Abstract
The relationship between obstructive sleep apnea (OSA) and chronic rhinosinusitis (CRS) has not yet been fully elucidated. Therefore, the objective of this study was to evaluate the connection between OSA risk and CRS by investigating associations between the STOP-Bang questionnaire and presence of CRS in a nationwide, population-based study. This is a cross-sectional study based on the Korean National Health and Nutrition Examination Survey (KNHANES). We evaluated 10,081 subjects who completed both the STOP-Bang and CRS-related questionnaires. Among the total subjects, 390 (3.9%) were CRS patients. The median STOP-Bang score was 3.0 [2.0; 4.0] in CRS patients, compared to 2.0 [1.0; 3.0] in subjects without CRS. In a low-risk group according to the STOP-Bang questionnaire, 3.1% of subjects were CRS patients. However, a gradual increase was observed among different risk groups. In the higher risk group, CRS patients accounted for 5.3% (P < 0.001). Among the four main symptoms of CRS (nasal obstruction, nasal discharge, facial pain/pressure, and decreased sense of smell), nasal obstruction (4.1 to 7.3%) and a decreased sense of smell (1.9 to 3.3%) increased with higher STOP-Bang scores. This study found that the proportion of patients with CRS was significantly higher in the group at a higher STOP-Bang score in the general population. Among symptoms of CRS, nasal obstruction and anosmia were found to be associated with an increased STOP-Bang score.
Keywords: Sinusitis, Obstructive sleep apnea, Risk, Questionnaire, KNHANES
Subject terms: Sleep disorders, Respiratory tract diseases
Introduction
Obstructive sleep apnea (OSA) is a disorder in which multiple upper airways are intermittently blocked during sleep1,2. The estimated prevalence of moderate to severe OSA requiring treatment varies depending on age group and sex, ranging from 3 to 50%3,4. OSA is known to be an independent risk factor for cardiovascular and cerebrovascular diseases, both of which are associated with an individual's mortality5. Consequently, the importance of OSA diagnosis is steadily increasing.
Recent studies have suggested that chronic intermittent hypoxia due to OSA can activate inflammatory pathways and increase oxidative stress, as indicated by high serum levels of interleukin (IL)-1, IL-6, IL-8, and tumor necrosis factor (TNF)-α6–8. The systemic inflammatory mechanisms of OSA are well-documented for many chronic diseases such as hypertension, diabetes mellitus (DM), and other metabolic syndromes. It has been suggested that OSA is also associated with chronic inflammatory diseases such as CRS9–11. Chronic rhinosinusitis (CRS) refers to the inflammation of the nose and paranasal sinuses. The role of the nose in OSA is already well known because people breathe through the nose during sleep12,13. Moreover, previous studies have reported that OSA and sleep disruption are highly prevalent in patients with CRS14,15. Currently, there is a lack of research assessing how the presence and symptoms of CRS in the general population are related to the STOP-Bang questionnaire.
The gold standard diagnostic tool for OSA is polysomnography16. However, due to limited access to sleep laboratories, OSA is underdiagnosed in the general population17. Therefore, the STOP-Bang questionnaire is widely used as an alternative screening test. The STOP-Bang questionnaire is a concise and easy-to-use screening tool for identifying patients at high risk of underlying moderate to severe OSA15,16. The STOP-Bang questionnaire was designed as a screening tool for OSA, comprising 4 self-reportable factors (STOP: snoring, tiredness, observed apnea, and high blood pressure) and four demographic variables (Bang: body mass index (BMI), age, neck circumference, and gender)18. A STOP-Bang score of 3 or higher demonstrates excellent sensitivity in discriminating mild, moderate, and severe OSA (sensitivity: 84%, 93%, and 100%, respectively in the original validation trial)18,19. The diagnostic accuracy of a STOP-Bang score of 3 or higher in detecting moderate to severe OSA was greater than 0.8018. The significance of STOP-Bang lies not only in its ability to measure the risk of OSA, but also in its role as a predictor of detrimental outcomes such as cardiovascular risk associated with OSA20.
