Abstract
Background
Selumetinib is the first approved treatment for pediatric patients with neurofibromatosis type 1 (NF1) and symptomatic, inoperable plexiform neurofibromas (PN) in the EU and US, as well as in multiple other countries. Evidence for the management of selumetinib-associated adverse events (AEs) is mostly limited to clinical trials and expanded-access programs. We gathered a panel of European healthcare practitioners with clinical experience prescribing selumetinib and/or managing pediatric patients with NF1-PN to provide recommendations on the prevention and management of AEs.
Methods
A modified Delphi approach was used to develop the recommendations among the group of experts. Initial statements were developed from a literature review of current management recommendations and regulatory reports. The panel refined the statements and rated the extent to which they agreed with them in 2 sessions and a follow-up survey. The panel comprised 2 pediatric neuro-oncologists, 1 pediatric oncologist, 1 pediatrician, 1 neuropediatrician, 1 oncologist, 1 neurologist, 2 psychologists, and 1 dermatologist.
Results
The experts agreed on the relative frequency and impact of AEs potentially associated with selumetinib. Consensus-level agreement was reached for 36 statements regarding the prevention and management of AEs potentially associated with selumetinib. Experts recommended treatments for AEs based on their experience.
Conclusions
The development of a variety of consensus statements indicates expert agreement on best practices for the prevention and management of AEs potentially associated with selumetinib in pediatric patients with NF1-PN. These events are generally manageable and should be considered alongside treatment benefit. Information sharing is warranted as further experience is gained.
Keywords: selumetinib, pediatric, neurofibromatosis type 1, plexiform neurofibromas, adverse event management
Neurofibromatosis type 1 (NF1) can be a debilitating condition that predisposes individuals to the development of tumors; with a birth incidence of between 1 in 2558 and 1 in 3333, it is one of the most common inherited genetic disorders.1,2 Of patients with NF1, up to 50% develop plexiform neurofibromas (PN), which may cause pain, motor dysfunction, and disfigurement.1 People with NF1 often have a reduced quality of life (QoL), including those with a mild phenotype.2 NF1-PN are associated with a range of physical morbidities; these morbidities can result in cognitive, social, and mental health complications, which can all substantially affect QoL.2–5 Pain caused by PN can disrupt daily activities such as school attendance, participation in daily activities, and sleep.6 People with PN are vulnerable to bullying, and can experience clinically significant anxiety and depression, as well as social withdrawal.6–8 This highlights the need for a multidisciplinary approach to the management of people with NF1-PN.
In June 2021, selumetinib (ARRY-142886, AZD6244), an oral, selective inhibitor of mitogen-activated protein kinase kinase (MEK) 1 and 2, was approved by the European Medicines Agency as monotherapy for the treatment of symptomatic, inoperable PN in pediatric patients with NF1 aged ≥3 years following FDA approval, in April 2020, for pediatric patients aged ≥2 years.9–11 For these patients, selumetinib is the first approved treatment for NF1-PN that would otherwise be managed symptomatically or with debulking surgery.12
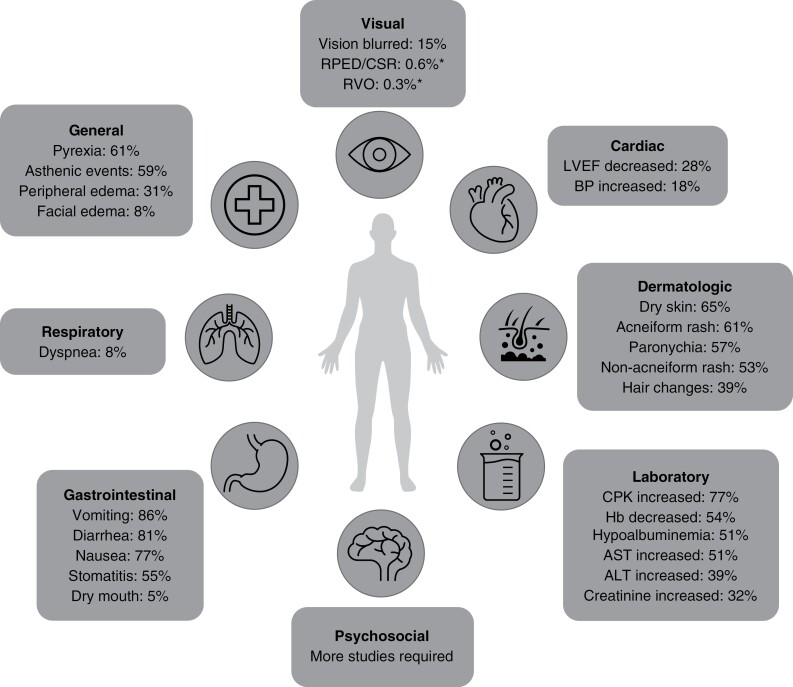
The approval of selumetinib was based on data from the SPRINT Phase II trial,13 in which selumetinib demonstrated a reduction in PN volume, extended progression-free survival compared with an indirect comparison with the National Cancer Institute Natural History study, and improved health-related QoL, pain, and functional outcomes in pediatric patients with NF1-PN.11 In terms of safety (Figure 1), the most frequent adverse events (AEs) in the trial were nausea, vomiting, diarrhea, asymptomatic creatine phosphokinase (CPK) elevation, acneiform rash, and paronychia.11 Most AEs were mild and there were no irreversible or cumulative adverse effects.11 Overall, 14 participants (28%) required a dose reduction and 5 (10%) discontinued treatment owing to adverse effects considered by the investigators to be possibly related to selumetinib.11 The safety profile of selumetinib is similar to that seen with other MEK inhibitors in patient populations, such as in those with melanoma.14–17
Figure 1.
Overall frequency of AEs reported in clinical trials of pediatric patients with NF1-PN and in adult patients with multiple tumor types.9,11,38 *Isolated cases of RPED, CSR, and RVO in adult patients with multiple tumor types, receiving treatment with selumetinib monotherapy and in combination with other anti-cancer agents, and in a single pediatric patient with pilocytic astrocytoma receiving selumetinib monotherapy have been observed.9 One report of CSR was made in a pediatric patient with NF1-PN during long-term follow-up (for up to 5 years) of patients enrolled in Phase I of SPRINT.38 A published case report noted development of outer retinal layer separation visualized by OCT in 2 children (aged 6 and 13 years, respectively) with optic pathway gliomas during selumetinib treatment.15
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BP, blood pressure; CPK, creatine phosphokinase; CSR, central serous retinopathy; Hb, hemoglobin; LVEF, left ventricular ejection fraction; NF1-PN, neurofibromatosis type 1 with plexiform neurofibromas; OCT, optical coherence tomography; RPED, retinal pigment epithelial detachment; RVO, retinal vein occlusion.
Given the therapeutic potential of selumetinib in treating pediatric patients with NF1-PN, it is important to minimize the impact of any potential AEs and avoid premature discontinuation or dose reductions that may lead to PN exacerbation. Currently, evidence for the management of AEs potentially associated with selumetinib is mostly limited to clinical trials. Physician experience of AE management for other MEK inhibitors in pediatric patients is likely to be limited, as published data are scarce. Expert recommendations on AE prevention and management are important for pediatric patients with NF1-PN, given they may be receiving treatment for several years, and a variety of practitioners are likely to be involved in providing their care. The psychosocial impact of AEs potentially associated with selumetinib should also be considered. A psychologist or specialist mental health nurse can provide support throughout the process and evaluate cognitive risk or fragility that may make implementation of measures to prevent and manage AEs more challenging; psychologists can evaluate the potential for and likely severity of psychosocial impacts of AEs. Furthermore, effective multidisciplinary management of AEs using dose modifications and patient or caregiver education have been shown to improve clinical outcomes.18,19
There is an unmet need for updated expert recommendations on the management of AEs potentially associated with selumetinib in patients with NF1-PN. Here we gathered a panel of European healthcare practitioners with clinical experience prescribing selumetinib (and/or other MEK inhibitors) and/or managing pediatric patients with NF1-PN to determine best practices and provide detailed strategies for the prevention and management of AEs potentially associated with selumetinib in this patient population.
Methods
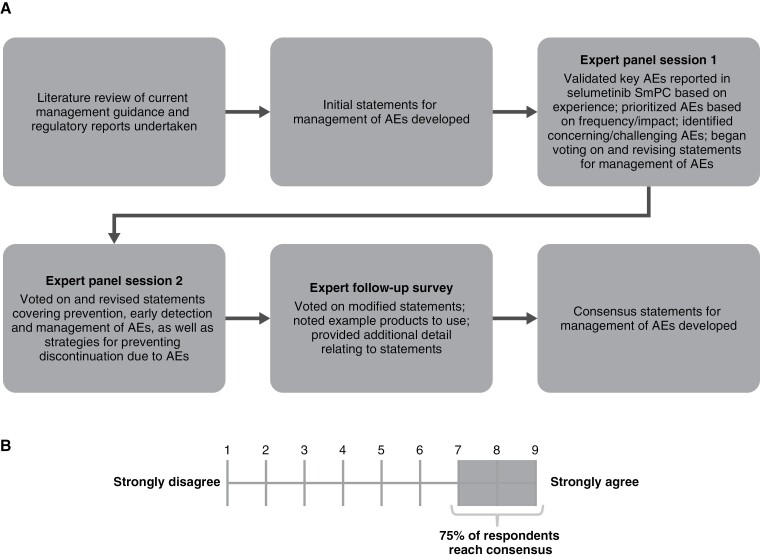
A modified Delphi approach was used to develop expert-led consensus statements for the prevention and management of AEs (Figure 2A). The panel of 10 experts refined the statements and rated the extent to which they agreed with them in 2 sessions and a follow-up survey. Unlike a traditional Delphi approach, initial statements were developed based on a literature review of current management recommendations and regulatory reports, in particular recommendations for MEK inhibitor use by Klesse et al. (2020) and the selumetinib Summary of Product Characteristics (SmPC).9,20
Figure 2.
