Abstract
Main conclusion
New findings are presented for Chaerophyllum coloratum L. on the volatile composition of the essential oil, based on data of hydrosol and fresh plant material, light and electron microscopy of leaves, and cytotoxic and antiviral activity.
Abstract
The widespread Apiaceae family includes many well-known and economically important plants that are cultivated as food or spices. Many produce essential oils and are generally a source of secondary metabolites and compounds that have numerous applications in daily life. In this study, the chemical composition of volatile organic compounds (VOCs), ultrastructure and biological activity of the Mediterranean endemic species Cheaerophyllum coloratum L. are investigated, as literature data for this plant species are generally very scarce. The essential oil and hydrosol were extracted from the air-dried leaves by hydrodistillation and the chemical composition of both extracts was analysed by GC–MS in conjunction with headspace solid-phase microextraction (HS-SPME) of VOCs from the hydrosol and the fresh plant material. In the composition of the essential oil, the oxygenated sesquiterpenes spathulenol and caryophyllene oxide were the most abundant components. In the fresh plant material, non-oxygenated sesquiterpenes dominated, with β-caryophyllene and germacrene D being the main components. The hydrosol was dominated by monoterpenes, with the oxygenated monoterpene p-cymen-8-ol being the most abundant. Light and electron micrographs of the leaf of C. coloratum show secretory structures, and we hypothesize that glandular leaf trichomes, secretory epidermal cells and secretory canals are involved in the production of volatiles and their secretion on the leaf surface. Since the biological potential of C. coloratum is poorly investigated, we tested its cytotoxic activity on cancer and healthy cell lines and its antiviral activity on plants infected with tobacco mosiac virus (TMV). Our results dealing with the composition, ultrastructure and biological activity show that C. coloratum represent a hidden valuable plant species with a potential for future research.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00425-024-04531-x.
Keywords: Antiviral activity, Cytotxic activity, Essential oil, GC–MS, Headspace solid-phase microextraction (HS-SPME), Hydrosol, Secretory structures, Trichomes, Tobacco mosiac virus (TMV), Volatile organic compounds (VOCs)
Introduction
The species Chaerophyllum coloratum L. belongs to the family Apiaceae, whose members are widely distributed, especially in the northern temperate zone. Plants from the Apiaceae are not only economically important, as they are used in industry and in the production of beverages (Sayed-Ahmad et al. 2017; Kamte et al. 2018), but are also known in folk medicine where they are used to treat various ailments (Ozhatay et al. 2008). The plants of the genus Cherophyllum are known for their strong aroma, which is why they are used in food production, for example, as flavoring agents in cheese preparations (Demirci et al. 2007). C. coloratum, a species endemic to the Dinarides, is widespread in Croatia, Bosnia and Herzegovina, Montenegro, Kosovo (Prokletije Mountains) and Albania. It grows in sunny and dry locations, on rocky meadows and similar soils in a Mediterranean climate (Šilić 1984) and belongs to species of international concern (Ozinga et al. 2005). In Croatia, it can be found along the coast and on the islands, from the Paklenica National Park (South Velebit) in the north to the Privlaka peninsula in the south (Nikolić et al. 2015). It grows mainly in heavily degraded habitats with poor soils, but also on flysch soil. The densest populations are found on stony pastures in the sub-Mediterranean and eu-Mediterranean zones.
In general, Chaerophyllum species were not traditionally used in the Dinaric region, although there are some reports of ethnobotany of some species, e.g. C. bulbosum in Turkey (Polat et al. 2013). Previous studies included the anatomy and morphology of Chaerophyllum species, the composition of essential oils, and the biological activity of their compounds. The relevant papers are listed in Mačukanović-Jocić et al. (2017). Despite the frequent occurrence of C. coloratum in the sub-Mediterranean part of the Dinaric Mountains, its phytochemical composition, anatomy and biologically active compounds have been insufficiently studied. As far as biological activities are concerned, we have found only one report on C. coloratum related to antibacterial activity (Božović et al. 2009). Stešević et al. (2016) investigated the composition of essential oils and phenolic compounds, while Vajs et al. (1995) reported on the analysis of essential oils from ripe fruits and umbels, both analysing C. coloratum from Montenegro. Pollen morphology and the flower visitors of this species were described by Mačukanović-Jocić et al. (2017). The main compounds previously described in the composition of the essential oil of C. coloratum were monoterpene hydrocarbons, especially in the roots and stems. A high content of myrcene was described in the root while β-(E)-ocimene, β-(Z)-ocimene and terpinolene were dominant compounds in the stem. In the oil of leaves spathulenol, p-cymene-8-ol and p-cymene were predominant compounds. In addition, monoterpenes terpinolene and p-(Z)-(E)-ocimene were identified as the main constituents of the flower oil, while in the fruits, the most abundant constituents were caryophyllene oxide, (Z)-β-farnesene, (E)-pinocarveol and myrtenol (Stešević et al. 2016).
The volatile substances of aromatic plants continue to be the subject of numerous scientific studies, which have led to the potential hidden in aromatic plants being recognised (Fredotovic et al. 2017, 2021; Vuko et al. 2021b, 2022). The composition of specialized plant metabolites and the fact that they often help plants to adapt and survive in very unfavorable environmental conditions point to the possibility of using these natural products in various fields such as medicine, pharmacy, plant protection, food industry, etc. The first objective of our study was therefore the phytochemical analysis of Croatian C. coloratum with the aim of describing its volatile composition and expanding the knowledge about this little-studied plant and volatiles in the Apiaceae family in general.
The anatomy of the root, stem, leaf and fruit of C. coloratum using light and fluorescence microscopy was investigated by Stešević et al. (2016), revealing that the clustering of C. coloratum and C. aureum based on anatomical features does not correspond to molecular features. However, apart from the staining and localization of the essential oils and phenols, the parts where the essential oil of C. coloratum is synthesized, accumulated and secreted are still unknown. As specialized metabolites with numerous functions, essential oils are complex mixtures of hydrocarbons and their oxygenated derivatives containing predominantly volatile low molecular weight terpenes, (Fahn 2000). VOCs are produced by glandular trichomes and other secretory structures and specialized secretory tissues that play a central ecological role in the plant (Sharifi-Rad et al. 2017). Secretory cells have been described in several plant families and can sometimes, as in the Piperaceae family, have diagnostic significance for the family (Marinho et al. 2011). The secretory tissues of plants are usually classified according to the substances they produce. However, one and the same tissue often secretes a number of different substances (Fahn 2000). All of the above prompted us to further investigate the secretory structures of C. coloratum at the ultrastructural level. Light and electron microscopy of the leaf of C. coloratum was therefore performed with the aim of expanding the knowledge of the secretion of volatiles at the cellular level in general and to reveal the structures involved in the secretion of aromatic compounds of this endemic species.
Essential oils as natural mixtures of phytochemicals have a number of beneficial biological effects for the plant that produces them. Numerous studies have shown that plant volatiles have antioxidant, antimicrobial, antiviral, anticancer and numerous other biological effects that are being actively researched to exploit the natural potential of plants and replace the use of synthetic substances (Fabricant and Farnsworth 2001; Urmi et al. 2013). Several studies conducted with different species of the genus Chaerophyllum have confirmed their significant pharmacological potential (Dall'Acqua et al. 2004; Ebrahimisadr et al. 2017; Khajehie et al. 2017; Köse and Elvan 2018; Zengin et al. 2020; Celikezen et al. 2022; Adil et al. 2024). Stešević et al. (2016) stated that their work on C. coloratum represents a valuable contribution to the plant anatomical features related to the chemical composition of the genus Chaerophyllum, providing a solid basis for further studies in the field of bioactivity of plant secondary metabolites. We followed up on these results and went one step further to explore the bioactivity that might be related to the VOCs and phenolic composition of C. coloratum. Natural plant substances can effectively suppress the progression of cancer development and are considered less toxic to the human body than chemotherapeutic agents currently used to treat various types of cancer (Dehelean et al. 2021). Therefore, we investigated the antiproliferative activity of C. coloratum for the first time to find new resources for biologically active anticancer agents. The excessive use of synthetic pesticides and plant protection products is a problem that we are constantly confronted with. The development of new pesticides and the search for natural pesticides are therefore the subject of numerous studies. Viral infections of plants are part of this current global problem. As wild plants have developed their own strategies to cope with different environmental conditions and fight viruses and other pathogens, we can learn from them and develop new natural protective agents by following the metabolites they use to survive. As we have previously identified plant volatiles as metabolites that protect plants against viral pathogens (Vuko et al. 2019, 2022), we investigated the antiviral potential of C. coloratum on plant hosts infected with TMV.
The study of the phytochemical composition of plant volatiles, leaf ultrastructure and biological potential of C. coloratum points to new plant species and their potential, to which our attention must be devoted in the future.