The Korea National Health and Nutrition Examination Survey (KNHANES) is an annual survey conducted by Korea Disease Control and Prevention Agency to assess nationwide health and nutrition statistics21. This nationally representative cross-sectional survey annually includes approximately 10,000 individuals and gathers data on socioeconomic status, health-related behaviors, quality of life, clinical profiles relevant to non-communicable diseases, and other related factors22. The increasing clinical importance of OSA prompted the inclusion of the STOP-Bang questionnaire as a survey item for adults aged 40 and above starting in 2019 (The KNHANES VIII)21.Therefore, the aim of this study was to assess the relationship between OSA risk and CRS by analyzing correlations between the STOP-Bang questionnaire and CRS presence and symptoms in the nationwide study.
Results
Relationship between presence of CRS and STOP-Bang questionnaire
The KNHANES survey period for the STOP-Bang and CRS-related questions was from 2019 to 2021. Out of the 22,559 individuals sampled during this period, 9,541 who did not complete the STOP-Bang questionnaire and an additional 2937 individuals who did not complete the CRS-related questionnaire were excluded. Therefore, a total of 10,081 subjects were included in this study. The final sample consisted of 390 (3.9%) individuals with CRS and 9691 individuals without CRS (Fig. 1). The median STOP-Bang score was 3.0 for patients with CRS (Mean ± standard deviation; 2.8 ± 1.6) and 2.0 for those without CRS (2.5 ± 1.5) (Fig. 2). The power size was 0.963. CRS patients exhibited higher percentages of snoring (25.4%), tiredness (46.2%), observed apnea (11.3%), and thick neck circumference (41.8%) compared to subjects without CRS (17.3%, 27.0%, 7.8%, 35.2%, respectively). However, age was significantly lower in patients with CRS (median age, with CRS: 58.0, without CRS: 60.0; P < 0.001). Additionally, the percentage of individuals over the age of 50 was significantly higher in the CRS group. This contrasts with the known association of increasing age with a higher risk of OSA (Table 1). Cigarette smoking was significantly (49.2% of CRS patients and 39.6% of subjects without CRS; P < 0.001) more prevalent in the CRS group, while alcohol use was not significantly (P = 0.161) different between the two groups. Asthma and AR were significantly more prevalent in the CRS group (Asthma: 9.4% of CRS patients and 3.0% of without CRS subjects; AR: 49.7% of CRS patients and 11.0% of without CRS subjects). There were no significant differences in house income (P = 0.212) or economic activity (P = 0.128). However, education level was higher in the CRS group (73.3% of CRS patients are highly educated level, compared to 65.5% of subjects without CRS; P = 0.003).
Fig. 1.
Flow diagram of participant recruitment.
Fig. 2.
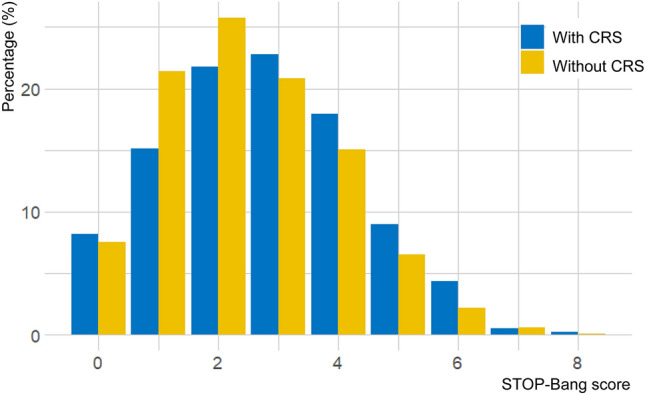
Distribution of STOP-Bang score by chronic rhinosinusitis status.
Table 1.