The development of expert consensus statements using (A) a modified Delphi approach and (B) the Likert scale. AE, adverse event; SmPC, Summary of Product Characteristics.
Ten experts participated: 2 pediatric neuro-oncologists, 1 pediatric oncologist, 1 pediatrician, 1 neuropediatrician, 1 oncologist, 1 neurologist, 2 psychologists, and 1 dermatologist. Three experts were from Austria, 2 were from Italy, and 1 each was from Germany, France, Spain, Portugal, and the UK.
The panel was divided into 2 sessions separated by 1 week. In the first session, experts validated and prioritized the key AEs reported in the initial approved selumetinib label based on their experience and began voting on and revising initial statements on AE management. The second session focused entirely on voting on and revising statements. When referring to the severity of an AE, these are described as per the Common Terminology Criteria for Adverse Events (CTCAE) (ie, grade 1, mild; grade 2, moderate; grade 3, severe; grade 4, life-threatening; grade 5, death related to an AE).21
The experts voted on closed statements using a Likert scale (1–9), whereby 1 indicated they “strongly disagree” with the statement, and 9 indicated they “strongly agree” (Figure 2B). Consensus was defined as ≥75% of the panel rating their agreement as 7 or higher.
In the follow-up survey, the panel voted on modified statements, noted example products to use, and provided additional detail relating to the statements. If they disagreed with the statements, they had an opportunity to elaborate on their rationale for disagreement.
Results
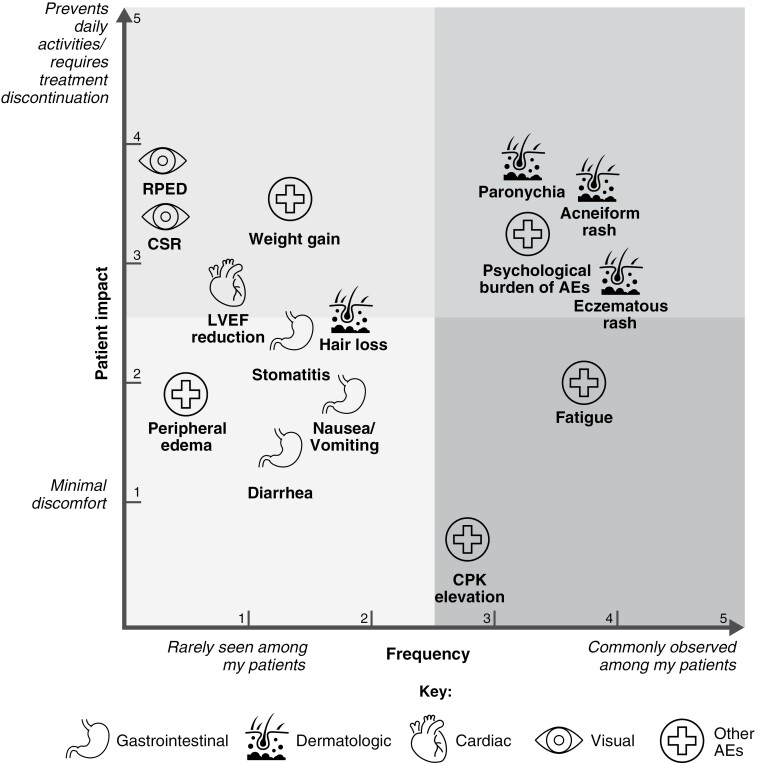
In the first session of the panel, the experts reached consensus on the relative observed frequency of AEs potentially associated with selumetinib, and their impact on patients (Figure 3), based on expert experience and as reported in the SPRINT Phase II trial (Stratum 1), in which AEs were assessed in 50 pediatric patients with symptomatic and inoperable PN.11
Figure 3.
Prioritization of AEs potentially associated with selumetinib in pediatric patients based on expert experience and as reported in the SPRINT Phase II trial. Experts were asked to add each AE to a co-ordinate system by considering the frequency at which each AE had been reported and the perceived impact of each on the QoL of pediatric patients, based on professional experience and patient-reported outcomes. AE, adverse event; CPK, creatine phosphokinase; CSR, central serous retinopathy; LVEF, left ventricular ejection fraction; QoL, quality of life; RPED, retinal pigment epithelial detachment.
On completion of the follow-up survey, consensus-level agreement had been reached for 36 statements regarding the prevention and management of AEs potentially associated with selumetinib (Table 1). Statements that did not reach consensus-level agreement are shown in Supplementary Table 1. Experts recommended treatments for AEs based on their experience. However, physicians should follow local guidelines and determine availability and accessibility when choosing a particular treatment for their patients, and use should reflect the severity of the AEs, as defined by CTCAE grade.21 When possible, management of AEs should be conducted using multidisciplinary consultation with relevant specialists. The selumetinib SmPC provides recommendations for dose reduction, interruption, and modification when required for management of AEs, and is considered the primary reference document for clinicians when prescribing selumetinib (Supplementary Table 2). The following sections reflect the recommendations of the expert panel and outline the consensus statements for each of the selected AEs. Specific examples of recommended treatment regimens for each AE (where applicable) are provided in Table 1. A Plain Language Summary can be found in the Supplementary Material.
Table 1.
Expert Recommendations for the Prevention and Management of AEs Potentially Associated with Selumetinib in Pediatric Patients with NF1-PN
| AE | Consensus statement | Level of consensusa | |
|---|---|---|---|
| Dermatologic | |||
| Paronychia | 1 | To prevent paronychia, patients should try to prevent potential traumas to the hands and feet (eg, avoiding tight shoes) and be advised on nail management (eg, not cutting the nails too short, avoiding cutting close to the nail fold) | 100% |
| • Soap substitutes: eg, Dermol® 500 lotion, Balneum Plus® bath oil, aqueous cream, Doublebase® emollient shower gel, Doublebase® bath additive, E45® bath additive, and Oilatum® shower gel | |||
| 2 | For all grades of paronychia, the affected nail should be softened with an antiseptic bath twice daily using recently opened antiseptic agents | 100% | |
| • Antiseptic agents: Diluted chlorhexidine, very diluted bleach soaks for nails, povidone–iodine solutions | |||
| 3 | Infection should be managed with an antiseptic with or without a topical antibiotic, according to local practice | 100% | |
| • Topical antibiotics: Mupirocin 2% w/w ointment, baneocin, topical clindamycin lotion (eg, Dalacin T® lotion) applied twice daily, clindamycin, and silver sulfadiazine 1% cream • Combined corticosteroid and topical antibiotic: Betamethasone valerate 0.1% and neomycin sulfate 0.5% (eg, Betnovate®-N) or betamethasone valerate 0.1% and fusidic acid 2% (eg, Fucibet®) • Topical antifungal in combination with a topical antibiotic • If a systemic antifungal is required, avoid fluconazole or itraconazole9 |
|||
| 4 | For CTCAE grade 2 paronychia, patients should use a high-potency steroid (topical) once infection is ruled out after collection of a sample from the affected site. A systemic antibiotic is recommended in case of infection. If such management is not successful, dose reduction then discontinuation of selumetinib should be considered | 100% | |
| • High-potency corticosteroids: Betamethasone valerate 0.1% (eg, Betnovate®) or fluocinolone acetamide 0.025% (eg, Synalar®) applied twice daily. Mometasone furoate 0.1% (eg, Elocon®) is a useful alternative and has the added advantage of only needing once daily application ○ Triamcinolone, fluocinonide, betamethasone dipropionate and gentamicin sulfate (eg, Diprogenta®), mometasone 0.1% (eg, Elocon®), and clobetasol, including clobetasol propionate 0.05% clobetasol propionate 0.05% (eg, Dermovate®), and clobetasol propionate 0.05%, neomycin sulfate 0.5%, nystatin 100 000 units/g (eg, Dermovate®-NN) • Systemic antibiotics (consider the age of the child and safety profiles when choosing the appropriate drug): Flucloxacillin, cefuroxime, ampicillin, and sulbactam (adjusted based on local antibiotic susceptibility), amoxicillin and clavulanic acid, azithromycin, minocycline, and clindamycin ○ Avoid systemic clarithromycin, erythromycin, or rifampicin9 |
|||
| 5 | For CTCAE grade 3 paronychia, if multiple nails are affected, patients should seek consultation for expert podiatry or surgical management and consider dose reduction or discontinuation of selumetinibb | 80% | |
| N/A | |||
| 6 | In the event of pus being present at the affected paronychia site, systemic antibiotics (tailored to the bacteria identified from a swab of the affected site) should be used in combination with a topical corticosteroid; corticosteroids should only be used as a standalone treatment once infection has been ruled out after collection of a sample from the affected site | 83% | |
| • Systemic antibiotics (consider the age of the child and safety profiles when choosing the appropriate drug): Flucloxacillin (when CTCAE grade 3 and superinfection is noted), cefuroxime, ampicillin, and sulbactam (adjusted based on local antibiotic susceptibility), flucloxacillin, and cefuroxime axetil (when CTCAE grade 3 and superinfection is noted) ○ Avoid systemic clarithromycin, erythromycin, or rifampicin9 • Corticosteroids: High potency products such as betamethasone valerate 0.1% (eg, Betnovate®) or fluocinolone acetamide 0.025% (eg, Synalar®) applied twice daily. Mometasone furoate 0.1% (eg, Elocon®) is a useful alternative and has the added advantage of only needing once daily application. Topical clobetasol propionate, triamcinolone, fluocinonide, mometasone 0.1% (eg, Elocon®), clobetasol propionate 0.05% (Dermovate®), and other ointments (eg, Dermovate®-NN: clobetasol propionate 0.05%, neomycin sulfate 0.5%, nystatin 100 000 units/g) |
|||
| 7 | To prevent further episodes of paronychia, the treating physician should consider referral to a specialist (eg, podiatrist, podologist, dermatologist, pediatric surgeon) | 88% | |
| N/A | |||
| Eczematous rash | 8 | Patients should moisturize twice daily with an emollient to prevent and manage an eczematous rash | 100% |
