Abstract
Objectives
To assess the diagnostic performance of ultrafast magnetic resonance imaging (UF-DCE MRI) in differentiating benign from malignant breast lesions.
Materials and methods
A comprehensive search was conducted until September 1, 2023, in Medline, Embase, and Cochrane databases. Clinical studies evaluating the diagnostic performance of UF-DCE MRI in breast lesion stratification were screened and included in the meta-analysis. Pooled summary estimates for sensitivity, specificity, diagnostic odds ratio (DOR), and hierarchic summary operating characteristics (SROC) curves were pooled under the random-effects model. Publication bias and heterogeneity between studies were calculated.
Results
A final set of 16 studies analyzing 2090 lesions met the inclusion criteria and were incorporated into the meta-analysis. Using UF-DCE MRI kinetic parameters, the pooled sensitivity, specificity, DOR, and area under the curve (AUC) for differentiating benign from malignant breast lesions were 83% (95% CI 79–88%), 77% (95% CI 72–83%), 18.9 (95% CI 13.7–26.2), and 0.876 (95% CI 0.83–0.887), respectively. We found no significant difference in diagnostic accuracy between the two main UF-DCE MRI kinetic parameters, maximum slope (MS) and time to enhancement (TTE). DOR and SROC exhibited low heterogeneity across the included studies. No evidence of publication bias was identified (p = 0.585).
Conclusions
UF-DCE MRI as a stand-alone technique has high accuracy in discriminating benign from malignant breast lesions.
Clinical relevance statement
UF-DCE MRI has the potential to obtain kinetic information and stratify breast lesions accurately while decreasing scan times, which may offer significant benefit to patients.
Key Points
• Ultrafast breast MRI is a novel technique which captures kinetic information with very high temporal resolution.
• The kinetic parameters of ultrafast breast MRI demonstrate a high level of accuracy in distinguishing between benign and malignant breast lesions.
• There is no significant difference in accuracy between maximum slope and time to enhancement kinetic parameters.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00330-024-10690-y.
Keywords: Breast cancer, Magnetic resonance imaging, Ultrafast, Kinetics, Artificial intelligence
Introduction
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is widely regarded as an excellent tool for evaluating inconclusive breast lesions, thanks to its high sensitivity and reliable negative predictive value in excluding malignancy [1–6]. However, a comprehensive breast MRI study protocol can be time-consuming. As a result, various efforts have been made to reduce scan times, aiming to make breast MRI more affordable and accessible. One such example is abbreviated breast MRI (AB-MRI), which substantially shortens the scan duration and necessitates only one post-contrast scan [7]. Several works have demonstrated the noninferiority of AB-MRI in cancer detection when compared to DCE-MRI [8–11]. However, one potential limitation of AB-MRI is the absence of kinetic information.
Ultrafast MRI (UF-DCE MRI) is a relatively novel technique which captures kinetic information within the first minute with very high temporal resolution (typically less than 7 s). Instead of relying on the conventional washout characteristics, the technique enables analysis of early contrast wash-in curves [12, 13]. Since UF-DCE MRI requires very high temporal resolution with an acceptable spatial resolution, several advanced MRI techniques have been used including view-sharing (VS), parallel imaging (PI), and compressed sensing (CS).
UF-MRI kinetic parameters, proposed as alternatives to those derived from conventional DCE-MRI, capture common pathophysiological processes in breast cancer, such as rapid contrast leakage [14] and tumor-associated vascular shunting [15]. Consequently, malignant lesions tend to exhibit more rapid enhancement on UF-MRI when compared to benign ones.
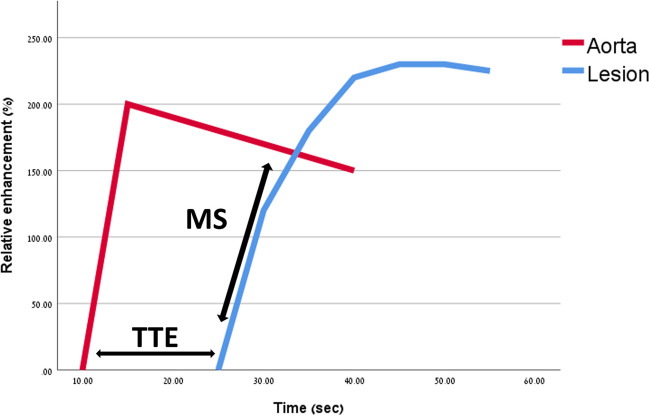
Maximum slope (MS), suggested by Mann et al in 2014 [16], is determined by drawing a tangent along the steepest part of the enhancing curve within the first minute, and calculating the relative enhancement percentage change divided by seconds (%/s). Time to enhancement (TTE), proposed by Mus et al [17], is calculated by the time point in which the lesion starts to enhance minus the time point where the aorta starts to enhance on the maximum intensity projection images (MIP). Figure 1 shows an illustration of MS and TTE.
Fig. 1.
Graphic illustration of MS and TTE. MS is defined as the steepest part of the lesion’s enhancing curve (%/s). TTE is calculated by the time point in which the lesion starts to enhance minus the time point where the aorta starts to enhance (s). MS, maximun slope. TTE, time to enhancement
Less frequently utilized kinetic parameters have been proposed, all grounded in the same fundamental pathophysiological mechanisms, showing variable success in differentiating benign from malignant processes. Examples include bolus arrival time (BAT) which closely resembles TTE [18], the time interval between arterial and venous visualization (AVI) [19], and the kinetic area under the curve (KAUC) [20].
While numerous studies have explored the diagnostic performance of UF-DCE MRI, a comprehensive quantitative review to consolidate the existing body of research has not been conducted until now. The goal of this systematic review and meta-analysis was to assess the diagnostic performance of UF-DCE MRI in differentiating benign from malignant breast lesions.
Materials and methods
Our protocol was constructed and registered at the Research Registry (Researchregistry1731, researtchregistry.com). Our work was performed by following the updated Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines (PRISMA 2020 [21]). A previous paper describing the approach to diagnostic test accuracy (DTA) meta-analysis in R software was used as a reference [22].
