Abstract
It has been suggested that having a reputation for being prosocial is a critical part of social status across all human societies. It has also been argued that prosocial behavior confers benefits, whether physiological, such as stress reduction, or social, such as building allies or becoming more popular. Here, we investigate the relationship between helping reputation (being named as someone others would go to for help), and hair-derived chronic stress (hair cortisol concentration). In a sample of 77 women and 62 men, we found that perceived helping reputation was not related to chronic stress. Overall, the results of our study suggest that, in an egalitarian society with fluid camp membership and widely practiced generosity such as the Hadza, helping reputation does not necessarily boost stress-related health benefits through prestige-signaling mechanisms observed in hierarchical, large-scale societies.
Keywords: Cooperation, Cortisol, Generosity, Hunter-gatherers, Prosociality
Subject terms: Evolution, Physiology
Introduction
Prosocial behavior is ubiquitous in humans. From an evolutionary perspective, there are several different potential causal factors that could explain prosociality in humans. One explanation is that there are payoffs to signaling a cooperative disposition to potential allies1. If gaining allies influences prosocial behavior, then types of generous or prosocial behavior that have the potential to be noticed by others should be more common. Signaling prosociality to others and maintaining a prosocial reputation explains generosity in some experimental contexts2–4. For example, in a dictator game it has been reported that study participants (n = 80) were giving out money to receivers for the sake of not violating others’ expectations rather than their wellbeing2 while agent-based simulations showed that one-shot economic games are purely based on direct short-term benefits unless repeated interactions are involved3. Studies in more naturalistic settings also show that altruistic behaviour promotes prosocial reputation5–7. For instance, a study of the Yora people n = 71), hunter-horticulturalists of Amazonian Peru, showed that individuals with strong reputations for being generous are more likely to be provisioned with food during periods of food shortages or illnes8. However, it is worth noting that, at least on the proximate level, the motivation behind helping others (or other form of inconspicuous behaviour; see9 for review) does not necessarily have to involve gaining long-term benefits (e.g., gaining allies or potential helpers) but, instead, may be based on more tangible and short-term benefits, such as direct reciprocity10,11 or feeling good while being generous12. Some studies show that prosocial behavior may not only help people gain allies but might also increase social status. For example, a study among the Tsimane, an Amazonian small-scale society, showed that men who exhibit high social status gain more cooperative hunting partners while individuals who cooperate with high status men, in turn, enhance their own social status13. Moreover, one study suggests that the causative direction of prosocial behavior and social status goes both ways14 (e.g., reputation can also boost pro-social behaviour15 and promote cooperation16). Other studies conducted among hunter-gatherers show that not only social status but also being prosocial is related to having more social partners, especially in activities requiring collaborative effort, such as some types of foraging. For instance, among Martu hunter-gatherers of Australia, individuals who invest more in sharing are preferred as hunting partners over those with greater skill at hunting17, suggesting that people prefer those who are willing to cooperate even over those that may be particularly good at the activity, although the exact direction of the causal inference cannot be derived from this observational study. Thus, cooperation, and prosociality in general, might not necessarily imply a competitive, status-seeking behavior but, rather, an efficient way of building trustful and meaningful relationships18. Indeed, the biological market hypothesis19 posits that in animal societies living in stable social groups partner choice has played a major role in the evolution of cooperation. However, status-seeking behaviour and building meaningful relationships might not necessarily be mutually exclusive as people might not necessarily compete with each other over cooperative partners directly but, by being prosocially active, attract preferred social partners and forming with them relationships based on mutualistic (and not necessarily convergent) needs20. In such a context, prosocial behavior might be considered to be a subtle but honest signal directed towards specific long-term and trusted partners rather than a social display aiming to attract new partners18. It has been suggested that, in small-scale societies, prosocial behavior might represent a desire to reaffirm the availability of cooperative partners in the future through signalling an honest willingness to help rather than an investment in immediate reciprocity18,21,22. The idea that costly, honest signals may be aimed at strengthening social relationships has been suggested to play a major role in the evolution of sociality in group-living animals23,24.
It has been argued that maintaining reliable relationships could be critical, especially among communities inhabiting unpredictable environments, mainly in terms of food security25. For example, agent-based models show that among Maasai and Maa-speaking pastoralists in Kenya and Tanzania prosocial behavior may lead to a limited form of risk pooling25. Such risk pooling benefits everyone living in a potentially volatile environment. For example, computer models show that having friends that can be relied on in times of need can help buffer individuals from the risks of living in an uncertain ecological environment26–28. Partnerships based on risk-pooling relationships have been ethnographically documented in Africa, especially among pastoralists, such as the Dassanetch of South-West Ethiopia, the Turkana of Kenya, and the Jie of Uganda, all of whom live in marginal environments that are prone to draught environments and among whom livestock are vulnerable to theft and diseases29,30. Managing risk with supportive relationships has also been noted by other researchers studying small-scale societies. For example, it has been noted that, among the Ache hunter-gatherers, social relationships function as a sort of social insurance for times when individuals may be in need (e.g., during illness). Indeed, individuals who shared and produced more than average experience better food provision when injured or sick compared to those produced and shared below average 5. Due to the year-round and seasonal unpredictability of food resources, such need-based prosocial behavior is especially important in highly mobile small-scale societies, such as the Hadza31.
Besides the tangible benefits of prosocial behavior explained by the risk-pooling mechanism, prosocial behavior can be a strategy for a long-term investment in social capital and, therefore, may enhance the social integration of an individual in a community32,33. Studies among both nonhumans and humans have shown that being well-integrated in a social network can positively affect health outcomes34–38. For example, studies of nonhuman primates have shown that individuals that are actively involved in affiliative interactions, such as grooming, experience lower levels of physiological stress34,39. However, the causative direction of such relationship is still debatable40. For example, studies among humans have shown that individuals that experienced acute stress were more likely to be engage in prosocial behavior such as sharing and helping41, though studies also show that induced stress can also elicit social withdrawal, antisocial behaviour and aggression42–45. Prosocial and antisocial behaviours resulting from acute stressors show the importance of context and, more specifically, social interdependence on the regulation of the acute stress responses.
Given that prosocial behavior may enhance the maintenance of reliable relationships18, and given that the latter have been shown to have stress-buffering effects40, it could be expected that individuals exhibiting such behavior may also experience stress-related health benefits. Indeed, it has been suggested that there are biological reward mechanisms that underpin human prosocial tendencies and that contribute to health46. For example, in large-scale hierarchical and industrialized populations, several different categories of prosocial behavior, including charitable spending, moral decision making, and cooperation in experimental economics games, have all been linked to lower physiological indicators of stress47–52 although it is unclear to what extent such prosocial bahaviour is confounded by socio-economic status and material wealth. Specifically, helping and supporting behaviors are associated with reduced morbidity and increased longevity for the helper53,54. For example, one study showed older individuals who reported providing more instrumental and emotional support experienced lower mortality rates, while receiving such support was unrelated to mortality53. Similarly, altruism and helping behaviors have been found to be correlated with wellbeing55, especially among older people56. It has been also shown that helping others buffers the association between stress and physical health57. Interestingly, acting prosocially has been linked to health also among nonhumans. For instance, one study of Barbary macaques (Macaca sylvanus) showed that individuals that provide rather than receive grooming experience less anxiety58. Although the above-described findings are rather suggestive, caution should be taken in interpreting these studies because the causative direction cannot be determined basing purely on observational methods.
