Abstract
We have studied the infection pathway of Autographa californica multinuclear polyhedrosis virus (baculovirus) in mammalian cells. By titration with a baculovirus containing a green fluorescent protein cassette, we found that several, but not all, mammalian cell types can be infected efficiently. In contrast to previous suggestions, our data show that the asialoglycoprotein receptor is not required for efficient infection. We demonstrate for the first time that this baculovirus can infect nondividing mammalian cells, which implies that the baculovirus is able to transport its genome across the nuclear membrane of mammalian cells. Our data further show that the virus enters via endocytosis, followed by an acid-induced fusion event, which releases the nucleocapsid into the cytoplasm. Cytochalasin D strongly reduces the infection efficiency but not the delivery of nucleocapsids to the cytoplasm, suggesting involvement of actin filaments in cytoplasmic transport of the capsids. Electron microscopic analysis shows the cigar-shaped nucleocapsids located at nuclear pores of nondividing cells. Under these conditions, we observed the viral genome, major capsid protein, and electron-dense capsids inside the nucleus. This suggests that the nucleocapsid is transported through the nuclear pore. This mode of transport seems different from viruses with large spherical capsids, such as herpes simplex virus and adenovirus, which are disassembled before nuclear transport of the genome. The implications for the application of baculovirus or its capsid proteins in gene therapy are discussed.
The study of host-virus interactions not only contributes to our basic knowledge of virology and cell biology but also is important in the further development of gene therapy vector systems (31). We (35) and others (reviewed in reference 4) have observed that the nuclear transport of vector DNA is a major barrier in the transfection of nondividing cells and hence in the application of nonviral gene therapy vectors in vivo. Several DNA-viruses have found an effective solution to this problem (33). The nucleocapsids of adenovirus (14, 15) and the enveloped herpes simplex virus (HSV) (1, 27) are actively transported toward the nucleus and subsequently dock at the nuclear pore. This triggers the release and nuclear transport of the viral genome. The mechanism of this process and the proteins involved are not known. In both cases a nucleocapsid residue is observed at the nuclear pore. However, it is likely that viral proteins are associated with the DNA during transport (14). Nuclear transport of the viral genome depends on the previous process of entry, which may include a passage through the acidic endosomal environment. During this process the viral capsid is modified to allow the next step in the infection sequence (15, 33). This entry-dependent modification of viral capsids allows the important functional distinction between an infecting capsid coming in and a newly formed capsid going out of the cell. Detailed knowledge of the nuclear transport process of viral DNA could lead to new insights into the nuclear transport of large complexes. It could also lead to new methods of intervention in viral infection and, finally, to novel solutions for the problem of efficient gene transfer in gene therapy.
An interesting example of a large DNA virus is the insect virus Autographa californica multinuclear polyhedrosis virus (AcMNPV), a baculovirus. It has a 130-kb double-stranded DNA genome, packaged in a cigar-shaped (25 by 260 nm) enveloped nucleocapsid. Baculovirus enters insect cells via receptor-mediated endocytosis (32, 34). The viral fusion protein gp64 is responsible for acid-induced endosomal escape (3). In the cytoplasm, the nucleocapsid probably induces the formation of actin filaments, which is a possible mode of transport toward the nucleus (8, 21). The exact molecular structure and protein composition of the nucleocapsid has been only partially described (7, 29); no information about modification of the capsid during entry is available. It has not been demonstrated directly that nondividing insect cells can be infected, although it seems likely on the basis of published data. With regard to the nuclear transport of the viral genome, two apparently contradictory reports were published. In one study, nucleocapsids of a related baculovirus species (Ploidia interpunctella granulosis virus) were observed docking at the nuclear pore of infected insect cells, at different stages of releasing their genome, but not inside the nucleus (28). This suggests a mechanism of DNA transport similar to HSV (1). Others detected AcMNPV nucleocapsids at the nuclear pore containing electron-dense material and inside the nucleus at various stages of releasing the genome (13). Here, a completely different mode of entry is implied, one involving transport of the entire capsid through the nuclear pore followed by release of the DNA. However, it was not excluded that the observed capsids had entered the nuclear compartment during mitosis, i.e., in the absence of a nuclear membrane. Interestingly, baculovirus can infect mammalian cells very efficiently, although it replicates only in insect cells. When a reporter gene under the control of a mammalian promoter was cloned into the baculovirus genome, it was expressed in hepatoma cells (Huh7 and HepG2), primary rat hepatocytes, and epithelioid cell lines (HeLa and Cos7) in vitro (6, 9, 17, 26). Therefore, it has been suggested that baculovirus could be used as a gene therapy vector. However, several important aspects of the interactions of the baculovirus virion with mammalian cells have not been studied in detail. A receptor has not been identified, and the mode and kinetics of entry and cellular transport are unknown. It has not been established whether nondividing mammalian cells can be infected and, if so, what the mechanism of entry of the viral genome into the nucleus is.
We show here that baculovirus can indeed infect nondividing mammalian cells through a mechanism apparently identical to that found in insect cells. The mode of nuclear entry of the viral genome appears to be different from what is known of other large DNA viruses. Our data suggest that the cigar-shaped nucleocapsid (25 nm in diameter) is transported through the nuclear pore, together with the viral genome.
MATERIALS AND METHODS
Cell culture and virus production.
HepG2- and Pk1-LLC (Pk1) cells were obtained from the American Type Culture Collection (ATCC) and cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS), penicillin (100 U/ml), and streptomycin (100 μg/ml). Huh7 cells, obtained from M. Nassal (Heidelberg, Germany), were cultured in the same medium supplemented with 1× nonessential amino acids (ICN, Zoetermeer, The Netherlands). H35 and HeLa cells were cultured in DMEM–F-10 (1:1) supplemented with 10% FCS and antibiotics. Sf21 cells were grown in SF900 serum-free medium supplemented with 2% FCS and antibiotics. The baculovirus strain AcMNPV was maintained by infecting Sf21 cells (2 × 106/ml) at a multiplicity of infection (MOI) of 0.01 to 0.1. Virus was harvested after 3 days by pelleting cells and debris at 1,000 × g for 10 min at 4°C. The titer of the supernatant was typically 107 to 108 active PFU/ml as determined by endpoint dilution assay. To concentrate baculovirus, the supernatant was pelleted at 80,000 × g for 30 min at 4°C and resuspended in phosphate-buffered saline (PBS). This suspension (5 × 108 PFU/ml) was routinely used for infection experiments. All cell culture media were from Life Technologies, Breda, The Netherlands.
GFP-baculovirus.
