Abstract
Background
Moyamoya disease (MMD) is a rare cerebrovascular disorder characterized by progressive steno-occlusive changes in the internal carotid arteries, leading to an abnormal vascular network. Hypertension is prevalent among MMD patients, raising concerns about its impact on disease outcomes. This study aims to compare the clinical characteristics and outcomes of MMD patients with and without hypertension.
Methods
We conducted a multicenter, retrospective study involving 598 MMD patients who underwent surgical revascularization across 13 academic institutions in North America. Patients were categorized into hypertensive (n=292) and non-hypertensive (n=306) cohorts. Propensity score matching (PSM) was performed to adjust for baseline differences.
Results
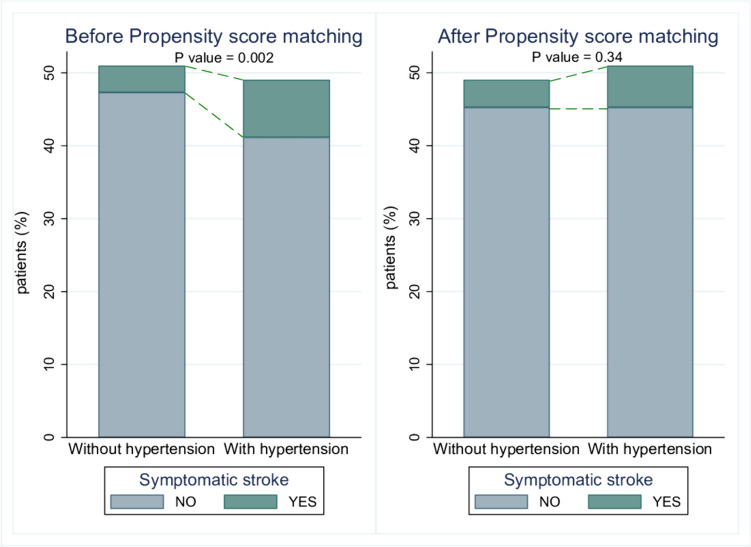
The mean age was higher in the hypertension group (46 years vs. 36.8 years, p < 0.001). Hypertensive patients had higher rates of diabetes mellitus (45.2% vs. 10.7%, p < 0.001) and smoking (48.8% vs. 27.1%, p < 0.001). Symptomatic stroke rates were higher in the hypertension group (16% vs. 7.1%; OR: 2.48; 95% CI: 1.39-4.40, p = 0.002) before matching. After PSM, there were no significant differences in symptomatic stroke rates (11.1% vs. 7.7%; OR: 1.5; CI: 0.64-3.47, p = 0.34), perioperative strokes (6.2% vs. 2.1%; OR 3.13; 95% CI: 0.83-11.82, p = 0.09), or good functional outcomes at discharge (93% vs. 92.3%; OR 1.1; 95% CI: 0.45-2.69, p = 0.82).
Conclusion
No significant differences in symptomatic stroke rates, perioperative strokes, or functional outcomes were observed between hypertensive and non-hypertensive Moyamoya patients. Appropriate management can lead to similar outcomes in both groups. Further prospective studies are required to validate these findings.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00701-024-06254-0.
Keywords: Moyamoya, HTN, Stroke, Multicenter
Introduction
Moyamoya disease (MMD) is a rare cerebrovascular disorder characterized by progressive steno-occlusive changes in the terminal portion of the internal carotid arteries and their main branches [23]. This pathological process leads to the formation of an abnormal vascular network at the base of the brain, which appears as a "puff of smoke" on angiography [23]. Although the etiology of MMD remains unclear, the disease manifests in a bimodal distribution, primarily affecting children aged 5-14 and adults aged 45-54 [12].