| • Emollients: eg, Daily baths (or showers) using mild cleansers or soap substitutes (such as standard emollients, eg, Dermol® 500 lotion and Dermol® cream). Bathing frequency of more than once per day will diminish skin hydration. Products with added fragrance including soaps, laundry detergent, and other scented products should be avoided. The choice of emollient should be guided by patient preference, lifestyle and practicality of application • Emollients include light (eg, Cetraben® lotion, QV® lotion), creamy (eg, Cetraben® cream, Diprobase® cream, Oilatum® cream), creamy with colloidal oatmeal (eg, Aveeno® cream), greasy (eg, Cetraben® ointment), very greasy (white soft paraffin ointment, 50:50 white soft paraffin: liquid paraffin, eg, Hydromol® ointment), urea-containing preparations (eg, Balneum Plus® cream), and preparations containing antimicrobials (eg, Dermol® 500 lotion, Dermol® cream). More than 1 emollient may be prescribed for use at different times of the day. Standard emollients can be used in the bath or shower as soap substitutes. Bath emollients containing antimicrobials (eg, Dermol® 600) are usually advocated for those with infected dermatitis. For dry lips consider plain petroleum jelly (eg, Vaseline®) or yellow soft paraffin. Proprietary preparations are also well tolerated by patients (eg, Nivea lip balm® and Labello® [Nivea®] lip butter®) |
|||
| 9 | If patients have a symptomatic eczematous rash, they should use a low-potency topical steroid | 100% | |
| • Low-potency steroids: eg, Low-potency steroids, such as 1% hydrocortisone (or other creams eg, Synalar® 1 in 10 dilution for those allergic to hydrocortisone), should be used twice daily in cases of mild/moderate (grade 1/2) dermatitis, and in sensitive areas such as the face and flexures (elbow/knee creases, skin folds, axillae) | |||
| 10 | If a symptomatic eczematous rash is not resolved by a low-potency topical steroid, patients should escalate to a more potent topical steroid. If it is not controlled with a more potent topical steroid, selumetinib dose reduction should be considered | 83% | |
| • Moderate potency steroids: Including clobetasone butyrate 0.05% (eg, Eumovate®), can be used on the trunk and limbs. These products can be used to induce symptom control in mild/moderate (grade 1–2) dermatitis (when prescribed regularly on a bd basis) and are also suitable for maintenance treatment (prescribed prn or ‘as required’ basis), and in those with moderate/severe (grade 2–3) dermatitis having stepped down from higher-potency products. Alternatives include “reduced strength” potent steroids such as betamethasone valerate 0.025% (eg, Betnovate-RD®) or fluocinolone acetamide 0.00625% (eg, Synalar® 1 in 4 dilution) • Higher-potency corticosteroids: eg, Betamethasone valerate 0.1% (eg, Betnovate®) or fluocinolone acetamide 0.025% (eg, Synalar®) applied twice daily ○ Clobetasol, triamcinolone, fluocinonide, betamethasone, clobetasone butyrate 0.05% (eg, Eumovate®), and betamethasone valerate 0.025% (eg, Betnovate-RD®) |
|||
| 11 | If an eczematous rash is present on the scalp, patients should consider using liquid or foam corticosteroids or coal tar shampoos | 100% | |
| • Dermatitis and/or dry scalp can usually be managed with over-the-counter shampoos—as per patient preference and affordability. Shampoos containing coal tar ± salicylic acid (eg, T-Gel®, Alphosyl 2 in 1®, Polytar®, Capasal®) can be prescribed. For moderate/severe (grade 2–3) scalp involvement consider adding a topical corticosteroid. Aqueous based products (eg, Synalar® gel) are often better tolerated (non-stinging) than topical corticosteroid lotions which may contain an alcohol base (betamethasone valerate 0.1% scalp application) • Shampoos designed for irritation: eg, Olsson® Sensitive Shampoo, E45® Dry Scalp Shampoo, and Eucerin DermoCapillaire® Calming Urea Shampoo • Medical emollients in lotion: eg, Diprobase® lotion, Doublebase® gel, and Emollin® spray-on oil • Examples of corticosteroids include Elocon® lotion, Bettamousse® gel, Synalar® gel, and liquid betamethasone • Coal tar shampoos: eg, Polytar® Scalp Shampoo Coal Tar Solution 4% ○ If systemic treatment is required, avoid ketoconazole, as this is a strong inhibitor of CYP3A49 |
|||
| 12 | If pruritus is present with an eczematous rash, an antihistaminergic agent should be added regardless of the grade of severity | 83% | |
| • Antihistaminergic agents: eg, Cetirizine and loratadine | |||
| Acneiform rash | 13 | For mild acneiform rashes, patients should use a topical antibiotic and/or low-potency topical corticosteroid | 100% |
| • Topical antibiotic: Clindamycin lotion (eg, Dalacin T lotion®) applied twice daily • Topical antifungal in combination with a topical antibiotic • If systemic treatment is needed, avoid fluconazole or itraconazole9 • Low-potency topical corticosteroids: eg, Hydrocortisone • Low-potency corticosteroids: eg, 1% hydrocortisone (or other creams eg, Synalar® 1 in 10 dilution for those allergic to hydrocortisone), should be used twice daily in cases of mild/moderate (grade 1–2) dermatitis, and in sensitive areas such as the face and flexures (elbow/knee creases, skin folds, axillae) • Low-potency topical corticosteroids: eg, Low-potency corticosteroids, such as 1% hydrocortisone (or other creams eg, Synalar® 1 in 10 dilution for those allergic to hydrocortisone), should be used twice daily in cases of mild/moderate (grade 1–2) dermatitis, and in sensitive areas such as the face and flexures (elbow/knee creases, skin folds, axillae) |
|||
| 14 | For patients with severe acneiform rashes, physicians should consider using semisynthetic oral tetracyclines. If the patient is aged <8 years, the use of topical antibiotics is preferred | 83% | |
| • Semisynthetic oral tetracyclines: Doxycycline. Minocycline may be used; however, some do not favor its use due to the AE profile ○ In children over the age of 12, lymecycline 408 mg can be used • Topical antibiotics: Mupirocin 2% w/w (eg, Bactroban®) and clindamycin 2% • If systemic treatment is required, avoid clarithromycin or erythromycin9 |
|||
| All dermatologic AEs | 15 | Patients should be advised to avoid excessive sun exposure or wear protective clothing (eg, hat, sunglasses, long sleeves, and trousers) in the sun to prevent or manage cutaneous rashes and hair discoloration | 85% |
| N/A | |||
| 16 | Psychosocial support should be available to reduce compulsive behavior leading to or exacerbating dermatologic AEs, such as obsessive or compulsive scratching or nail biting | 75% | |
| N/A | |||
| Gastrointestinal | |||
| Stomatitis | 17 | Treatment with an analgesic or topical nystatin is recommended for early-stage stomatitis | 100% |
| • Analgesics: Paracetamol and lidocaine (depending on severity) • Oral rinses: 0.9% saline or sodium bicarbonate ○ Avoid alcoholic mouth washes |
|||
| 18 | To help manage pain as a result of stomatitis, oral mucositis gels can be used | 100% | |
| • Oral mucositis gels: eg, Gelclair® and 2% viscous lidocaine | |||
| 19 | To prevent stomatitis, patients are recommended to follow an oral healthcare regimen including brushing teeth twice daily, brushing the tongue, and replacing toothbrushes at least every 3 months | 100% | |
| • Antiseptic mouth washes or saline or sodium bicarbonate rinses | |||
| 20 | T he patient and healthcare professional should regularly inspect the mouth for stomatitis | 100% | |
| N/A | |||
| Nausea and diarrhea |
21 | To manage nausea, diarrhea, and constipation, patients should avoid fried, fatty, and high-salt foods, in consultation with a dietitian if possible | 85% |
| N/A | |||
| 22 | If patients require treatment for nausea or vomiting due to gastritis, proton pump inhibitors should be used (excluding omeprazole) | 85% | |
| Proton pump inhibitors: Lansoprazole | |||
| 23 | If patients cannot manage nausea or vomiting with dietary changes alone, antiemetics can be used | 85% | |
| Antiemetics: Ondansetron, dimenhydrinate, metoclopramide, and granisetron | |||
| Cardiac | |||
| LVEF reduction | 24 | LVEF reduction often resolves spontaneously, so if it is within a reasonable range for the patient's age and they are not symptomatic, no management approaches are needed (aside from regular echocardiography) | 100% |
| N/A | |||
| 25 | If patients have discontinued selumetinib due to LVEF reduction, but LVEF recovers to the LLN (per local institution) within 3 months, selumetinib may be restarted at a reduced dose | 100% | |
| N/A | |||
| 26 | Although cardiac AEs are infrequent, patients should be monitored with echocardiograms approximately every 3 months, with more frequent monitoring at the start of treatment | 83% | |
| N/A | |||
| 27 | If patients have other cardiovascular risk factors, consider monitoring with echocardiograms more frequently, with periodic readings in intervals <3 months | 100% | |
| N/A | |||
| Visual | |||
| RPED/CSR | 28 | An ophthalmologic evaluation, including fundoscopy and OCT, should be performed prior to treatment initiation to establish a baseline for future evaluations | 100% |
| N/A | |||
