Abstract
Background:
Protein-tyrosine-phosphatase CD45 is exclusively expressed in all nucleated cells of the hematopoietic system but is rarely expressed in endothelial cells. Interestingly, our recent study indicated that activation of the endogenous CD45 promoter in human endothelial colony forming cells (ECFCs) induced expression of multiple EndoMT marker genes. However, the detailed molecular mechanisms underlying CD45 that drive EndoMT and the therapeutic potential of manipulation of CD45 expression in atherosclerosis are entirely unknown.
Method:
We generated a tamoxifen-inducible EC-specific CD45 deficient mouse strain (EC-iCD45KO) in an ApoE-deficient (ApoE−/−) background and fed with a Western diet (C57BL/6) for atherosclerosis and molecular analyses. We isolated and enriched mouse aortic endothelial cells with CD31 beads to perform single-cell RNA sequencing. Biomedical, cellular, and molecular approaches were utilized to investigate the role of endothelial CD45-specific deletion in the prevention of EndoMT in ApoE−/− model of atherosclerosis.
Results:
Single-cell RNA sequencing revealed that loss of endothelial CD45 inhibits EndoMT marker expression and transforming growth factor-β signaling in atherosclerotic mice. which is associated with the reductions of lesions in the ApoE−/− mouse model. Mechanistically, the loss of endothelial cell CD45 results in increased KLF2 expression, which inhibits transforming growth factor-β signaling and EndoMT. Consistently, endothelial CD45 deficient mice showed reduced lesion development, plaque macrophages, and expression of cell adhesion molecules when compared to ApoE−/− controls.
Conclusions:
These findings demonstrate that the loss of endothelial CD45 protects against EndoMT-driven atherosclerosis, promoting KLF2 expression while inhibiting TGFβ signaling and EndoMT markers. Thus, targeting endothelial CD45 may be a novel therapeutic strategy for EndoMT and atherosclerosis.
Introduction:
Atherosclerotic cardiovascular disease is the leading cause of morbidity and mortality in the United States and most developed countries1,2. It is generally believed that endothelial dysfunction caused by the cholesterol and oxidized phospholipids in lipoproteins (ox-LDL) is one of the major initiating events3. Trapped lipoproteins activate endothelial cells and vascular smooth muscle cells to increase their expression of adhesion molecules and production of chemokines4 5. In turn, this leads to the recruitment of monocytes/lymphocytes into the arterial vessel wall6. Lesion progression involves the migration and proliferation of resident smooth muscle cells (SMCs)2, extracellular matrix deposition7, and lipid or necrotic core formation8. In advanced stages of atherosclerosis, increased endothelial dysfunction has been identified, and advanced plaque ruptures associated with thin, collagen-poor fibrous caps9,10.
Endothelial-to-mesenchymal transition (EndoMT) is the process whereby an endothelial cell undergoes a series of molecular events that lead to progressively losing endothelial-specific markers and gaining mesenchymal phenotypes11. EndoMT plays a fundamental role in forming the cardiac valves of the developing heart, and mounting evidence indicates that EndoMT is involved in atherosclerosis12–14. ECs can be distinguished by the expression of cell–cell adhesion molecules, including platelet/EC adhesion molecule-1 (CD31/PECAM-1)15, vascular endothelial (VE)-cadherin16, tyrosine kinase with immunoglobulin-like and epidermal growth factor (EGF)-like domains 1 (TIE1), and TIE217,18. During EndoMT, endothelial cells gain mesenchymal markers such as α-SMA19, collagen20, and SM2221.
TGFβ signaling has been identified as the central player in driving EndoMT during atheroma progression 22, but the processes leading to activation of its signaling remain poorly defined. TGFβ-induced gene expression in ECs during EndoMT revealed induction of expression of a number of pro-inflammatory chemokines and cytokines and their receptors22 , leucocyte adhesion molecules (such as ICAM-1 and VCAM-1)23,24, pro-inflammatory extracellular matrix (ECM) component long linked to inflammation7. Endothelial phenotype and function can be maintained by FGFR signaling pathways that counter TGFβ-driven EndoMT during atherosclerosis 25,26. A reduction in endothelial FGF-mediated signaling 27,28 or the primary FGF receptor in the endothelium (FGF receptor 1 [FGFR1]) activates TGFβ signaling, leading to EndoMT29. FGFR1’s and transcriptional factor KLF2 expression in ECs is affected by certain inflammatory stimuli that induce a profound reduction in their expression30.
Over the past few decades, findings indicated that CD45, a receptor-type protein tyrosine phosphatase, is exclusively expressed in all nucleated cells of the hematopoietic system except mature red blood cells and platelets. CD45, receptor type, C (PTPRC), was the prototypic receptor-like protein tyrosine phosphatase (PTP) and an essential regulator of signal transduction pathways in lymphocyte cells since it dephosphorylates, activating phospho-tyrosine sites and thereby dampening signaling pathways31. CD45 should rarely be expressed on endothelial cells. However, recent studies indicated that CD45 is expressed in umbilical vein endothelial cultures when stimulated with IL-132, and lung endothelial cells expressed both CD31 and CD45 in a pulmonary arterial hypertension rat model33. Importantly, we recently reported that activation of the endogenous CD45 promoter in human endothelial colony forming cells induced expression of EndoMT marker genes34 Also, the CD45 phosphatase inhibitor blocked EndoMT activities in TGFβ1-treated ovine mitral valve endothelial cells35. However, whether endothelial CD45-specific deletion can prevent EndoMT-driven atherosclerosis remains entirely unknown. Thus, we propose that CD45 may also mediate EndoMT in atherosclerotic mouse models through new mechanisms, highlighting that endothelial CD45 may be a new drug target for preventing or treating atherosclerosis.
Since we have reported that activation of the endogenous CD45 promoter in human endothelial colony forming cells induced migration, a hallmark of EndoMT, increased collagen gel contraction, a hallmark of mesenchymal cells, and decreased cell-cell barrier integrity, indicating reduced endothelial function34; it brings us the interest to uncover molecular mechanisms that CD45 drives EndoMT and promotes atherosclerotic progression. The principal objective of this study was to determine whether endothelial CD45-specific deletion could prevent EndoMT in atherosclerosis. To investigate the role of endothelial CD45 in EndoMT during atherosclerosis, we generated a tamoxifen-inducible EC-specific CD45-deficient mouse strain (EC-iCD45KO) into an atherosclerotic (ApoE−/−) background (C57BL/6) and fed these mice with the western diet. Second, we tried single-cell RNA sequencing and biomedical methods to determine the molecular mechanisms behind CD45-regulation of EndoMT involved in endothelial CD45-mediated TGFβ1–3 and FGFR1 and its in vivo relevance. Our findings demonstrate that the loss of endothelial CD45 protects against EndoMT-driven atherosclerosis, promoting KLF2 expression while inhibiting TGFβ signaling and EndoMT markers.
Materials and Methods:
An expanded Materials and Methods section is available in the Supplement Materials and Methods and Supplement Table 1.
