Abstract
Witnessing violent or traumatic events is common during childhood and adolescence and could cause detrimental effects such as increased risks of psychiatric disorders. This stressor could be modeled in adolescent laboratory animals using the chronic witnessing social defeat (CWSD) paradigm, but the behavioral consequences of CWSD in adolescent animals remain to be validated for cognitive, anxiety-like, and depression-like behaviors and, more importantly, the underlying neural mechanisms remain to be uncovered. In this study, we first established the CWSD model in adolescent male mice and found that CWSD impaired cognitive function and increased anxiety levels and that these behavioral deficits persisted into adulthood. Based on the dorsal-ventral functional division in hippocampus, we employed immediate early gene c-fos immunostaining after behavioral tasks and found that CWSD-induced cognition deficits were associated with dorsal CA3 overactivation and anxiety-like behaviors were associated with ventral CA3 activity reduction. Indeed, chemogenetic activation and inhibition of dorsal CA3 neurons mimicked and reversed CWSD-induced recognition memory deficits (not anxiety-like behaviors), respectively, whereas both inhibition and activation of ventral CA3 neurons increased anxiety-like behaviors in adolescent mice. Finally, chronic administration of vortioxetine (a novel multimodal antidepressant) successfully restored the overactivation of dorsal CA3 neurons and the cognitive deficits in CWSD mice. Together, our findings suggest that dorsal CA3 overactivation mediates CWSD-induced recognition memory deficits in adolescent male mice, shedding light on the pathophysiology of adolescent CWSD-induced adverse effects and providing preclinical evidence for early treatment of stress-induced cognitive deficits.
Subject terms: Depression, Stress and resilience
Introduction
Witnessing stress is common during childhood and adolescence. A considerable number of children and teenagers have witnessed various stressors such as frequent parent quarrels or friends being physically or verbally bullied [1–4]. Witnessing stress has been associated with a wide range of adverse effects, such as a higher frequency of withdrawal, increased levels of anxiety, aggressiveness and delinquency, academic failure, and sleep disturbance [5–10]. Some of these adverse effects could persist many years later [4]. Importantly, this stressor has been partially reproduced in the animal model of chronic witnessing social defeat (CWSD) in adult rodents, i.e., witnessing a conspecific being beaten by a CD-1 mouse or Long–Evans rat. After several sessions of CWSD, the witnessing mice showed increased anxiety-like and depression-like behaviors and cognitive deficits [11–18]. On the other hand, only a few CWSD animal studies focused on adolescent animals. Regarding behavioral effects, these studies reported controversial results in depression-like behaviors [19–21] and did not observe alterations in cognition [19] or anxiety-like behaviors [19, 21], which are not consistent with the majority of the adolescent stress studies showing cognitive deficits and depression-like behaviors [22], as well as increased anxiety-like behaviors [23–27]. Therefore, the behavioral consequences of CWSD in adolescent animals remain to be validated for cognitive, anxiety-like, and depression-like behaviors, and more importantly, the underlying neural mechanisms remain to be uncovered.
Hippocampus is one of the brain regions consistently showing vulnerability to stress effects [28–31], including adolescent stress exposure [22]. The functional division along the dorsal-ventral axis is well established, with the dorsal hippocampus more closely related to cognitive functions and the ventral hippocampus to emotional behaviors [32–34]. The effects of CWSD on hippocampus have been examined in two studies, which focused on the dentate gyrus and reported new-born neuronal cell survival attenuation and microglial overactivation [17, 35]. Besides, dorsal and ventral hippocampus are involved in the generation and re-activation of fear memory engram cells, respectively, in this paradigm [36]. Nevertheless, it is largely unknown how CWSD in adolescence affects dorsal and ventral hippocampus and whether hippocampal alterations play causal roles in the behavioral deficits induced by this stressor.
To address these issues, in this study, we first established the CWSD model in adolescent male mice and carried out a series of behavioral tests to validate the effects of the CWSD paradigm on cognitive and emotional domains. Then, using immediate early gene c-fos immunostaining (which reflects neural activation [37]), we investigated how CWSD affected neuronal activation in the dorsal and ventral CA3, a hippocampal subregion that we found to be highly vulnerable to CWSD (see Results section). Using chemogenetic techniques, we then manipulated dCA3 and vCA3 neuronal activity to investigate their causal involvement in CWSD-induced cognitive and emotional behavioral alterations. Finally, we examined whether chronic administration of vortioxetine, a novel multimodal antidepressant [38], could restore CWSD-induced behavioral and neural alterations, which was to provide preclinical evidence for the potential use of this drug in adolescents.
Materials and methods
Below is a brief summary of the “Materials and Methods”. See Supplementary Information for details.
Animals
Adolescent (3 weeks old) male C57BL/6N mice and male CD-1 mice (8–12 months old) were purchased from Vital River Laboratories in Beijing, China. All experiments were approved by the Peking University Committee on Animal Care and Use and performed in compliance with the National Institute of Health’s Guide for the Use and Care of Laboratory Animals.
Witnessing social defeat stress
The witnessing social defeat stress procedure was carried out following previous description with slight modification [18]. Briefly, the cage of a CD-1 mouse (aggressor) was divided into two halves using a perforated divider. A young adult male C57 mice (intruder) was introduced into the cage to confront with the CD-1 mouse for 10 min. A “witness” C57 mouse (stressed) was placed on the other side of the divider during this encounter. For single WSD (S-WSD), after 10 min, the “witness” animals were returned to their home cages for subsequent behavioral tests or sacrifice. For chronic WSD (CWSD), the stress process repeated 10 days (PND35-44).
Behavioral testing
A series of behavioral tests were carried out, including cognitive behaviors (Novel Object Recognition, Spatial Object Recognition, Y-maze Spontaneous Alternation), anxiety-like behaviors (Light-Dark Box, Elevated Plus Maze, Open Field test), and depression-like behaviors (Sucrose Preference Test, Forced Swimming Test, Tail Suspension Test, and Sucrose Splash Test).
Stereotaxic surgery and virus injection
For chemogenetic activation or inhibition of dCA3 and vCA3 neurons, AAV2/9-hSyn-hM4D (Gi)-mcherry-WPREs (1.66 × 1012 viral genomes/mL) or AAV2/9-hSyn-hM3D (Gq)-mcherry (3.43 × 1012 viral genomes/mL) were bilaterally injected to the dCA3 or vCA3 on PND28. Quantitative analyses of the extent of viral transfection showed that, on average, in dCA3 AAV injection experiments, 66.26 ± 4.56% of dCA3, 15.44 ± 6.24% of dCA1, and 2.18 ± 1.5% of dDG were infected (Figs. S5A, S6A) and, in vCA3 AAV injection experiments, 70.12 ± 5.89% of vCA3, 10.44 ± 2.48% of vCA1, and 1.58 ± 1.09% of vDG were infected (Figs. S7A, S8A). These results indicate that the infection of AAV was mainly restricted in dCA3 or vCA3.
Drugs
Vortioxetine (Lundbeck, Denmark) was dissolved in 10% aqueous hydroxypropyl-β-cyclodextrin. Control and stressed mice were randomly assigned to one of four groups: CT+Veh, CWSD+Veh, CT+Vort, and CWSD+Vort. The Veh groups received treatment with vehicle, while the Vort groups received vortioxetine (i.p., 10 mg/kg) [39, 40] once daily for 28 days (PND 45–72).
Statistical analysis
SPSS 26.0 (SPSS, Chicago, IL, USA) and GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA) were used to perform statistical analyses. Statistical significance was defined as p < 0.05. The exact statistical methods, p and F/t values for each figure are provided in Supplementary Table S1.
