Abstract
Motivation
Large language models, trained on enormous corpora of biological sequences, are state-of-the-art for downstream genomic and proteomic tasks. Since the genome contains the information to encode all proteins, genomic language models (gLMs) hold the potential to make downstream predictions not only about DNA sequences, but also about proteins. However, the performance of gLMs on protein tasks remains unknown, due to few tasks pairing proteins with the coding DNA sequences (CDS) that can be processed by gLMs.
Results
In this work, we curated five such datasets and used them to evaluate the performance of gLMs and proteomic language models (pLMs). We show that gLMs are competitive and even outperform their pLMs counterparts on some tasks. The best performance was achieved using the retrieved CDS compared to sampling strategies. We found that training a joint genomic-proteomic model outperforms each individual approach, showing that they capture different but complementary sequence representations, as we demonstrate through model interpretation of their embeddings. Lastly, we explored different genomic tokenization schemes to improve downstream protein performance. We trained a new Nucleotide Transformer (50M) foundation model with 3mer tokenization that outperforms its 6mer counterpart on protein tasks while maintaining performance on genomics tasks. The application of gLMs to proteomics offers the potential to leverage rich CDS data, and in the spirit of the central dogma, the possibility of a unified and synergistic approach to genomics and proteomics.
Availability and implementation
We make our inference code, 3mer pre-trained model weights and datasets available.
1 Introduction
Large language models (LLMs) have revolutionized the field of NLP thanks to their ability to learn through self-supervision on unlabeled data (Devlin et al. 2018, Brown et al. 2020, Raffel et al. 2020). Recently, the same techniques have been applied to learn from biological data. The discrete and sequential nature of biological sequences, such as proteins or DNA and RNA, paired with the abundance of unlabeled data, obtained through high-throughput sequencing, make it a perfect application for these methods to thrive. This effort started first in proteomics, where several works showed that training large Transformer models to recover masked amino acids in protein sequences leads to powerful representations that can then be used to solve diverse downstream tasks with state-of-the-art performance (Elnaggar et al. 2021, Jumper et al. 2021, Lin et al. 2023). More recently, similar models were developed for genomics and trained over the human reference genome as well as hundreds of reference genomes from different species to recover masked consecutive nucleotides in chunks (Ji et al. 2021, Benegas et al. 2022, Dalla-Torre et al. 2023, Nguyen et al. 2023, Zhou et al. 2023, Nguyen et al. 2024). These DNA models, while more recent and still less mature than their protein counterparts, have also showed the ability to build strong representations of nucleic acid sequences to solve downstream tasks with improved performance, including predicting diverse DNA molecular phenotypes related to splicing, regulatory elements, and chromatin profiles.
Motivated by the central dogma of biology, which states that the genome encodes all protein information, and by the fact that codon usage can influence protein structure and function (Liu 2020), a third class of models, codon language models (cLMs), was recently introduced (Hallee et al. 2023, Li et al. 2024, Outeiral and Deane 2024). These models were trained on large datasets made of coding sequences (CDS) by reconstructing masked codons—rather than masked amino acids. Notably, the Codon Adaptation Language Model (CaLM) showed that cLMs can outperform their amino acid-based counterparts on several downstream tasks of interest such as species recognition, prediction of protein, and transcript abundance or melting point estimation when controlling for model size (Outeiral and Deane 2024). This improved performance seems to be related to the ability of codon-based language models to capture patterns of codon usage across DNA sequences.
Inspired by these recent results, we aimed to study to what extent genomic language models (gLMs) can be used as a general unified approach to solve tasks in both domains—genomics and proteomics. In opposition to cLMs, gLMs have been trained over full raw genomes and as such can be used to analyze noncoding regions as well as full genes including exons and intronic regions. While this makes gLMs widely capable for genomics tasks, their capacity to solve protein tasks from their corresponding CDS has not been explored. Since they have never seen “true” CDS per se during training, as exons are always separated by introns in eukaryotic species genomes, and coding sequences represent on average only of the human genome data used for training (Lander 2011), it is unclear to what extent these models can be competitive with protein language models (pLMs).
In this article, we present a comprehensive analysis of gLMs applied to protein-related tasks. We established a benchmark of five common protein analysis tasks and curated CDS sequences for a fair comparison between pLMs and gLMs. Our evaluation of two state-of-the-art pLMs and gLMs revealed that gLMs outperformed or matched pLMs on three out of five tasks, while underperforming on the remaining two. Notably, careful curation of matched CDS sequences was crucial for optimal gLM performance. The two tasks where pLMs significantly outperformed gLMs required sensitivity to codon-level changes. This observation led us to train a new gLM with a 3mer tokenization scheme, using full genomes as with our other gLMs. While our 3mer model slightly improved performance on the two codon-sensitive tasks, it did not enhance on the other proteins tasks and showed identical performance on genomic-specific tasks compared to its 6mer counterpart, suggesting that tokenization changes may not be the most fruitful path for improvement.
Intriguingly, gLMs significantly outperformed pLMs in predicting protein melting points—a trend also observed with cLMs. Further investigation revealed that gLMs achieve this by leveraging GC-content and species information from nucleotide sequences, features not captured by protein-based models. To better understand the representations learned by gLMs and pLMs, we designed a novel experimental protocol for systematically analyzing how these models represent sequences with single mutations within their embedding spaces. Interestingly, in the case of predicting beta-lactamase enzyme activity, gLMs clustered CDS by mutated positions, while pLMs grouped the corresponding proteins based on the mutated amino acid. These distinct representations motivated us to explore how to combine gLMs and pLMs to leverage their complementary strengths. We developed a new approach for combining both model classes. Our joint model recovers current state-of-the-art performance on four out of five tasks while setting a new state-of-the-art for protein stability prediction, demonstrating strong additive effects between their different sequence representations. We made the weights of our 3mer pre-trained model and the curated datasets available on HuggingFace.
2 Materials and methods
2.1 Models
Language models (LMs) are a statistical method for modeling language. These methods create a probability distribution over words, giving the likelihood that a sequence of words exists. A common way to train LM is with so-called cloze tests where the model is tasked with predicting a masked word (or words) given the context of the sentence. This is known as masked language modeling (MLM). Masked language modeling has seen incredible adoption and success because it allows one to leverage enormous amounts of unlabeled data to learn good representations. Models trained in an unsupervised manner with the masked language objective can then be fine-tuned for particular tasks.
This same approach has been widely adopted in biology where there is a large amount of unlabeled sequence data and relatively few labels. These sequences can be treated much like sentences. First applied to proteins, this approach has now seen success in modeling genomic sequences. The most powerful and common architecture for language modeling is the transformer. The transformer architecture operates on sets of tokens which are vector embeddings of components of the input sequences. If the input is an image, a token could be the vector of pixels in a patch. If the input is a sentence, the token might be a vector embedding of a sequence of characters, or a word. Indeed, this approach has seen success in both natural language processing (NLP) and computer vision.
When using the MLM objective to train language models, the most common approach is to use Bidirectional Encoder Representations from Transformers (BERT) (Devlin et al. 2018). This approach allows the language model to condition on the entire context of a masked token in pre-training, rather than perhaps just the left-context which as approach commonly used in auto-regressive human language modeling. Since biological sequences do not have the left-to-right structure of human sentences, it is integral to utilize bidirectional information. Embeddings representations of masked tokens from BERT are mapped to a probability distribution over the token vocabulary by a language modeling head.
2.1.1 Architecture
All models considered in this work were encoder-only transformers. The genomic language models considered were closely related to the ESM family of architectures (Lin et al. 2023), allowing a fair comparison between the genomic and proteomic approaches. All architectures share some main components. The first is an encoding layer which transforms tokens into an embedding space. The second is a transformer stack, which accepts this numerical representation and is trained to refine token representations in the context of the surrounding sequence. All models also have a head which transforms the refined embedding to some desired output. In pre-training models utilize a language model head which takes the final embedding representations of the model and converts it into a probability distribution over the vocabulary of tokens. In fine-tuning, the language model head is ignored and a single-layer task-specific head is responsible for transforming these final representations into predictions. As we are primarily studying existing pre-trained models and their fine-tuned performance and embeddings, we are predominantly concerned with the second setting.
Transformer block. The transformer block is the key component of the ESM architecture. Each transformer component consists of layer normalization followed by multi-headed self-attention. Self-attention is the mechanism that is central to the transformer architecture and allows all tokens to attend to one another in data-dependent dynamic manner. This is implemented via scaled dot-product attention.