Genetic Mouse Models and Aortic Lesion Evaluation
In this study, all animal procedures were performed in compliance with institutional guidelines, and mouse protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Boston Children’s Hospital, MA, USA. Both male and female mice were used. C57BL/6 mice (stock #00664), ApoE−/− mice (stock #002052), EC-specific Cre deleter mice (iCDH5CreERT2). Mice with tamoxifen-inducible deletion of endothelial CD45 on an ApoE−/− background (EC-iCD45KO/ApoE−/−) (7 injections every other day)36. For controls, wild-type C57BL/6 mice bearing iCDH5 CreERT2 (denoted as WT). These mice crossed to the ApoE−/− (C57BL/6) background to obtain control ApoE−/−/WT (ApoE−/−) mice. WT and EC-iCD45KO/ApoE−/− were fed the western diet (WD) for 12 to 16 weeks beginning at 8 months age. We then evaluated atherosclerosis lesion size at the aortic sinus, abdominal aorta, and face entire aorta. The mouse heart and aorta were perfused, dissected, and subjected to quantification of atherosclerosis as previously described37.
Cell culture
To isolate murine aortic endothelial cells (MAECs), aortas were collected and washed twice with PBS at 4 °C and then carefully stripped of fat and connective tissue. Aortas were cut into 3 mm long sections, and segments were put on the Matrigel-coated (Corning) plate with endothelial cells medium38. After 4 days, vascular networks were visible under the light microscope and tissue segments were removed. ECs were detached and then cultured in fresh endothelial cells medium. The identity of isolated ECs was confirmed by immunofluorescent staining using EC markers CD31. A full list of reagents including antibodies and primers is included in the supplementary information. Cultured MAECs were treated with 5 μM tamoxifen for 3 days to induce the deletion of CD45 gene from EC-iCD45KO/ApoE−/−; iCDH5-Cre mice, MAECs isolated from ApoE−/− mice as controls. Cells were treated with 100 μg/mL ox-LDL or 10ng/mL TGFβ1 at the indicated time while maintaining 2 μM tamoxifen in the medium.
Aortic sample preparation for scRNA-seq
Aortic cells were isolated as described before36. Mouse aortas were cut into fine pieces and placed in 5 mL of enzyme solution at 37° C. (Collected the supernatant every 10 min until all the fine pieces were digested). (Enzyme solution: 5 mL of DMEM, Collagenase type I: 5mg/mL (Gibco), Collagenase type IV:5mg/mL (Gibco), Liberase :5mg/mL (Roche). This solution was then filtered with a 40-μm strainer, centrifuged (400g for 5 minutes), and the cell pellet was suspended in 90 μL of buffer containing 0.5% BSA and 2 mmol/L EDTA in PBS. Then, 20 μL of CD31 microbeads (Miltenyi Biotec) was added to the suspension at 4° C for 15 minutes. Microbeads were passed through an LS column (Miltenyi Biotec). Cells were processed for scRNA-seq with the 10x Genomics platform, and sequencing was performed by MedGenome (Figure S1).
Data Analysis of scRNA-seq
The raw scRNA-seq data were processed using Cell Ranger (version 6.1.2) (10x Genomics). We used the Cell Ranger and R package Seurat version 4.0.2 to perform scRNA-seq analysis. In brief, we generated a normalized gene expression matrix harboring between ApoE and EC-iCD45KO/ApoE−/− mice. The high-quality data after these filtering steps was then used at additional processing steps including log normalization data scaling, principal component analysis (PCA), cell clustering, and UMAP visualization. Next, marker genes in each cell cluster were used to annotate cell types based on known marker genes in the database and literature. The marker gene expressions were visualized by DotPlot and VlnPlot functions. Pathway enrichment analysis for group comparison was performed with R package ReactomeGSA. The trajectory inference for single-cell data from EndoMT models was performed with the R package Slingshot39.
Hematoxylin and Eosin staining
Cryostat sections of mouse aortic root, abdominal aorta, and BCA were washed in PBS for 5 minutes, then fixed in 4% paraformaldehyde for 15 minutes. Next, slides were stained with hematoxylin for 3 minutes, followed by running tap water washes for 10 minutes. Slices were then dipped in eosin working solution for 30 seconds, quickly rinsed with tap water, dehydrated using graded ethanol (95% and 100% ethanol), followed by transparentizing by xylene. Slices were mounted in synthetic resin40.
Flow cytometry analysis
Flow cytometry analysis of aortic cells from normal diet ApoE−/− and WD diet ApoE−/− (16–20 weeks) was isolated as described above. Cell pellets were resuspended in FACS buffer (2%HBSS, 0.004%FBS, 2mM EDTA in PBS) at 4°C for 30 min to block unspecific binding of antibodies to Fc receptors. Cells were incubated for antibodies for 30 min; flow cytometry analysis was performed using the BD instrument.
Oil Red O staining
Cryostat sections:
cryostat sections were fixed in 4% paraformaldehyde. Slides were washed with PBS, and rinsed with 60% isopropanol, followed by staining with freshly prepared 0.5% Oil red O solution in isopropanol for 20 minutes at 37°C. Slices were then put in 60% isopropanol for 30 sec, followed by 3 washes in water, and then dried slides mounted with glycerin. Imaging was processed with a Zeiss LSM880 microscope and analyzed with ZEN-Lite software and NIH ImageJ software.
En face Oil Red O Staining:
Whole aortae were dissected symmetrically, pinned to parafilm to allow the en face exposed and fixed in formalin for 24hrs. The aortae were washed in PBS 3 times and rinsed in 100% propylene glycol, followed by staining with 0.5% Oil Red O solution in propylene glycol for 20 mins at 65°C. The samples were then put in 85% propylene glycol for 2 mins, followed by three washes in DD Water and then dried slides. The image was performed using a Nikon SMZ1500 stereomicroscope, SPOT Insight 2Mp Firewire digital camera, and SPOT Software 5.1.
Van Gieson’s staining
Sections were fixed in 10% buffered formalin for 15 min and washed in water. Slides were stained with 3 min in Van Gieson’s solution, dehydrated in 95% alcohol and 100% alcohol two times. Then, slides were cleared in xylene two times and mounted with Permount. Staining results were presented as: Collagen fibers–Red and Other tissue elements–Yellow.
RNA isolation, quantitative real-time PCR
Total RNA was extracted from primary MAECs using the RNeasy kits (Qiagen), respectively. On-column DNA digestion (Qiagen) was carried out during RNA extraction. For the synthesis of the first strand of cDNA, 1 μg of total RNA after DNase treatment was reverse transcribed using cDNA Synthesis Kit (Vazyme). Quantitative real-time PCR was performed on a CFX96 detection system (StepOnePlus Real-Time PCR Systems) with SYBR Green reagents (Vazyme). All PCR primers’ sequences are presented in online supplemental table 1.
Evaluation of EndoMT Markers by Immunostaining In Vivo and In Vitro
Samples from ApoE−/− and EC-iCD45KO/ ApoE−/− were evaluated by co-staining CD31 (or VE-cadherin) with α-SMA, KLF2, and ICAM-1. EndoMT is expressed by the overlapping percentile of CD31 (or VE-cadherin) with other markers. For in vitro EndoMT marker staining, mouse aortic EC (MAEC) cultures were treated with Ox-LDL (100 μg/mL) or TGFβ1 (10 ng/mL) on the indicated days.