Clinical presentations of MMD are diverse, including strokes, transient ischemic attacks (TIA), seizures, aphasia, headaches, cognitive impairments in children, dysarthria, and hemiparesis [20]. Diagnostic modalities for MMD include CT angiography (CTA), magnetic resonance imaging (MRI), and MR angiography (MRA), with conventional angiography remaining the gold standard for both diagnosis and surgical planning [4]. Management of MMD primarily involves revascularization procedures, which aim to prevent stroke by enhancing cerebral blood flow in affected areas [1, 6, 15, 17].
It has been observed that a subset of MMD patients presents with renovascular hypertension, a condition associated with renal artery lesions [13, 19, 21]. The prevalence of renovascular hypertension in MMD patients is estimated to be around 2% [12, 27]. However, clinical observations suggest a higher prevalence of hypertension among MMD patients, raising concerns about its impact on disease outcomes [16].
Therefore, we aim to compare the clinical characteristics and outcomes of Moyamoya disease patients with and without hypertension using a multicenter, institutional, propensity score-matched analysis.
Methods
We conducted a multicenter, retrospective study in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [5]. Institutional review board approval was obtained at all centers. No identifiable patient information was presented in the study and, thus, informed consent was not required.
Patient population
This study involved Moyamoya-affected hemispheres treated with surgical revascularization across 13 academic institutions predominantly in North America. Inclusion criteria were standardized across centers and included all patients with Moyamoya disease who underwent surgical revascularization treatment. Data were collected and analyzed on a per-hemisphere basis, categorizing hemispheres into hypertensive (above 139/89) and non-hypertensive (120/80 to 139/89) cohorts based on patient medical history. Hypertension was defined as a documented history of hypertension (systolic blood pressure >139 mmHg or diastolic blood pressure >89 mmHg) or the use of antihypertensive medications at the time of admission [26]. Patients with secondary causes of systemic hypertension, such as renal artery stenosis or endocrine disorders, were excluded from the analysis to focus on primary hypertension.
Data collected included patient demographics (age, gender, race, hypertension, diabetes mellitus, smoking status, sickle cell disease), presenting symptoms (TIA, stroke, subarachnoid hemorrhage (SAH), intraparenchymal hemorrhage, intraventricular hemorrhage (IVH), incidental finding), disease characteristics (laterality, Suzuki grade), procedural details (DR vs IR), complications (major, minor, hemorrhagic, ischemic, periprocedural), follow-up (length of follow-up), and angiographic and functional outcomes (modified Rankin Score (mRS) and National Institute of Health Stroke Scale (NIHSS)).
Study endpoints
Study outcomes included major (ischemic or hemorrhagic with >4 change in NIHSS score) and minor (ischemic or hemorrhagic with <4 change in NIHSS score) symptomatic strokes (confirmed by imaging), good functional outcome (mRS 0-2) at discharge, NIHSS at discharge, length of hospital stay (days), perioperative strokes (including minor and major strokes confirmed by imaging), and follow-up strokes, categorized into ischemic and hemorrhagic after discharge. A stroke was defined by a new hypodensity on CT or a diffusion-weighted imaging hit on MRI not present on admission. A TIA was defined by a transient acute neurological deficit lasting less than 24 hours without radiographic evidence of stroke.
Statistical analysis
All statistical analyses were conducted using Stata (V.17.0; StataCorp). Baseline characteristics of hypertensive and non-hypertensive cohorts were compared using Pearson’s chi-squared or Fisher’s exact tests for categorical variables, and Student’s t-test or Mann-Whitney U tests for continuous variables, as appropriate. Given the significant baseline differences between hypertensive and non-hypertensive patients—such as age, diabetes mellitus, and smoking status—we used propensity score matching (PSM) to control for these confounders [2]. PSM was performed in a 1:1 ratio without replacement, using a caliper of 0.2 standard deviations of the logit of the propensity score. Propensity scores were derived using a logistic regression model that accounted for all baseline characteristics. The PSMATCH2 package for Stata was utilized for the propensity score derivation [14].