Here, each token submits a query and key vectors of dimension dk, as well as a value vector. Each are stacked in matrices Q, K, and V respectively. The dot product of the query matrix Q and the key matrix K, calculates the similarity between each pair of query and key vectors. These attention values are normalized to a probability distribution with softmax and then used to dynamically scale the set of value vectors, V. Intuitively, the process asks how much any two tokens should attend to one another (the attention value), and does a weighted sum of the tokens value vectors according to the calculated attention.
2.1.2 Tokenization and token embedding
Although these models are quite similar, they do differ in the way in which they tokenize an input sequence, and the process by which they encode these tokens into a numerical representation.
ESM: Both ESM architectures tokenize on amino acids which is a natural way to break up protein sequences. The ESM-1b model uses learned positional embeddings to embed tokens. The ESM-2 architecture utilizes Rotary Position Embeddings (RoPE) (Su et al. 2021) which, unlike learned embeddings, allow the extrapolation to longer sequences than trained on.
Nucleotide transformer v2: The Nucleotide Transformer v2 (NT-v2) uses 6mer tokenization on DNA sequences. This coarser tokenization allows the model to accept sequences as long as 12kbp, which is particularly important for capturing long range genomic interactions. As described in the Nucleotide Transformer paper (Dalla-Torre et al. 2023), the second version of the model, similar to ESM, makes use of the RoPE embeddings scheme.
DNABERT2: DNABERT2 uses the Byte Pair Encoding scheme, rather than k-mers to tokenize DNA sequences (Zhou et al. 2023). BPE is a method first developed for encoding strings of text which iterative combines the most frequently occurring n-gram of length 2. The authors argue that this approach is more computationally efficient. DNABERT2 also replaces the learned positional embeddings of its predecessor with Attention with Linear Biases (ALiBi, Press et al. 2021), a method which, similar to RoPE, removes the constraint on input length.
2.1.3 Pre-training
We pre-trained the 3mer 50 million parameter NT-v2 identically to its 6mer counterpart described in the original Nucleotide Transformer paper (Dalla-Torre et al. 2023). The model was trained on 64 TPUv4s for 8 days on the Multispecies dataset (described in detail in the same paper) (Dalla-Torre et al. 2023). We used batch size of 8 sequences and a fixed length of 2048 tokens. Sampled sequences had 15% of their tokens altered. Of this subset, 80% were masked. We added noise by replacing an additional 15% of tokens with randomly selected standard tokens.
We utilized the batch sum of the cross-entropy between the predicted probabilities and the target tokens as the loss. The effective batch size was chosen to be 512, corresponding to million tokens. We used Adam optimizer (Kingma and Ba 2014) and learning rate scheduling as described in the Nucleotide Transformer paper (Dalla-Torre et al. 2023), with and . Over the first 16 000 steps, we utilized a warmup schedule which increased the learning rate linearly from to , and then decreasing as the square-root decay over the rest of pre-training. Validation was performed on a held-out set every tokens, and checkpoints were saved every tokens.
The 3mer model was trained until 600 billion tokens to match the number of base pairs which the 6mer model (trained to 300 billion tokens) had seen. However, we report the 3mer results for the checkpoint at 300B tokens as we did not see a significant increase in performance on the downstream tasks between the two checkpoints (Supplementary Table S3).
2.1.4 Fine-tuning
Fine-tuning of the models was done using IA3 (Liu et al. 2022) parameter-efficient fine-tuning, along with a single-layer classification or regression head. IA3 scales activation by a learnable vector, introducing a number of parameters approximately 0.1% of the total number of parameters. Models were fine-tuned with a batch size of eight. Adam optimizer was used with a learning rate of 0.003. Models were evaluated at fixed intervals over the validation set during training. For all models and tasks we used early stopping on the task’s respective test metric with a patience of 30 steps. In the case that early stopping does not occur we set a task-specific maximum for the number of training steps which we report in Supplementary Table S4. Checkpoints with the best performance in the validation set (highest R2 for regression tasks or lowest cross-entropy loss for classification tasks) were saved and evaluated on the respective test set. For robustness, we performed five different runs per task and model, using distinct random initialization of the fine-tuning parameters as well as different training and validation splits. With the exception of the Stability task, training and validation splits were resampled with replacement in each fold. For Stability, the training and validation splits used in the TorchProtein benchmark (Xu et al. 2022) came from different distributions, and so in order to be able to make a fair comparison of our methods, we shuffle the respective training and validation sets rather than resampling.
2.1.5 Evaluation methodology
The two pre-trained gLMs, DNABERT2 and NT-v2, and the two pre-trained pLMs, ESM-1b and ESM-2, were respectively evaluated with corresponding CDS and protein sequences as input and fine-tuned in similar conditions for a fair comparison. In opposition to all the other tasks that are regression tasks at the sequence level, the SSP task is a classification task at the amino acid level. This is simply performed by pLMs by predicting for each amino acid embedding a secondary structure from the eight possible classes (Supplementary Table S7). For the Nucleotide Transformer, as tokens represent 6-mers, each token embedding is mapped to two classification predictions corresponding to the two amino acids that the 6-mer represents. As DNABERT2 uses Byte Pair Encoding to tokenize nucleotides sequences, we could not retrieve any systematic mapping from tokens to amino acids and thus could not evaluate this model over the SSP task.
In addition to the primary evaluations, we conducted a comparative analysis of NT-v2 and both ESM models against two classical secondary structure prediction (SSP) algorithms. Specifically, we assessed PSIPRED (Buchan and Jones 2019), which utilizes BLAST searches to identify homologous sequences in conjunction with a neural network, and s4pred (Moffat and Jones 2021), a single-sequence method that operates independently of evolutionary or homologous data. Since PSIPRED does not support the 8-class SSP task, all models were instead evaluated on the 3-class SSP task, which only categorizes amino acids as helix, sheet, or coil.
2.2 Protein downstream tasks datasets
We study five protein tasks of interest that are frequent in the literature. This collection includes sequence- and residue-level tasks, spanning regression and multi-label classification. We detail and motivate below these five tasks. See Supplementary Table S1 for an overview of these tasks.
2.2.1 Tasks
Secondary structure prediction (SSP): Understanding the structure of proteins is integral to understanding their function. This task tests a model’s ability to learn local secondary structure. The task is a multi-label classification task where each input amino acid is associated with one of eight labels denoting which secondary structure that residue is a part of. All secondary structures were empirically derived using crystallography or NMR.
The structural data for the training and validation sets were collected by Klausen et al (Klausen et al. 2019). Crystal structures were retrieved from Protein Data Bank and filtered with a 25% sequence similarity threshold, a resolution at least as fine as 2.5 angstrom and a length of at least 20 amino acids (Klausen et al. 2019). Following the work of Klausen (Klausen et al. 2019), we used splits filtered at 25% sequence identity to ensure generalization, and evaluated the models on three independent test datasets: TS115 (Yang et al. 2018), CB513 (Cuff and Barton 1999), and CASP12 (Abriata et al. 2018). TS115 consists of 115 proteins whose structure has been determined by X-ray crystallography, filtered to a resolution of 3 Angstroms (Yang et al. 2018). CB513 is composed of 513 nonredundant protein regions from 434 proteins whose structures are determined by X-ray crystallography (Cuff and Barton 1999). CASP12 is a curated list of 21 proteins whose structures have been determined through crystallography or NMR (Abriata et al. 2018).
Melting point prediction (MPP): It is often desirable to design protein with specific thermostability profiles. However, predicting protein melting point can be a challenging task as even single residue mutations can have large impacts (Pinney et al. 2021). Melting point prediction (MPP) is a sequence-level regression task that evaluates a model’s ability to predict a measure of melting temperature.
The data originates from the thermostability atlas, which was originally measured and compiled using a mass spectrometry-based proteomic approach (Jarzab et al. 2020). We follow the same “mixed” splits described in FLIP (Dallago et al. 2021) which seek to avoid over-emphasis of large clusters. Sequences are clustered at 20% identity with 80% of clusters assigned to the train dataset and 20% of clusters assigned to the test dataset.
Beta-lactamase activity prediction: It is also important for models to have the precision to accurately predict the effects of single amino acid mutations (Xu et al. 2022). Beta-Lactamase is a regression task consisting of sequences from a study exploring the fitness landscape of all single codon substitutions in the TEM-1 gene (Firnberg et al. 2014). Labels indicate the ability of mutant genes to confer ampicillin resistance.
The data for this task is from Firnberg et al. (2014) which systematically examined the fitness landscape of all single codon mutations in the TEM-1 Beta-lactamase gene synthesized in native host Escherichia coli. The TEM-1 gene is known to confer antibiotic resistance, and fitness is taken to be a function of this resistance. In particular, gene fitness was measured by splitting the library of mutants onto thirteen sub-libraries exposed to increasing levels of ampicillin concentration. Using deep sequencing, the resistant alleles were counted in each sub-library. Unnormalized fitness fi of allele i was calculated as a weighted average of the allele counts on each plate by the log Amp concentration (Firnberg et al. 2014). Fitness values were then normalized by wildtype fitness, fWT, such that normalized fitness indicates a mutation which is more fit than wildtype (Firnberg et al. 2014).