Immunofluorescence staining
Human Tissue Paraffin Sections:
Human healthy control and diseased aortic arch samples from atherosclerosis patients were purchased from Maine Medical Center Biobank (Sample ID: RA06–1040A1)39. The paraffin sections were de-paraffinized and performed antigen retrieval to unmask the antigenic epitope with 10 mM Sodium Citrate, pH 6.0, with 0.5% Tween 20 at 90 °C for 30 minutes. Allow the slides to cool before proceeding with staining procedure40. Immunofluorescence staining of the slides was performed with the standard protocol described below. Samples were blocked in PBS with donkey serum, 3% BSA, and 0.3% triton X-100 at 4°C overnight and incubated with primary antibodies VE-cadherin with CD45 at 4°C overnight41,42. The sections were washed three times, and respective secondary antibodies conjugated to fluorescent labels (Alexa Flour 594, 488, or 647) were added for 2 h at room temperature. The sections were mounted with Fluoroshield mounting medium containing DAPI (1:100).
Mouse aortic root and BCA cryosections:
Sections were fixed by 4% paraformaldehyde for 15 min at room temperature and blocked in PBS solution containing 3% donkey and/or 10% goat serum, 3% BSA, and 0.3% Triton X-100 for 1 hour41,42. Samples were then incubated with primary antibody at 4°C overnight, followed by incubation with the respective secondary antibodies conjugated to fluorescent labels (Alexa Flour 594, 488, or 647; 1:200) for 2 h at room temperature. The sections were mounted with Fluoroshield (R & D Systems) mounting medium containing DAPI (1:100).
En Face Preparation and Immunofluorescence Labeling
En face immunofluorescence was performed as described 43. Thoracic aorta were isolated and further fixed in 2% paraformaldehyde solution at 4 °C for 1.5 hours. Fixed tissues were permeabilized in a permeabilization buffer (PBS containing 0.2% Triton X-100) for 1 hour at room temperature and subsequently incubated with a blocking buffer (5.5% FBS in permeabilization buffer) for 1 hour at room temperature. Primary antibodies were diluted in a staining buffer (2.75% FBS in permeabilization buffer) and incubated with tissue for 16 hours at 4 °C with gentle agitation. Tissues were subsequently washed 3× in the permeabilization buffer in 30-minute intervals. Secondary antibodies (conjugated with Alexa Fluor 647, Alexa Fluor 594, or Alexa Fluor 488) diluted as 1:200 working solution (10 μg/mL) in the staining buffer were added after the third wash and incubated at room temperature for 3 hours. Tissues were subsequently washed 3× in the permeabilization buffer and 1× in PBS with 30 minutes for each step. After the PBS wash, vascular tissues were bisected along the direction of flow and mounted with Fluoroshield (R & D Systems). Images were obtained using Zeiss LSM 880 with the manufacturer’s software (Zen Black for microscope operation and Zen Blue for image export and analysis).
Data Analysis:
All data were analyzed by Prism 8 (GraphPad Software Inc). Data are expressed as the mean ± SEM. The means of two groups were compared using Student’s t-test (unpaired, two-tailed), and one-way analysis of variance was used for comparison of more than two groups with P < 0.05 considered to be statistically significant. Unless otherwise indicated, all experiments were repeated at least three times.
Results:
Assess CD45 expression in atherosclerotic lesions
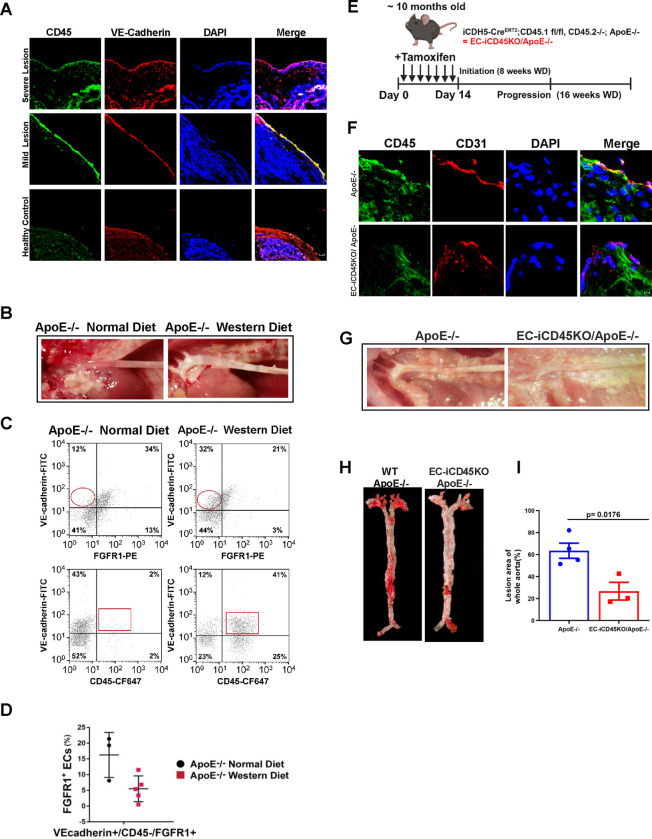
To confirm the localization of CD45 protein in the mouse aortic endothelial cells, we performed an immunofluorescence study on both samples in the patient atherosclerotic arch sample and mouse aortic root. We found that CD45 co-expression with VE-cadherin in the patient’s aorta arch samples with severe and mild atherosclerotic lesions (Figure 1A); we also detected that CD45 protein expression co-expressed with CD31 in the aortic root with the atheroma of 12-week-old ApoE-null mice (Figure 1F). As ApoE−/− mice fed a normal diet also develop lesions, albeit at a slower rate compared to WD-fed ApoE−/− mice, we chose to use WT mice that were fed a normal chow as our true negative control for atherosclerosis occurrence to rule out the potential effect of high cholesterol content in WD-fed mice and lesion development in normal chow-fed ApoE−/− mice. (Figure 1B). In addition, we performed en face immunostaining of the thoracic aorta with antibodies to VE-Cadherin, CD31, DAPI, and CD45 (Figure 4E). It showed CD45 co-expression with VE-Cadherin and CD31 in wild-type ApoE-null mice but a significant reduction of CD45 expression in EC-iCD45KO ApoE-null mice. Furthermore, flow cytometry analyses of total aortic cells for VE-cadherin, FGFR1, and CD45 showed a significant reduction in VE-cadherin+/FGFR1+ ECs in WD-fed ApoE−/− mice, compared to normal diet-fed ApoE−/− mice (Figure. 1C, D). In contrast, VE-cadherin+/CD45+ ECs were greatly increased in WD-fed, ApoE−/− mice compared to normal diet-fed ApoE−/− mice (Figure. 1C), suggesting loss of FGFR1 may contribute EndoMT in lesions of WD-fed ApoE−/− mice.
Figure 1. Endothelial CD45 dependent EndoMT during atherosclerosis.
Immunostaining of aortic arch section from atherosclerosis patients with VE-cadherin (endothelial junctions, red), and CD45 protein(green) (A), and aortic root with CD31(red) and CD45 protein(green) from ApoE−/− and EC-iCD45KO/ApoE−/− mice fed with western diet (WD) for 12 weeks (F). B, Aortas from ApoE−/− mice fed a normal diet versus WD, —lesions are apparent in ApoE−/− WD-fed aortas. C, Representative flow cytometry data from ECs labeled with anti-VE-cadherin-FITC, anti-CD45-CF647, and anti-FGFR1-PE. Circles highlight increased FGFR1-ECs in ApoE−/− WD aortas. Squares indicate increased CD45 ECs in WD-fed aortas from ApoE−/− mice. D, Cytometric analysis from ApoE−/− normal diet (circles, n=3) and ApoE−/− WD (squares, n=5) mice. FGFR1+ECs are decreased (p=0.0324) in ApoE−/− WD-fed mice aortas. E, Diagram showing the timeline for the deletion induction by tamoxifen injections and atherosclerotic progression. G, Aortas from ApoE−/− mice versus EC-iCD45KO/ApoE−/− mice fed with western diet (WD) for 16 weeks (—lesions are apparent in ApoE−/−WD-fed aortas). H, En face aortas from ApoE−/− and EC-iCD45KO / ApoE−/− female mice stained with Oil Red O (16-week WD). I, Statistics for En face ORO staining (A: Scale bars = 10 μM), F: Scale bars = 5 μM).