It has been argued that being compassionate towards others and the resulting helping behavior have strong evolutionary roots in fostering social integration and a sense of belonging59. A growing body of evidence suggests that being integrated socially is associated with physiological health indices, such as physiological stress levels60, especially in relation to daily contacts with regular social partners40,61. Long-term chronic stress measured by hair cortisol has been linked to other measures of social status in humans (e.g., socio-economic status and self-perceived social status)62–64. Even though hair cortisol concentration (HCC) is not necessarily a synonym of stress per se1–4, it is heightened among individuals experiencing ongoing chronic stress66, among children brought up in high-risk environments67, and among people with dementia exhibiting low levels of social engagement68. Importantly, in recent studies HCC has been successfully applied as a measure of wellbeing among both humans and nonhuman primates40,69–71. However, most studies linking prosocial behavior with stress among humans have been conducted in industrialized, large-scale hierarchical populations72–75. Moreover, studies of the association between prosocial behavior and physiological stress commonly test short-term measurements of changes in salivary cortisol76 or neuroimaging for stress-responses following self-reported prosocial activity or a laboratory-based prosociality test77. As such, there are not yet studies testing whether having a reputation for being prosocial within an actual community is related to social status in ways that affect long-term cortisol levels.
Here we investigate the extent to which cortisol levels are related to perceived prosociality in a hunter-gatherer society, the Hadza. Specifically, we tested the association between long-term cortisol levels and one of the (prosociality-based) facets of reputation15: helping reputation. If prosociality is valued within a particular cultural context, one would expect the reputation for prosociality, as measured by helping reputation, to play a role in overall social status and thereby affect long-term physiological markers of stress, such as hair cortisol. However, using the same dataset we have previously shown that among the Hadza social status based on friendship popularity and foraging reputation is not related to HCC69,78. This is because prosocial behavior within the context of risk-pooling theory does not imply direct, prestige-based physiological rewards resulting from prosocial behavior because, as opposed to large-scale hierarchical societies, in small-scale egalitarian hunter-gatherer groups it is expected that people will engage in such a need-based behavior25,79.
The Hadza are perfectly suited for our study because they live in a relatively egalitarian society exhibiting neither structured hierarchy nor formal leadership. Although the Hadza do not engage in norm enforcement to the degree seen in many other societies80, prosocial behavior, such as food sharing, is widespread and commonly practiced81,82. Hadza social preferences while directing prosocial behavior have also been documented82–84 as has been social prestige resulting from either friendship popularity or foraging reputation69,78,85. Moreover, the unpredictability of food resources, especially those acquired by men such as highly valued large game and honey86,87, make it very plausible that risk pooling plays a major role in prosocial behavior among the Hadza25. We therefore hypothesized that, as opposed to large-scale and hierarchical societies with social inequality in terms of socio-economic (SES) status, among the Hadza where generosity is widely expected, helping reputation will be not related to indices of stress.
Results
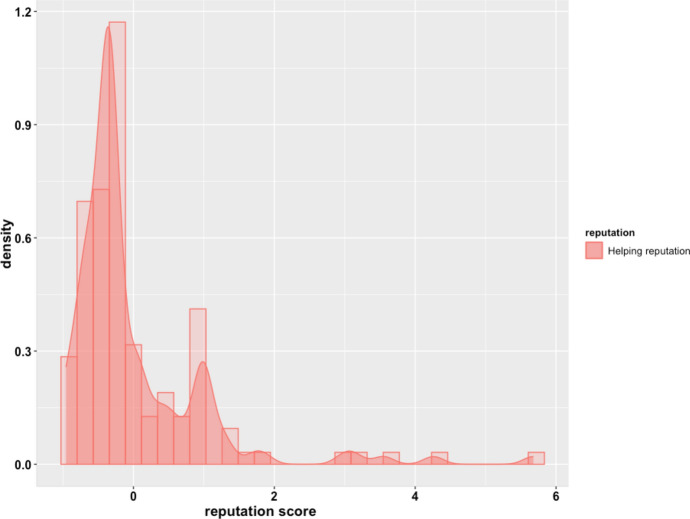
Mean hair cortisol concentration for women (n = 77) was 78.25 pg/mg (median = 64.38, range 15.04–229.36), and for men (n = 62) it was 96.82 pg/mg (median = 80.73, range 32.26–257.14). The Wilcoxon Rank Sum Test found significant differences in HCC between the two data collection field seasons (W = 2917.5; p = 0.006). Perceived helping reputation scores exhibited a positive skew (Fig. 1), especially in bigger camps (Table S2, Fig. 1a–h; Supplementary Material)). For example, in the most extreme example, in camp 2 one individual received 18 nominations while 28 individuals received none (Fig. 1b; Supplementary Material).
Fig. 1.
Histogram and density of perceived helping reputation scores. The X axis is the within-camp z score for the reputation metric. Positively skew distribution of the helping reputation scores suggests that a large proportion of individuals received none or very few helping nominations while a very small proportion of individuals received many helping nominations.
Although Kendall Tau showed that two variables included in the model, age and helping reputation, were significantly correlated with each other (tau = 0.16, p = 006), all VIF values were generally low (age VIF = 1.09, sex VIF = 1.07, perceived helping reputation VIF = 1.12) so all variables were included in the full model.
Perceived helping reputation was not associated significantly with hair cortisol concentrations (Table 1; Fig. 2), although sex and age were significantly associated with HCC (Table 1; Fig. 2).
Table 1.
LMM results explaining power-transformed cortisol concentration variance among the Hadza.
| B ± SE | t value | Pr( >|t|) | AIC | R2 | |
|---|---|---|---|---|---|
| Intercept | − 0.595 ± 0.009 | 63.281 | < 0.001 | − 488.60 | Marginal: 0.068 |
| Age | 0.0004 ± 0.0001 | 2.531 | 0.013 | Conditional: 0.211 | |
| Sex | − 0.014 ± 0.006 | 2.350 | 0.020 | ||
| Perceived helping status | − 0.0001 ± 0.003 | 0.49 | 0.961 |
Fig. 2.
Predicted lmm output for relationship between power transformed picograms of hair cortisol concentrations and within-camp z score of the reputation metric; Dotes indicate cortisol levels of the study participants with respect to helping status (A) and age (B). The shaded areas represent 95% confidence intervals.
Null results were retained while controlling for foraging reputation (i.e., digging status for women and hunting reputation for men) and popularity (Table S4 and S5, Supplementary Material). Bayes Factors calculated for the model (BF01 = 3.11 ± 3.5%) showed that, given the data included in the model, the relationship between helping reputation and HCC was ~ 3 times more likely under the null hypothesis compared to the alternative hypothesis.
Discussion
The results of our study show that prosocial reputation is not related to HCC, suggesting that, in a small society representing an egalitarian social model where generosity is widely expected and practiced, helping reputation might not necessarily translate into stress-related health benefits.