Materials and methods
Plant material
The plant material (leaves of Cheaerophyllum coloratum L.) was collected in May 2019 in Majdan, Municipality of Klis, Croatia (43° 32′ 41.49″ N; 16° 31′ 6.74″ E) on degraded, stony pastures, habitat. The identity of the plant material was confirmed by PhD Juraj Kamenjarin based on the literature (Nikolić 2020). Voucher specimens of the plant material were deposited at the Faculty of Science, Department of Biology, University of Split, Split, Croatia. The plant material was air-dried in a single layer for two weeks and the mixture was packed in paper bags and stored in a dry place protected from light until hydrodistillation. The randomized mixture of fresh samples was used the day after harvest for HS-SPME analysis of volatiles.
Hydrodistillation
An amount of 20.00 g of the dried plant material was mixed with 500 mL of water in the flask of the Clevenger apparatus; then, 35 mL of water and 2 mL of pentane (VWR Chemicals, Radnor, PA, USA) were added to the inner tube of the Clevenger apparatus. After hydrodistillation for 3 h, the fractions of hydrophobic (essential oil) and hydrophilic volatile compounds (extracted in pentane (used as the solvent trap) and water fractions, respectively) were removed from the apparatus separately and stored at − 20 °C and + 4 °C, respectively.
Methanol extract
An amount of 1.00 g of dried plant material was freeze-dried and homogenized in 80% methanol–water followed by extraction in an ultrasonic bath at room temperature for 20 min. Then, 15 mL of 80% methanol–water was added and the mixture was centrifuged at 5000g for 5 min. The supernatant was transferred to a new Eppendorf tube, centrifuged again at 7000g for 20 min, transferred to a new tube, evaporated to dry in a rotary evaporator, dissolved in 10% DMSO and stored at − 20 °C until use.
Headspace solid-phase microextraction (HS-SPME)
DVB/CAR/PDMS (divinylbenzene/carboxene/polydimethylsiloxane) SPME fibre (Agilent Technologies, Palo Alto, Santa Clara, CA, USA) was set up on the PAL Auto Sampler System (PAL RSI 85, CTC Analytics AG, Schlieren, Switzerland) and conditioned according to the manufacturer's instructions. Approximately 1 g of the plant or hydrosol samples were added to the 20 mL glass vials, which were sealed with a stainless-steel cap and polytetrafluorethylene (PTFE)/silicone septa. The equilibration of the sample lasted 15 min at 60 °C. The extraction was then continued for 45 min. Thermal desorption lasted 6 min at an injection temperature of 250 °C directly into the GC column.
Gas chromatography–mass spectrometry analysis (GC–MS)
The VOCs isolated from C. coloratum were analysed using a gas chromatograph (Type 8890 Agilent Technologies) and a tandem mass spectrometer detector (Type 5977E MSD, Agilent Technologies). The VOCs were separated using an HP-5MS capillary column (30 m × 0.25 mm, 0.25 µm film thickness, Agilent Technologies). The conditions for GC–MS analysis and the procedure for compound identification were described in detail by Radman et al. (2022). The retention indices are determined using the retention times of the compounds of interest and comparing them to the retention times of a series of n-alkanes under the same chromatographic conditions. The values are compared to the reported ones in the literature from the National Institute of Standards and Technology (NIST). Their mass spectra were matched against the spectra from the NIST 17 (D-Gaithersburg) and Wiley 9 (Wiley, New York, NY, USA) mass spectral libraries. The percentage composition of the samples was determined using the normalization method without correction factors. The results for all samples were measured in three independent analyses and expressed as the average area percentage (%) of each compound.
NADI and Sudan III staining
Hand-cut cross sections of the leaf were treated with NADI reagent (0.5 mL of 0.1% α-naphthol (Merck KGaA, Darmstadt, Germany), 0.5 mL of 1% N,N-dimethyl-p-phenylenediamine (Merck), and 49 mL of 0.1 M sodium phosphate buffer, pH 7.2 (Merck) to detect terpenes. Sudan III (Merck) was used to detect lipids (Ivanova et al. 2023). The slides were observed using a light microscope (Leica DM 3000 LED, Leica Microsystems, Wetzlar, Germany) and the images were captured with Leica DMC 4500 camera and image processing software (Leica Application Suite, LAS 4.9). All microphotgraphs were prepared Using Adobe Photoshop (Adobe, San Jose, CA, USA).
Transmission electron microscopy (TEM)
The leaf tissue samples of C. coloratum were fixed in 2.5% glutaraldehyde (in 0.1 M phosphate buffer solution, PBS) for two hours at 4 °C and then postfixed in 1% OsO4 for two more hours. After postfixation, the samples were washed twice in distilled water for 10 min. En bloc staining with 2% uranyl acetate was performed overnight at room temperature. An ascending series (30–100%) of acetone was used for dehydration. After dehydration, the samples were impregnated with 1:3 Spurr resin (SPI Supplies, West Chester, PA, USA)/100% acetone, then in 1:1 Spurr resin/100% acetone and finally in 3:1 Spurr resin/100% acetone; each step for 30 min. The samples were kept in pure Spurr resin for one hour and then embedded using silicone molds and left for 48 h at 60 °C. Tissue sections were first cut at 0.5 μm (RMC Boeckeler, PowerTome XL, ultramicrotome) and stained with Toluidine blue O (ThermoFisher Scientific Inc., Waltham, MA, USA) to choose the area of interest. The semithin sections were observed under light microscope Leica DM 3000 LED and the images were captured with Leica DMC 4500 camera. The ultrathin sections were cut at 0.06 μm and were contrasted with uranyl acetate and Reynold’s solution. Transmission electron microscope JEM JEOL 1400 Flash (JEOL Ltd., Tokyo, Japan) was used for the observation of ultrathin sections.
Cytotoxic activity
The cytotoxic activity of the methanolic extract of C. coloratum on three cancer cell lines, the human cervical cancer cell line (HeLa), the human colon cancer cell line (HCT116) and the human osteosarcoma (U2OS) as well as on a healthy cell line, the retinal pigmented epithelial cells (RPE1) (the cells were donated by Prof. Janoš Terzić from the School of Medicine, University of Split) was performed using the MTS-based CellTiter 96® Aqueous Assay (Promega, Madison, WI, USA). Cells were grown in a CO2 incubator at 37 °C and 5% CO2 until they reached 80%, whereupon they were counted using an automated handheld cell counter (Merck). Approximately 5000 cells were seeded in 96-well plates and treated with serially diluted extracts. The cells were grown for a further 48 h. Then 20 µL of MTS tetrazolium reagent (Promega) was added to each well and left in the incubator at 37 °C and 5% CO2 for 3 h. The absorbance was measured at 490 nm using a 96-well plate reader (Infinite M Plex, Tecan, AG, Switzerland). IC50 values were calculated from three independent experiments and four replicates of each concentration.
Antiviral activity
Plants of Nicotiana tabacum L. cv. Samsun that were systemically infected with TMV were used to prepare the viral inoculum. A piece of the leaf was crushed with a pestle and a phosphate buffer (0.06 M, pH 6.979) at a concentration that resulted in 5–20 lesions per inoculated leaf of the local host plant, Datura stramonium L. Care was taken to ensure that the test plants were as uniform in size as possible. Hydrosol was applied as a spray solution to the leaves of the local host plants on two consecutive days before virus inoculation. On the second treatment day, one hour after spraying, the test plants were rubbed with a virus inoculum prepared as described above. The leaves were dusted with silicon carbide (Sigma-Aldrich, St. Louis, MO, USA) prior to virus inoculation and washed with distilled water after inoculation The antiviral activity of the hydrosol was evaluated on the fourth day after inoculation when clear and visible local lesions had developed. The number of local lesions on the leaves of treated and control plants was compared as described by Vuko et al. (2019). All data are expressed as mean ± SD (n = 3). Statistical significance was determined using a t test.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 10 (GraphPad Software, Inc., Boston, MA, USA).
Results and discussion
Composition of VOCs obtained by hydrodistillation—essential oil
The percentage of compounds identified in the essential oil obtained by hydrodistillation from the air-dried leaves was 92.75% of the total compounds detected, with the sesquiterpenes group dominating at 41.75% (Fig. 1).
Fig. 1.

VOCs of Chaerophyllum coloratum essential oil sorted by structural groups
The oxygenated sesquiterpene spathulenol (14.65%) was the most abundant of all constituents (Suppl. Table S1). The dominance of oxygenated sesquiterpenes was also evident in the overall percentage: 85% of all identified sesquiterpenes were oxygenated (35.62% of all identified compounds) (Fig. 2b). This was mainly due to the high abundance of spathulenol and caryophyllene oxide (12.94%). Salvial-4(14)-en-1-one (1.50%), humulene epoxide II (1.30%), and cadina-1(10),4-dien-8α-ol (1.69%) were detected in significant amounts. The dominance of spathulenol was also found in the essential oil of the leaves of C. coloratum (10.19%) (Stešević et al. 2016), C. macropodum (10.4%) (Ebrahimabadi et al. 2010), but also in high amounts in the essential oil of the fruits of C. macropodum (7.3%) (Başer et al. 2006) and in the essential oil of the seeds of C. macrospermum (8.8%) (Razavi and Nejad-Ebrahimi 2010). A high proportion of caryophyllene oxide was found in the essential oil of C. aksekiense (6.0%) from Turkey (Başer et al. 2000), C. bulbosum (6.6%) from Iran (Masoudi et al. 2011) and in the essential oil of the fruits of C. coloratum (6.93%) was the dominant compound (Stešević et al. 2016). Humulene epoxide II (7.8%) was the second most abundant constituent in the essential oil of C. aksekiense (7.8%) (Başer et al. 2006).