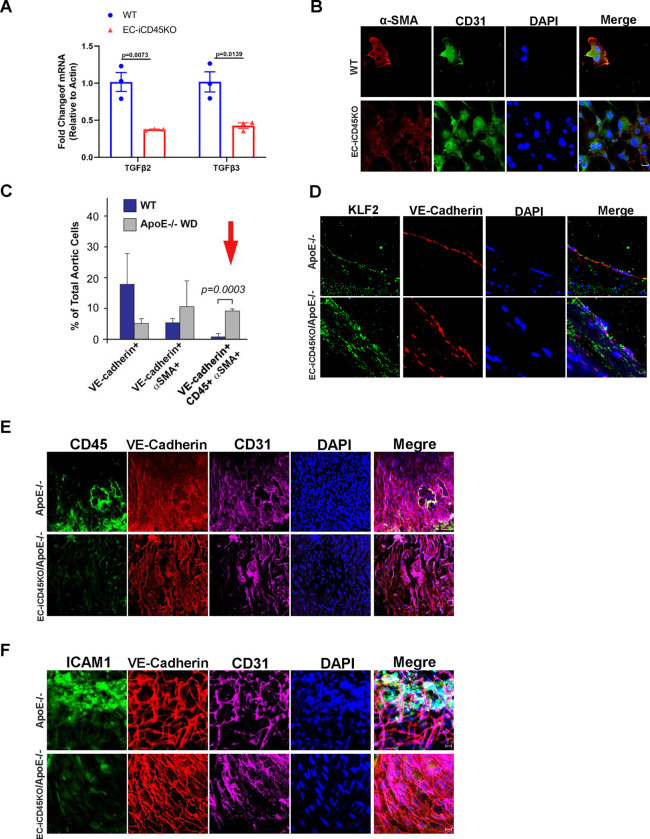
Figure 4. Endothelial CD45- deficiency inhibits EndoMT by reducing TGFβ signaling and upregulates KLF2 expression during atherosclerosis.
A, Mouse aortic endothelial cells (MAECs) of wild-type (WT/ApoE−/−) and EC-iCD45KO were treated with 5 μmol/L tamoxifen for 3 days, followed by treatment with 100 μg/mL oxidized low-density lipoprotein, and then collected cell lysis for quantitative polymerase chain reaction to detect endothelial-to-mesenchymal transition (EndoMT) markers TGFβ2 and TGFβ3(n=3). B, IF staining to detect CD31 and α-SMA. Similarly, MAECs of wild-type and EC-iCD45KO were isolated and were treated with 5 μmol/L tamoxifen for 3 days, followed by treatment with 10 ng/mL transforming growth factor-β (TGFβ1) for 4 days, with a change to fresh medium and TGFβ1 every other day. C, Aortic ECs from WT and ApoE−/− mice fed a western diet (WD) were labeled with fluorescently-conjugated antibodies to VE-cadherin, CD45, and α-SMA and then analyzed by flow cytometry. The red arrow indicates significantly more triple-positive cells in ApoE−/−WD mice, which is suggestive of a CD45+ EndoMT during atherosclerosis. D, IF staining for KLF-2 (green), VE-cadherin (red), and DAPI (blue) on the luminal surface of mouse aortic root sections. (Scale bars = 5 μM). E, En face immunostaining of the thoracic aorta was performed with antibodies to VE-Cadherin, CD31, DAPI (nuclei), and either (E) CD45 or (F) ICAM-1 (B, D Scale bars = 5 μM; E, Scale bars = 50 μM; F: Scale bars = 10 μM).
Single-Cell RNA-Seq revealed that endothelial CD45 plays a critical role in EndoMT in an atherosclerosis mouse model
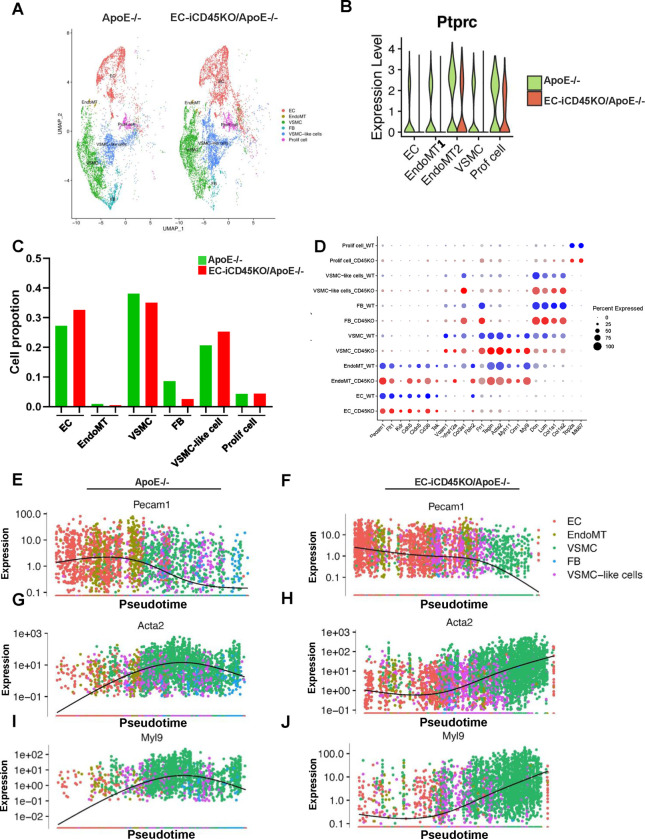
To investigate whether endothelial CD45 plays a role in EndoMT, we performed scRNA-seq analysis in the atherosclerotic ApoE−/− mouse model. ApoE−/− and EC-iCD45KO/ApoE−/− male mice were fed a WD for 16 weeks, followed by isolation of aortas, enzymatic digestion, and scRNA-seq (Figure S4). Overall, 2 individual samples in the compared groups yielded a similar number of cells for the subsequent analysis (cells for ApoE−/− and EC-iCD45KO/ApoE−/−) after processing the data quality control underlying filtering of cells with low genes and UMIs (Figure S5), as well as dead cells as described in the Methods. We confirmed the efficacy of the CD45 knockout in aortic endothelial cells (Figure 2B). Using specific cell markers, we grouped EndoMT-related cell populations ECs, EndoMT (these cells express both EC and SMC markers), VSMCs, SMCs- like cells, fibroblasts, and proliferation cells. In the clusters, the ratio of ECs is higher in the EC-iCD45KO/ApoE−/− versus ApoE−/− group, whereas the ratio of EndoMT cells, VSMCs, VSMCs- like cells, and fibroblasts are higher in ApoE−/−mice (Figure 2C), suggesting that loss of endothelial CD45 attenuated EndoMT. The ratio of EndoMT, SMCs, SMCs-like cells, and fibroblasts cells (Figure 2C) confirmed the phenotype of EC-iCD45KO/ApoE−/− mice in atherosclerosis. Cell type–specific markers were defined relative to all other cells (Figure 2D). Next, we showed the expression dynamics of cell-specific markers in pseudotime. The trajectory plot in Figure 2E–2J clearly demonstrates the gene expression of representative markers specific to ECs (Pecam1) and SMCs (Acta2 and Myl9) through the EC→EndoMT→SMC process, which is shown as normalized expression. Similarly, we used the trajectory plot tested EC (Cdh5, Cldn5, Egfl7 and Tek) and SMC markers (Myh11, Tagln and Fbln5) in ApoE−/− and EC-iCD45KO/ApoE−/− aortic cells (Figure S7).