Results
Behavioral and neural effects of acute witnessing social defeat stress in adolescent male mice
We first examined whether a single session of witnessing social defeat (S-WSD) could induce acute stress responses in adolescent mice (Fig. S1A). During the 10-min witness period, stressed mice showed increased freezing time (t7.966 = 4.234, p = 0.012; Fig. S1B). After S-WSD, naïve mice spent significantly more time with stressed mice than with control mice in an emotion discrimination test (p < 0.001; Tukey’s post hoc; Fig. S1C), consistent with previous findings that stressed mice could be discriminated from control mice by naïve mice possibly based on altered behaviors due to negative emotional states, a social recognition process supported by oxytocin signaling [41, 42]. S-WSD also significantly increased serum CORT levels (t8.563 = 5.775, p < 0.001; Fig. S1D). These results are consistent with previous studies in adult animals [16, 18, 43] and support WSD as a valid and severe stressor for adolescent mice.
We then examined the effects of S-WSD on neuronal activation (measured by the density of c-fos+ neurons) in the three subregions (CA1, CA3, and DG) of the dorsal and ventral hippocampus (Fig. S1E, F), respectively. S-WSD significantly increased the density of c-fos+ neurons in dorsal (p < 0.001) and ventral (p < 0.001) CA3, not other subregions of hippocampus, indicative of high vulnerability of CA3 to S-WSD. The increased c-fos+ neuron density could not be attributed to witnessing CD1 mice, as shown in a control experiment of witnessing CD-1 mice alone (not the social defeat process) (Fig. S2).
Adolescent CWSD impairs cognition and increases anxiety-like behaviors
We continued to examine the effects of chronic witnessing social defeat stress (CWSD, 10-min stress exposure daily for 10 days, PND35-44) in adolescent mice (Fig. 1A) on cognitive, anxiety-like, and depression-like behaviors. CWSD mice exhibited significantly less weight gain during stress exposure (Fig. 1B) and showed significantly less social interaction with CD-1 mice 24 h after stress cessation (Fig. 1C), similar to chronic social defeat stress [44].
Fig. 1. Effects of chronic witnessing social defeat stress on cognition and anxiety-like behaviors in adolescent mice.
A The experimental timeline of the cognitive and anxiety-like behavioral tests after chronic witnessing social defeat stress (CWSD). B During the 10-day stress exposure, CWSD mice exhibited less weight gain than control mice. C CWSD mice showed reduced social interaction with CD-1 mice. CWSD mice showed significantly lower discrimination index than control mice in novel object recognition (D) and spatial object recognition (E) tests. Left, representative trajectory maps for CT and CWSD mice during the test phase in each task; warm colors represent more time. F CWSD mice had a lower spontaneous alternation ratio in the Y-maze test than control mice. Left, diagram of Y-maze apparatus; middle, spontaneous alternation ratio (SA); right, percentage of erroneous responses: alternative arm return (AAR) and same arm return (SAR). G In the open field test, CWSD mice spent less time in the center zone and traveled significantly less than control mice. Left, representative motion path in the open field test; middle, time spent in center zone; right, total distance traveled in 10 min. H In the light-dark box test, CWSD mice spent significantly less time in the light box. Left, representative motion path in the light chamber of the light-dark box; right, time spent in the light chamber. I In the elevated-plus maze test, CWSD did not affect the time spent in the open arm. Left, representative trajectory map in the elevated plus maze test; warm colors represent more time; right, time spent in open arms. AAR alternative arm return, CT control, CWSD chronic witnessing social defeat stress, EPM elevated plus maze, LDB light-dark box, NOR novel object recognition, OFT open field test, PND postnatal day, SA spontaneous alteration, SAR same arm return, SOR spatial object recognition. * p < 0.05, ** p < 0.01, *** p < 0.001; ### p < 0.001.
The effects of CWSD on cognition were assessed in three behavioral tests. In the novel object recognition (NOR) test (Fig. 1D), CWSD mice failed to discriminate the novel object from the familiar one and showed significantly lower discrimination index than the control mice (t37 = 4.545, p < 0.001). Similar findings were observed in the spatial object recognition (SOR) test in that CWSD mice could not discriminate the displaced object from the stationary one and showed lower discrimination index than control mice (t38 = 2.762, p = 0.008; Fig. 1E). The total exploration time did not significantly differ between groups during the acquisition or test stage in the two tasks (Fig. S3A, B). In the Y-maze spontaneous alternation task, CWSD mice showed decreased spontaneous alternation ratio (t37 = 2.069, p = 0.046; Fig. 1F), indicative of impaired spatial working memory. The alternative arm return (AAR), same arm return (SAR) or the total number of entries (Fig. S3C) were not significantly affected.
For anxiety-like behaviors (Fig. 1G), CWSD mice spent less time in the center zone (t38 = 2.971, p = 0.005) and traveled significantly less (t38 = 2.852, p = 0.007) than control mice in the open field (OF) test. In the light-dark box (LDB) test, CWSD mice spent significantly less time in the light box (t38 = 3.474, p = 0.001; Fig. 1H), despite of lack of group differences in the latency to the light box (Fig. S3D). No group differences were observed in the elevated-plus maze (EPM) test (Fig. 1I and Fig. S3E).
Intriguingly, CWSD did not induce depression-like behaviors (Fig. S3F). No group differences were observed for sucrose preference (SPT, Fig. S3G), immobility time in forced swimming (FST, Fig. S3H) or tail suspension tests (TST, Fig. S3I). We retested FST or TST tests 4 h later, and did not observe the CWSD effects or its interaction with the testing time.
These behavioral results indicate that CWSD impairs hippocampus-dependent memory and increases anxiety-like behaviors in the open field and light-dark box tests in adolescent male mice.
Adolescent CWSD differentially alters neuronal activation in dorsal and ventral CA3
To examine the effects of CWSD on neuronal activation of the three subregions (CA3, CA1, and DG) in dorsal and ventral hippocampus in adolescent mice, we quantified the density of c-fos+ neurons in the brain tissues obtained 90 min after SOR and LDB tests, respectively. After SOR, CWSD significantly increased the density of c-fos+ neurons in dCA3 (t10 = 2.488, p = 0.032; Fig. 2A, upper). No significant changes were observed in vCA3 (Fig. 2B, lower), or other hippocampal subregions (Fig. S4A). After LDB, CWSD significantly reduced the density of c-fos+ neurons in vCA3 (t20 = 2.701, p = 0.014; Fig. 2B, lower). No significant alternations were found in dCA3 (Fig. 2B, upper) or other hippocampal subregions (Fig. S4B). These results extend our observations in S-WSD and support the vulnerability of CA3 neuronal activity following adolescent WSD.
Fig. 2. Neural alterations in dorsal and ventral CA3 in adolescent mice exposed to chronic witnessing social defeat stress (CWSD).
CWSD increased the number of c-fos+ neurons in dCA3 (not vCA3) after the spatial object recognition test (A) and decreased the number of c-fos+ neurons in vCA3 (not in dCA3) after the light-dark box test (B). Representative images showing the expression of c-fos and DAPI in the dCA3 (upper) and vCA3 (lower) of control and CWSD mice. Scale bar, 30 µm. CWSD significantly upregulated VGluT1 and VGAT expression in dCA3 (C), and significantly downregulated the VGluT1/VGAT ratio in vCA3 (D). Upper, representative images show the expression of VGluT1 and VGAT in dCA3 and vCA3 in CT and CWSD groups. Scale bar, 50 µm or 10 µm. Lower, the relative optical density of VGluT1 (left), VGAT (middle), and their ratio (right) in dCA3 and vCA3 in both groups. Adolescent CWSD reduced the length and complexity of apical dendrites of pyramidal neurons in dCA3 (E–G) and vCA3 (I–K). E, I representative tracings of neurons from the two groups. Scale bar, 50 µm. Adolescent CWSD reduced the spine density on apical dendrites in dCA3 (H) and vCA3 (L), especially in thin and stubby spines. The total spine density was calculated as the sum of three spine types. Scale bar, 10 µm. CT control, CWSD chronic witnessing social defeat stress, dCA3 dorsal CA3, LDB light-dark box, SOR spatial object recognition, vCA3 ventral CA3, VGAT vesicle GABA transporter, VGluT1 vesicle glutamate transporter-1. * p < 0.05, ** p < 0.01, *** p < 0.001; & p < 0.05, &&& p < 0.001.