Demographics and STOP-Bang questionnaire for study subjects with and without chronic rhinosinusitis.
| Patients, by presence of CRS | |||
|---|---|---|---|
| with CRS (N = 390) | without CRS (N = 9691) | P value | |
| STOP-Bang (0–8) | 3.0 [2.0; 4.0] | 2.0 [1.0; 3.0] | < 0.001 |
| STOP | |||
| Snore | 99 (25.4%) | 1680 (17.3%) | < 0.001 |
| Tired | 180 (46.2%) | 2616 (27.0%) | < 0.001 |
| Observed | 44 (11.3%) | 754(7.8%) | 0.016 |
| Pressure | 129 (33.1%) | 3392 (35.0%) | 0.467 |
| Bang | |||
| BMI | 24.1 [21.6;26.3] | 24.0 [21.9;26.2] | 0.709 |
| BMI (> 30 kg/m2) | 24 (6.2%) | 538 (5.6%) | 0.692 |
| Age | 58.0 [48.0;68.0] | 60.0 [50.0;69.0] | < 0.001 |
| Age (> 50 years) | 250 (64.1%) | 7324 (75.6%) | < 0.001 |
| Neck circumferential (> 36.3 cm) | 163 (41.8%) | 3407 (35.2%) | 0.008 |
| Sex (male) | 186 (47.7%) | 4179 (43.1%) | 0.083 |
| Smoking | 191(49.2%) | 3807 (39.6%) | < 0.001 |
| Alcohol | 0.161 | ||
| Regular user | 161 (41.4%) | 4358 (45.1%) | |
| Non-user | 227 (58.4%) | 5275 (54.6%) | |
| Comorbidity | |||
| Asthma | 34 (9.4%) | 273 (3.0%) | < 0.001 |
| AR | 179 (49.7%) | 1000 (11.0%) | < 0.001 |
| House income | 0.212 | ||
| High | 90 | 2549 | |
| Middle-high | 103 | 2393 | |
| Middle-low | 95 | 2424 | |
| Low | 101 | 2281 | |
| Economic activity | 0.128 | ||
| Employed | 196 (54.6%) | 5335 (58.8%) | |
| Unemployed | 163 (35.4%) | 3742 (41.2%) | |
| Educated level | 0.003 | ||
| High (> 9 years) | 263 (73.3%) | 5939 (65.5%) | |
| Low (≤ 9 years) | 96 (26.7%) | 3135 (34.5%) | |
CRS chronic rhinosinusitis, OSA obstructive sleep apnea, AR allergic rhinitis.
Relationship between OSA risk and CRS state or symptoms
In this study, those with STOP Bang scores of 0–2, 3–4, and 5–8 were categorized into low, intermediate, and high OSA risk groups, respectively. Based on this categorization, we found that all parameters of STOP-Bang questionnaire increased significantly in a stepwise manner (Supplementary Table 1). Moreover, the presence of CRS significantly increased as the risk of OSA increased from low to high (3.1%, 3.8%, and 5.3%, respectively; P < 0.001; Table 2). There were trend differences in the timing of CRS diagnosis among the groups (median age: 39.0, 41.0, and 35.0 years; P = 0.054). However, there was no significant difference in the presence of nasal polyps among the three OSA risk groups (P = 0.463). Additionally, there was no significant difference in whether individuals were currently on treatments (P = 0.302).
Table 2.
Chronic rhinosinusitis states with high risk for OSA according to the STOP-Bang questionnaire.
| CRS state | Total N | Low | Intermediate | High | P value |
|---|---|---|---|---|---|
| Patients with CRS | 10,081 | 169/5475 (3.1%) | 137/3630 (3.8%) | 52/976 (5.3%) | < 0.001 |
| Diagnosis of nasal polyps | 498 | 46/248 (18.5%) | 42/179 (23.5%) | 15/71 (21.1%) | 0.463 |
| Age at diagnosis of CRS | 679 | 39.0 [23.0;49.0] | 41.0 [23.0;55.0] | 35.0 [17.0;52.5] | 0.054 |
| Currently receiving treatment for CRS | 683 | 23/340 (6.8%) | 27/263 (10.3%) | 7/80 (8.8%) | 0.302 |
CRS Chronic rhinosinusitis. Method: Cochran-Armitage trend test, Kruskal–Wallis rank sum test.