Literature search
A comprehensive search of Medline, Embase, and Cochrane was conducted for relevant articles published from each database’s inception up to September 1, 2023. Both controlled vocabulary terms and text words were used. No age restrictions were applied. Details of the search strategy are included in the Supplemental materials. Reference lists of included studies were manually searched to identify additional relevant studies. The search strategy is shown in Table S1.
Eligibility criteria
Studies were included if they met the following criteria: (a) The full text was available in English. (b) Published in a peer-reviewed journal (abstracts and conference presentations excluded). (c) MRI acquisition data was available. (d) The purpose of the study was to assess the diagnostic performance of UF-DCE MRI in differentiating benign from malignant breast lesions. Only studies using UF-MRI kinetic parameters exclusively to stratify lesions were used in this work. Studies using artificial intelligence (AI) methods, such as convolutional neural networks or radiomics, were excluded. (e) The study reported sufficient data to calculate the number of true-positive (TP), false-positive (FP), true-negative (TN), and false-negative (FN). If these values could not be obtained, the study was excluded.
Study selection
We executed a strategy that encompassed three distinct phases.
In phase 1, the results of the literature search were imported into reference manager software (EPPI Reviewer 6, University of London, England) for independent title and abstract review by multiple investigators (Y.A., T.M., O.G., and R.K.). Prior to commencing the independent review of titles and abstracts, reviewers conducted a preliminary screening of 20 studies in duplicate, aimed at enhancing consistency among the reviewers.
In phase 2, the full-text articles from potentially eligible sources were retrieved, and three investigators (Y.A., T.M., O.G., and R.K.) independently assessed them for inclusion. Any discrepancies were resolved by consensus.
In phase 3, three reviewers (Y.A., T.M., and O.G.) independently assessed the risk of bias using Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2 [23]). Any discrepancies were again resolved by consensus.
Data extraction
Data were extracted into a spreadsheet program (Microsoft Excel 2021; Microsoft, Redmond, USA) independently by multiple investigators (Y.A., T.M., O.G., and R.K.). Prior to commencing the data extraction, reviewers extracted data from three studies in duplicate, aimed at enhancing consistency among the reviewers. Any discrepancies were resolved by consensus.
For studies that used multiple readers, readers’ data was averaged for analysis [24]. For the construction of the main contingency table, we used the model in each study that achieved the best results using only UF-DCE MRI criteria, either a single UF-MRI kinetic parameter or a combination of UF-DCE MRI parameters. When a dataset was used for multiple studies, we extracted values from only one of the studies.
The following data were extracted: title of study, first author, country of first author, journal of publication, study design, patient demographics (mean age, indication for imaging, sample size BIRADS cutoff for inclusion if available), number of cancer cases, cancer definition, reference standard used (histopathology vs imaging follow-up), MRI parameters (MRI system and magnetic field strength, temporal resolution, slice thickness, acceleration method, injection speed), UF-DCE MRI parameters (kinetic parameters used, a priori vs post hoc cutoff used to stratify lesions), number of readers for each lesion, and 2 × 2 contingency table (FP, FN, TP, TN).
Quality assessment
Independent quality assessment of included studies was performed using the revised tool for QUADAS-2 [23]. Three investigators (Y.A., T.M., and O.G.) assessed all studies for the following criteria: patient selection, index test, reference standard, flow, and timing. The following criteria were defined as being high risk for bias: consecutive patient selection was not used (patient selection 1), inclusion limited to a particular group of patients, e.g., those who had undergone surgery or individuals with non-mass enhancement (NME) lesions (patient selection 2), radiologists were not blinded to previous clinical or imaging tests (index test), and the histopathological testing was not used for all lesions (reference standard and flow and timing). Any discrepancies were resolved by consensus.
Outcomes
The primary outcome was to estimate the diagnostic test accuracy of UF-DCE MRI in differentiating benign from malignant lesions. In the primary analysis, we assessed diagnostic accuracy using the most accurate model from each study. Additionally, in a separate analysis, we evaluated the diagnostic accuracy of MS and TTE individually with direct comparison between them. Several covariates were used in the meta-regression models when there was sufficient variability within the data.
Statistical analysis
Each meta-analysis was conducted to calculate the combined sensitivity, specificity, and diagnostic odds ratio (DOR), along with their respective 95% confidence intervals (CIs). Coupled forest plots and hierarchic summary operating characteristics (SROC) curves were created using the estimated model parameters. The bivariate random-effects model was used for the analysis [22, 25]. The following meta-analyses were conducted:
The best UF-DCE MRI kinetic parameter within each study (main analysis).
Studies assessing MS kinetic parameter individually.
Studies assessing TTE kinetic parameter individually.
Meta-regression models were created for the main analysis. To compare diagnostic accuracy between MS and TTE, “head-to-head” meta-regression models were performed with the inclusion of all studies assessing either MS or TTE individually and the kinetic parameters as covariates.
Heterogeneity for sensitivity, specificity, and DOR was assessed using the I2 test, with values greater than 50% considered at risk for significant variability [26]. Heterogeneity for SROC curve was assessed using the correlation coefficient between sensitivity and specificity, with values larger than 0 indicating high heterogeneity [27].
The publication bias was evaluated by a funnel plot and Egger’s test.
Analysis was performed using the “mada,” “meta,” and “dmetatools” packages in R (R version 3.6.3; R Foundation for Statistical Computing). A two-sided p value less than 0.05 was considered statistically significant.