An expression cassette containing the cytomegalovirus (CMV) immediate-early promoter and a gene encoding a modified green fluorescent protein (hGFP-S65T; Clontech, Palo Alto, Calif.) was cloned into the baculovirus genome using the Bac-to-Bac Expression System (Life Technologies). Briefly, the polyhedrin promoter was removed from the pFASTBAC plasmid by cutting with SnaBI and StuI, blunting and ligation. A CMV-GFP cassette was removed as a SpeI-NotI fragment from the pGFP-S65T plasmid (Clontech) and ligated into the multiple-cloning site of the modified pFASTBAC plasmid (Gibco, Breda, The Netherlands). The CMV-GFP-containing baculovirus (GFP-bac) was generated according to the protocol provided by the manufacturer (Bac-to-Bac system; Gibco).
Baculovirus infection of mammalian cells.
Routinely, Pk1 cells were seeded on six-well plates (104/cm2, 10 cm2/well, containing 2 ml of medium, with a plating efficiency of >90%) and infected by adding GFP-bac at different MOIs. GFP expression was routinely analyzed by fluorescence cell sorter analysis (FACS; see below) at different time points after infection. To arrest Pk1 cells in the G1/S phase of the cell cycle, cells were seeded and cultured in the presence of 50 ng of aphidicolin (Sigma, Zwijndrecht, The Netherlands) per ml. For the experiments with cellular toxins, the cells were preincubated for 1 h in medium supplemented with either nocodazole at 2 μM, vinblastine at 10 μM, colchicine at 2 μM, cytochalasin D at 0.5 μM, chloroquine at 0.1 mM, or bafilomycin A1 at 1 μM (all from Sigma) or ammonium chloride (Life Technologies) at 25 mM. The effect of the cytochalasin D treatment on the integrity of actin filaments was tested by staining with rhodamine-phalloidin (Molecular Probes, Leiden, The Netherlands). The effect of ammonium chloride on lysosomal pH was tested with the fluorescent weak base Syto 17 (Molecular Probes). The effect of nocodazole on the integrity of the tubulin network was tested by staining microtubules with an antitubulin antibody (Sigma). These tests showed that all treatments were effective within 1 h, and reversible within 4 h by replacing the medium, without apparent toxicity. In some experiments (see Fig. 4, 7, and 8) GFP-bac infection was synchronized by infection at an MOI of 500 for 30 min, followed by washing with fresh medium. This results 18 h later in a GFP expression equivalent to a continuous infection at an MOI of 50 (data not shown).
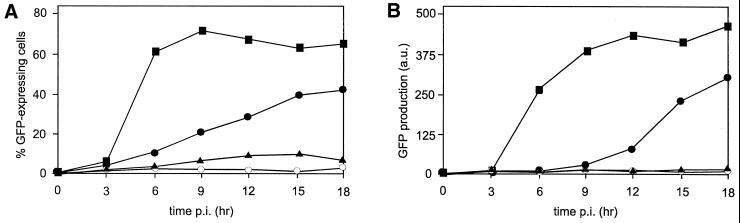
FIG. 4.
Effect of toxins on GFP-bac infection. Pk1 cells were seeded on six-well plates (104/cm2); after 12 h, the cells were pretreated with the toxins indicated for 1 h and then infected with GFP-bac at an MOI of 500 for 30 min, followed by a wash and further incubation with toxin-supplemented medium as described in Materials and Methods. Cells were harvested at different time points and analyzed by FACS. (A) Percentage of GFP+ cells. (B) Amount of GFP produced, expressed as the mean fluorescence value of positive cells minus the mean fluorescence value of negative cells and multiplied by the percentage of positive cells in arbitrary units. Symbols: ●, untreated cells; ▴, cytochalasin D; ■, nocodazole; ○, ammonium chloride.
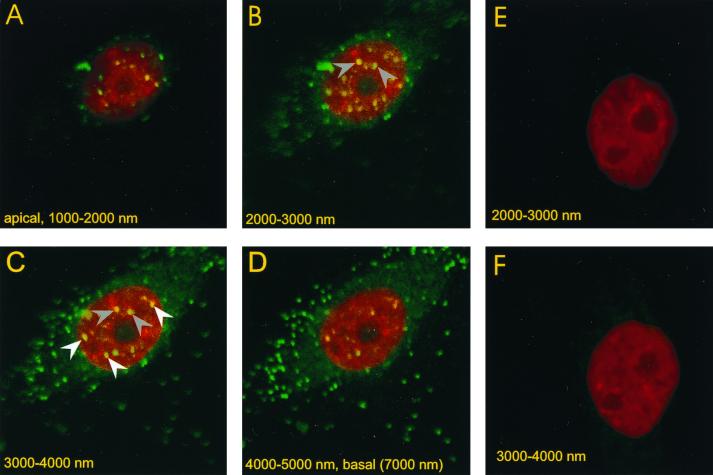
FIG. 7.
Detection of viral genome in nuclei of Pk1 cells by FISH analysis. Pk1 cells were seeded on glass chamber slides (104/cm2) in the presence of aphidicolin. The cells were infected with GFP-bac after 12 h at an MOI of 500 for 30 min, followed by a wash, as in Fig. 4. Cells were fixed and analyzed by FISH 4 h after infection, as described in Materials and Methods. (A to D) Four successive, apical- to basal-side confocal images are shown, each 1 μm thick. Viral genome is detected as green fluorescent spots inside the nucleus, which is stained red with propidium iodide. The spots marked with gray arrows are visible in panels B and C; spots marked with white arrows are visible only in panel C, which establishes their nuclear localization. (E and F) Successive confocal sections, each 1 μm thick, of uninfected control cells.
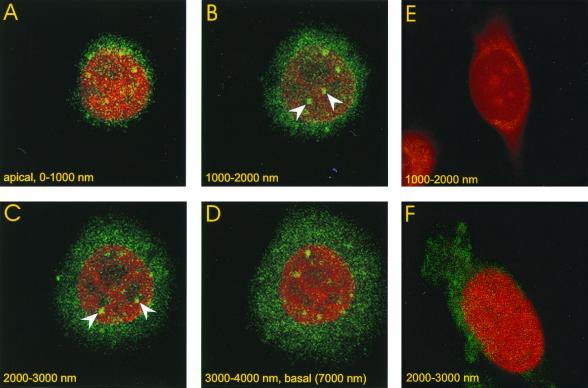
FIG. 8.
Detection of the major capsid protein in the nuclei of Pk1 cells by immune fluorescence. Pk1 cells were seeded on glass chamber slides (104/cm2) in the presence of aphidicolin. The cells were infected with GFP-bac after 12 h at an MOI of 500 for 30 min, followed by a wash, as in Fig. 4 and 7. Cells were fixed 4 h after infection and analyzed by immune fluorescence using an antibody against the major capsid protein p39 as described in Materials and Methods. Four successive, apical- to basal-side confocal images are shown, each 1 μm thick. Spots of green fluorescence indicate the presence of viral capsids in the nucleus, which is stained red with propidium bromide. Nuclear spots not visible in adjacent sections, which establishes their nuclear localization, are marked with arrows. (E) Confocal section, 1 μm, of infected cell treated with secondary antibody only. (F) Confocal section, 1 μm, of uninfected cell treated with primary and secondary antibody.