Fig. 2.
The distribution of non-oxygenated and oxygenated monoterpenes (a) and sesquiterpenes (b) of Chaerophyllum coloratum. The first value is the proportion of the total identified components, the second value is the proportion of monoterpenes/sesquiterpenes among terpenes
Aliphatic compounds were the second most abundant group, among which 98% were saturated compounds (26.49% of all identified compounds). The saturated alcohol dodecan-1-ol (13.18%), the aldehyde tetradecanal (9.22%) and the alcohol tetradecan-1-ol (2.18%) were the most abundant. In the previous studies of various Chaerophyllum species, aliphatic compounds were also discovered among a variety of terpenes (Başer et al. 2000, 2006; Ağalar et al. 2021).
The distribution of non-oxygenated and oxygenated monoterpenes (14.52%) was different from the distribution in the sesquiterpenes group (Fig. 2a). 64% of the monoterpenes were non-oxygenated (9.22% of all identified compounds), with p-cymene (5.13%), limonene (1.55%) and β-pinene (1.03%) being the most important. The most important compound in the essential oil of C. macropodum from Turkey was p-cymene (39.3%) (Başer et al. 2000), but it was also among the dominant components in the essential oil of C. macrospermum (14.3%) from Iran (Razavi and Nejad-Ebrahimi 2010), C. villosum (10.0%) from India (Joshi 2013a, b) and in the essential oil of C. coloratum (16.53%, flowers; 7.60%, leafs; 6.70%, stems) from Montenegro (Stešević et al. 2016). p-Cymene as well as limonene were among the dominant compounds in C. aureum from Serbia (Lakušić et al. 2009). Limonene was the second most abundant compound in C. libanoticum (15.9%) from Turkey (Demirci et al. 2007), and very abundant in C. macropodum (leaf 6.7%, flower 12.0%) from Iran (Nematollahi et al. 2005), C. crinitum (5.8%) from Turkey (Hayta and Celikezen 2016) and C. temulum (5.4%) from Serbia (Stamenković et al. 2015). Only four diterpenes (2.71%) were identified, 67% of which are oxygenated. (E)-Phytol, a diterpene alcohol, was the dominant constituent at 1.41%. Phytol was found to be one of the dominant components in the stem essential oil of C. temulum with a greater amount during flowering (Stamenković et al. 2015).
Among the benzene derivatives (5.00%), myristicin (2.69%) and benzyl benzoate (1.43%) dominated. Myristicin (18.9%) was characterised as the second most abundant component in C. macrospermum essential oil from Turkey (Ağalar et al. 2021).
The other compounds (1.66%) were mainly esters and a C13-norisoprenoid β-ionone.
Headspace composition of the VOCs isolated by HS-SPME
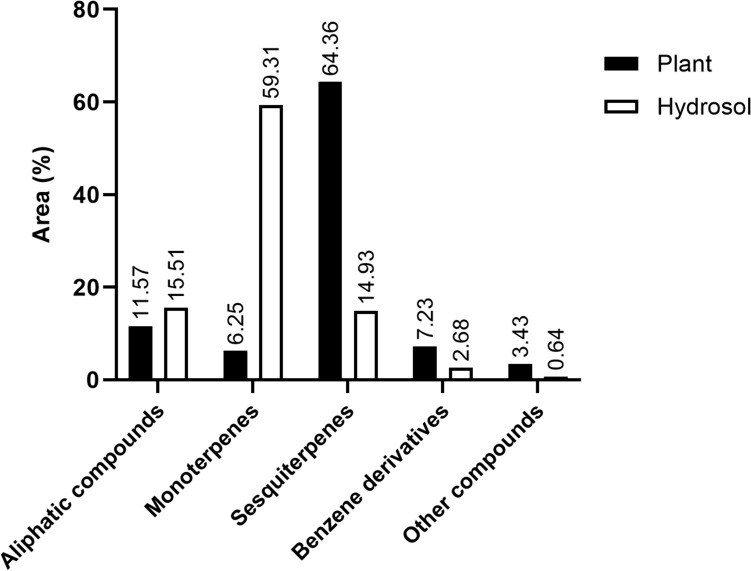
The samples of fresh plant (HS-P) and hydrosol obtained by hydrodistillation of air-dried plant (HS-H), were isolated by HS-SPME using divinylbenzene/carboxene/polydimethylsiloxane (DVB/CAR/PDMS). In HS-P 92.85% and in HS-H 93.07% of the total VOCs detected were identified. The classification of the identified compounds was based on five structural groups: aliphatic compounds, monoterpenes, sesquiterpenes, benzene derivatives and other compounds (Fig. 3).
Fig. 3.
VOCs of Chaerophyllum coloratum plant and hydrosol isolated by HS-SPME, sorted by structural groups
Plant
Sesquiterpenes (64.36%) were the dominant group, with non-oxygenated sesquiterpens accounting for 81% of the detected sesquiterpens (51.88% of total compounds) in HS-P (Fig. 2b). This was due to β-caryophyllene (32.39%) and germacrene D (8.69%) (Suppl. Table S2). The most abundant oxygenated sesqiterpenes were spathulenol (3.45%) and caryophyllene oxide (5.52%).
Of the monoterpenes (6.25%), 67% belong to the non-oxygenated monoterpenes (4.17% of the total compounds) (Fig. 2a). The most abundant of them was limonene (3.76%). Petrović et al. (2017) analysed the headspace composition of roots, shoots and flowering parts of C. hirsutum from Serbia and recorded monoterpenoids as dominant compounds in all samples.
Aliphatic compounds accounted for 11.57% of the total compounds, with the unsaturated ketone oct-1-en-3-one (3.98%) and unsaturated alcohol (2.07%) being the most abundant. Benzaldehyde (2.34%) and benzyl alcohol (1.80%) were one of the most abundant compounds in the benzene derivatives group (7.23%). One alcohol, two esters and norisoprenoids (C9 and C13) were in the group of other compounds (3.43%).
Hydrosol
In HS-H, monoterpenes dominated (59.31%) (Fig. 3), with the oxygenated monoterpene p-cymen-8-ol (26.94%) being the most abundant compound (Suppl. Table S2), which was not detected in HS-P but was present in 4.03% in essential oil (Suppl. Table S1). Berbenone (7.47%) and terpinen-4-ol (4.25%) were two abundant oxygenated monoterpenes that contributed significantly to the dominance of oxygenated monoterpenes in HS-H, where they accounted for 75% (11.21% of total compounds). p-Cymen (4.04%) and α-phellanderene (2.10%) were two predominant non-oxygenated monoterpenes. Studies have shown that up to 30% of essential oil compounds can be found in the hydrosol once hydrodistillation is complete (Jensch and Strube 2022). These are mainly oxygenated terpenes (Aćimović et al. 2020). In the group of sesquiterpenes 75% were oxygenated sesquiterpenes (11.21% of the total compounds). Spathulenol (7.60%) and carophyllene oxide (1.07%) were the most abundant.
Light and electronmicroscopy of the leaf of Cheaerophyllum coloratum L.
To date, no in-depth studies have been conducted on the oil secretion by C. coloratum. Essential oils can be produced in external and internal secretory structures which include secretory glandular trichomes, secretory cavities, ducts, and individual essential oil cells (Fahn 2000). In this study, in addition to Sudan III, NADI and toluidine blue staining in conjunction with light microscopy, transmission electron microscopy was used to investigate the secretory structures of the leaves, the synthesis and accumulation of the essential oil of C. coloratum. The results were compared with studies on oil-secreting structures of other aromatic plants to shed light on this endemic species, its biology and the potential hidden in its bioactive products.
Hand-cut transverse sections of C. coloratum leaves were stained with NADI reagent to detect terpenes and Sudan III to detect lipids. The NADI reagent produces a differentiated staining for the detection of the different terpene classes. The staining is blue for essential oils and red for resins (diterpenes, triterpenes and derivatives) (Ivanova et al. 2023). The NADI reaction resulted in blue staining of the epidermal cells and secretory cells associated with the ducts, confirming the presence of essential oil (Fig. 4a, b). In the Sudan III test, in addition to the red–orange staining of the cuticle, the lipid nature of the secretory ducts was confirmed by positive red–orange staining of the epitelial cells grouped as a layer around the duct and of the secretory epidermal cells (Fig. 4c, d). In addition, numerous red–orange lipid droplets were observed in the leaf mesophyll cells near the duct (Fig. 4c). Due to the lipophilic nature of the essential oil compounds, their presence was confirmed in both analyses. In the histochemical analysis of E. tenuifolia subsp. sibthorpiana, internal secretory ducts and secreted droplets in the leaf and stem were stained with reagents NADI and Sudan III, confirming the presence of essential oil (Ivanova et al. 2023).
Fig. 4.
