Abstract
Detecting microsecond structural perturbations in biomolecules has wide relevance in biology, chemistry and medicine. Here we show how MHz repetition rates at X-ray free-electron lasers can be used to produce microsecond time-series of protein scattering with exceptionally low noise levels of 0.001%. We demonstrate the approach by examining Jɑ helix unfolding of a light-oxygen-voltage photosensory domain. This time-resolved acquisition strategy is easy to implement and widely applicable for direct observation of structural dynamics of many biochemical processes.
Subject terms: Molecular biophysics, Proteins, Structure determination
The MHz repetition rates available at second-generation X-ray free-electron lasers enable the collection of microsecond time-resolved X-ray scattering data with exceptionally low noise, providing insights into protein structural dynamics.
Main
Biomolecular transformations, reactions and interactions are at the basis of all life. Deciphering these mechanisms in a time-resolved manner and with submolecular precision opens new dimensions of biological understanding. Access to submillisecond timescales in near-native environments is particularly important, but remains challenging.
There are two primary acquisition schemes to acquire time-resolved data. In ‘pump-probe’ mode, each reaction trigger is followed by a probe pulse at a defined time delay and time-series are constructed by repeated measurement of many time points. This mode enables femtosecond time resolution and has been used at X-ray free-electron lasers (XFELs) for time-resolved protein crystallography and protein solution scattering1–3. In practice, this method limits acquisition rates leading to larger sample consumption. An alternative approach is to read out a series of probe pulses following a single trigger event. In this way, the efficiency of data collection is vastly improved, reducing sample consumption and suppressing experimental noise through massive averaging4. Here, the time resolution is limited by the X-ray repetition and detector acquisition rates.
MHz repetition rates at second-generation XFELs now open up the opportunity to use the latter scheme for time-resolved studies in the microsecond range. The European XFEL (EuXFEL) is the first in this class and delivers trains at 10 Hz containing up to 2,700 X-ray pulses with a variable repetition rate up to 4.5 MHz (Fig. 1b)5. Thus far, the high repetition rate has posed severe technical challenges for single-pulse detection of scattering and diffraction images, due to electronic noise and nonlinear gain in the detector readout, as well as shockwaves or explosions in the jet6. For these reasons, this unique timing capability has only been used in X-ray microscopy, dynamic compression experiments and X-ray photon correlation spectroscopy7–9, but not yet in the pursuit of biomolecular structural dynamics through protein scattering. Here, we demonstrate the realization of this approach through time-resolved wide-angle X-ray scattering (TR-WAXS) at the EuXFEL.
Fig. 1. Microsecond TR-WAXS utilizing the MHz repetition rate at the EuXFEL.
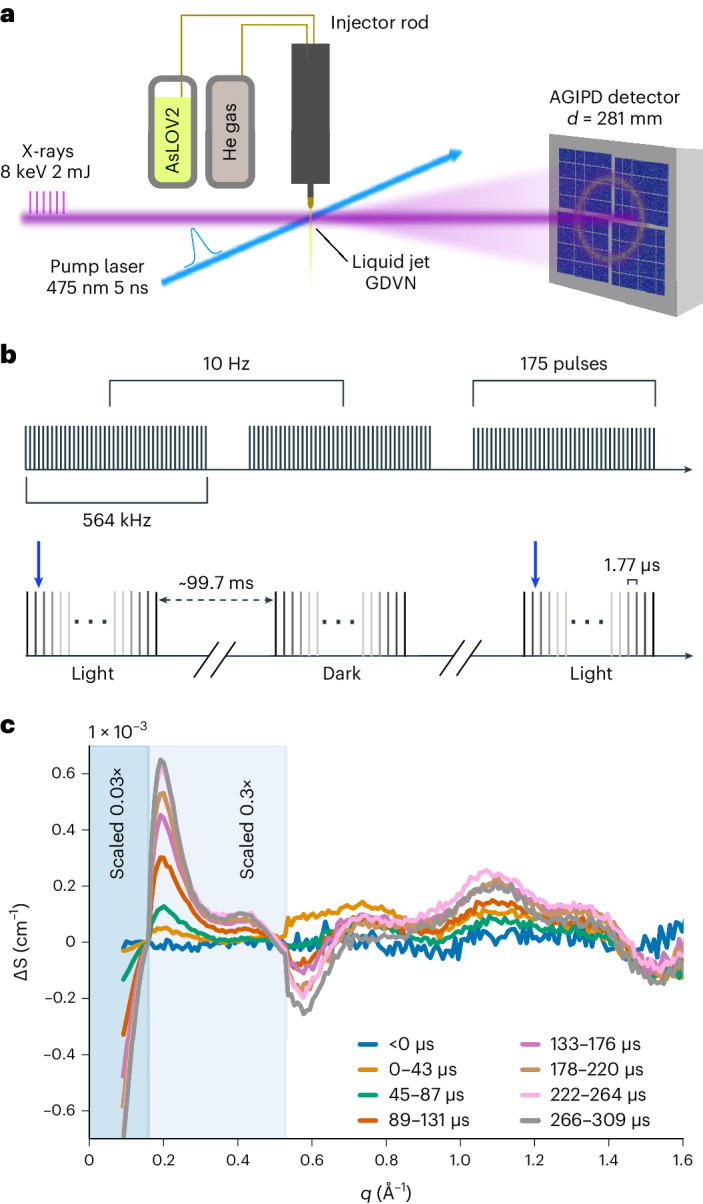
a, Schematic depiction of the X-ray and optical laser path, GDVN liquid jet and recorded scattering with the AGIPD detector (not drawn to scale). b, Pulse train structure and laser excitation scheme used to obtain microsecond time resolution. The 10 Hz trains comprise 175 pulses at 564 kHz (1.77-µs interval). The blue arrow depicts the timing of optical excitation of every other pulse train. c, TR-WAXS data of AsLOV2. The momentum transfer is defined as , with 2θ and λ as the scattering angle and the X-ray wavelength, respectively. The data were normalized in the q-range 1.6 Å−1 > q > 1.4 Å−1 and scaled for better visualization as indicated in the panel.
TR-WAXS can resolve structural changes of biomolecules and chemicals in solution, providing an ~atomic-scale glimpse of their function under near-native conditions4,10,11. We investigate the phototropin light-oxygen-voltage (LOV)2 domain from Avena sativa (AsLOV2), which features a prototypical signaling mechanism, where a C-terminal helix (Jɑ, 22 residues; Extended Data Fig. 1) detaches from the core in response to photoexcitation12,13. This unique photoactivity has been exploited in a broad range of optogenetic applications and has been the subject of intense experimental investigation14–19. Despite this interest, the mechanism and timing of Jɑ unfolding and the structure of the unfolded state are not definitively known.
Extended Data Fig. 1. UV-VIS absorption spectrum.