Outcome differences between hypertensive and non-hypertensive cohorts, both before and after matching, were assessed using univariable binary logistic and linear regression analyses, as appropriate. Results were reported as odds ratios (ORs) or beta coefficients with corresponding 95% confidence intervals (CIs). Fisher’s exact test was used for comparing outcomes with zero frequencies. Statistical significance was set at p<0.05, and all tests were two-tailed. Because the number of missing data points was minimal, no imputation was performed to avoid introducing bias [11]. The analysis was conducted using available data only.
We also used a Cox Proportional Hazard Model to determine the effect of hypertension in both symptomatic stroke and follow-up stroke. The model was adjusted to age, smoking, Suzuki grade, procedure type, diabetes mellitus, underlying disease, surgery side, and incidental MMD.
Results
Baseline characteristics
A total of 598 patients were included, with 292 patients having hypertension and 306 patients without hypertension (Fig. 1). The mean age was significantly higher in the hypertension group (46 years, SD 12.5) compared to the non-hypertension group (36.8 years, SD 15.1) (p < 0.001). The gender distribution was similar between the two groups, with 29.7% of males in the hypertension group and 29% in the non-hypertension group (p = 0.84).
Fig. 1.
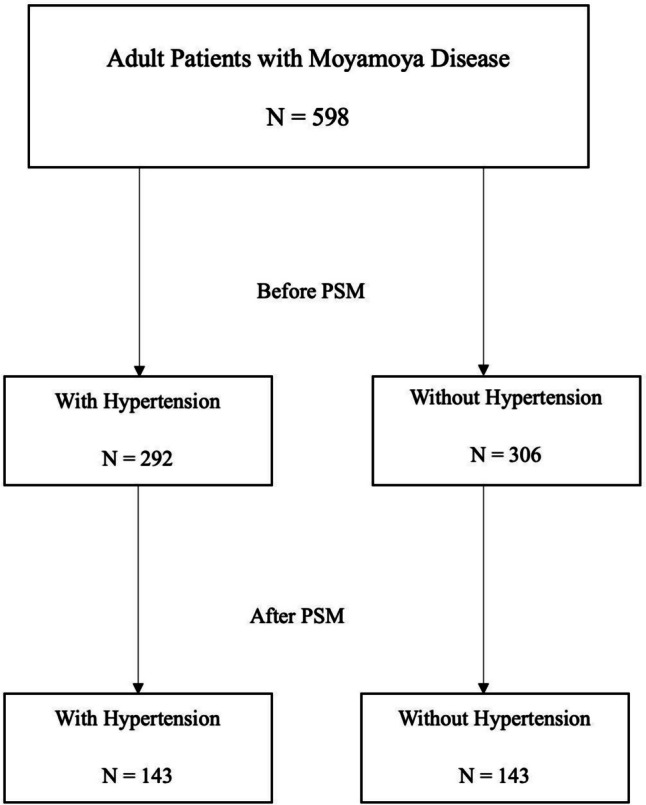
Flowchart shows the inclusion for patients in this study
Comorbid conditions were more prevalent in the hypertension group, with higher rates of diabetes mellitus (45.2% vs. 10.7%, p < 0.001) and smoking (48.8% vs. 27.1%, p < 0.001). There was no significant difference in the family history of Moyamoya disease (2% vs. 1.3%, p = 0.53), but the prevalence of sickle cell disease was lower in the hypertension group (3.4% vs. 7.5%, p = 0.03). The rates of indirect revascularization were similar between the two groups (60.2% vs. 57.1%, p = 0.45). However, hypertensive moyamoya patients’ group had a higher rate of combined revascularization compared to the non-hypertensive moyamoya patients’ group (13.3% vs. 6.2%, p = 0.004) (Table 1).
Table 1.