FACS analysis.
At different times after infection, cells were harvested by trypsinization, fixed for 15 min in 2% (wt/vol) paraformaldehyde in PBS (PFA-PBS), washed by centrifugation in 5 g of bovine serum albumin per liter in PBS (PBS-BSA), and analyzed by a Becton Dickinson FACScan. Forward scatter and GFP fluorescence (detected in the fluorescein isothiocyanate [FITC] fluorescence channel) were analyzed using the Cellquest computer program (Becton Dickinson, Leiden, The Netherlands). For analysis of the asialoglycoprotein receptor activity, cells were seeded in six-well plates (104/cm2). After overnight incubation, 10 ng of FITC-asialofetuin (FITC-ASF) per ml was added for 3 h. Cells were harvested and analyzed by FACS as described above. The endocytotic activity was measured using the fluid-phase marker FITC-dextran (molecular weights, 20,000; Sigma). Cells were seeded on six-well plates (104/cm2) and incubated the following day for 3 h with 100 μM FITC-dextran. Cells were harvested and analyzed by FACS as described above. The total uptake of FITC-dextran or FITC-ASF was expressed as the mean fluorescence value of treated cells minus the mean fluorescence value of untreated cells. For determination of the DNA content, the cells were harvested by trypsinization, washed with PBS-BSA and fixed in ice-cold 70% (vol/vol) ethanol for 30 min at 4°C, treated with 1 g of Triton X-100 per liter for 10 min, 1 g of sodium citrate per liter in PBS for 10 min, and 0.1 g of RNase per liter in PBS for 15 min at room temperature. Finally, the cells were resuspended in 100 μl of PBS-BSA. Just prior to FACS analysis, 4 mg of propidium iodide and 2 g of spermine-HCl per liter was added.
Immunofluorescence.
Pk1 cells were seeded in the presence of aphidicolin on chamber slides (Lab-Tek II; Life Technologies) and infected 12 h later (see above). At 4 h after infection, cells were washed and fixed in PFA-PBS for 20 min at room temperature. Cells were treated with 1 g of Triton X-100 per liter in PBS for 5 min at 4°C and subsequently with 5% FCS in PBS for 30 min at 37°C. Primary antibody (monoclonal antibody p39p10) against the baculovirus major capsid protein p39 (a generous gift of J. S. Manning, University of California, Davis) was added at a 1/100 dilution in PBS-FCS for 1 h at 37°C. Cells were washed with PBS three times and then incubated with 5% FCS in PBS for 30 min at 37°C. FITC-conjugated rabbit anti-mouse antibody (Sigma) was added for 1 h at 37°C. Cells were washed three times in PBS and mounted in Vectashield (Vector, Bethesda, Md.) with propidium iodide. Analysis was done on a 410LSM Zeiss confocal microscope.
FISH.
Pk1 cells were seeded in the presence of aphidicolin on chamber glasses (Lab-Tek II; Life Technologies) and infected 12 h later (see immunochemistry section). The fluorescence in situ hybridization (FISH) protocol was essentially as described by Greber et al. (14). Cells were incubated in ice-cold methanol for 5 min, followed by incubation in 1% PFA-PBS for 30 min. The PFA was quenched by treatment with 25 mM ammonium chloride for 5 min. GFP-bac DNA was isolated from purified virus by proteinase K treatment and phenol-chloroform extraction. The isolated DNA was checked by restriction analysis. GFP-bac DNA was labeled with biotin. Hybridization to cells of biotin-labeled probe and FITC-avidin was performed as described by Mulder et al. (23). Cells were analyzed by using the confocal microscopy equipment described above in the immunohistochemistry section.
Time-lapse video experiments.
Pk1 cells were incubated, in the presence of aphidicolin, on 3-amino-propyl-triethoxysilane (TESPA; Sigma) coated 23-mm-diameter coverslips. Cells were infected with GFP-bac at an MOI of 100 and incubated in a tissue culture chamber as described previously (19). This tissue culture chamber was mounted on an IX70 inverted fluorescence microscope (Olympus, Zoeterwoude, The Netherlands). Time-lapse fluorescence images were taken with a charge-coupled device camera coupled to a DKR-700P Digital Still Recorder (both from Sony, Zoeterwoude, The Netherlands). The digital still recorder and the mercury lamp of the microscope were controlled by a custom-made time-lapse device (Paes, Zoeterwoude, The Netherlands). During the entire incubation, the cells were monitored by low-level phase-contrast illumination via a time-lapse video recorder (Nikon, Amsterdam, The Netherlands) taking 50 frames per min.
Electron microscopy.
Pk1 cells were seeded on six-well plates and pretreated with aphidicolin, nocodazole, cytochalasin D, or ammonium chloride as described above. Cells were infected at an MOI of 1,000 for 4 h, washed, and fixed overnight in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.3). Next, the cells were washed with 0.1 M cacodylate buffer (pH 7.3) and postfixed in 1% OsO4 with 50 mM K3Fe(CN)6 in cacodylate buffer (pH 7.3) and dehydrated in an ethanol series. After they were embedded in Epon, ultrathin (80-nm) sections were cut and stained in 7% uranyl actetate in water, followed by treatment with lead citrate. Sections were viewed in a Philips CM100 electron microscope.
RESULTS
Infection of mammalian cells by baculovirus.
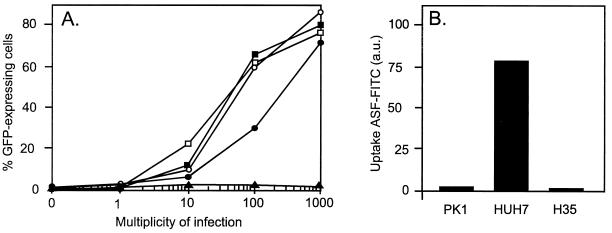
We have cloned the humanized enhanced GFP gene (hGFP-S65T) under the control of a CMV promoter into the baculovirus AcMNPV (GFP-bac; see Materials and Methods). To establish the relative infection efficiency on different mammalian cell lines, we titrated this GFP-bac on two human hepatoma cell lines, HepG2 and Huh7, a rat hepatoma cell line H35, an epithelial human cervix carcinoma cell line HeLa, and an epithelial pig kidney cell line Pk1. FACS analysis showed that all cell types tested here are infected and expressed GFP with comparable efficiency, with the notable exception of H35 (Fig. 1A). Initial reports showed only marker gene expression in liver-derived cells (6, 17). It was therefore suggested that baculovirus infects mammalian cells via the asialoglycoprotein receptor. However, Pk1 cells can be successfully infected but do not show significant uptake of FITC-ASF in contrast to Huh7 cells (Fig. 1B). Apparently, interaction with the asialoglycoprotein receptor is not required for the infection of mammalian cells.