Figure 2. scRNA-seq analysis reveals that endothelial CD45 mediated EndoMT during atherosclerosis.
A, Cell type clusters of ApoE−/− and EC-iCD45KO/ApoE−/− mice, including endothelial cells (ECs), endothelial-to-mesenchymal transition (EndoMT) cells, vascular smooth muscle cells (VSMCs), fibroblasts, VSMC-like cells, and proliferation cell types. B, Expression level of CD45 (PTPRC) among the different cell populations. C, Proportion of ECs, EndoMT, VSMCs, fibroblasts, VSMC-like cells, and proliferation cells. D, Dot plot of selected marker genes for each cell cluster. Dot size indicates the percentage of cells expressing each gene, and dot color indicates the expression level. E-J, Gene expression of representative markers specific to ECs (Pecam1) and SMCs (Acta2 and Myl9) through the EC→EndoMT→SMC process is shown as normalized expression vs UMAP_1 (also refer to Figure S7 for more marker genes).
Single-Cell RNA-Seq indicated that endothelial CD45 plays a critical role in the specific EndoMT cell populations
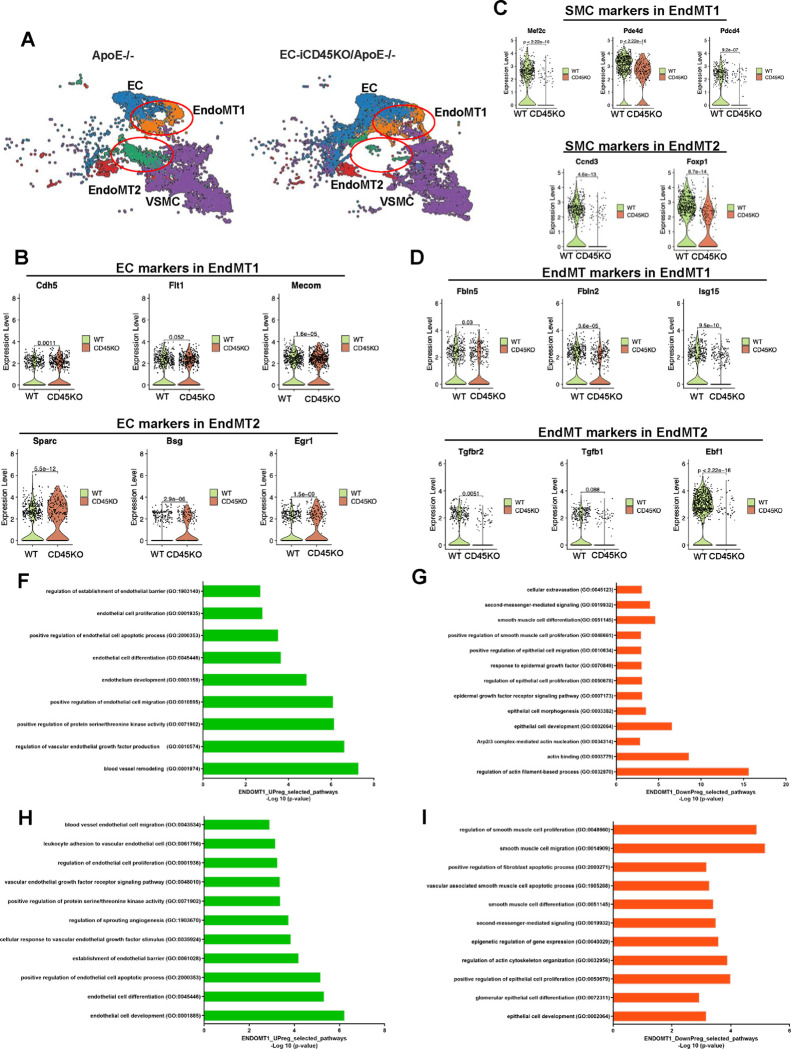
We continue to characterize EndoMT cells into two subclusters: EndoMT1 and EndoMT2 (Figure 3A). This result is supported by measuring specific EC, SMC, and EndoMT markers in the EndoMT1 and EndoMT2 clusters. The violin plots in Figure 3 (B–D) show that EC markers (Cdh5, Flt1, Mecom) increased in EC-iCD45KO/ApoE−/− aortas compared with ApoE−/− aortas, but SMC markers (Mef2c, pde4d, pdcd4) and EndoMT markers (Fbln2, Fbln5, Isg15)are downregulated in EC-iCD45KO/ApoE−/− mice in EndoMT1 subcluster. In EndoMT2 cells, EC markers (Sparc, Bsg, Egr1) increased, but SMC markers (Ccdn3 and Foxp1) and EndoMT markers (TGFβ1, TGFβ2, Ebf1 ) decreased. Pathway analysis with genes coordinately differentially expressed in ApoE−/− and EC-iCD45KO/ApoE−/− mice (Figure 3F–I). Actin-binding pathways were decreased in EndoMT1. Endothelial cell migration, proliferation, and development pathways were enriched, while smooth muscle cell proliferation, epithelial cell development pathways were decreased in both clusters EndoMT1 and 2. Similarly, we used violin plots plot to test the other EC, EndoMT and SMC markers in EndoMT 1 and 2 clusters in ApoE−/− and EC-iCD45KO/ApoE−/− aortic cells (Figure S7).
Figure 3. scRNA-seq analysis differential CD45-driven EndoMT on distinct cell populations.
A, Cell type clusters of ApoE−/− (6476 cells) and EC-ICD45KO/ApoE−/− (7021 cells) mice, including endothelial cells (ECs), endothelial-to-mesenchymal transition (EndoMT) cells, and smooth muscle cells (SMCs). EndoMT clusters were further divided into 2 subgroups: EndoMT1 and EndoMT2. Violin plots of EC (B), SMC (C), and EndoMT(D) markers differentially expressed in ApoE−/− and EC-ICD45KO/ApoE−/− mice. In the EndoMT1 subgroups of EC marker–positive cells (Cdh5, Flt1, Mecom), SMC marker–positive cells (Mef2c, Pde4d, Pdcd4) and EndoMT marker-positive cells (Fbln2, Fbln5, Lsg15) were analyzed. In the EndoMT2 subgroups of EC marker–positive cells (Sparc, Bsg, Egr1), SMC marker–positive cells (Ccnd3, Foxop1) and EndoMT marker- positive cells (TGFβ1, TGFβ2, Ebf1) were analyzed. Pathway analysis with genes coordinately differentially expressed in ApoE−/− and EC-ICD45KO/ApoE− mice (F-I).