Next, to corroborate the effects of CWSD on neuronal activation, we measured the expression levels of the vesicular transporters of glutamate (vesicular glutamate transporter-1, VGluT1) and GABA (vesicular GABA transporter, VGAT) using immunofluorescence (Fig. 2C, D), which could reflect excitatory and inhibitory neurotransmission and their balance to some extent [45, 46]. In dCA3 (Fig. 2C), CWSD significantly upregulated VGluT1 and VGAT expression, not affecting the VGluT1/VGAT ratio. In vCA3 (Fig. 2D), CWSD significantly downregulated the VGluT1/VGAT ratio, despite of nonsignificant alterations of VGluT1 and VGAT expression alone. No significant alterations were found in other subregions of hippocampus (except for vDG, where CWSD significantly reduced the VGluT1/VGAT ratio) (Fig. S4C, D). These results suggested that CWSD specifically disturbed the excitatory and inhibitory neurotransmission in CA3.
Finally, we assessed the effects of CWSD on dendritic morphology in CA3 using Golgi-Cox staining (Fig. 2E–H and I–L). Here we only examined CA3, not CA1 or DG, based on the abovementioned results that S-WSD and CWSD mainly affected CA3 functional activity. We found that CWSD significantly reduced the length of apical dendrites (Fig. 2F, J) and complexity (Fig. 2G, K) in both dCA3 and vCA3. Spine loss in stressed mice was also observed (Fig. 2H, L), especially in thin spines.
These results suggest that adolescent CWSD differentially alters functional activation of dCA3 and vCA3 and impairs neuronal structural plasticity throughout the CA3. Based on the functional segregation along the hippocampal dorsal-ventral axis, we hypothesized that CWSD-induced cognitive deficits may be associated with the neuronal over-activation in dCA3, whereas CWSD-induced anxiety-like behaviors may be associated with the neuronal de-activation of vCA3. To test these hypotheses, we carried out the following chemogenetic experiments on dCA3 and vCA3, respectively.
CWSD-induced cognitive deficits in adolescent mice are causally mediated by dCA3 neurons
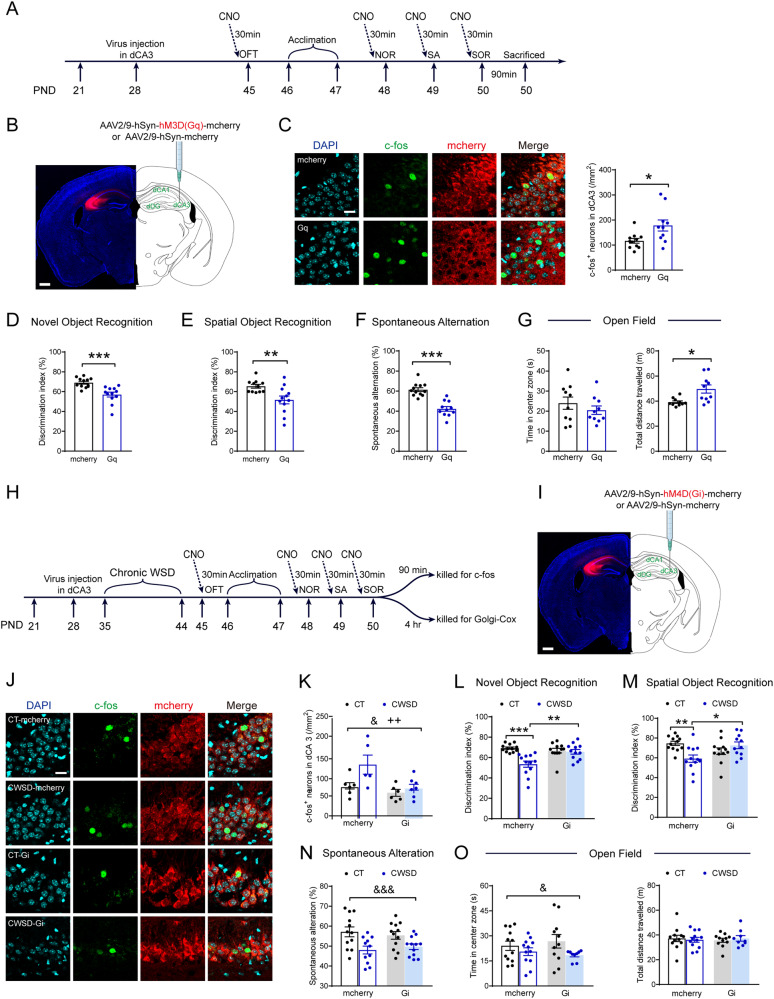
We carried out two experiments to activate or inhibit dCA3 neurons, respectively, to examine the causal link between dCA3 neuronal activity and CWSD-induced cognitive deficits. First, to mimic the effects of CWSD, we activated dCA3 neurons by bilateral injection of the AAV vector carrying the hM3D (Gq) or mcherry into dCA3 at PND28 (Fig. 3A, B, S5A). After 2-week recovery and transfection, behavioral tests on cognition and anxiety were carried out and CNO (1 mg/kg) was delivered i.p. 30 min prior to each test to activate virus-infected neurons in dCA3. C-fos staining quantification showed increased c-fos expression in Gq mice (t19 = 2.629, p = 0.017; Fig. 3C), supporting valid chemogenetic manipulation. For cognitive behaviors (Fig. 3D–G and Fig. S5B–D), Gq mice exhibited significantly lower discrimination indices in the NOR (t22 = 4.269, p < 0.001; Fig. 3D) and SOR tests (t21 = 3.033, p = 0.006; Fig. 3E) and showed reduced spontaneous alternation ratio (t21 = 6.495, p < 0.001; Fig. 3F) and increased erroneous alternation ratios (Fig. S5D) in the Y-maze test. The two groups did not significantly differ in anxiety-like behaviors in the open field (Fig. 3G, left), despite of increased distance traveled in Gq mice (Fig. 3G, right). These results indicated that activating the dCA3 neurons impaired cognition, not anxiety-like behaviors, in adolescent mice.
Fig. 3. Dorsal CA3 neuronal activity mediates witnessing stress-induced recognition memory deficits in adolescent mice.
A The experimental timeline of the behavioral tests, CNO injection, and brain tissue acquisition after hM3D(Gq) viral injection. B Left panel, an image showing region-specific expression of mcherry in dCA3; right panel, a schematic of activation virus (AAV-Gq) microinjection into the dCA3 of adolescent mice. Scale bar, 500 µm. C Representative images show the expression of DAPI, c-fos, and mcherry in the dCA3 of control and Gq mice. Immunostaining analyses confirmed that Gq-induced an increase in the expression of c-fos in the dCA3. Scale bar, 20 µm. Gq mice exhibited impaired recognition memory in the novel object recognition task (D), in the spatial object recognition task (E), and in the Y-maze spontaneous alternation (F). G In the open field test, the two groups spent similar time in the central zone, despite Gq mice traveling a greater total distance. H The experimental timeline of the behavioral tests, CNO injection, and brain tissue acquisition after hM4D(Gi) viral injection. I Left panel, an image shows region-specific expression of mcherry in the dCA3; right panel, schematic of inhibition virus (AAV-Gi) microinjection into the dCA3 of adolescent mice. Scale bar, 500 µm. J Representative images show the expression of DAPI, c-fos, and mcherry in the dCA3 of four groups. Scale bar, 20 µm. K During the spatial object recognition test, CWSD increased the number of c-fos+ neurons in dCA3, while Gi virus reduced it. CWSD-induced deficits of novel object recognition memory (L) and spatial object recognition memory (M) were reversed by inhibition of dCA3 neurons. N In the Y-maze test, CWSD-induced spatial working memory deficits were not reversed by inhibition of dCA3 neurons. O In the open field test, CWSD-induced higher anxiety levels were not relieved by inhibition of dCA3 neurons. CNO Clozapine N-oxide, CT control, CWSD chronic witnessing social defeat stress, NOR novel object recognition, OFT open field test, PND postnatal day, SA spontaneous alteration, SOR spatial object recognition. * p < 0.05, ** p < 0.01, *** p < 0.001; # p < 0.05, ## p < 0.01, ### p < 0.001; & p < 0.05, &&& p < 0.001; ++ p < 0.01.