Though the Hadza have no explicit leaders, it is possible that social influence still impacts the perception of social status. Given that among the Hadza there is no institutionalized authority, this influence may be more related to more nuanced forms of social status related to prestige, such as popularity and foraging status85. Having a reputation for being someone that others can go to in times of need may be an indicator of social prestige, and indeed there is some evidence that men in some societies engage in ‘competitive helping’ or signaling their generosity to attractive potential mates88. Some studies show that engaging in prosocial behavior is associated with better indices of health48,49,51,89. However, the results of our study show that, among the Hadza, physiological stress is not associated with helping reputation.
One of the reasons why we did not detect any relationship between prosociality attributes and stress levels could be methodological issues related to the difficulty of detecting such nuanced helping behaviors in our study. Most of previous research on prosociality and cortisol has focused on larger, more conspicuous prosociality such as grand gestures that are above and beyond what may be expected in a generally cooperative society (which can have a substantial amount of helping behavior)18. It remains to be seen whether such grand-gesture prosocial behaviors are contributing to lower cortisol levels through enhancing reputation or social prestige (mediated by self-perception). Smaller, daily or regular prosocial behaviors that are expected by members of a particular society have been less well-documented in relation to perceived well-being, but in some studies these behaviors actually seem to be related to higher cortisol levels90. However, some types of help might go unnoticed. This could be the case when asking many people who they have helped, in which case many Hadza (particularly women) said they have not helped anyone. When followed up with suggestions of types of help (such as giving food or helping with children), the study participants tend to say, ‘oh yes, we do that every day.’ These small, unnoticed prosocial behaviors may not be associated with lower cortisol. Indeed, some studies show that in large-scale, hierarchical societies the effects of prosocial behavior are dependent on the impact of the behavior, which is whether they make a major difference in the life of another person. Though opportunities for these sorts of life-changing prosocial behaviors do arise in small-scale, non-Industrialized societies, they do so less frequently than in societies with great disparities in personal wealth. Indeed, social stratification has been found to be a significant predictor of prosocial behavior. For instance, people of lower socioeconomic status (SES) in industrialized societies demonstrate more prosocial behavior across a range of behaviors (e.g. charitability, trust, generosity, and helpfulness) than do higher SES individuals91, but see92.
The lack of a relationship between perceived prosociality and cortisol levels observed in our study could be because the latter might be related to other social characteristics that were not considered in this study, such as social prestige. Indeed, some studies have shown that social prestige is related to social status, which affects cortisol levels93,94, although one study of Garisakang forager-horticulturalists of Papua New Guinea found that cortisol levels were not associated with prosociality95. Therefore, if, despite their being quite egalitarian, social status exists among the Hadza and is related to their cortisol measures, then perhaps prosociality is not a major determinant of Hadza social status. Interestingly, using the same dataset, we have previously showed that among the Hadza social prestige is not related to stress in both men and women,69,78 which further suggests that social prestige or reputation derived from both foraging and other social characteristics of an individual, such as prosocial behavior, are driven by deeply embedded social norms rather than self-interest and competitive behavior31,96. Alternatively, prosocial reputation among the Hadza might also be driven by low cost and high reward in a foraging economy, especially when it comes to food sharing86. It is also worth entertaining the possibility that stress-related health benefits accrued by high helping reputation status might be traded off by the physiological or energetic costs related to maintaining such status. In other words, costs and benefits related to helping reputation might cancel each other out when examined by biomarkers, such as HCC. We have previously shown that among the Hadza higher HCC levels related to high levels of proximity contacts with other camp members might be mitigated by proximity to friends40.
It could be argued that the apparent absence of an association between prosocial reputation and stress levels observed in our study might be that Hadza men do not tend to get stressed to the degree that would be sufficiently reflected in physiological chronic stress indices, such as HCC40. However, it has been widely reported that, in spite of the fact that their lifestyle is often described as ‘relaxed,’ Hadza do experience a lot of pressures and anxieties related to limited access to potable water (personal observation) and diseases97–100. Indeed, deaths resulting from falls from trees, infected wounds, and other diseases are not infrequent while a lack of large game due to the influx of pastoralist Datooga people in their land is also a common cause of concern78.
It also could be argued that the apparent lack of a relationship between prosocial reputation and stress observed in our study might be due to the fact that glucocorticoids levels, especially markers of chronic stress, such as HCC, might not be synonymous with of stress65,78. Moreover, animal studies have shown that HCC to be associated with energetic expenditure101,102 so, provided that even moderate exercise increases cortisol levels, HCC levels linked to physically demanding workload of the Hadza, such as foraging, can mask the association between HCC with less energetically demanding but more subtle activities, such as maintaining a prosocial reputation. Thus, controlling for physical demands of daily activities among the Hadza, as well as other important factors influencing HCC, such as illness and sleep disruption, would potentially enable to detect a relationship between social status/prestige and stress levels. Still, previous studies have shown that HCC can be reliably used as an indicator of stress103,104, and we have previously successfully used it as such in studies of nonhuman primates71 as well as, using the same dataset used in this study, in our previous research among the Hadza40,69,78. Our null results do not, however, exclude the possibility that helping reputation is not associated with health-related measures other than stress, such as those related to nutritional status or cardiovascular indices.
Alternatively, although some have claimed that prosocial behavior is underpinned by physiological rewards55, physiological rewards are not actually necessary for promoting generosity. Need-based transfers have been demonstrated to pay off in and of themselves in many environments26. Thus, the physiological rewards documented in relation to one-shot charitable giving or helping may not be present in an accepted, institutionalized system of prosociality that reduces risk28. Competitive signaling of prosociality may even be discouraged in these contexts. Osotua (“umbilical cord”) relationship among Maasai pastoralists, for example, involve restraint in helping. Osotua partners should help only when they are asked to do so and only if they are able to do so, and they are expected to give only the amount that is actually needed105. Moreover, a recent study showed that unpredictable needs are associated with lower expectations of repayment106. The cultivation of long term, reliable relationships likely require a different technique for signaling prosociality than ostentatious, non-targeted acts of prosociality. Likewise, it has been argued that prosocial food sharing among Meriam hunter-gatherer women is a subtle signal of relational commitment, rather than an attempt to signal personal attributes, such as foraging ability18. It has been also suggested that helping behavior (or, rather, pro-social behavior in general) in early egalitarian societies, as well as among extant small-scale hunter-gatherers is driven by social norms99,107 rather than competitive practices observed in large-scale, hierarchical societies108. This might explain an apparent lack of an association between helping reputation and HCC observed in our study.
An association between social status and health indices has been reported in other hunter-gather groups, such as the Tsimane109,110. However, this study, as well as our previous research, might suggest that in a small-scale society on the extreme range of egalitarianism, such as the Hadza, the pattern of social interactions rather than social status per se is related to physiological stress levels. For example, using the same data we have previously shown that, although among the Hadza social status/prestige drives proximity networks (at least among men85), foraging reputation and popularity are not related to physiological stress69,78. Studies on stress and social status among two groups of hunter-gatherers exhibiting different levels of egalitarianism: the Hadza and Tsimane, suggest that lack of such associations should not be considered as lack of capacity. Rather, these studies show that people have the evolutionary capacity linking prosocial behavior with status and thus, stress reactivity, but it is less expressed in societies where helping behaviour is a baseline expectation.