In the analysis of CRS symptoms, no significant association was found between the STOP-Bang classification and anterior/posterior nasal drip or presence of facial pain (P = 0.131 or P = 0.803, respectively, Table 3). However, a notable relationship was observed between higher-risk OSA groups and increased likelihood of experiencing symptoms such as nasal congestion (low OSA risk group: 4.1%, intermediate risk OSA group: 5.7%, high risk OSA group: 7.3%; P < 0.001) and reduced sense of smell (low risk OSA group: 1.9%, intermediate risk OSA group: 3.3%, high risk OSA group: 3.3%; P < 0.001).
Table 3.
Chronic rhinosinusitis symptoms with high risk for OSA according to the STOP-Bang questionnaire.
| Symptoms related with CRS | Low (n = 5475) | Intermediate (n = 3630) | High (n = 976) | P value |
|---|---|---|---|---|
| Anterior/posterior nasal drip for more than 3 months | 373 (6.8%) | 275 (7.6%) | 80 (8.4%) | 0.131 |
| Nasal obstructions for more than 3 months | 225 (4.1%) | 206 (5.7%) | 71 (7.3%) | < 0.001 |
| Facial pain or pressure for more than 3 months | 25 (0.5%) | 18 (0.5%) | 6 (0.6%) | 0.803 |
| Anosmia or hyposmia for more than 3 months | 106 (1.9%) | 120 (3.3%) | 32 (3.3%) | < 0.001 |
CRS chronic rhinosinusitis; Method: Cochran-Armitage trend test.
Discussion
The goal of this study was to examine the association between CRS and OSA risk through an analysis of the relationship between the CRS and STOP-Bang questionnaire based on the KNHANES. We confirmed that subjects with CRS had higher STOP-Bang questionnaire scores through a national survey, thereby providing real-world evidence from the general population. Previous studies have reported that OSA and sleep disruption are highly prevalent in patients with CRS14,15. A study on World Trade Center Responders reported CRS is an independent risk factor for OSA, with an odds ratio (OR) of 1.8014. Furthermore, these studies have examined the relationship between CRS and OSA using internally developed sleep quality questionnaires or home sleep testing. However, to the best of our knowledge, no studies have demonstrated the relationship between CRS and the STOP-Bang questionnaire using a large nationwide database.
Additionally, our study is the first to investigate specific symptoms of CRS in relation to OSA, while also identifying which components of the STOP-Bang questionnaire are associated with CRS in the general population. Among the four symptoms of CRS (nasal congestion, discharge, facial pain/pressure, and decreased sense of smell), nasal congestion and decreased sense of smell were increased in the high risk group. According to a previous study, participants with these typical CRS symptoms had an increased risk of various sleep-related problems such as snoring, difficulties in initiating sleep, difficulties in maintaining sleep, early morning awakening, and excessive daytime sleepiness compared to those without CRS symptoms15. Interestingly, within the STOP-Bang questionnaire, we found positive correlations of CRS with snoring, tiredness, and observed apnea. This finding suggests that airflow disturbance can increase the risk of OSA in CRS patients, providing additional information beyond previous studies that suggest a potential link between the two conditions through shared systemic inflammatory pathophysiological mechanisms9. We did not observe significant differences in hypertension, BMI, or sex among components of the STOP-Bang questionnaire for CRS patients. Similarly, a previous study has shown that patients with CRS tend to exhibit a higher risk of OSA even with lower BMI levels23. Our data showed that patients with CRS were relatively younger in age, despite the well-established association between increasing age and a higher risk of OSA24. Nevertheless, CRS patients still had a higher risk of OSA.