FIG. 1.
GFP-bac infection of mammalian cells. (A) Titration on different cell lines. Cells were seeded on six-well plates (104/cm2) and immediately infected with GFP-bac at different MOIs as indicated. The cells were harvested and analyzed by FACS 18 h later, as described in Materials and Methods. Datum points (percentage of GFP-expressing cells) represent the average (±2%) of two independent titrations. Symbols: ●, HeLa cells; ■, Huh7 cells; □, Pk1 cells; ▴, H35 cells; ○, HepG2 cells. (B) Uptake of FITC-ASF by different cell lines. Cells were seeded on six-well plates (104/cm2) and incubated the next day with 10 ng of FITC-ASF per ml for 3 h. Cells were washed and harvested, and FITC-ASF uptake was analyzed by FACS. FITC-ASF uptake is expressed as the mean fluorescence value of treated cells minus the mean fluorescence value of untreated cells.
Baculovirus can infect nondividing mammalian cells.
To establish whether baculovirus is able to infect nondividing mammalian cells, we analyzed baculovirus infection of Pk1 cells arrested in S phase with aphidicolin, a reversible blocker of DNA polymerase. To show that this treatment was effective, we performed FACS analysis of aphidicolin-arrested cells. At 6 h after release from a 12-h aphidicolin treatment, a dramatic decrease of cells in G1/S (85 to 5%) and a concomitant increase of cells in G2 (from 5 to 86%) was observed. At 12 h after release, the majority of the cells had progressed through mitosis into G1 (81%). This was not observed in untreated cells or in cells continuously incubated with aphidicolin. In addition, during time-lapse video recordings in the presence of aphidicolin, only a few (two per 100 cells) mitotic events were recorded 12 to 24 h after seeding. In contrast, untreated cells were actively cycling (56 mitoses per 100 cells). These results are evidence of an effective and reversible G1/S block by aphidicolin under these conditions.
Pk1 cells were infected with GFP-bac 12 h after seeding in the presence or absence of aphidicolin and analyzed for GFP expression 12 h after infection (Fig. 2). The results show that cells arrested in G1/S can be infected as efficiently as untreated cells. Further illustration of this point was obtained by time-lapse video recording of infected cells in the presence of aphidicolin (Fig. 3). The videotape showed no more than three mitotic events (arrows). All other cells changed position but did not divide, as determined by analysis of the video recordings. A GFP signal can be observed 9 h after infection in many cells that did not undergo mitosis in the previous hours. These results show that baculovirus can infect nondividing mammalian cells, which implies that the viral genome is able to penetrate the mammalian nuclear membrane.
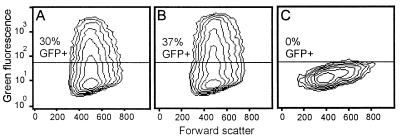
FIG. 2.
GFP-bac infection of Pk1 cells arrested in S phase. Pk1 cells were seeded on six-well plates (104/cm2) in the presence or absence of aphidicolin, infected 12 h later at an MOI of 50 and analyzed for GFP expression by FACS 24 h after seeding. (A) Untreated and infected cells. (B) Aphidicolin-treated and infected cells. (C) Untreated and uninfected cells.
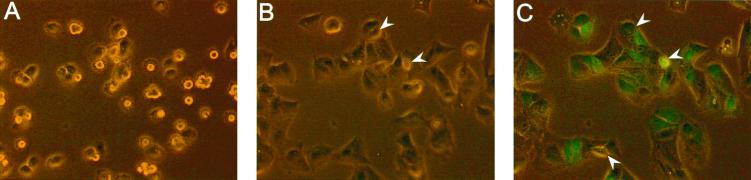
FIG. 3.
GFP-bac infection of Pk1 cells does not require a mitotic event. Pk1 cells (2 × 104/cm2) were seeded in the presence of aphidicolin on a coated 23-mm-diameter coverglass in a culture chamber mounted on an inverted fluorescence microscope connected to a video recorder and a digital still recorder. The cells were infected with GFP-bac at an MOI of 100. Mitotic events were scored by analysis of a continuous time-lapse video recording, as described in Materials and Methods. At 90-min intervals phase-contrast fluorescence images were captured to detect GFP expression, i.e., at 1.5 h (A), 6 h (B), and 12 h (C) after infection. Arrows indicate cells that have gone through mitosis; all other cells did not go through mitosis as determined by analysis of the video recordings.
Baculovirus enters via endocytosis; acidification of the endosome is required for escape.
As described in the introduction, nuclear transport of an adenovirus capsid is dependent on previous activation during entry (14). Therefore, we investigated the mode and kinetics of baculovirus entry into mammalian cells. Baculovirus is taken up in insect cells by receptor-mediated endocytosis, followed by pH-dependent fusion of the envelope with the endosome (3, 34). It has been suggested that baculovirus enters mammalian cells by the same pathway because chloroquine strongly inhibits marker gene expression (6, 17). We also observed that chloroquine (data not shown), bafilomycin A1 (data not shown), and ammonium chloride (Fig. 4) strongly inhibit infection of Pk1 cells (5, 25). However, this inhibition of infection could be due to a reduction in receptor recycling or a reduction in endocytosis (25). Indeed, the uptake of FITC-dextran by Pk1 cells is reduced to 30 to 70% of the control by ammonium chloride, chloroquine, or bafilomycin A1 (FACS analysis; data not shown). To clarify this issue, we studied viral uptake directly by quantitative electron microscopy of Pk1 cells infected at a high MOI (Fig. 5, Table 1). In untreated cells, a quarter of the virus particles observed in the cells was found as enveloped virus in the process of entry at the plasma membrane (Table 1, Fig. 5A). About half of the virus particles observed were enveloped and inside cytoplasmic vesicles (Table 1, Fig. 5B and C). A quarter of the internalized virus particles was found in the cytoplasm as unenveloped nucleocapsids (Table 1, Fig. 5D and E). In ammonium chloride-treated cells we observed enveloped virus particles in cytoplasmic vesicles with a frequency comparable to that of untreated cells, whereas nucleocapsids were almost absent from the cytoplasm (Table 1). This shows that not endocytosis but endosomal escape was blocked by ammonium chloride. By adding ammonium chloride at different time points after a synchronized infection, we established that the halftime of endosomal escape in Pk1 cells is about 50 min (Fig. 6). These results support the conclusion that baculovirus enters the mammalian cell via endocytosis and is released into the cytoplasm by acid-induced fusion of the envelope with the endosomal membrane. We cannot determine from our data whether the nucleocapsids are modified by passage through the acidic endosomal environment.
