Abstract
Purpose: S-flurbiprofen (SFP) plaster, a non-steroidal anti-inflammatory drug preparation that penetrates effectively into deep tissue, is currently used as a conservative treatment for osteoarthritis. We investigated the analgesic and adverse effects of SFP plaster after total hip arthroplasty (THA).
Methods: A retrospective comparative study identified 100 patients who underwent primary THA in our department. Group A consisted of 50 patients who received the selective cyclooxygenase-2 inhibitor celecoxib for 14 days after surgery, while Group B consisted of 50 patients who received SFP plaster for 14 days after surgery. We noted the numerical rating pain intensity scale (NRS) score, body temperature, and adverse effects of the analgesics.
Results: Groups A and B showed no significant difference in NRS scores (p > 0.05). The body temperature was significantly higher in Group B than in Group A on days one, two, three, and five (p < 0.01). In Group A, two patients (4%) showed drug-induced renal dysfunction, and one patient (2%) showed gastrointestinal disturbance. Patients in Group B showed no systemic or local adverse effects.
Conclusions: The application of SFP plaster after THA provided an analgesic effect similar to that obtained with oral celecoxib without causing obvious side effects. Applying an SFP plaster may be an effective solution for postoperative analgesia.
Keywords: s-flurbiprofen, postoperative analgesia, osteoarthritis, numerical rating pain intensity scale, non-steroidal anti-inflammatory drug
Introduction
Total hip arthroplasty (THA) is a highly effective procedure for relieving pain and restoring function in patients with hip disease. Cases of THA have been increasing number every year worldwide [1]. The increasing popularity of muscle-sparing minimally invasive surgery (MIS) [2] has enabled patients to leave the hospital and return to normal life earlier after surgery [3], during which time postoperative pain control is important. The use of oral nonsteroidal anti-inflammatory drugs (NSAIDs) is highly prevalent in Japan. However, as NSAIDs express their anti-inflammatory effects by suppressing the cyclooxygenase (COX) system and decreasing prostaglandin production [4], they may induce adverse effects such as gastrointestinal disturbances [5,6] and renal dysfunction [7].
As conventional topical NSAIDs do not readily enter plasma [8], they show minimal systemic side effects such as gastrointestinal disturbances or renal dysfunction [9,10] and have been recommended as a conservative treatment option for osteoarthritis [11,12]. However, these topical analgesics are rarely used for postoperative analgesia since they are considered to have a low analgesic effect against acute or severe pain due to their low systemic permeability [13]. To the best of our knowledge, no previous report has evaluated the effect of topical NSAIDs on post-THA pain.
S-flurbiprofen (SFP) plaster (40 mg/140 cm2, LOQOAⓇ tape; Taisho Pharmaceutical Co., Tokyo, Japan) is a topical NSAID that effectively penetrates deep tissues and plasma and has been commercially available in 2016. SFP contains the active optical enantiomer (S isomer) of FP, a powerful COX inhibitor that exhibits good skin penetration. Sugimoto et al. calculated the transdermal absorption rate by determining the amount of drug remaining in tapes detached after application to rat skin for 24 hours and found that the rate for SFP was 92.9%, much greater than the rates for ketoprofen (67.8%) and loxoprofen (32.4%) [14], which are widely used as topical NSAIDs. In a study of 19 patients scheduled to undergo total knee arthroplasty (TKA), Yataba et al. applied 40 mg of SFP plaster to 10 knees and 40 mg of FP plaster to nine knees for 12 hours and compared the synovial membrane tissue, synovial fluid, and plasma samples collected during surgery. They found that, in comparison with the corresponding drug concentrations for FP plaster, the drug concentration after using SFP plaster was 14.8-fold greater in synovial membrane tissue, 32.7-fold greater in the synovial fluid, and 34.5-fold greater in plasma, indicating that SFP penetrates tissues significantly more effectively [15]. In clinical practice, a randomized blinded trial on 633 patients with osteoarthritis of the knee also showed that, when SFP plaster and FP plaster were each applied for two weeks, SFP plaster provided significantly better pain relief [16]. Moreover, a study examined the analgesic effect of SFP plaster after TKA [17]; however, no previous study has compared the postoperative analgesic effect of SFP plasters with oral analgesics. We hypothesized that an SFP plaster would be effective for post-THA pain with minimal adverse effects and studied the analgesic and adverse effects of applying an SFP plaster after THA.