One of the limitations of our study is that it does not consider inter-camp dynamics in prosocial behavior as it is based on in-camp nominations (following78,85). It has been previously suggested that than inter-camp relationships are important in shaping the social structure of small-scale hunter-gatherer societies111–113, especially in terms of prosocial activities, such as food sharing114. It could be also argued that individuals’ social reputations cannot be established reliably because of the Hadza’s flexible residence patterns. Indeed, over the course of a year, Hadza change camps on average six times99. Therefore, limiting our study to in-camp nominations could potentially distort the relationship between prosocial behavior and stress40. However, it has been also suggested that Hadza do not choose their camps randomly78 but, rather, their choices to a great extent are based on social preferences based on friendships and social characteristics of their campmates33.
Overall, our findings do not support the idea that physiological rewards for prosocial behaviors are a human universal and are consistent with helping behavior described by other ethnographers as serving to maintain long-term, reliable relationships among the Hadza members that is deeply embedded within highly egalitarian social norms. We believe that, although helping behavior is ubiquitous among the Hadza, it is not generally related to prestige. This separation of helping behavior from prestige may, in fact, be necessary for helping to serve as an honest signal of commitment to particular relationships in a society where generosity and reciprocity are expected norms driven by the unpredictability of resources.
Methods
Subject population
The Hadza are hunter-gatherers who number approximately 1,000 but only about 300 of them continue to exhibit hunter-gatherer lifestyle99. They live in a savanna-woodland habitat that encompasses about 4000 km2 around Lake Eyasi in northern Tanzania. They live in mobile camps that average thirty individuals. Camp membership often changes as people move in and out of camps. These camps move about every six weeks on average. Hadza men hunt and gather other resources, foraging on average 6.3 h per day. The Hadza are politically egalitarian, with no official big men, chiefs, or leaders79,115,116 (but for a more detailed discussion of forms of inequality in ‘egalitarian’ societies see117). The Hadza also have very little disparity in material wealth (although men do own and treasure their hunting equipment, such as bows and arrows99).
Data collection
This study is a part of a larger project on proximity networks and social status among the Hadza, and some of the data used here (e.g., on cortisol levels or social status) have been previously reported in previous publications40,69,78,85,118,119. Data collection was conducted in 2016 and 2017 for roughly four months each year. Data were collected in camps that were actively foraging for most of their calories, although visiting and some limited trade with neighbouring food-producing groups like the Datooga pastoralists did occur as it has in all camps since CB’s first visit in 2007. Hair samples and reputational data on helping status were collected from eight camps with sizes ranging from six to 38 adults (mean = 19) during the last stay of the research visit at each camp (n = 8). Only adult individuals were included in the study. On average, we collected reputational data from 100% of adults per camp (83 women and 64 men, although 22 individuals did not nominate anyone as a helper) and hair samples from 77 of those same women and 62 of the men. Of the 147 study participants, two men and three women were sampled twice because they happened to be in two different study camps when the study was conducted. The mean age for men that provided hair samples was 38 years (median = 38, range 18–71 years, n = 62). The mean age for women that provided hair samples was 42 years (median = 38, range 18–89, n = 77).
Conducting interviews
We interviewed camp members about helping behavior using open-ended questions, including questions about who should be helped, who has recently helped them, and what kinds of helping were commonplace among the Hadza. We also asked participants in each camp to name, in order, the people to whom they would go for help in the camp they were living in at the time.
Scores obtained from the questionnaires where then used to construct a scale of perceived helping status, which was calculated as the total number of nominations an individual received. One person nominated 2 individuals and was unable to decide on one nominee, so the two nominated persons received 0.5 points (i.e., instead of 1). Several Hadza named locations of camps where various kin lived, rather than specific people they would go to for help; however, most were able to name specific people after some prompting. Interviews with the Hadza were conducted by the PI in Swahili, in which the majority of the Hadza are fluent, although some older Hadza required the assistance of an interpreter (i.e., a Hadza research assistant fluent in the Hadza language) during the interviews because their command of Swahili was basic40,69. Research was conducted a few weeks at each camp, and the interviews were conducted during the last day of the study in a camp in a secluded area just outside of the camp so that interviewees were out of earshot of other people in the camp40.
Obtaining hair samples for HCC
We were mainly interested in chronic long-term levels of stress rather than the acute stress response because we are interested in the mediation of chronic stress (following40,69,85). Hair cortisol has the advantage of allowing the back-tracking of average levels of cortisol over a longer time-frame (often several months, based on the assumption of an average hair growth rate of 1 cm/month)120. Determining cortisol from hair also has the benefit of being a biological sampling procedure that causes minimal discomfort to the participants 40. Thus, unlike other methods of sampling, the stress of the sampling method itself does not affect the cortisol measurement from the sample, as hair reflects chronic stress over weeks or months rather than the acute stress of the moment (which can be elevated due to anxiety of having hair cut)121. It is worth noting that many Hadza women keep their hair short (often shorter than men), so that the time period of cortisol levels detected in hair is estimated to be approximately one month in women and 2–3 months in men69. The popularity of short hair style for Hadza women was a limiting factor in getting a larger sample size, as some women had such short hair that there was not enough for analysis69. Some women that provided hair were pregnant (n = 14) or lactating (n = 4), but we have previously shown that reproductive state is not a significant predictor of HCC69.
Hair preparation
The Hadza do not dye or bleach their hair. They occasionally use soap (generally hand soap) to wash their hair69. Hair cortisol was extracted according to the procedures outlined in Sauve et al.122. Briefly, hair samples from the 1 cm closest to the scalp end were cut into small pieces using sterile small surgical scissors123, weighed (to around 10–15 mg), and placed into 1.5 ml reaction tubes. Prior to extraction, hair samples were ground using the IKA ULTRA TURRAX Tube Drive System (following124).
For extraction, 1.5 mL of methanol was added, and the vial was sealed and incubated overnight for 18 hours at room temperature while gently shaking69. After incubation, samples were centrifuged, and the methanol extract was transferred to a disposable glass vial and evaporated to dryness under nitrogen. The samples were dissolved in 250 μL phosphate-buffered saline (pH 8.0). Samples were vortexed for one minute, and then again for 30 s before the assay40,69,78
Hair analysis
Cortisol levels were measured using the Salimetrics® Cortisol Enzyme-linked Immunoassay (ELISA) Kit (Salimetrics Europe, Suffolk, UK) as per the manufacturer’s instructions69. In principle, the assay measures competitive binding to a capture antibody between hair-extracted cortisol and cortisol conjugated to horseradish peroxidase, which converts 3,3′,5,5′-tetramethylbenzidine (TMB) to 3,3′,5,5′-tetramethylbenzidine diimine in a chromogenic reaction. After termination of the reaction by the addition of sulphuric acid, absorbance was measured at 450 nm, and cortisol levels were calculated based on a standard curve (following69). The intra- and inter-assay coefficient of variance was < 9% and < 10% respectively.