Since beta-lactamase task consists of all single codon mutations, the dataset contains many degenerate coding sequences. In PEER (Xu et al. 2022) labels were averaged over degenerate coding sequences in the original dataset. This process removes much data and does not allow us to study gLMs on degenerate sequences, as well as the impact of synonymous mutation on fitness. Consequently, we propose two training datasets, sharing a single test set. The Complete set contains all CDS samples except those that are degenerate with respect to any CDS in the test set. The Unique set contains a random, maximal, subset of the nondegenerate coding sequences. This Unique set allows comparison between the gLMs and pLMs since all translated sequences are unique, while the Complete set demonstrates the impact of data availability on gLM performance. Notably, although the unique dataset is nondegenerate we use the raw fitness values of the CDS, rather than those averaged over degenerate sequences.
Fluorescence prediction: Estimating the fitness landscape of proteins which are many mutations away from the wildtype sequence is one of the core challenges of protein design. This task evaluates a model’s ability to predict log-fluorescence of higher-order mutant green fluorescent protein (avGFP) sequences.
Original data are from an experimental study of the avGFP fitness landscape (Sarkisyan et al. 2016). The library was generated via random mutagenesis of the wildtype sequence and synthesized in E.coli. Inspired from the TAPE and PEER benchmarks (Rao et al. 2019, Xu et al. 2022), we restrict the training set to amino acid sequences with three or fewer mutations from parent GFP sequences, while the test set is all sequences with four or more mutations.
Like the Beta-lactamase task, random mutagenesis of the avGFP gene led to CDS which were degenerate. However, since this process was much less systematic, this was true of a much smaller fraction of the sequences. In the training set and validation sets there were 54 025 uniquely translating CDS of the 58 417 total sequences. Since most sequences were nondegenerate we selected a random maximal subset and did not study the degenerate sequences. Notably, since the test set was higher order mutants, there was only one amino acid sequence (SS26C:SN168H:SD188V:SS200G) of the 27 217 which did not have a unique coding sequence. We removed randomly one of the two corresponding CDS. Notably, this means the test set is near identical to that used in TAPE (Rao et al. 2019) and PEER (Xu et al. 2022) benchmarks.
Protein stability prediction: It is important for models trained on diverse sequences to be able to accurately predict a small region of the fitness landscape. This regression task evaluates how well models predict stability around a small region of high-fitness sequences. The train and validation originate from a multi-round experiment and consist of a broad selection of de novo computationally designed proteins composing a small number of topologies. The test set consists of the neighborhoods of single codon mutations around a few of the most stable candidates (Rocklin et al. 2017).
The data for this task originates from Rocklin et al. (2017) in which stability is measured as a function of the resistance to increasing levels of protease. In particular, the designed libraries were synthesized in yeast and exposed to different concentrations of protease. At each level of protease the fraction of proteins remaining folded was measured, and these values were used to infer the EC50: the value at which half of cells express proteins that pass a defined stability threshold. The stability of a protein is then defined as the difference between the EC50 value of the protein and that of the predicted EC50 in the unfolded state, calculated in a scale (Rocklin et al. 2017).
2.2.2 Performance metrics
Since we have chosen tasks frequent in the literature, we follow the standard performance metrics that have been used in order to be able to compare our methods to previous results. For Beta-Lactamase Activity, Fluorescence and Stability we use Spearman’s ρ. For melting point prediction, we use the coefficient of determination (R2), and for secondary structure prediction, we use per amino acid accuracy.
2.3 Retrieving and curating coding sequences
One main contribution of this work is to retrieve, curate, and share consolidated CDS datasets for the five protein tasks of interest to allow the comparison of nucleic acid- and amino acid-based models. We detail in this paragraph how these CDS were collected for each task.
Melting point prediction: For MPP, we used the Uniprot Consortium (2023) ID mapping tool to map the Uniprot ID’s associated with each protein, available from the TAPE benchmark (Rao et al. 2019), to the DNA sequence database of EMBL CDS (Kanz et al. 2005). Any retrieved CDS from EMBL whose translation did not match the original amino acid sequences were filtered out.
Secondary structure prediction: In SSP, we used protein sequences with associated PDB ID’s (Berman et al. 2000) from the dataset hosted by NetsurfP-3.0 (Høie et al. 2022). To collect the CDS we first used the RCSB 1D Coordinate Server (Berman et al. 2000) which assembles alignments between structure and sequence databases, to find alignments to protein sequences from the Uniprot database. Returned alignments to Uniprot were filtered out if there was not complete coverage. The remaining Uniprot id’s were then mapped to the sequence database EMBL CDS using the same process as for MPP described above.
Beta-lactamase activity prediction: For the beta-lactamase task, all sequences corresponded to the same gene. We obtained the TEM-1 reference gene as well as the mutations from the supplementary material of reference (Rocklin et al. 2017).
Stability prediction: For the stability prediction task, coding sequences were taken from supplementary material of the original experimental study (Rocklin et al. 2017). Since all CDS translate into unique amino acids, we are able to match the dataset splits presented in TAPE (Rao et al. 2019).
Fluorescence prediction: Finally, for the fluorescence task we obtained the reference GFP gene, as well as its mutations from the reference of the original data (Sarkisyan et al. 2016). We chose to take the Unique subset as described above since the dataset was mostly nondegenerate.
2.4 Additional analysis
2.4.1 Nearest neighbor analysis
We define the neighborhood of a sequence s to be the set of sequences whose embeddings are the k-nearest neighbors of s’s embedding. More formally, let be a model mapping sequences in Vm to some d-dimensional embedding space. Here V is the token vocabulary of the model, m the fixed length of the input, and θ the model’s parameterization.
We can define the k-nearest neighbors of with respect some model , to be the set such that and
where dist is some metric on the embeddings space. In this work we take this metric to be the Euclidean distance. We also experiment with cosine similarity and find similar results. It is important to note that cosine similarity is not truly a distance metric as it does not satisfy the triangle inequality. There are also some concerns regarding fidelity and reliability of cosine similarity in high dimensions (Steck et al. 2024). For these reasons we choose to use the Euclidean distance.
2.4.2 TSNE dimensionality reduction
For the study of model embedding spaces, we chose to use TSNE to reduce the embedding dimensions so that they could be visualized in two dimensions. Although we experimented with other dimensionality reduction methods, we chosen TSNE because our study focused on local neighborhoods of embeddings and TSNE primarily maintains the relative distances between neighboring points. A future study looking at global organizing principles of embeddings spaces may choose to use another reducing method that respects global relationships between points (e.g. UMAP).
2.4.3 A comment on the meaning of “true CDS”
In this work, we use True CDS both to mean the transcript which really encoded a protein of interest, but also to mean a natural transcript that could have encoded the protein of interest. It is not always possible to retrieve the coding sequence which truly encoded the measured protein, as the study of interest may not have recorded in it. In the cases in which the coding sequence were recorded (Fluorescence, Beta-Lactamase, Stability) we were able evaluate the genomic language models on these sequences.
In the cases where the coding sequences were not available (Secondary Structure, Melting Point), we followed the aforementioned procedure detailed in methods. That is, we retrieved a biological transcript, present in a real reference genome, which encoded the protein of interest. While this does not guarantee that the transcript is identical to that which truly produced the protein, we argue that it the closest you can recover, and most importantly, we know with certainty that it is biological plausible. Research has shown that codon bias can vary within genes of the same species, and that certain codon may be important at specific locations in a gene. Sampling strategies generally do not respect these codon relationships, while our method does.
3 Results
3.1 Genomic language models are competitive on protein tasks
In this work we studied the performance of gLMs (Fig. 1a) and pLMs (Fig. 1b) on five protein tasks of interest which are frequent in the literature (Rao et al. 2019, Xu et al. 2022). This collection includes sequence- and residue-level tasks, spanning regression and multi-label classification. Two of the tasks focus on a single protein and aim to predict the impact of a small number of mutations on the protein fitness, while the three other encompass different proteins coming from multiple topologies and species. We report the tasks along with the size of the respective datasets in Supplementary Table S1.
Figure 1.