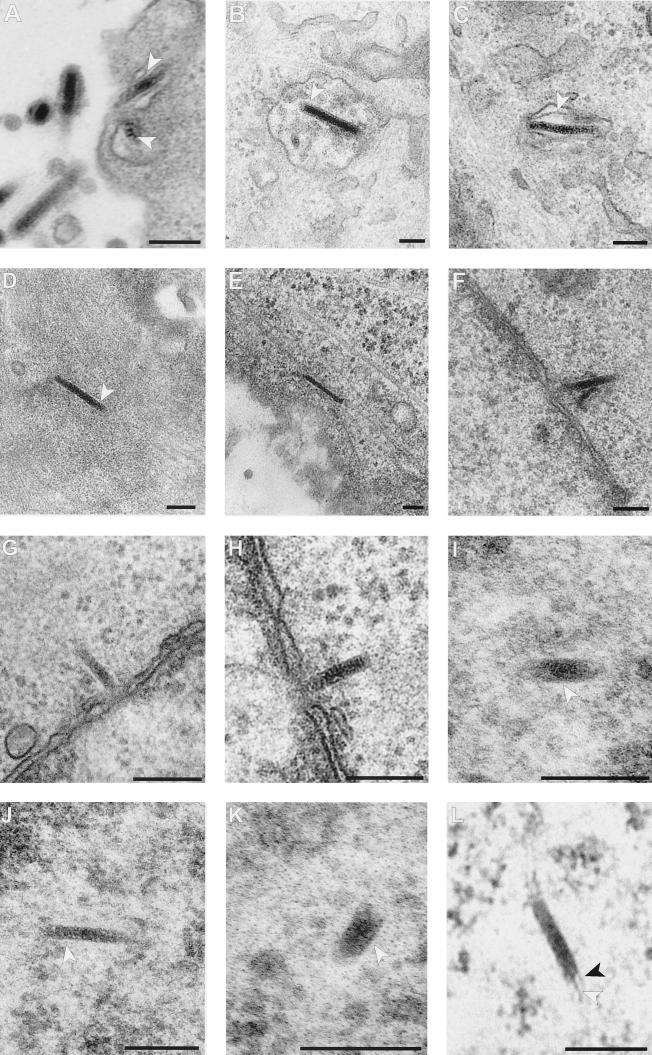
FIG. 5.
Electron micrographic analysis of GFP-bac infection. Pk1 cells were seeded on six-well plates (5 × 104/cm2) and infected with GFP-bac after 12 h at an MOI of 1,000; 4 h later the cells were fixed and processed. (A) Enveloped virus particles (indicated by arrows) entering Pk1 cells. (B and C) Enveloped virus particles inside cytoplasmic vesicles (the viral envelope is indicated by arrows). (D and E) GFP-bac nucleocapsid inside the cytoplasm. Note the dark (electron-dense) core of the capsid corresponding to the DNA and the lighter capsid wall around it (arrow). (F, G, and H) Nucleocapsids docking at the cytoplasmic side of the nuclear pores. (I, J, and K) Nucleocapsids (arrows) inside the nucleus. (L) Partially filled nucleocapsid. The thread leaving the capsid is indicated by a white arrow; the capsid shell is indicated by a black arrow. Bars, 100 nm.
TABLE 1.
Quantitative electron microscopic analysis of GFP-bac infection of Pk1 cellsa
| Treatment | Cells (no. of sections analyzed) | No. of enveloped viruses
|
No. of nucleocapsids
|
|||
|---|---|---|---|---|---|---|
| Plasma membrane | Vesicle | Cytoplasm | Nuclear pore | Nucleoplasm | ||
| None | 80 | 28 | 58 | 32 | 8 | 8 |
| Ammonium chloride | 35 | 18 | 30 | 1 | 0 | 0 |
| Cytochalasin D | 30 | 16 | 16 | 32 | 0 | 0 |
| Nocodazole | 80 | 35 | ND | ND | 6 | 4 |
| Aphidicolinb | 80 | NDc | ND | ND | 10 | 15 |
Pk1 cells were seeded on six-well plates (5 × 104/cm2) and infected at an MOI of 1,000 after 12 h in the presence of different toxins as indicated. Toxin concentrations, their efficacy, and the absence of apparent toxicity are described in Materials and Methods. The cells were fixed 4 h after infection and analyzed by electron microscopy, as described in the Materials and Methods. The table shows for each treatment condition in column 1 the total number of 80-nm sections analyzed (Cells), the number of enveloped viruses attached to the plasma membrane or inside vesicles, and the number of nucleocapsids in the cytoplasm, on the nuclear membrane, or inside the nucleus. Each selected field showed a complete section of a different cell covering nucleus, cytoplasm, and plasma membrane. Since the depolymerization of microtubules by nocodazole results in a highly disorganized cytoplasm, we were not able to accurately determine the number of nucleocapsids inside vesicles or in the cytoplasm of Pk1 cells treated with nocodazole.
In a separate experiment, the nuclei of aphidicolin-treated cells were analyzed. Due to possible differences in titer and infection conditions, we do not consider the difference from untreated control data to be significant.
ND, not determined.
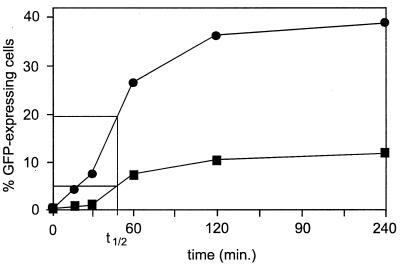
FIG. 6.
Endosomal escape of GFP-bac in Pk1 cells. To determine the rate of endosomal escape, ammonium chloride was added at different intervals after the infection of Pk1 cells. Pk1 cells seeded on six-well plates (104/cm2). The next day, untreated (■) or nocodazole-treated (●) cells were infected with GFP-bac at an MOI of 500 for 1 h at 0°C. Cells were washed and further incubated at 37°C. Ammonium chloride was added to a final concentration of 25 mM at different time points, as indicated. Cells were harvested and analyzed by FACS (percentage of GFP-expressing cells) 18 h after infection.
Baculovirus infection is inhibited by depolymerization of actin filaments.
It has been suggested that cytoplasmic transport of baculovirus nucleocapsid in insect cells is a result of capsid-induced actin polymerization (8, 21). We studied the role of the actin and tubulin filament system during GFP-bac infection of mammalian cells. One-third of the capsids observed in the cytoplasm colocalized with filaments of different type in electron microscopic sections (Fig. 5D and E), which allows no conclusion concerning their mode of transport. Cytochalasin D, causes reversible depolymerization of actin filaments (see Materials and Methods), and strongly inhibits GFP expression of infected Pk1 cells (Fig. 4). By electron microscopy we observed that cytochalasin D neither prevents the uptake of enveloped virions inside cytoplasmic vesicles nor prevents their escape into the cytoplasm (Table 1). This is consistent with a role of actin filaments in cytoplasmic transport of baculovirus nucleocapsids in mammalian cells (see Discussion).