Statistical analysis
Because we collected reputational data from eight camps of different sizes with different numbers of potential nominators, we standardized these raw values by deducting a mean camp value from each individual score in a camp and then dividing it by the standard deviation of the camp, which resulted in a within-camp z score for each reputation metric for each study participant (following69). Because cortisol concentrations were highly skewed, we transformed these measurements using the Tukey Ladder of Powers procedures125 available in the ‘rcompanion’ package126 for R (but we also report results based on log-transformed HCC (Table 1S, Supplementary Material). Following that, the visual inspections of normality and homogeneity of error variances did not indicate a violation of model assumptions.
We analysed the data using a linear mixed-effects model (LMM) fit by the restricted-maximum likelihood estimation (REML) with cortisol levels as a dependent variable while perceived helping reputation was used as predictors. Regarding the three women and two men that participated in the study twice in two different camps, for the LMM analysis we used only data from the camps they participated first in order to avoid pseudo-replication (although their nominations were included in quantifying helping status of other participants, they nominated in both camps they participated). We also included in the model sex and age as fixed independent variables and camp nested within field season as a random variable (because the Wilcoxon Rank Sum Test showed that HCC values differed between the two field seasons (e.g., 2016 and 2017) during which the hair samples were collected). In addition, Bayes Factors (BF01) was calculated to determine whether our data support the null (i.e., helping reputation are not related to HCC) or alternative (i.e., helping reputation is related to HCC) hypothesis.
In Supplementary Material we also report lmm models that included additional two social status domains that were significantly correlated with helping reputation (Table S3, Supplementary Material): foraging reputation and popularity (which relationship with HCC we reported in our previous articles 69,78. These models were run separately for men and women. Quantifying popularity and foraging reputation is described in Supplementary Material.
To minimize the problem of collinearity, we first ran Kendal Tau correlations on all variable combinations and excluded highly correlated variables (Kendall tau > 0.8). We also calculated variance inflation factors (VIF) for all the variables, including only variables with VIF < 4. LMM was performed using the ‘lme4’package127 for R128. We calculated marginal (i.e., for fixed effects only) and conditional (i.e., for both fixed and random effects for camp) R2 for the LMM model using the ‘lmerTest’ package129 for R. Figure 2 was generated using the ‘ggeffect’ package130 for R.
Ethical approval
Informed, verbal (as the vast majority of the Hadza cannot read and write) consent was obtained from all study participants. This research was approved by University of Roehampton’s ethics committee (LSC 16/ 172) as well as by the Tanzanian authorities COSTECH and NIMR. This research was also approved by local authorities, and researchers stayed in camps only by invitation. The study was performed in accordance with the Declaration of Helsinki as well as other relevant guidelines and regulations.
Supplementary Information
Acknowledgements
We would like to thank the Leakey Foundation for their support of CB and PF and the John Templeton Foundation for their support for AA and LC. The opinions expressed in this publication are those of the authors and do not necessarily reflect the views of the John Templeton Foundation. We thank Costech and NIMR for permission to conduct research. We would like to thank Daudi and Trude Peterson for all of their help and support. Finally, we thank the Hadza for their enduring tolerance and hospitality.
Author contributions
CB conceived the study. PF and CB designed the study. PF and CB wrote the original manuscript. PF collected, and analysed the data, with help from IB and JM for field assistance and LL for the ELISA work. PF, AA, LC, DD, JL and CB all contributed to writing and editing the manuscript.
Funding
This work was supported by the Leakey Foundation Grant awarded to CB and PF and the John Templeton Foundation Grant awarded to AA and LC.
Data availability
In order to protect the anonymity of health data from the Hadza, a simulated dataset using the R package synthpop will be provided131. Data, along with R codes used in this study, will be available in Figshare from the date of publication.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original HTML version of this Article contained an error in the protocol for HCC extraction. Full information regarding the correction made can be found in the correction for this Article.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/6/2024
A Correction to this paper has been published: 10.1038/s41598-024-77749-0
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-72238-w.
References
- 1.Jensen, K. Prosociality. Curr. Biol.26, R748–R752 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Dana, J., Cain, D. M. & Dawes, R. M. What you don’t know won’t hurt me: Costly (but quiet) exit in dictator games. Organ. Behav. Hum. Decis. Process.100, 193–201 (2006). [Google Scholar]
- 3.Delton, A. W., Krasnow, M. M., Cosmides, L. & Tooby, J. Evolution of direct reciprocity under uncertainty can explain human generosity in one-shot encounters. Proc. Natl. Acad. Sci.108, 13335–13340 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haley, K. J. & Fessler, D. M. T. Nobody’s watching?. Evol. Hum. Behav.26, 245–256 (2005). [Google Scholar]
- 5.Gurven, M., Allen-Arave, W., Hill, K. & Hurtado, M. “It’s a wonderful life”: Signaling generosity among the Ache of Paraguay. Evol. Hum. Behav.21, 263–282 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Peterson, N. Demand sharing: Reciprocity and the pressure for generosity among foragers. Am. Anthropol.95, 860–874 (1993). [Google Scholar]
- 7.Smith, E. A. & Bird, R. L. B. Turtle hunting and tombstone opening. Evol. Hum. Behav.21, 245–261 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Sugiyama, L. S. & Chacon, R. Effects of illness and injury on foraging among the Yora and Shiwiar: Pathology risk as adaptive problem. In Adaptation and Human Behavior 371–396 (Routledge, 2017). [Google Scholar]
- 9.Al-Shawaf, L. Levels of analysis and explanatory progress in psychology: Integrating frameworks from biology and cognitive science for a more comprehensive science of the mind. Psychol. Rev.10.1037/rev0000459 (2024). [DOI] [PubMed] [Google Scholar]
- 10.Axelrod, R. & Hamilton, W. D. The evolution of cooperation. Science211, 1390–1396 (1981). [DOI] [PubMed] [Google Scholar]
- 11.Trivers, R. L. The evolution of reciprocal altruism. Q. Rev. Biol.46, 35–57 (1971). [Google Scholar]
- 12.Schwartz, C. E. et al. Doing good, feeling good, and having more: Resources mediate the health benefits of altruism differently for males and females with lumbar spine disorders. Appl. Res. Qual. Life7, 263–279 (2012). [Google Scholar]