Genomic language models are competitive on protein tasks. (a, b) We outline key differences between gLMs and pLMs pre-training that make the task of building robust protein representations more difficult for gLMs. Unlike pLMs and cLMs, gLMs pre-training is predominantly () on noncoding regions of the genome, the vast majority of which (barring prokaryotic genomes) are noncontiguous, while fine-tuning and inference are carried out with contiguous CDS. In addition, coding regions during pre-training are not tokenized on codons, making amino acid representations nontrivial. (c) Evaluation results of Nucleotide Transformer v2 500M (NT-v2), DNABERT2, ESM-2 650M, and ESM-1b 650M on the test datasets of the proposed tasks. The metrics used to measure performance were chosen to match previous benchmarks and include Spearman correlation ρ, R2, and accuracy, with a higher value indicating better performance for all metrics. Error bars represent the standard deviation across five independent model runs. Notably NT-v2, matches, or supersedes pLMs on two of the five tasks.
To apply gLMs to protein tasks, it is necessary to create the DNA sequence-based version of the protein datasets, meaning the CDS sequence that encodes the proteins of interest (Fig. 1a). However, due to the degeneracy of the genetic code and the lack of protein identifiers in datasets, obtaining the true CDS of a given protein sequence is not trivial. Here, we have retrieved, curated and released these associated CDS for each of the aforementioned tasks. We detail process of retrieving these sequences in the Section 2. This dataset should enable further research into how gLMs may be applied to protein tasks.
For a comparison of pLMs with gLMs we decided to focus our attention on the ESM-2 (Lin et al. 2023) (650M parameters) and ESM-1b (Rives et al. 2021) (650M) models for proteins and NT-v2 (Dalla-Torre et al. 2023) (500M) and DNABERT2 (Zhou et al. 2023) (117M) for genomics, as these are considered as the state-of-the-art models in their respective fields. We evaluated these models by finetuning each on the five downstream protein tasks using the curated CDS sequences as inputs for gLMs and the respective amino acid sequences for pLMs (Fig. 1a and b). To ensure a fair comparison, we used the same methodology, number of steps and hyperparameters across all models, performed five independent runs for every model training, and report their mean performance and standard deviations. As recommended in recent work, we used a parameter-efficient finetuning approach (Dalla-Torre et al. 2023). See Methods for more details about our experimental protocol.
We compared the four aforementioned models over the five protein tasks (Fig. 1c and Supplementary Table S2). We excluded the secondary structure prediction task analysis for the DNABERT2 model as its tokenization technique yields tokens representing variable numbers of nucleotides, thus making it challenging to link tokens to amino acids. For NT-v2 we simply made two predictions per embedding as the model uses a 6mer tokenization. First, we observed that the NT-v2 matches or outperforms its DNABERT2 gLM counterpart on all the protein downstream tasks, confirming the recently published results on genomics downstream tasks (Dalla-Torre et al. 2023). Interestingly, ESM-2 and ESM-1b seem to have comparable performance over these five tasks.
Our results show that NT-v2 matches or exceeds the performance of its pLMs counterparts on three of the five tasks: Stability prediction, fluorescence prediction and melting point prediction (Fig. 1c and Supplementary Table S2). On melting point prediction in particular, NT-v2 and DNABERT2 models outperform significantly the ESM models. Interestingly, this result was also reported for cLMs (Outeiral and Deane 2024). We further explore these results later in this work. These results are noteworthy given the strong distribution shift between the genomic data used to train gLMs and the curated CDS sequences. In particular, the vast majority of pre-training sequences are noncoding and the small fraction (∼1% to 2%) which are coding, are genes rather than CDS, and so coding exons are separated by noncoding introns. Furthermore, genomic tokenization yields tokens that, unlike proteomic or codon tokenization, are not identifiable with codons. These differences are summarized in Fig. 1a and b. The results of Nucleotide Transformer on the protein tasks suggest that despite a significant distribution shift between pre-training and fine-tuning, as well as suboptimal protein tokenization, gLMs are able to capture protein features to the same extent than protein models.
However, we find that NT-v2 and DNABERT2 models under-perform pLMs on the beta-lactamase activity prediction and secondary structure prediction tasks (Fig. 1c). We have also compared the different gLMs and pLMs with two additional baselines: the state-of-the-art classic secondary structure prediction tool PSIPRED that uses BLAST search for homologous sequences (Buchan and Jones 2019), and S4Pred, the successor of PSIPRED single-sequence mode that only takes protein sequence as input (Moffat and Jones 2021). We observed that NT-v2 achieves a performance comparable with S4Pred but worse than full PSIPRED, while ESM-2 outperforms S4Pred in all and PSIPRED in two of the three test datasets (Supplementary Table S6). These results suggest that gLMs can capture global patterns in protein sequences but fail to capture finer-grained effects such as per amino acid structure or the impact of single point mutations, as assessed by secondary structure prediction and beta-lactamase respectively. We report here the results for the beta-lactamase complete dataset which contains all single codon substitutions of the TEM-1 gene. We also explored whether synonymous codon information is improving gLM performance by evaluating all models on a maximal, nondegenerate, unique set and found lower performance (Supplementary Fig. S1). Results for the unique set as well as the procedure for generating both dataset variants is described in methods.
3.2 True CDS sequences are essential to the performance of genomic language models
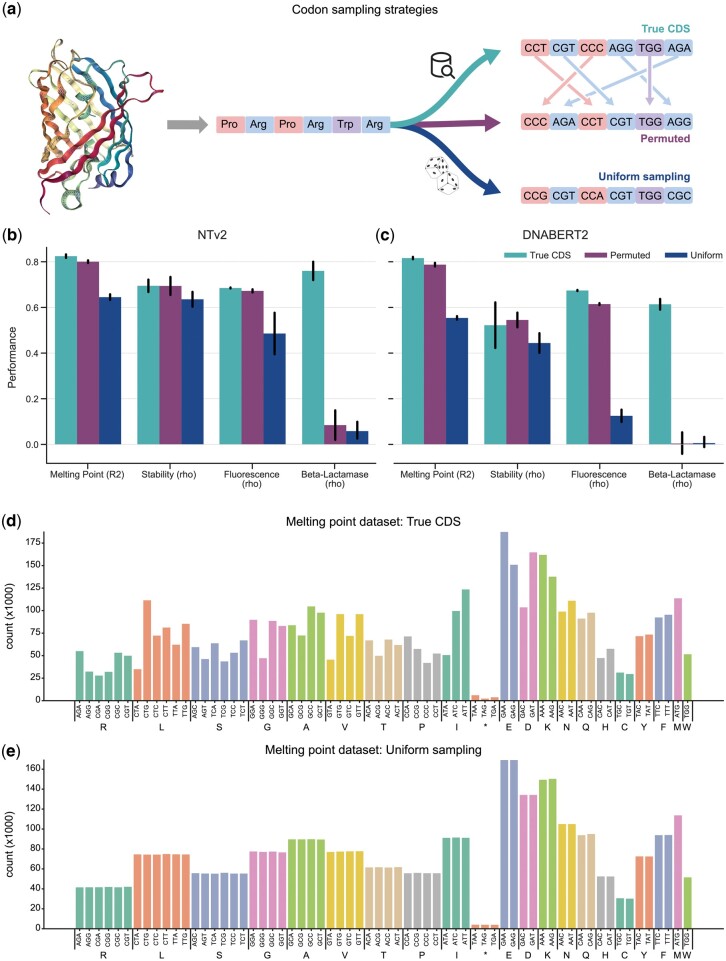
Since a growing body of biological literature indicates that codon bias influences protein folding (Saunders and Deane 2010, Liu 2020), there is a strong motivation to evaluate gLMs on the true CDS encoding the proteins of interest. However, this information is not always readily available, and when it is not, it can be a time-consuming, costly, or intractable process to retrieve the original CDS. As a result, in evaluating gLMs on proteins, it is common to approximate the desired CDS by sampling according to a given strategy. However, sampling from a global codon distribution may not recover biological plausible codon usage for a particular protein. Codon frequencies are known to vary not only between species (Supplementary Fig. S3), but also between genes of the same species (Parvathy et al. 2022), as we show for our five protein tasks (Fig. 2d and e, Supplementary Fig. S2). The implication is that it is unlikely that statistical sampling strategies can reconstruct the particular codon bias and relationships of a specific protein. The sampled CDS, although it may be drawn from some real codon distribution, may be unrealistic in the particular gene that it is sampled. While such studies have been conducted for codon language models, the effects of such imperfect codon sampling strategies on gLMs performance have not yet been studied.
Figure 2.