Next, we examined whether inhibiting dCA3 neuronal activity (by injection of hM4D (Gi) virus) could restore CWSD-induced cognitive deficits (Fig. 3H, I and S6A). Two-way ANOVA on c-fos staining results (Fig. 3J, K) revealed that c-fos+ neuron density in dCA3 was significantly increased by CWSD, replicating our previous results, and was significantly decreased by Gi virus (F (1, 20) = 8.901, p = 0.007), proving the validity of viral manipulation. Inhibition of dCA3 neurons did not ameliorate the spine loss induced by CWSD (Fig. S6B). For the behavioral effects (Fig. 3L–O and Fig. S6C–E), inhibiting dCA3 neuron activity specifically reversed CWSD-induced recognition memory deficits in adolescent mice as detailed below.
First, in the NOR task, all but the CWSD-mcherry group (Fig. 3L) discriminated the novel object from the familiar one. A CWSD × virus interaction was observed (F (1, 44) = 10.34, p = 0.002) and inhibition of dCA3 neurons ameliorated CWSD-induced decreases of the discrimination index (p = 0.003, Tukey’s post hoc). Second, in the SOR task (Fig. 3M and S6D), although all groups successfully recognized the object placed in novel location (Fig. 3M), Tukey’s post hoc tests following two-way ANOVA (interaction effect: F (1, 43) = 11.24, p = 0.002) showed that the discrimination index of the CWSD-mcherry group was significantly lower than that of the control mice (p = 0.006), indicating CWSD-induced deficits, and than that of the CWSD-Gi group (p = 0.025), indicating that inhibition of dCA3 neurons reversed CWSD-induced spatial memory loss. Finally, inhibition of dCA3 neurons did not reverse CWSD-induced spatial working memory deficits in the Y-maze test (Fig. 3N and S6E) and anxiety-like behaviors in the OF test (Fig. 3O).
By manipulating dCA3 neuronal activity in two experiments, we found that activating dCA3 neurons reproduced CWSD-induced cognitive deficits and that inhibiting dCA3 neurons successfully restored CWSD-induced deficits in recognition memory, not in spatial working memory and anxiety-like behaviors. These results support the causal involvement of dCA3 neuronal over-activation in CWSD-induced recognition memory deficits.
Abnormal vCA3 neuronal activity leads to increased anxiety-like behaviors in adolescent mice
We carried out two experiments to activate or inhibit vCA3 neurons, respectively, to examine the causal link between vCA3 neuronal activity and CWSD-induced increased anxiety-like behaviors. First, we injected the AAV vector carrying the Gi or mcherry into the vCA3 bilaterally to inhibit vCA3 (Figs. 4A, B, S7A). Chemogenetic manipulation significantly decreased the density of c-fos+ neurons in vCA3 (t23 = 2.434, p = 0.023; Fig. 4C), indicative of valid neuronal manipulation. For anxiety-like behaviors, inhibiting vCA3 decreased the time spent in the central zone in the OF (t18 = 2.183, p = 0.042; Fig. 4D, left) and the time spent in the light box in the LDB test (t22 = 1.961, p = 0.063; Fig. 4E), suggesting increased anxiety-like behaviors. No group differences were found for the EPM (Fig. 4F), the total distance traveled in the OF (Fig. 4D, right) or the latency to the light box (Fig. S7B). Inhibiting vCA3 did not affect the SOR performance (Figs. 4G, S7C).
Fig. 4. Inhibition or activation of vCA3 neuronal activity increases anxiety in adolescent male mice.
A The experimental timeline of the behavioral tests, CNO injection, and brain tissue acquisition after hM4D(Gi) viral injection. B Left panel, an image shows region-specific expression of mcherry in vCA3; right panel, schematic of activation virus (AAV-Gi) microinjection into the vCA3 of adolescent mice. Scale bar, 500 µm. C Representative images show the expression of DAPI, c-fos, and mcherry in the vCA3 of control and Gi mice. Immunostaining analyses confirmed Gi-induced reduction the number of c-fos+ neurons in the vCA3. Scale bar, 20 µm. D In the open field test, inhibition of vCA3 neurons decreased the time spent in the central zone. E Compared with control mice, Gi mice spent less time in the light box in the light-dark box (LDB) test. F In the elevated-plus maze test, inhibition of vCA3 neurons did not affect the time spent in the open arm. G In the spatial object recognition test, the two groups showed comparable spatial memory. H The experimental timeline of the behavioral tests, CNO injection, and brain tissue acquisition after hM3D(Gq) viral injection. I Left panel, an image showing region-specific expression of mcherry in vCA3; right panel, schematic of inhibition virus (AAV-Gq) microinjection into the vCA3 of adolescent mice. Scale bar, 500 µm. J Representative images show the expression of DAPI, c-fos, and mcherry in the vCA3 of four groups. Scale bar, 20 µm. K During the LDB test, CWSD decreased the number of c-fos+ neurons in vCA3, while the two Gq groups exhibited increased number of c-fos+ neurons. L Activation of vCA3 neurons decreased the time spent in the center zone, increased the total distance traveled, and did not reverse CWSD-induced higher anxiety levels in the open field test. M In the LDB test, activation of vCA3 neurons significantly reduced the time spent in the light box, increased the latency to the light box, and did not reverse CWSD-induced higher anxiety levels in adolescent mice. N Activation of vCA3 neurons did not restore CWSD-induced spatial memory loss, but instead impaired spatial memory. CNO Clozapine N-oxide, CT control, CWSD chronic witnessing social defeat stress, EPM elevated plus maze, LDB light-dark box, OFT open field test, PND postnatal day, SOR spatial object recognition. * p < 0.05, ** p < 0.01, *** p < 0.001; ## p < 0.01, ### p < 0.001; & p < 0.05, &&& p < 0.001; + p < 0.05, +++ p < 0.001.
We then examined whether activating vCA3 neuronal activity (by injection of Gq virus) could reverse CWSD-induced anxiety-like behaviors (Fig. 4H, I, Fig. S8A). The two Gq groups exhibited increased density of c-fos+ neurons in vCA3 than the control-mcherry groups (p < 0.05; Fig. 4J, K), indicative of over-activated status of vCA3 by chemogenetic manipulation. Activation of vCA3 neurons did not ameliorate the spine loss induced by CWSD (Fig. S8B). For the behavioral effects, activating vCA3 neuron activity did not reverse CWSD-induced emotional deficits and instead resulted in increased anxiety-like behaviors and cognitive deficits as we explained below.
In the OF test, two-way ANOVA analysis on time in the center zone (Fig. 4L, left) revealed a significant stress × virus interaction (F (1, 43) = 7.559, p = 0.009). Tukey’s post hoc comparisons showed that CWSD significantly decreased the time in the center zone (p = 0.002) and so did vCA3 neuronal over-activation (p < 0.001). For the total distance traveled (Fig. 4L, right) in the open field, a significant interaction between CWSD and virus was also observed, which was driven by the significant difference between CT-mcherry and CT-Gq mice (p = 0.009) and lack of differences among other groups. In the LDB test, two-way ANOVA on the time in the light box only revealed a main effect of virus in that chemogenetic activation of vCA3 neurons significantly reduced this index (F (1, 40) = 5.229, p = 0.028; Fig. 4M, left). For the latency to the light box (Fig. 4M, right), the interaction between CWSD and Gq reached significance (F (1, 40) = 10.15, p = 0.003). Post hoc comparisons showed that CWSD significantly increased the latency to the light box (p < 0.001) and so did vCA3 neuronal over-activation (p < 0.001); no group differences were found between CWSD-mcherry and CWSD-Gq groups. In the SOR test, both CWSD and activation of vCA3 neurons impaired spatial memory (Fig. 4N), as CWSD-mcherry and CT-Gq groups failed to recognize the object placed in novel location. Two-way ANOVA on the discrimination index revealed significant main effects of CWSD (F (1, 41) = 23.02, p < 0.001) and Gq (F (1, 41) = 17.56, p < 0.001), without any interaction. During the acquisition phase, the total probe time were not affected by CWSD or Gq (Fig. S8C), while CT-Gq mice showed more total distance traveled than CT-mcherry mice (Fig. S6D), which is largely consistent with the index in the open field.