Comparison of baseline characteristics between unmatched patients with and without hypertension
| Total = 598 | With hypertension = 292 | Without hypertension = 306 | P-value | |
|---|---|---|---|---|
| Age, mean years (SD) | 41.3 (14.6) | 46.0 (12.5) | 36.8 (15.1) | < 0.001 |
| Male, n (%) | 176/598 (29.4) | 87/292 (29.7) | 90/306(29.0) | 0.84 |
| Race, n (%) | ||||
| Caucasian | 318/598 (53.1) | 148/292 (50.6) | 170/306 (55.5) | 0.23 |
| African-American | 170/598 (28.4) | 93/292(31.8) | 77/306 (25.1) | 0.07 |
| Asian | 70/598 (11.7) | 33/292 (11.3) | 37/306 (12.0) | 0.80 |
| Hispanic | 27/598 (45) | 10/292 (3.4) | 17/306 (9.1) | 0.24 |
| other | 13/598 (2.1) | 8/292 (2.7) | 5/306 1.6) | 0.40 |
| Diabetes mellitus, n (%) | 165/598 (27.5) | 132/292 (45.2) | 33/306 (10.7) | < 0.001 |
| Smoker, n (%) | 214/598 (35.7) | 131/292 (48.8) | 83/306 (27.1) | < 0.001 |
| Family history of moyamoya, n (%) | 10/598 (1.6) | 6/292 (2.0) | 4/306 (1.3) | 0.53 |
| Underlying disease, n (% | ||||
| Sickle cell disease | 33/598 (5.5) | 10/292 (3.4) | 23/306 (7.5) | 0.03 |
| Sickle cell trait | 5/598 (0.8) | 3/292 (1.3) | 2/306 (0.6) | 0.67 |
| Neurofibromatosis | 4/598 (0.67) | 3/292 (1.0) | 1/306 (0.3) | 0.36 |
| Procedure type, n (%) | ||||
| Indirect Revascularization | 351/598 (58.7) | 176/292 (60.2) | 175/306 (57.1) | 0.45 |
| Direct Revascularization | 305/598 (51.0) | 155/292 (53.0) | 150/306 (49.0) | 0.32 |
| Combined | 58/598 (9.7) | 39/292 (13.3) | 19/306 (6.2) | 0.004 |
| Suzuki grade, n (%) | ||||
| I | 24/598 (4.0) | 12/289 (4.1) | 12/303 (3.9) | 1.0 |
| II | 71/598 (11.8) | 31/292 (10.6) | 40/306 (13.0) | 0.37 |
| III | 181/598 (30.2) | 95/292 (32.5) | 86/306 (28.1) | 0.23 |
| IV | 178/598 (29.7) | 83/292 (28.4) | 95/306 (31.0) | 0.48 |
| V | 95/598 (15.8) | 43/292 (14.7) | 52/306 (16.9) | 0.50 |
| VI | 41/598 (6.8) | 24/292 (8.2) | 17/306 (5.5) | 0.25 |
| Surgery side, n (%) | ||||
| Right hemisphere | 306/598 (51.1) | 148/292 (50.6) | 158/306 (51.6) | 0.81 |
| Left hemisphere | 292/598 (48.8) | 144/292 (49.3) | 148/306 (48.3) | 0.81 |
| Follow-up (months), median months (IQR) | 17 (7-54) | 16 (7-50) | 17 (7-57) | 0.64 |
| mRS (0-2) on admission, n (%) | 529/590 (89.6) | 254/287 (88.5) | 275/303 (90.7) | 0.41 |
| Incidental, n (%) | 163/598 (27.2) | 68/292 (23.2) | 95/306 (31.0) | 0.03 |
| Stroke, n% | ||||