True CDS outperforms alternative sampling strategies. (a) A schematic showing the three codon sampling strategies studied, all of which preserve the amino acid sequence. The uniform mutation describes uniformly sampling a codon from the set of its synonymous codons, the permutation mutation describes randomly shuffling codons of the same amino acid in the sequence, and the third using the true CDS. (b, c) The impact of three codon sampling strategies on NT-v2 (b) and DNABERT2 (c) performance over four tasks. Error bars indicate the standard deviation across five independent model runs. We present the results for NT-v2 on SSP in the appendix since we could not evaluate DNABERT2 on SSP due to its BPE tokenization. Performance is measured as Spearman correlation for Fluorescence, beta-lactamase, and Stability, R2 for Melting Point, and accuracy for SSP classification task. (d, e) Disparity of codon frequencies between the true CDS distribution and uniform distribution for the melting point prediction task. We report the codon usage frequencies for each dataset in Supplementary Fig. S2.
To investigate this question, we compared the performance of gLMs finetuned on the true CDS or on two other codon sampling strategies (Fig. 2a): (i) we permuted codons of like amino acids to perturb codon usage while respecting codon frequencies from the true CDS and (ii) we sampled uniformly codons for each amino acid (note the difference in codon frequency between true and uniform sampling in Fig. 2d and e). We observed that on all tasks, having access to the “true” CDS improves the performance over sequences obtained by sampling codons from their natural frequencies (Fig. 2b and c), thus justifying the need for our curated dataset. We also show that randomly sampling codons yields degraded and close to zero performance on the beta-lactamase prediction task. These results were true for both NT-v2 and DNABERT2. In addition, we observed that NT-v2 is more robust than DNABERT2 to the codon distributions shift, which might be related to the different tokenizations, 6mers tokenization and byte-pair encoding (BPE), respectively.
3.3 3mer tokenization is not enough to close the gap between gLMs and pLMs
Given that pLMs were only superior to gLMs on the two protein tasks that required fine-grained amino acid-level resolution (beta-lactamase and Secondary Structure Prediction; Fig. 1c), and as codons are represented by groups of three nucleotides, we investigated whether 3mer tokenization in NT-v2 could be more suitable for those tasks than the current 6mer tokenization scheme. To address this, we pre-trained a 50M parameter NT-v2 model but replacing the 6mer tokenization by a 3mer one, while respecting the exact same training data (full genomes) and hyperparameters than NT-v2 models (Dalla-Torre et al. 2023) (Fig. 3a, Supplementary Fig. S4a and b). We then compared the performance of this newly pre-trained model to its 6mer counterpart as well as to ESM-2 150M, the closest ESM-2 model in terms of number of parameters.
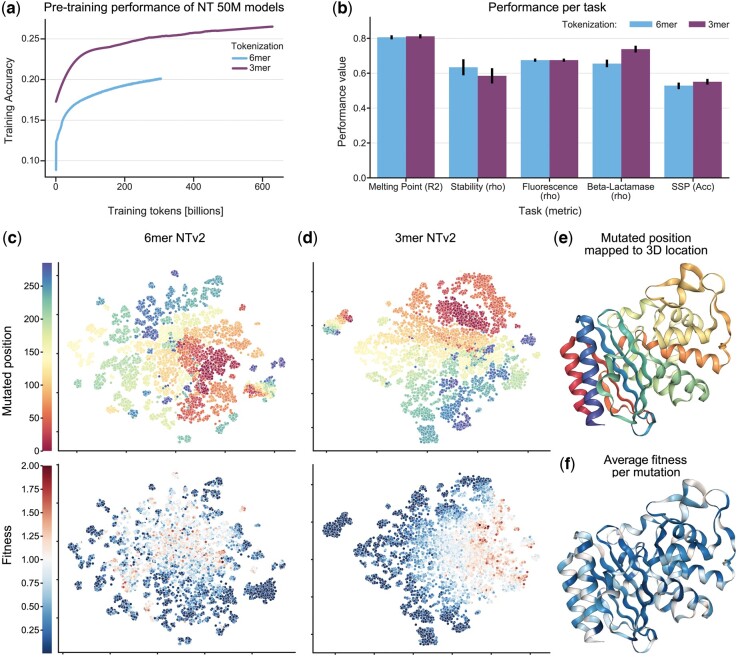
Figure 3.
3mer tokenization achieves better performance than 6-mer on specific protein tasks. (a) Pre-training validation accuracy of the 3-mer and 6-mer model on 600B tokens and 300B tokens respectively. (b) Test set performance of 3-mer model at the 300B checkpoint and 6-mer model at the 300B checkpoint. Error bars demonstrate the standard deviation across five independent model runs. The 3-mer tokenized model outperforms or equals the 6-mer on all tasks but one, despite seeing half of the nucleotide base pairs in pre-training. (c, d) Dimensionality reduction via t-SNE on the training set of the beta-lactamase enzyme activity task reveals that the 3-mer model better organizes sequences in relation to fitness and organizes similarly to the 6-mer model in regards to mutated position. Here, we take WT to be fitness 1.0. This result may suggest that the more fine-grained 3-mer resolution allows NT-v2 to better predict the single codon changes that compromise this dataset. (e) Mapping mutated position to the 3D structure of the protein reveals clustering by secondary structure. (f) Fitness averaged over synonymous mutation shows that most variants perform worse than WT.
We observed that the 3mer tokenization improves performance over 6mer tokenization on the beta-lactamase activity and structure prediction tasks (Fig. 3b). These two tasks require codon-level precision, which can explain why models that tokenize codons individually may have an advantage. However, this improved performance was not enough to close the gap with pLMs. In addition, 3mer tokenization does not improve over two other tasks and even decreases performance on the third task.
We note that as a result of coarser tokenization, the 6mer model has seen twice as many nucleotide base pairs in training as the 3mer model. For completeness, we trained the 3mer model up to the same number of base pairs (600 billion tokens). We find that despite consistent improvement measured by the pre-training objective, we see little change in performance on the protein downstream tasks (Supplementary Fig. S4c and Supplementary Table S3).
A t-SNE dimensionality reduction of beta-lactamse embeddings of the finetuned 3mer and 6mer models shows that the 3mer model organizes better the embeddings with respect to sequence fitness, supporting the evaluation results and the hypothesis that fine-grained tokenization is valuable for isolating the effects of single codon mutations (Fig. 3c–e). We also note that under the reduction, both models cluster embeddings of sequences which are mutated at similar locations in the pimrary structure of the protein (Fig. 3c–e). However, the 3mer model seems to globally organizes embeddings by position mutated, while the 6mer model only locally clusters sequences with similar mutated positions.
We also evaluated the 3mer tokenization model on the 18 downstream genomics tasks from the NT study (Dalla-Torre et al. 2023) to evaluate the impact of the tokenization on genomics datasets. Here we observed identical performance, within the margin of error, with its 6mer counterpart (Supplementary Fig. S4d and e). Grouping more nucleotides per token is beneficial to gLMs as it allows to extend their perception field, showed to improve performance (Avsec et al. 2021, de Almeida et al. 2024), while keeping the number of tokens constant and hence preventing the quadratic scaling of compute of Transformer models. As such, 6mer tokenization reduces compute time and cost compared to 3mer tokenization. As the 3mer tokenization does not yield significant overall improvement on protein downstream tasks, only on the ones that require codon-level precision, 6mer tokenization is to be preferred for gLMs. This also suggests that changing the tokenization scheme of gLMs might not be the most fruitful path toward advancing these models.
3.4 gLMs dominate for melting point prediction through the identification of GC-content, species, and codon usage
We showed that gLMs outperforms significantly their pLM counterpart on the melting point prediction task. A similar behavior has been reported for cLMs (Outeiral and Deane 2024). This motivated us to analyze the disparity between gLMs and pLMs performance on this task. In particular, we explored whether the superior performance of gLMs can be attributed to a biological phenomenon such as codon usage, or whether it is exploiting a “superficial” feature unique to CDS data. Here we define superficial as information readily available that does not contribute to a better understanding of proteins.
In investigating the impact of codon usage reported in Fig. 2a and b, we found that in the absence of codon usage information the NT-v2 performance drops below that of ESM, the gLM achieving an accuracy of only 0.64 compared to the pLM’s 0.72 (Fig. 1c). This result suggests that NT-v2 is utilizing codon frequencies. We next explored if the improved performance on the melting point prediction task could be related to additional sequence features. One indication that the NT-v2 might be exploiting superficial features of CDS would be if it can achieve the similar performance using only global sequence information. The motivation is that a biological phenomenon regarding codon usage would likely depend on its absolute and relative locations. To test this we developed two hypotheses around the use of global sequence information.
The GC-content hypothesis. We hypothesized that the NT-v2 may use GC-content to influence protein melting point prediction. The GC-content of a genomic sequence indicates the proportion of guanine (G) or cytosine (C) bases. G-C base pairs, featuring three hydrogen bonds, are more stable than A-T base pairs with two hydrogen bonds. Higher GC-content leads to higher melting temperatures in equal-length sequences. To test this hypothesis, we augmented both ESM-2 and NT-v2 with the sequence’s GC-content information by appending the normalized GC-content to the embeddings before making the melting point prediction. Although this addition moderately improves performance with an increase in R2 from 0.72 to 0.74, the model still lags behind NT-v2 (Fig. 4a). Augmenting NT-v2 with the same information does not lead to any increase in performance. This suggests that NT-v2 already has access to GC-content information.