In contrast, toxins that cause depolymerization of microtubules, such as colchicine (data not shown), vinblastine (data not shown), or nocodazole strongly increase both the percentage of GFP-expressing Pk1 cells (Fig. 4A) and the amount of GFP that is produced per cell (Fig. 4B). Nocodazole does not affect the kinetics of endosomal escape (t1/2, Fig. 6). Apparently, intact microtubules are not required for the intracellular transport of nucleocapsids.
Nucleocapsids locate at the nuclear pore and in the nucleus of nonmitotic cells.
The ability of baculovirus to infect nondividing mammalian cells implies nuclear transport of the viral genome. We therefore analyzed the nuclear transport of baculovirus capsids. At 4 h after the infection of Pk1 cells at a high MOI, about 8% of the internalized nucleocapsids was found localized at the cytoplasmic side of a nuclear pore (Fig. 5F to H, Table 1). Also, in nocodazole- or aphidicolin-treated cells, nucleocapsids were found localized at the nuclear pores (Table 1). All of these capsids contained electron-dense material, indicating that the genome is still present inside (11).
The next step of the infection sequence is the transport of the viral genome into the nucleus. At 4 h after infection, aphidicolin-arrested Pk1 cells were analyzed by FISH and confocal microscopy. Half of the analyzed nuclei (n = 20) contained 5 to 10 GFP-bac genomes (Fig. 7). This corresponds well with the observed infection efficiency (percentage of GFP-expressing cells) under these conditions (data not shown). To establish whether capsid proteins are transported into the nucleus as well, we analyzed the localization of the major capsid protein p39 in Pk1 cells by confocal immunofluorescence microscopy. The aphidicolin-arrested cells were infected as described for the FISH experiments. Inside about half of the nuclei (n = 16) we observed 5 to 10 p39 capsid protein spots by confocal analysis (Fig. 8). Under these conditions, cytoplasmic p39 spots are observed mainly in the basal area of Pk1 cells, which is not visible in the selected confocal sections, but not in the nonsusceptible H35 cells (not shown). These results indicate that both the viral genome and the capsid are transported into the nucleus of nonmitotic cells.
Analysis of electron micrographs of Pk1 cells 4 h after infection revealed nucleocapsids inside the nucleus of untreated and nocodazole- or aphidicolin-treated cells (Fig. 5I, J, and K, Table 1). When Pk1 cells were arrested by aphidicolin in G1/S, as described earlier, one in five 80-nm sections of different cells did show a nuclear nucleocapsid (Table 1). In the presence of nocodazole no mitosis takes place, and the nuclear membrane stays intact. Under this condition, four nucleocapsids were found in 80 sections of different nuclei (Table 1). We conclude that the capsids did not enter the nucleus as a result of a mitotic event but are transported through the nuclear pore. Of 27 nucleocapsids observed inside the nucleus, 25 were filled with electron-dense material, indicating that the DNA was still present inside the capsid. Two nucleocapsids were only partially filled. One of these showed a thread-like structure which appears to represent DNA with associated proteins leaving the nucleocapsid (Fig. 5L). No empty capsids were observed, but if present they would be difficult to identify against the granular background of the chromatin.
When endosomal escape was inhibited by ammonium chloride or actin filaments were disrupted by cytochalasin D, we did not observe any p39 capsid protein, or GFP-bac genome in the nucleus by confocal microscopy (n = 15 for each condition; data not shown). Moreover, no nucleocapsids were observed at the nuclear membrane or inside the nucleus by electron microscopy under these conditions (Table 1). This is in accordance with the strong inhibition of infection by both ammonium chloride and cytochalasin D.
These data are consistent with an infection mechanism in mammalian cells in which the baculovirus nucleocapsid, after escape from the endosomal compartment, docks at the nuclear pore and is subsequently transported into the nuclear lumen.
DISCUSSION
We have studied the infection pathway of baculovirus (AcMNPV) in mammalian cells, in particular, the transport of the viral genome into the nucleus. In agreement with previous reports (6, 9, 17, 26), we show that baculovirus can successfully infect different mammalian cell types (Fig. 1). At first it was suggested that infection was liver specific and that the asialoglycoprotein receptor could be involved (6, 17). However, more recent data show that baculovirus infection is not restricted to liver-derived cells (9, 26). Our results confirm and extend this last observation and show that Pk1 cells, which do not express the asialoglycoprotein receptor, can be successfully infected (Fig. 1). We cannot infer from our data whether or not a specific receptor is involved in the infection of mammalian cells. However, a nonspecific interaction seems unlikely since H35 cells do not express GFP after infection with GFP-bac (Fig. 1). Baculovirus capsids were not detected inside these cells by immunohistochemistry and confocal analysis (data not shown), whereas they are active in endocytosis (FITC-dextran uptake; data not shown). This is consistent with the hypothesis that H35 cells lack the as-yet-unidentified receptor required for baculovirus uptake in mammalian cells.
In insect cells, it has been shown that the acidification of the endosomes induces a structural change in the viral fusion protein gp64, which enables endosomal escape (3, 24). Recent results suggest that also in mammalian cells the pH-dependent gp64 fusion protein function is essential in infection (16). Our data extend these findings by showing that baculovirus infection of Pk1 cells starts with endocytosis (Fig. 5, Table 1), followed within 1 h by acid-induced endosomal escape from the endosome into the cytoplasm (Fig. 4 and 6, Table 1). During this process, the virus loses its envelope (Fig. 5). After endosomal escape the nucleocapsid has to be transported through the cytoplasm toward the nucleus.
Several large viruses are dependent on the cytoskeleton for transport through the cytoplasm toward the nucleus (15, 27). In insect cells, the involvement of actin filaments in cytoplasmic transport of baculovirus nucleocapsids has been suggested (8, 21). Our data show that in Pk1 cells cytochalasin D, which causes a reversible disintegration of intracellular actin filements, inhibits baculovirus infection (Fig. 4). It does not interfere with endocytosis or endosomal escape, but it does prevent transfer of the nucleocapsid to the nucleus (Table 1). While this is consistent with a role of actin filaments in the cytoplasmic transport of baculovirus nucleocapsids in mammalian cells, the exact nature of this interaction remains to be established. The large increase of GFP expression in the presence of substances that disrupt microtubules (Fig. 4 and 6) is remarkable but not readily explained. Nocodazole does not significantly increase endocytotic activity (FITC-dextran uptake; data not shown) and does not affect the t1/2 of endosomal escape of the virus (Fig. 6). Also the number of nucleocapsids in the nucleus is not increased compared to the untreated cells (Table 1 and confocal immunohistochemistry on 20 cells; also data not shown). Therefore, it appears that nocodazole has a yet-unexplained effect on the expression of the CMV-GFP marker gene.