CRS is a heterogeneous multifactorial disease25. It is known to be associated with microbial infections, smoking, pollutants, allergens, and other social factors such as a correlation with low socioeconomic status26,27. However, in our study, it was not related to household income or economic activity. The prevalence of CRS was higher in the group with higher education. Regarding comorbidities of CRS, previous research has indicated that the presence of persistent AR in addition to CRS can further amplify the risk of sleep problems15. Moreover, conditions that are well-known risk factors for OSA, such as AR and asthma, were found to be more prevalent in patients with CRS. The presence of these conditions, which are frequently observed in CRS, is considered to increase the risk of OSA. CRS is classified into type 2 and non-type 2 according to endotypes28. In Korea, there has been an eosinophilic shift, and the prevalence of type 2 CRS is increasing29,30. Comorbidities such as AR and asthma are also on the rise, which may increase the risk of OSA.
The association between CRS and OSA is complex. Although such association is not fully established yet, some studies have suggested that these two conditions might be bidirectionally related. As mentioned earlier, CRS has been identified as a risk factor for OSA. However, some studies also suggest that OSA may increase the development of CRS31. According to Garvey et al., patients with both CRS and OSA show higher rates of antibiotic and steroid usage than those with CRS alone31. In particular, among the group of patients with both OSA and CRS who did not receive continuous positive airway pressure or sleep surgery, the likelihood of using antibiotics or steroids remained higher even when compared to groups receiving similar rates of endoscopic sinus surgery31.
A limitation of our study was that it was designed as a cross-sectional study, making it difficult to establish causal relationships. However, in our research, CRS treatment status did not significantly correlate with the OSA risk. Additional study is needed to further investigate this. Another limitation of our study was that the KNHANES included the STOP-Bang questionnaire only for individuals aged 40 and above. Consequently, our study was limited to participants within this age group, and the findings may not be representative of all age groups. Additionally, we did not diagnose OSA through objective tests but relied on a questionnaire-based approach. To enhance the reliability of our findings, we utilized various indicators from multiple questionnaires. Furthermore, since this study relied on survey data, defining CRS through endoscopic or CT findings was not possible, nor was obtaining nasal tissue for endotyping. Future prospective studies should incorporate endoscopic examinations, CT scans, and tissue sampling from participants to address these limitations.
Conclusions
Our study showed that the subjects with CRS had significantly higher STOP-Bang questionnaire score. This finding was associated with increased occurrences of snoring, tiredness, and apnea. Among symptoms of CRS, nasal obstruction and decreased sense of smell were found to be linked to an increased risk of OSA.
Methods
Ethics statement
The 2019-2021 KNHANES was approved by the Institutional Review Board (IRB) of the Korea Disease Control and Prevention Agency (IRB No. 2018-01-03-C-A, 2018-01-03-2C-A, and 2018-01-03-3C-A). All participants signed informed consent forms during the survey. This study was also approved by the IRB of Soonchunhyang University Cheonan Hospital (IRB No. 2023-09-056). The study was performed in accordance with the approved guidelines and the Declaration of Helsinki.
Study population
This was a cross-sectional study based on nationwide population data. It was conducted using the Korean National Health and Nutrition Examination Survey (KNHANES) data from 2019 to 2021. KNHANES is an annual national survey project assessing the health and nutrition status of Korean since 199822. It is carried out by the Korea Disease Control and Prevention Agency22. Each year, KNHANES samples approximately 10,000 nationally representative individuals using a multi-stage clustered probability design22. The KNHANES data were obtained from the official KNHANES website (https://knhanes.kdca.go.kr/knhanes). A flow chart of the participant inclusion and exclusion criteria is provided in Fig. 1. Individuals who completed both the STOP-Bang and CRS-related questionnaires were included in the study, while those who did not complete either questionnaire were excluded.
STOP-Bang questionnaire
The STOP-Bang questionnaire was conducted for individuals aged 40 years and older in KNHANES after the year 2019 to assess the risk of obstructive sleep apnea (OSA). The STOP-Bang questionnaire was utilized as the assessment tool. It consisted of eight items, namely: (1) Snoring: Do you snore loudly (louder than talking or loud enough to be heard through closed doors)?; (2) Tired: Do you often feel tired, fatigued, or sleepy during daytime?; (3) Observed: Has anyone observed you stop breathing during your sleep?; (4) Pressure: Do you have or are you being treated for high blood pressure?; (5) BMI > 30 kg/m2; (6) Age > 50 years old; (7) Neck circumference > 36.3 cm; and (8) Gender (if male)32. The risk of OSA was categorized based on the STOP-Bang score, with the following classifications: (1) high risk of OSA, affirmative responses to 5–8 items; (2) intermediate risk of OSA, affirmative responses to 3–4 items; and (3) low risk of OSA, affirmative responses to 0–2 items. The total count of "yes" responses was calculated as the sum of affirmative answers across all questionnaire items33.