Figure 4.
gLMs use species codon usage bias to outperform pLMs on melting point prediction. (a) The results of appending combinations of GC-content and Species to NT-v2 and ESM-2 embeddings during fine-tuning on the melting point prediction task. Error bars show the standard deviation of five independent folds. We find that species information accounts for the majority of the disparity of performance between ESM-2 and NT-v2. We also augment NT with the same information but see no change in performance indicating NT-v2 already has access to this information. We report full results in Supplementary Table S5. (b) The distribution of melting points for each species in the dataset shows distinct profiles. (c) Dimensionality reduction via t-SNE of the pre-trained and fine-tuned NT-v2 and ESM-2 models demonstrates that the gLM captures the structure of species information to a greater degree than pLM and initially acquired this knowledge from its pre-training. (d) We train ESM-2 and NT-v2 models to predict the species from sequence via fine-tuning with a single layer classification head. For each fold, we plot the f1-score, precision, and recall across species, weighted by the number of sequences in each species. (e) Bar plot for the overall species classification accuracy with error bars demonstrating the standard deviation across five independent folds. Results from both (d) and (e) confirm that NT is superior at identifying species. We present full confusion matrices in Supplementary Figs S5 and S6.
The species-level conditioning hypothesis. We next explored if the NT-v2 may exploit codon usage information to condition on the species the sequence was derived from. The melting point prediction dataset consists of proteins from thirteen different species ranging from unicellular E.coli, to mice and humans. Proteins of different species have distinct melting point profiles and identifiable codon preferences (Fig. 4b). Codon bias across species is a well-documented phenomenon that reflects mutational and selective pressures (Sharp et al. 2010), and is evident in the melting point prediction dataset (Supplementary Fig. S3). To test this hypothesis, first we verify that gLM can better identify species from sequence. We finetuned NT-v2 and ESM-2 on the task of species identification and found that NT achieves an accuracy of 0.95 while ESM-2 achieves an accuracy of only 0.81 (Fig. 4d and e; Supplementary Figs S5 and S6). In addition, we showed that the t-SNE for pretrained embeddings of models reveal that gLM embeddings are strongly structured by species while pLM are not (Fig. 4c).
To test whether species information may account for the difference in performance we augmented both ESM-2 and NT-v2 with the species information of each sequence and evaluated test set performance. This augmentation was done by appending a one-hot species-identifying vector to the embeddings of each model. We find that augmenting ESM-2 with species information increases performance from an R2 value of 0.72 to 0.79 (Fig. 4a). This closes most of the gap with the NT-v2 trained from curated CDS and brings the model to the performance of NT-v2 trained with permutated codons (no local information) which has an R2 of 0.80. In contrast, augmenting NT-v2 with species does not result in an improvement in performance (Supplementary Table S5), suggesting that NT-v2 achieves the majority of its advantage via conditioning on species information, which it learned during pre-training.
Using these findings, we finally tested if augmenting ESM-2 with both global attributes (GC-content and species) could recover the performance of NT-v2. Our results show that although there are additive benefits for ESM-2 from having both features, the majority of the information appears to come from species identification, and there still exists a gap in performance with NT-v2 (Fig. 4a, Supplementary Table S5). We presume this remaining advantage is coming from local codon interaction information present in the coding sequences.
3.5 gLMs and pLMs organize local embedding structure by different principles
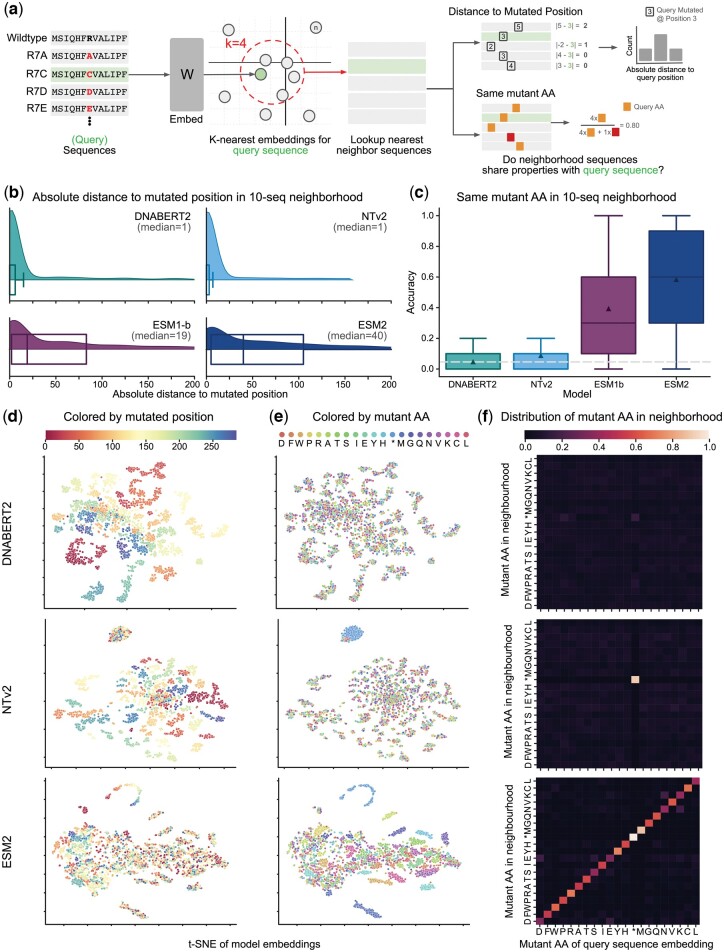
Following the melting point prediction analysis and the different performances between gLMs and pLMs, we aimed to better understand the differences in sequence representation between the different model types. To do so, we systematically visualized through t-SNEs the embeddings spaces of finetuned NT-v2 and ESM-2 models on all tasks. We have previously observed clustering by species in the embedding space of NT-v2 for the melting point prediction task (Fig. 4c). We also observed clustering by mutated positions and amino acids at mutated positions for models finetuned on the beta-lactamase activity prediction task (Fig. 5d and e). In order to confirm numerically this finding, we derived a novel experimental protocol to investigate local structure in the embedding space of each type of model (Fig. 5a). Our protocol aims to determine whether mutated sequences whose embeddings are in the same neighborhood in the finetuned model latent space, share similar properties with one another. To do so, we determined for each sequence in the dataset the k-nearest neighbors in each finetuned model latent space, using mean embeddings over tokens, and computed similarity metrics over the sequences properties. In the case of beta-lactamase, we looked specifically at the distance between mutated positions as well as similarities of amino acids in the mutated sequence that we named here amino acid accuracy.
Figure 5.
gLM and pLM organize protein embeddings via different sequence properties. (a) A schematic depicting the experimental procedure used to probe the model embedding spaces. The beta-lactamase enzyme activity task consists of all single codon mutations of the TEM1 gene, however we limit ourselves to a maximal nondegenerate subset. The procedure first embeds sequences, searches for nearest neighbors using Euclidean distance, and then determines whether neighbors share certain properties with the query sequence. (b) Distributions of the absolute difference between the mutated position of the query sequence and that of its 10 sequence neighborhood. Results show that the median absolute distance between the position of the query sequence and its neighbors is much larger for pLMs than gLMs. (c) Boxplot of the percent of sequences in each 10 sequence neighborhood which share the same mutated amino acid. The dotted line indicates the expected accuracy under a uniformly random distribution of embeddings, while the white triangles indicate the mean accuracy. (d) t-SNE of model embeddings averaged across the sequence length and colored by the location of the mutation. (e) The same t-SNE of model embeddings but now colored by the mutant amino acid. (f) Heatmaps where the x axis denotes the mutant amino acid of the query sequence and the column is the distribution of mutated amino acids of sequences whose embeddings are in the 10-neighborhood of that query vector.
Our method demonstrates that gLMs locally organize their sequences by the location of the mutation (Fig. 5d–f). In particular, we find that the median distance between the mutated position of a sequence and its 10 nearest neighbors is only 1 for gLMs, while for pLMs it is significantly larger (Fig. 5b). We report results for a neighborhood of size 10, but found that the results held for larger neighborhoods (Supplementary Fig. S7a).