By these experiments, we found that while inhibiting vCA3 neuronal activity reproduced CWSD-induced anxiety-like behaviors, activating vCA3 neuronal activity also increased anxiety-like behaviors and impaired cognition. These results suggest that anxiety-like behaviors are associated with the abnormal vCA3 neuronal activity, which warrants to be more carefully corrected to reverse CWSD-induced anxiety-like behaviors in adolescent mice.
Vortioxetine reverses CWSD-induced cognitive deficits by restoring dCA3 neuronal activity
In this section, we evaluated the therapeutic effects of vortioxetine on CWSD-induced behavioral and neural alterations. As vortioxetine would be administered after CWSD and for 28 days, we first demonstrated in an independent cohort of animals that CWSD-induced cognitive deficits and anxiety-like behaviors persisted 1 month (>PND73) after stress cessation (Fig. S9).
We then carried out the experiment of chronic vortioxetine administration (10 mg/kg/day, 28 days, i.p.; Fig. 5A). Vortioxetine successfully reversed CWSD-induced recognition memory deficits. In the NOR test, while all groups discriminated the novel object from the familiar one (Fig. 5C), two-way ANOVA on the discrimination index revealed a significant interaction between CWSD and drug (F (1, 41) = 13.54, p < 0.001), with the CWSD-Veh group having significantly lower discrimination index when compared with the control-Veh group (p < 0.001, indicating CWSD-induced deficits) and with the CWSD-Vort group (p < 0.001, indicating successful reversal). Similar results were obtained in the SOR test. All but the CWSD-Veh group (Fig. 5D) were able to discriminate the displaced object from the stationary one. Post hoc group comparisons following the two-way ANOVA (interaction effect: F (1, 41) = 9.300, p = 0.004) on discrimination index revealed that vortioxetine reversed CWSD-induced spatial memory deficits (p = 0.007). Vortioxetine did not reverse CWSD-induced behavioral alterations in other tasks, where only the main effect of CWSD reached significance, including social avoidance (Fig. 5B), Y-maze spontaneous alternation (Figs. 5E, S10C), and the LDB test (Fig. 5F).
Fig. 5. Chronic systemic administration of vortioxetine reverses CWSD-induced recognition memory deficits and dCA3 neuronal over-activation.
A The experimental timeline of the vortioxetine injection and behavioral tests after stress exposure. B Vortioxetine did not reverse CWSD-induced social avoidance. CWSD-induced deficits of novel object recognition memory (C) and spatial object recognition memory (D) were reversed by chronic vortioxetine administration, while spatial working memory deficits were not restored (E). F In the LDB test, CWSD-induced higher anxiety levels were not alleviated by vortioxetine. G, H Vortioxetine reversed CWSD-induced dCA3 neuronal over-activation during the spatial object recognition test, not vCA3 neuronal de-activation during the LDB test. Representative images show the expression of c-fos and DAPI in the dCA3 in the four groups of animals. Scale bar, 30 µm. CT control, CWSD chronic witnessing social defeat stress, LDB light-dark box, NOR novel object recognition, PND postnatal day, SA spontaneous alteration, SOR spatial object recognition, Veh vehicle, Vort vortioxetine. * p < 0.05, ** p < 0.01, *** p < 0.001; & p < 0.05, && p < 0.01, &&& p < 0.001.
Finally, we examined whether the reversal effects of vortioxetine were associated with the dCA3 neuronal activity. For the density of c-fos+ neurons in dCA3 after SOR (Fig. 5G, H), there was a significant interaction between CWSD and vortioxetine (F (1, 20) = 5.275, p = 0.033). CWSD-induced dCA3 neuronal over-activation was reversed by vortioxetine administration (p = 0.018). For the VGluT1/VGAT expression, vortioxetine marginally reversed (F (1, 19) = 3.867, p = 0.066; Fig. S11D) CWSD-induced increasing trend of the VGluT1/VGAT ratio (uncorrected p = 0.063; Fig. S11D). No significant results were found for VGluT1 or VGAT levels (Fig. S11B, C). In vCA3, vortioxetine did not reverse CWSD-induced negative effects, including the reduction of c-fos+ neuron density after LDB (F (1, 17) = 17.77, p < 0.001; Fig. 5J, K), VGAT increases (Fig. S11G), and VGluT1/VGAT ratio decreases (Fig. S11H). No significant results were found for VGluT1 levels (Fig. S11F).
Taken together, we observed long-lasting cognitive and emotional deficits following CWSD in adolescent mice. Chronic vortioxetine treatment specifically reversed the recognition memory deficits as well as the dCA3 neuronal hyperactivity, not anxiety-like behaviors or vCA3 neuronal hypoactivity.
Discussion
In this study, we examined CWSD-induced behavioral alterations in adolescent mice and the underlying neural mechanisms. We found that CWSD during adolescence impaired cognitive function and increased anxiety-like behaviors in adolescent mice, which could persist into adulthood. The c-fos immunostaining revealed that CWSD resulted in over-activation of dCA3 neurons and hypo-activation of vCA3 neurons. The chemogenetic experiments showed that activating and inhibiting dCA3 neuron activity specifically mimicked and reversed CWSD-induced recognition memory deficits, not anxiety-like behaviors. In comparison, both inhibiting and activating vCA3 neurons increased anxiety-like behaviors and CWSD-induced anxiety-like behaviors were not reversed by vCA3 neuronal activation. Finally, vortioxetine successfully restored dCA3 neuron activity and recognition memory in CWSD mice, not affecting vCA3 neuronal activation and anxiety-like behaviors. Together, our findings suggest that dCA3 neuronal activity causally mediates CWSD-induced recognition memory deficits in adolescent male mice.
For the effects of CWSD on cognitive deficits and anxiety-like behaviors, while CWSD resulted in increased anxiety-like and cognitive deficits in adult animals [11, 14–16, 18], the few CWSD studies in adolescent animals did not observe alterations in cognition [19] or anxiety-like behaviors in EPM [19, 21]. By adding more cognitive paradigms, we show that CWSD in adolescence has detrimental effects on cognition across several tasks including novel object recognition, spatial object recognition, and Y-maze spontaneous alternation. For the discrepancy between the previous study evaluating cognition [19] and our study, there are several methodological differences including rodent strain (rat vs. mouse), CWSD procedure (witnessing mother being defeated, 30 min/day × 7 days vs. witnessing peer being defeated, 10 min/day × 10 days), and behavioral tests (radial arm water maze vs. three cognitive tests). Which of these factors play crucial roles for CWSD to induce cognitive deficits remain to be tested in future studies. For anxiety-like behaviors, we did not observe changes in the EPM, which are consistent with previous reports [19, 21], but we found that CWSD increased anxiety-like behaviors in the light-dark box and open field tests, which indicate that CWSD-induced anxiety phenotype may be more pronounced in specific environmental contexts. Additionally, our findings that the CWSD-induced cognitive deficits and anxiety behaviors still sustained in adulthood echo with clinical observations [47] and further highlight the robustness of the detrimental effects of adolescent CWSD on cognition and anxiety.