Chronic rhinosinusitis and clinical factor measurements
CRS was defined based on the survey questionnaire as the presence of two or more symptoms persisting for at least 12 weeks, with at least one being nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip)28. Optional symptoms included facial pain/pressure and reduction or loss of smell28. The survey questionnaire included items related to CRS symptoms such as anterior/posterior nasal drip, nasal obstruction, facial pain or pressure, and anosmia or hyposmia persisting for more than three months. To address potential errors, a comprehensive analysis was conducted on relevant survey items, including questions about the timing of CRS diagnosis, ongoing treatment for CRS, and the presence of nasal polyps.
Additional clinical factors included in the analysis were asthma, allergic rhinitis, smoking status, alcohol usage, household income, economic activity, and educational level. Alcohol usage was defined as regular if individuals consumed one or more drinks per month in the past year, while non-users were defined as those who had never consumed alcohol or had consumed less than one drink per month in the past year.
Statistical analysis
Categorical variables are presented as N (%). They were assessed using a chi-square test for two-group comparisons. For three or more groups, a Cochran-Armitage trend test was employed to identify any observed trends. Continuous variables are presented as median [Q1; Q3] values. If the number of data points was less than 5000, a Shapiro–Wilk Normality test was performed to assess normality. If the number of data points exceeded 5000, an Anderson-Daring test was utilized. Following the normality test, two-group comparisons utilized either a t-test or Mann–Whitney test based on the normality assumption, while comparison between three or more groups employed either Analysis of Variance (ANOVA) or Kruskal–Wallis Rank Sum Test depending on the normality assumption. A P-value of less than 0.05 was considered statistically significant. All statistical analyses and data visualization were conducted using SAS version 9.4 for Windows (SAS Institute Inc., Cary, NC, USA) and R (version 4.1.2; R Foundation for Statistical Computing, Vienna, Austria).
Supplementary Information
Acknowledgements
This study was supported by the Soonchunhyang University Research Fund.
Author contributions
Conceptualization and study design: HKC, JHC; data acquisition: HKC, DK, HWL, YL; Statistical analysis and visualization: HKC; the original draft of the manuscript: HKC, JHC; supervision or mentorship: BB, JYL, JHC. All authors reviewed the manuscript.
Data availability
All the data generated and/or analyzed during the current study are included in this article and are available from the Korea National Health and Nutrition Examination Survey homepage (https://knhanes.kdca.go.kr/knhanes/sub03/sub03_02_05.do).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-71923-0.