Unlike gLMs, we observed that finetuned pLMs organize their sequences by the identity of the mutated amino acid (Fig. 5d–f). That is, sequences embeddings tend to be close if the mutant codon translates to the same amino acid. We find that for ESM-2 and ESM-1b, in a 10-embedding neighborhood sequences share the same mutant amino acid as the query sequences with accuracy 58.4% and 39.4% respectively (Fig. 5c). This is significantly above the expected accuracy of 4.6% where the embeddings arrange uniformly randomly with respect to mutant amino acid identity (Fig. 5c). The NT-v2 and DNABERT2 models have an amino acid accuracy of 8.8% and 4.8% respectively. Similarly to the mutated position, we find that this analysis is robust with respect to neighborhood size (Supplementary Fig. S7b).
3.6 Genomic and proteomic language models are additive
Finally, motivated by the competitive, and in some cases superior, performance of gLMs on our benchmark, as well as by the differences observed in gLMs and pLMs sequence latent spaces, we studied whether the two approaches may be complementary for solving protein tasks. To this end, we finetuned and evaluated a joint genomic-protein language model across all regression tasks. The joint model consists of NT-v2 and ESM-2 models with their final embedding concatenated together followed by a single layer regression head (Fig. 6a). The NT-v2 component uses the true CDS while the ESM-2 component uses the respective translated protein. The joint model is trained identically to each of its sub-models using identical training strategy and hyperparameters.
Figure 6.
Genomic and proteomic language models are additive. (a) Schematic depicting how the joint model was constructed. The true CDS and amino acid sequences were given as input to NT-v2 and ESM-2, respectively. Output embeddings from each model were averaged over the sequence length, concatenated together, and fed to a predictor head. (b) Radar plot of the test set performance on the four regression tasks proposed in the work. Results show that the joint model matches over outperforms its single model counterparts. (c) Spearman correlation on the test set for the protein stability task reveals that the joint genomic-proteomic outperforms state of the art models from literature. Error bars represent the standard deviation across independent model runs. We ran five folds for NT-v2, ESM-2, and Joint models, while remaining results were taken from the TorchProtein (Xu et al. 2022) leader board and are averaged over three seeds.
We found that on all tasks the joint model performs at least as well as genomic and proteomic sub-models (Fig. 6b). In particular, on the task of the stability prediction, the joint model outperforms each individual approach and achieves a new state-of-the art performance against the PEER Benchmark for Protein Sequence Understanding (Xu et al. 2022), even outperforming approaches which utilize additional external data and multi-task learning (Fig. 6c). Overall, our results show that each type of model captures different but complementary sequence representations that can be combined in joint genomic-proteomic models to achieve improved performance on protein tasks. The success of this joint genomic-proteomic approaches motivates continued research into how we might build protein models that leverage the best of both approaches.
4 Discussion
To the best of our knowledge, this work represents the first attempt to evaluate the potential of gLMs to solve protein tasks. In order to do that, we retrieved, curated, consolidated, and published protein datasets with the respective CDS sequences that encode these proteins. We show that evaluating gLMs on true CDS is the fairest way to compare them to pLM on protein tasks, offering gLM the potential to leverage codon information that may influence protein structure (Saunders and Deane 2010, Liu 2020). We show that gLMs perform consistently better on true CDS than on sequences generated from other, even quite similar, sampling strategies. We release the datasets publicly with the hope that they will encourage further research to build general models that can be widely used across fields in biology including genomics and proteomics.
We evaluated two state-of-the-art gLMs (NT-v2 & DNABERT2) and two pLMs (ESM-2 & ESM-1b) across this benchmark, offering the first preliminary comparison of the two approaches on protein downstream tasks. We demonstrate that gLMs are surprisingly capable on protein downstream tasks, especially given that their pre-training on whole genomes and noncontiguous coding regions are not conducive to learning protein representations. In particular, we find that gLMs match or exceed pLMs on 2 of the 5 tasks of interest, even outperforming pLMs on melting point prediction.
On melting point prediction, we find that gLMs greatly outperform their protein counterparts, as previously reported for the cLM CaLM (Outeiral and Deane 2024). We investigate this disparity in performance and explore whether such models represent better protein sequences or whether they capture other superficial features of the CDS not present in the translated amino acid sequence. We find that it is largely explainable by gLMs’ ability to identify and condition on the species of a sequence based on the codon bias fingerprint present in the CDS.
We note that NT, and gLMs in general, underperform relative to pLMs on the two tasks that require fine-grained codon-level precision. We investigate whether this performance difference may, in part, be due to the NTs coarser 6mer tokenization. To this end we pre-trained from scratch a 3mer tokenized NT-v2 model and compared it to an identically trained and sized 6mer model, finding that the finer tokenization consistently performs better on those two downstream protein tasks. This suggests that while the 6mer tokenization may be an ideal trade-off for gLMs on genomic tasks, which often benefit from longer context windows, for protein tasks the coarseness of multi-residue tokenization may come at a cost.
In addition to the tasks that require codon-level resolution, we do not expect these sequence-based gLMs to be competitive on tasks that require structural information, like solvent accessibility prediction, or that require residue-residue contact maps. However, gLMs can be used as general sequence encoders and used together with models that capture such protein structural properties. As future work, it would be interesting to see a comparison between gLMs and pLMs on this type of tasks and their potential mutual complementarity.
While we tried to standardize all hyperparameters and training steps, there are inherent differences between the models that were not possible to account for on our analyses and can influence the conclusions about which class of models is superior. These include different model architectures, input dimensions, tokenizations, and pre-training data. To mitigate this limitation we have benchmarked different models within each gLMs/pLMs category that differ in some of those aspects, in order to achieve robust conclusions about the different model classes. This benchmark is readily available to evaluate future models with different architectures and should foster the development of models that improve on the technical limitations of the current models described in this work.
In addition, we explored whether gLMs and pLMs represent proteins in a fundamentally different way. We offer some preliminary analysis that focuses on how each model type organizes its sequence embedding space. Our results suggest that the two methods structure local neighborhoods of embeddings according to different principles. In particular, local regions of protein embedding space shared common mutated amino acids, while local regions of genomic embedding spaces shared a common location of mutation in the primary structure of the protein. These differences in structure exist in the embeddings space, but continue to be apparent when the dimensionality is reduced.
Since gLMs achieved state-of-the-art performance on several of the protein downstream tasks and capture different sequence representations than pLMs, we explored whether gLMs and pLMs may be additive. Indeed, we found that each type of model captured different but complementary sequence representations and that a joint genomic-proteomic model supersedes both individual approaches. This results suggests that the synergy of gLMs and pLMs may be a promising future direction for improving the performance on protein tasks.
The demonstrated successes of gLMs in modeling protein sequences indicate that they are a good starting point for building unified foundational models for biology, but there is still much work needed to understand how to improve these models. Our findings suggest that gLMs and pLMs have different strengths and represent proteins in different ways. This observation not only further motivates research into the interpretability of gLMs for protein tasks, but also into joint methods that may leverage the advantages of both approaches. We expect that the collection and release of the five CDS datasets will help the community to make progress in this direction.
Supplementary Material
Acknowledgements
We would like to thank Patrick Bordes for help on assembling the CDS dataset for the MPP task.
Contributor Information
Sam Boshar, InstaDeep, Cambridge, MA 02142, United States.
Evan Trop, InstaDeep, Cambridge, MA 02142, United States.
Bernardo P de Almeida, InstaDeep, Paris 75010, France.
Liviu Copoiu, InstaDeep, London W2 1AY, United Kingdom.
Thomas Pierrot, InstaDeep, Cambridge, MA 02142, United States.
Author contributions
Sam Boshar (Conceptualization [equal], Data curation [equal], Formal analysis [equal], Investigation [equal], Methodology [equal], Software [equal], Validation [equal], Visualization [equal], Writing—original draft [equal]), Evan Trop (Conceptualization [equal], Formal analysis [equal], Investigation [equal], Methodology [equal], Resources [equal], Software [equal], Supervision [equal], Validation [equal], Visualization [equal], Writing—original draft [equal], Writing—review & editing [equal]), Bernardo P. de Almeida (Conceptualization [equal], Formal analysis [equal], Investigation [equal], Methodology [equal], Software [equal], Supervision [equal], Validation [equal], Visualization [equal], Writing—original draft [equal], Writing—review & editing [equal]), Liviu Copoiu (Conceptualization [equal], Data curation [equal], Resources [equal], Writing—review & editing [equal]), and Thomas Pierrot (Conceptualization [equal], Funding acquisition [equal], Investigation [equal], Methodology [equal], Project administration [equal], Supervision [equal], Writing—original draft [equal], Writing—review & editing [equal]).
Supplementary data
Supplementary data are available at Bioinformatics online.
Conflict of interest
None declared.
Funding
None declared.
Data availability
We make available our inference code, 3mer pre-trained model weights and datasets.