Leaf sections of Chaerophyllum coloratum histochemically stained for terpenes with NADI reagent (a, b) and lipids stained with Sudan III (c, d). ue, upper epidermis; le, lower epidermis; sc, epithelial (secretory) cells around the duct; dt, non-glandular trichome; c, cavity of the duct; arrowheads (black and white), NADI staining; arrows, Sudan III staining
Unicellular and non-glandular trichomes on the leaf epidermis of C. coloratum were previously described by Stešević et al. (2016). On semi-thin leaf sections of C. coloratum obtained with a microtome, we observed the presence of glandular trichomes (Fig. 5a). The round glandular cell at the distal end is connected to the epidermis by a non-glandular, flattened cell, the basal cell. The cells involved in secretion are usually characterized by small vacuoles and a relatively dense cytoplasm containing numerous mitochondria, and the abundance of other cell compartments and organelles varies depending on the substance secreted (Fahn 2000; Caissard et al. 2004). In the glandular cell of C. coloratum, dense cytoplasm and numerous vesicles as well as a reddish content were observed. The corresponding reddish content is abundant on the upper surface of the epidermis and its occurrence on the leaf surface is consistent with the reddish content in the subcuticular space and within the secretory epidermal cells (Fig. 5a, b). In addition to the glandular trichomes, elongated, unicellular, non-glandular trichomes were observed on both leaf surfaces of C. coloratum in agreement with the work of Stešević et al. (2016) (Figs. 4a–c, 5c).
Fig. 5.
a–f Semithin sections through the C. coloratum leaf, Toluidine blue. ue, upper epidermis, le lower epidermis, pc parenchymal cells, sec secretory epidermal cell, gt grandular trichome, ngt non-glandular trichome; arrowheads, secretory content within the glandular trichome, in subcuticular space, in secretory epidermal cells and on the leaf surface; xy xylem, ph phloem; arrow, secretory content inside the cavity, c cavity of the duct, sc epithelial (secretory) cells around the duct
Secretory cells are also observed in the epidermal layer on the upper leaf surface (Fig. 5a, b, d). These cells are squeezed between epidermal cells, as shown on TEM micrographs (Fig. 6a). In addition to small vacuoles, a dense cytoplasm and numerous mitochondria, sometimes leucoplasts, plastoglobules and unusual structures of reticulum or dictyosomes, like periplastidal reticulum, smooth tubular reticulum and osmiophilic vesicules or cisternae are sometimes observed in secretory tissues (Caissard et al. 2004). The secretory epidermal cells of C. coloratum contain numerous plastids and periplastidal reticulum surrounded by vesicles and oil bodies, as well as abundant ER cisternae that accumulate in the apical cytoplasm towards the subcuticular space. Plastids (leucoplasts) closely surrounded by ER cisternae and secretory vesicles of different sizes in the cytoplasm of the secretory cells of C. coloratum are directly involved in secretion (Fig. 6a, b). As the secretory process progresses, the number of plastids increases and they become surrounded by endoplasmic reticulum cisternae (Bosabalidis 1996). The involvement of plastids, especially leucoplasts, in the secretion of monoterpenes has also been demonstrated biochemically (Ascensão and Pais 1998). It is also known that the large amounts of smooth endoplasmic reticulum may play a role in the synthesis and transport of sesquiterpenes and steroids or in the conversion of phenylalanine to cinnamic acid for the synthesis of phenylpropanoids (Ascensão and Pais 1998). We assume that secretory epidermal cells secrete volatiles into the subcuticular space before they penetrate the cuticle and reach the leaf surface (Fig. 6a) based on the observation that the osmiophilic material in the cytoplasm and lumen of the ER cisternae has the same appearance as the osmiophilic substance in the subcuticular space and on the outer surface of the leaf (Fig. 6a–c). It is known that essential oils can be stored in a subcuticular space that expands during secretion and is then released by rupturing the cuticle (Caissard et al. 2004). Volatiles that transiently accumulate in the subcuticular space of glandular trichomes of L. leonurus are probably released via cuticular micropores, as described by Ascensão and Pais (1998). Volatiles of C. coloratum are observed as osmiophilic substances with high electron density and gray oil droplets (oil bodies) which were caught in the cuticle as well as on the leaf surface (Fig. 6b, c). This proposed pathway of C. coloratum volatiles can be compared with similar observations by Wei et al. (2019), who describe the developmental process of the secretory epidermal cells of the petala of Rosa rugosa and the associated secretory pathway. It is also known that volatiles can be stored in conjugated form in vacuoles or in specialized channels; precursors that are deconjugated to release volatiles and lytic enzymes are mixed with the contents of these storage compartments (Baldwin 2010). As already described for other aromatic plant species, the thickness and ultrastructure of the cuticle are not involved in the permeability for volatiles, but the chemical composition is (Caissard et al. 2004). Since volatiles have been observed on the leaf surface of C. coloratum, tightly enclosed in membrane vesicles (Fig. 6c), we assume according to Paiva (2016) that the nature of their secretion to the outside is granulocrine.
Fig. 6.
a–d Ultrathin sections through the C. coloratum leaf. a ec epidermal cell, sec secretory epidermal cell, cu cuticle, scs subcuticular space, er endoplasmic reticulum, p plastid, vocs volatile organic compounds; arrowhead, lighter secretory content; *, osmiophylic secretory content. b The enlargement of the part of the secretory epidermal cell shown on the a. ob oil body. c White arrowheads show grayish osmiophilic content, and asterisks show osmiophilic secretory content in the cuticular space. Inset: a higher magnification of the leaf surface area. d sc, epithelial (secretory) cells around the duct; c, cavity of the duct; yellow arrowhead, cell wall protuberance
The secretory ducts in the plant family Apiaceae are a typical example of shizogenic cavities (Ivanova et al. 2023). The lumen of these structures is usually lined with several layers of secretory cells. The innermost layer is the most active in the process of secretion (Fahn 2000). Secretory ducts described in C. coloratum by Stešević et al. (2016) are also observed on semithin and ultrathin sections of the leaf (Figs. 5e, 6d). These cavities are arranged near the phloem part of the vascular bundle in a central midrib (Fig. 5e), but are also located among the mesophyll cells, accompanying the vascular bundles (Fig. 5f). The cavities are surrounded by secretory cells with osmiophilic content that passes from the secretory cells into the cavity. These osmiophilic substances are abundant along the wall protuberance of the secretory cell towards the cavity (Fig. 6d). As described above, the common ultrastructural feature of cells secreting a lipophilic substance is the presence of electron-dense material in the plastids. In the centre of the cavity, grayish oil bodies outlined by electron-dense droplets are visible (Fig. 6d). This is similar to grayish osmiphilic oil droplets and clumpy black osmiophilic substance without obvious boundaries described in the secretory epidermal cells of rose petals (Wei et al. 2019). As the authors describe, these droplets are processed into black grains with a high electron density. The electron density of the oil bodies in the cavity of C. coloratum corresponds to that of the oil bodies in the secretory epidermal cells (Fig. 6b, d). In the resin ducts of Magnifera indica L., the lipophilic droplets associated with the dictyosome also appeared less osmiophilic than those in the plastids and ER, and both types of droplets were also found outside the plasmalemma (Fahn 2000). The occurrence of osmiophilic material with different electron densities in one and the same cell suggests that different components of the lipophilic material are synthesized by different cellular compartments.
Based on a detailed analysis of the secretory structures of the leaf of C. coloratum we concluded that the secretory activity of this plant is mediated by glandular trichomes, secretory epidermal cells and secretory ducts resulting in an efficient secretory system that produces volatiles to enable efficient adaptation and rapid response to environmental conditions.
Cytotoxic activity of Cheaerophyllum coloratum L.