To understand the underlying neural mechanisms for the CWSD-induced cognitive deficits, we carried out a series of chemogenetic experiments, providing direct, causal evidence that CWSD-induced deficits in recognition memory are mediated by dCA3 neuronal hyperactivation. This was supported by the vortioxetine experiment in that vortioxetine successfully restored cognitive performance in recognition tests and normalized the density of c-fos+ neurons in the dCA3. Finally, we performed correlation analyses between the density of c-fos+ neurons in dCA3 and the discrimination index in the spatial object recognition test across three experiments (Fig. S12A–C): dCA3-Gq, dCA3-Gi, and vortioxetine treatment. Negative correlations were observed in three experiments and two of them reached significance (dCA3-Gq: r = −0.600, p = 0.007; dCA3-Gi: r = −0.268, p = 0.228; vortioxetine treatment: r = −0.493, p = 0.027), which are consistent with group comparison results and support the association between dCA3 activity and recognition memory. These results are in line with the primary efficacy of vortioxetine for cognitive improvement as an antidepressant [48, 49] and highlight the dCA3 neuronal activity as one of the therapeutic targets of vortioxetine.
It is worth noting that inhibiting dCA3 neurons and administering vortioxetine successfully restored CWSD-induced deficits in recognition memory in novel object recognition and spatial object recognition tests, not in the Y-maze spontaneous alternation test. The dissociation between recognition memory tests and Y-maze spontaneous alternations has been documented in previous studies examining nectin-3 knockdown in dCA3 [50] or in dDG [51]. Recognition memory tests evaluate animals’ long-term (>1 h) memory toward objects or spatial locations and have been proposed to rely relatively specifically on the hippocampus [52, 53], whereas the Y-maze spontaneous alternation task evaluates spatial working memory and recruits multiple brain regions, including the basal forebrain, hippocampus, thalamus, prefrontal cortex, and others [54]. These findings suggest that CWSD-induced damage to spatial working memory in adolescent mice could not be simply attributed to dCA3 neuronal over-activation and warrant future investigation on other brain regions.
Our investigation about the involvement of the vCA3 in CWSD-induced increased anxiety-like behaviors yields a more complex picture. While adolescent CWSD reduced the density of c-fos+ neurons in vCA3 and chemogenetic inhibition of vCA3 neurons indeed increased anxiety-like behaviors, chemogenetic activation of vCA3 neurons failed to ameliorate CWSD-induced anxiety-like behaviors and instead increased anxiety-like behaviors in control mice. In previous studies, anxiety has been causally and consistently linked to vCA3 excitatory neurons or COCH (coagulation factor c homolog gene) positive neurons in that activating these neurons in vCA3 promotes anxiety and inhibiting them reduces anxiety [55–57]. Our results show that the abnormal neuronal activity of vCA3 (activation or inhibition) promotes anxiety. As our chemogenetic manipulation is not specific to particular cell types, our results might reflect the summed effects of excitatory and inhibitory neurons. We speculate that there may be a U-shaped relationship between vCA3 overall activity and anxiety such that maintaining neuronal activity at certain medium levels may help to reduce anxiety levels, which invites future investigation. Finally, we observed that spatial object recognition performances were significantly impaired following chemogenetic excitation of vCA3 neurons, consistent with the notion that ventral hippocampus could also contribute to cognition [58, 59].
It should be noted that one limitation of our chemogenetic manipulation is using the virus with Syn promoter, which targeted at both excitatory and inhibitory neurons. Future studies could use viruses with specific types of neuronal promoters to clarify which and how specific type(s) of neurons in dCA3 or vCA3 mediate CWSD-induced behavioral alterations.
While the main focus of our study is to examine how CA3 neuronal activity mediates CWSD-induced behavioral deficits, we further investigated the CWSD and intervention effects on excitatory/inhibitory neurotransmission and neuronal morphology. For excitatory/inhibitory neurotransmission, in vCA3, CWSD downregulated the VGluT1/VGAT ratio, which was replicated in the vortioxetine experiment (along with VGAT decreases), and vortioxetine failed to restore these changes, in line with the absence of vortioxetine treatment effects on anxiety-like behaviors and c-fos expression. In dCA3, CWSD upregulated VGluT1 and VGAT expression, not affecting VGluT1/VGAT ratio, whereas in the later vortioxetine experiment CWSD increased the VGluT1/VGAT ratio, not affecting VGluT1 and VGAT expression. While the inconsistent stress effects across experiments may be due to different time points of molecular detection (PND53 vs. PND78) and warrant future investigation, the changes are largely in line with CWSD-induced increased neuronal activity in dCA3. Furthermore, we found that vortioxetine marginally restored dCA3 VGluT1/VGAT ratio, which is consistent with the treatment effects on cognition and c-fos expression and lends further support to vortioxetine’s neural mechanisms of restoring neuronal activity in dCA3.
For neuronal morphology, we found that CWSD simplified apical dendritic structure and reduced spine density throughout CA3 and that CWSD-induced spine loss was replicated in the subsequent manipulation experiments (Figs. S6B, S8B). Intriguingly, inhibition of dCA3 neurons and activation of vCA3 neurons could not reverse CWSD-induced spine loss, suggesting that cognition-enhancing effects of dCA3 neuronal inhibition do not require the restoration of spine loss and that the association between vCA3 spine loss and anxiety-like behaviors remains unclear. We did not examine whether vortioxetine could reverse CWSD-induced spine loss; future studies are needed to answer this question and to clarify whether and how spine loss relates to behavioral impairments. Our results also reveal an interesting dissociation between neuronal structural plasticity and functional activity (structural simplification was observed in both dCA3 and vCA3, yet the two subregions exhibited neuronal over-activation and inhibition, respectively). Another intriguing observation is that the alteration directions of spine density and VGluT1 following CWSD could be similar or different. They both showed decreasing alterations in vCA3 (despite of not approaching significance for VGluT1). This may be because VGluT1 is expressed in excitatory pre-synaptic terminals and partly reflects the density of excitatory synapses. The two biomarkers were altered in different directions in dCA3 (see also [60, 61]). This may be because upregulation of VGluT1 could indicate increased glutamate release, which further leads to excitatory toxicity and results in spine loss. Why the two biomarkers exhibit different alteration patterns in vCA3 and dCA3 remains unclear and warrants future investigation.
Finally, the effects of CWSD on depression-like behaviors warrant some clarification. While it is well established that CWSD exposure induces a depression-like phenotype in adult rodents [18, 62], studies in adolescent mice report controversial results: one study using young adult mice (PND 49–58) reported increased depression-like behaviors [21]; a previous study [20] and our study here using young juvenile mice (PND35-44) did not observe depression-like behaviors. Similarly, some widely used stress models such as chronic social defeat and chronic restraint stress report increased depression-like behaviors in adulthood, but not in adolescent mice [63–65]. Together, these results suggest that adolescent mice may be less vulnerable than adult mice in developing depression-like behaviors, which may be related to the immature function of hypothalamic-pituitary-adrenal axis: prepubertal rodents have a delayed rise of glucocorticoid levels and prolonged glucocorticoid release in response to several types of stressors compared with adult rodents [29]. Importantly, considering the possibility of delayed effects following adolescent stress [66, 67], we carried out an experiment evaluating depression-like behaviors in adulthood (>PND73) after adolescent CWSD exposure. We found that adult mice exposed to CWSD during adolescence showed increased immobility time in FST (t19 = 5.665, p < 0.001; Fig S7J) and decreased sucrose grooming time in SST (t20 = 2.373, p = 0.027; Fig S7H). The sucrose preference was also reduced, which did not approach significance (t19 = 1.565, p = 0.134; Fig S7I). These results indicate that adolescent CWSD could induce depression-like behaviors, despite of delayed onsets in adult mice. Unfortunately, the vortioxetine experiment was designed based on the short-term behavioral effects of CWSD (i.e., cognition and anxiety), and we did not test the treatment effects of vortioxetine on depression-like behaviors. Now with the delayed depression-like phenotype of CWSD, future studies could investigate the prevention and treatment effects of vortioxetine on depression-like behaviors to assess its antidepressant efficacy.
To conclude, our study highlights the detrimental and long-lasting effects of CWSD on cognitive and anxiety-like behaviors in adolescent mice. Importantly, converging evidence across chemogenetic and pharmacological experiments suggest that the dCA3 neuronal hyperactivation mediates CWSD-induced recognition memory deficits, and provide preclinical evidence for early treatment of cognitive impairment by vortioxetine in adolescents suffering from CWSD.