| 29 | Although visual AEs are infrequent, patients should have ophthalmologic evaluations, including fundoscopy and OCT, every 3–6 months. More frequent assessments may be warranted in younger children | 83% | |
| N/A | |||
| 30 | If patients are diagnosed with RPED or CSR, regardless of whether visual acuity is affected, interruption selumetinib treatment should be considered until the RPED or CSR is resolved | 100% | |
| N/A | |||
| Other | |||
| CPK elevation | 31 | Physicians should ensure that CPK elevation is a resting measurement and not related to other muscle injury or vigorous exercise | 100% |
| N/A | |||
| 32 | To monitor for CPK elevation, physicians should perform blood tests every 1–3 months at the start of treatment, later transitioning to tests every 4–6 months | 85% | |
| N/A | |||
| 33 | If CPK elevation is <5 times the upper limit of normal (ie, CTCAE grade 1 or 2) and patients are asymptomatic, selumetinib should not be discontinued | 100% | |
| N/A | |||
| 34 | If patients are diagnosed with rhabdomyolysis, permanent selumetinib discontinuation is recommended | 85% | |
| N/A | |||
| Accumulation of lower-grade AEs | 35 | Ensure appropriate education of patients and caregivers on the potential AEs and the need to control them with proactive AE management | 100% |
| N/A | |||
| Psychological AEs | 36 | Reinitiate selumetinib on a trial basis, after a guided (interdisciplinary team) break, to assess if the patient can cope better with the benefit or risk of treatment | 100% |
| N/A | |||
All branded products listed are examples only.
aProportion of valid votes from experts (N = 10) who rated 7+ on the Likert scale. bNote that the selumetinib SmPC makes no specific recommendations on timelines for restarting the drug and such recommendations are based on the expert opinion of the panel.
AE, adverse event; bd, twice daily; CPK, creatine phosphokinase; CSR, central serous retinopathy; CTCAE, Common Terminology Criteria for Adverse Events; LLN, lower limit of normal; LVEF, left ventricular ejection fraction; N/A, not available; NF1-PN, neurofibromatosis type 1 with plexiform neurofibromas; OCT, optical coherence tomography; prn, pro re nata; RPED, retinal pigment epithelial detachment; SmPC, Summary of Product Characteristics.
Dermatologic Adverse Events
Dermatologic AEs potentially associated with selumetinib treatment include acneiform and maculopapular rash, pruritis (itchy skin), dry skin, paronychia, and eczematous rash; the most commonly reported are dry skin and acneiform rash.11 The psychological burden of these AEs can be high, with the potential to negatively impact treatment compliance if the patient’s caregiver and physician decide to discontinue treatment. The burden of these AEs may also affect a patient’s ability to perform daily activities,22 potentially leading to feelings of worry, frustration, and depression.23 Paronychia, defined as inflammation of the nail fold, has been indicated as a reason for treatment discontinuation or interruption and may be associated with pain and functional impairment; therefore, management must be considered.22,24 Eczematous rash causes inflammation and irritation of the skin, and studies have indicated that its severity is related to QoL.23,25,26 Acneiform rashes are characterized by erythematous papular and pustular lesions.27 Facial rashes are often a cause for discontinuation in adolescent patients, resulting from the social burden of this AE.22 Panel recommendations on the management of these and other dermatologic AEs are provided here.
Paronychia
To prevent paronychia, patients should try to prevent potential traumas to the hands and feet (eg, avoiding tight shoes) and be advised on nail management (eg, not cutting the nails too short, avoiding cutting close to the nail fold).
Other nail management tips include avoidance of biting or chewing nails, picking cuticles, scratching, manicuring, and finger sucking. Antiseptics may be used before cutting nails and the use of soap substitutes can be considered.
2. For all grades of paronychia, the affected nail should be softened with an antiseptic bath twice daily using recently opened antiseptic agents.
Nails should be soaked for 5–20 min once or twice daily in lukewarm or warm water. In the literature, softening of nails using antiseptic baths is noted as often being sufficient for the treatment of paronychia without pus.28 After soaking, nails should be rinsed and thoroughly dried. Such treatment can continue until recovery, but if no improvement is observed after 1 week, therapeutic escalation can be considered.
3. Infection should be managed with an antiseptic with or without a topical antibiotic, according to local practice.
The dosage and duration of treatment depend on the specific topical antibiotic used. In general, the use of a topical antibiotic 2–3 times daily is recommended, although this may depend on severity and tolerance. Topical antibiotics may only be used for mild infections and there is some disagreement on the use of occlusion with the topical antibiotic. If possible, the choice of topical antibiotic should be tailored to the bacteria identified from a swab of the affected site. A combined preparation of a steroid and topical antibiotic could be considered to manage the chronic inflammatory reaction seen with MEK inhibition.29 If there is evidence of a fungal infection, a topical antifungal could be used in combination with a topical antibiotic. When selecting a systemic antifungal, fluconazole (a strong cytochrome P450 2 C19 [CYP2C19]/moderate CYP3A4 inhibitor) or itraconazole (a strong CYP3A4 inhibitor) should be avoided due to their potential to increase the maximum plasma concentration of selumetinib.9
4. For CTCAE grade 2 paronychia, patients should use a high-potency steroid (topical) once infection is ruled out after collection of a sample from the affected site. A systemic antibiotic is recommended in case of infection. If such management is not successful, dose reduction then discontinuation of selumetinib should be considered.
If just 1 finger or toe is involved, this may not warrant the use of systemic antibiotics, and treatment should be left to the physician’s best practices and judgment. Occlusive bandages could be used alongside topical steroids; for this, fludroxycortide tape for chronic non-infected paronychia was recommended, as it provides the dual effect of topical steroid application and absorption and occlusion.9 The specific choice of antibiotic should be guided by swab results, if available.
5. For CTCAE grade 3 paronychia, if multiple nails are affected, patients should seek consultation for expert podiatry or surgical management and consider dose reduction or discontinuation of selumetinib.
A few of the experts noted that some dermatologists may not be in favor of podiatry or surgical management and that topical or oral treatment may be sufficient for grade 3 (severe) paronychia. Dose reduction or discontinuation may also be considered if all treatment measures have been exhausted, or if symptoms of systemic infection appear (eg, fever, reduced general condition). Before restarting selumetinib, the panel recommends waiting at least 2 weeks and until the paronychia has healed.
Note that the selumetinib SmPC makes no specific recommendations on timelines for restarting the drug and such recommendations are based on the expert opinion of the panel.
6. In the event of pus being present at the affected paronychia site, systemic antibiotics (tailored to the bacteria identified from a swab of the affected site) should be used in combination with a topical or systemic corticosteroid; corticosteroids should only be used as a standalone treatment once infection has been ruled out after collection of a sample from the affected site.
If the responsible bacteria cannot be identified, Staphylococcus can be targeted as it is the most likely to be involved. Systemic steroids are rarely used and there is a strong preference for topical steroids.
7. To prevent further episodes of paronychia, the treating physician should consider referral to a specialist (eg, podiatrist, podologist, dermatologist, pediatric surgeon).
Referral to a specialist should be considered both to treat and prevent paronychia; however, this may be unnecessary for mild manifestations. Such referrals could be more strongly considered for prevention of paronychia if a patient has experienced previous episodes.
Eczematous Rash
8. Patients should moisturize twice daily with an emollient to prevent and manage an eczematous rash.
The whole body should be moisturized, with attention to exposed areas and skin folds. Moisturizing is particularly recommended following showers or baths.
9. If patients have a symptomatic eczematous rash, they should use a low-potency topical steroid.
Treatment should be used for 1–2 weeks and can be stopped earlier if the rash has resolved within this time frame.