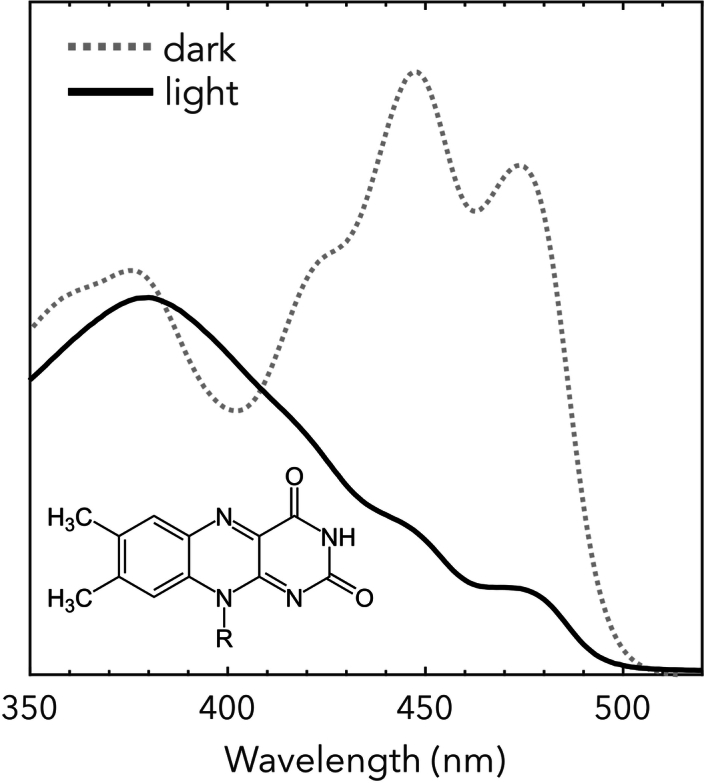
AsLOV2 UV-VIS absorption spectrum in dark and light states together with FMN cofactor chemical structure.
To record microsecond TR-WAXS at the EuXFEL, the sample was carried in a liquid jet via three-dimensional (3D)-printed gas dynamic virtual nozzle (GDVN)20 to the interaction point of the optical and X-ray beams at the single particles, clusters, and biomolecules and serial femtosecond crystallography (SPB/SFX) endstation (Fig. 1a)21. Photoexcitation was conducted through the transparent GDVN nozzle with nanosecond laser pulses timed to the start of every second X-ray pulse train (Fig. 1b). Careful consideration was given to ensure sufficient excitation volume to span the entire X-ray probe train (~1 nl). The scattering was recorded on the AGIPD detector for each probe pulse, covering a q-range from 2.1 Å−1 > q > 0.08 Å−1 (corner resolution). The two-dimensional (2D) scattering was integrated into rings as a function of the momentum transfer (q) and delay time (t) along the pulse train. Approximately 30% of the data were excluded, because the shape of the scattering was affected by fluctuations in experimental conditions (Methods). After averaging over several repeats, the difference scattering ΔS = Slight(q,t) − Sdark(q,t) was computed (Fig. 1c). We found that it was crucial to subtract entire laser-on from the laser-off trains from each other, reducing the effect of systematic noise from the detector and fluctuations in jet thickness and X-ray intensity (Extended Data Fig. 2). This reduction was effective as the noise of the ΔS signal (Extended Data Fig. 3) was comparable to the estimated Poisson noise (Extended Data Fig. 4). The experimental time resolution of 1.77 µs corresponds to the inverse of the repetition rate of the XFEL (564 kHz) and the data span a time window of ~300 µs reflecting the length of the X-ray pulse train.
Extended Data Fig. 2. Comparison of different ways to compute difference scattering.
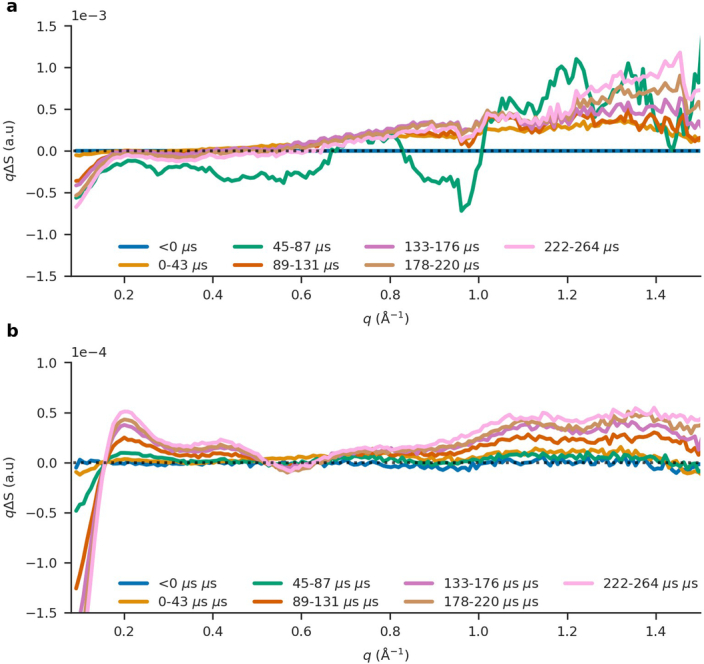
a. Here, we present an alternative approach that relies solely on the dark frames recorded during laser-induced trains. The difference scattering is calculated by averaging the dark measurements in each of the light-induced trains and using this average as the dark reference. b. Our more reliable approach of calculating the difference scattering is to subtract an entire dark train from a light train. This removes systematic errors from the data acquisition in the detector. Each time point in the plots is composed of 50 pulses binned together, and the averaged dark scattering is subtracted to produce the difference scattering. The difference scattering has been scaled to match the theoretical scattering from Pepsi-SAXS10.
Extended Data Fig. 3. Time-resolved WAXS data collected with low noise levels recorded at the SPB/SFX instrument of the European XFEL.
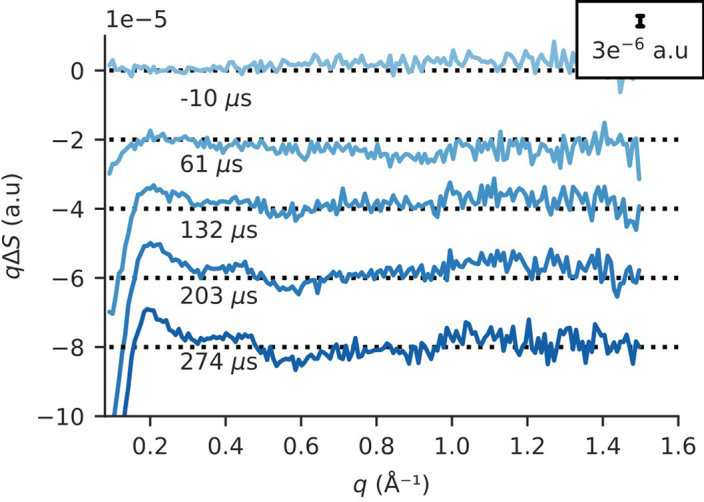
Our data shows an exceptional signal-to-noise ratio, allowing for difference signals of ~10−6 to be recorded. As the normalization conditions alter the scale of the difference signal, the data is typically normalized around 1.5 Å−1 or 2.3 Å−1. Here, the data is normalized to the scattering in the range between 1.6 Å−1 and 1.4 Å−1 to match the data treatment of previously published time-resolved solution scattering studies performed at a synchrotron, where best-case noise levels are ~10−4 and ~5*10−5 in pump-probe and sequential acquisition schemes. The data shown here,ΔI(q), have a q-bin size of 0.00836 Å−1. The red lines at the pre-excitation time point show ± 10−5, respectively; this boundary corresponds to the noise levels in the data.
Extended Data Fig. 4. Estimated relative poisson noise of the experiment.
