Abstract
Background:
Pyogenic granuloma (PG), or lobular capillary hemangioma, poses a clinical challenge with its uncertain etiology and treatment options. Although the clinical features and prevalence of PGs are well established, definitive evidence-based treatments remain elusive. This practical review aims to illuminate the complexities of PG management by analyzing surgical interventions based on literature analysis.
Methods:
A PubMed/Medline search of “pyogenic granuloma” and “surgery” yielded 1171 studies. Inclusion criteria targeted intervention-associated PG complications over 5% and treatment modalities, excluding nonclinical studies and topics unrelated to plastic and reconstructive surgery. Screening involved Oxford level of evidence, patient data extraction, complications, intervention types, success rates, sessions, follow-ups, and treatments.
Results:
Thirty-one studies met inclusion criteria. Most studies were retrospective (67.7%). Ten studies satisfied intervention-linked eruptions, primarily oculoplastic, whereas 21 investigated both surgical and nonsurgical treatment modalities. Across interventions, 3579 patients (age: 34.2–85.7 years) were involved. Postsurgical PG complications averaged 15.1% and were treated predominantly with surgical excision, achieving nearly complete resolution. Surgical and nonsurgical treatment studies included 1233 patients (age: 3–46.5 years), demonstrating a 68.2% average resolution after a single session, with surgical excision exhibiting the highest success rate (96.2%) and minimal complications.
Conclusions:
This practical review highlights the complexities of managing PG, emphasizing a spectrum of effective treatments and potential postoperative complications. Ophthalmologic procedures showed PG incidences of 9%–24.4%. Surgical excision proved highly effective, surpassing methods like lasers and injectables that exhibited varied success rates requiring multiple treatment sessions. Challenges included study diversity and varying evidence levels, warranting further comparative research for PG management strategies.
Takeaways
Question: What are the surgical interventions and treatment modalities available for addressing pyogenic granuloma (PG), and how effective are these interventions in achieving resolution of the lesions?
Findings: Surgical excision is effective, achieving near-complete resolution in a single session with minimal complications, whereas alternative treatments such as lasers and injectables show varying success, requiring multiple sessions for resolution. The study also demonstrates the importance of refining reconstructive techniques to minimize postoperative issues following ophthalmologic procedures, particularly due to intervention-linked eruptions of PGs.
Meaning: This review explores managing PG, highlighting the effectiveness of surgical excision and calling for further research to refine treatment strategies, especially in ophthalmologic procedures.
INTRODUCTION
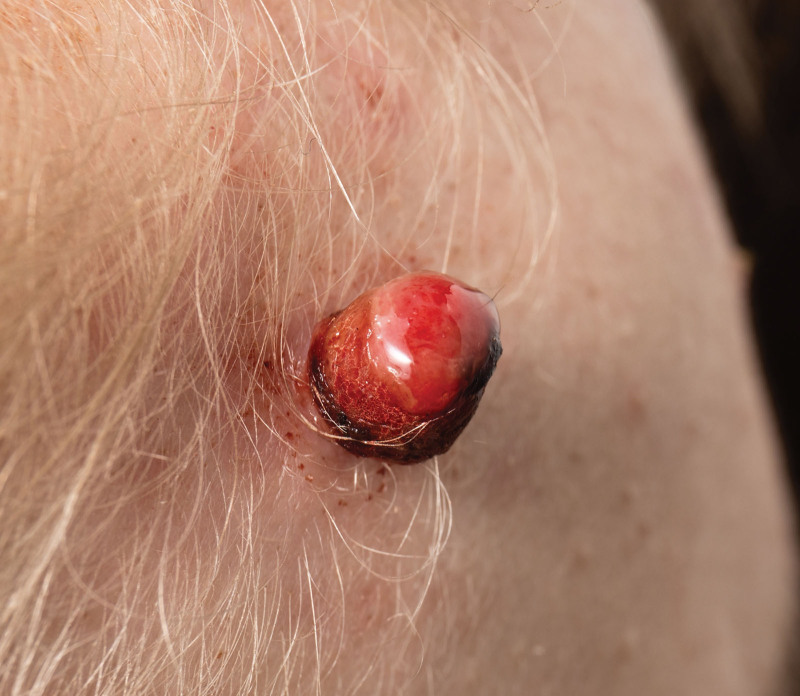
Pyogenic granuloma (PG), also known as lobular capillary hemangioma, is a common, acquired, benign vascular proliferation of the skin and mucous membranes that was first described in 1897.1 Clinical features of this lesion are characterized by its small size, rubicund appearance, and frequent tendency to ulcerate (Fig. 1).2 The etiology of PG is unknown; however, the histopathologic and molecular complexity shows a distinctive capillary arrangement with plump endothelial cells and notable mitotic activity, supported by immunohistochemical markers like glucose transporter type 1 and Wilms tumor 1, among other trauma-induced genetic mutations in B-Raf proto-oncogene serine/threonine kinase, serine/threonine kinase, and guanine nucleotide-binding protein (G protein), Q polypeptide.3–8 These histological differentiations suggest its potential clinical utility, aiding in decision-making and treatment strategies. Further, local trauma, irritation, and systemic conditions have all been implicated in the eruption of PG.9–20
Fig. 1.
Clinical presentation of a PG on the frontal region of the head. Photograph attribution: Penny Jane Williamson, ID: 2246820423.
Epidemiological data show PGs prevalence across all age groups, spanning diverse anatomical locations, and distinct demographic patterns with varied associations.21,22 Notably, PGs show a predilection to pregnancy, suggestive of hormonal influences, with reactive localized hyperplastic gingival lesions concentrating during the second to fourth decades of life (Fig. 2).23–41
Fig. 2.
Clinical presentation of a PG on the maxillary interdental gingiva. Photograph attribution: Kasama Kanpittaya, ID: 1421389106.
Differential diagnoses of PG may be challenging due to its potential resemblance to various other vascular lesions. It is important to distinguish lobular capillary hemangiomas from benign lesions like hemangiomas, nevi, warts, fibrokeratomas, granulation tissue, glomus tumors, and others on histopathological examination.42 Although rare, malignancies should be considered, as demonstrated by several case reports of metastatic renal clear cell carcinoma, basal and squamous cell carcinoma, primary cutaneous anaplastic large cell lymphoma, hepatocellular carcinoma, and breast cancer cutaneous metastases masquerading as PGs.43–48 Malignant melanoma, including amelanotic variants, and malignant fibrous histiocytoma also closely mimics the appearance of PGs.49–53 Cautious evaluation, histopathological confirmation, and thorough understanding of diverse presentations and potential masqueraders of PG are essential for effective clinical management. Complications are minimal but may include ulceration, bleeding from trauma, infections, and cosmetic disfigurement; especially when lesions are facial, treatment may be required.42
Treatment options remain vast and include surgical excision, curettage, laser therapies, or topical agents, all with varying efficacies.2,8,54,55 This practical review aims to comprehensively analyze the spectrum of surgical interventions that have led to the development and eruption of PGs as it relates to the field of plastic and reconstructive surgery, as well as review evidence behind clinically effective treatment modalities and their associated potential complications.