The final phase of infection requires the transfer of the viral genome to the nuclear compartment. We have shown that baculovirus can infect nondividing mammalian cells (Fig. 2 and 3), which implies transport of the viral genome through the nuclear membrane. First, nucleocapsids appear to dock on the nuclear pore of infected cells (Fig. 5, Table 1). Our data imply that the next step involves the transport of the nucleocapsid through the nucleopore, with the condensed genome inside. This is based on the observation that not only the viral genome (Fig. 7) but also the major capsid protein (Fig. 8) is observed in the nuclei of infected nonmitotic cells. Moreover, all of the nucleocapsids observed at the nucleopore were electron dense, and electron-dense nucleocapsids were observed inside the nucleus of growth-arrested cells (Fig. 5, Table 1). High electron density in electron micrographs is evidence that the capsids were filled with DNA, as has been shown for baculovirus (11, 12, 28) and herpesvirus (27). Further studies are required to establish which elements of the nuclear transport machinery interact with the baculovirus capsid to allow passage through the nuclear pore.
The mode of entry of baculovirus into the nucleus appears to be different from that of other DNA viruses. Adenovirus and HSV have spherical capsids that are not transported through a nuclear pore (33). The HSV nucleocapsid coat is left empty at the nuclear pore after release and nuclear transport of the viral genome (1, 27, 33). The adenovirus capsid partially dissociates upon docking at the nuclear pore, and the genome is transported into the nucleus together with at least one capsid protein, leaving other components behind (14, 15). In contrast, the small diameter of the cigar-shaped baculovirus nucleocapsid (25 by 260 nm) allows its transport through the nuclear pore, since it has been shown that at least 23-nm-diameter gold particles can be transported (10).
During the last step of the infection process, the baculovirus genome is presumably released from the capsid inside the nucleus to allow transcription and replication of the condensed DNA (28). The mechanism of genome release of P. interpunctella granulosis virus (PiGV), a virus closely related to AcMNPV, has been studied in vitro (30). The nucleocapsid is stabilized by zinc ions; their removal by EDTA treatment results in release of the genome at the apical cap (12). We have observed the same phenomenon in AcMNPV (N.D.V.L., data not shown). It is not known what triggers release of the viral genome in the nuclei of either insect or mammalian cells.
For insect cells, conflicting results on the issue of nuclear transport of viral genome have been published. Our results are not in agreement with the nuclear transport mechanism suggested by Summers for PiGV infection of insect cells, i.e., genome release from the capsid docking at the nuclear pore (28). Our data do support the mechanism suggested by Granados and Lawler, who observed electron-dense AcMNPV capsids at the nuclear pore and inside the nucleus of infected insect cells (13). However, in this study the mitotic activity of the infected cells was not rigorously excluded. Although it seems unlikely, we cannot exclude the possibility that different strains of baculovirus (PiGV and AcMNPV) use different modes of nuclear genome transport in insect cells.
Modification of the infecting viral particle occurs during HSV infection, where tegument is shedded during cytoplasmic transport (1), and in adenovirus infection, where the nucleocapsid is sequentially dismantled during endosomal, cytoplasmic, and nuclear transport (14, 15). From our data it cannot be concluded whether such modifications are required for baculovirus nucleocapsid nuclear transport. At present it is not known how many proteins constitute the baculovirus capsid or which domains are exposed to interact with cellular proteins. We are presently performing studies to clarify this point.
The ability of baculovirus to infect mammalian cells has suggested its use as a gene therapy vector. We show here that baculovirus has the ability to infect nondividing cells efficiently, which is an important feature for in vivo gene transfer vectors (4, 35). The large and variable size of the capsid and genome (11, 20) suggests that it should be possible to package large expression cassettes in a baculovirus. The virus is not pathogenic in humans, and patients are not expected to have circulating antibodies against it. Moreover, it may be possible to make patients tolerant to baculovirus without serious risk, in contrast to virus vectors based on modified human pathogens. So far, in vivo infection experiments have been hampered by the fact that the virus is rapidly inactivated by serum complement (18). Furthermore, interaction of baculovirus with Kupffer cells in vitro was shown to elicit a cytokine response, which may result in an inflammatory reaction in vivo (2). Modification of the envelope membrane, e.g., with complement inhibitors and receptor ligands, can potentially solve these problems. Changing the receptor specificity of the virus may also be possible by modification of envelope fusion protein gp64 (22) or by linking receptor targeted elements to the viral envelope. Finally, baculovirus may prove to be a useful and safe vector for the transfer of large genomic constructs in applications that allow an ex vivo approach. However, further studies are required to establish whether low-level production of viral proteins occurs in mammalian cells infected with baculovirus, which could elicit an immune response after transplantation.
A different approach would be to identify the capsid proteins involved in intracellular and nuclear transport and to use this knowledge to develop a synthetic gene transfer system that will not only target a transgene to the nucleus but also transport it into the nucleus.
ACKNOWLEDGMENTS
We thank Pim Visser for help with electron microscopic analysis, An Langeveld for FISH analysis, André Houtsmüller for help with confocal analysis, and Ron A. M. Fouchier for critically reading the manuscript. The p39 monoclonal antibody was a generous gift from J. S. Manning (Department of Microbiology, University of California, Davis).