Loss of CD45 in ECs attenuates the expression of EndoMT markers
We measured EndoMT markers in MAECs from WT and EC-iCD45KO after treating them with 100 μg/mL of oxidized low-density lipoprotein for 24 hours. It showed that endothelial CD45 knock-out attenuated the TGFβ2–3 mRNA expression in MAECs (Figure. 4A). Consistently, MAECs from WT and EC-iCD45KO mice were treated with (TGFβ1 10ng/mL) for 4 days. We found that WT expressed α-SMA, but endothelial CD45 deletion leads to a reduction of α-SMA in MAECs (Figure. 4B). In addition, we applied the triple-label flow cytometric method we developed for mitral valve analyses to total aortic ECs from age and gender-matched wild-type (WT) and ApoE−/− mice fed a WD for 16–20 weeks (Figure. 4C). (arrow) shows CD45+/VE-cadherin+/αSMA+ cells were significantly increased from 0.8 ± 1.0% in WT mice (n=3) to 9.2 ± 0.7% in ApoE−/− WD-fed mice (n=3) (p = 0.0003). The presence of α-SMA+ in ApoE−/− WD-fed mice indicates CD45+ ECs underwent an EndoMT in an aortic atherosclerotic lesion. Furthermore, it showed a significant upregulation of KLF2 in aortic root from EC-iCD45KO/ApoE−/− mice fed with WD 12 weeks, compared to normal CD45 wild-type ApoE−/− mice (Figure. 4D).
Endothelial Loss of CD45 Prevents Inflammation and Atherosclerosis
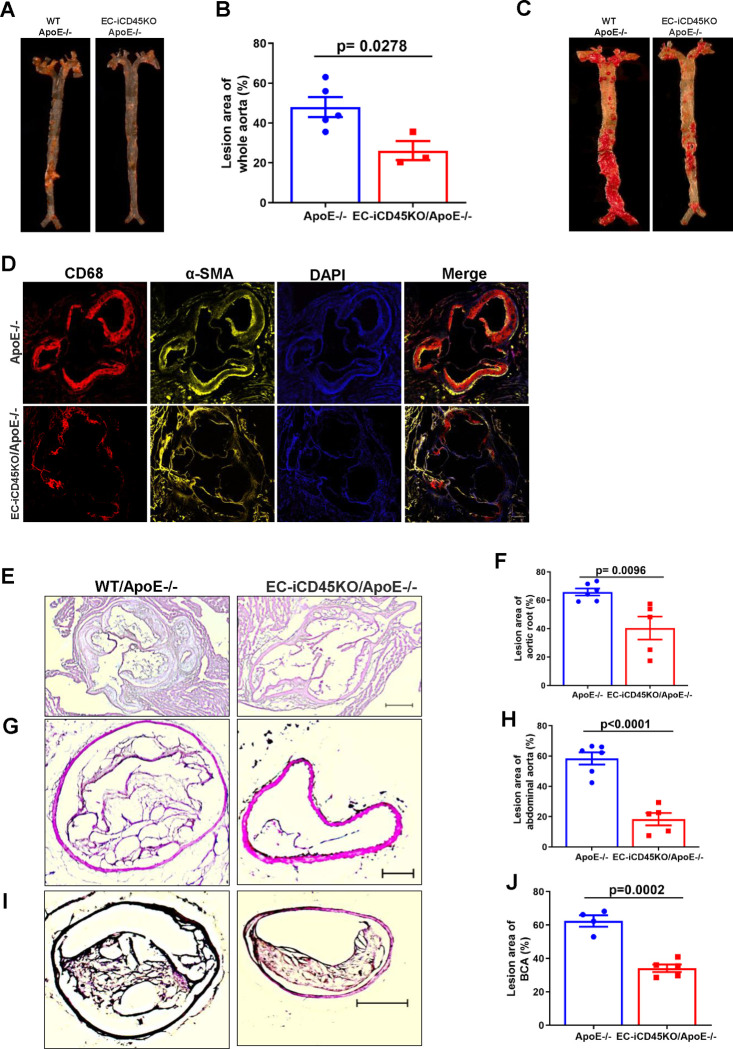
Aortic sinus, abdominal aorta, and en face whole aorta staining were performed to evaluate lesion ween wild-type ApoE-null and endothelial CD45 deficient WD mice. We observed a markedly reduced atherosclerotic lesion formation using aortic root (12-week WD), BCA (16-week WD), and abdominal aorta (16-week WD) in endothelial CD45 deficiency mice (Figure 5 E–I). Whole mount en face aortas from ApoE−/− and EC-iCD45KO/ApoE−/− mice were stained with Oil Red O (Figure 1H–I: 16-week WD; Figure 5A–B: 16-week WD, Figure 5C: 13-week WD). CD45 depletion significantly reduced atherosclerotic burden along the entire aorta in ApoE-null mice. Mice of the 2 genotypes fed with WD diet for 16 weeks showed no difference in body weights for males or females. Serum total cholesterol as well as triglycerides, HDL, LDL, levels showed no significant difference among the 2 groups of the male or female mice (data not shown). Next, we found a significant reduction of ICAM-1 expression on thoracic aorta (Figure 4F) and aortic root in EC-iCD45KO/ApoE−/− mice (Figure S3B). We did not detect the VCAM-1 expression has a significant difference between ApoE−/− and EC-iCD45KO/ApoE−/− mice in the aortic root (Figure S3A). In addition, aortic root sections from ApoE−/− and EC-iCD45KO/ApoE−/− mice (16-week WD) were co-stained with CD68 (a marker for lesion macrophages) and α-SMA. Diminished foam cell formation (Oil red o stained) and α-SMA were seen because of CD45 deficiency (Figure S2; Figure 5D).
Figure 5. Endothelial CD45 deletion reduces atherosclerosis in the ApoE−/− mouse model.
A, En face aortas from ApoE−/− and EC-iCD45KO /ApoE−/− female (B, 12-week WD) and male mice (C, 13-week WD) stained with Oil Red O. B, Statistics for en face oil red o staining in female mice. D, aortic root sections from ApoE−/− and EC-iCD45KO ApoE−/− mice were stained with the macrophage marker CD68 and α-SMA. Hematoxylin and Eosin (H&E) staining for aortic root (E); aortic aneurysm sections (G); and brachiocephalic artery (I). Statistics for H&E staining in the aortic root (F), aortic aneurysm sections (H), and brachiocephalic artery (J). (Scale bars = 20 μM).
Discussion
In the present study, we show for the first time that endothelial CD45 genetic deletion of the reduced atheroma burden in ApoE-null mice fed with the western diet. Our single-cell data indicated that EC-specific knock-out CD45 reduces a novel sub-population of EndoMT cells-EndMT2. We also found that CD45 deficiency led to decreased TGFβ2–3 expression on MAECs treated with ox-LDL. In addition, we unexpectedly observed that the absence of endothelial CD45 led to the reduction of α-smooth muscle actin, which is typical of EndoMT in the aortic plaque. Finally, we found that endothelial CD45 deficiency positively correlated with KLF2 expression, which counters EndoMT.