10. If a symptomatic eczematous rash is not resolved by a low-potency topical steroid, patients should escalate to a more potent topical steroid. If it is not controlled with a more potent topical steroid, selumetinib dose reduction should be considered.
In general, the panel recommends waiting 3–7 days before escalating to a more potent topical steroid. If an improvement is seen with a more potent topical steroid, subsequent use of emollients and low-potency topical steroids can help control the rash. In the event of a potential secondary infection (eg, from excoriation, impetiginized eczema, or eczema herpeticum) more urgent dermatological assessment and escalation of treatment may be required.
11. If an eczematous rash is present on the scalp, patients should consider using liquid or foam corticosteroids or coal tar shampoos.
Patients should first use emollient shampoos and those designed for irritation before using corticosteroids or coal tar shampoos. If topical corticosteroids are added for moderate−severe scalp involvement, they should be used for a maximum of 2 weeks.
12. If pruritus is present with an eczematous rash, an antihistaminergic agent should be added regardless of the grade of severity.
An antihistamine should only be used if the rash is symptomatic. The frequency of use depends on the specific antihistamine and extent of symptoms. If the patient’s sleep is disturbed due to the rash, an antihistamine with a sedative effect may be used.
Acneiform Rash
13. For mild acneiform rashes, patients should use a topical antibiotic and/or low-potency topical corticosteroid.
Overall, topical antibiotics are typically the first choice of treatment. Topical corticosteroids are then added only if notable inflammation is present with no sign of infection. Swabs should be considered to guide therapy if appropriate.
14. For patients with severe acneiform rashes, physicians should consider using semisynthetic oral tetracyclines. If the patient is aged <8 years, the use of topical antibiotics is preferred.
Oral antibiotics can be considered in more severe cases. Empirical treatment in line with acne management guidelines is recommended, unless bacterial swab sensitivities are available to guide treatment choice. Oral tetracyclines should be used for 2–6 weeks, as long as the patient is symptomatic. Minocycline is sometimes used; however, it is not recommended due to its associated AE profile. Systemic doxycycline treatment has been used to successfully treat a case of iatrogenic MEK1/2 inhibitor-induced acneiform rash following a slight worsening of the skin lesions after initial treatment with topical erythromycin cream and fusidic acid plus betamethasone cream.27 Resolution of a case of recalcitrant selumetinib-induced paronychia with doxycycline following treatment with various antibiotics, topical medications, and surgeries that were only deemed to be minimally effective, has also been reported for a 12-year-old patient with NF1.30 Therefore, doxycycline may be an acceptable therapeutic agent for patients aged ≥8 years with dermatologic AEs that have otherwise been difficult to treat.
All Dermatologic Events
15. Patients should be advised to avoid excessive sun exposure or wear protective clothing (eg, hat, sunglasses, long sleeves, and trousers) in the sun to prevent or manage cutaneous rashes and hair discoloration.
16. Psychosocial support should be available to reduce compulsive behavior leading to or exacerbating dermatologic AEs, such as obsessive or compulsive scratching or nail biting.
If feasible, a multidisciplinary team including a psychologist should be involved in treatment. If a psychologist is included in initial meetings, they can provide support throughout the treatment process and evaluate cognitive risk or fragility that may make implementation of measures to prevent and manage AEs more challenging, in addition to evaluating the potential for and likely severity of psychosocial impacts of AEs. Psychologists can provide and coordinate any necessary psychosocial support and inform patients and caregivers of preventative strategies for burdens resulting from AEs. This approach may be applicable to and have value for other AEs aside from those that are dermatologic.
Gastrointestinal Adverse Events
Although most gastrointestinal AEs potentially associated with selumetinib treatment have been reported as mild–moderate (grade 1 or 2) in severity, they are some of the most commonly reported.31 Abdominal pain, diarrhea, nausea, and vomiting are reported more frequently than constipation and oral mucositis.11 Stomatitis has also been reported, which is defined by the presence of ulcerative erosive lesions in the mouth;32 this AE can cause pain and may have a greater impact on some patient’s daily activities than nausea, vomiting, and diarrhea. Stomatitis has been associated with reduced QoL in both physical and social functions, as well as with depression and anger.32 Although diarrhea may not have as considerable an impact on daily life as stomatitis, it is a far more common AE that is potentially associated with selumetinib treatment, and leads to patients needing to adapt their school, family, and social lives.33 A significant decrease in QoL may be experienced by patients with severe diarrhea,34 and, therefore, prevention and treatment are critical. Panel recommendations for the management of stomatitis, nausea, and diarrhea are outlined here.
Stomatitis
17. Treatment with an analgesic or topical nystatin is recommended for early-stage stomatitis.
Early-stage stomatitis consists of redness and possible swelling or drooling, but no ulcerations. Analgesics or oral rinses are recommended; alcoholic mouth washes should be avoided.
18. To help manage pain as a result of stomatitis, oral mucositis gels can be used.
Oral mucositis gels should be used for as long as symptoms are present.
19. To prevent stomatitis, patients are recommended to follow an oral healthcare regimen including brushing teeth twice daily, brushing the tongue, and replacing toothbrushes at least every 3 months.
Soft toothbrushes should be used (eg, finger brushes). Since adherence to brushing teeth may be an issue in young children, a psychologist could advise caregivers on how to motivate children and manage brushing more easily. Regular antiseptic mouth washes or saline or sodium bicarbonate rinses may also be suitable. Patients should follow other good dental care regimens, including regular flossing, as dental issues may result in traumas to the mouth.
20. The patient and healthcare professional should regularly inspect the mouth for stomatitis.
Healthcare professionals should look for redness, swelling, drooling, and ulcerations when examining the mouth. If feasible, this inspection should occur at every visit, especially during the first 6 months of therapy. Since stomatitis is not common, it may not necessitate frequent inspection unless the patient or caregiver notes any symptoms. Therefore, caregivers and older patients should be encouraged to self-inspect the mouth and report any concerns to the relevant healthcare professionals.
Nausea and diarrhea
21. To manage nausea, diarrhea, and constipation, patients should avoid fried, fatty, and high-salt foods, in consultation with a dietitian if possible.
Although these dietary recommendations may be obvious to some, not all patients and caregivers will have the same understanding, so effective communication will be needed. Dietary restrictions should not be so strict that they conflict with general nutrition best practices, as they could be perceived as excessive and lead to non-compliance. Psychologists may support caregivers to motivate children to follow the dietary recommendations and prevent non-compliance.
22. If patients require treatment for nausea or vomiting due to gastritis, proton pump inhibitors should be used (excluding omeprazole).
Gastritis can be suspected by clinical history or symptoms and confirmed by an endoscopy. The use of omeprazole should be avoided because it is a CYP2C19 inhibitor and, therefore, may increase plasma concentrations of selumetinib.9 If nausea or vomiting is recurrent, input from a gastroenterologist may be needed when selecting a treatment.
23. If patients cannot manage nausea or vomiting with dietary changes alone, antiemetics can be used.
Antiemetics should only be used if patients have had serious nausea or vomiting. If used, patients may trial antiemetics (eg, selective serotonin receptor [5HT3] antagonists such as ondansetron) for 2–3 weeks to assess if treatment is effective.
Cardiac Adverse Events
Hypertension, left ventricular ejection fraction (LVEF) reduction (reduction in the amount of blood ejected during a ventricular contraction of the heart compared with the amount present prior to the contraction21), sinus tachycardia (heart beating faster than normal), and right ventricular dysfunction were reported in SPRINT.11 In SPRINT, cardiac AEs (eg, hypertension, 8%; asymptomatic decreased ejection fraction, 14%) were less frequently reported than dermatologic (eg, acneiform rash, 60%; pruritis, 32%) and gastrointestinal (eg, abdominal pain, 56%; diarrhea, 66%) AEs.11 However, the potential burden they can have on patients is high. Panel recommendations for the management of LVEF reduction are outlined here.
Left Ventricular Ejection Fraction Reduction
24. LVEF reduction often resolves spontaneously, so if it is within a reasonable range for the patient’s age and they are not symptomatic, no management approaches are needed (aside from regular echocardiography).
A reasonable range for LVEF reduction is considered above the lower limit of normal (LLN) for the patient’s age and if there are no other functional or anatomical changes on echocardiography or clinical symptoms of low cardiac output. In patients who develop symptomatic LVEF reduction or grade 3 or 4 LVEF reduction, selumetinib should be discontinued and prompt cardiology referrals should be carried out as per guidance in the SmPC.9
25. If patients have discontinued selumetinib due to LVEF reduction, but LVEF recovers to the LLN (per local institution) within 3 months, selumetinib may be restarted at a reduced dose.
If selumetinib is restarted at a reduced dose, it should be at the first recommended dosage reduction.9 Restarting selumetinib at a reduced dose is consistent with recommendations provided in clinical trials involving selumetinib (Supplementary Table 2). In some instances, physicians may only restart selumetinib at the full dosage once the AE is resolved. For more information, please refer to the SmPC.9
26. Although cardiac AEs are infrequent, patients should be monitored with echocardiograms approximately every 3 months, with more frequent monitoring at the start of treatment.