METHODS
Literature Search
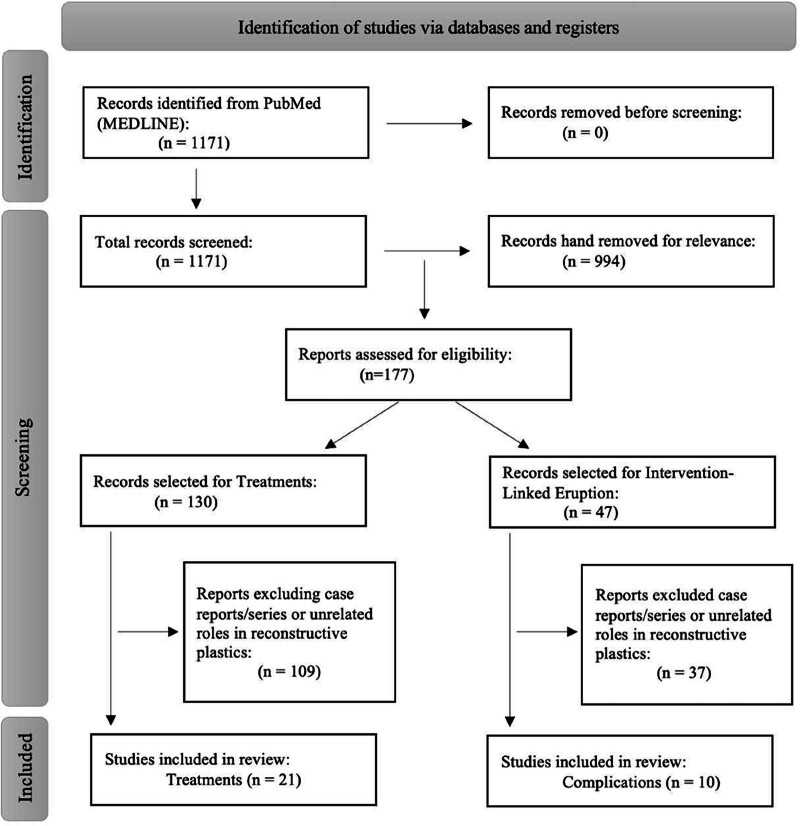
A search of the PubMed/Medline database was performed in April of 2024 to evaluate intervention-linked PG eruptions and to assess treatment modalities for PG using the following query: “pyogenic granuloma” and “surgery.” The search identified 1171 studies published between 1964 and 2024. Inclusion criteria included those of management and intervention-associated PG complications over 5%. Exclusion criteria and subsequent eliminations were those that centered on misdiagnoses of other diseases, differential diagnoses, nonhuman subjects, nonclinical articles, case reports, and articles not accessible in English. Studies were further subdivided and eliminated as to whether plastic and reconstructive surgery may have an impactful role. A flowchart of the rigorous, stepwise selective process is detailed in Figure 3.
Fig. 3.
Flowchart of studies included in the practical review.
Data Extraction
Data were extracted from included studies using a standardized data extraction form. All articles pulled data for author, publication year, the number and mean age of patients, and study design Oxford level of evidence. We used the Oxford Center for Evidence-Based Medicine levels of evidence framework to categorize the included articles based on their strength of evidence.56 Among the separate categories of publications, more specific data were extracted accordingly. For articles on complications, intervention type, percentage of patients with PG eruption, and treatments were extracted. Specific for articles on surgical and nonsurgical treatment, data were collected on modality type, success rate, number of sessions, last follow-up, and any complications, if applicable.
Ethical Considerations
This study involved the analysis of previously published data; no ethical approval was required. All data were retrieved from publicly available sources, and confidentiality of study participants was maintained throughout the analysis.
Data Synthesis and Analysis
The study used descriptive statistics to summarize the dataset, calculating measures such as mean, SD, and range. A meta-analysis was also conducted to evaluate the efficacy of the treatment group, both surgical and nonsurgical interventions for PG eradication, using Stata/BE 18.0 software. Single proportion estimation was used for effect size calculation. Homogeneity testing was performed to evaluate heterogeneity among included studies, with the I² index. Publication bias was assessed using Egger test in conjunction with a corresponding funnel plot. Additionally, a leave-one-out meta-analysis was conducted to evaluate the stability of the pooled effect size estimate. Subgroup meta-analyses were conducted based on the type of intervention used and the location of the PG to explore potential variations in effect sizes across different subgroups. Bias assessment tests were carried out to detect small-study effects. Additionally, a trim-and-fill analysis was performed to evaluate the impact of publication bias on the observed results.
RESULTS
After inclusion and exclusion criteria were met, 31 articles published between 1997 and 2024 were included in this review. Ten intervention-linked eruption articles and 21 surgical and nonsurgical treatment modality studies for PG were selected. Within the intervention group, all studies discussed ophthalmologic/oculoplastic eyelid reconstructive procedures and techniques. The total number of patients was 3579, with individual study populations ranging from six to 1917. The overall mean age of patients was 58.4 years, ranging from 34.2 to 85.7 years. The mean PG complication postsurgical intervention was 15.1%, with a range between 9% and 24.4%. Six studies were retrospective chart reviews, two were randomized clinical trials, and two were prospective, single-arm, noncomparative cohort studies. Most PGs were treated with surgical excision, topical steroids, or triamcinolone injections with near-complete resolution at an average follow-up of 11.1 months, with a range between 6 weeks and 24 months.
In the treatment group, three were considered for surgical excision, nine for laser therapy, and five for injectables and other options such as cryotherapy/liquid nitrogen. Four studies were considered for conservative therapy, which included topical therapies of various eye drops and observation. Fifteen articles were retrospective chart reviews, two were noncomparative prospective trials, one was a prospective observational study, and one was a prospective controlled comparative study. There were two randomized clinical trials. The total number of patients was 1233, with a range from five to 388. The mean age of patients was 29.1 years, with a range between 3 and 46.5 years. The mean time for the last follow-up of 8.7 months. Across all studies, PG lesions arose and were treated across all areas with the most frequent in the head/neck, oral cavity, and extremities. Minimal complications from erythema, mild pain, swelling, and dyspigmentation were shown across most modalities.
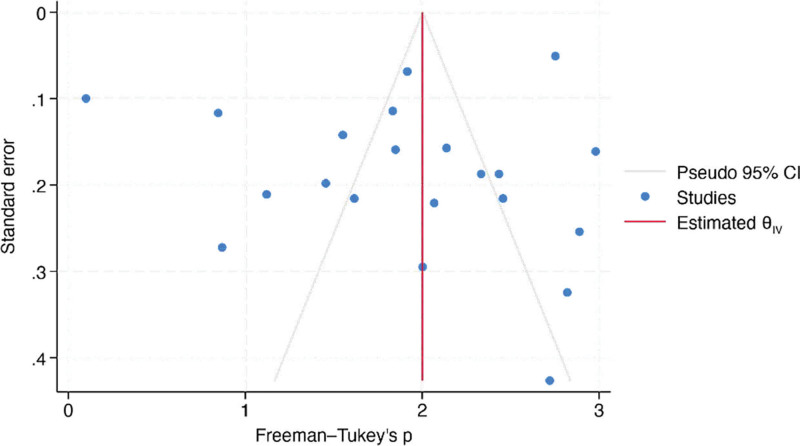
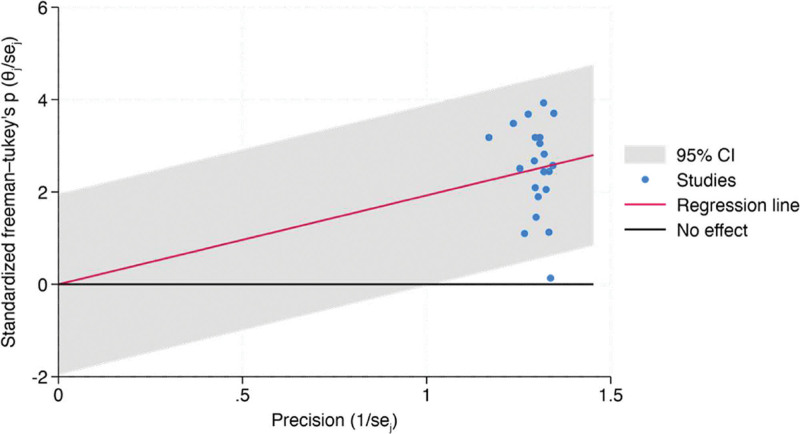
Meta-analysis synthesized data from 21 studies investigating the resolution rates of PG following a single treatment. The overall proportion of patients reporting complete resolution was estimated to be 68.2% (95% CI, 51.4%–83.1%). Significant heterogeneity was observed among the studies (I² = 96.68%). A leave-one-out meta-analysis remained stable at 68.2% (95% CI, 51.4%–83.1%), with all P values associated with the omitted studies being significant (P < 0.001). The test of theta yielded a statistically significant effect (z = 10.20, P < 0.001), indicating an overall positive treatment effect. Likewise, the test of homogeneity was significant (Q = 813.30, P < 0.001). The regression-based Egger test suggests no significant evidence of publication bias in the meta-analysis of PG resolution rates (P = 0.2222) as well as a fill-and-trim analysis, which remained consistent at 1.926 (95% CI, 1.597–2.255). Corresponding Galbraith and funnel plots are represented by Figures 4 and 5.
Fig. 4.
Funnel plot of meta-analysis.
Fig. 5.
Galbraith plot of meta-analysis.