Materials and methods
This study was approved by the Ethics Committee of our institution. The need for informed consent was waived by the ethics committee due to the retrospective nature of the study. Of 112 patients who underwent primary THA by the same surgeon at our department between November 2015 and December 2017, 100 patients who met the following criteria were included. The study population consisted of 86 women and 14 men, of which 89 had experienced osteoarthritis of the hip and 11 showed idiopathic femoral head necrosis. All patients underwent muscle-sparing joint replacement with the anterolateral approach using cementless implants on both the acetabular and femoral sides (age at surgery, 62.3 ± 8.9 years (mean ± standard deviation)). Patients who underwent THA with other approaches, such as lateral or posterolateral approaches with muscle-tendon dissection, were excluded. We also excluded patients who had undergone non-elective surgery and those who received urgent or semi-urgent THA for trauma, such as femoral neck fracture. Moreover, patients with preoperative gastrointestinal disturbance, renal dysfunction, or cardiovascular disorders were excluded.
Post-THA analgesia in our department consisted of intraoperative local infiltration of anesthesia with 100 mg of levobupivacaine after implant replacement and intravenous patient-controlled analgesia at 1 mL/hour of a mixture of fentanyl 10 mL, droperidol 1 mL, and physiological saline 39 mL for two days after surgery. Group A consisted of 50 patients who underwent treatment between November 2015 and November 2016 and received oral administration of the selective COX-2 inhibitor celecoxib 400 mg/day (200 mg each after breakfast and after dinner) and the potassium-competitive acid blocker vonoprazan 10 g/day for 14 days from the day after surgery. Group B consisted of 50 patients who underwent treatment between December 2016 and December 2017 and received the SFP plaster (LOQOAⓇ tape 40 mg; 40 mg/140 cm2) once daily (after breakfast) for 14 days from the day after surgery. The patients were instructed to apply one plaster at the most painful site, which was usually the anterior surface of the proximal thigh, although some applied it to the buttocks.
We investigated the changes in pain using the numerical rating pain intensity scale (NRS) score, changes in body temperature, and any adverse effects of analgesics. The pain was graded by the NRS score on an 11-point scale from 0 to 10, with 0 representing no pain and 10 the maximum pain imaginable. Patients were asked to grade their pain verbally three times a day (morning, noon, and night) from postoperative day one to day 14, and the highest value for each day was used. Their temperature was also taken three times a day preoperatively and from postoperative days 1-14. The highest values of body temperature were used. Serum creatinine concentration was measured preoperatively and on postoperative days one, three, seven, and 14. Renal function was evaluated by measuring serum creatinine concentration preoperatively and on postoperative days one, three, seven, and 14 and by calculating each patient’s estimated glomerular filtration rate (eGFR) using their sex, age, and serum creatinine level; postoperative renal dysfunction was defined by an eGFR ≤80% of the preoperative value during the postoperative course. Adverse effects at the application site, gastrointestinal disturbances, cardiovascular disorders, and other systemic side effects were diagnosed on the basis of medical histories and physical examinations. Oral acetaminophen was administered as a rescue antipyretic analgesic, and the number of patients who required the rescue medication was counted.
Differences in the mean age, height, weight, preoperative eGFR, and surgery time between Groups A and B were analyzed using the Mann-Whitney U test. The repeated-measure endpoints (NRS score and body temperature) were analyzed with linear mixed models that included the group (A or B), dummy variables for time, group-by-time interactions, and the baseline value of each endpoint as covariates (NRS score on day one, body temperature preoperatively) and the patients as a random effect. The covariance structure was a completely general covariance matrix. The results were reported as the least-squares means with a 95% confidence interval at each time point. A p-value of <0.05 was considered statistically significant, and all p-values were two-sided without multiplicity adjustment. All statistical analyses were performed using SPSS Statistics for Windows/Macintosh (IBM Corp., IBM SPSS Statistics for Windows, version 23.0, Armonk, NY).