- 13.von Rueden, C. R., Redhead, D., O’Gorman, R., Kaplan, H. & Gurven, M. The dynamics of men’s cooperation and social status in a small-scale society. Proc. R. Soc. B286, 20191367 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kafashan, S., Sparks, A., Griskevicius, V. & Barclay, P. Prosocial behavior and social status. In The Psychology of Social Status 139–158 (Springer, 2014). [Google Scholar]
- 15.Garfield, Z. H. et al. The content and structure of reputation domains across human societies: A view from the evolutionary social sciences. Philos. Trans. R. Soc. B376, 20200296 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romano, A. et al. Reputation and socio-ecology in humans. Philos. Trans. R. Soc. B376, 20200295 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bird, R. B. & Power, E. A. Prosocial signaling and cooperation among Martu hunters. Evol. Hum. Behav.36, 389–397 (2015). [Google Scholar]
- 18.Bliege Bird, R., Ready, E. & Power, E. A. The social significance of subtle signals. Nat. Hum. Behav.2, 452–457 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Noë, R. & Hammerstein, P. Biological markets: Supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behav. Ecol. Sociobiol.35, 1–11 (1994). [Google Scholar]
- 20.Noë, R. & Hammerstein, P. Biological markets. Trends Ecol. Evol.10, 336–339 (1995). [DOI] [PubMed] [Google Scholar]
- 21.Gurven, M. To give and to give not: The behavioral ecology of human food transfers. Behav. Brain Sci.27, 543–560 (2004). [Google Scholar]
- 22.Gurven, M., Hill, K. & Jakugi, F. Why do foragers share and sharers forage? Explorations of social dimensions of foraging. In Socioeconomic Aspects of Human Behavioral Ecology Vol. 23 19–43 (Emerald Group Publishing Limited, 2004). [Google Scholar]
- 23.Zahavi, A. The testing of a bond. Anim. Behav.25, 246–247 (1977). [Google Scholar]
- 24.Zahavi, A. & Zahavi, A. The Handicap Principle: A Missing Piece of Darwin’s Puzzle (Oxford University Press, 1999). [Google Scholar]
- 25.Cronk, L. & Aktipis, A. Design principles for risk-pooling systems. Nat. Hum. Behav.44, 1–9 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Aktipis, A. et al. Cooperation in an uncertain world: For the Maasai of East Africa, need-based transfers outperform account-keeping in volatile environments. Hum. Ecol.44, 353–364 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campenni, M., Cronk, L. & Aktipis, A. Need-based transfers enhance resilience to shocks: An agent-based model of a maasai risk-pooling system. Hum. Ecol.50, 1–14 (2021). [Google Scholar]
- 28.Cronk, L. et al. Managing risk through cooperation: Need-based transfers and risk pooling among the societies of the Human Generosity Project. In Global Perspectives on Long Term Community Resource Management 41–75 (Springer, 2019). [Google Scholar]
- 29.Almagor, U. Pastoral Partners: Affinity and Bond Partnership among the Dassanetch of South-West Ethiopia Vol. 1 (Manchester University Press, 1978). [Google Scholar]
- 30.Gulliver, P. H. The Family Herds: A Study of Two Pastoral Tribes in East Africa, The Jie and T (Routledge, 2013). [Google Scholar]
- 31.Lewis, H. M., Vinicius, L., Strods, J., Mace, R. & Migliano, A. B. High mobility explains demand sharing and enforced cooperation in egalitarian hunter-gatherers. Nat. Commun.5, 5789 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lightner, A. D., Pisor, A. C. & Hagen, E. H. In need-based sharing, sharing is more important than need. Evol. Hum. Behav.44, 474 (2023). [Google Scholar]
- 33.Smith, K. M., Larroucau, T., Mabulla, I. A. & Apicella, C. L. Hunter-gatherers maintain assortativity in cooperation despite high levels of residential change and mixing. Curr. Biol.28, 3152–3157 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Brent, L., Semple, S., Dubuc, C., Heistermann, M. & MacLarnon, A. Social capital and physiological stress levels in free-ranging adult female rhesus macaques. Physiol. Behav.102, 76–83 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Carter, G. G., Farine, D. R. & Wilkinson, G. S. Social bet-hedging in vampire bats. Biol. Lett.13, 20170112 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kornienko, O. & Santos, C. E. The effects of friendship network popularity on depressive symptoms during early adolescence: Moderation by fear of negative evaluation and gender. J. Youth Adolesc.43, 541–553 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Silk, J. B. et al. The benefits of social capital: Close social bonds among female baboons enhance offspring survival. Proc. R. Soc. B Biol. Sci.276, 3099–3104 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor, S. E. Tend and befriend: Biobehavioral bases of affiliation under stress. Curr. Dir. Psychol. Sci.15, 273–277 (2006). [Google Scholar]
- 39.Engh, A. L. et al. Behavioural and hormonal responses to predation in female chacma baboons (Papio hamadryas ursinus). Proc. R. Soc. B Biol. Sci.273, 707–712 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fedurek, P. et al. Relationship between proximity and physiological stress levels in hunter-gatherers: The Hadza. Horm. Behav.147, 105294 (2023). [DOI] [PubMed] [Google Scholar]
- 41.Von Dawans, B., Fischbacher, U., Kirschbaum, C., Fehr, E. & Heinrichs, M. The social dimension of stress reactivity: acute stress increases prosocial behavior in humans. Psychol. Sci.23, 651–660 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Almuqrin, A. et al. The association between psychosocial stress, interpersonal sensitivity, social withdrawal and psychosis relapse: A systematic review. Schizophrenia9, 22 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Repetti, R. L. Social withdrawal as a short-term coping response to daily stressors. (1992).
- 44.Greenberg, G. D. et al. Sex differences in stress-induced social withdrawal: Role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Front. Behav. Neurosci.7, 223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simons, R. L., Conger, R. D. & Chao, W. Stress, support, and antisocial behaviour trait as determinants of emotional well-being and parenting practices among single mothers. J. Marriage Fam.55, 385 (1993). [Google Scholar]
- 46.Von Dawans, B., Fischbacher, U., Kirschbaum, C. & Fehr, E. The Social dimension of stress reactivity in humans. Psychol. Sci.23, 651–660 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Aknin, L. B. et al. Prosocial spending and well-being: Cross-cultural evidence for a psychological universal. J. Pers. Soc. Psychol.104, 635–652 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Dunn, E. W., Ashton-James, C. E., Hanson, M. D. & Aknin, L. B. On the costs of self-interested economic behavior: How does stinginess get under the skin?. J. Health Psychol.15, 627–633 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Margittai, Z. et al. A friend in need: Time-dependent effects of stress on social discounting in men. Horm. Behav.73, 75–82 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Sollberger, S., Bernauer, T. & Ehlert, U. Stress influences environmental donation behavior in men. Psychoneuroendocrinology63, 311–319 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Starcke, K., Polzer, C., Wolf, O. T. & Brand, M. Does stress alter everyday moral decision-making?. Psychoneuroendocrinology36, 210–219 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Youssef, F. F. et al. Stress alters personal moral decision making. Psychoneuroendocrinology37, 491–498 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Brown, S. L., Nesse, R. M., Vinokur, A. D. & Smith, D. M. Providing social support may be more beneficial than receiving it: Results from a prospective study of mortality. Psychol. Sci.14, 320–327 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Brown, S. L. & Brown, R. M. Connecting prosocial behavior to improved physical health: Contributions from the neurobiology of parenting. Neurosci. Biobehav. Rev.55, 1–17 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Post, S. G. Altruism, happiness, and health: It’s good to be good. Int. J. Behav. Med.12, 66–77 (2005). [DOI] [PubMed] [Google Scholar]