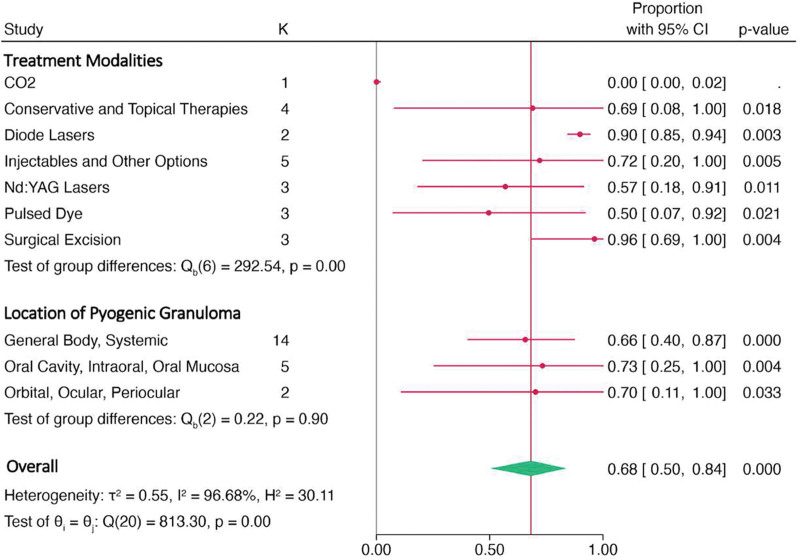
A subgroup analysis forest plot on treatment modalities and location of PG is demonstrated by Figure 6. Surgical excision seems to be the most effective therapy for resolving PG, with a high proportion of patients reporting complete resolution (96.2%, 95% CI, 86.9%–100.0%). Conservative interventions also show promising results, with a proportion of 69.0% (95% CI, 28.6%–97.8%), and injectables and Nd:YAG laser therapies demonstrating relatively high-resolution rates as well (72.0%, 95% CI, 34.4%–98.3% and 56.9%, 95% CI, 38.4%–74.6%, respectively). Resolution rates among affected areas (general body, ocular, or oral) were not statistically significant.
Fig. 6.
Forest plot of subgroup analysis among various types of treatment modalities and different lesion locations.
INTERVENTION-LINKED PG COMPLICATIONS IN EYELID RECONSTRUCTION
Flaps and Grafts
PG eruption emerges as a common postoperative complication following eyelid reconstructive surgery. In 41 patients who underwent a semicircular flap repair without posterior lamellar reconstruction, PG eruptions were observed in 24.4% of cases, all of which received subsequent treatment of topical steroids and triamcinolone injections, according to a retrospective chart review.57 A review of dermis-fat grafting for anophthalmic socket reconstruction showed a PG occurrence at a rate of 12.9% in eight patients.58 Although infrequent, in a new technique of lower eyelid reconstruction using a transverse facial artery perforator flap, PG presented in one case at 9% during follow-up.59 The one-stage free tarsoconjunctival graft and musculocutaneous transposition flap approach also showed an isolated case with an incidence of 16.7%, which was treated with surgical exicison.60 Despite generally positive outcomes with less-invasive procedures, PG complications underscore the necessity for continued vigilance in reconstructive techniques to ensure optimal results and minimal postoperative issues (Table 1).
Table 1.
Eyelid Reconstruction, Flaps, and Grafts
| Author | Year | Level of Evidence | Patients (n) | Mean Age (y) | Treatment Intervention/Modality | PG Percentage | Treatment to Resolve PG | Follow-up (mo) | |
|---|---|---|---|---|---|---|---|---|---|
| McNutt, et al57 | 2015 | III | 41 | 74 | Semicircular rotational flap closure | 10/41 | 24.4% | Topical steroids, triamcinolone injections, and excision | 9.8 |
| Galindo-Ferreiro, et al58 |
2018 | III | 62 | 34.2 | Dermis-fat graft in anophthalmic sockets | 8/62 | 12.9% | Not specified | 6 |
| Yamakawa, et al59 |
2022 | III | 11 | 85.7 | Transverse facial artery perforator flap | 1/11 | 9.0% | Not specified | 13 |
| Pham, et al60 | 2022 | III | 6 | 61.3 | One-stage free tarsoconjunctival graft and musculocutaneous transposition flap | 1/6 | 16.7% | Surgical excision | 8.5 |
Surgical Techniques
Several reconstructive techniques aimed at addressing eyelid conditions have also revealed PG as an important complication. A retrospective review of the lateral canthal “V” incision with a lateral tarsal strip for floppy eyelid syndrome showed an 11% PG formation, affecting a single patient.61 The internal cantholysis technique for closure of moderate and large full-thickness eyelid defects also demonstrated an 11% incidence of PG in two patients, despite achieving favorable cosmetic outcomes.62 In a noncomparative prospective study of 32 patients, using a combined technique of amniotic membrane and oral mucosa transplantation for severe symblepharon-related fornix reconstruction, a notable occurrence of PGs was observed at a rate of 12.5%.63 A 2011 randomized clinical trial of 1452 patients evaluating first-time trachomatous trichiasis surgery revealed an overall PG incidence rate of 10.5%.64 A later randomized clinical trial in 2013 of 1917 patients by the same author showed varied rates of PG formation for the tarsal/tarsorrhaphy clamp and the bilamellar tarsal rotation at 16.8% and 22.4%, respectively.65 Additionally, a 2024 noncomparative prospective study evaluating cryopreserved ultra-thick human amniotic membrane for anophthalmic socket contracture management reported that eight of 42 eyelids (19.0%) developed PGs.66 These studies emphasize the need for heightened awareness and refining approaches to mitigate PG complications in reconstructive techniques for optimal postoperative outcomes (Table 2).
Table 2.
Eyelid Reconstruction, Surgical Techniques
| Author | Year | Level of Evidence | Patients (n) | Mean Age (y) | Treatment Intervention/Modality | PG Percentage | Treatment to Resolve PG | Follow-up (mo) | |
|---|---|---|---|---|---|---|---|---|---|
| Gower, et al61 | 2011 | I | 1452 | 47.2 | First-time trichiasis surgery | 198/1881 | 10.5% | Surgical excision | 1.5 |
| Gower, et al62 | 2013 | I | 1917 | 55.2 | Trachomatous trichiasis, with tarsal/tarsorrhaphy clamp | 281/1669 | 16.8% | Not specified | 24 |
| Trachomatous trichiasis, Standard bilamellar tarsal rotation instrumentation | 375/1674 | 22.4% | |||||||
| Perry, et al63 | 2013 | III | 18 | 73 | Internal cantholysis for closure of larger full-thickness eyelid defects, transconjunctival approach | 2/18 | 11.1% | Conservative measures | 4.6 |
| Kheirkhah, et al64 | 2013 | II | 32 | 47.3 | Combined method: oral mucosal transplantation, and amniotic membrane transplantation for severe symblepharon | 4/32 | 12.5% | Triamcinolone injection and surgical excision | 16.4 |
| Phillips, et al65 | 2019 | III | 7 | 65 | Lateral canthal “V” incision with a lateral tarsal strip | 1/9 | 11.1% | Not specified | 17 |
| AlSemari, et al66 | 2024 | I | 33 | 40.9 | Cryopreserved ultra-thick human amniotic membrane for anophthalmic socket contracture | 8/42 | 19% | Excision and topical antibiotic with steroids | 10.9 |
CLINICALLY EFFECTIVE PG TREATMENT OPTIONS
Surgical Excision
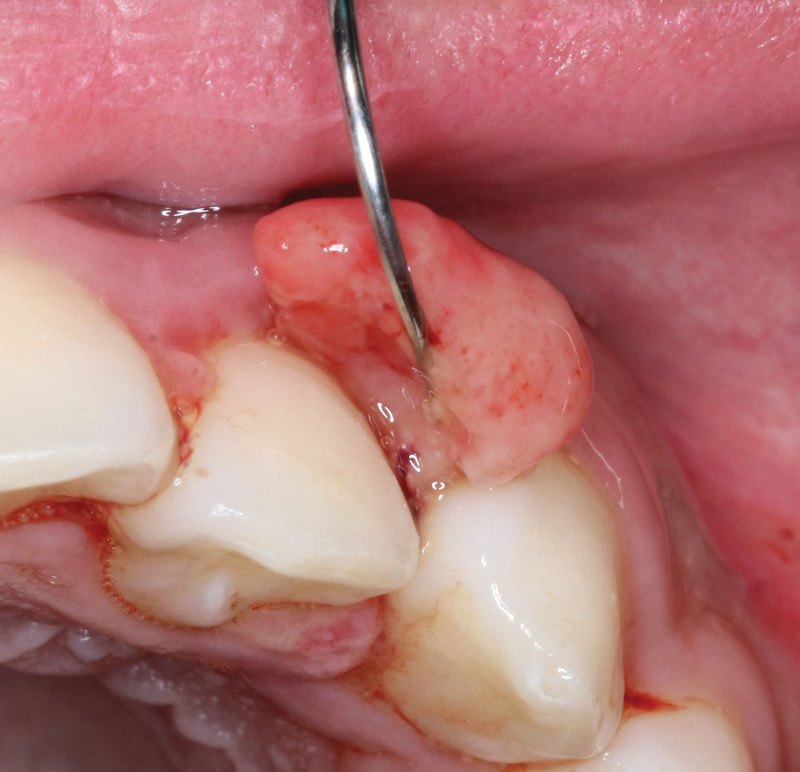
Surgical excision, particularly in intraoral lesions, head and neck, upper extremities, and digits proves highly effective, achieving a 98% resolution after a single session (Fig. 7). This outperforms curettage, cautery, or shave excision, as substantiated by both retrospective and prospective studies.67,68 In cases of gingival PGs, a retrospective study demonstrated that modified excision with deep curettage surpasses simple excision, achieving a nearly 15% higher success rate in 28 patients.69 Alternatively, the combination of shave excision with silver nitrate cauterization, while offering advantages such as shorter procedure times, cost-effectiveness, higher patient comfort, and superior scar assessment scores demonstrates a lower resolution rate at 90%, with a risk for temporary skin staining.68 The average follow-up after removal in these cases was approximately 11 months (Table 3).