This recommendation is consistent with those for other MEK inhibitors; for example, echocardiograms are recommended before treatment, after the first month, then approximately every 3 months (and after any dose modifications) for dabrafenib and trametinib.35 Less frequent monitoring (eg, every 4–6 months) may be appropriate as treatment progresses. However, constant monitoring places a high burden on patients and may not be warranted due to the low frequency of cardiac AEs in real-world clinical practice and clinical trials. When to transition patients to less frequent monitoring is highly variable, with some transitioning after 3 months and others after 1–2 years, depending on prior physician experience.
27. If patients have other cardiovascular risk factors, consider monitoring with echocardiograms more frequently, with periodic readings in intervals <3 months.
Cardiovascular risk factors include hypertension, congenital heart disease, hypercholesterolemia, and diabetes mellitus. If feasible, cardiologists can also be consulted to understand the relevant risk factors for and management of LVEF reduction, if encountered.
Visual Adverse Events
Although visual AEs may be less frequently reported or diagnosed during selumetinib treatment of NF1 than those already discussed, there is potential for these to place a high burden on patients.11,20 Furthermore, visual AEs associated with MEK inhibitor treatment of a variety of conditions are well documented in the literature.20,36 Notably, 86 serous retinopathy events in 70 patients were observed in a study of 493 patients with BRAF-mutated melanoma who were treated with cobimetinib, a MEK inhibitor, and vemurafenib, a BRAF inhibitor.37
Cases of mild–moderate (grade 1 or 2) blurred vision, dry eye, ocular hypertension, cataract, photophobia, and watering eyes have previously been reported as AEs potentially associated with selumetinib treatment, all of which were relatively uncommon.11 One documented event of an ophthalmologic AE of special interest was identified from long-term (up to 5 years) SPRINT Phase I safety data; this participant developed shallow bilateral central serous retinopathy (CSR) without vision changes that was diagnosed by ocular coherence tomography (OCT) only (not present on clinical examination) on their post-cycle 94 evaluation, which had resolved at repeat evaluation 3 weeks later without discontinuation of selumetinib.38 Another publication documented the development of outer retinal layer separation visualized by OCT in 2 children (aged 6 and 13 years, respectively) with optic pathway gliomas during selumetinib treatment; in both cases, discontinuation of selumetinib resulted in resolution of the AE without sequelae but 1 patient experienced recurrence following retreatment with selumetinib.15 These cases highlight the importance of ophthalmologic screening during MEK inhibitor treatment. In SPRINT, ophthalmologic evaluation was performed after 4 and 12 months of treatment and then annually; this monitoring frequency appeared to be adequate for the patient population enrolled in SPRINT when conducted within the context of provision of appropriate education on concerning signs or symptoms of possible toxicities (eg, exercise intolerance or blurred vision).31 Consensus-level agreement was reached on the management of retinal pigment epithelial detachment (RPED) and CSR, as these are likely to have a major impact on a patient’s QoL. RPED is defined by the separation of the retinal pigment epithelium from the inner collagenous layer of Bruch’s membrane, and CSR by the serous detachment of the neurosensory retina secondary to 1 or more focal lesions of the retinal pigment epithelium.39,40 As young children may not be able to correctly or promptly report symptoms related to RPED or CSR, ophthalmologic screening is especially warranted in this population.
Retinal Pigment Epithelial Detachment and Central Serous Retinopathy
28. An ophthalmologic evaluation, including fundoscopy and optical coherence tomography (OCT), should be performed prior to treatment initiation to establish a baseline for future evaluations.
Evaluations should also be performed if patients note visual disturbances or symptoms (eg, squinting or blurred vision). However, since reporting such disturbances may be challenging for pediatric patients, caregivers should be educated to report symptoms if possible. OCT may not be feasible in very young patients due to a lack of cooperation.
29. Although visual AEs are infrequent, patients should have ophthalmologic evaluations, including fundoscopy and OCT, every 3–6 months. More frequent assessments may be warranted in younger children.
Some experts noted that ophthalmologic evaluations may typically be conducted more frequently at the beginning of treatment, with monitoring later transitioning to every 3–6 months. However, 1 study of 2 children with optic pathway gliomas reported the development of retinal edema after 6 and 7 months of selumetinib treatment, respectively, indicating the importance of more research to determine when retinal complications are most likely to develop during selumetinib treatment.15 Therefore, it is important to note that monthly monitoring may be warranted in some cases, including in children aged <6 years; in patients with limited verbal skills, who may not be able to accurately report symptoms associated with visual AEs; and in patients who have pre-existing abnormal fundoscopy results, decreased visual acuity, or ongoing progressing optical gliomas. Separate monitoring recommendations exist for patients with PNs involving the eyelid, orbit, periorbital, and facial structures, as they are at risk of vision loss even if selumetinib is not used.41
30. If patients are diagnosed with RPED or CSR, regardless of whether visual acuity is affected, interruption of selumetinib treatment should be considered until the RPED or CSR is resolved.
To determine if RPED or CSR has resolved, patients should be monitored with an ophthalmologic evaluation every 3–4 weeks. More frequent monitoring may be advisable in some cases, including in patients who have evidence of more advanced retinopathy. Determination of the most appropriate monitoring period should be made on an individual basis. Retinopathy potentially associated with MEK inhibitors typically has no or mild symptoms and, if diagnosed early (ie, before full retinal detachment occurs), any impact on visual acuity has the potential to resolve within 1–4 months. This highlights the importance of screening for this AE, particularly in younger children who may be unable to accurately report or verbalize any associated symptoms until the later stages of retinopathy.37
Other Adverse Events
Headache, dizziness, fatigue, and anemia, as well as psychological AEs such as depression and insomnia, may be considered potentially associated with selumetinib treatment.11 Panel recommendations for the management of CPK elevation, accumulation of lower-grade AEs, and psychological AEs are outlined here.
Creatine Phosphokinase Elevation
31. Physicians should ensure that CPK elevation is a resting measurement and not related to other muscle injury or vigorous exercise.
32. To monitor for CPK elevation, physicians should perform blood tests every 1–3 months at the start of treatment, later transitioning to tests every 4–6 months.
33. If CPK elevation is <5 times the upper limit of normal (ie, CTCAE grade 1 or 2) and patients are asymptomatic, selumetinib should not be discontinued.
Although CPK elevation may be frequent, it is often asymptomatic, and, therefore, monitoring should not be excessive to avoid unnecessary burden on patients.
34. If patients are diagnosed with rhabdomyolysis, permanent selumetinib discontinuation is recommended.
Some patients have rapidly progressing, inoperable PN, and, therefore, the ultimate decision on discontinuation should be made by the physician based on a risk–benefit analysis.
Accumulation of Lower-Grade Events
35. Ensure appropriate education of patients and caregivers on the potential AEs and the need to control them with proactive AE management.
Education should be provided by an interdisciplinary team including physicians, nurses, and a psychosocial caregiver. Patients should be closely monitored to detect small changes that may warrant intervention. Patients should be taught coping techniques, and age-appropriate visualization tools should be used that contain neutral information (eg, no emotional pictures). Finally, patients and caregivers should be made aware of patient organizations that can support them and reduce feelings of loneliness and isolation with the burden of their disease.
Psychological Events
36. Reinitiate selumetinib on a trial basis, after a guided (interdisciplinary team) break, to assess if the patient can cope better with the benefit or risk of treatment.
If feasible, patients and their families should receive psychological counseling and social support from the start of selumetinib treatment, as this may improve patient wellbeing as well as ensuring treatment compliance. During a patient’s guided break, they should have appointments with a psychologist to evaluate therapy motivation, the burden associated with their AEs, and treatment compliance.
Discussion
Selumetinib is an oral MEK1/2 inhibitor approved for the treatment of pediatric patients with NF1-PN,9,10 and has demonstrated a clinically meaningful reduction in PN volume, extended progression-free survival, and improved health-related QoL and other patient-reported outcomes.11 Selumetinib has a manageable safety profile, in line with that of other MEK inhibitors, and associated AEs are mostly mild to moderate, with few patients requiring dose modifications or discontinuation due to AEs.11 The most common AEs in the SPRINT Phase II trial were nausea, vomiting, diarrhea, asymptomatic CPK elevation, acneiform rash, and paronychia.11 However, expert recommendations on how to appropriately and effectively prevent and manage AEs during selumetinib treatment are lacking. It is known that by improving tolerability through appropriate AE management, the clinical benefit of a drug can be increased. Here, we used a modified Delphi approach with a multidisciplinary panel to develop consensus statements on the prevention, early detection, and management of AEs that frequently occur during selumetinib treatment, and/or those that have the greatest impact on patients.
Although it is important to have recommendations on all AEs that can occur during selumetinib treatment, the panel agreed that particular focus should be given to AEs that, in their experience, have the highest frequency and/or patient impact. When discussing the prioritization of AEs, the panel experts agreed that physicians are typically concerned about high-impact AEs that may lead to discontinuation, whereas patients are likely most concerned about dermatologic AEs, which can affect their appearance and have a social impact. Therefore, it is important to consider these different perspectives when determining an approach to managing and preventing AEs. In the first part of this panel discussion, the experts agreed on the relative frequency and impact of AEs potentially associated with selumetinib. From this, consensus statements and expert recommendations were provided for priority AEs.