Results
Study demographics and risk of bias
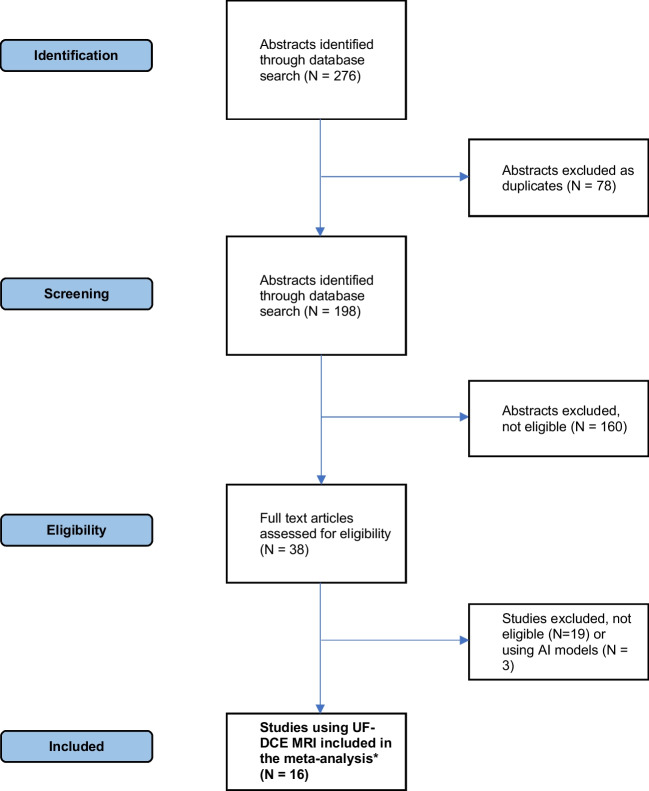
Figure 2 illustrates the study flow diagram. In the initial stage, 198 studies underwent screening based on their titles and abstracts, and from this pool, 38 were identified as potentially meeting the eligibility criteria. Following a thorough review of their full texts, 16 studies analyzing 2090 lesions were found to meet the inclusion criteria and were subsequently incorporated into the meta-analysis [16–18, 20, 28–40]. Table 1 provides a summary of the included studies.
Fig. 2.
Prefered Reporting Items for Systematic Reviewes and Meta-Analysis (PRISMA) flowchart. UF-MRI, ultrafast magnetic resonance imaging. AI, artificial intelligence. *Typically, one or a combination of the following parameters is used: maximum slope, time to enhancement, bolus arrival time, arterial venous visualization, kinetic area under curve, or peak enhancement
Table 1.
Characteristics of included studies
| Study | Year | Study design | Study population | Number of lesions | Number of cancers | MRI system | Magnetic field strength |
Acceleration method | Contrast agent /injection speed (ml/s) | UF-MRI best model | Reference standard | TN | FN | TP | FP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abe et al | 2016 | Retrospective | D + S | 62 | 33 | Philips | 3 T | PI (SENSE) | Gadobenate dimeglumine /2 | KAUC | Histology + FU | 23 | 5 | 28 | 6 |
| Cao et al | 2023 | Prospective | D | 150 | 117 | Siemens | 3 T | CS (VIBE) | Gadobenate dimeglumine /2 | MS + TTP + TTE + KAUC | Histology | 27 | 11 | 106 | 6 |
| Goto et al | 2018 | Retrospective | D | 107 | 81 | Siemens | 3 T | VS (TWIST) | Gadoterate meglumine/2 | MS + TTE | Histology + FU | 20 | 9 | 72 | 6 |
| Honda et al | 2019 | Retrospective | D | 90 | 61 | Siemens | 3 T | CS (VIBE) | Gadobutrol/2 | TTE | Histology | 15 | 9 | 52 | 14 |
| Kim et al | 2022 | Retrospective | Post operative | 58 | 10 | Philips | 3 T | PI (SENSE) | Gadobutrol/2 | TTE | Histology + FU | 29 | 1 | 9 | 19 |
| Lee et al | 2019 | Retrospective | Preoperative | 202 | 140 | GE | 3 T | VS (DISCO) | Gadoterate meglumine/3 | IEP | Histology + FU | 56 | 35 | 105 | 6 |
| Mann et al | 2014 | Retrospective | D | 199 | 104 | Philips | 3 T | VS (TWIST) | Gadoterate meglumine/2.5 | MS | Histology + FU | 62 | 10 | 93 | 33 |
| Mori et al | 2020 | Retrospective | D (NME) | 77 | 54 | Philips | 3 T | PI (SENSE) | Gadobenate dimeglumine /2.5 | KAUC | Histology + FU | 17 | 10 | 44 | 6 |
| Mus et al | 2017 | Retrospective | D | 195 | 99 | Siemens | 3 T | VS (TWIST) | Gadoterate meglumine/3 | TTE | Histology + FU | 75 | 8 | 91 | 21 |
| Ohashi et al | 2019 | Retrospective | D | 139 | 90 | Siemens | 3 T | VS (KWIC) | Gadobutrol/2.5 | MS | Histology + FU | 31 | 8 | 81 | 19 |
| Onishi et al | 2020 | Retrospective | D + S | 125 | 26 | GE | 3 T | VS (DISCO) | Gadobutrol/2 | MS + BAT | Histology | 87 | 13 | 13 | 12 |
| Pelissier et al | 2021 | Retrospective | D | 210 | 150 | Siemens | 1.5 T | VS (TWIST) | Gadoterate meglumine/2 | MS | Histology + FU | 54 | 24 | 126 | 6 |
| Peter et al | 2020 | Retrospective | D (masses) | 83 | 60 | Siemens | 3 T | VS (TWIST) | Gadoterate meglumine/2 | Peak enhancement | Histology + FU | 14 | 9 | 51 | 9 |
| Ramli et al | 2023 | Prospective | D | 83 | 49 | Siemens | 3 T | VS (TWIST) | Gadoterate meglumine/2 | MS | Histology | 29 | 13 | 36 | 5 |
| Van zeist et al | 2018 | Prospective | S (high risk) | 201 | 31 | Siemens | 3 T | VS (TWIST) | Gadoterate meglumine/2.5 | MS + TTE | Histology | 139 | 5 | 26 | 31 |
| Yamaguchi et al | 2022 | Retrospective | D | 110 | 88 | Siemens | 3 T | CS (VIBE) | Gadobutrol/2.5 | TTE | Histology + FU | 20 | 32 | 56 | 2 |
AI artificial intelligence, BAT bolus arrival time, CNN convoluted neural network, D diagnostic, CS compression sensing, FN false negative, FP false positive, FU follow-up, GE General Electric, IEP initial enhancement phase, KAUC kinetic area under curve, MS maximum slope, MRI magnetic resonance imaging, PI parallel imaging, S screening, TN true negative, TP true positive, TTE time to enhancement, TTP time to peak, VS view sharing
Table 2S provides a risk of bias summary. Three studies were at low risk of bias [28, 37, 38], while the remaining 13 studies were at high risk or unknown risk of bias. The main sources of bias included no indication as to whether the patients were selected consecutively, radiologists unblinded to previous imaging or clinical data, and histopathology not employed as the reference standard for all lesions.