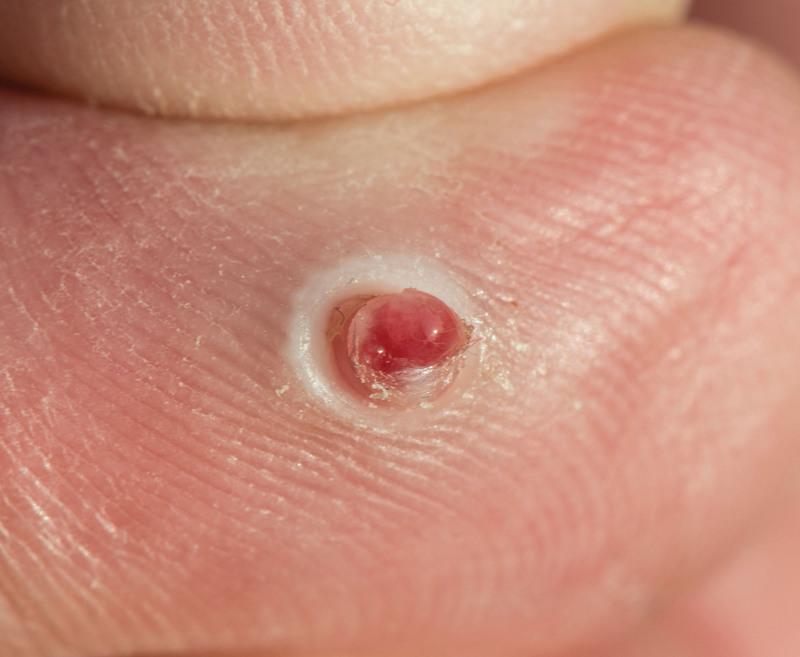
Fig. 7.
Clinical presentation of a PG on the second digit of the hand. Photograph attribution: CLS Digital Arts, ID: 182431496.
Table 3.
Clinically Effective PG Treatment: Surgical Excision
| Author | Year | LOE | Patients (n) | Age (y) | Areas Affected | Treatment Type | Resolution after 1 Session | Sessions to Resolve | Last Follow-up (m) | Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| Giblin et al67 | 2007 | III | 388 | 40.5 | Head/neck, intraoral areas | Surgical excision | 96.4% | 1 | Not specified | Scar formation and aesthetically unpleasant results |
| Curettage, shave excision, or cautery | 90% | 1 | ||||||||
| Al-Noaman68 | 2020 | III | 28 | 35.7 | Gingiva, mandibular and maxillary | Simple excision, root planning | 85.2% | 1 | 12 | Not specified |
| Modified excision with deep curettage | 100% | 1 | ||||||||
| Çelik et al69 | 2023 | II | 38 | 38.4 | Head/neck, upper extremities, digits | Shave excision with silver nitrate cauterization | 90% | 1.1 | 9.4 | Wound dehiscence, temporary skin staining with silver nitrate treatment |
| Surgical excision | 100% | 1 |
Nd:YAG Laser Therapy
Nd:YAG laser therapy exhibits diverse success rates, as highlighted in various studies. In two retrospective case series studies, success rates of approximately 50% were attained after a single session, often leading to complete resolution after two sessions.70,71 A 2012 noncomparative prospective study demonstrated a 74% success rate after a single session, requiring an average of 1.5 sessions for resolution, with follow-up conducted at 22 months.72 Despite these successes, Nd:YAG lasers, across all studies, often necessitated multiple sessions and were associated with crusting, pain during and after treatment, and the potential for bleeding and scarring (Table 4). In contrast, a 2022 prospective observational study using the combined continuous-wave/pulsed CO2 laser approach eradicated PGs in a single session with a 98% success rate; occasional cases of transient dyspigmentation and erythema were reported.73 Further studies are necessary to validate the efficacy of CO2 lasers (Table 4).
Table 4.
Clinically Effective PG Treatment: Nd:YAG and CO2 Lasers
| Author | Year | LOE | Patients (n) | Age (y) | Areas Affected | Treatment Type | Resolution after 1 Session | Sessions to Resolve | Last Follow-up (m) | Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| Raulin et al70 | 2002 | II | 100 | 26.8 | Head/neck, digits, integument | CO2/continuous wave | 98% | 1 | 6 | Transient hypopigmentation, hyperpigmentation, erythema |
| Bédard et al71 | 2009 | III | 25 | 39.3 | Not specified | Nd:YAG | 44% | 2.28 | 2 | Pain during treatment, transient swelling, bleeding, hypopigmentation, induration |
| Hammes et al72 | 2012 | II | 20 | 35.5 | Head/neck, extremities, torso, groin | Nd:YAG | 74% | 1.5 | 22 | Crusting |
| Dong et al73 | 2019 | III | 21 | 40 | Digits | Nd:YAG | 53% | 1.5 | 12 | Pain during and after treatment, swelling, bleeding, scaring |
Pulsed-dye and Diode Lasers
Pulsed-dye lasers exhibit differing success rates across three retrospective reviews in the literature. In one study, a modest clearance of 25% on the head/neck and digits was observed after initial therapy, requiring two repeat sessions over 36 months.74 A more recent 2022 study reported a 66.8% clearance with a single session, observing increased responsiveness in smaller lesions (2.2 mm in diameter and 1.3 mm in height) and nonorbital areas during a year of follow-up.75 The effectiveness of pulsed-dye lasers, when combined with shave excision, surpasses the efficacy of either therapy alone, however.76 Diode lasers, as demonstrated in a 2018 randomized clinical trial involving 21 patients showed faster incision speeds, shorter intervention times, reduced bleeding, and superior healing in gingival lesions, exceeding surgical excision by 8.2% after a single session.77 Further, a retrospective case series study on potassium-titanyl-phosphate diode lasers revealed a nearly 90% clearance rate with minimal scarring and few complications in treating PG (Table 5).78 The available literature on both pulsed-dye and diode lasers is currently limited; further large-scale research to validate the safety and efficacy of outcomes in PG removal is needed.
Table 5.