Results
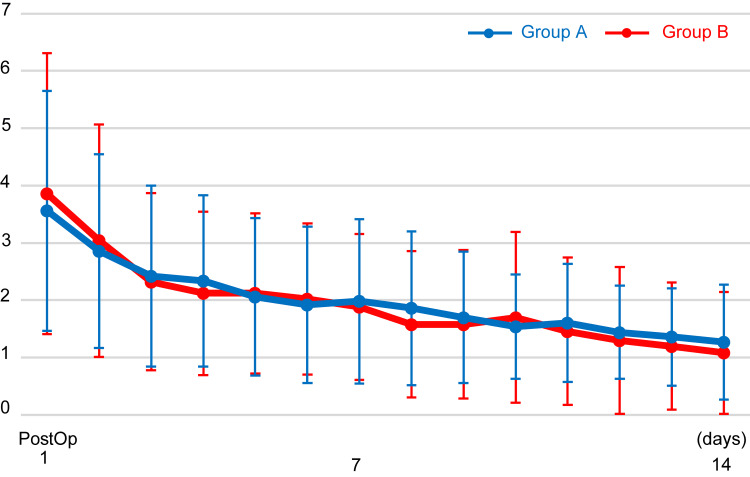
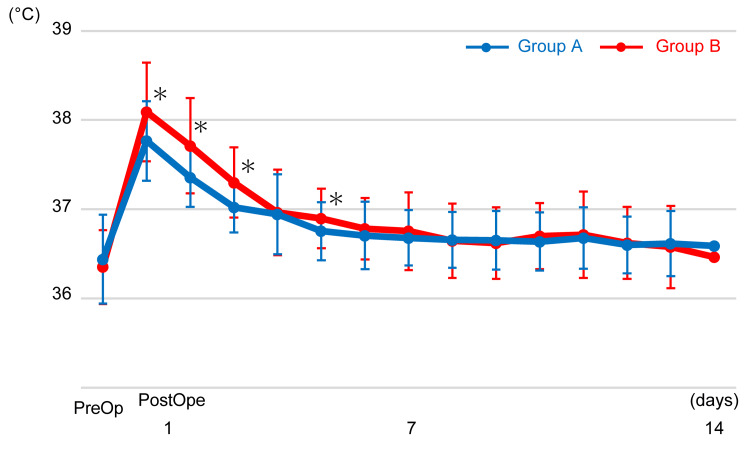
There were no significant differences in the mean age, height, weight, preoperative eGFR, and surgery time between Groups A and B (p > 0.05) (Table 1). Groups A and B showed no significant differences in the transition of NRS scores (p > 0.05) (Figure 1). The mean NRS score dropped to below 3 points on day two in Group A and on day three in Group B and to below 2 points on day six in Group A and day seven in Group B. The transition of body temperature showed a significant intergroup effect (p < 0.001). Body temperature was significantly higher in Group B than in Group A on days one, two, three, and five (p < 0.01) (Figure 2).
Table 1. Patient demographics.
Data are presented as mean ± standard deviation. eGFR, estimated glomerular filtration rate
| Group A | Group B | |
| Sex | ||
| Male | 7 (14%) | 7 (14%) |
| Female | 43 (86%) | 43 (86%) |
| Disease | ||
| Osteoarthritis | 44 (88%) | 45 (90%) |
| Femoral head necrosis | 6 (12%) | 5 (10%) |
| Age (years) | 61.3 ± 9.9 | 62.6 ± 7.8 |
| Height (cm) | 156.6 ± 6.4 | 156.6 ± 6.9 |
| Weight (kg) | 59.0 ± 9.3 | 59.1 ± 11.2 |
| Preoperative eGFR (mL/min) | 87.6 ± 22.9 | 86.1 ± 17.9 |
| Surgery time (min) | 114.6 ± 21.0 | 108.3 ± 26.2 |
Figure 1. NRS scores from postoperative days 1-14.