- 56.Kumar, A. & Dixit, V. Altruism, happiness and health among elderly people. Indian J. Gerontol.31, 480 (2017). [Google Scholar]
- 57.Poulin, M. J. & Holman, E. A. Helping hands, healthy body? Oxytocin receptor gene and prosocial behavior interact to buffer the association between stress and physical health. Horm. Behav.63, 510–517 (2013). [DOI] [PubMed] [Google Scholar]
- 58.Shutt, K., MacLarnon, A., Heistermann, M. & Semple, S. Grooming in Barbary macaques: Better to give than to receive?. Biol. Lett.3, 231–233 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seppala, E., Rossomando, T. & Doty, J. R. Social connection and compassion: Important predictors of health and well-being. Soc. Res. Int. Q.80, 411–430 (2013). [Google Scholar]
- 60.Dickman, K. D., Thomas, M. C., Anderson, B., Manuck, S. B. & Kamarck, T. W. Social integration and diurnal cortisol decline: The role of psychosocial and behavioral pathways. Psychosom. Med.82, 568 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stetler, C. A. & Miller, G. E. Social integration of daily activities and cortisol secretion: A laboratory based manipulation. J. Behav. Med.31, 249–257 (2008). [DOI] [PubMed] [Google Scholar]
- 62.O’Brien, K. M., Meyer, J., Tronick, E. & Moore, C. L. Hair cortisol and lifetime discrimination: Moderation by subjective social status. Health Psychol. Open4, 2055102917695176 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Serwinski, B., Salavecz, G., Kirschbaum, C. & Steptoe, A. Associations between hair cortisol concentration, income, income dynamics and status incongruity in healthy middle-aged women. Psychoneuroendocrinology67, 182–188 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ursache, A., Merz, E. C., Melvin, S., Meyer, J. & Noble, K. G. Socioeconomic status, hair cortisol and internalizing symptoms in parents and children. Psychoneuroendocrinology78, 142–150 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.MacDougall-Shackleton, S. A., Bonier, F., Romero, L. M. & Moore, I. T. Glucocorticoids and “stress” are not synonymous. Integr. Org. Biol.1, Obz017 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stalder, T. et al. Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology77, 261–274 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Fuchs, A. et al. Link between children’s hair cortisol and psychopathology or quality of life moderated by childhood adversity risk. Psychoneuroendocrinology90, 52–60 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Kim, E., Bolkan, C., Crespi, E. & Madigan, J. The relationship between hair cortisol, chronic stress, and well-being among older adults with dementia. Innov. Aging3, S468 (2019). [Google Scholar]
- 69.Fedurek, P. et al. Status does not predict stress: Women in an egalitarian hunter–gatherer society. Evol. Hum. Sci.2, e44 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heimbürge, S., Kanitz, E. & Otten, W. The use of hair cortisol for the assessment of stress in animals. Gen. Comp. Endocrinol.270, 10–17 (2019). [DOI] [PubMed] [Google Scholar]
- 71.Sadoughi, B., Lacroix, L., Berbesque, C., Meunier, H. & Lehmann, J. Effects of social tolerance on stress: hair cortisol concentrations in the tolerant Tonkean macaques (Macaca tonkeana) and the despotic long-tailed macaques (Macaca fascicularis). Stress24, 1–9 (2021). [DOI] [PubMed] [Google Scholar]
- 72.Beck, A., Hastings, R. P., Daley, D. & Stevenson, J. Pro-social behaviour and behaviour problems independently predict maternal stress. J. Intellect. Dev. Disabil.29, 339–349 (2004). [Google Scholar]
- 73.Faber, N. S. & Häusser, J. A. Why stress and hunger both increase and decrease prosocial behaviour. Curr. Opin. Psychol.44, 49–57 (2022). [DOI] [PubMed] [Google Scholar]
- 74.Nitschke, J. P., Forbes, P. A. & Lamm, C. Does stress make us more—or less—prosocial? A systematic review and meta-analysis of the effects of acute stress on prosocial behaviours using economic games. Neurosci. Biobehav. Rev.142, 104905 (2022). [DOI] [PubMed] [Google Scholar]
- 75.Plenty, S., Östberg, V. & Modin, B. The role of psychosocial school conditions in adolescent prosocial behaviour. Sch. Psychol. Int.36, 283–300 (2015). [Google Scholar]
- 76.Azulay, H., Guy, N., Pertzov, Y. & Israel, S. Empathy modulates the effect of stress reactivity on generous giving. Front. Neurosci.16, 814789 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Inagaki, T. K. et al. The neurobiology of giving versus receiving support: The role of stress-related and social reward-related neural activity. Psychosom. Med.78, 443 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fedurek, P. et al. Status does not predict stress among Hadza hunter-gatherer men. Sci. Rep.13, 1327 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woodburn, J. Sharing is not a form of exchange: An analysis of property-sharing in immediate-return hunter-gatherer societies. Prop. Relat. Renewing Anthropol. Tradit.275, 48–63 (1998). [Google Scholar]
- 80.Marlowe, F. W. et al. More ‘altruistic’punishment in larger societies. Proc. R. Soc. B Biol. Sci.275, 587–592 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hawkes, K., O’Connell, J. F. & Jones, N. B. Hadza meat sharing. Evol. Hum. Behav.22, 113–142 (2001). [DOI] [PubMed] [Google Scholar]
- 82.Wood, B. M. & Marlowe, F. W. Household and kin provisioning by Hadza men. Hum. Nat.24, 280–317 (2013). [DOI] [PubMed] [Google Scholar]
- 83.Apicella, C. L., Marlowe, F. W., Fowler, J. H. & Christakis, N. A. Social networks and cooperation in hunter-gatherers. Nature481, 497–501 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stibbard-Hawkes, D. N., Smith, K. & Apicella, C. L. Why hunt? Why gather? Why share? Hadza assessments of foraging and food-sharing motive. Evol. Hum. Behav.43, 257–272 (2022). [Google Scholar]
- 85.Fedurek, P. et al. Social status does not predict in-camp integration among egalitarian hunter-gatherer men. Behav. Ecol.33, 65–76 (2022). [Google Scholar]
- 86.Berbesque, J. C., Wood, B. M., Crittenden, A. N., Mabulla, A. & Marlowe, F. W. Eat first, share later: Hadza hunter–gatherer men consume more while foraging than in central places. Evol. Hum. Behav.37, 281–286 (2016). [Google Scholar]
- 87.Berbesque, J. C. & Marlowe, F. W. Sex differences in food preferences of Hadza hunter-gatherers. Evol. Psychol.7, 147470490900700400 (2009). [Google Scholar]
- 88.Raihani, N. J. & Smith, S. Competitive helping in online giving. Curr. Biol.25, 1183–1186 (2015). [DOI] [PubMed] [Google Scholar]
- 89.Aknin, L. B., Dunn, E. W. & Norton, M. I. Happiness runs in a circular motion: Evidence for a positive feedback loop between prosocial spending and happiness. J. Happiness Stud.13, 347–355 (2012). [Google Scholar]