Clinically Effective PG Treatment: Pulsed-dye and Diode Lasers
| Author | Year | LOE | Patients (n) | Age (y) | Areas Affected | Treatment Type | Resolution after 1 session | Sessions to Resolve | Last Follow-up (mo) | Complications Reported |
|---|---|---|---|---|---|---|---|---|---|---|
| Pulsed-dye | ||||||||||
| Tay et al74 | 1997 | III | 22 | 3.4 | Head/Neck, fingers | Pulsed-dye | 25% | 2.2 | 36 | Not specified |
| Sud et al75 | 2010 | III | 49 | 23.5 | Head/Neck, limbs, and trunk | Pulsed-dye | Not specified | 1.8 | Not specified | Not specified |
| Surgical excision | 1.7 | |||||||||
| Shave- excision and pulsed-dye | 1.1 | |||||||||
| Wu et al76 | 2022 | III | 212 | 3 | Head/neck, orbital | Pulsed-dye | 66.8% | 1 | 12 | Edematous erythema, slight bleeding, hyperpigmentation, and hypopigmentation |
| Diode Lasers | ||||||||||
| Isola et al77 | 2018 | I | 21 | 46.5 | Gingiva, mandibular and maxillary | Diode Laser | 90% | 1 | 1 | Postoperative discomfort and pain |
| Surgical excision | 81.8% | 1 | ||||||||
| Just et al78 | 2019 | III | 28 | 30.4 | Head/neck, upper extremities, trunk | potassium-titany-phosphate Laser | 89.3% | 1.1 | 3 | Postoperative pain, minimal scaring |
Sclerotherapy and Injectables
Sclerotherapy and injectables show considerable disparity in treating PG. Monoethanolamide oleate injections achieved 100% efficacy without recurrence in nine patients, but pain on injection and postinflammatory pigmentation were reported.79 A review of 3% sodium tetradecyl sulfate showed only a 16% resolution, with multiple sessions, worse pain management, more side effects, and lower resolution after a single treatment compared with diode lasers at 81.3% in oral lesions.80 Polidocanol, a foam sclerosing agent, achieved a 73% elimination rate after a single session in a retrospective review involving 11, but adverse effects such as swelling, fever, and skin rashes were observed.81 Ethanol injections resulted in complete resolution of all PG cases in the head/neck and digits with a single session, albeit a small sample of five patients and a short follow-up at one month.82 In a 2006 randomized clinical trial, cryotherapy/liquid nitrogen had increased sessions and lower rates of resolution (63%) compared with curettage and electrodesiccation (97%) in cutaneous and labial lesions (Table 6).83
Table 6.
Clinically Effective PG Treatment: Injectables and Other Options
| Author | Year | LOE | Patients (n) | Age (y) | Areas Affected | Treatment Type | Resolution after 1 Session | Sessions to Resolve | Last Follow-up (m) | Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| Matsumoto et al79 | 2001 | III | 9 | 25.2 | Head/neck, oral cavity | Sclerotherapy, monoethanolamide oleate | 100% | 1 | 3 | Pain during injection, postinflammatory pigmentation |
| Ichimiya et al80 | 2004 | III | 5 | 38.2 | Head/neck, digits | Ethanol injection | 100% | 1 | 1 | Pain and swelling |
| Ghodsi et al81 | 2006 | I | 76 | 34.8 | Cutaneous or labial | Cryotherapy/liquid nitrogen | 63% | 1.42 | 4 | Scaring, dyspigmentation |
| Curettage and electrodesiccation | 97% | 1.03 | ||||||||
| Shivhare et al82 | 2022 | III | 73 | 36.9 | Oral cavity | Diode Laser | 81.3% | 1 | 3 | Pain, edema, ulceration, ecchymosis, infections, and scarring |
| Sclerotherapy 3% sodium tetradecyl sulfate | 16.6% | >1 | ||||||||
| Yang et al83 | 2023 | III | 11 | 14.8 | Head/neck, trunk, extremities | Sclerotherapy, polidocanol | 73% | 1.45 | 6 | Swelling, fever, skin rash, and red rash |
Conservative and Topical Therapies
Conservative and topical therapies represent viable options for PG management. A recent prospective study highlighted the efficacy of topical 0.5% timolol eye drops, particularly in ophthalmic PG cases, with 77.5% achieving an excellent response, complete resolution over a 6-month follow-up, and no complications (Fig. 8).84 Topical timolol/propranolol was revealed to be less effective, but showing only a 15% resolution in periungual PGs at a short follow-up of 1 month.85 In cases of PGs arising from burns, a retrospective review indicated that conservative approaches resulted in increased healing, whereas surgical interventions showed mixed outcomes.86 Additionally, conservative periodontal therapy in a retrospective review addressing gingival pregnancy tumors contributed to tumor regression in 64% of patients.87 Collectively, these studies emphasize the potential of conservative and topical therapies as effective, noninvasive alternatives for treating PG, with favorable clinical outcomes with minimal adverse effects (Table 7).
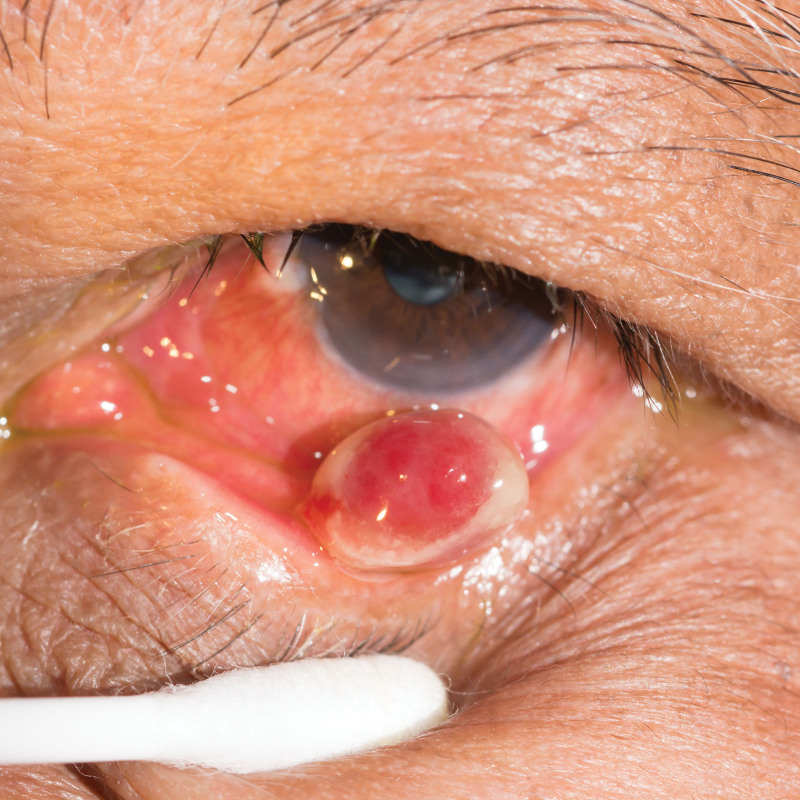
Fig. 8.
Clinical presentation of a PG on the inferior palpebral margin. Photograph attribution: ARZTSAMUI, ID: 364003601.
Table 7.