Great efforts are performed to investigate the potential therapeutic effects of oils against several diseases especially those characterized by excessive cell growth and proliferation such as cancer or bacterial infections (Ivanova et al. 2023). Natural products could play an important role in the development of new chemotherapeutic agents that might be less toxic than current chemotherapy, which has severe side effects. Since we have already established that methanolic extracts of some aromatic plants have cytotoxic potential against cancer cells, we found it interesting to test this activity of C. colaratum extract (Vuko et al. 2021a, b, 2022). Therefore, we performed the first in vitro study of the cytotoxic effect of the methanolic extract of C. coloratum on three cancer cell lines, cervical cancer cell line (HeLa), colon cancer (HCT116) and osteosarcoma cell line (U2OS), as well as on the healthy retinal pigmented epithelial cells (RPE1), using the MTS assay. The results showed that this plant extract has significant cytotoxic activity on cancer cell lines and that it is significantly less toxic to the healthy cell line RPE1. C. coloratum showed higher toxicity on HeLa cells (IC50 = 151, 35 µg/mL) than on U2OS and HCT116 cells (IC50 = 256.45 µg/mL; IC50 = 298.34 µg/mL) (Fig. 7). It is important to emphasize that the extract has low toxicity to the healthy cell line RPE1 (IC50 = 677.40 µg/mL) compared with cancer cells. To date, there is no information on the cytotoxic activity of this aromatic plant species, but there are limited data on the cytotoxic activity of other species of the genus Chaerophyllum, such as C. villosum (Adil et al. 2024), C. macropodium (Celikezen et al. 2022), C. macrospermum (Zengin et al. 2020), and C. hirustum (Dall'Acqua et al. 2004). Adil et al. (2024) investigated the cytotoxic activity of methanolic, chloroform and aqueous extracts of C. villosum using brine shrimp lethality assay. The extracts showed a dose-dependent toxicity for brine shrimp. The chloroform extract was the most toxic (96.67%) to brine shrimp with an LD50 value of 14.81 µg/mL. The authors believe that the toxicity of C. villosum is due to the richness of cytotoxic compounds in the extracts such as saponins, alkaloids, tannins and flavonoids (Huang et al. 2012; Mungenge et al. 2014). Celikezen et al. (2022) showed that aqueous extracts of C. macropodium had significant cytotoxic effects on neuroblastoma cells at all concentrations tested. Of note, the extract showed no toxicity toward the human fibroblast cell line. This was the first study to show that C. macropodium has a selective cytotoxic effect on neuroblastoma cells without affecting healthy human fibroblasts. The authors hypothesized that the cytotoxic effects of C. macropodium species are due to phenolic compounds that have been shown to have anticancer properties. It was reported that o-coumaric acid and its compounds are able to inhibit the growth of glioblastoma cells as well as lung and colon cancer cells (Nasr Bouzaiene et al. 2015; Gutiérrez Mercado et al. 2022). In addition to coumaric acid, the anticancer activity of other dominant compounds in plants of the genus Chaerophyllum, such as rosmarinic and chlorogenic acids, has been demonstrated (Hou et al. 2017; Ma et al. 2020). The chloroform extract of C. hisutum showed cytotoxic activity against the human intestinal adenocarcinoma (LoVo) cell line (Dall'Acqua et al. 2004), while C. macrospermum was more toxic to human hepatocarcinoma cells (HepG2) than to healthy murine bone marrow stromal cells (S17) (Zengin et al. 2020).
Fig. 7.

Cytotoxic activity of the methanolic extract of C. coloratum on HeLa, HCT116, U2OS and RPE1 cells using the MTS cell proliferation assay. Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparisons test. IC50 values are means of three independent experiments performed in groups of four ± SD (standard deviation), and statistical significances are labelled *P < 0.05 and ***P < 0.001
Although there are only a few reports on the cytotoxic activity of the genus Chaerophyllum, a considerable number of papers were found on the biological activity of other Apiaceae species. Vuko et al. (2022) showed that the methanolic extract of Portenschlagiella ramosissima moderately inhibited the growth of HeLa, HCT116 and U2OS cell line. Studies by Fredotović et al. (2020) on the same cell lines HeLa, HCT116 and U2OS showed a significant cytotoxic activity of the methanolic extract of two onions A. × cornutum (IC50 values for Hela 24.15 µg/mL, HCT116 24.73 µg/mL and U2OS 47.8 µg/mL) and A. cepa (IC50 values for HeLa 24.79 µg/mL, HCT116 25.97 µg/mL and U2OS 36.6 µg/mL). Compared to the results of the present study, these extracts were more effective in inducing cell death. Some recent studies with extracted polyphenols from the olive varieties Leccino and Istrarska Bjelica, treated and nontreated with silicon, showed significant cytotoxic activity against the cell lines HeLa, HCT116 and U2OS. The extracted polyphenols showed comparable inhibition of HeLa cell growth (IC50 values of 239.689 µg/mL for Leccino, 175.142 µg/mL for Leccino with Si, 175.142 µg/mL for Istarska Bjelica) as the methanolic extracts of C. coloratum (IC50 = 151.35 µg/mL). HCT116 was the most sensitive cell line (IC50 values for the extracted polyphenols varied from 95.497 µg/mL to 36.075 µg/mL). The cytotoxic activity of the extracts on the U2OS cell line was similar to that on HCT116 (Pasković et al. 2024). The essential oil of Tropaeolum majus L. showed extremely high cytotoxic activity on HeLa, HCT116 and U2OS cells with IC50 values below 5 µg/mL (IC50 values for HeLa 4.91 µg/mL, for HCT116 1.49 µg/mL and for U2OS 4.53 µg/mL) (Vrca et al. 2023). Cytotoxic effects of five traditionally cultivated species of the Apiaceae family, Falcaria vulgaris, Smyrniopsis aucheri, Smyrniopsis munzurdagensis, Smyrnium cordifolium, and Actinolema macrolema were tested on murine macrophage (RAW 264.7), human embryonic kidney (HEK 293), and human hepatocellular carcinoma (HepG2) cell lines. Methanolic extracts of most tested species showed no toxicity in all tested cell lines, with the exception of Falcari a vulgaris and Smyrniopsis munzurdagensis, suggesting that further testing of their biological activity, as well as analyses of the activity of phytochemicals contained in species of the Apiaceae family, is needed (Zengin et al. 2019). Heo et al. (2009) showed that the methanolic extract of Angelica gigas strongly inhibited the growth of breast cancer cells. The authors hypothesize that the bioactive compounds present in the extract, mostly polyphenols, are mainly responsible for inhibiting tumor growth, but further research is needed on the potential of bioactive compounds that could be used for pharmaceutical and medicinal purposes. The results of the current study show that C. coloratum can be considered a promising source of bioactive compounds with selective cytotoxic activity that could be used in the future for the development of natural chemotherapeutics.
Antiviral activity of Cheaerophyllum coloratum L.
In a world where we are struggling with climate change and the resulting plant diseases and pest infestations, lower crop yields and a growing population, crop improvement is essential to reduce the overuse of synthetic pesticides and crop protection products. Crop protection programs are constantly striving to increase crop resistance and tolerance to diseases and pests, thereby increasing crop yields. We have focused our attention on under-researched plants as renewable sources of compounds with positive effects on humans and the environment. We hypothesize that aromatic plants and their volatile compounds can help us find out how wild plants cope with environmental and pathogen challenges. We believe that there is a hidden potential in the plants of the eu-Mediterranean zones, which are often exposed to harsh weather conditions such as excessive sunlight, prolonged drought and poor soils, that helps them to defend themselves against environmental influences with their specialized metabolites and to survive despite the above-mentioned factors. Viruses are important plant pathogens that attack agricultural crops, and the search for new antiviral agents is particularly welcome for ecological reasons. Therefore, we wanted to investigate whether the volatiles of C. coloratum could have an impact on plant protection against viral pathogens. We focused on TMV, a model virus in the history of plant virology, since we still do not have adequate protection against TMV and burning the plants is the only solution in case of infection. The results of the efficacy of the hydrosol of C. coloratum on local host plants infected with TMV are shown in Fig. 8. The hydrosol applied to the leaves of the host plants before virus inoculation significantly reduced the number of local lesions. The treated plants were compared with the corresponding control groups. On average, the hydrosol-treated plants developed 4.97 lesions per leaf compared to 9.27 lesions per leaf in the control plants. A promising inhibition of local infection of 46.40% confirmed our hypothesis about the antiviral potential of C. coloratum volatiles. Based on the results presented, we concluded that hydrosol has antiviral activity and may activate the plant defense response and increase plant resistance to viral pathogens. This activity of C. coloratum hydrosol could be mediated by its volatile components, namely p-cymene-8-ol as the main component as well as by berbenone, (3E,5Z)-2,6-dimethylocta-1,3,5,7-tetraene, p-cymene and terpinen-4-ol, which were also detected as very abundant components in hydrosol (Suppl. Table S2). Synergistic activity with other, less represented compounds is also a possible mode of antiviral action, as both monoterpenes and sesquiterpenes have been described as antiviral compounds (Vuko et al. 2019). Various biological activities of plant volatiles have already been described, for example, p-cymene is one of the main components of extracts and essential oils used in traditional medicine as antimicrobial agents (Marchese et al. 2017). Spathulenol is also known as an antimicrobial agent and terpinen-4-ol provides the broad antimicrobial activity and anti-inflammatory properties of tea tree oil (Cordeiro et al. 2020; Elkiran et al. 2023). In addition, the bark oil of Protium confusum with p-cymen-8-ol as the main component showed larvicidal activity against Aedes aegypti (Santana et al. 2009).
Fig. 8.