Supplementary information
Acknowledgements
Special thanks to Xiao-Dong Wang, PhD, for technical assistance and feedback.
Author contributions
TS, JL, and Y-AS designed research; XL, RL, Y-XS, H-LW, and HW performed research; RL, XL, TW, YM, X-XL, and QW analyzed data; JT, TS, and XL wrote the manuscript.
Funding
This work was supported by the Beijing Natural Science Foundation (grant No., 7222236), the National Natural Science Foundation of China (grant No., 82271569, 82171529, 82071528, and 82001418), the Capital Medical Development Research Fund (2020-2-4113 and 2022-1-4111). The funders have no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xiao Liu, Rui Liu.
Contributor Information
Ji-Tao Li, Email: ljt_102124@163.com.
Tian-Mei Si, Email: si.tian-mei@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-024-01848-9.
References
- 1.Howard R, Spivak ELJ, VanAudenhove K, Lee D, Kelly M, Iskander J. CDC grand rounds: a public health approach to prevention of intimate partner violence. MMWR Morb Mortal Wkly Rep. 2014;63:38–41. [PMC free article] [PubMed] [Google Scholar]
- 2.Zolotor AJ, Denham AC, Weil A. Intimate partner violence. Obstet Gynecol Clin N Am. 2009;36:847–60. 10.1016/j.ogc.2009.10.011 [DOI] [PubMed] [Google Scholar]
- 3.Cooper A, Smith EL. Homicide trends in the United States, 1980–2008. NCJ 236018. Washington, DC: Bureau of Justice Statistics; 2011.
- 4.Teicher MH, Samson JA. Annual research review: enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry Allied Discip. 2016;57:241–66. 10.1111/jcpp.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teicher MH, Vitaliano GD. Witnessing violence toward siblings: an understudied but potent form of early adversity. PLoS One. 2011;6:e28852. 10.1371/journal.pone.0028852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teicher MH, Samson JA. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry. 2013;170:1114–33. 10.1176/appi.ajp.2013.12070957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:113–23. 10.1001/archgenpsychiatry.2009.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flaherty EG, Stirling J Jr, American Academy of Pediatrics. Committee on Child A, Neglect. Clinical report-the pediatrician’s role in child maltreatment prevention. Pediatrics. 2010;126:833–41. 10.1542/peds.2010-2087 [DOI] [PubMed] [Google Scholar]
- 9.Christie D, Viner R. Adolescent development. Bmj. 2005;330:301–4. 10.1136/bmj.330.7486.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wamser-Nanney R. Types of childhood maltreatment, posttraumatic stress symptoms, and indices of fertility. Psychol Trauma. 2022;14:1263–71. 10.1037/tra0001169 [DOI] [PubMed] [Google Scholar]
- 11.Warren BL, Sial OK, Alcantara LF, Greenwood MA, Brewer JS, Rozofsky JP, et al. Altered gene expression and spine density in nucleus accumbens of adolescent and adult male mice exposed to emotional and physical stress. Dev Neurosci. 2014;36:250–60. 10.1159/000362875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patki G, Solanki N, Salim S. Witnessing traumatic events causes severe behavioral impairments in rats. Int J Neuropsychopharmacol. 2014;17:2017–29. 10.1017/S1461145714000923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patki G, Salvi A, Liu H, Salim S. Witnessing traumatic events and post-traumatic stress disorder: insights from an animal model. Neurosci Lett. 2015;600:28–32. 10.1016/j.neulet.2015.05.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sial OK, Warren BL, Alcantara LF, Parise EM, Bolanos-Guzman CA. Vicarious social defeat stress: bridging the gap between physical and emotional stress. J Neurosci Methods. 2016;258:94–103. 10.1016/j.jneumeth.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iniguez SD, Flores-Ramirez FJ, Riggs LM, Alipio JB, Garcia-Carachure I, Hernandez MA, et al. Vicarious Social defeat stress induces depression-related outcomes in female mice. Biol Psychiatry. 2018;83:9–17. 10.1016/j.biopsych.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carnevali L, Montano N, Tobaldini E, Thayer JF, Sgoifo A. The contagion of social defeat stress: insights from rodent studies. Neurosci Biobehav Rev. 2020;111:12–18. 10.1016/j.neubiorev.2020.01.011 [DOI] [PubMed] [Google Scholar]
- 17.Yoshioka T, Yamada D, Segi-Nishida E, Nagase H, Saitoh A. KNT-127, a selective delta opioid receptor agonist, shows beneficial effects in the hippocampal dentate gyrus of a chronic vicarious social defeat stress mouse model. Neuropharmacology. 2023;232:109511. 10.1016/j.neuropharm.2023.109511 [DOI] [PubMed] [Google Scholar]
- 18.Warren BL, Vialou VF, Iniguez SD, Alcantara LF, Wright KN, Feng J, et al. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73:7–14. 10.1016/j.biopsych.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H, Patki G, Salvi A, Kelly M, Salim S. Behavioral effects of early life maternal trauma witness in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:80–87. 10.1016/j.pnpbp.2017.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sial OK, Gnecco T, Cardona-Acosta AM, Vieregg E, Cardoso EA, Parise LF, et al. Exposure to vicarious social defeat stress and Western-style diets during adolescence leads to physiological dysregulation, decreases in reward sensitivity, and reduced antidepressant efficacy in adulthood. Front Neurosci. 2021;15:701919. 10.3389/fnins.2021.701919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakatake Y, Furuie H, Yamada M, Kuniishi H, Ukezono M, Yoshizawa K, et al. The effects of emotional stress are not identical to those of physical stress in mouse model of social defeat stress. Neurosci Res. 2020;158:56–63. 10.1016/j.neures.2019.10.008 [DOI] [PubMed] [Google Scholar]
- 22.Borsini A, Giacobbe J, Mandal G, Boldrini M. Acute and long-term effects of adolescence stress exposure on rodent adult hippocampal neurogenesis, cognition, and behaviour. Mol Psychiatry. 2023;28:4124–37. 10.1038/s41380-023-02229-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iniguez SD, Riggs LM, Nieto SJ, Dayrit G, Zamora NN, Shawhan KL, et al. Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress. 2014;17:247–55. 10.3109/10253890.2014.910650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwarteng F, Wang R, Micov V, Hausknecht KA, Turk M, Ishiwari K, et al. Adolescent chronic unpredictable stress leads to increased anxiety and attention deficit/hyperactivity-like symptoms in adulthood. Psychopharmacology. 2022;239:3779–91. 10.1007/s00213-022-06242-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caruso MJ, Crowley NA, Reiss DE, Caulfield JI, Luscher B, Cavigelli SA, et al. Adolescent social stress increases anxiety-like behavior and alters synaptic transmission, without influencing nicotine responses, in a sex-dependent manner. Neuroscience. 2018;373:182–98. 10.1016/j.neuroscience.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page CE, Coutellier L. Adolescent stress disrupts the maturation of anxiety-related behaviors and alters the developmental trajectory of the prefrontal cortex in a sex- and age-specific manner. Neuroscience. 2018;390:265–77. 10.1016/j.neuroscience.2018.08.030 [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Teng T, Li X, Fan L, Xiang Y, Jiang Y, et al. Impact of inosine on chronic unpredictable mild stress-induced depressive and anxiety-like behaviors with the alteration of gut microbiota. Front Cell Infect Microbiol. 2021;11:697640. 10.3389/fcimb.2021.697640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22. 10.1146/annurev.neuro.22.1.105 [DOI] [PubMed] [Google Scholar]
- 29.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- 30.McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, et al. Mechanisms of stress in the brain. Nat Neurosci. 2015;18:1353–63. 10.1038/nn.4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomar A, McHugh TJ. The impact of stress on the hippocampal spatial code. Trends Neurosci. 2022;45:120–32. 10.1016/j.tins.2021.11.005 [DOI] [PubMed] [Google Scholar]