References
- 1.Kapur, V. K. et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med.13, 479–504. 10.5664/jcsm.6506 (2017). 10.5664/jcsm.6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim, S. D. et al. Relationship between allergic rhinitis and nasal surgery success in patients with obstructive sleep apnea. Am. J. Otolaryngol.42, 103079. 10.1016/j.amjoto.2021.103079 (2021). 10.1016/j.amjoto.2021.103079 [DOI] [PubMed] [Google Scholar]
- 3.Heinzer, R. et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med.3, 310–318. 10.1016/S2213-2600(15)00043-0 (2015). 10.1016/S2213-2600(15)00043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peppard, P. E. et al. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol.177, 1006–1014. 10.1093/aje/kws342 (2013). 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jehan, S. et al. Obstructive sleep apnea and stroke. Sleep Med. Disord.2, 120–125 (2018). [PMC free article] [PubMed] [Google Scholar]
- 6.Mullington, J. M., Haack, M., Toth, M., Serrador, J. M. & Meier-Ewert, H. K. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog. Cardiovasc. Dis.51, 294–302. 10.1016/j.pcad.2008.10.003 (2009). 10.1016/j.pcad.2008.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhadriraju, S., Kemp, C. R. Jr., Cheruvu, M. & Bhadriraju, S. Sleep apnea syndrome: Implications on cardiovascular diseases. Crit. Pathw. Cardiol.7, 248–253. 10.1097/HPC.0b013e31818ae644 (2008). 10.1097/HPC.0b013e31818ae644 [DOI] [PubMed] [Google Scholar]
- 8.Selmi, C., Montano, N., Furlan, R., Keen, C. L. & Gershwin, M. E. Inflammation and oxidative stress in obstructive sleep apnea syndrome. Exp. Biol. Med. (Maywood)232, 1409–1413. 10.3181/0704-MR-103 (2007). 10.3181/0704-MR-103 [DOI] [PubMed] [Google Scholar]
- 9.Kao, L. T. et al. Obstructive sleep apnea and the subsequent risk of chronic rhinosinusitis: A population-based study. Sci. Rep.6, 20786. 10.1038/srep20786 (2016). 10.1038/srep20786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drager, L. F., Togeiro, S. M., Polotsky, V. Y. & Lorenzi-Filho, G. Obstructive sleep apnea: A cardiometabolic risk in obesity and the metabolic syndrome. J. Am. Coll. Cardiol.62, 569–576. 10.1016/j.jacc.2013.05.045 (2013). 10.1016/j.jacc.2013.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu, S. et al. The association between obstructive sleep apnea and metabolic syndrome: A systematic review and meta-analysis. BMC Pulm. Med.15, 105. 10.1186/s12890-015-0102-3 (2015). 10.1186/s12890-015-0102-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferris, B. G. Jr., Mead, J. & Opie, L. H. Partitioning of respiratory flow resistance in man. J. Appl. Physiol.19, 653–658. 10.1152/jappl.1964.19.4.653 (1964). 10.1152/jappl.1964.19.4.653 [DOI] [PubMed] [Google Scholar]
- 13.Park, C. Y. et al. Clinical effect of surgical correction for nasal pathology on the treatment of obstructive sleep apnea syndrome. PLoS One9, e98765. 10.1371/journal.pone.0098765 (2014). 10.1371/journal.pone.0098765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunderram, J. et al. Chronic rhinosinusitis is an independent risk factor for OSA in world trade center responders. Chest155, 375–383. 10.1016/j.chest.2018.10.015 (2019). 10.1016/j.chest.2018.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bengtsson, C. et al. Chronic rhinosinusitis impairs sleep quality: Results of the GA2LEN study. Sleep10.1093/sleep/zsw021 (2017). 10.1093/sleep/zsw021 [DOI] [PubMed] [Google Scholar]
- 16.Kim, J. W. et al. Polysomnographic phenotyping of obstructive sleep apnea and its implications in mortality in Korea. Sci. Rep.10, 13207. 10.1038/s41598-020-70039-5 (2020). 10.1038/s41598-020-70039-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng, S. S. et al. Use of Berlin questionnaire in comparison to polysomnography and home sleep study in patients with obstructive sleep apnea. Respir. Res.20, 40. 10.1186/s12931-019-1009-y (2019). 10.1186/s12931-019-1009-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pivetta, B. et al. Use and performance of the STOP-Bang questionnaire for obstructive sleep apnea screening across geographic regions: A systematic review and meta-analysis. JAMA Netw. Open4, e211009. 10.1001/jamanetworkopen.2021.1009 (2021). 10.1001/jamanetworkopen.2021.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung, F. et al. STOP questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology108, 812–821. 10.1097/ALN.0b013e31816d83e4 (2008). 10.1097/ALN.0b013e31816d83e4 [DOI] [PubMed] [Google Scholar]