No significant difference was observed between Groups A and B. NRS: numerical rating pain intensity scale
Figure 2. Body temperature obtained preoperatively and from postoperative days 1-14.
Body temperature was significantly higher in Group B on postoperative days one, two, three, and five (*p < 0.01).
In Group A, two patients (4%) experienced renal dysfunction, and one patient (2%) experienced gastrointestinal disturbance necessitating withdrawal of the medication. However, patients in Group B did not experience any systemic or local adverse effects. Neither group did not show any cardiovascular or other systemic adverse effects.
In Group B, 18 patients (36%) required 400 mg of rescue acetaminophen for fever (17 cases) or headache (one case) management. Acetaminophen for fever was administered only a few times, at most once a day from days one to three, and no patient required its regular use. No patient in Group A required rescue acetaminophen (Table 2).
Table 2. Systemic and local adverse effects in both groups.
In Group A, two patients experienced renal dysfunction, and one patient experienced gastrointestinal disturbance. However, patients in Group B did not experience any systemic or local adverse effects.
| Adverse effects | Group A | Group B |
| Application site | 0 | 0 |
| Renal dysfunction | 2 (4%) | 0 |
| Gastrointestinal disturbance | 1 (2%) | 0 |
| Cardiovascular disorders | 0 | 0 |
| Rescue acetaminophen | 0 | 18 (36%) |
Discussion
In this study, we compared the analgesic effect of SFP plaster 40 mg after THA with that of a dose of 400 mg/day of oral celecoxib and found that both were almost equivalent. On a visual analogue pain intensity scale, a score of 30 mm or more is considered to represent moderate pain [18], and the ideal goal is to control pain to below a score of 20 mm. In both groups in the present study, the scores declined to below 3 points within three days and below 2 points within a week, indicating good pain control in both groups. Although several researchers have reported their experience of using an SFP plaster on the knee [15,16,19], there are no studies describing its use on the hip. Our study suggests that the SFP plaster may provide adequate tissue penetration and analgesia even in areas with comparatively thicker subcutaneous fat, such as the hip.
The adverse effects of SFP plaster include its local effects at the site of application, such as dermatitis, erythema, and rash, and systemic adverse effects attributable to its characteristic effective penetration into plasma. No local adverse effects of SFP plaster were observed in this study. In a study of local adverse effects in patients who applied SFP plaster continuously for 52 weeks, 47% showed skin symptoms [20], but the rate was not significantly different from that for the placebo [19]. Plaster application is generally considered to cause severe physical irritation when it is pulled off [21], and rather than the active ingredient SFP, skin symptoms are believed to be due to the physical irritation caused by repeated application and removal; thus, patients must be instructed to pull the tape off gently. Since the present study only covered two weeks, no patient developed local adverse effects. Attention must be paid to skin symptoms when the tape is used for longer periods.
The most important systemic side effects of SFP plaster application are the same as those of oral NSAIDs, including gastrointestinal disturbances, renal dysfunction, and cardiovascular disorders [22]. The reported risk of upper gastrointestinal bleeding due to oral nonselective NSAIDs is four- to fivefold greater than that in patients not taking them [6,23]. Although the selective COX-2 inhibitor celecoxib causes fewer gastrointestinal problems than nonselective NSAIDs [23,24], administration of gastrointestinal protective agents is recommended in patients with high gastrointestinal risk [25]. In this study, despite the use of the potassium-competitive acid blocker vonoprazan to prevent gastrointestinal disturbance during celecoxib use, one patient (2%) experienced gastrointestinal disturbance sufficiently severe to require celecoxib withdrawal. Meanwhile, there were no cases of gastrointestinal disturbance among the patients who used SFP plaster. The reported incidence of gastrointestinal adverse effects associated with long-term use of oral FP is 9.1% [26], in comparison with the 3% and 6% reported for the long-term use of 40 mg and 80 mg, respectively, of SFP plaster [19]. Since the present study only covered two weeks, no patient developed gastrointestinal disturbance. However, the occurrence of a serious bleeding gastric ulcer requiring laparoscopic hemostasis during the use of SFP plaster has been reported [19], and therefore, caution is required.