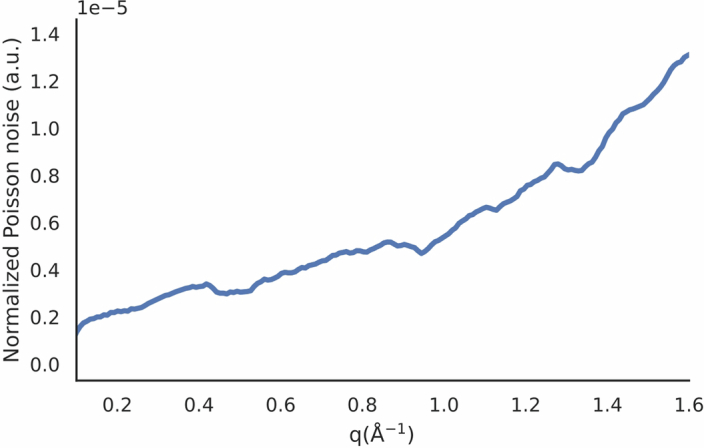
The relative poisson noise was estimated from , where I(q) represents the total number of photons per q-bin for a single time delay, using a bin size of 0.00836 Å−1. The data shown here, was normalized to the q-range between 1.6 Å−1 and 1.4 Å−1. The estimated noise is at the same level as the difference scattering of the pre-excitation time point, where there is no signal by definition, shown in Extended Data Fig. 3, indicating that most noise sources were avoided or eliminated through subtraction when calculating the difference scattering.
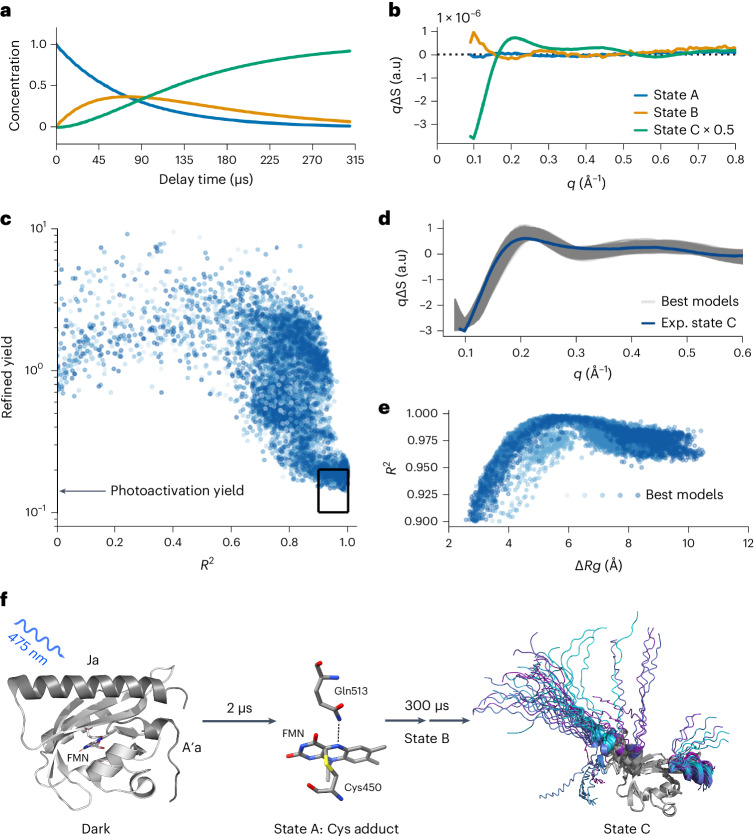
The TR-WAXS response of AsLOV2 shows microsecond evolution with oscillations extending beyond q-values of 1.5 Å−1, which translates into a spatial resolution of 4.2 Å. The data have an exceptionally low noise floor corresponding to 0.001% as determined from noise fluctuations from a pre-excitation time point (Extended Data Fig. 3), which is at least one order of magnitude lower than previous accounts for this method22. Deconvolution of the data using spectral decomposition with exponential conversion laws indicated that the data are best fit to a sequential model of type A → B → C, yielding base patterns for the three states (Fig. 2b, Extended Data Fig. 5). In TR-WAXS, large difference signals at low q < 0.15 Å−1 typically indicate changes of the radius of gyration (Rg) of the protein3. From this we deduce that the structural change in state C is sizable, but that changes in states A and B are comparably smaller. We assign state C to the unfolded state (vide infra), which is further underpinned by its timescale, emerging within ~300 µs (Fig. 2a), in agreement with kinetics inferred from infrared spectroscopy17,18. States A and B could only be resolved because of the low noise floor of the new scattering method approach. State A forms within the first time point of our measurement at 1.77 µs, in agreement with previous reports of FMN-cysteinyl adduct formation23. We assign state B to a previously unrecognized intermediate state, which occurs subsequent to Cys adduct formation and before large changes in the Jɑ helix. Notably, intermediate states in Jɑ unfolding have been previously proposed through a long MD simulation24, but not clearly observed experimentally.
Fig. 2. TR-WAXS uncovers a new intermediate state and the structure of the unfolded Jɑ helix in the AsLOV2 photoreceptor domain.
a,b, The time evolution of constituent states (a) and their spectral components derived from kinetic decomposition of the TR-WAXS data (b). c, Structural modeling results generated using our adapted AlphaFold method. R² is used as an indicator of a good fit between experimental and theoretical difference signals. Darker blue shades correspond to increasing numbers of mutations in the Jɑ helix. Structures with mutations in the N-terminal helix are also included. The best models were selected by choosing those that have both a photoactivation yield of 15 ± 5% (as derived in Extended Data Fig. 9) and R2 > 0.9, resulting in 6,032 candidate models (black box). d, The theoretical difference scattering of the best fits (gray) and the scaled experimental scattering profile of state C (blue) are shown. e, R2 of the top candidate structures versus change in radius of gyration (ΔRg). f, The structural dynamics results are shown in the canonical photoactivation mechanism of AsLOV2.
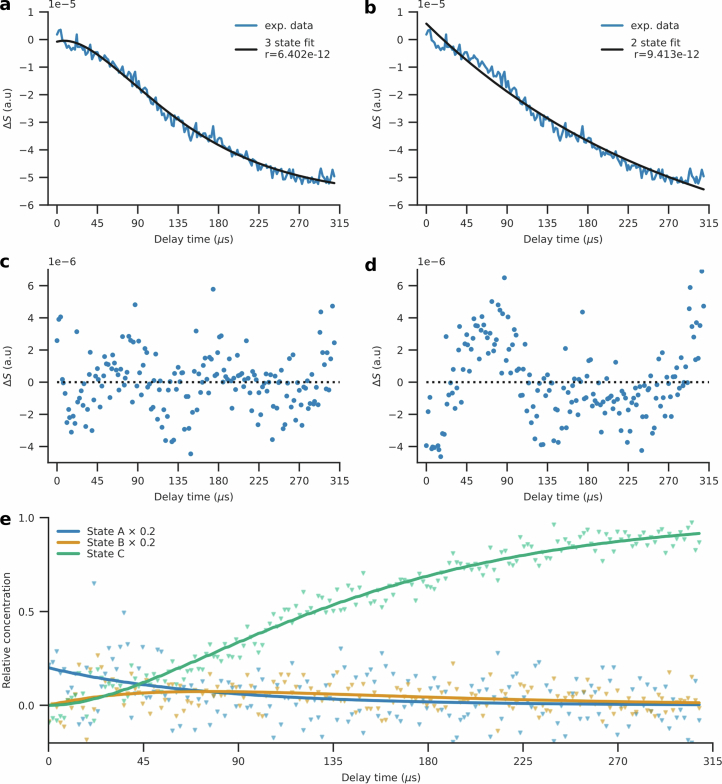
Extended Data Fig. 5. A three-state model is required for kinetic fitting of the difference WAXS data.
