroth K. (2017). Ancestral Circuits for the Coordinated Modulation of Brain State. Cell 171, 1411-+. 10.1016/j.cell.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stednitz S.J., McDermott E.M., Ncube D., Tallafuss A., Eisen J.S., and Washbourne P. (2018). Forebrain Control of Behaviorally Driven Social Orienting in Zebrafish. Curr Biol 28, 2445–2451.e3. 10.1016/j.cub.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mueller T., Dong Z., Berberoglu M.A., and Guo S. (2011). The dorsal pallium in zebrafish, Danio rerio (Cyprinidae, Teleostei). Brain Res 1381, 95–105. 10.1016/j.brainres.2010.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.