On the other hand, selective COX-2 inhibitors can cause renal dysfunction [27] and cardiovascular side effects [28] with prolonged use. Because COX-2 is permanently expressed in the kidneys, the incidence of renal dysfunction caused by selective COX-2 inhibitors and nonselective NSAIDs is not different, and a similar level of caution with respect to this adverse effect is required for both categories of drugs [29]. In this study, the eGFR decreased to ≤ 80% of its preoperative value in two patients (4%) taking celecoxib. Because prophylactic antibacterial agents were also administered postoperatively, we could not determine which agent was the primary cause of renal dysfunction. However, this is an important side effect of NSAIDs and must always be considered during their use. A significant elevation in creatinine concentration in week 44 during a 52-week continuous use of SFP plaster has been reported, but the elevated concentration was still within normal limits and did not pose a clinical threat [30]. In addition, no case of serious renal dysfunction was reported.
In this study, we also investigated the body temperature, and the mean temperature from postoperative days one to five was higher in patients who used the SFP plaster than in those who took celecoxib, with 17 patients (34%) in Group B requiring rescue acetaminophen. This suggests that the SFP plaster has minimal systemic antipyretic effects. Although the systemic exposure during the use of SFP plaster 80 mg is roughly equivalent to that associated with the normal dose of oral FP, the maximum plasma concentration is only 66% [22,26], and the gradual and continuous absorption may help mitigate the systemic adverse effects. The problem of postoperative fever can be solved by taking oral acetaminophen regularly for several days after the surgery.
The most critical limitation of this study is the retrospective design. However, there were no significant differences in the mean age, height, weight, preoperative eGFR, and surgery time between the two groups. Moreover, both groups were treated by the same process, except for postoperative analgesic drugs. Although rescue acetaminophen was used for fever or headache in Group B, we considered that it did not affect the highest value of NRS scores because it was used for once a day at most. Another limitation is that only THA by the anterolateral approach was included in this study. The comparison between SFP plaster and celecoxib may have shown significant differences in NRS scores in cases involving more invasive THA, including the muscle-tendon dissection approach or revision surgery. However, the anterolateral approach is the most commonly performed procedure in our department, and the postoperative analgesic effect achieved using SFP plaster after this procedure was almost the same as that achieved with oral administration of celecoxib. Therefore, we consider that SFP plaster is an effective dosage form for postoperative analgesia.
Conclusions
To our knowledge, this is the first study to compare the postoperative analgesic effect of SFP plasters with oral analgesics. In post-THA patients, its analgesic effect was similar to that of the widely used celecoxib. Additionally, there were no obvious side effects. Therefore, we consider that the SFP plaster application is an effective approach for postoperative analgesia.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study. Ethics Committee of Kawasaki Medical School issued approval 3376. This study was approved by the Ethics Committee of our institution. The need for informed consent was waived by the ethics committee owing to the retrospective nature of the study.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Yoshiaki Miyake, Shigeru Mitani