The reconstructed kinetics and residuals (r, see Eq. 7) of the AsLOV2 difference scattering data for a decomposition with a three-state model (a,c) are compared to those from a two-state kinetic model (b, d). The scattering data and residuals are shown as an average over the q-range (0.08 Å−1 < q < 1.2 Å−1). The three-state model is preferred over the two-state model, because the fits and residuals of the three-state model show a very good agreement over the entire time-series (panels a and c), whereas the two-state model shows systematic deviations at early and late times (panels b and d). Moreover, the r value was better for the three-state model (6.4*10−12) compared to the two-state model (9.413*10−12). (e) The time evolution of the constituent states derived from spectral decomposition are shown including experimental data points. States A and B exhibit high noise because of the low signal amplitude (Fig. 2b), however, as demonstrated, a three-state model is needed to correctly capture the kinetics. States A and B are scaled by a factor of 0.2 for better visualization.
Focusing on state C and to assess the extent of Jɑ unfolding, we refined structural models predicted by AlphaFold25, where a large variability was obtained through sampling with dropout enabled inference (20,000 structures predicted)26 and a number of glycine mutations in the Jɑ helix. We then determined best fits against the predicted structures by comparison of the root-mean-square of residuals (R2) between theoretical and experimental difference scattering curves (Fig. 2d). Since we compare the curves on absolute scales, this selection is also based on appropriate computed activation factors of the structural pairs (further described in the Methods, the boxed region in Fig. 2c includes 6,032 structures). All of the selected structures show unfolded Jɑ helices (subset shown in Fig. 2f), with an increase of Rg by 5–7 Å yielding the best fits (Fig. 2e). Notably, an inspection of the best-fitting models shows that the residues directly preceding the Jɑ segment, which form a loop segment in the dark, now form an ordered helical domain (Extended Data Fig. 6). Finally, we find that the N-terminal A′ɑ helix is unfolded in most structures. Our data establishes that the Jɑ helix unfolds in a two-step mechanism within 300 μs, and also suggests that it completely unfolds and that additional structural changes accompany this process. This concludes a long series of investigations into Jɑ unfolding14–19,24, and demonstrates the promising capability of this new time-resolved X-ray scattering method.
Extended Data Fig. 6. Comparison of loop region in folded/unfolded state.
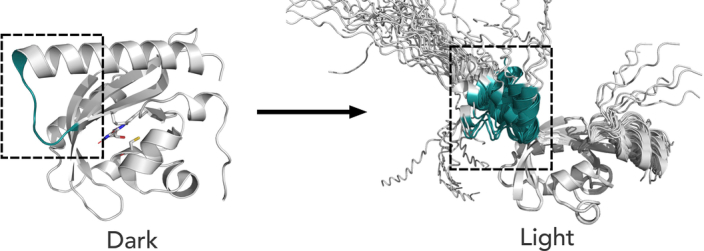
The loop region (shown in green) in the dark structure (PDB 7GPX) converts to a helix in the light following from AlphaFold modeling of State C.
Our new implementation of TR-WAXS realizes the unused potential of MHz XFELs to provide unique structural information about transient states on the important microsecond timescale. The additional timing information is gained with only minor adjustments of existing XFEL acquisition schemes and is highly compatible with other methods that use short X-ray pulses, for example serial crystallography1–3 or X-ray emission spectroscopy27. The method rests on the high X-ray fluence per pulse at the EuXFEL, which is about three orders of magnitude higher than a fourth-generation synchrotron (Extended Data Fig. 7). Paired with the fast readout rate of the AGIPD detector, exceptionally low noise levels are obtained. Currently, this is a unique advantage of second-generation high repetition rate XFELs; however, advances in detector technologies may make synchrotrons competitive in the future. The excellent data quality enabled identification of a new transient state in AsLOV2 Jɑ unfolding and opens the door for investigating microsecond reaction dynamics with dilute samples of proteins, peptides, RNA or DNA28, especially when combined with ongoing development of ultrastable liquid sheet jet sample injection technology29. It also permits detection of difference scattering signals to very high scattering angles (q > 1.5 Å−1; Fig. 1c), suggesting that time-resolved and high-resolution structural information can be obtained in crystallography30–32 or single-particle diffraction experiments33. Overall, we anticipate that our method will accelerate knowledge gain for dynamic enzymatic and chemical mechanisms.
Extended Data Fig. 7. Comparison of data acquisition configurations between the European XFEL and a modern synchrotron configuration.
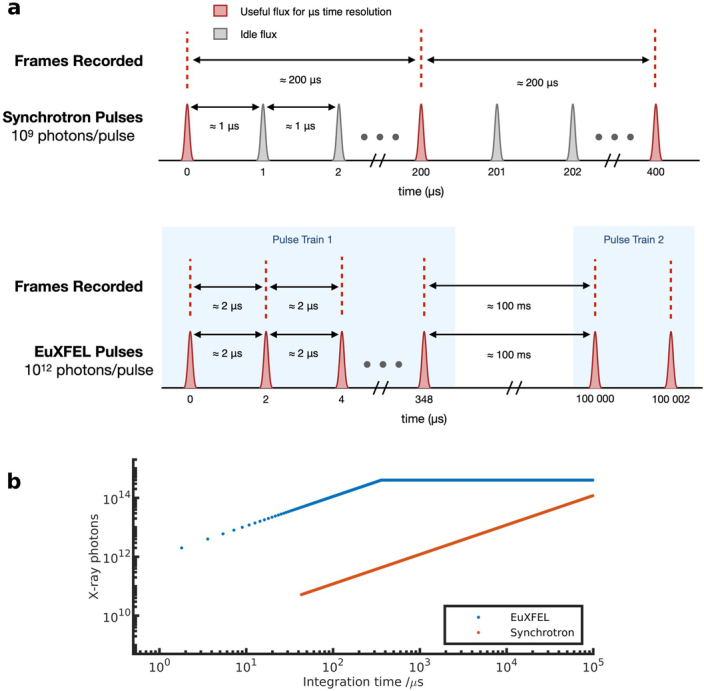
(a) Synchrotrons nowadays can achieve average fluxes approaching the European XFEL. However, the available detectors, such as the Eiger 2, have maximum continuous frame rates on the order of 5000 frames per second. For microsecond time resolution, so detector integration below a microsecond, the usable flux is only about 0.5% of the total. At the European XFEL, the unusual pulse structure makes it possible to use all the available flux. (b) Total detectable photon flux at a 4th generation synchrotron versus the EuXFEL source as a function of integration time. The EuXFEL was assumed to have a repetition rate of 564 kHz and 2 × 1012 photons/pulse and the synchrotron 1.2 MHz and 1 × 109 photons/pulse. For the synchrotron we assumed an Eiger detector in burst mode at 23k fps, giving a minimum integration time of 43. As timescales increase the performance of a synchrotron approaches that of an XFEL.
Methods
Sample delivery at EuXFEL SPB/SFX
The sample was delivered to the X-ray interaction region using a liquid jet generated from a 3D-printed type C GDVN provided by the EuXFEL Sample Environment Group. The nozzles used are part of the standard 3D-printed suite offered by the EuXFEL34. Fused silica capillaries (0.360 mm outer diameter (o.d.) and 0.150 mm internal diameter (i.d.), Polymicro) were fastened to the liquid and gas inlets using a small dab of epoxy glue (Devcon). The standard SPB/SFX liquid injector rod was used to mount the GDVNs. The rod was assembled as follows: a 3.175 mm o.d. stainless steel tube was first glued to the capillaries ~5 mm above the nozzle. This tube was fastened into a stainless-steel nozzle adaptor using a 10–32 PEEK fitting (Idex). The capillaries were then fed through the entire length of the rod and the end piece was screwed into the tip.