Table 2.
Different meta-analysis models and their pooled accuracy measurements
| Meta-analysis model | Number of studies | Sensitivity (95%CI) | Specificity (95%CI) | DOR (95%CI) | AUC of the ROC curve (95%CI) |
|---|---|---|---|---|---|
| Best UF-DCE MRI kinetic parameter | 16 | 83% (79–88%) | 77% (72–83%) | 18.9 (13.7–26.2) | 0.876 (0.830–0.887) |
| MS individually | 7 | 80% (70–90%) | 77% (68–89%) | 17.1 (11.4–25.6) | 0.865 (0.805–0.891) |
| TTE individually | 7 | 71% (57–86%) | 80% (69–82%) | 15.5 (8.3–28.9) | 0.857 (0.763–0.889) |
AUC area under curve, DOR diagnostic odds ratio, MS maximum slope, ROC receiver operating characteristic, TTE time to enhancement, UF-DCE MRI ultrafast magnetic resonance imaging
Data synthesis and pooling
Best UF-MRI kinetic parameter analysis
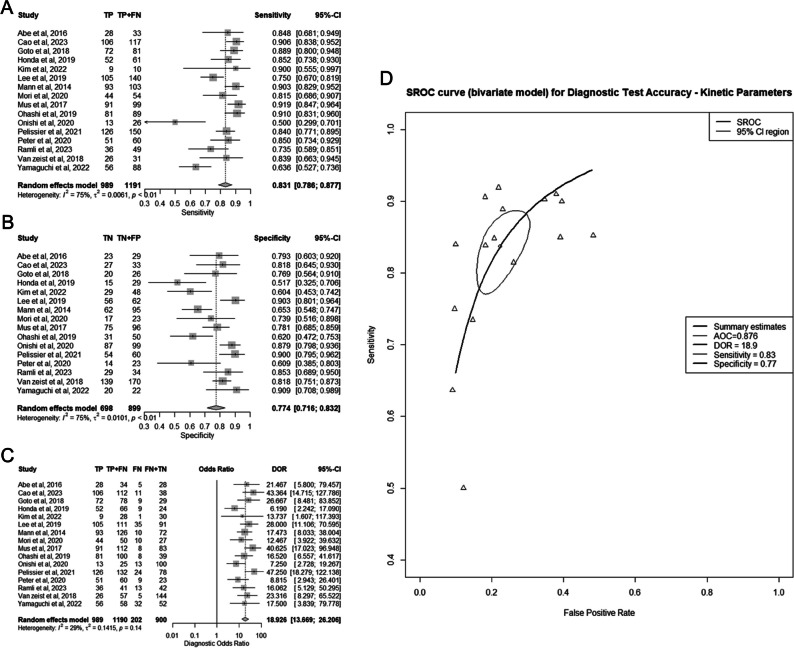
Sixteen studies encompassing 2090 lesions were included in this meta-analysis [11, 16–18, 20, 28–39] (Fig. 3A–D). The best model in each study is specified in Table 1. The pooled sensitivity was 83% (95% CI 79–88%), the pooled specificity was 77% (95% CI 72–83%), the pooled DOR was 18.9 (95% CI 13.7–26.2), and the AUC of the SROC curve was 0.876 (95% CI 0.830–0.887).
Fig. 3.
Diagnostic performance of best ultrafast magnetic resonance imaging (UF-DCE MRI) kinetic parameters for differentiating benign from malignant breast lesions. A Summary forest plot of sensitivity. B Summary forest plot of specificity. C Summary forest plot of diagnostic odds ratio (DOR). D Hierarchic summary operating characteristics (SROC) curve
Based on the I2 test, there was a high risk for substantial heterogeneity for sensitivity and specificity, and a low risk of substantial variability for DOR (Fig. 3A–C). The correlation coefficient between sensitivity and specificity was −0.62, indicating low heterogeneity of the SROC curve.
In multivariate meta-regression models (as shown in Table 3S), we observed that a lower mean age and the use of Siemens MRI systems were linked to increased sensitivity, with corresponding p values of 0.034 and 0.008, respectively. Additionally, MRI temporal resolution greater than 5 s was found to be associated with greater specificity (p = 0.037), while studies involving more than 110 lesions were associated with a higher DOR with a p value of 0.021.
Individual UF-DCE MRI parameter analysis—MS vs TTE
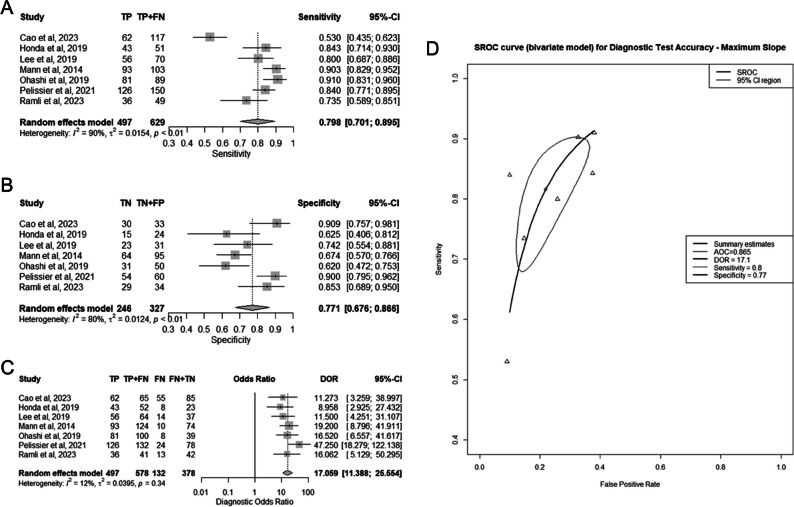
Seven studies incorporating 956 lesions were included in the MS meta-analysis [16, 28, 30, 32, 34, 35, 37] (Fig. 4A–D). The pooled sensitivity was 80% (95% CI 70–90%), the pooled specificity was 77% (95% CI 68–89%), the pooled DOR was 17.1 (95% CI 11.4–25.6), and the AUC of the SROC curve was 0.865 (95% CI 0.805–0.891).