Acquisition, analysis, or interpretation of data: Yoshiaki Miyake, Shigeru Mitani, Yoshifumi Namba, Norifumi Umehara, Toyohiro Kawamoto , Shuro Furuichi
Drafting of the manuscript: Yoshiaki Miyake, Shigeru Mitani, Yoshifumi Namba, Norifumi Umehara, Toyohiro Kawamoto , Shuro Furuichi
Critical review of the manuscript for important intellectual content: Yoshiaki Miyake, Shigeru Mitani
References
- 1.Global perspectives on arthroplasty of hip and knee joints. Abdelaal MS, Restrepo C, Sharkey PF. Orthop Clin North Am. 2020;51:169–176. doi: 10.1016/j.ocl.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Anterolateral mini-incision hip replacement surgery: a modified Watson-Jones approach. Bertin KC, Röttinger H. Clin Orthop Relat Res. 2004;429:248–255. [PubMed] [Google Scholar]
- 3.A comparison of hospital length of stay and short-term morbidity between the anterior and the posterior approaches to total hip arthroplasty. Martin CT, Pugely AJ, Gao Y, Clark CR. J Arthroplasty. 2013;28:849–854. doi: 10.1016/j.arth.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 4.Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Vane JR. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 5.Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Huang JQ, Sridhar S, Hunt RH. Lancet. 2002;359:14–22. doi: 10.1016/S0140-6736(02)07273-2. [DOI] [PubMed] [Google Scholar]
- 6.Case-control study on the association of upper gastrointestinal bleeding and nonsteroidal anti-inflammatory drugs in Japan. Sakamoto C, Sugano K, Ota S, et al. Eur J Clin Pharmacol. 2006;62:765–772. doi: 10.1007/s00228-006-0171-6. [DOI] [PubMed] [Google Scholar]
- 7.Individual non-steroidal anti-inflammatory drugs and risk of acute kidney injury: a systematic review and meta-analysis of observational studies. Ungprasert P, Cheungpasitporn W, Crowson CS, Matteson EL. Eur J Intern Med. 2015;26:285–291. doi: 10.1016/j.ejim.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Oral versus topical NSAIDs in rheumatic diseases: a comparison. Heyneman CA, Lawless-Liday C, Wall GC. Drugs. 2000;60:555–574. doi: 10.2165/00003495-200060030-00004. [DOI] [PubMed] [Google Scholar]
- 9.Tolerability of topical diclofenac sodium 1% gel for osteoarthritis in seniors and patients with comorbidities. Baraf HS, Gold MS, Petruschke RA, Wieman MS. Am J Geriatr Pharmacother. 2012;10:47–60. doi: 10.1016/j.amjopharm.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Safety and efficacy of topical diclofenac sodium gel for knee osteoarthritis in elderly and younger patients: pooled data from three randomized, double-blind, parallel-group, placebo-controlled, multicentre trials. Baraf HS, Gloth FM, Barthel HR, Gold MS, Altman RD. Drugs Aging. 2011;28:27–40. doi: 10.2165/11584880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Kolasinski SL, Neogi T, Hochberg MC, et al. Arthritis Care Res (Hoboken) 2020;72:149–162. doi: 10.1002/acr.24131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Zhang W, Nuki G, Moskowitz RW, et al. Osteoarthritis Cartilage. 2010;18:476–499. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Efficacy of topical non-steroidal anti-inflammatory drugs in the treatment of osteoarthritis: meta-analysis of randomised controlled trials. Lin J, Zhang W, Jones A, Doherty M. BMJ. 2004;329:324. doi: 10.1136/bmj.38159.639028.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Analgesic effect of the newly developed S(+)-flurbiprofen plaster on inflammatory pain in a rat adjuvant-induced arthritis model. Sugimoto M, Toda Y, Hori M, et al. Drug Dev Res. 2016;77:20–28. doi: 10.1002/ddr.21288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plasma pharmacokinetics and synovial concentrations of S-flurbiprofen plaster in humans. Yataba I, Otsuka N, Matsushita I, et al. Eur J Clin Pharmacol. 2016;72:53–59. doi: 10.1007/s00228-015-1960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Efficacy of S-flurbiprofen plaster in knee osteoarthritis treatment: results from a phase III, randomized, active-controlled, adequate, and well-controlled trial. Yataba I, Otsuka N, Matsushita I, Matsumoto H, Hoshino Y. Mod Rheumatol. 2017;27:130–136. doi: 10.1080/14397595.2016.1176624. [DOI] [PubMed] [Google Scholar]