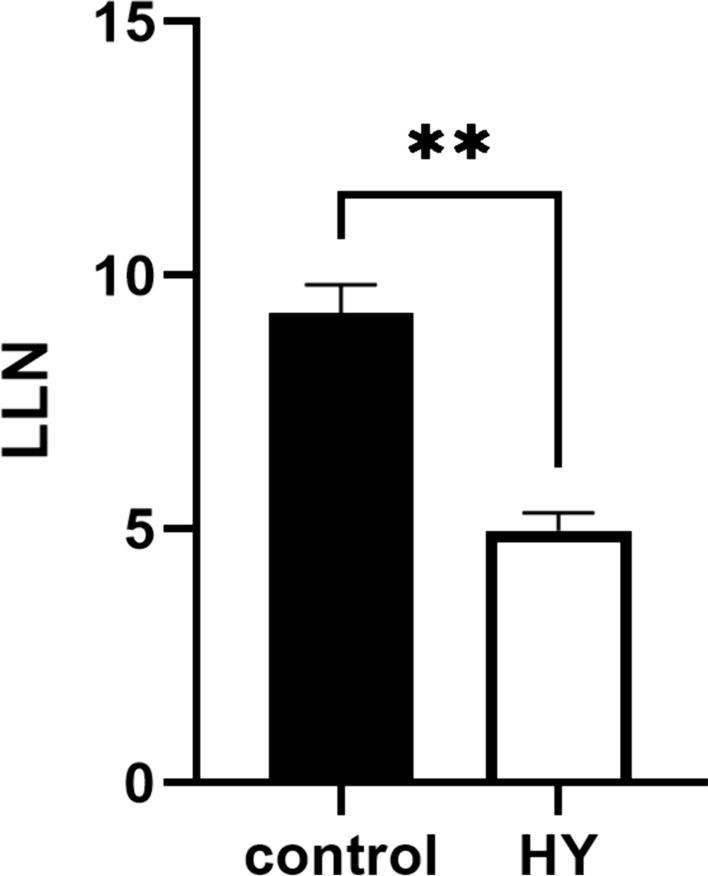
Number of local lesions (LLN) on the plants treated with hydrosol of C. coloratum for two consecutive days before TMV inoculation. Control plants were treated with distilled water. The error bars show the standard deviation of the triplicate analyses. **Statistically significant difference between control and treatment data (P < 0.01)
These effects of secondary metabolites of aromatic plants are a promising area that deserves further investigation of Chaerophyllum and other aromatic species and the potential use of volatiles in plant protection. Although they have not been tested as antiviral compounds, aromatic Apiaceae plants and their compounds have a different biological potential according to literature data. Anethum graveolens L. (dill) could be used as a biopesticide (Najafzadeh et al. 2019), while caraway (Carum carvi L.), which contains carvone, has been used in agriculture as a sprout inhibitor (Bouwmeester and Kuijpers 1993), antifungal agent and insect repellent (Toxopeus and Bouwmeester 1992). Our results on antiviral activity thus complement the so far sparse knowledge on the biological potential of C. coloratum, which to our knowledge mainly relates to its antibacterial activity (Božović et al. 2009). This promising activity should be further investigated to clarify how plants cope with viral pathogens. Although chemical substances and pesticides are still irreplaceable in the control of plant diseases due to their ease of use and economic benefits, we must not stop looking for natural resources as environmentally friendly means of eradicating and/or preventing various pathogens.
Conclusion
For the species Chaerophyllum coloratum L. (Apiaceae), new data on the volatile composition, leaf ultrastructure and biological potential are presented, showing that endemic plants and their volatile products are not yet sufficiently studied. The essential oil is dominated by oxygenated sesquiterpenes, the hydrosol by monoterpenes and the fresh plant material by non-oxygenated sesquiterpenes. Light and electron micrographs of the leaf show secretory ducts located near vascular elements in the vascular bundle. On the leaf surface, there are unbranched, air-filled, non-glandular trichomes and glandular trichomes with volatile contents. In addition to glandular trichomes, the secretory cells located not only around the secretory duct but also between the cells of the upper epidermis, are responsible for the production of volatiles of C. coloratum. Initial data on the bioactivity of C. coloratum show that the plant is a promising source of compounds with selective cytotoxic activity that could be used in the future for the development of natural chemotherapeutics. Its volatile compounds are effective as natural antiviral agents, as plants treated with hydrosol prior to virus inoculation developed a reduced number of local lesions. Our results dealing with the composition, ultrastructure and biological activity of C. coloratum show that the Apiaceae are still far from being explored and that they still represent a hidden potential for the future.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The CO2 incubator EC 160 (Nuve), Microbiological protection cabinet type KTB-NS II Bio protection class II according to EN12,469 (Klimaoprema), and the Leica DMIL LED Inverted Routine Microscope (Leica Microsystems) used for cell culture and the Infinite M Plex plate reader (Tecan), used for measuring absorbance for calculating cytotoxic activity and JEOL JEM 1400 Flash transmission electron microscope were procured as part of the project “Functional integration of the University of Split through the development of scientific research infrastructure in the building of the three faculties”, funded by the European Commission through the European Regional Development Fund.
Abbreviations
- HS-H
Headspace composition of volatile organic compounds isolated by HS-SPME from hydrosol
- HS-P
Headspace composition of volatile organic compounds isolated by HS-SPME from fresh plants
- HS-SPME
Headspace solid-phase microextraction
- TMV
Tobacco mosaic virus
- VOCs
Volatile organic compounds
Author contributions
All authors contributed to the study's conception and design. EV—conceptualization, methodology, data analysis, writing—original draft preparation, writing—review and editing, supervision. SR—methodology, conducted GC–MS and HSPME analysis, writing—original draft preparation. Ibo—methodology, conducted electron microscopy, electron micrographs editing, data analysis, writing—review and editing. JK—methodology, resources, harvesting and identification of plant material. Ibe—conducted antiviral activity, and data analysis. ŽF—methodology, conducted cytotoxic activity, visualization, writing—review and editing, supervision. All authors read and approved the manuscript.
Funding
This study was funded by Institutional support of the Faculty of Science, University of Split.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aćimović M, Tešević V, Smiljanić K, Cvetković M, Stanković J, Kiprovski B, Sikora V (2020) Hydrolates:by-products of essential oil distillation: chemical composition, biological activity and potential uses. Adv Technol 9(2):54–70. 10.5937/savteh2002054a [Google Scholar]
- Adil M, Dastagir G, Ambrin SAA, Rahim F, Quddoos A, Filimban FZ, Izhar Ul H (2024) Cytotoxic, phytotoxic and insecticidal potential of Achillea millefolium L. and Chaerophyllum villosum wall. ex dc. Braz J Biol 84:e262479. 10.1590/1519-6984.262479 [DOI] [PubMed] [Google Scholar]
- Ağalar HG, Altintaş A, Demirci B (2021) The essential oil profiles of Chaerophyllum crinitum and C. macrospermum growing wild in Turkey. Nat Volatiles Essent Oils 8(1):39–48 [Google Scholar]
- Ascensão L, Pais MS (1998) The leaf capitate trichomes of Leonotis leonurus: histochemistry, ultrastructure and secretion. Ann Bot 81(2):263–271. 10.1006/anbo.1997.0550 [Google Scholar]
- Baldwin IT (2010) Plant volatiles. Curr Biol 20(9):R392–R397. 10.1016/j.cub.2010.02.052 [DOI] [PubMed] [Google Scholar]
- Başer KHC, Tabanca N, Özek T, Demirci B, Duran A, Duman H (2000) Composition of the essential oil of Chaerophyllum aksekiense A. Duran et Duman, a recently described endemic from Turkey. Flavour Fragr J 15(1):43–44. 10.1002/(SICI)1099-1026(200001/02)15:13.0.CO;2 [Google Scholar]
- Başer KHC, Özek G, Özek T, Duran A (2006) Composition of the essential oil of Chaerophyllum macropodum Boiss. fruits obtained by microdistillation. J Essent Oil Res 18(5):515–517. 10.1080/10412905.2006.9699157 [Google Scholar]
- Bosabalidis AM (1996) Ontogenesis, ultrastructure and morphometry of the petiole oil ducts of celery (Apium graveolens L.). Flavour Fragr J 11(5):269–274. 10.1002/(sici)1099-1026(199609)11:5%3c269::aid-ffj584%3e3.0.co;2-z [Google Scholar]
- Bouwmeester HJ, Kuijpers A-M (1993) Relationship between assimilate supply and essential oil accumulation in annual and biennial caraway (Carum carvi L.). J Essent Oil Res 5(2):143–152. 10.1080/10412905.1993.9698193 [Google Scholar]
- Božović M, Damjanović-Vratnica BŠ, Perović S (2009) Chemical composition and antibacterial activity of essential oil of Chaerophyllum coloratum L. (Apiaceae). Paper presented at the 2nd Symposium of Chemistry and Environment, Bar, Montenegro
- Caissard J-C, Joly C, Ve B, Hugueney P, Me M, Baudino S (2004) Secretion mechanisms of volatile organic compounds in specialized cells of aromatic plants. Recent Res Dev Cell Biol 2:1–15 [Google Scholar]
- Celikezen FC, Turkez H, Firat M, Arslan ME, Oner S (2022) In vitr evaluation of selective cytotoxic activity of Chaerophyllum macropodum Boiss. on cultured human SH-SY5Y neuroblastoma cells. Neurotox Res 40(5):1360–1368. 10.1007/s12640-022-00537-z [DOI] [PubMed] [Google Scholar]