Fig. 4.
Diagnostic performance of maximum slope (MS) individually for differentiating benign from malignant breast lesions. A Summary forest plot of sensitivity. B Summary forest plot of specificity. C Summary forest plot of diagnostic odds ratio (DOR). D Hierarchic summary operating characteristics (SROC) curve
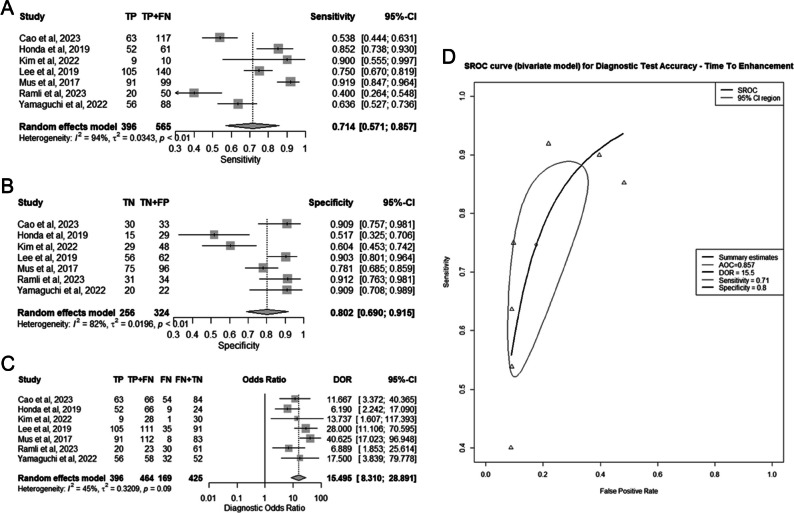
Seven studies comprising a total of 889 lesions were included in the TTE meta-analysis [17, 28, 30–32, 37, 39] (Fig. 5A–D). The pooled sensitivity was 71% (95% CI 57–86%), the pooled specificity was 80% (95% CI 69–82%), the pooled DOR was 15.5 (95% CI 8.3–28.9), and the AUC of the SROC curve was 0.857 (95% CI 0.763–0.889).
Fig. 5.
Diagnostic performance of time to enhancement (TTE) individually for differentiating benign from malignant breast lesions. A Summary forest plot of sensitivity. B Summary forest plot of specificity. C Summary forest plot of diagnostic odds ratio (DOR). D Hierarchic summary operating characteristics (SROC) curve
For both the MS and TTE analysis, there was a high risk for substantial heterogeneity for sensitivity and specificity, and a low risk of substantial variability for DOR (Figs. 4A–C, 5A–C). The correlation coefficients between sensitivity and specificity were −0.77 and −0.75 for MS and TTE respectively, suggesting low heterogeneity of the SROC curves.
In the “head-to-head” meta-regression analysis, as presented in Table 4S, there were no statistically significant differences observed between MS and TTE in terms of sensitivity, specificity, or DOR, with corresponding p values of 0.346, 0.638, and 0.880, respectively.
Table 2 provides an overview of various models along with their corresponding pooled accuracy measurements.
Publication bias
The shape of the funnel plot is generally symmetrical, which indicates low publication bias (Figure 1S). Egger’s test also supports low publication bias (p = 0.585).
Discussion
UF-DCE MRI is a promising new technique which has the potential to obtain kinetic information while reducing scan time. To the best of our knowledge, this is the first meta-analysis exploring the diagnostic accuracy of this novel approach. This comprehensive meta-analysis aggregated 16 studies and 2090 lesions to investigate the discriminatory power of UF-DCE MRI in differentiating benign from malignant breast lesions.
We found that when using UF-DCE MRI kinetic parameters, the pooled diagnostic sensitivity, specificity, DOR, and area under SROC for differentiating benign from malignant lesions are 83% (95% CI 79–88%), 77% (95% CI 72–83%), 18.9 (95% CI 13.7–26.2), and 0.876 (95% CI 0.83–0.887), respectively. Prior meta-analyses assessing the diagnostic accuracy full protocol DCE-MRI have found a pooled sensitivity, specificity, DOR, and area under the SROC of 87–93%, 74–85%, 18.8–91, and 0.86–0.96 respectively [41–43]. Except for sensitivity, which is slightly lower, our pooled figures fall within the range of these values, which is highly encouraging. Our work focused on the diagnostic accuracy of UF-DCE MRI as a stand-alone technique. In contrast, the previous abovementioned meta-analyses [41–43] examined the diagnostic accuracy of full-protocol breast MRI, which encompasses morphological data, kinetic curve data, and diffusion sequences. It is probable that by combining UF-DCE MRI with DCE-MRI sequences, we can expect an additive effect that will further enhance diagnostic accuracy. Many studies included in this meta-analysis lend support to this hypothesis. A few works have shown that the addition of UF-DCE MRI to DCE-MRI increased the diagnostic accuracy compared to DCE-MRI alone [29, 31, 32]. Other works have shown that UF-DCE MRI as a stand-alone technique is as good or even better than standard curve type analysis [17, 20, 30, 33, 34]. This suggests the possibility of reducing scan time while maintaining or potentially enhancing diagnostic accuracy. An option to consider involves integrating AB-MRI and UF-DCE MRI, allowing the utilization of kinetic data while simultaneously reducing examination time by eliminating the requirement for delayed scans. Researchers are encouraged to conduct more in-depth examinations in this direction in their future work.