- 20.Valdivia, G. et al. Association between cardiovascular mortality and STOP-Bang questionnaire scores in a cohort of hospitalized patients: A prospective study. J. Bras. Pneumol.47, e20210039. 10.36416/1806-3756/e20210039 (2021). 10.36416/1806-3756/e20210039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh, K. et al. Korea National Health and Nutrition Examination Survey, 20th anniversary: Accomplishments and future directions. Epidemiol. Health43, e2021025. 10.4178/epih.e2021025 (2021). 10.4178/epih.e2021025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kweon, S. et al. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol.43, 69–77. 10.1093/ije/dyt228 (2014). 10.1093/ije/dyt228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahdavinia, M. et al. Patients with chronic rhinosinusitis and obstructive sleep apnea have increased paroxysmal limb movement. Am. J. Rhinol. Allergy32, 94–97. 10.1177/1945892418762843 (2018). 10.1177/1945892418762843 [DOI] [PubMed] [Google Scholar]
- 24.Glasser, M., Bailey, N., McMillan, A., Goff, E. & Morrell, M. J. Sleep apnoea in older people. Breathe7, 248–256. 10.1183/20734735.021910 (2011). 10.1183/20734735.021910 [DOI] [Google Scholar]
- 25.Cha, H. et al. Effects of neutrophil and eosinophil extracellular trap formation on refractoriness in chronic rhinosinusitis with nasal polyps. Allergy Asthma Immunol. Res.15, 94–108. 10.4168/aair.2023.15.1.94 (2023). 10.4168/aair.2023.15.1.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geramas, I., Terzakis, D., Hatzimanolis, E. & Georgalas, C. Social factors in the development of chronic rhinosinusitis: A systematic review. Curr. Allergy Asthma Rep.18, 7. 10.1007/s11882-018-0763-0 (2018). 10.1007/s11882-018-0763-0 [DOI] [PubMed] [Google Scholar]
- 27.Peeters, S. et al. Association between outdoor air pollution and chronic rhinosinusitis patient reported outcomes. Environ. Health21, 134. 10.1186/s12940-022-00948-7 (2022). 10.1186/s12940-022-00948-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fokkens, W. J. et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology58, 1–464. 10.4193/Rhin20.600 (2020). 10.4193/Rhin20.600 [DOI] [PubMed] [Google Scholar]
- 29.Kim, S. J. et al. Changes in histological features of nasal polyps in a Korean population over a 17-year period. Otolaryngol. Head Neck Surg.149, 431–437. 10.1177/0194599813495363 (2013). 10.1177/0194599813495363 [DOI] [PubMed] [Google Scholar]
- 30.Shin, S. H., Ye, M. K., Kim, J. K. & Cho, C. H. Histological characteristics of chronic rhinosinusitis with nasal polyps: Recent 10-year experience of a single center in Daegu, Korea. Am. J. Rhinol. Allergy28, 95–98. 10.2500/ajra.2014.28.4003 (2014). 10.2500/ajra.2014.28.4003 [DOI] [PubMed] [Google Scholar]
- 31.Garvey, E. et al. Obstructive sleep apnea and chronic rhinosinusitis: Understanding the impact of OSA on CRS disease burden?. J. Neurol. Surg. B Skull Base84, P093. 10.1055/s-0043-1762314 (2023). 10.1055/s-0043-1762314 [DOI] [Google Scholar]
- 32.Byun, J.-I., Kim, D.-H., Kim, J.-S. & Shin, W. C. Usefulness of using alternative body-mass index and neck circumference criteria for STOP-Bang questionnaire in screening South Korean obstructive sleep apnea patients. Sleep Med. Res.11, 38–43. 10.17241/smr.2020.00591 (2020). 10.17241/smr.2020.00591 [DOI] [Google Scholar]
- 33.Chung, F., Liao, P. & Farney, R. Correlation between the STOP-Bang score and the severity of obstructive sleep apnea. Anesthesiology122, 1436–1437. 10.1097/ALN.0000000000000665 (2015). 10.1097/ALN.0000000000000665 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data generated and/or analyzed during the current study are included in this article and are available from the Korea National Health and Nutrition Examination Survey homepage (https://knhanes.kdca.go.kr/knhanes/sub03/sub03_02_05.do).