Clinically Effective PG Treatment: Conservative and Topical Therapies
| Author | Year | LOE | Patients (n) | Age (y) | Areas Affected | Treatment Type | Resolution after 1 Session | Sessions to Resolve | Last Follow-up (mo) | Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| Zhu et al84 | 2016 | III | 39 | 27.1 | Maxillary, mandibular, gingival pregnancy tumors | Conservative, periodontal therapy | 64% | NA | 20 | Not specified |
| Surgical excision after failed conservative therapy | 100% | NA | ||||||||
| Sibaud et al85 | 2019 | III | 13 | NA | Paronychia/periungual | Beta blocker, timolol/propranolol | 15% | NA | 1 | Not specified |
| Zhao et al86 | 2019 | III | 15 | 19.1 | Head/neck, trunk, limbs; burn associated | Conservative, wound debridement, dressings, antibiotics | 100% | NA | 6 | Scar contracture deformity |
| Full-thickness excision | 66% | NA | ||||||||
| Shave excision | 66% | NA | ||||||||
| Jaiswal et al87 | 2021 | II | 40 | 23.5 | Eyes, palpebral or bulbar conjunctiva | Topical 0.5% timolol eye drops | 77.5% | NA | 6 | Not specified |
DISCUSSION
This practical review aims to analyze the spectrum of surgical interventions linked to the development and eruption of PG, along with evaluating clinically effective treatment modalities, both surgical and nonsurgical, documented in the existing literature. The findings uncovered an incidence of postoperative PG ranging from 9% to 24.4% across ophthalmologic and oculoplastic procedures in eyelid reconstruction. Multiple techniques, including semicircular flap repair, dermis-fat grafting, and transverse facial artery perforator flap repair, have reported PG rates between 9% and 16.7%. Although less frequent in some procedures, occurrences in techniques like lateral canthal incision and amniotic membrane with oral mucosa transplantation highlight the need for refining reconstructive approaches to minimize PG-related complications for optimal postoperative outcomes.57–65,88–91
In evaluating treatment modalities for PG, the meta-analysis synthesized data from 21 studies investigating resolution rates showed that the overall proportion of patients achieving complete resolution was 68.2% (95% CI, 51.4%–83.1%). Notably, resolution rates did not significantly differ across affected areas, suggesting consistent treatment efficacy regardless of lesion location. Surgical excision emerged as the most effective therapy, with 96.2% (95% CI, 86.9%–100.0%) achieving resolution. The advantages of surgical excision, notably minimal complications and high success rates with fewer required treatments overshadowed alternative techniques like curettage, cautery, and shave excisions.67–69 The application of lasers, specifically Nd:YAG, CO2, pulsed-dye, and diode lasers, revealed varying success rates in PG resolution. CO2 and diode lasers, however, demonstrated clear considerable success comparably, outperforming in both eliminations after a single treatment and mean sessions to complete resolve.70–78 Injectable therapies like sclerotherapy and ethanol injections also proved effective.79–83 Alternatively, conservative and topical therapies, including topical beta-blockers, emerged as noninvasive options in PG management, highlighting their safety, especially in those unwilling to pursue surgical options.84–87
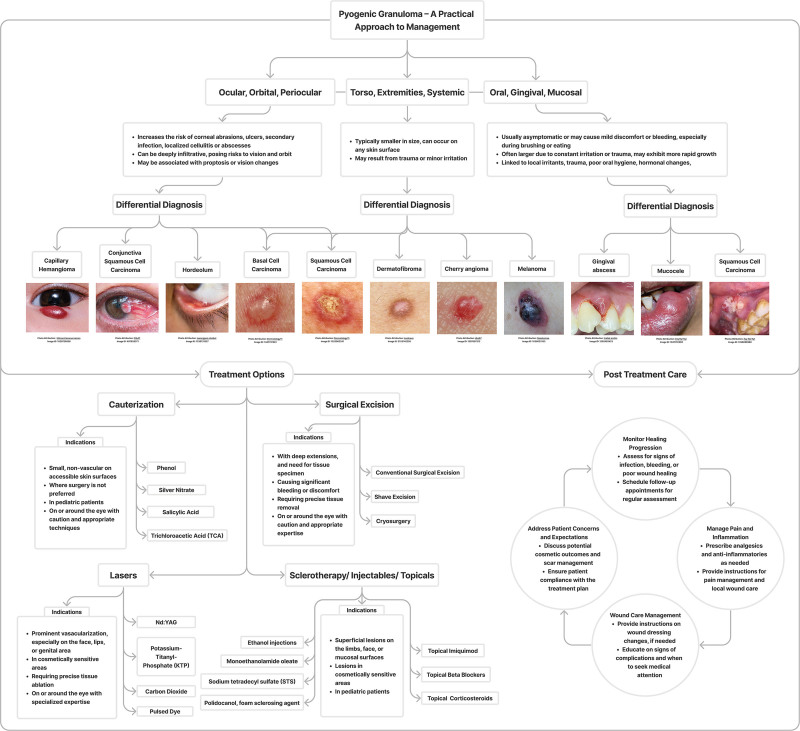
As clinicians face the challenge of navigating the complexities of PG management, including a comprehensive flowchart diagram (Fig. 9) depicting potential differential diagnoses and available treatment options is a valuable tool. This visual aid provides a systematic approach to decision-making, enhancing diagnostic accuracy and guiding therapeutic strategies for improved patient outcomes.
Fig. 9.
Flowchart diagram for a practical approach on PG management.
LIMITATIONS
Key challenges due to the diverse array and heterogeneity of the studies make direct comparisons of surgical approaches for PG formation and treatment difficult. Varying levels of evidence and differing follow-up periods in these studies may have impacted the reported complication rates, emphasizing the need for higher-level evidence trials. Potential selection biases cannot be disregarded. Limitations of conducting a meta-analysis should also be acknowledged. These may include heterogeneity in study methodologies, populations, and outcomes, which can affect the generalizability of findings. The quality of included studies and potential publication bias could influence the reliability of the meta-analytic results as well as the availability of the data may have restricted the scope of the analysis, potentially overlooking relevant studies or subgroups.
CONCLUSIONS
This comprehensive practical review accentuates the multifaceted nature of PG interventions, showcasing successes and challenges across diverse surgical techniques, conservative therapies, lasers, surgical excision, and injectables. The significance of meticulous surgical approaches to minimize complications and the promising outcomes of laser therapies in managing PG underscore the need for tailored treatment strategies. Further comparative studies are imperative to refine therapeutic choices and enhance clinical decision-making for optimal PG management.
DISCLOSURES
Dr. Janis receives royalties from Springer Publishing and Thieme Publishers. The other author has no financial interest to declare in relation to the content of this article.
Footnotes
Published online 13 September 2024.
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.Poncet A, Dor L. Botryomycosis humaine. Rev de Chir. 1897;18:996–997. [Google Scholar]
- 2.Goss JA, Greene AK. Congenital vascular tumors. Otolaryngol Clin North Am. 2018;51:89–97. [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro JL, Moraes RM, Carvalho BFC, et al. Oral pyogenic granuloma: an 18-year retrospective clinicopathological and immunohistochemical study. J Cutan Pathol. 2021;48:863–869. [DOI] [PubMed] [Google Scholar]
- 4.Bejjanki KM, Mishra DK, Kaliki S. Periocular lobular capillary hemangioma in adults: a clinicopathological study. Middle East Afr J Ophthalmol. 2019;26:138–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rastogi K, Singh L, Khan NA, et al. Benign vascular anomalies: a transition from morphological to etiological classification. Ann Diagn Pathol. 2020;46:151506. [DOI] [PubMed] [Google Scholar]
- 6.Tobouti PL, Olegário I, de Sousa SC. Benign vascular lesions of the lips: diagnostic approach. J Cutan Pathol. 2017;44:451–455. [DOI] [PubMed] [Google Scholar]
- 7.Al Dhaybi R, Powell J, McCuaig C, et al. Differentiation of vascular tumors from vascular malformations by expression of Wilms tumor 1 gene: evaluation of 126 cases. J Am Acad Dermatol. 2010;63:1052–1057. [DOI] [PubMed] [Google Scholar]
- 8.Atherton K, Hinen H. Vascular anomalies: other vascular tumors. Dermatol Clin. 2022;40:401–423. [DOI] [PubMed] [Google Scholar]
- 9.Koo MG, Lee SH, Han SE. Pyogenic granuloma: a retrospective analysis of cases treated over a 10-year. Arch Craniofac Surg. 2017;18:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagerman GF, Silva-Velazco J, Molina-Lopez JF. Miscellaneous perianal afflictions. Clin Colon Rectal Surg. 2019;32:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richert B, Lecerf P, Caucanas M, et al. Nail tumors. Clin Dermatol. 2013;31:602–617. [DOI] [PubMed] [Google Scholar]
- 12.Alomari MH, Kozakewich HPW, Kerr CL, et al. Congenital disseminated pyogenic granuloma: characterization of an aggressive multisystemic disorder. J Pediatr. 2020;226:157–166. [DOI] [PubMed] [Google Scholar]
- 13.Walker JL, Wang AR, Kroumpouzos G, et al. Cutaneous tumors in pregnancy. Clin Dermatol. 2016;34:359–367. [DOI] [PubMed] [Google Scholar]
- 14.Lopez A, Tang S, Kacker A, et al. Demographics and etiologic factors of nasal pyogenic granuloma. Int Forum Allergy Rhinol. 2016;6:1094–1097. [DOI] [PubMed] [Google Scholar]