- 32.Anacker C, Hen R. Adult hippocampal neurogenesis and cognitive flexibility—linking memory and mood. Nat Rev Neurosci. 2017;18:335–46. 10.1038/nrn.2017.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, et al. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013;77:955–68. 10.1016/j.neuron.2012.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. 10.1016/j.neuron.2009.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshioka T, Yamada D, Kobayashi R, Segi-Nishida E, Saitoh A. Chronic vicarious social defeat stress attenuates new-born neuronal cell survival in mouse hippocampus. Behav Brain Res. 2022;416:113536. 10.1016/j.bbr.2021.113536 [DOI] [PubMed] [Google Scholar]
- 36.Terranova JI, Yokose J, Osanai H, Marks WD, Yamamoto J, Ogawa SK, et al. Hippocampal-amygdala memory circuits govern experience-dependent observational fear. Neuron. 2022;110:1416–31.e13. 10.1016/j.neuron.2022.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Q, He Q, Wang J, Fu C, Hu H. Use of TAI-FISH to visualize neural ensembles activated by multiple stimuli. Nat Protoc. 2018;13:118–33. 10.1038/nprot.2017.134 [DOI] [PubMed] [Google Scholar]
- 38.Sanchez C, Asin KE. Artigas F. Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther. 2015;145:43–57. 10.1016/j.pharmthera.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 39.Felice D, Guilloux JP, Pehrson A, Li Y, Mendez-David I, Gardier AM, et al. Vortioxetine improves context discrimination in mice through a neurogenesis independent mechanism. Front Pharmacol. 2018;9:204. 10.3389/fphar.2018.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torrisi SA, Geraci F, Tropea MR, Grasso M, Caruso G, Fidilio A, et al. Fluoxetine and vortioxetine reverse depressive-like phenotype and memory deficits induced by Abeta1-42 oligomers in mice: a key role of transforming growth factor-beta1. Front Pharmacol. 2019;10:693. 10.3389/fphar.2019.00693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferretti V, Maltese F, Contarini G, Nigro M, Bonavia A, Huang H, et al. Oxytocin signaling in the central amygdala modulates emotion discrimination in mice. Curr Biol. 2019;29:1938–53.e6. 10.1016/j.cub.2019.04.070 [DOI] [PubMed] [Google Scholar]
- 42.Smith ML, Asada N, Malenka RC. Anterior cingulate inputs to nucleus accumbens control the social transfer of pain and analgesia. Science. 2021;371:153–59. 10.1126/science.abe3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finnell JE, Lombard CM, Padi AR, Moffitt CM, Wilson LB, Wood CS, et al. Physical versus psychological social stress in male rats reveals distinct cardiovascular, inflammatory and behavioral consequences. PLoS One. 2017;12:e0172868. 10.1371/journal.pone.0172868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Li JT, Wang H, Niu WP, Zhang CC, Zhang Y, et al. Role of trace amineassociated receptor 1 in the medial prefrontal cortex in chronic social stress-induced cognitive deficits in mice. Pharmacol Res. 2021;167:105571. 10.1016/j.phrs.2021.105571 [DOI] [PubMed] [Google Scholar]
- 45.Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, et al. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci. 2005;25:6221–34. 10.1523/JNEUROSCI.3003-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, et al. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci US A. 2004;101:7158–63. 10.1073/pnas.0401764101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker-Descartes I, Mineo M, Condado LV, Agrawal N. Domestic violence and its effects on women, children, and families. Pediatr Clin N Am. 2021;68:455–64. 10.1016/j.pcl.2020.12.011 [DOI] [PubMed] [Google Scholar]
- 48.Frampton JE. Vortioxetine: a review in cognitive dysfunction in depression. Drugs. 2016;76:1675–82. 10.1007/s40265-016-0655-3 [DOI] [PubMed] [Google Scholar]
- 49.Bennabi D, Haffen E, Van Waes V. Vortioxetine for cognitive enhancement in major depression: from animal models to clinical research. Front Psychiatry. 2019;10:771. 10.3389/fpsyt.2019.00771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang XD, Su YA, Wagner KV, Avrabos C, Scharf SH, Hartmann J, et al. Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss. Nat Neurosci. 2013;16:706–13. 10.1038/nn.3395 [DOI] [PubMed] [Google Scholar]
- 51.Wang XX, Li JT, Xie XM, Gu Y, Si TM, Schmidt MV, et al. Nectin-3 modulates the structural plasticity of dentate granule cells and long-term memory. Transl Psychiatry. 2017;7:e1228. 10.1038/tp.2017.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31:10721–31. 10.1523/JNEUROSCI.6413-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27:2948–57. 10.1523/JNEUROSCI.5289-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104. 10.1016/S0149-7634(01)00041-0 [DOI] [PubMed] [Google Scholar]
- 55.Jing W, Zhang T, Liu J, Huang X, Yu Q, Yu H, et al. A circuit of COCH neurons encodes social-stress-induced anxiety via MTF1 activation of Cacna1h. Cell Rep. 2021;37:110177. 10.1016/j.celrep.2021.110177 [DOI] [PubMed] [Google Scholar]
- 56.Parfitt GM, Nguyen R, Bang JY, Aqrabawi AJ, Tran MM, Seo DK, et al. Bidirectional control of anxiety-related behaviors in mice: role of inputs arising from the ventral hippocampus to the lateral septum and medial prefrontal cortex. Neuropsychopharmacology. 2017;42:1715–28. 10.1038/npp.2017.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vo BN, Marron Fernandez de Velasco E, Rose TR, Oberle H, Luo H, Hopkins CR, et al. Bidirectional influence of limbic GIRK channel activation on innate avoidance behavior. J Neurosci. 2021;41:5809–21. 10.1523/JNEUROSCI.2787-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci. 2002;116:884–901. 10.1037/0735-7044.116.5.884 [DOI] [PubMed] [Google Scholar]
- 59.Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus-memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–83. 10.1016/j.neubiorev.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 60.Khalil OS, Pisar M, Forrest CM, Vincenten MC, Darlington LG, Stone TW. Prenatal inhibition of the kynurenine pathway leads to structural changes in the hippocampus of adult rat offspring. Eur J Neurosci. 2014;39:1558–71. 10.1111/ejn.12535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Papadogiannis A, Dimitrov E. A possible mechanism for development of working memory impairment in male mice subjected to inflammatory pain. Neuroscience. 2022;503:17–27. 10.1016/j.neuroscience.2022.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Finnell JE, Muniz BL, Padi AR, Lombard CM, Moffitt CM, Wood CS, et al. Essential role of ovarian hormones in susceptibility to the consequences of witnessing social defeat in female rats. Biol Psychiatry. 2018;84:372–82. 10.1016/j.biopsych.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mouri A, Ukai M, Uchida M, Hasegawa S, Taniguchi M, Ito T, et al. Juvenile social defeat stress exposure persistently impairs social behaviors and neurogenesis. Neuropharmacology. 2018;133:23–37. 10.1016/j.neuropharm.2018.01.016 [DOI] [PubMed] [Google Scholar]
- 64.Han Y, Zhang L, Wang Q, Zhang D, Zhao Q, Zhang J, et al. Minocycline inhibits microglial activation and alleviates depressive-like behaviors in male adolescent mice subjected to maternal separation. Psychoneuroendocrinology. 2019;107:37–45. 10.1016/j.psyneuen.2019.04.021 [DOI] [PubMed] [Google Scholar]
- 65.Gorbunova AA, Kudryashova IV, Manolova AO, Novikova MR, Stepanichev MY, Gulyaeva NV. Effects of individual stressors used in a battery of “chronic unpredictable stress” on long-term plasticity in the hippocampus of juvenile rats. Acta Neurobiol Exp. 2017;77:244–53. 10.21307/ane-2017-058 [DOI] [PubMed] [Google Scholar]
- 66.Tzanoulinou S, Gantelet E, Sandi C, Marquez C. Programming effects of peripubertal stress on spatial learning. Neurobiol Stress. 2020;13:100282. 10.1016/j.ynstr.2020.100282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–48. 10.1002/hipo.10207 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.