- Cordeiro L, Figueiredo P, Souza H, Sousa A, Andrade-Júnior F, Medeiros D, Nóbrega J, Silva D, Martins E, Barbosa-Filho J, Lima E (2020) Terpinen-4-ol as an antibacterial andantibiofilm agent against Staphylococcus aureus. Int J Mol Sci 21(12):4531. 10.3390/ijms21124531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Acqua S, Viola G, Piacente S, Cappelletti EM, Innocenti G (2004) Cytotoxic constituents of roots of Chaerophyllum hirsutum. J Nat Prod 67(9):1588–1590. 10.1021/np040046w [DOI] [PubMed] [Google Scholar]
- Dehelean CA, Marcovici I, Soica C, Mioc M, Coricovac D, Iurciuc S, Cretu OM, Pinzaru I (2021) Plant-derived anticancer compounds as new perspectives in drug discovery and alternative therapy. Molecules 26(4):1109. 10.3390/molecules26041109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirci B, Kosar M, Demirci F, Dinc M, Baser KHC (2007) Antimicrobial and antioxidant activities of the essential oil of Chaerophyllum libanoticum Boiss. et Kotschy. Food Chem 105(4):1512–1517. 10.1016/j.foodchem.2007.05.036 [Google Scholar]
- Ebrahimabadi AH, Djafari-Bidgoli Z, Mazoochi A, Kashi FJ, Batooli H (2010) Essential oils composition, antioxidant and antimicrobial activity of the leaves and flowers of Chaerophyllum macropodum Boiss. Food Control 21(8):1173–1178. 10.1016/j.foodcont.2010.01.014 [Google Scholar]
- Ebrahimisadr P, Majidiani H, Bineshian F, Jameie F, Ghasemi E, Ghaffarifar F (2017) Evaluation of the cytotoxicity effect of Chaerophyllum extract on Leishmania major and J774 cell line in vitro. Jundishapur J Nat Pharm Prod. 10.5812/jjnpp.38948 [Google Scholar]
- Elkiran O, Avsar C, Veyisoglu A, Bagci E (2023) The chemical composition and biological activities of essential oil from the aerial parts of Eryngium maritimum L. (Apiaceae). J Essent Oil Bear Plants 26(3):566–575. 10.1080/0972060x.2023.2208169 [Google Scholar]
- Fabricant DS, Farnsworth NR (2001) The value of plants used in traditional medicine for drug discovery. Environ Health Perspect 109:69–75. 10.2307/3434847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn A (2000) Structure and function of secretory cells. Adv Bot Res 31:37–75. 10.1016/s0065-2296(00)31006-0 [Google Scholar]
- Fredotovic Z, Samanic I, Kamenjarin J, Puizina J (2017) The triparental triploid onion Allium × cornutum Clementi ex Visiani, 1842, possesses a sterile S-type of cytoplasm. Genet Resour Crop Evol 64(8):1971–1983. 10.1007/s10722-017-0489-1 [Google Scholar]
- Fredotović Ž, Soldo B, Šprung M, Marijanović Z, Jerković I, Puizina J (2020) Comparison of organosulfur and amino acid composition between triploid onion Allium cornutum Clementi ex Visiani, 1842, and common onion Allium cepa L., and evidences for antiproliferative activity of their extracts. Plants (Basel) 9(1):98. 10.3390/plants9010098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredotovic Z, Puizina J, Nazlic M, Maravic A, Ljubenkov I, Soldo B, Vuko E, Bajic D (2021) Phytochemical characterization and screening of antioxidant, antimicrobial and antiproliferative properties of Allium × cornutum Clementi and two varieties of Allium cepa L. peel extracts. Plants (Basel) 10(5):832. 10.3390/plants10050832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez Mercado YK, Mateos Diaz JC, Ojeda Hernandez DD et al (2022) Ortho-coumaric acid derivatives with therapeutic potential in a three-dimensional culture of the immortalised U-138 MG glioblastoma multiforme cell line. Neurol Perspect 2(Suppl 1):S19–S30. 10.1016/j.neurop.2021.09.006 [Google Scholar]
- Hayta S, Celikezen FC (2016) Evaluation of essential oil composition, antioxidant and antimicrobial properties of Chaerophyllum crinitum Boiss (Apiaceae) from Turkey: a traditional medicinal herb. J Biol Sci 16(3):72–76. 10.3923/jbs.2016.72.76 [Google Scholar]
- Heo BG, Chon SU, Park YJ, Bae JH, Park SM, Park YS, Jang HG, Gorinstein S (2009) Antiproliferative activity of Korean wild vegetables on different human tumor cell lines. Plant Food Hum Nutr 64(4):257–263. 10.1007/s11130-009-0138-8 [DOI] [PubMed] [Google Scholar]
- Hou N, Liu N, Han J, Yan Y, Li J (2017) Chlorogenic acid induces reactive oxygen species generation and inhibits the viability of human colon cancer cells. Anticancer Drugs 28(1):59–65. 10.1097/cad.0000000000000430 [DOI] [PubMed] [Google Scholar]
- Huang W-Y, Zhang H-C, Liu W-X, Li C-Y (2012) Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J Zhejiang Univ Sci B 13(2):94–102. 10.1631/jzus.b1100137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova S, Dyankov S, Karcheva-Bahchevanska D, Todorova V, Georgieva Y, Benbassat N, Ivanov K (2023) Echinophora tenuifolia subsp. sibthorpiana—study of the histochemical localization of essential oil. Molecules. 10.3390/molecules28072918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensch C, Strube J (2022) Proposal of a new green process for waste valorization and cascade utilization of essential oil plants. Sustainability 14(6):3227. 10.3390/su14063227 [Google Scholar]
- Joshi RK (2013a) Antimicrobial activity of leaf essential oil of Chaerophyllum villosum Wall. ex DC. from Kumaun Himalayan of Uttrakhand. Indo Am J Pharm Res 3(2):1503–1509 [Google Scholar]
- Joshi RK (2013b) Root essential oil composition of Chaerophyllum villosum Wall. ex DC. From Uttarakhand, India. Am J Essent Oils Nat Prod 1(1):34–36 [Google Scholar]
- Kamte SLN, Ranjbarian F, Cianfaglione K, Sut S, Dall’Acqua S, Bruno M, Afshar FH, Iannarelli R, Benelli G, Cappellacci L, Hofer A, Maggi F, Petrelli R (2018) Identification of highly effective antitrypanosomal compounds in essential oils from the Apiaceae family. Ecotoxicol Environ Saf 156:154–165. 10.1016/j.ecoenv.2018.03.032 [DOI] [PubMed] [Google Scholar]
- Khajehie N, Golmakani MT, Eblaghi M, Eskandari MH (2017) Evaluating the effects of microwave-assisted hydrodistillation on antifungal and radical scavenging activities of Oliveria decumbens and Chaerophyllum macropodum essential oils. J Food Protect 80(5):783–791. 10.4315/0362-028x.Jfp-16-428 [DOI] [PubMed] [Google Scholar]
- Köse ŞO, Elvan O (2018) Antimicrobial and antioxidant properties of Sirmo (Allium vineale L.), Mendi (Chaerophyllum macropodum Boiss.) and Siyabo (Ferula rigidula DC.). GIDA J Food 43:294–302. 10.15237/gida.GD17099 [Google Scholar]
- Lakušić B, Slavkovska V, Pavlović M, Milenković M, Antić Stanković J, Couladis M (2009) Chemical composition and antimicrobial activity of the essential oil from Chaerophyllum aureum L. (Apiaceae). Nat Prod Commun 4(1):115–118 [PubMed] [Google Scholar]
- Ma ZJ, Yang JJ, Yang Y, Wang XX, Chen GH, Shi AC, Lu YB, Jia SN, Kang XW, Lu L (2020) Rosmarinic acid exerts an anticancer effect on osteosarcoma cells by inhibiting DJ-1 via regulation of the PTEN-PI3K-Akt signaling pathway. Phytomedicine 68:153186. 10.1016/j.phymed.2020.153186 [DOI] [PubMed] [Google Scholar]
- Mačukanović-Jocić M, Stešević D, Rančić D, Stevanović ZD (2017) Pollen morphology and the flower visitors of Chaerophyllum coloratum L. (Apiaceae). Acta Bot Croat 76(1):1–8. 10.1515/botcro-2016-0039 [Google Scholar]
- Marchese A, Arciola C, Barbieri R, Silva A, Nabavi S, Tsetegho Sokeng A, Izadi M, Jafari N, Suntar I, Daglia M, Nabavi S (2017) Update on monoterpenes as antimicrobial agents: a particular focus on p-cymene. Materials 10(8):947. 10.3390/ma10080947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinho CR, Zacaro AA, Ventrella MC (2011) Secretory cells in Piper umbellatum (Piperaceae) leaves: a new example for the development of idioblasts. Flora 206(12):1052–1062. 10.1016/j.flora.2011.07.011 [Google Scholar]
- Masoudi S, Faridchehr A, Alizadehfard S, Zabarjadshiraz N, Chalabian F, Taghizadfarid R, Rustaiyan A (2011) Chemical composition and antibacterial activity of the essential oils of Semenovia frigida and Chaerophyllum bulbosum from Iran. Chem Nat Compd 47(5):829–832. 10.1007/s10600-011-0076-1 [Google Scholar]