Since CD45 is commonly used as a negative marker for ECs, the existence of CD45-positive ECs may have been overlooked in previous studies. Protein tyrosine phosphatase receptor type C (CD45)-positive endothelium has been observed in mitral valves at 6 months post-myocardial infarction and exhibited EndoMT phenotype in adult sheep35. Also, the CD45 phosphatase inhibitor blocked EndoMT activities in TGFβ1-treated ovine mitral valve endothelial cells 35. We detected CD45-positive ECs on aortic arch from human patients with atherosclerosis. It indicated that endothelial CD45 plays a vital role in human atherosclerosis. The results of the present study reveal that loss of endothelial CD45 results in a favorable outcome in an animal model of atherosclerosis. In the absence of endothelial CD45 mice on the ApoE-null background on a WD diet for 12 weeks had a 25.39% reduction in lesion size in the aortic sinus compared with ApoE-null mice expressed with CD45. On the other hand, we found that CD45-deficient ApoE-null female mice fed with the WD diet exhibited a reduction of en face lesion area of the entire aorta (21.82 %, 12-week WD; 36.79%, 16-week WD), compared with ApoE-null mice expressed with CD45. This notion is supported by our finding that when significant lesions (28.28≈%) were reduced at brachiocephalic artery (BCA) and a reduction of 40.05% at abdominal aorta in endothelial CD45 deficiency mice. Overall, these results clearly indicate that the lack of endothelial CD45 prevents atherosclerosis not only in the aortic sinus but also in the entire aorta.
EndoMT contributes to atherosclerotic pathobiology and is associated with more complex plaques13. However, the detailed molecular mechanisms underlying CD45 that drive EndoMT and the therapeutic potential of manipulating CD45 expression in atherosclerosis are entirely unknown. The single-cell RNA-seq analysis provides an unbiased overview of how the transcriptome changes in the aortic cells. Cells populated 15 major clusters after integrating the single-cell transcriptomic profiles. In the EC-iCD45KO group, the population of endothelial, resident macrophage, and mesenchymal-endothelial transition cells increased while vascular smooth muscle, EndoMT, and fibroblast cells decreased (Figure S6). In EndoMT cells, EC markers (Cdh5, Cldn5, Pecam1) increased, but SMC markers (Acta2 and Myl9), and EndoMT markers (Col1a2 and Col3a1)decreased. In addition, we characterized EndoMT cells into two sub-clusters: 1 and 2. EC-specific CD45 deletions reduced the cell numbers in the EndoMT2 sub-cluster in atherosclerotic mice but had less impact on the EndoMT1 sub-cluster. CD45 is not normally expressed in ECs, and the details of how it might regulate normal or partial EndoMT needs to be addressed. It is reported that the partial endothelial-to-mesenchymal transition is mediated by HIF-induced CD45 upon carotid artery ligation44. The single-cell results of the present study identified a CD45-dependent EndoMT population in aortic endothelial cells (EndoMT2 sub-cluster), and it indicated that endothelial CD45 may mediate partial EndoMT in advanced atherosclerosis45.
It is well established that atheroma progression is involved in inflammation during EndoMT46. In line with this concept, our single-cell study showed that loss of endothelial CD45 led to decreases of multiple proinflammatory cytokines in EC-iCD45KO mice, including ICAM-1(EndoMT1 sub-cluster) and CCl5 (in both EndoMT1 and EndoMT2 sub-cluster). Oxidatively modified LDL (ox-LDL) is a critical pathogenic factor in atherogenesis47. It demonstrated that oxidized low-density lipoprotein reduces TGFβ1 synthesis with a feedback upregulation of TGFβ1 receptors and concurrently increases the expression of ICAM-1 in human coronary artery endothelial cells48. Consistently, we observed a reduction of ICAM-1 expression in mouse thoracic aorta and aortic sinus in the EC-iCD45KO/ApoE−/− mice. These data are associated and consistent with the reduced atherosclerosis results. Strikingly, the endothelial CD45-specific endothelial knockout ablate TGFβ signaling genetically confined to the MAECs, reduced ox-LDL-induced gene expression, including TGFβ2–3. Our flow cytometry data from ECs highlight a significant reduction of FGFR1- ECs in ApoE−/− WD aortas which indicates ECs undergo EndoMT in atherosclerosis since FGFR1 inhibits TGFβ during EndoMT49. In contrast, a marked increase in the expression of KLF2 in CD45 EC-iCD45KO/ApoE−/− at the aortic root. Unlike KLF4, KLF2 is not directly regulated by TGFβ signaling. Nevertheless, KLF2 appear functionally important given a known interaction among other KLFs. TGFβR2 disruption, may lead to a small rise in KLF4 levels and thereby increasing KLF250–52.
In summary, we report the first evidence that in ApoE-null mice fed with WD, the absence of endothelial CD45 leads to reduced lesion development, foam cells, plaque macrophages, and expression of adhesion molecules, which is negatively associated with the degree of atherosclerosis. In addition, endothelial CD45 deficiency led to a downregulation of the expression of TGFβ2–3, cytokines CCl5, and α-SMA while an upregulation of KLF2 expression; coincides with CD45-deficiency-mediated reduction of EndoMT and inflammation in atherosclerosis. These findings highlight the novel function of CD45 in endothelial cells in relation to EndoMT-driven atherosclerosis, the leading cause of death in the United States.
Supplementary Material
References
- 1.Martin SS, Aday AW, Almarzooq ZI, et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation. Feb 20 2024;149(8):e347–e913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorokin V, Vickneson K, Kofidis T, et al. Role of Vascular Smooth Muscle Cell Plasticity and Interactions in Vessel Wall Inflammation. Front Immunol. 2020;11:599415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khatana C, Saini NK, Chakrabarti S, et al. Mechanistic Insights into the Oxidized Low-Density Lipoprotein-Induced Atherosclerosis. Oxid Med Cell Longev. 2020;2020:5245308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorey MB, Oorni K, Kovanen PT. Modified Lipoproteins Induce Arterial Wall Inflammation During Atherogenesis. Front Cardiovasc Med. 2022;9:841545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gencer S, Evans BR, van der Vorst EPC, Doring Y, Weber C. Inflammatory Chemokines in Atherosclerosis. Cells. Jan 25 2021;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farahi L, Sinha SK, Lusis AJ. Roles of Macrophages in Atherogenesis. Front Pharmacol. 2021;12:785220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gialeli C, Shami A, Goncalves I. Extracellular matrix: paving the way to the newest trends in atherosclerosis. Curr Opin Lipidol. Oct 1 2021;32(5):277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. Apr 29 2011;145(3):341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jebari-Benslaiman S, Galicia-Garcia U, Larrea-Sebal A, et al. Pathophysiology of Atherosclerosis. Int J Mol Sci. Mar 20 2022;23(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gimbrone MA Jr., Garcia-Cardena G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res. Feb 19 2016;118(4):620–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovacic JC, Dimmeler S, Harvey RP, et al. Endothelial to Mesenchymal Transition in Cardiovascular Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. Jan 22 2019;73(2):190–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease. Circulation. Apr 10 2012;125(14):1795–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evrard SM, Lecce L, Michelis KC, et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat Commun. Jun 24 2016;7:11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen PY, Qin L, Baeyens N, et al. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest. Oct 26 2015;125(12):4514–4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lertkiatmongkol P, Liao D, Mei H, Hu Y, Newman PJ. Endothelial functions of platelet/endothelial cell adhesion molecule-1 (CD31). Curr Opin Hematol. May 2016;23(3):253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breviario F, Caveda L, Corada M, et al. Functional properties of human vascular endothelial cadherin (7B4/cadherin-5), an endothelium-specific cadherin. Arterioscler Thromb Vasc Biol. Aug 1995;15(8):1229–1239. [DOI] [PubMed] [Google Scholar]