- 90.Davis, J. D. R. & Cowen, P. J. Biochemical stress of caring. Psychol. Med.31, 1475–1478 (2001). [DOI] [PubMed] [Google Scholar]
- 91.Piff, P. K., Kraus, M. W., Côté, S., Cheng, B. H. & Keltner, D. Having less, giving more: The influence of social class on prosocial behavior. J. Pers. Soc. Psychol.99, 771 (2010). [DOI] [PubMed] [Google Scholar]
- 92.Stamos, A., Lange, F., Huang, S. & Dewitte, S. Having less, giving more? Two preregistered replications of the relationship between social class and prosocial behavior. J. Res. Personal.84, 103902 (2020). [Google Scholar]
- 93.Decker, S. A. Salivary cortisol and social status among Dominican men. Horm. Behav.38, 29–38 (2000). [DOI] [PubMed] [Google Scholar]
- 94.Von Rueden, C., Gurven, M. & Kaplan, H. The multiple dimensions of male social status in an Amazonian society. Evol. Hum. Behav.29, 402–415 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Konečná, M. & Urlacher, S. S. Male social status and its predictors among Garisakang forager-horticulturalists of lowland Papua New Guinea. Evol. Hum. Behav.38, 789–797 (2017). [Google Scholar]
- 96.Woodburn, J. Egalitarian societies revisited. In Property and Equality 18–31 (Berghahn Books, 2005). [Google Scholar]
- 97.Crittenden, A. N. et al. Oral health in transition: The Hadza foragers of Tanzania. PloS One12, e0172197 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones, N. B. Demography and Evolutionary Ecology of Hadza Hunter-Gatherers Vol. 71 (Cambridge University Press, 2016). [Google Scholar]
- 99.Marlowe, F. The Hadza: Hunter-Gatherers of Tanzania Vol. 3 (University of California Press, 2010). [Google Scholar]
- 100.Bennett, F. J., Barnicot, N. A., Woodburn, J. C., Pereira, M. S. & Henderson, B. E. Studies on viral, bacterial, rickettsial and treponemal diseases in the Hadza of Tanzania and a note on injuries. Hum. Biol.45, 243–272 (1973). [PubMed] [Google Scholar]
- 101.Lavergne, S. G. et al. Hair cortisol as a reliable indicator of stress physiology in the snowshoe hare: Influence of body region, sex, season, and predator–prey population dynamics. Gen. Comp. Endocrinol.294, 113471 (2020). [DOI] [PubMed] [Google Scholar]
- 102.Rakotoniaina, J. H. et al. Hair cortisol concentrations correlate negatively with survival in a wild primate population. BMC Ecol.17, 1–13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ibar, C. et al. Evaluation of stress, burnout and hair cortisol levels in health workers at a University Hospital during COVID-19 pandemic. Psychoneuroendocrinology128, 105213 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rajcani, J., Vytykacova, S., Solarikova, P. & Brezina, I. Stress and hair cortisol concentrations in nurses during the first wave of the COVID-19 pandemic. Psychoneuroendocrinology129, 105245 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aktipis, C. A., Cronk, L. & de Aguiar, R. Risk-pooling and herd survival: An agent-based model of a Maasai gift-giving system. Hum. Ecol.39, 131–140 (2011). [Google Scholar]
- 106.Beltran, D. G. et al. Unpredictable needs are associated with lower expectations of repayment. Curr. Res. Ecol. Soc. Psychol.4, 100095 (2023). [Google Scholar]
- 107.Lee, R. D. Rethinking the evolutionary theory of aging: Transfers, not births, shape senescence in social species. Proc. Natl. Acad. Sci.100, 9637–9642 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Putnam, R. D. Bowling Alone: The Collapse and Revival of American Community (Simon and schuster, 2000). [Google Scholar]
- 109.Alami, S. et al. Mother’s social status is associated with child health in a horticulturalist population. Proc. R. Soc. B287, 20192783 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gurven, M. et al. The Tsimane health and life history project: Integrating anthropology and biomedicine. Evol. Anthropol. Issues News Rev.26, 54–73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bird, D. W., Bird, R. B., Codding, B. F. & Zeanah, D. W. Variability in the organization and size of hunter-gatherer groups: Foragers do not live in small-scale societies. J. Hum. Evol.131, 96–108 (2019). [DOI] [PubMed] [Google Scholar]
- 112.Hill, K. R. et al. Co-residence patterns in hunter-gatherer societies show unique human social structure. Science331, 1286–1289 (2011). [DOI] [PubMed] [Google Scholar]
- 113.Hill, K. R., Wood, B. M., Baggio, J., Hurtado, A. M. & Boyd, R. T. Hunter-gatherer inter-band interaction rates: Implications for cumulative culture. PloS One9, e102806 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Salali, G. D. et al. Knowledge-sharing networks in hunter-gatherers and the evolution of cumulative culture. Curr. Biol.26, 2516–2521 (2016). [DOI] [PubMed] [Google Scholar]
- 115.Woodburn, J. Egalitarian societies revisited. Prop. Equal.1, 18–31 (2005). [Google Scholar]
- 116.Woodburn, J. Egalitarian Societies. Man17, 431–451 (1982). [Google Scholar]
- 117.Speth, J. D. Seasonality, resource stress, and food sharing in so-called “egalitarian” foraging societies. J. Anthropol. Archaeol.9, 148–188 (1990). [Google Scholar]
- 118.Fedurek, P. et al. The relationship between perceived friendship and proximity networks among Tanzanian Hadza. Hunt. Gatherer Res.7, 107–142 (2021). [Google Scholar]
- 119.Fedurek, P. et al. Height and integration in proximity networks among Tanzanian Hadza men. Am. J. Hum. Biol.10.1002/ajhb.24129 (2024). [DOI] [PubMed] [Google Scholar]
- 120.Wennig, R. Potential problems with the interpretation of hair analysis results. Forensic Sci. Int.107, 5–12 (2000). [DOI] [PubMed] [Google Scholar]
- 121.Russell, E., Koren, G., Rieder, M. & Van Uum, S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology37, 589–601 (2012). [DOI] [PubMed] [Google Scholar]
- 122.Sauvé, B., Koren, G., Walsh, G., Tokmakejian, S. & Van Uum, S. H. M. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin. Investig. Med. Med. Clin. Exp.30, E183-191 (2007). [DOI] [PubMed] [Google Scholar]
- 123.Slominski, R., Rovnaghi, C. R. & Anand, K. J. S. Methodological considerations for hair cortisol measurements in children. Ther. Drug Monit.37, 812–820 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xiang, L., Sunesara, I., Rehm, K. E. & Marshall, G. D. Jr. A modified and cost-effective method for hair cortisol analysis. Biomarkers21, 200–203 (2016). [DOI] [PubMed] [Google Scholar]
- 125.Tukey, J. W. Exploratory data analysis. vol. 2 (Reading, MA, 1977).
- 126.Mangiafico, S. & Mangiafico, M. S. Package ‘rcompanion’. Cran Repos 1–71 (2017).
- 127.Bates, D. M. lme4: Mixed-effects modeling with R. (2010).
- 128.R Development Core, team. A language ans environment for statistical computing. R Found Stat Comput Vienna Austria2, (2018).
- 129.Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw.82, 1–26 (2017). [Google Scholar]
- 130.Lüdecke, D. ggeffects: Tidy data frames of marginal effects from regression models. J. Open Sour. Softw.3, 772 (2018). [Google Scholar]
- 131.Nowok, B., Raab, G. M. & Dibben, C. synthpop: Bespoke creation of synthetic data in R. J. Stat. Softw.74, 1–26 (2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In order to protect the anonymity of health data from the Hadza, a simulated dataset using the R package synthpop will be provided131. Data, along with R codes used in this study, will be available in Figshare from the date of publication.