In our analysis, we observed a considerable degree of heterogeneity in sensitivity and specificity assessments, while there was comparatively lower heterogeneity in the analysis of DOR and SROC. This outcome aligns with the conventional expectation of a negative correlation between sensitivity and specificity. As one of these values increases, the other tends to decrease, resulting in what is known as the “threshold effect” [44], which likely contributed to variations in heterogeneity. We used the random-effects model to enable pooling of the results within this context. For the DOR and SROC analysis, which provide a single measure merging the results of each diagnostic study thereby eliminating the “threshold effect,” heterogeneity was low.
We investigated additional sources of heterogeneity through a meta-regression analysis. Studies of larger scale that utilized Siemens MRI systems, featuring a temporal resolution exceeding 5 s and including younger women, tended to yield slightly better accuracy. The correlation between different technical parameters and diagnostic performance is particularly important, as there is no standardized protocol for UF-DCE MRI; each vendor and institution employ different MRI sequences. However, our findings were inconsistent across various analyses, making it challenging to draw firm conclusions. With the accumulation of more data, it is expected that a more standardized UF-DCE MRI sequence with optimal technical parameters can be established.
MS and TTE are the two most frequently utilized kinetic parameters in UF-DCE MRI, and most studies within this meta-analysis incorporated either one or both parameters. Based on our results, although MS achieved slightly higher accuracy than TTE individually, there is no statistically significant difference between the two methods. The parameters capture different kinetic values—MS is a measurement of the time-intensity curve and TTE measures the earliest time of lesion enhancement (Fig. 1). However, as both parameters reflect the same basic pathophysiological mechanism (AV shunting and capillary leakage), their yielding of similar outcomes is not unexpected. This observation might also explain why our meta-regression analysis failed to reveal any evidence supporting an increase in accuracy when using models combining more than one parameter, in contrast to our initial expectation. It is possible that these parameters are not additive but instead redundant.
In addition to its role in classifying breast lesions as benign or malignant, UF-DCE MRI may have other applications in the realm of breast cancer. These could include improving lesion conspicuity compared to DCE-MRI [45, 46], differentiating invasive breast carcinoma from ductal carcinoma in situ (DCIS) [30, 47, 48], predicting tumor prognostic markers and receptor status [29, 47–49], and predicting pathological complete response (pCR) after neoadjuvant treatment [50–52]. These potential roles warrant further exploration in upcoming dedicated systematic reviews and meta-analyses.
Our study had several limitations. First, many of the studies had suboptimal quality with regard to patient selection, index test, and reference standard. Second, we included only studies using UF-DCE MRI parameters exclusively, thereby limiting the number of available studies and affecting its statistical power. Third, there was significant variability among the included studies with respect to the kinetic parameter used in each study. This limitation is relevant specifically to our main analysis using the best model in each study. Nevertheless, as discussed previously, all kinetic parameters reflect a similar pathophysiological mechanism of rapid contrast leakage and AV shunting, resulting in faster wash-in within the first minute, and thus potentially allowing for pooling of the results. This is further supported by the low heterogeneity of DOR and SROC curves between the studies, the similar accuracy values of the two main kinetic parameters when used individually (MS and TTE), and the similar accuracy of models using one or multiple parameters. Furthermore, our analysis did not encompass studies utilizing artificial intelligence methods, primarily due to their scarcity. However, such an investigation could be undertaken in the future as more data become available. Finally, several of the studies did not report TP, FP, TN, and FN values directly, necessitating extraction of these values indirectly from the sensitivity, specificity, and total number of malignancies values, which may decrease accuracy.
In conclusion, based on our meta-analysis, UF-DCE MRI as a stand-alone technique has high accuracy in discriminating benign from malignant breast lesions. Our findings did not reveal a notable distinction in accuracy between the two primary UF-DCE MRI kinetic parameters, MS and TTE. We recommend further research, particularly focusing on the utilization of AI techniques and the integration of UF-DCE MRI with DCE-MRI sequences, to enhance diagnostic precision.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- AB-MRI
Abbreviated breast MRI
- AI
Artificial intelligence
- AUC
Area under curve
- CI
Confidence interval
- CS
Compressed sensing
- DCE-MRI
Dynamic contrast-enhanced magnetic resonance imaging
- DOR
Diagnostic odds ratio
- FN
False-negative
- FP
False-positive
- KAUC
Kinetic area under the curve
- MS
Maximum slope
- PI
Parallel imaging
- QUADAS-2
Quality Assessment of Diagnostic Accuracy Studies-2
- SROC
Hierarchic summary operating characteristics
- TN
True-negative
- TP
True-positive
- TTE
Time to enhancement
- UF-DCE
MRI ultrafast MRI
- VS
View sharing
Funding
Open access funding provided by Tel Aviv University. The authors state that this work has not received any funding.
Declarations
Guarantor
The scientific guarantor of this publication is Dr. Yoav Amitai.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because it is a review.
Ethical approval
Institutional Review Board approval was not required because it is a review.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported because it is a meta-analysis.