References
- Abriata LA, Tamò GE, Monastyrskyy B. et al. Assessment of hard target modeling in casp12 reveals an emerging role of alignment-based contact prediction methods. Proteins 2018;86:97–112. [DOI] [PubMed] [Google Scholar]
- Avsec Ž, Agarwal V, Visentin D. et al. Effective gene expression prediction from sequence by integrating long-range interactions. Nat Methods 2021;18:1196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benegas G, Batra SS, Song YS. DNA language models are powerful zero-shot predictors of non-coding variant effects. PNAS2023;120(44):e2311219120. [DOI] [PMC free article] [PubMed]
- Berman HM, Westbrook J, Feng Z. et al. The protein data bank. Nucleic Acids Res 2000;28:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TB, Mann B, Ryder N. et al. Language models are few-shot learners. In: Proceedings of the Neural Information Processing Systems (NeurIPS 2020), 2020.
- Buchan DWA, Jones DT.. The PSIPRED protein analysis workbench: 20 years on. Nucleic Acids Res 2019;47:W402–7. 10.1093/nar/gkz297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuff JA, Barton GJ.. Evaluation and improvement of multiple sequence methods for protein secondary structure prediction. Proteins 1999;34:508–19. [DOI] [PubMed] [Google Scholar]
- Dalla-Torre H, Gonzalez L, Mendoza-Revilla J. et al. The nucleotide transformer: building and evaluating robust foundation models for human genomics. bioRxiv, 10.1101/2023.01.11.523679, 2023, preprint: not peer reviewed. [DOI]
- Dallago C, Mou J, Johnston KE. et al. Flip: benchmark tasks in fitness landscape inference for proteins. Proceedings of the Neural Information Processing Systems Track on Datasets and Benchmarks 1 (NeurIPS 2021), 2021.
- de Almeida BP, Dalla-Torre H, Richard G. et al. SegmentNT: annotating the genome at single-nucleotide resolution with DNA foundation models. bioRxiv, 10.1101/2024.03.14.584712, 2024, preprint: not peer reviewed. [DOI]
- Devlin J, Chang M-W, Lee K. et al. Bert: pre-training of deep bidirectional transformers for language understanding. arXiv, arXiv:1810.04805, 2018, preprint: not peer reviewed.
- Elnaggar A, Heinzinger M, Dallago C. et al. ProtTrans: toward understanding the language of life through self-supervised learning. IEEE Trans Pattern Anal Mach Intell 2021;44:7112–27. [DOI] [PubMed] [Google Scholar]
- Firnberg E, Labonte JW, Gray JJ. et al. A comprehensive, high-resolution map of a gene’s fitness landscape. Mol Biol Evol 2014;31:1581–92. 10.1093/molbev/msu081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallee L, Rafailidis N, Gleghorn JP. cdsBERT-extending protein language models with codon awareness. bioRxiv, 10.1101/2024.05.20.594989, 2023, preprint: not peer reviewed. [DOI]
- Høie MH, Kiehl EN, Petersen B. et al. Netsurfp-3.0: accurate and fast prediction of protein structural features by protein language models and deep learning. Nucleic Acids Res 2022;50:W510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarzab A, Kurzawa N, Hopf T. et al. Meltome atlas—thermal proteome stability across the tree of life. Nat Methods 2020;17:495–503. 10.1038/s41592-020-0801-4. [DOI] [PubMed] [Google Scholar]
- Ji Y, Zhou Z, Liu H. et al. DNABERT: pre-trained bidirectional encoder representations from transformers model for DNA-language in genome. Bioinformatics 2021;37:2112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J, Evans R, Pritzel A. et al. Highly accurate protein structure prediction with alphafold. Nature 2021;596:583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanz C, Aldebert P, Althorpe N. et al. The EMBL nucleotide sequence database. Nucleic Acids Res 2005;33:D29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingma DP, Ba J. Adam: a method for stochastic optimization. International Conference on Learning Representations (ICLR), 2015.
- Klausen MS, Jespersen MC, Nielsen H. et al. Netsurfp-2.0: improved prediction of protein structural features by integrated deep learning. Proteins Struct Funct Bioinf 2019;87:520–7. [DOI] [PubMed] [Google Scholar]
- Lander E. Initial impact of the sequencing of the human genome. Nature 2011;470:187–97. 10.1038/nature09792. [DOI] [PubMed] [Google Scholar]
- Li S, Moayedpour S, Li R. et al. CodonBERT large language model for mRNA vaccines. Gen Research 2024;34:1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Akin H, Rao R. et al. Evolutionary-scale prediction of atomic-level protein structure with a language model. Science 2023;379:1123–30. [DOI] [PubMed] [Google Scholar]
- Liu H, Tam D, Muqeeth M. et al. Few-shot parameter-efficient fine-tuning is better and cheaper than in-context learning. Adv Neural Inf Process Syst 2022;35:1950–65. [Google Scholar]
- Liu Y. A code within the genetic code: codon usage regulates co-translational protein folding. Cell Commun Signal 2020;18:145. 10.1186/s12964-020-00642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat L, Jones DT.. Increasing the accuracy of single sequence prediction methods using a deep semi-supervised learning framework. Bioinformatics 2021;37:3744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen E, Poli M, Faizi M. et al. HyenaDNA: long-range genomic sequence modeling at single nucleotide resolution. Proceedings of the International Conference on Neural Information Processing Systems (NeurIPS 2023), 2023.
- Nguyen E, Poli M, Durrant MG. et al. Sequence modeling and design from molecular to genome scale with evo. bioRxiv, 10.1101/2024.02.27.582234, 2024, preprint: not peer reviewed. [DOI]
- Outeiral C, Deane CM.. Codon language embeddings provide strong signals for use in protein engineering. Nat Mach Intell 2024;6:170–9. 10.1038/s42256-024-00791-0. [DOI] [Google Scholar]
- Parvathy S, Udayasuriyan V, Bhadana V.. Codon usage bias. Mol Biol Rep 2022;49:539–65. 10.1007/s11033-021-06749-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney MM, Mokhtari DA, Akiva E. et al. Parallel molecular mechanisms for enzyme temperature adaptation. Science 2021;371:eaay2784. [DOI] [PubMed] [Google Scholar]
- Press O, Smith NA, Lewis M. Train short, test long: attention with linear biases enables input length extrapolation. International Conference on Learning Representations (ICLR), 2022.
- Raffel C, Shazeer N, Roberts A. et al. Exploring the limits of transfer learning with a unified text-to-text transformer. J Mach Learn Res 2020;21:1–67.34305477 [Google Scholar]
- Rao R, Bhattacharya N, Thomas N. et al. Evaluating protein transfer learning with tape. Adv Neural Inf Process Syst 2019; 32:9689–9701. [PMC free article] [PubMed]
- Rives A, Meier J, Sercu T. et al. Biological structure and function emerge from scaling unsupervised learning to 250 million protein sequences. Proc Natl Acad Sci USA 2021;118:e2016239118. 10.1073/pnas.2016239118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocklin GJ, Chidyausiku TM, Goreshnik I. et al. Global analysis of protein folding using massively parallel design, synthesis, and testing. Science 2017;357:168–75. 10.1126/science.aan0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisyan K, Bolotin D, Meer M. et al. Local fitness landscape of the green fluorescent protein. Nature 2016;533:397–401. 10.1038/nature17995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders R, Deane CM.. Synonymous codon usage influences the local protein structure observed. Nucleic Acids Res 2010;38:6719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PM, Emery LR, Zeng K.. Forces that influence the evolution of codon bias. Philos Trans R Soc Lond B Biol Sci 2010;365:1203–12. 10.1098/rstb.2009.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck H, Ekanadham C, Kallus N. Is cosine-similarity of embeddings really about similarity? WWW '24: Companion Proceedings of the ACM Web Conference 2024.
- Su J, Lu Y, Pan S. et al. RoFormer: enhanced transformer with rotary position embedding. arXiv, arXiv:2104.09864, 2021, preprint: not peer reviewed.
- Uniprot Consortium. Uniprot: the universal protein knowledgebase in 2023. Nucleic Acids Res 2023;51:D523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Zhang Z, Lu J. et al. Peer: a comprehensive and multi-task benchmark for protein sequence understanding. In: Proceedings of the Neural Information Processing Systems Track on Datasets and Benchmarks (NeurIPS 2022) 2022.
- Yang Y, Gao J, Wang J. et al. Sixty-five years of the long march in protein secondary structure prediction: the final stretch? Brief Bioinf 2018;19:482–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Ji Y, Li W. et al. DNABERT-2: efficient foundation model and benchmark for multi-species genome. In: International Conference on Learning Representations (ICLR) 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We make available our inference code, 3mer pre-trained model weights and datasets.