- 15.Ferry AP. Pyogenic granulomas of the eye and ocular adnexa: a study of 100 cases. Trans Am Ophthalmol Soc. 1989;87:327–343; discussion 343. [PMC free article] [PubMed] [Google Scholar]
- 16.Wu D, Qian T, Nakao T, et al. Medically uncontrolled conjunctival pyogenic granulomas: correlation between clinical characteristics and histological findings. Oncotarget. 2017;8:2020–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tukenmez Demirci G, Atis G, Kivanc Altunay I, et al. The epidemiology of non-melanocytic benign and malignant skin tumors in pediatric patients attending to the dermatology department. J Clin Med Res. 2015;7:770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SC, Patel RM, Lucas DR, et al. Sinonasal lobular capillary hemangioma: a clinicopathologic study of 34 cases characterizing potential for local recurrence. Head Neck Pathol. 2013;7:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagliai KA, Cohen BA. Pyogenic granuloma in children. Pediatr Dermatol. 2004;21:10–13. [DOI] [PubMed] [Google Scholar]
- 20.Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267–276. [DOI] [PubMed] [Google Scholar]
- 21.Wollina U, Langner D, França K, et al. Pyogenic granuloma—a common benign vascular tumor with variable clinical presentation: new findings and treatment options. Open Access Maced J Med Sci. 2017;5:423–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong CHL, Dean DR, Hull K, et al. World workshop on oral medicine VII: relative frequency of oral mucosal lesions in children, a scoping review. Oral Dis. 2019;25:193–203. [DOI] [PubMed] [Google Scholar]
- 23.Soyele OO, Ladeji AM, Adebiyi KE, et al. Pattern of distribution of reactive localised hyperplasia of the oral cavity in patients at a tertiary health institution in Nigeria. Afr Health Sci. 2019;19:1687–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saleh SM, Idris AM, Vani NV, et al. Retrospective analysis of biopsied oral and maxillofacial lesions in South-Western Saudi Arabia. Saudi Med J. 2017;38:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatem M, Abdulmajid ZS, Taher EM, et al. Benign orofacial lesions in Libyan population: a 17 years retrospective study. Open Dent J. 2015;9:380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aly MM, Abdul-Aziz MAM, Elchaghaby MA. A retrospective analysis of oral and maxillofacial pathological lesions in a group of Egyptian children over 21 years. BMC Oral Health. 2022;22:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prosdócimo ML, Agostini M, Romañach MJ, et al. A retrospective analysis of oral and maxillofacial pathology in a pediatric population from Rio de Janeiro-Brazil over a 75-year period. Med Oral Patol Oral Cir Bucal. 2018;23:e511–e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapthanasupkul P, Juengsomjit R, Klanrit P, et al. Oral and maxillofacial lesions in a Thai pediatric population: a retrospective review from two dental schools. J Med Assoc Thai. 2015;98:291–297. [PubMed] [Google Scholar]
- 29.Zuñiga MD, Méndez CR, Kauterich RR, et al. Paediatric oral pathology in a Chilean population: a 15-year review. Int J Paediatr Dent. 2013;23:346–351. [DOI] [PubMed] [Google Scholar]
- 30.Daif ET. Correlation of age, sex, and location with recurrence of oral giant pyogenic granuloma after surgical excision. J Craniofac Surg. 2016;27:e433–e435. [DOI] [PubMed] [Google Scholar]
- 31.Tamiolakis P, Chatzopoulou E, Frakouli F, et al. Localized gingival enlargements. A clinicopathological study of 1187 cases. Med Oral Patol Oral Cir Bucal. 2018;23:e320–e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy V, Saxena S, Reddy M. Reactive hyperplastic lesions of the oral cavity: a ten year observational study on North Indian Population. J Clin Exp Dent. 2012;4:e136–e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saravana GH. Oral pyogenic granuloma: a review of 137 cases. Br J Oral Maxillofac Surg. 2009;47:318–319. [DOI] [PubMed] [Google Scholar]
- 34.Buchner A, Shnaiderman-Shapiro A, Vered M. Relative frequency of localized reactive hyperplastic lesions of the gingiva: a retrospective study of 1675 cases from Israel. J Oral Pathol Med. 2010;39:631–638. [DOI] [PubMed] [Google Scholar]
- 35.Al-Khateeb T, Ababneh K. Oral pyogenic granuloma in Jordanians: a retrospective analysis of 108 cases. J Oral Maxillofac Surg. 2003;61:1285–1288. [DOI] [PubMed] [Google Scholar]
- 36.Al-Khateeb TH. Benign oral masses in a Northern Jordanian population—a retrospective study. Open Dent J. 2009;3:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishnapillai R, Punnoose K, Angadi PV, et al. Oral pyogenic granuloma—a review of 215 cases in a South Indian Teaching Hospital, Karnataka, over a period of 20 years. Oral Maxillofac Surg. 2012;16:305–309. [DOI] [PubMed] [Google Scholar]
- 38.Montazer Lotf-Elahi MS, Farzinnia G, Jaafari-Ashkavandi Z. Clinicopathological study of 1000 biopsied gingival lesions among dental outpatients: a 22-year retrospective study. BMC Oral Health. 2022;22:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manjunatha BS, Sutariya R, Nagamahita V, et al. Analysis of gingival biopsies in the Gujarati population: a retrospective study. J Cancer Res Ther. 2014;10:1088–1092. [DOI] [PubMed] [Google Scholar]
- 40.Shamim T, Varghese VI, Shameena PM, et al. A retrospective analysis of gingival biopsied lesions in South Indian population: 2001–2006. Med Oral Patol Oral Cir Bucal. 2008;13:E414–E418. [PubMed] [Google Scholar]
- 41.Effiom OA, Adeyemo WL, Soyele OO. Focal reactive lesions of the Gingiva: an analysis of 314 cases at a tertiary Health Institution in Nigeria. Niger Med J. 2011;52:35–40. [PMC free article] [PubMed] [Google Scholar]
- 42.Sarwal P. Pyogenic granuloma. In: StatPearls. Treasure Island, Fla.: StatPearls Publishing. Available at https://www.ncbi.nlm.nih.gov/books/NBK556077/. Updated October 23, 2023. [Google Scholar]
- 43.Williams J, Depcik-Smith N, Williams T, et al. Metastatic renal clear cell carcinoma masquerading as a pyogenic granuloma on the lip. Dermatol Online J. 2021;27. [DOI] [PubMed] [Google Scholar]
- 44.Yadav S, Thami GP, Bhatnagar A, et al. Polypoid basal cell carcinoma masquerading as pyogenic granuloma. Indian J Dermatol. 2010;55:296–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khullar G, Singh S, Saikia UN, et al. Squamous cell carcinoma of the nail fold masquerading as pyogenic granuloma. Indian J Dermatol Venereol Leprol. 2016;82:555–557. [DOI] [PubMed] [Google Scholar]
- 46.Bains A, Vedant D, Shanker V, et al. Primary cutaneous anaplastic large cell lymphoma masquerading as large pyogenic granuloma. Indian Dermatol Online J. 2016;7:526–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee MC, Huang YL, Yang CH, et al. Cutaneous seeding of hepatocellular carcinoma due to percutaneous ethanol injection and masquerading as a pyogenic granuloma. Dermatol Surg. 2004;30:438–440. [DOI] [PubMed] [Google Scholar]
- 48.Cohen PR. Pleomorphic appearance of breast cancer cutaneous metastases. Cureus. 2021;13:e20301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moshe M, Levi A, Ad-El D, et al. Malignant melanoma clinically mimicking pyogenic granuloma: comparison of clinical evaluation and histopathology. Melanoma Res. 2018;28:363–367. [DOI] [PubMed] [Google Scholar]
- 50.Rao AG, Babu VA, Koppada D, et al. Amelanotic melanoma in the vicinity of acquired melanocytic nevi and not arising from agminated melanocytic nevi: masquerading as pyogenic granuloma. Indian J Dermatol. 2016;61:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tashiro J, Perlyn CA, Melnick SJ, et al. Non-pigmented melanoma with nodal metastases masquerading as pyogenic granuloma in a 1-year old. J Pediatr Surg. 2014;49:653–655. [DOI] [PubMed] [Google Scholar]
- 52.So NL, Chan CF, Ho KW, et al. Amelanotic melanoma masquerading as a pyogenic granuloma: caution warranted. Hong Kong Med J. 2014;20:265.e1–265.e2. [DOI] [PubMed] [Google Scholar]
- 53.Gounder P, Lam M, Vinciullo C, et al. Malignant fibrous histiocytoma masquerading as pyogenic granuloma. Orbit. 2017;36:122–123. [DOI] [PubMed] [Google Scholar]