Dermatologic AEs with MEK inhibitors are well documented,14,27,42 and the panel agreed that, because of their frequency and impact on patients, these should be prioritized for selumetinib. The frequency of specific dermatologic AEs was noted to vary by age group, with acneiform rash more commonly seen in adolescents than young children. Of all dermatologic AEs, paronychia was seen as the most challenging to address, with the panel noting that it should be managed aggressively and be a key focus for healthcare practitioners. To prevent dermatologic AEs such as rashes or paronychia, patients should use emollients, practice sun safety, and prevent potential traumas to the hands or feet, for example by avoiding tight shoes. The psychological burden of AEs was also highlighted because it is high in most patients, can be worsened if there are underlying psychological issues, and has the potential to affect treatment compliance, especially in certain age groups.
Gastrointestinal AEs were also considered a priority because they are one of the most commonly reported AEs potentially associated with selumetinib, including nausea, vomiting, and diarrhea.11 As of February 2024, the FDA and EMA approvals state that selumetinib can be taken with or without food because studies have shown that taking selumetinib with a meal has no clinically relevant impact on GI-related AEs.9 Although GI-related AEs are mostly mild (grade 1 or 2), such symptoms can negatively impact QoL, disrupting school, family, and social activities.33 Stomatitis was considered an important AE to manage since it can be associated with considerable pain and have a great impact on a patient’s daily life.32 Alongside strategies including oral healthcare regimens and topical treatments, oral examinations are recommended if the patient shows any symptoms. For nausea and vomiting, it is important for patients to maintain a healthy, low-fat diet.
The panel agreed that visual and cardiac AEs have a high burden if encountered. While there may not be any measures to prevent cardiac or visual AEs, patients should try to adhere to the monitoring frequencies outlined in this report to ensure that AEs are detected and managed early if they do occur. However, little can be done to manage such AEs aside from considering dose reduction or discontinuation. This is consistent with authors of other studies of MEK inhibitors, who recommend periodic evaluations at regular intervals to facilitate early detection.37
Other AEs included fatigue, peripheral edema, and weight gain. However, consensus statements were not developed for these AEs, as the panel agreed that they are either relatively rare or low grade; for example, nearly all incidences of fatigue are grade 1 or 2 in severity.
This report presents a strong level of consensus from a multidisciplinary group to support previous recommendations20 regarding several aspects of the prevention and management of AEs potentially associated with selumetinib. It is worth noting that, although it would have been beneficial to include representatives from all countries where selumetinib is approved, it would have been logistically difficult to reach a consensus on each statement between all representatives. Therefore, discussions were limited to the 10 European experts, which may limit worldwide application of these recommendations. As an additional limitation, although the Delphi consensus included recommendations on managing a wide range of AEs associated with selumetinib, it was not exhaustive of all possible scenarios (eg, issues of weight gain, liver toxicity in children with fatty liver disease, the need for prophylactic management of conditions pre-existing before selumetinib initiation such as acneiform rash). When pediatric patients start selumetinib treatment, various preventative measures and monitoring for early signs of toxicity should be taken to reduce the risk of AEs, to ensure treatment can continue, and to minimize the need for dose reduction (and potential reduced efficacy). To proactively prevent discontinuation due to AEs, it is important that patients and caregivers are informed about potential AEs and are aware of the various prevention and management strategies. If selumetinib is discontinued, it is important that patients can cope with the psychological burden of AEs upon reinitiation. For this reason, reinitiation can occur but the patient should be monitored for AEs after a guided break by an interdisciplinary team or upon new data available on rechallenge.
Conclusions
The development of a variety of consensus statements indicates expert agreement on best practices for the prevention and management of AEs potentially associated with selumetinib in pediatric patients with NF1-PN. These events are generally manageable43 and should be considered alongside the overall treatment benefit. Information must be shared among healthcare professionals as further selumetinib experience is gained.
Supplementary material
Supplementary data are available at Neuro-Oncology Practice online.
Acknowledgments
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Victoria Jones, PhD, and Jen Shepherd, PhD, of Infinity, OPEN Health Communications, London, UK and was funded by Alexion, AstraZeneca Rare Disease, in accordance with Good Publications Practice (GPP 2022) guidelines. D.H. is supported by the NIHR Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Contributor Information
Amedeo A Azizi, Department of Pediatrics and Adolescent Medicine, Division of Neonatology, Pediatric Intensive Care and Neuropediatrics; Comprehensive Center for Pediatrics; Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Darren Hargrave, University College London Great Ormond Street Institute for Child Health, London, UK.
João Passos, Instituto Português Oncologia de Lisboa Francisco Gentil, Lisbon, Portugal.
Pierre Wolkenstein, Henri-Mondor Hospital, APHP, UPEC, Créteil, France.
Thorsten Rosenbaum, Department of Pediatrics and Adolescent Medicine, Sana Kliniken Duisburg, Duisburg, Germany.
Claudia Santoro, Neurofibromatosis Referral Center, Department of Women’s and Children’s Health and General and Specialized Surgery, University of Campania “Luigi Vanvitelli”, Naples, Italy; Clinic of Child and Adolescent Psychiatry, Department of Mental and Physical Health and Preventive Medicine, University of Campania “Luigi Vanvitelli”, Naples, Italy.
Verena Rosenmayr, Medical University of Vienna and Department of Pediatrics and Adolescent Medicine, Vienna General Hospital, Vienna, Austria.
Thomas Pletschko, Department of Pediatrics and Adolescent Medicine, Division of Neonatology, Pediatric Intensive Care and Neuropediatrics; Comprehensive Center for Pediatrics; Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Paolo A Ascierto, Melanoma, Cancer Immunotherapy and Innovative Therapies Unit, Istituto Nazionale Tumori IRCCS Fondazione G. Pascale, Naples, Italy.
Héctor Salvador Hernández, Department of Pediatric Oncology and Hematology, Sant Joan de Déu Barcelona Hospital, Barcelona, Spain.
Funding
The Delphi panel mentioned in this manuscript was funded by Alexion. Editorial assistance was funded by Alexion AstraZeneca Rare Disease.
Conflict of interest statement
A.A.A.: Member of advisory boards, and received scientific grants, speaker honoraria, travel support, and payments for workshop participation from Alexion. Member of Novartis advisory board. C.S.: Received payment for scientific consultation and speaker honoraria from Alexion/AstraZeneca. Participated on a data safety monitoring/advisory board for Alexion/AstraZeneca. Received payment for scientific consultation from IQVIA. Held a leadership or fiduciary role for ANF patient advocacy. D.H.: Member of advisory boards, and received research grants, speaker honoraria, materials for clinical trials, institutional clinical trial support, and travel support from Alexion/AstraZeneca. H.S.H.: Alexion/AstraZeneca advisory board, speaker honoraria, travel support, and consulting fees. J.P.: Alexion consulting fees, honoraria, travel expenses, participation on a data safety monitoring/advisory board. P.A.A.: Grants/contracts from Bristol Myers Squibb, Roche-Genentech, Pfizer/Array, and Sanofi. Consulting fees from Bristol Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, Merck Serono, Pierre-Fabre, Sun Pharma, Sanofi, Idera, Sandoz, 4SC, Italfarmaco, Nektar, Pfizer/Array, Lunaphore, Medicenna, Bio-Al Health, ValoTx, Replimmune, and Bayer. Support for attending meetings and/or travel from Pfizer, Bio-Al Health, and Replimmune. Participation on data safety monitoring/advisory boards from Bristol Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, AstraZeneca, Immunocore, Boehringer Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Oncosec, Nouscom, Seagen, iTeos, and Erasca. Member of advisory boards for Alexion. P.W.: Consulting fees, speaker payment/honoraria and logistical support from, and member of advisory boards for Alexion. Received consulting fees and speaker honoraria from AstraZeneca. Received consulting fees from SpringWorks. T.P.: Received consulting fees from, and was a member of advisory boards for, Alexion. Received grants from NF Kinder. T.R.: Member of advisory boards and participated on a data safety monitoring/advisory board for, and received speaker honoraria, travel support, personal payments, grants/contracts, and consulting fees from Alexion/AstraZeneca. Received payment/honoraria from the Taiwan Society for Pediatric Neurosurgery. Unpaid leadership/fiduciary role for the German Society for Neuropediatrics, NF Working Group Germany. V.R.: Received consulting fees and was a member of advisory boards for Alexion. An unpaid board member of psychosocial working group for Psychosoziale Arbeitsgemeinschaft in der Gesellschaft für Pädiatrische Onkologie und Hämatologie (PSAPOH) Gesellschaft für Pädiatrische Onkologie und Hämatologie (GPOH).
Authorship statement
Conceptualization: A.A., C.S., D.H., P.A., P.W., T.R. Resources: C.S., P.W. Data curation: C.S., P.A., P.W. Formal analysis: D.H., P.W. Supervision: A.A., D.H., H.S.H., P.A., P.W. Validation: A.A., C.S., P.W., T.P., V.R. Investigation: A.A., D.H., H.S.H., T.R. Visualization: A.A., C.S., H.S.H., P.A. Methodology: A.A., D.H., T.P., V.R. Writing—original draft: C.S., D.H., T.P., V.R. Writing—review and editing: all authors. Other: T.R. provided patient and best practice data, and A.A. provided expert opinion.