Liquid and gas were delivered to the nozzle as previously described34. In short, liquid reservoirs were connected to the nozzle inlets via PEEK tubing (Idex, 0.250 µm i.d.). Multiple reservoirs were connected in parallel to facilitate fast switching between sample, buffer and wash solutions using a high-speed electronic valve (Rheodyne). Liquid flow was regulated using an HPLC pump (Shimadzu LC-20AD), while helium gas flow was regulated with an electronic pressure regulator (Proportion Air GP1). Gas and liquid flow rates of 23 mg min−1 and 30 µl min−1, respectively were typical during the measurement and monitored with in-line flow meters (Bronkhorst F-111B-2K0-TGD-33-V, 0–700 mg min−1 and Bronkhorst ML120V00-TGD-CC-0-S, 0–100 µl min−1 respectively). This resulted in jet velocities on the order of 10 s of meters per second, which is sufficient to outrun the radiation-induced explosion caused by the ultrafast X-ray pulse and to replenish the sample for each X-ray exposure.
Alignment of the nozzle tip with respect to the interaction region was carried out by manipulating the position of the injector rod using motorized stages. This placement was aided by visualization with the side-view microscope camera illuminated with the EuXFEL femtosecond laser coupled into the sample chamber via a fiber bundle laser synchronized with the X-ray pulse35,36.
Optical excitation scheme
Actinic excitation of the sample was carried out with an optical parametric oscillator (Opolette 355, Opotek) tuned to 475 nm with pulse duration of ~5 ns and pulse energy of 2 mJ mm−2 (ref. 35). The output beam (~325 × 338 µm FWHM) was aligned to overlap with the lower half of the GDVN to facilitate excitation of a sufficient sample volume to span the entire X-ray pulse train (~1 nl). Given the slower fluid velocity within the inner GDVN channel, it is possible to excite sufficient volume within the ~325 × 325 µm focal spot. Careful evaluation of the illumination volume was carried out to ensure sufficient sample excitation. The optical pump laser was modulated at half the XFEL intertrain repetition rate (5 Hz) yielding alternating light and dark trains to enable robust extraction of the light-induced scattering response. In this way, the experimental time range and resolution were directly defined by the XFEL pulse bunch length and intra-train repetition rate (~300 µs and 1.77 µs, respectively). This strategy represents a convenient means to access dense temporal sampling on the micro- to millisecond scale that does not require complex electronic triggering or changes to optical beam alignment.
EuXFEL SPB/SFX beamline configuration
The data were collected at the SPB/SFX instrument of the EuXFEL in September 2022, under the proposal p3046. The EuXFEL delivered bunch trains at 10 Hz with an intra-train pulse repetition rate of 564 kHz. The photon energy was 8,000 eV, which corresponds to a wavelength of 1.55 Å. From previous measurements, the focal spot was estimated at around 300 × 300 nm FWHM. The energy of every X-ray pulse was measured by a gas monitor detector upstream and was close to 2 mJ. With this beamline configuration and photon energy the beamline transmission between the gas monitor detector and the interaction region is estimated to be 65%. The AGIPD 1 M detector was placed 0.281 m downstream from the interaction region37. The experiment was monitored online with Hummingbird38.
Data acquisition and computation of time-resolved difference X-ray scattering
We recorded 175 images per pulse train with a time spacing of 1.77 µs between each acquisition. For practical reasons, the data collection was split up into runs, where each run comprised a few thousand trains. Data were collected at two different sample concentrations: 15 and 11 mg ml−1. Data filtering was performed to account for intermittent liquid jet instability. This was conducted by comparing the correlation between the absolute integrated scattering intensity of individual trains within the run against the train-average for a run; trains below a threshold of 0.99995 were considered low quality and omitted from the averaging of the scattering curves. A total of 7.75 million images were retained or ~70% of usable frames. The averaging of the scattering curves was conducted for the two concentrations separately over repeats and runs of light and dark absolute scattering, resulting in Slight(q,t) and Sdark(q,t). Here, t is the delay time of the probe pulse with respect to the arrival time of the excitation laser pulse. If not indicated otherwise (Fig. 1c and Extended Data Fig. 3), the filtered data were then normalized over the entire q-range (2.1 Å−1 > q > 0.08 Å−1) by dividing each scattering point by the sum of the total scattering within the selected q-range. Once normalized, difference scattering curves were calculated, ΔS = Slight(q,t) − Sdark(q,t). Subsequently, the low concentration was scaled to match the high concentration, a small offset was also applied to account for systematic detector errors, and the difference curves (ΔS) of the two concentrations were then merged using a weighted average, where the weights correspond to the number of light frames in each dataset. The data displayed in Fig. 1c were recorded during 8 h of total experiment time and the duration of pure data collection was 3 h 15 min with a total sample consumption of ~75 mg. Such a quantity is accessible for a wide range of biological materials. Furthermore, a reduction might be possible using a flow segmentation scheme as described by Echelmeier et al.39.
Kinetic modeling
Kinetic decomposition of the experimental data was performed to better understand the reaction dynamics of the AsLOV2 photocycle. Global fitting was carried out assuming a sequential reaction scheme with a variable number of states. The TR-WAXS scattering data can be expressed as a linear combination of time-independent basis spectra:
| 1 |
Where is the measured transient intensity, are the time-independent basis spectra and are the time-dependent concentrations of the components i. In the fitting procedure, the time-dependence of a two-state (A → B) and three-state (A → B → C) model were expressed as exponential functions as:
| 2 |
| 3 |
and
| 4 |
| 5 |
| 6 |
The constants were optimized using simplex minimization of the target function r using ‘fminsearch.m’ of MATLAB v.2019:
| 7 |
whereby was determined on each iteration by the least-square solution of equation (1) using the backslash operator (‘mldivide’) in MATLAB. The goodness of the fits was judged by plotting the refined kinetics against time-slices of the data in (Extended Data Fig. 4). The three-state model gave a lower compared to the two-state model and a better agreement in terms of shape of the fit.
AlphaFold models
To simulate unfolding of the Jɑ helix, 2,000 initial AlphaFold models were created using AFsample with dropout enabled, to determine whether the aggressive sampling could capture the unfolding25,26. The initial 2,000 models did not display any unfolding and showed only small differences in the last two residues of the Jɑ helix.
A second approach was taken to ensure the models would have the unfolded helix. By substituting every second amino acid in the helix it can be destabilized artificially, making AlphaFold unable to find any similar sequence in its database. Therefore, it classifies it as a disordered loop instead. The input sequence was modified by introducing 3, 5, 7, 9 and 11 glycine mutations starting from the second-to-last residue and substituting every second until the desired number of mutations was reached. These mutated sequences were then used to run AFsample again, generating 2,000 models for each sequence, resulting in a total of 10,000 models. In addition, we also introduced mutation in the N-terminal helix to investigate its possible effects on the scattering, five glycine inserts were introduced on top of the Jɑ inserts resulting in an additional 10,000 models.
AFsample was run using the following settings: 1,000 models with dropout templates enabled, 500 models with dropout enabled and no templates, each with a maximum of 21 recycles, and 500 models with dropout enabled and no templates, each with a maximum of 9 recycles. The mutated amino acids were then reverted back to the original residue using coot40. From the initial 2,000 models, which showed very small variation in structure, one was chosen to represent the native state of the protein.