- 54.Plachouri KM, Georgiou S. Therapeutic approaches to pyogenic granuloma: an updated review. Int J Dermatol. 2019;58:642–648. [DOI] [PubMed] [Google Scholar]
- 55.Lee J, Sinno H, Tahiri Y, et al. Treatment options for cutaneous pyogenic granulomas: a review. J Plast Reconstr Aesthet Surg. 2011;64:1216–1220. [DOI] [PubMed] [Google Scholar]
- 56.Howick J, Chalmers I, Glasziou P, et al. Oxford Centre for Evidence-Based Medicine 2011 levels of evidence. Available at https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence. Published 2011. Accessed November 20, 2023. [Google Scholar]
- 57.McNutt SA, Weber AC, Costin BR, et al. Rotational flap repair of full thickness eyelid defects without a posterior lamellar graft or flap. Orbit. 2015;34:268–273. [DOI] [PubMed] [Google Scholar]
- 58.Galindo-Ferreiro A, Khandekar R, Hassan SA, et al. Dermis-fat graft for anophthalmic socket reconstruction: indications and outcomes. Arq Bras Oftalmol. 2018;81:366–370. [DOI] [PubMed] [Google Scholar]
- 59.Yamakawa S, Suda S, Hayashida K. A new lower eyelid reconstruction using transverse facial artery perforator flap based on an anatomical study. J Plast Reconstr Aesthet Surg. 2023;77:39–45. [DOI] [PubMed] [Google Scholar]
- 60.Pham CM, Heinze KD, Mendes-Rufino-Uehara M, et al. Single-stage repair of large full thickness lower eyelid defects using free tarsoconjunctival graft and transposition flap: experience and outcomes. Orbit. 2022;41:178–183. [DOI] [PubMed] [Google Scholar]
- 61.Phillips ME, Fowler BT, Dryden SC, et al. Canthal V-plasty for floppy eyelid surgery. Plast Reconstr Surg Glob Open. 2019;7:e2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perry JD, Mehta MP, Lewis CD. Internal cantholysis for repair of moderate and large full-thickness eyelid defects. Ophthalmology. 2013;120:410–414. [DOI] [PubMed] [Google Scholar]
- 63.Kheirkhah A, Ghaffari R, Kaghazkanani R, et al. A combined approach of amniotic membrane and oral mucosa transplantation for fornix reconstruction in severe symblepharon. Cornea. 2013;32:155–160. [DOI] [PubMed] [Google Scholar]
- 64.Gower EW, Merbs SL, Munoz BE, et al. Rates and risk factors for unfavorable outcomes 6 weeks after trichiasis surgery. Invest Ophthalmol Vis Sci. 2011;52:2704–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gower EW, West SK, Harding JC, et al. Trachomatous trichiasis clamp vs standard bilamellar tarsal rotation instrumentation for trichiasis surgery: results of a randomized clinical trial. JAMA Ophthalmol. 2013;131:294–301. [DOI] [PubMed] [Google Scholar]
- 66.AlSemari MA, AlZahrani F, Ahad M, et al. Clinical use of cryopreserved ultra-thick human amniotic membrane for anophthalmic socket contracture. Eur J Ophthalmol. 2024;34:672–677. [DOI] [PubMed] [Google Scholar]
- 67.Giblin AV, Clover AJ, Athanassopoulos A, et al. Pyogenic granuloma—the quest for optimum treatment: audit of treatment of 408 cases. J Plast Reconstr Aesthet Surg. 2007;60:1030–1035. [DOI] [PubMed] [Google Scholar]
- 68.Çelik M, Kara M, Özdemir AG, et al. Comparison of the efficacy of combined silver nitrate coagulation and shave excision with surgical excision and linear closure in the treatment of pyogenic granuloma. Dermatol Surg. 2023;49:473–478. [DOI] [PubMed] [Google Scholar]
- 69.Al-Noaman AS. Pyogenic granuloma: clinicopathological and treatment scenario. J Indian Soc Periodontol. 2020;24:233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bédard MS, Boulanger J. Treatment of lobular capillary hemangioma with the Nd:YAG laser: retrospective case series of 25 patients. J Cutan Med Surg. 2009;13:181–182. [DOI] [PubMed] [Google Scholar]
- 71.Dong J, Peng SG, Zhang XY, et al. Efficacy of Nd-YAG laser for treatment of pyogenic granuloma on the fingers and toes. Lasers Med Sci. 2019;34:41–45. [DOI] [PubMed] [Google Scholar]
- 72.Hammes S, Kaiser K, Pohl L, et al. Pyogenic granuloma: treatment with the 1,064-nm long-pulsed neodymium-doped yttrium aluminum garnet laser in 20 patients. Dermatol Surg. 2012;38:918–923. [DOI] [PubMed] [Google Scholar]
- 73.Raulin C, Greve B, Hammes S. The combined continuous-wave/pulsed carbon dioxide laser for treatment of pyogenic granuloma. Arch Dermatol. 2002;138:33–37. [DOI] [PubMed] [Google Scholar]
- 74.Tay YK, Weston WL, Morelli JG. Treatment of pyogenic granuloma in children with the flashlamp-pumped pulsed dye laser. Pediatrics. 1997;99:368–370. [DOI] [PubMed] [Google Scholar]
- 75.Wu JP, Dong LP, Lu XY, et al. Treatment of pyogenic granuloma in children with a 595 nm pulsed dye laser: a retrospective study of 212 patients. Lasers Surg Med. 2022;54:835–840. [DOI] [PubMed] [Google Scholar]
- 76.Sud AR, Tan ST. Pyogenic granuloma-treatment by shave-excision and/or pulsed-dye laser. J Plast Reconstr Aesthet Surg. 2010;63:1364–1368. [DOI] [PubMed] [Google Scholar]
- 77.Isola G, Matarese G, Cervino G, et al. Clinical efficacy and patient perceptions of pyogenic granuloma excision using diode laser versus conventional surgical techniques. J Craniofac Surg. 2018;29:2160–2163. [DOI] [PubMed] [Google Scholar]
- 78.Just U, Hinterhuber G, Knobler R, et al. A potassium-titanyl-phosphate laser is an efficacious tool in the treatment of pyogenic granulomas. A retrospective study in 28 patients. Photochem Photobiol Sci. 2019;18:343–348. [DOI] [PubMed] [Google Scholar]
- 79.Matsumoto K, Nakanishi H, Seike T, et al. Treatment of pyogenic granuloma with a sclerosing agent. Dermatol Surg. 2001;27:521–523. [DOI] [PubMed] [Google Scholar]
- 80.Shivhare P, Haidry N, Sah N, et al. Comparative evaluation of efficacy and safety of the diode laser (980 nm) and sclerotherapy for the treatment of oral pyogenic granuloma. Int J Dent. 2022;2022:8269221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang C, Li M, Li X, et al. Foam sclerotherapy in the treatment of hemangiomas and venous malformations. Dermatol Surg. 2023;49:855–861. [DOI] [PubMed] [Google Scholar]
- 82.Ichimiya M, Yoshikawa Y, Hamamoto Y, et al. Successful treatment of pyogenic granuloma with injection of absolute ethanol. J Dermatol. 2004;31:342–344. [DOI] [PubMed] [Google Scholar]
- 83.Ghodsi SZ, Raziei M, Taheri A, et al. Comparison of cryotherapy and curettage for the treatment of pyogenic granuloma: a randomized trial. Br J Dermatol. 2006;154:671–675. [DOI] [PubMed] [Google Scholar]
- 84.Jaiswal H, Patidar N, Shah C, et al. Topical timolol 0.5% as the primary treatment of ophthalmic pyogenic granuloma: a prospective, single-arm study. Indian J Ophthalmol. 2021;69:1155–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sibaud V, Casassa E, D’Andrea M. Are topical beta-blockers really effective “in real life” for targeted therapy-induced paronychia. Support Care Cancer. 2019;27:2341–2343. [DOI] [PubMed] [Google Scholar]
- 86.Zhao H, Huang S, Fu X. Should pyogenic granulomas following burns be excised? Burns. 2015;41:431–436. [DOI] [PubMed] [Google Scholar]
- 87.Zhu YQ, Wang YQ, Tang YC, et al. Initial periodontal therapy for the treatment of gingival pregnancy tumor. Genet Mol Res. 2016;15:gmr.15028119. [DOI] [PubMed] [Google Scholar]
- 88.Dadeya S, Kamlesh, Khurana C, et al. Intraoperative daunorubicin versus conjunctival autograft in primary pterygium surgery. Cornea. 2002;21:766–769. [DOI] [PubMed] [Google Scholar]
- 89.Kheirkhah A, Nazari R, Nikdel M, et al. Postoperative conjunctival inflammation after pterygium surgery with amniotic membrane transplantation versus conjunctival autograft. Am J Ophthalmol. 2011;152:733–738. [DOI] [PubMed] [Google Scholar]
- 90.Liu HY, Chen YF, Chen TC, et al. Surgical result of pterygium extended removal followed by fibrin glue-assisted amniotic membrane transplantation. J Formos Med Assoc. 2017;116:10–17. [DOI] [PubMed] [Google Scholar]
- 91.Kothari EA, Tenewitz JE, Jayman JR, et al. Pilot study of a glue-less, suture-less amniotic membrane for pterygium excision. Int Ophthalmol. 2022;42:2933–2938. [DOI] [PubMed] [Google Scholar]