- 17.Effect of flurbiprofen and S-flurbiprofen patches on multimodal pain management after total knee arthroplasty: a prospective randomized controlled trial. Tsuji M, Kobayashi N, Yukizawa Y, Oishi T, Takagawa S, Inaba Y. J Arthroplasty. 2020;35:2033–2038. doi: 10.1016/j.arth.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Time course of subjective pain ratings, and wound and leg tenderness after hysterectomy. Møiniche S, Dahl JB, Erichsen CJ, Jensen LM, Kehlet H. Acta Anaesthesiol Scand. 1997;41:785–789. doi: 10.1111/j.1399-6576.1997.tb04784.x. [DOI] [PubMed] [Google Scholar]
- 19.The efficacy and safety of S-flurbiprofen plaster in the treatment of knee osteoarthritis: a phase II, randomized, double-blind, placebo-controlled, dose-finding study. Yataba I, Otsuka N, Matsushita I, Matsumoto H, Hoshino Y. J Pain Res. 2017;10:867–880. doi: 10.2147/JPR.S131779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The long-term safety of S-flurbiprofen plaster for osteoarthritis patients: an open-label, 52-week study. Yataba I, Otsuka N, Matsushita I, Matsumoto H, Hoshino Y. Clin Drug Investig. 2016;36:673–682. doi: 10.1007/s40261-016-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comparison of poultice-type and tape-type patches containing ketoprofen on human skin irritation. Kawamura N, Shinkai N, Yamauchi H, Takayama S. J Toxicol Sci. 2003;28:415–425. doi: 10.2131/jts.28.415. [DOI] [PubMed] [Google Scholar]
- 22.[Medicine interview form of LOQOA® tape] Japan, vol. 40. [ Aug; 2024 ]. 2016. https://www.pmda.go.jp/ https://www.pmda.go.jp/
- 23.Variability among nonsteroidal antiinflammatory drugs in risk of upper gastrointestinal bleeding. Massó González EL, Patrignani P, Tacconelli S, García Rodríguez LA. Arthritis Rheum. 2010;62:1592–1601. doi: 10.1002/art.27412. [DOI] [PubMed] [Google Scholar]
- 24.The effectiveness of five strategies for the prevention of gastrointestinal toxicity induced by non-steroidal anti-inflammatory drugs: systematic review. Hooper L, Brown TJ, Elliott R, Payne K, Roberts C, Symmons D. BMJ. 2004;329:948. doi: 10.1136/bmj.38232.680567.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.OARSI guidelines for the non-surgical management of knee osteoarthritis. McAlindon TE, Bannuru RR, Sullivan MC, et al. Osteoarthritis Cartilage. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 26.[Medicine interview form of Froben®] [ Aug; 2024 ]. 2012. https://www.pmda.go.jp/ https://www.pmda.go.jp/
- 27.Adverse effects of cyclooxygenase 2 inhibitors on renal and arrhythmia events: meta-analysis of randomized trials. Zhang J, Ding EL, Song Y. JAMA. 2006;296:1619–1632. doi: 10.1001/jama.296.13.jrv60015. [DOI] [PubMed] [Google Scholar]
- 28.Onset of acute myocardial infarction after use of non-steroidal anti-inflammatory drugs. Hammad TA, Graham DJ, Staffa JA, Kornegay CJ, Dal Pan GJ. Pharmacoepidemiol Drug Saf. 2008;17:315–321. doi: 10.1002/pds.1560. [DOI] [PubMed] [Google Scholar]
- 29.Comparison of rofecoxib, celecoxib, and naproxen on renal function in elderly subjects receiving a normal-salt diet. Schwartz JI, Vandormael K, Malice MP, et al. Clin Pharmacol Ther. 2002;72:50–61. doi: 10.1067/mcp.2002.126182. [DOI] [PubMed] [Google Scholar]
- 30.A minimal impact of long-term S-flurbiprofen plaster application on kidney function in osteoarthritis patients. Otsuka N, Yataba I, Matsushita I, Matsumoto H, Hoshino Y, Terada Y. Clin Exp Nephrol. 2017;21:1060–1067. doi: 10.1007/s10157-017-1406-9. [DOI] [PMC free article] [PubMed] [Google Scholar]