Computation of scattering profiles
Theoretical scattering profiles were calculated using Pepsi-SAXS from the AlphaFold models, a sample concentration of 15 mg ml−1 was assumed for the theoretical scattering profiles41. We used Pepsi-SAXS, because it computes the scattering of the solvation shell from a grid, which we expect to lead to accurate results for partially unfolded proteins and because the software is very efficient in computing the scattering of the candidate compounds. The scattering was computed for 170 points, in the range between 0.08 Å−1 < q < 1.5 Å−1. The theoretical difference scattering (ΔSmodel) was calculated by subtracting scattering of a native predicted model from each of the unfolded models.
Structural fitting
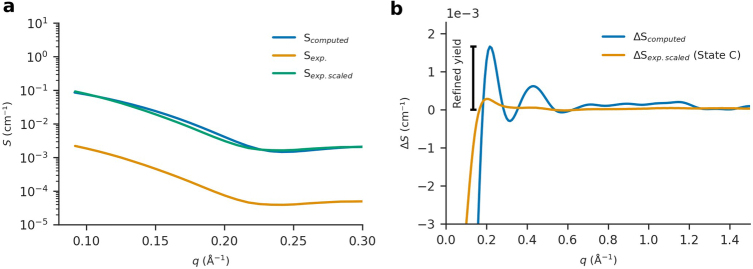
To compare the theoretical and experimental scattering, the experimental scattering was put on an absolute scale by scaling the experimental dark scattering to the theoretical dark scattering equation (8), this scale was then applied to the difference scattering (Extended Data Fig. 8). For comparing the models to the experiment, we optimized a projected photoactivation yield c for each candidate structural pair according to:
| 8 |
| 9 |
c corresponds to the photoactivation yield, which a certain structural pair would require the difference X-ray scattering to have for an optimal fit. By performing a least-square optimization on the numerator in equation (9), c could be estimated for each structural pair. The q-scale used for fitting was q < 0.16 Å−1. We used it to discriminate good fits. We selected 6,032 models with the highest R2 and which had a projected activation yield of 15 ± 5% (see Supplementary Data).
Extended Data Fig. 8. Scaling procedure to assign units to the experimental difference X-ray scattering.
(a) The buffer-subtracted and averaged experimental X-ray scattering in dark is scaled to the scattering computed from the dark model of AsLOV2 (Scomputed). (b) The difference scattering (ΔSexp) was scaled using the same factor to ensure comparability with the theoretical scattering (ΔScomputed). In this context, ΔScomputed represents the computed difference scattering between a candidate structure for state C and the ground state structure. Now, the scaling factor between the experimental and theoretical difference scattering, which is shown for one candidate structural pair in the panel, will correspond to the refined photoactivation yield for a candidate structural pair. The factor c was determined by Eq. 8 in the structural fit procedure for each difference scattering curve from candidate structural pairs. The computed scattering was calculated assuming a sample concentration of 15 mg/ml.
Determination of the photoexcitation yield by auxiliary SAXS experiments
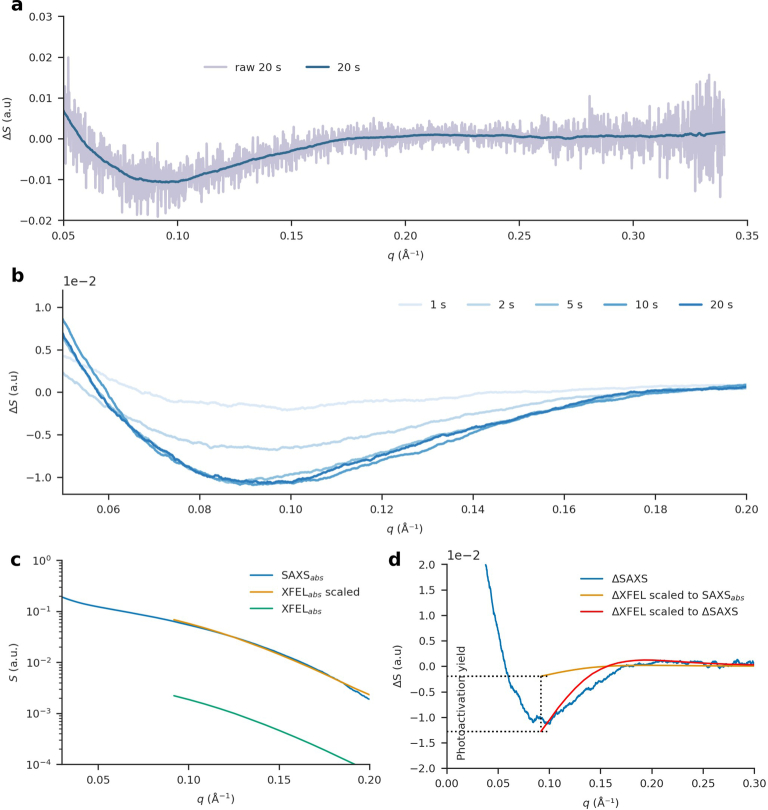
To determine the photoexcitation (activation) yield, we performed a separate SAXS experiment with AsLOV2. The data was recorded at the Diamond Light Source (beamline B21) at room temperature (20 °C). First, we recorded SAXS data in complete darkness followed by illumination of the sample for one second using a laser diode (wavelength, 470 nm; average power, 68 W m−2; spot size was an ellipse of 4.5 × 1.9 mm with an area of 6.7 mm2). The protein was allowed to dark-revert for 5 min and the procedure was repeated four more times with increasing illumination time for each cycle (2, 5, 10 and 20 s). Saturation of the difference signal (light–dark) was observed from 5 s of illumination onwards (Extended Data Fig. 9b). By comparing the signal height of this SAXS difference data for full photoconversion to ΔSexp from the XFEL, we determined the excitation yield at the EuXFEL to be 15 ± 5% (Extended Data Fig. 9d).
Extended Data Fig. 9. Determination of the photoactivation yield from steady state difference SAXS.
(a.) Smoothed difference SAXS curves (light–dark) on top of raw difference SAXS curves for 20 s illumination time. (b.) Zoom-in and overlay of the difference SAXS curves (light–dark) at all illumination times of AsLOV2. The difference signal saturated for illuminations of more than five seconds, indicating that the maximum photoactivation yield of 100% had been achieved. (c.) The XFEL scattering was then scaled to the SAXS scattering from Diamond to facilitate direct comparison between them. (d.) The photoactivation of the XFEL was subsequently determined by comparing the ratio between the scaled XFEL and the saturated SAXS difference scattering. From this analysis, we determined that the photoexcitation yield at the XFEL was approximately 15%. The scattering obtained from the SAXS experiment was smoothed using a Savitzky–Golay filter with a window length of 120 and 1st degree polynomial if nothing else is stated. The SAXS data was recorded at Diamond Light Source, beamline B21.