Methodology
• review and meta-analysis
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sarica O, Uluc F (2014) Additional diagnostic value of MRI in patients with suspicious breast lesions based on ultrasound. Br J Radiol 87:20140009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oztekin PS, Kosar PN (2014) Magnetic resonance imaging of the breast as a problem-solving method: to be or not to be? Breast J 20:622–631 [DOI] [PubMed] [Google Scholar]
- 3.Amitai Y, Scaranelo A, Menes TS et al (2020) Can breast MRI accurately exclude malignancy in mammographic architectural distortion? Eur Radiol 30:2751–2760 [DOI] [PubMed] [Google Scholar]
- 4.Xie Z, Xu W, Zhang H, Li L, An Y, Mao G (2023) The value of MRI for downgrading of breast suspicious lesions detected on ultrasound. BMC Med Imaging 23:72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennani-Baiti B, Bennani-Baiti N, Baltzer PA (2016) Diagnostic performance of breast magnetic resonance imaging in non-calcified equivocal breast findings: results from a systematic review and meta-analysis. PLoS One 11:e0160346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spick C, Szolar DHM, Preidler KW, Tillich M, Reittner P, Baltzer PA (2015) Breast MRI used as a problem-solving tool reliably excludes malignancy. Eur J Radiol 84:61–64 [DOI] [PubMed] [Google Scholar]
- 7.Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB (2014) Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol 32:2304–2310 [DOI] [PubMed] [Google Scholar]
- 8.Panigrahi B, Mullen L, Falomo E, Panigrahi B, Harvey S (2017) An abbreviated protocol for high-risk screening breast magnetic resonance imaging: impact on performance metrics and BI-RADS assessment. Acad Radiol 24:1132–1138 [DOI] [PubMed] [Google Scholar]
- 9.Borthakur A, Weinstein SP, Schnall MD, Conant EF (2019) Comparison of study activity times for “full” versus “fast MRI” for breast cancer screening. J Am Coll Radiol 16:1046–1051 [DOI] [PubMed] [Google Scholar]
- 10.Petrillo A, Fusco R, Sansone M et al (2017) Abbreviated breast dynamic contrast-enhanced MR imaging for lesion detection and characterization: the experience of an Italian oncologic center. Breast Cancer Res Treat 164:401–410 [DOI] [PubMed] [Google Scholar]
- 11.Kim SY, Cho N, Hong H et al (2022) Abbreviated screening mri for women with a history of breast cancer: comparison with full-protocol breast MRI. Radiology 305:36–45 [DOI] [PubMed] [Google Scholar]
- 12.Kataoka M, Honda M, Ohashi A et al (2022) Ultrafast dynamic contrast-enhanced MRI of the breast: how is it used? Magn Reson Med Sci 21:83–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Y, Heller SL (2020) Abbreviated and ultrafast breast MRI in clinical practice. Radiographics 40:1507–1527 [DOI] [PubMed] [Google Scholar]
- 14.Cuenod CA, Balvay D (2013) Perfusion and vascular permeability: basic concepts and measurement in DCE-CT and DCE-MRI. Diagnos Interven Imaging 94:1187–1204 [DOI] [PubMed] [Google Scholar]
- 15.Pries AR, Höpfner M, le Noble F, Dewhirst MW, Secomb TW (2010) The shunt problem: control of functional shunting in normal and tumour vasculature. Nat Rev Cancer 10:587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mann RM, Mus RD, van Zelst J, Geppert C, Karssemeijer N, Platel B (2014) A novel approach to contrast-enhanced breast magnetic resonance imaging for screening: high-resolution ultrafast dynamic imaging. Invest Radiol 49:579–585 [DOI] [PubMed] [Google Scholar]
- 17.Mus RD, Borelli C, Bult P et al (2017) Time to enhancement derived from ultrafast breast MRI as a novel parameter to discriminate benign from malignant breast lesions. Eur J Radiol 89:90–96 [DOI] [PubMed] [Google Scholar]
- 18.Onishi N, Sadinski M, Gibbs P et al (2020) Differentiation between subcentimeter carcinomas and benign lesions using kinetic parameters derived from ultrafast dynamic contrast-enhanced breast MRI. Eur Radiol 30:756–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onishi N, Kataoka M, Kanao S et al (2018) Ultrafast dynamic contrast-enhanced mri of the breast using compressed sensing: breast cancer diagnosis based on separate visualization of breast arteries and veins. J Magn Reson Imaging 47:97–104 [DOI] [PubMed] [Google Scholar]
- 20.Abe H, Mori N, Tsuchiya K et al (2016) Kinetic analysis of benign and malignant breast lesions with ultrafast dynamic contrast-enhanced MRI: comparison with standard kinetic assessment. AJR Am J Roentgenol 207:1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shim SR, Kim SJ, Lee J (2019) Diagnostic test accuracy: application and practice using R software. Epidemiol Health 41:e2019007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whiting PF, Rutjes AW, Westwood ME et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536 [DOI] [PubMed] [Google Scholar]
- 24.McGrath TA, McInnes MDF, Langer FW, Hong J, Korevaar DA, Bossuyt PMM (2017) Treatment of multiple test readers in diagnostic accuracy systematic reviews-meta-analyses of imaging studies. Eur J Radiol 93:59–64 [DOI] [PubMed] [Google Scholar]
- 25.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH (2005) Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 58:982–990 [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558 [DOI] [PubMed] [Google Scholar]
- 27.Shim S, Shin I, Bae J (2015) Meta-analysis of diagnostic tests accuracy using STATA software. J Health Info Stat 40:190–199 [Google Scholar]
- 28.Cao Y, Wang X, Shi J et al (2023) Multiple parameters from ultrafast dynamic contrast-enhanced magnetic resonance imaging to discriminate between benign and malignant breast lesions: comparison with apparent diffusion coefficient. Diagn Interv Imaging 104:275–283 [DOI] [PubMed] [Google Scholar]
- 29.Goto M, Sakai K, Yokota H et al (2019) Diagnostic performance of initial enhancement analysis using ultra-fast dynamic contrast-enhanced MRI for breast lesions. Eur Radiol 29:1164–1174 [DOI] [PubMed] [Google Scholar]