Data Availability
All data generated during development of these consensus recommendations are presented in the main manuscript text and the supplementary materials.
References
- 1. Dombi E, Baldwin A, Marcus LJ, et al. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med. 2016;375(26):2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergqvist C, Servy A, Valeyrie-Allanore L, et al. NF France Network. Neurofibromatosis 1 French national guidelines based on an extensive literature review since 1966. Orphanet J Rare Dis. 2020;15(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44(2):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamoy-Jimenez G, Kim R, Suppiah S, et al. Quality of life in patients with neurofibromatosis type 1 and 2 in Canada. Neurooncol Adv. 2020;2(Suppl 1):i141–i149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang X, Yoo HK, Amin S, et al. Clinical and humanistic burden among pediatric patients with neurofibromatosis type 1 and plexiform neurofibroma in the USA. Childs Nerv Syst. 2022;38(8):1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolters PL, Burns KM, Martin S, et al. Pain interference in youth with neurofibromatosis type 1 and plexiform neurofibromas and relation to disease severity, social-emotional functioning, and quality of life. Am J Med Genet A. 2015;167A(9):2103–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Copley-Merriman C, Yang X, Juniper M, et al. Natural history and disease burden of neurofibromatosis type 1 with plexiform neurofibromas: A systematic literature review. Adolesc Health Med Ther. 2021;12:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fjermestad KW. Health complaints and work experiences among adults with neurofibromatosis 1. Occup Med (Lond). 2019;69(7):504–510. [DOI] [PubMed] [Google Scholar]
- 9. European Medicines Agency. 2024. Koselugo Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/koselugo-epar-product-information_en.pdf. Accessed February 12, 2024. [Google Scholar]
- 10. Food and Drug Administration. 2020. Koselugo Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213756s000lbl.pdf. Accessed November 3, 2023. [Google Scholar]
- 11. Gross AM, Wolters PL, Dombi E, et al. Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med. 2020;382(15):1430–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fisher MJ, Blakeley JO, Weiss BD, et al. Management of neurofibromatosis type 1-associated plexiform neurofibromas. Neuro Oncol. 2022;24(11):1827–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. AstraZeneca. Koselugo approved in the EU for children with neurofibromatosis type 1 and plexiform neurofibromas. https://www.astrazeneca.com/media-centre/press-releases/2021/koselugo-approved-in-the-eu-for-children-with-neurofibromatosis-type-1-and-plexiform-neurofibromas.html#. Accessed February 12, 2024. [Google Scholar]
- 14. Abdel-Rahman O, ElHalawani H, Ahmed H.. Risk of selected dermatological toxicities in cancer patients treated with MEK inhibitors: A comparative systematic review and meta-analysis. Future Oncol. 2015;11(24):3307–3319. [DOI] [PubMed] [Google Scholar]
- 15. Avery RA, Trimboli-Heidler C, Kilburn LB.. Separation of outer retinal layers secondary to selumetinib. J AAPOS. 2016;20(3):268–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flaherty KT, Robert C, Hersey P, et al. METRIC Study Group. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367(2):107–114. [DOI] [PubMed] [Google Scholar]
- 17. Weber ML, Liang MC, Flaherty KT, Heier JS.. Subretinal fluid associated with MEK inhibitor use in the treatment of systemic cancer. JAMA Ophthalmol. 2016;134(8):855–862. [DOI] [PubMed] [Google Scholar]
- 18. McGregor B, Mortazavi A, Cordes L, et al. Management of adverse events associated with cabozantinib plus nivolumab in renal cell carcinoma: A review. Cancer Treat Rev. 2022;103:102333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ravaud A. Treatment-associated adverse event management in the advanced renal cell carcinoma patient treated with targeted therapies. Oncologist. 2011;16(Suppl 2):32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klesse LJ, Jordan JT, Radtke HB, et al. The use of MEK inhibitors in neurofibromatosis type 1-associated tumors and management of toxicities. Oncologist. 2020;25(7):e1109–e1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. NIH NCI DCTD Cancer Evaluation Program. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_8.5x11.pdf. Accessed November 3, 2023. [Google Scholar]
- 22. Joshi SS, Ortiz S, Witherspoon JN, et al. Effects of epidermal growth factor receptor inhibitor-induced dermatologic toxicities on quality of life. Cancer. 2010;116(16):3916–3923. [DOI] [PubMed] [Google Scholar]
- 23. Wagner LI, Lacouture ME.. Dermatologic toxicities associated with EGFR inhibitors: the clinical psychologist’s perspective. Impact on health-related quality of life and implications for clinical management of psychological sequelae. Oncol (Williston Park). 2007;21(11 Suppl 5):34–36. [PubMed] [Google Scholar]
- 24. Wollina U. Systemic drug-induced chronic paronychia and periungual pyogenic granuloma. Indian Dermatol Online J. 2018;9(5):293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sur M, Boca AN, Ilies RF, et al. Correlation between quality of life and disease severity of pediatric patients with atopic dermatitis. Exp Ther Med. 2020;20(6):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Atopic dermatitis - National Institute of Arthritis and Musculoskeletal and Skin Diseases. https://www.niams.nih.gov/health-topics/atopic-dermatitis. Accessed January 25, 2024. [Google Scholar]
- 27. Volontè M, Isoletta E, Gordon S, et al. Acneiform rash as a side effect of selumetinib in a child with neurofibromatosis type 1 treated for inoperable plexiform neurofibromas: Good results with doxycycline. Dermatol Ther. 2022;35(8):e15607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee DK, Lipner SR.. Optimal diagnosis and management of common nail disorders. Ann Med. 2022;54(1):694–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schad K, Baumann Conzett K, Zipser MC, et al. Mitogen-activated protein/extracellular signal-regulated kinase kinase inhibition results in biphasic alteration of epidermal homeostasis with keratinocytic apoptosis and pigmentation disorders. Clin Cancer Res. 2010;16(3):1058–1064. [DOI] [PubMed] [Google Scholar]
- 30. Sun Q, Antaya RJ.. Treatment of MEK inhibitor-induced paronychia with doxycycline. Pediatr Dermatol. 2020;37(5):970–971. [DOI] [PubMed] [Google Scholar]
- 31. Gross AM, Dombi E, Wolters PL, et al. Long-term safety and efficacy of selumetinib in children with neurofibromatosis type 1 on a phase 1/2 trial for inoperable plexiform neurofibromas. Neuro Oncol. 2023;25(10):1883–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Al-Rudayni AHM, Gopinath D, Maharajan MK, Menon RK.. Impact of oral mucositis on quality of life in patients undergoing oncological treatment: A systematic review. Transl Cancer Res. 2020;9(4):3126–3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jivraj F, Kang S, Reedie S, et al. The patient and clinician assessment of gastrointestinal (GI) related adverse events associated with oral disease-modifying therapies in multiple sclerosis: A qualitative study. Adv Ther. 2022;39(11):5072–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lui M, Gallo-Hershberg D, DeAngelis C.. Development and validation of a patient-reported questionnaire assessing systemic therapy induced diarrhea in oncology patients. Health Qual Life Outcomes. 2017;15(1):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. NHS. 2020. Dabrafenib and trametinib. https://www.swagcanceralliance.nhs.uk/wp-content/uploads/2020/09/Dabrafenib-and-Trametinibv2.pdf. Accessed November 3, 2023. [Google Scholar]
- 36. Méndez-Martínez S, Calvo P, Ruiz-Moreno O, et al. Ocular adverse events associated with MEK inhibitors. Retina. 2019;39(8):1435–1450. [DOI] [PubMed] [Google Scholar]
- 37. de la Cruz-Merino L, Di Guardo L, Grob JJ, et al. Clinical features of serous retinopathy observed with cobimetinib in patients with BRAF-mutated melanoma treated in the randomized coBRIM study. J Transl Med. 2017;15(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gross AM, Dombi E, Wolters PL, et al. Long-term safety and efficacy of selumetinib in children with neurofibromatosis type 1 on a phase 1/2 trial for inoperable plexiform neurofibromas. Neuro Oncol. 2023;25(10):1883–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karampelas M, Malamos P, Petrou P, et al. Retinal pigment epithelial detachment in age-related macular degeneration. Ophthalmol Ther. 2020;9(4):739–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang M, Munch IC, Hasler PW, Prünte C, Larsen M.. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86(2):126–145. [DOI] [PubMed] [Google Scholar]
- 41. Avery RA, Katowitz JA, Fisher MJ, et al. OPPN Working Group. Orbital/periorbital plexiform neurofibromas in children with neurofibromatosis type 1: Multidisciplinary recommendations for care. Ophthalmology. 2017;124(1):123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dávila Osorio VL, Vicente MA, Baselga E, et al. Adverse cutaneous effects of mitogen-activated protein kinase inhibitors in children. Pediatr Dermatol. 2021;38(2):420–423. [DOI] [PubMed] [Google Scholar]
- 43. Anderson MK, Johnson M, Thornburg L, Halford Z.. A review of selumetinib in the treatment of neurofibromatosis type 1-related plexiform neurofibromas. Ann Pharmacother. 2022;56(6):716–726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during development of these consensus recommendations are presented in the main manuscript text and the supplementary materials.