Protein expression and purification
AsLOV2 expression and purification followed from previously reported protocol12. The expression plasmid (6His-Gb1-AsLOV2) was obtained from the group of K. Gardner at CUNY. The AsLOV2 was expressed in Escherichia coli BL21 (DE3) STAR (Thermo Fisher Scientific). This culture was propagated in 11 l of the LB medium, induced with 1 mM IPTG at OD600 = 0.8–0.9 and then incubated at 18 °C, 180 rpm for 16 h. The cells were centrifuged at 6,000g, 4 °C for 20 min. The cell pellet was washed with 30 ml Tris buffer (50 mM Tris-HCl, 500 mM NaCl, 0.5 mM dithiothreitol and 5% (v/v) glycerol, pH 8). The washed pellet was then resuspended in 60 ml Tris buffer and sonicated for 2 min with cycles of 15 s of sonication separated by 45-s intervals at 50% pulse amplitude using a Branson 450 Digital Sonifier (Branson, BBU13119802A). The sonicated lysate was then cleared at 15,000g, 4 °C for 35 min and filtered with a 0.45-µm filter. This was then equilibrated within a Ni-NTA resin column (88222, Thermo Scientific) for further purification. The resin was washed with Tris buffer and 50 mM imidazole. Then, elution was performed in steps up to 500 mM imidazole. The 6×His tag was cleaved with TEV protease (T4455, Sigma Aldrich) in a 1:50 molar ratio of TEV:AsLOV2, and the mixture was dialyzed twice in Tris buffer at 4 °C. The dialyzed AsLOV2 sample was applied to another Ni-NTA resin column to remove the cleaved 6×His tag and residual TEV. The AsLOV2 sample was further purified using a HiPrep 26/60 Sephacryl S-100 HR (17119401, Cytiva) size-exclusion column. The final yield was 325 mg (from 11 l culture) and it was stored at −80 °C before the experiment at 13 mg ml−1.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41592-024-02344-0.
Supplementary information
Best fitted AlphaFold models of unfolded state of AsLOV2.
Source data
Binned microsecond time-resolved difference scattering data.
Result from kinetic modeling as well as result from structural modeling (Fig. 2d is incomplete due to size).
Difference scattering curves derived from two different ways of calculating a difference signal.
Time-resolved WAXS curves from five different time points.
Integrated kinetics from a two- and three-state model as well as residuals from the modeling and the reconstructed kinetics.
Scaled and unscaled absolute and difference scattering curves from experimental and theoretical from one dark and one light model.
Raw and smoothed absolute SAXS curves of AsLOV2 as well as difference SAXS curves obtained at different illumination times. Scaled and unscaled absolute and difference WAXS curves.
Acknowledgements
S.W., F.M. and H.G. acknowledge support by the Swedish Research Council through research award 2019-06092 and the BMBF for this research. F.M. acknowledges further support by the Swedish Research Council through research award 2018-00234. The sample reservoirs employed in parts of the measurements presented here were designed and fabricated by the Max Planck Institute for Medical Research, Heidelberg, which also provided instruction in its use. P.A. and A.V.M. acknowledge the support of the Universities Australia and the German Academic Exchange Service for this research. We thank K. Gardner at CUNY for sharing the AsLOV2 plasmid. We acknowledge the use of the EuXFEL biological sample preparation laboratory, enabled by the XBI User Consortium. We acknowledge the EuXFEL in Schenefeld, Germany for provision of X-ray free-electron laser beamtime at Scientific Instrument SPB/SFX and thank the SPB/SFX instrument group and facility staff for their assistance. J.V. acknowledges funding from the European Union’s Horizon 2020 research and innovation program under grant agreement no. 101004728. R.K. acknowledges funding from the National Science Foundation, BioXFEL Science and Technology Center (award no. 1231306) and Directorate for Biological Sciences (award no. 1943448). AlphaFold computations were enabled by resources provided by the National Academic Infrastructure for Supercomputing in Sweden at NSC Berzelius partially funded by the Swedish Research Council through grant agreement no. 2022-06725.
Extended data
Author contributions
S.W., F.M. and P.K. designed the study. D.F. prepared the protein samples. L.M., F.M. and A.B. reduced the data. P.K., L.M., A.B., D.F., P.A., R.A., R.B., J.B., S.B., G.D., H.G., R.K., M.K., J.P., F.K., T.L., R.L., F.L., M.M., A.M., P.M., J.M., A.N., K.O., A.R., T.S., J.V., D.W., A.W., T.V.Y., T.Y., F.M. and S.W. collected the scattering data at the EuXFEL. L.M. analyzed the time-resolved scattering data. L.M., S.W., P.K. and F.M. wrote the paper with input from all authors.
Peer review
Peer review information
Nature Methods thanks Marco Cammarata and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Allison Doerr, in collaboration with the Nature Methods team.
Funding
Open access funding provided by Uppsala University.
Data availability
The raw experimental data are available at the EuXFEL repository at 10.22003/XFEL.EU-DATA-003046-00 and the radial profiles are available at the Coherent X-ray Imaging Data Bank, CXIDB ID 225. The refined AlphaFold models are available as Supplementary Data. Source data are provided with this paper.
Code availability
The code utilized in this research is solely based on established equations and no new central algorithm was developed or utilized in the process; however, the code can be provided upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Patrick E. Konold, Leonardo Monrroy.
Contributor Information
Filipe Maia, Email: filipe.maia@icm.uu.se.
Sebastian Westenhoff, Email: sebastian.westenhoff@kemi.uu.se.
Extended data
is available for this paper at 10.1038/s41592-024-02344-0.
Supplementary information
The online version contains supplementary material available at 10.1038/s41592-024-02344-0.
References
- 1.Pandey, S. et al. Time-resolved serial femtosecond crystallography at the European XFEL. Nat. Methods17, 73–78 (2020). 10.1038/s41592-019-0628-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barends, T. R. M. et al. Direct observation of ultrafast collective motions in CO myoglobin upon ligand dissociation. Science350, 445–450 (2015). 10.1126/science.aac5492 [DOI] [PubMed] [Google Scholar]
- 3.Levantino, M. et al. Ultrafast myoglobin structural dynamics observed with an X-ray free-electron laser. Nat. Commun.6, 6772 (2015). 10.1038/ncomms7772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westenhoff, S. et al. Rapid readout detector captures protein time-resolved WAXS. Nat. Methods7, 775–776 (2010). 10.1038/nmeth1010-775c [DOI] [PubMed] [Google Scholar]
- 5.Decking, W. et al. Author Correction: a MHz-repetition-rate hard X-ray free-electron laser driven by a superconducting linear accelerator. Nat. Photonics14, 650 (2020). 10.1038/s41566-020-0680-3 [DOI] [Google Scholar]
- 6.Grünbein, M. L. et al. Effect of X-ray free-electron laser-induced shockwaves on haemoglobin microcrystals delivered in a liquid jet. Nat. Commun.12, 1672 (2021). 10.1038/s41467-021-21819-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vagovič, P. et al. Megahertz x-ray microscopy at x-ray free-electron laser and synchrotron sources. Optica6, 1106 (2019). 10.1364/OPTICA.6.001106 [DOI] [Google Scholar]
- 8.Dallari, F. et al. Microsecond hydrodynamic interactions in dense colloidal dispersions probed at the European XFEL. IUCrJ8, 775–783 (2021). 10.1107/S2052252521006333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Husband, R. J. et al. A MHz X-ray diffraction set-up for dynamic compression experiments in the diamond anvil cell. J. Synchrotron Radiat.30, 671–685 (2023). 10.1107/S1600577523003910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cammarata, M. et al. Tracking the structural dynamics of proteins in solution using time-resolved wide-angle X-ray scattering. Nat. Methods5, 881–886 (2008). 10.1038/nmeth.1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ki, H., Oang, K. Y., Kim, J. & Ihee, H. Ultrafast X-ray crystallography and liquidography. Annu. Rev. Phys. Chem.68, 473–497 (2017). 10.1146/annurev-physchem-052516-050851 [DOI] [PubMed] [Google Scholar]