- 17.Peng Q, Shan D, Cui K, et al. The Role of Endothelial-to-Mesenchymal Transition in Cardiovascular Disease. Cells. Jun 3 2022;11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korhonen EA, Lampinen A, Giri H, et al. Tie1 controls angiopoietin function in vascular remodeling and inflammation. J Clin Invest. Sep 1 2016;126(9):3495–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piera-Velazquez S, Jimenez SA. Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases. Physiol Rev. Apr 1 2019;99(2):1281–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. Sep 2011;179(3):1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ursoli Ferreira F, Eduardo Botelho Souza L, Hassibe Thome C, et al. Endothelial Cells Tissue-Specific Origins Affects Their Responsiveness to TGF-beta2 during Endothelial-to-Mesenchymal Transition. Int J Mol Sci. Jan 22 2019;20(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen PY, Qin L, Li G, et al. Endothelial TGF-beta signalling drives vascular inflammation and atherosclerosis. Nat Metab. Sep 2019;1(9):912–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross MD, Bielinski SJ, Suarez-Lopez JR, et al. Circulating soluble intercellular adhesion molecule 1 and subclinical atherosclerosis: the Coronary Artery Risk Development in Young Adults Study. Clin Chem. Feb 2012;58(2):411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickett JR, Wu Y, Zacchi LF, Ta HT. Targeting endothelial vascular cell adhesion molecule-1 in atherosclerosis: drug discovery and development of vascular cell adhesion molecule-1-directed novel therapeutics. Cardiovasc Res. Oct 24 2023;119(13):2278–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo W, Liu A, Chen Y, et al. Inhibition of accelerated graft arteriosclerosis by gene transfer of soluble fibroblast growth factor receptor-1 in rat aortic transplants. Arterioscler Thromb Vasc Biol. Jun 2004;24(6):1081–1086. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Liaw L, Prudovsky I, et al. Fibroblast growth factor signaling in the vasculature. Curr Atheroscler Rep. Jun 2015;17(6):509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen PY, Simons M. Fibroblast growth factor-transforming growth factor beta dialogues, endothelial cell to mesenchymal transition, and atherosclerosis. Curr Opin Lipidol. Oct 2018;29(5):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen PY, Qin L, Barnes C, et al. FGF regulates TGF-beta signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Rep. Dec 27 2012;2(6):1684–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma J, Sanchez-Duffhues G, Goumans MJ, Ten Dijke P. TGF-beta-Induced Endothelial to Mesenchymal Transition in Disease and Tissue Engineering. Front Cell Dev Biol. 2020;8:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lingrel JB, Pilcher-Roberts R, Basford JE, et al. Myeloid-specific Kruppel-like factor 2 inactivation increases macrophage and neutrophil adhesion and promotes atherosclerosis. Circ Res. May 11 2012;110(10):1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Justement LB. The Role of CD45 in Signal Transduction. In: Dixon FJ, ed. Advances in Immunology. Vol 66: Academic Press; 1997:1–65. [DOI] [PubMed] [Google Scholar]
- 32.Forsyth KD, Chua KY, Talbot V, Thomas WR. Expression of the leukocyte common antigen CD45 by endothelium. The Journal of Immunology. 1993;150(8):3471–3477. [PubMed] [Google Scholar]
- 33.Hong J, Wong B, Huynh C, et al. Tm4sf1-marked Endothelial Subpopulation Is Dysregulated in Pulmonary Arterial Hypertension. Am J Respir Cell Mol Biol. Apr 2023;68(4):381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nasim S, Wylie-Sears J, Gao X, et al. CD45 Is Sufficient to Initiate Endothelial-to-Mesenchymal Transition in Human Endothelial Cells-Brief Report. Arterioscler Thromb Vasc Biol. May 2023;43(5):e124–e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bischoff J, Casanovas G, Wylie-Sears J, et al. CD45 Expression in Mitral Valve Endothelial Cells After Myocardial Infarction. Circ Res. Nov 11 2016;119(11):1215–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong Y, Wang B, Du M, et al. Targeting Epsins to Inhibit Fibroblast Growth Factor Signaling While Potentiating Transforming Growth Factor-beta Signaling Constrains Endothelial-to-Mesenchymal Transition in Atherosclerosis. Circulation. Feb 21 2023;147(8):669–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong Y, Lee Y, Cui K, et al. Epsin-mediated degradation of IP3R1 fuels atherosclerosis. Nat Commun. Aug 7 2020;11(1):3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J-M, Chen AF, Zhang K. Isolation and Primary Culture of Mouse Aortic Endothelial Cells. JoVE. 2016/December/19 2016(118):e52965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui K, Gao X, Wang B, et al. Epsin Nanotherapy Regulates Cholesterol Transport to Fortify Atheroma Regression. Circ Res. Jan 6 2023;132(1):e22–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu B, Gupta K, Cui K, et al. Targeting Liver Epsins Ameliorates Dyslipidemia in Atherosclerosis. bioRxiv. 2024. [Google Scholar]
- 41.Lin Y, Li LL, Nie W, et al. Brain activity regulates loose coupling between mitochondrial and cytosolic Ca(2+) transients. Nat Commun. Nov 21 2019;10(1):5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan H, Xue C, Auerbach BJ, et al. Single-Cell Genomics Reveals a Novel Cell State During Smooth Muscle Cell Phenotypic Switching and Potential Therapeutic Targets for Atherosclerosis in Mouse and Human. Circulation. Nov 24 2020;142(21):2060–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu YW, Martino N, Gerlach BD, et al. MEF2 (Myocyte Enhancer Factor 2) Is Essential for Endothelial Homeostasis and the Atheroprotective Gene Expression Program. Arterioscler Thromb Vasc Biol. Mar 2021;41(3):1105–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamashiro Y, Ramirez K, Nagayama K, et al. Partial endothelial-to-mesenchymal transition mediated by HIF-induced CD45 in neointima formation upon carotid artery ligation. Cardiovasc Res. Jul 4 2023;119(7):1606–1618. [DOI] [PubMed] [Google Scholar]
- 45.Alvandi Z, Bischoff J. Endothelial-Mesenchymal Transition in Cardiovascular Disease. Arterioscler Thromb Vasc Biol. Sep 2021;41(9):2357–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen PY, Schwartz MA, Simons M. Endothelial-to-Mesenchymal Transition, Vascular Inflammation, and Atherosclerosis. Front Cardiovasc Med. 2020;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steinberg D. The LDL modification hypothesis of atherogenesis: an update. J Lipid Res. Apr 2009;50 Suppl(Suppl):S376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen H, Li D, Saldeen T, Mehta JL. Transforming growth factor-beta(1) modulates oxidatively modified LDL-induced expression of adhesion molecules: role of LOX-1. Circ Res. Dec 7 2001;89(12):1155–1160. [DOI] [PubMed] [Google Scholar]
- 49.Goumans MJ, Ten Dijke P. TGF-beta Signaling in Control of Cardiovascular Function. Cold Spring Harb Perspect Biol. Feb 1 2018;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen PY, Qin L, Li G, et al. Smooth Muscle Cell Reprogramming in Aortic Aneurysms. Cell Stem Cell. Apr 2 2020;26(4):542–557 e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen PY, Qin L, Simons M. TGFbeta signaling pathways in human health and disease. Front Mol Biosci. 2023;10:1113061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boon RA, Fledderus JO, Volger OL, et al. KLF2 suppresses TGF-beta signaling in endothelium through induction of Smad7 and inhibition of AP-1. Arterioscler Thromb Vasc Biol. Mar 2007;27(3):532–539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.