REFERENCES
- 1.Batterson W, Furlong D, Roizman B. Molecular genetics of herpes simplex virus. VIII. Further characterization of a temperature-sensitive mutant defective in release of viral DNA and in other stages of the viral reproductive cycle. J Virol. 1983;45:397–407. doi: 10.1128/jvi.45.1.397-407.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck N B, Sidhu J S, Omiecinski C J. Baculovirus vectors repress phenobarbital-mediated gene induction and stimulate cytokine expression in primary cultures of rat hepatocytes. Gene Ther. 2000;7:1274–1283. doi: 10.1038/sj.gt.3301246. [DOI] [PubMed] [Google Scholar]
- 3.Blissard G W, Wenz J R. Baculovirus gp64 envelope glycoprotein is sufficient to mediate pH-dependent membrane fusion. J Virol. 1992;66:6829–6835. doi: 10.1128/jvi.66.11.6829-6835.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulikas T. Nuclear localization signal peptides for the import of plasmid DNA in gene therapy. Int J Oncol. 1997;10:301–309. doi: 10.3892/ijo.10.2.301. [DOI] [PubMed] [Google Scholar]
- 5.Bowman E J, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyce F M, Bucher N L. Baculovirus-mediated gene transfer into mammalian cells. Proc Natl Acad Sci USA. 1996;93:2348–2352. doi: 10.1073/pnas.93.6.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braunagel S C, Summers M D. Autographa californica nuclear polyhedrosis virus, PDV, and ECV viral envelopes and nucleocapsids: structural proteins, antigens, lipid and fatty acid profiles. Virology. 1994;202:315–328. doi: 10.1006/viro.1994.1348. [DOI] [PubMed] [Google Scholar]
- 8.Charlton C A, Volkman L E. Penetration of Autographa californica nuclear polyhedrosis virus nucleocapsids into IPLB Sf 21 cells induces actin cable formation. Virology. 1993;197:245–254. doi: 10.1006/viro.1993.1585. [DOI] [PubMed] [Google Scholar]
- 9.Condreay J P, Witherspoon S M, Clay W C, Kost T A. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc Natl Acad Sci USA. 1999;96:127–132. doi: 10.1073/pnas.96.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dworetzky S I, Feldherr C M. Translocation of RNA-coated gold particles through the nuclear pores of oocytes. J Cell Biol. 1988;106:575–584. doi: 10.1083/jcb.106.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraser M J. Ultrastructural observations of virion maturation in Autographa californica nuclear polyhedrosis virus-infected Spodoptera frugiperda cell cultures. J Ultrastruct Mol Struct Res. 1986;95:189–195. [Google Scholar]
- 12.Funk C J, Consigli R A. Evidence for zinc binding by two structural proteins of Plodia interpunctella granulosis virus. J Virol. 1992;66:3168–3171. doi: 10.1128/jvi.66.5.3168-3171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granados R R, Lawler K A. In vivo pathway of Autographa californica baculovirus invasion and infection. Virology. 1981;108:297–308. doi: 10.1016/0042-6822(81)90438-4. [DOI] [PubMed] [Google Scholar]
- 14.Greber U F, Suomalainen M, Stidwill R P, Boucke K, Ebersold M W, Helenius A. The role of the nuclear pore complex in adenovirus DNA entry. EMBO J. 1997;16:5998–6007. doi: 10.1093/emboj/16.19.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greber U F, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann C, Lehnert W, Strauss M. The baculovirus vector system for gene delivery into hepatocytes. Gene Ther Mol Biol. 1998;1:231–239. [Google Scholar]
- 17.Hofmann C, Sandig V, Jennings G, Rudolph M, Schlag P, Strauss M. Efficient gene transfer into human hepatocytes by baculovirus vectors. Proc Natl Acad Sci USA. 1995;92:10099–10103. doi: 10.1073/pnas.92.22.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann C, Strauss M. Baculovirus-mediated gene transfer in the presence of human serum or blood facilitated by inhibition of the complement system. Gene Ther. 1998;5:531–536. doi: 10.1038/sj.gt.3300607. [DOI] [PubMed] [Google Scholar]
- 19.Ince C, van Dissel J T, Diesselhoff M M. A Teflon culture dish for high-magnification microscopy and measurements in single cells. Pfluegers Arch. 1985;403:240–244. doi: 10.1007/BF00583594. [DOI] [PubMed] [Google Scholar]
- 20.Kool M, Voncken J W, van Lier F L, Tramper J, Vlak J M. Detection and analysis of Autographa californica nuclear polyhedrosis virus mutants with defective interfering properties. Virology. 1991;183:739–746. doi: 10.1016/0042-6822(91)91003-y. [DOI] [PubMed] [Google Scholar]
- 21.Lanier L M, Volkman L E. Actin binding and nucleation by Autographa californica M nucleopolyhedrovirus. Virology. 1998;243:167–177. doi: 10.1006/viro.1998.9065. [DOI] [PubMed] [Google Scholar]
- 22.Mottershead D. Baculoviral display of the green fluorescent protein and rubella virus envelope proteins. Biochem Biophys Res Commun. 1997;238:717–722. doi: 10.1006/bbrc.1997.7372. [DOI] [PubMed] [Google Scholar]
- 23.Mulder M P, Wilke M, Langeveld A, Wilming L G, Hagemeijer A, van Drunen E, Zwarthoff E C, Riegman P H, Deelen W H, van den Ouweland A M, et al. Positional mapping of loci in the DiGeorge critical region at chromosome 22q11 using a new marker (D22S183) Hum Genet. 1995;96:133–141. doi: 10.1007/BF00207368. [DOI] [PubMed] [Google Scholar]
- 24.Plonsky I, Zimmerberg J. The initial fusion pore induced by baculovirus GP64 is large and forms quickly. J Cell Biol. 1996;135:1831–1839. doi: 10.1083/jcb.135.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seglen P O. Inhibitors of lysosomal function. Methods Enzymol. 1983;96:737–764. doi: 10.1016/s0076-6879(83)96063-9. [DOI] [PubMed] [Google Scholar]
- 26.Shoji I, Aizaki H, Tani H, Ishii K, Chiba T, Saito I, Miyamura T, Matsuura Y. Efficient gene transfer into various mammalian cells, including non-hepatic cells, by baculovirus vectors. J Gen Virol. 1997;78:2657–2664. doi: 10.1099/0022-1317-78-10-2657. [DOI] [PubMed] [Google Scholar]
- 27.Sodeik B, Ebersold M W, Helenius A. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol. 1997;136:1007–1021. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Summers M D. Electron microscopic observations on granulosis virus entry, uncoating and replication processes during infection of the midgut cells of Trichoplusia ni. J Ultrastruct Res. 1971;35:606–625. doi: 10.1016/s0022-5320(71)80014-x. [DOI] [PubMed] [Google Scholar]
- 29.Summers M D, Smith G E. Baculovirus structural polypeptides. Virology. 1978;84:390–402. doi: 10.1016/0042-6822(78)90257-x. [DOI] [PubMed] [Google Scholar]
- 30.Tweeten K A, Bulla L A, Jr, Consigli R A. Characterization of an extremely basic protein derived from granulosis virus nucleocapsids. J Virol. 1980;33:866–876. doi: 10.1128/jvi.33.2.866-876.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verma I M, Somia N. Gene therapy: promises, problems and prospects. Nature. 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 32.Wang P, Hammer D A, Granados R R. Binding and fusion of Autographa californica nucleopolyhedrovirus to cultured insect cells. J Gen Virol. 1997;78:3081–3089. doi: 10.1099/0022-1317-78-12-3081. [DOI] [PubMed] [Google Scholar]
- 33.Whittaker G R, Helenius A. Nuclear import and export of viruses and virus genomes. Virology. 1998;246:1–23. doi: 10.1006/viro.1998.9165. [DOI] [PubMed] [Google Scholar]
- 34.Wickham T J, Granados R R, Wood H A, Hammer D A, Shuler M L. General analysis of receptor-mediated viral attachment to cell surfaces. Biophys J. 1990;58:1501–1516. doi: 10.1016/S0006-3495(90)82495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilke M, Fortunati E, van den Broek M, Hoogeveen A T, Scholte B J. Efficacy of a peptide-based gene delivery system depends on mitotic activity. Gene Ther. 1996;3:1133–1142. [PubMed] [Google Scholar]