- 12.Harper, S. M., Neil, L. C. & Gardner, K. H. Structural basis of a phototropin light switch. Science301, 1541–1544 (2003). 10.1126/science.1086810 [DOI] [PubMed] [Google Scholar]
- 13.Harper, S. M., Neil, L. C., Day, I. J., Hore, P. J. & Gardner, K. H. Conformational changes in a photosensory LOV domain monitored by time-resolved NMR spectroscopy. J. Am. Chem. Soc.126, 3390–3391 (2004). 10.1021/ja038224f [DOI] [PubMed] [Google Scholar]
- 14.Chen, E., Swartz, T. E., Bogomolni, R. A. & Kliger, D. S. A LOV story: the signaling state of the phot1 LOV2 photocycle involves chromophore-triggered protein structure relaxation, as probed by far-UV time-resolved optical rotatory dispersion spectroscopy. Biochemistry46, 4619–4624 (2007). 10.1021/bi602544n [DOI] [PubMed] [Google Scholar]
- 15.Nakasone, Y., Eitoku, T., Matsuoka, D., Tokutomi, S. & Terazima, M. Dynamics of conformational changes of Arabidopsis phototropin 1 LOV2 with the linker domain. J. Mol. Biol.367, 432–442 (2007). 10.1016/j.jmb.2006.12.074 [DOI] [PubMed] [Google Scholar]
- 16.Eitoku, T., Nakasone, Y., Matsuoka, D., Tokutomi, S. & Terazima, M. Conformational dynamics of phototropin 2 LOV2 domain with the linker upon photoexcitation. J. Am. Chem. Soc.127, 13238–13244 (2005). 10.1021/ja052523i [DOI] [PubMed] [Google Scholar]
- 17.Konold, P. E. et al. Unfolding of the C-terminal Jα helix in the LOV2 photoreceptor domain observed by time-resolved vibrational spectroscopy. J. Phys. Chem. Lett.7, 3472–3476 (2016). 10.1021/acs.jpclett.6b01484 [DOI] [PubMed] [Google Scholar]
- 18.Gil, A. A. et al. Femtosecond to millisecond dynamics of light induced allostery in the Avena sativa LOV domain. J. Phys. Chem. B121, 1010–1019 (2017). 10.1021/acs.jpcb.7b00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao, X., Rosen, M. K. & Gardner, K. H. Estimation of the available free energy in a LOV2-J α photoswitch. Nat. Chem. Biol.4, 491–497 (2008). 10.1038/nchembio.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DePonte, D. P. et al. Gas dynamic virtual nozzle for generation of microscopic droplet streams. J. Phys. D Appl. Phys.41, 195505 (2008). 10.1088/0022-3727/41/19/195505 [DOI] [Google Scholar]
- 21.Mancuso, A. P. et al. The single particles, clusters and biomolecules and serial femtosecond crystallography instrument of the European XFEL: initial installation. J. Synchrotron Radiat.26, 660–676 (2019). 10.1107/S1600577519003308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berntsson, O. et al. Sequential conformational transitions and α-helical supercoiling regulate a sensor histidine kinase. Nat. Commun.8, 284 (2017). 10.1038/s41467-017-00300-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennis, J. T. M. et al. Primary reactions of the LOV2 domain of phototropin, a plant blue-light photoreceptor. Biochemistry42, 3385–3392 (2003). 10.1021/bi034022k [DOI] [PubMed] [Google Scholar]
- 24.Iuliano, J. N. et al. Unraveling the mechanism of a LOV domain optogenetic sensor: a glutamine lever induces unfolding of the jα helix. ACS Chem. Biol.15, 2752–2765 (2020). 10.1021/acschembio.0c00543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature596, 583–589 (2021). 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallner, B. AFsample: improving multimer prediction with AlphaFold using aggressive sampling. Bioinformatics39, btad573 (2023). 10.1093/bioinformatics/btad573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kern, J. et al. Structures of the intermediates of Kok’s photosynthetic water oxidation clock. Nature563, 421–425 (2018). 10.1038/s41586-018-0681-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zielinski, K. A. et al. RNA structures and dynamics with Å resolution revealed by x-ray free-electron lasers. Sci. Adv.9, eadj3509 (2023). 10.1126/sciadv.adj3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konold, P. E. et al. 3D-printed sheet jet for stable megahertz liquid sample delivery at X-ray free-electron lasers. IUCrJ10.1107/S2052252523007972 (2023). [DOI] [PMC free article] [PubMed]
- 30.Chapman, H. N. et al. Femtosecond X-ray protein nanocrystallography. Nature470, 73–77 (2011). 10.1038/nature09750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grünbein, M. L. et al. Megahertz data collection from protein microcrystals at an X-ray free-electron laser. Nat. Commun.9, 3487 (2018). 10.1038/s41467-018-05953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiedorn, M. O. et al. Megahertz serial crystallography. Nat. Commun.9, 4025 (2018). 10.1038/s41467-018-06156-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sobolev, E. et al. Megahertz single-particle imaging at the European XFEL. Commun. Phys.3, 97 (2020). 10.1038/s42005-020-0362-y [DOI] [Google Scholar]
- 34.Vakili, M. et al. 3D printed devices and infrastructure for liquid sample delivery at the European XFEL. J. Synchrotron Radiat.29, 331–346 (2022). 10.1107/S1600577521013370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koliyadu, J. C. P. et al. Pump-probe capabilities at the SPB/SFX instrument of the European XFEL. J. Synchrotron Radiat.29, 1273–1283 (2022). 10.1107/S1600577522006701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmer, G. et al. Pump-probe laser system at the FXE and SPB/SFX instruments of the European X-ray free-electron laser facility. J. Synchrotron Radiat.26, 328–332 (2019). 10.1107/S160057751900095X [DOI] [PubMed] [Google Scholar]
- 37.Allahgholi, A. et al. The adaptive gain integrating pixel detector at the European XFEL. J. Synchrotron Radiat.26, 74–82 (2019). 10.1107/S1600577518016077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daurer, B. J., Hantke, M. F., Nettelblad, C. & Maia, F. R. N. C. Hummingbird: monitoring and analyzing flash X-ray imaging experiments in real time. J. Appl. Crystallogr.49, 1042–1047 (2016). 10.1107/S1600576716005926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Echelmeier, A. et al. Segmented flow generator for serial crystallography at the European X-ray free electron laser. Nat. Commun.11, 4511 (2020). 10.1038/s41467-020-18156-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta. Crystallogr. D Biol. Crystallogr.66, 486–501 (2010). 10.1107/S0907444910007493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grudinin, S., Garkavenko, M. & Kazennov, A. Pepsi-SAXS: an adaptive method for rapid and accurate computation of small-angle X-ray scattering profiles. Acta Crystallogr. D Struct. Biol.73, 449–464 (2017). 10.1107/S2059798317005745 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Best fitted AlphaFold models of unfolded state of AsLOV2.
Binned microsecond time-resolved difference scattering data.
Result from kinetic modeling as well as result from structural modeling (Fig. 2d is incomplete due to size).
Difference scattering curves derived from two different ways of calculating a difference signal.
Time-resolved WAXS curves from five different time points.
Integrated kinetics from a two- and three-state model as well as residuals from the modeling and the reconstructed kinetics.
Scaled and unscaled absolute and difference scattering curves from experimental and theoretical from one dark and one light model.
Raw and smoothed absolute SAXS curves of AsLOV2 as well as difference SAXS curves obtained at different illumination times. Scaled and unscaled absolute and difference WAXS curves.
Data Availability Statement
The raw experimental data are available at the EuXFEL repository at 10.22003/XFEL.EU-DATA-003046-00 and the radial profiles are available at the Coherent X-ray Imaging Data Bank, CXIDB ID 225. The refined AlphaFold models are available as Supplementary Data. Source data are provided with this paper.
The code utilized in this research is solely based on established equations and no new central algorithm was developed or utilized in the process; however, the code can be provided upon request.