- 30.Honda M, Kataoka M, Onishi N et al (2020) New parameters of ultrafast dynamic contrast-enhanced breast MRI using compressed sensing. J Magn Reson Imaging 51:164–174 [DOI] [PubMed] [Google Scholar]
- 31.Kim ES, Cho N, Kim SY et al (2022) Added value of ultrafast sequence in abbreviated breast MRI surveillance in women with a personal history of breast cancer: a multireader study. Eur J Radiol 151:110322 [DOI] [PubMed] [Google Scholar]
- 32.Lee SJ, Ko KH, Jung HK, Koh JE, Park AY (2020) The additional utility of ultrafast MRI on conventional DCE-MRI in evaluating preoperative MRI of breast cancer patients. Eur J Radiol 124:108841 [DOI] [PubMed] [Google Scholar]
- 33.Mori N, Sheth D, Abe H (2020) Nonmass enhancement breast lesions: diagnostic performance of kinetic assessment on ultrafast and standard dynamic contrast-enhanced MRI in comparison with morphologic evaluation. AJR Am J Roentgenol 215:511–518 [DOI] [PubMed] [Google Scholar]
- 34.Ohashi A, Kataoka M, Kanao S et al (2019) Diagnostic performance of maximum slope: a kinetic parameter obtained from ultrafast dynamic contrast-enhanced magnetic resonance imaging of the breast using k-space weighted image contrast (KWIC). Eur J Radiol 118:285–292 [DOI] [PubMed] [Google Scholar]
- 35.Pelissier M, Ambarki K, Salleron J, Henrot P (2021) Maximum slope using ultrafast breast DCE-MRI at 1.5 Tesla: a potential tool for predicting breast lesion aggressiveness. Eur Radiol 31:9556–9566 [DOI] [PubMed] [Google Scholar]
- 36.Peter SC, Wenkel E, Weiland E et al (2020) Combination of an ultrafast TWIST-VIBE Dixon sequence protocol and diffusion-weighted imaging into an accurate easily applicable classification tool for masses in breast MRI. Eur Radiol 30:2761–2772 [DOI] [PubMed] [Google Scholar]
- 37.Ramli Hamid MT, Ab Mumin N, Wong YV, Chan WY, Rozalli FI, Rahmat K (2023) The effectiveness of an ultrafast breast MRI protocol in the differentiation of benign and malignant breast lesions. Clin Radiol 78:444–450 [DOI] [PubMed] [Google Scholar]
- 38.van Zelst JCM, Vreemann S, Witt HJ et al (2018) Multireader study on the diagnostic accuracy of ultrafast breast magnetic resonance imaging for breast cancer screening. Invest Radiol 53:579–586 [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi K, Nakazono T, Egashira R et al (2023) Time to enhancement of breast lesions and normal breast parenchyma in light of menopausal status and menstrual cycle for ultrafast dynamic contrast-enhanced MRI using compressed sensing. Magn Reson Imaging 96:102–107 [DOI] [PubMed] [Google Scholar]
- 40.Jing X, Wielema M, Cornelissen LJ et al (2022) Using deep learning to safely exclude lesions with only ultrafast breast MRI to shorten acquisition and reading time. Eur Radiol 32:8706–8715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu CR, Chen KY, Li P, Xia ZY, Wang B (2021) Accuracy of multiparametric MRI in distinguishing the breast malignant lesions from benign lesions: a meta-analysis. Acta Radiol 62:1290–1297 [DOI] [PubMed] [Google Scholar]
- 42.Dong H, Kang L, Cheng S, Zhang R (2021) Diagnostic performance of dynamic contrast-enhanced magnetic resonance imaging for breast cancer detection: an update meta-analysis. Thorac Cancer 12:3201–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L, Tang M, Min Z, Lu J, Lei X, Zhang X (2016) Accuracy of combined dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging for breast cancer detection: a meta-analysis. Acta Radiol 57:651–660 [DOI] [PubMed] [Google Scholar]
- 44.Trikalinos TA, Balion CM, Coleman CI et al (2012) AHRQ methods for effective health care meta-analysis of test performance when there is a “gold standard”. In: Chang SM, Matchar DB, Smetana GW, Umscheid CA, (eds) Methods guide for medical test reviews. Agency for Healthcare Research and Quality (US), Rockville (MD)
- 45.Honda M, Kataoka M, Iima M et al (2020) Background parenchymal enhancement and its effect on lesion detectability in ultrafast dynamic contrast-enhanced MRI. Eur J Radiol 129:108984 [DOI] [PubMed] [Google Scholar]
- 46.Kim S-Y, Cho N, Choi Y et al (2020) Ultrafast dynamic contrast-enhanced breast MRI: lesion conspicuity and size assessment according to background parenchymal enhancement. Korean J Radiol 21:561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamaguchi K, Nakazono T, Egashira R et al (2021) Maximum slope of ultrafast dynamic contrast-enhanced MRI of the breast: comparisons with prognostic factors of breast cancer. Jpn J Radiol 39:246–253 [DOI] [PubMed] [Google Scholar]
- 48.Shin SU, Cho N, Kim SY, Lee SH, Chang JM, Moon WK (2020) Time-to-enhancement at ultrafast breast DCE-MRI: potential imaging biomarker of tumour aggressiveness. Eur Radiol 30:4058–4068 [DOI] [PubMed] [Google Scholar]
- 49.Onishi N, Kataoka M (2021) Breast cancer screening for women at high risk: review of current guidelines from leading specialty societies. Breast Cancer 28:1195–1211 [DOI] [PubMed] [Google Scholar]
- 50.Cao Y, Wang X, Li L et al (2023) Early prediction of pathologic complete response of breast cancer after neoadjuvant chemotherapy using longitudinal ultrafast dynamic contrast-enhanced MRI. Diagn Interv Imaging. 10.1016/j.diii.2023.07.003 10.1016/j.diii.2023.07.003 [DOI] [PubMed] [Google Scholar]
- 51.Kim JH, Park VY, Shin HJ, Kim MJ, Yoon JH (2022) Ultrafast dynamic contrast-enhanced breast MRI: association with pathologic complete response in neoadjuvant treatment of breast cancer. Eur Radiol 32:4823–4833 [DOI] [PubMed] [Google Scholar]
- 52.Ramtohul T, Tescher C, Vaflard P et al (2022) Prospective evaluation of ultrafast breast MRI for predicting pathologic response after neoadjuvant therapies. Radiology 305:565–574 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.