- Mungenge C, Zimudzi C, Zimba M, Nhiwatiwa T (2014) Phytochemical screening, cytotoxicity and insecticidal activity of the fish poison plant Synaptolepis alternifolia Oliv. (Thymelaeaceae). J Pharmacog Phytochem 2(5):15–19 [Google Scholar]
- Najafzadeh R, Ghasemzadeh S, Mirfakhraie S (2019) Effect of essential oils from Nepeta crispa, Anethum graveolens and Satureja hortensis against the stored-product insect “Ephestia kuehniella (Zeller)”. J Med Plants Byprod 8(2):163–169 [Google Scholar]
- Nasr Bouzaiene N, Kilani Jaziri S, Kovacic H, Chekir-Ghedira L, Ghedira K, Luis J (2015) The effects of caffeic, coumaric and ferulic acids on proliferation, superoxide production, adhesion and migration of human tumor cells in vitro. Eur J Pharmacol 766:99–105. 10.1016/j.ejphar.2015.09.044 [DOI] [PubMed] [Google Scholar]
- Nematollahi F, Akhgar MR, Larijani K, Rustaiyan A, Masoudi S (2005) Essential oils of Chaerophyllum macropodum Boiss. and Chaerophyllum crinitum Boiss. from Iran. J Essent Oil Res 17(1):71–72. 10.1080/10412905.2005.9698834 [Google Scholar]
- Nikolić T (2020) Flora Croatica: Vaskularna flora Republike Hrvatske; Ključevi za determinaciju s pratećim podatcima: Equisetidae, Lycopodiidae, Ophyoglossidae, Polypodidae, Cycadidae, Ginkgooidae, Gnetidae, Pinidae, Magnoliidae—porodice A—FAB, vol 2. Alfa d.d., Zagreb, Croatia
- Nikolić T, Milović M, Bogdanović S, Jasprica N (2015) Endemi u hrvatskoj flori, 1 edn. Alfa d.d., Zagreb
- Ozhatay N, Akalin E, Ozhatay E, Unlu S (2008) Rare and endemic taxa of Apiaceae in Turkey and their conservation significance. Istanb J Pharm 40:1–15 [Google Scholar]
- Ozinga WA, de Heer M, Hennekens SM et al (2005) Target species—species of European concern; a database driven selection of plant and animal species for the implementation of the Pan European Ecological Network. Wageningen, Alterra [Google Scholar]
- Paiva EAS (2016) How do secretory products cross the plant cell wall to be released? A new hypothesis involving cyclic mechanical actions of the protoplast. Ann Bot 117(4):533–540. 10.1093/aob/mcw012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasković I, Franić M, Polić Pasković M, Talhaoui N, Marcelić Š, Lukić I, Fredotović Ž, Žurga P, Major N, Goreta Ban S, Vidović N, Rončević S, Nemet I, Džafić N, Soldo B (2024) Silicon foliar fertilisation ameliorates olive leaves polyphenolic compounds levels and elevates its potential towards different cancer cells. Appl Sci 14(11):4669. 10.3390/app14114669 [Google Scholar]
- Petrović GM, Stamenković JG, Stojanović GS, Mitić VD, Zlatković BK (2017) Chemical profile of essential oils and headspace volatiles of Chaerophyllum hirsutum from Serbia. Nat Prod Commun 12(9):1513–1515. 10.1177/1934578x1701200932 [Google Scholar]
- Polat R, Cakilcioglu U, Satıl F (2013) Traditional uses of medicinal plants in Solhan (Bingöl—Turkey). J Ethnopharmacol 148(3):951–963. 10.1016/j.jep.2013.05.050 [DOI] [PubMed] [Google Scholar]
- Radman S, Čagalj M, Šimat V, Jerković I (2022) Seasonal variability of b volatilome from Dictyota dichotoma. Molecules 27(9):3012. 10.3390/molecules27093012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi SM, Nejad-Ebrahimi S (2010) Essential oilcomposition of Chaerophyllum macrospermum (Spreng.) Fisch C.A. Mey seeds. J Essent Oil Bear Plants 13(2):205–210. 10.1080/0972060x.2010.10643813 [Google Scholar]
- Santana AI, Vila R, Espinosa A, Olmedo D, Gupta MP, Cañigueral S (2009) Composition and biological activity of essential oils from Protium confusum. Nat Prod Commun 4(10):1401–1406 [PubMed] [Google Scholar]
- Sayed-Ahmad B, Talou T, Saad Z, Hijazi A, Merah O (2017) The Apiaceae: ethnomedicinal family as source for industrial uses. Ind Crops Prod 109:661–671. 10.1016/j.indcrop.2017.09.027 [Google Scholar]
- Sharifi-Rad J, Sureda A, Tenore G, Daglia M, Sharifi-Rad M, Valussi M, Tundis R, Sharifi-Rad M, Loizzo M, Ademiluyi A, Sharifi-Rad R, Ayatollahi S, Iriti M (2017) Biological activities of essential oils: from plant chemoecology to traditional healing systems. Molecules 22(1):70. 10.3390/molecules22010070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šilić Č (1984) Endemične biljke. 3rd edn. Svjetlost, Zavod za udž̌benike i nastavna sredstva, Sarajevo, Beograd, Sarajevo
- Stamenković JG, Stojanović GS, Radojković IR, Petrović GM, Zlatković BK (2015) Chemical composition of the essential oil from Chaerophyllum Temulum (Apiaceae). Nat Prod Commun 10(8):1934578X1501000. 10.1177/1934578x1501000832 [PubMed] [Google Scholar]
- Stešević D, Božović M, Tadić V, Rančić D, Stevanović ZD (2016) Plant-part anatomy related composition of essential oils and phenolic compounds in Chaerophyllum coloratum, a Balkan endemic species. Flora 220:37–51. 10.1016/j.flora.2016.01.006 [Google Scholar]
- Toxopeus H, Bouwmeester HJ (1992) Improvement of caraway essential oil and carvone production in the Netherlands. Ind Crops Prod 1(2–4):295–301. 10.1016/0926-6690(92)90031-p [Google Scholar]
- Urmi KF, Mostafa S, Begum G, Kaiser H (2013) Comparative brine shrimp lethality bioassay of different plant parts of Bauhinia Purpurea L. J Pharm Sci Res 5(10):190–192 [Google Scholar]
- Vajs V, Milosavljevic S, Tesevic V, Zivanovic P, Jancic R, Todorovic B, Slavkovska V (1995) Chaerophyllum coloratum L.: essential oils of ripe fruits and umbels. J Essent Oil Res 7(5):529–531. 10.1080/10412905.1995.9698578 [Google Scholar]
- Vrca I, Jug B, Fredotović Ž, Vuko E, Brkan V, Šestić L, Juretić L, Dunkić V, Nazlić M, Ramić D, Smole Možina S, Kremer D (2023) Significant benefits of environmentally friendly hydrosols from Tropaeolum majus L. seeds with multiple biological activities. Plants 12(22):3897. 10.3390/plants12223897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuko E, Rusak G, Dunkić V, Kremer D, Kosalec I, Rađa B, Bezić N (2019) Inhibition of satellite RNA associated cucumber mosaic virus infection by essential oil of Micromeria croatica (Pers.) Schott. Molecules 24(7):1342. 10.3390/molecules24071342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuko E, Dunkić V, Maravić A, Ruščić M, Nazlić M, Radan M, Ljubenkov I, Soldo B, Fredotović Ž (2021a) Not only a weed plant-biological activities of essential oil and hydrosol of Dittrichia viscosa (L.) Greuter. Plants 10(9):1837. 10.3390/plants10091837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuko E, Dunkić V, Ruščić M, Nazlic M, Mandić N, Soldo B, Šprung M, Fredotović Ž (2021b) Chemical composition and new biological activities of essential oil and hydrosol of Hypericum perforatum L. ssp. veronense (Schrank) H. Lindb. Plants (Basel) 10(5):1014. 10.3390/plants10051014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuko E, Radman S, Jerković I, Kamenjarin J, Vrkić I, Fredotović Ž (2022) A plant worthy of further study-volatile and non-volatile compounds of Portenschlagiella ramosissima (Port.) Tutin and its biological activity. Pharmaceuticals 15(12):1454. 10.3390/ph15121454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Zhu M, Zhou J, Chen L, Sheng L, Feng L (2019) Cytological evidence for the pathway of synthesis, accumulation, and secretion of Rose essential oil. J Essent Oil Bear Plants 22(2):301–310. 10.1080/0972060x.2019.1605938 [Google Scholar]
- Zengin G, Mahomoodally MF, Paksoy MY, Picot-Allain C, Glamocilja J, Sokovic M, Diuzheva A, Jeko J, Cziaky Z, Rodrigues MJ, Sinan KI, Custodio L (2019) Phytochemical characterization and bioactivities of five Apiaceae species: natural sources for novel ingredients. Ind Crops Prod 135:107–121. 10.1016/j.indcrop.2019.04.033 [Google Scholar]
- Zengin G, Sinan KI, Ak G, Mahomoodally MF, Paksoy MY, Picot-Allain C, Glamocilja J, Sokovic M, Jeko J, Cziaky Z, Rodrigues MJ, Pereira CG, Custodio L (2020) Chemical profile, antioxidant, antimicrobial, enzyme inhibitory, and cytotoxicity of seven Apiaceae species from Turkey: a comparative study. Ind Crops Prod. 10.1016/j.indcrop.2020.112572 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.