| Ischemic stroke | 339/598 (56.6) | 172/292 (58.9) | 167/306 (54.5) | 0.28 |
| TIA | 129/598 (21.5) | 58/292 (19.8) | 71/306 (23.2) | 0.37 |
| Intraventricular hemorrhage | 15/598 (2.5) | 10/292 (3.4) | 5/306 (1.6) | 0.19 |
| Intracerebral hemorrhage | 38/598 (6.3) | 19/292 (6.5) | 19/306 (6.2) | 1.0 |
| Subarachnoid hemorrhage | 35/598 (5.8) | 18/292 (6.1) | 17/306 (5.5) | 0.86 |
Outcomes
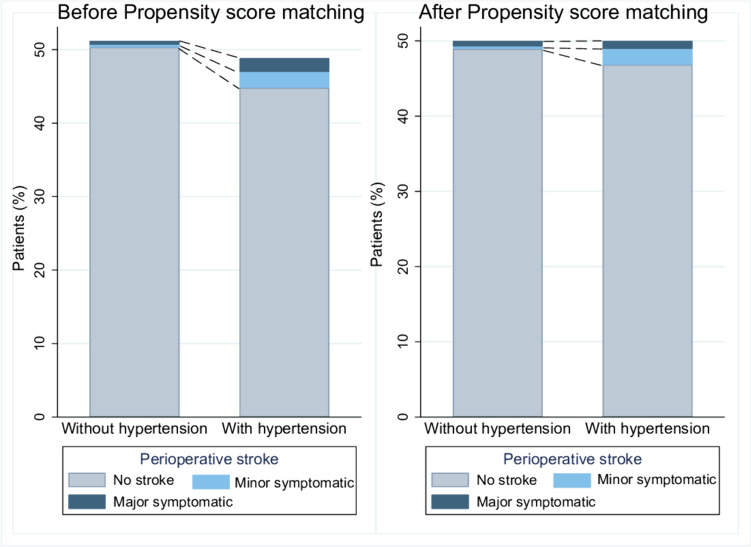
The overall symptomatic stroke occurred more frequently in the hypertension group (16% vs. 7.1%; OR: 2.48; 95% CI: 1.39-4.40, p = 0.002) (Fig. 2). Symptomatic ischemic strokes were more common in the hypertension group (14.5% vs. 6%; OR: 2.62; CI: 1.41-4.48, p = 0.002). Moreover, the hypertension group had a higher rate of perioperative stroke (8.2% vs. 2.2%; OR: 3.82; CI: 1.62-9.02, p = 0.002) including minor symptomatic (4.4% vs. 0.6%; OR: 7.08; CI: 1.58-31.66, p = 0.01) and major symptomatic (3.7% vs. 0.9%; OR: 3.95; CI: 1.09-14.31, p = 0.036) compared to the non-hypertension group (Fig. 3).
Fig. 2.
Incidence of symptomatic stroke in Moyamoya disease patients with and without hypertension, before and after PSM
Fig. 3.
Perioperative stroke outcomes in Moyamoya disease patients with and without hypertension, before and after PSM
Good functional outcome at discharge, measured by the mRS score, was similar between the groups (91% in hypertension vs. 92.4% in non-hypertension; OR 0.83; 95% CI: 0.47-1.50, p = 0.55). NIHSS scores at discharge were comparable (median 0 in both groups, p = 0.76). Length of hospital stay was slightly longer in the hypertension group but did not reach statistical significance (median 4 days vs. 3 days, p = 0.06). Follow-up stroke rates were higher in the hypertension group but were not statistically significant (9.5% vs. 6.8%; OR 1.45; 95% CI: 0.79-2.67, p = 0.22) (Table 2).
Table 2.
Comparison of outcomes between unmatched patients with and without hypertension
| With hypertension = 292 | Without hypertension = 306 | Effect variable | Value (95% CI) | P-value | |
|---|---|---|---|---|---|
| Symptomatic stroke, n (%) | 41/252 (16.0) | 19/263 (7.1) | OR | 2.48 (1.39 to 4.40) | 0.002 |
| Symptomatic ischemic stroke, n (%) | 37/255 (14.5) | 16/263 (6.0) | OR | 2.62 (1.41 to 4.48) | 0.002 |
| Symptomatic hemorrhagic stroke, n (%) | 5/252 (1.9) | 1/263 (0.3) | OR | 5.30 (0.61 to 45.71) | 0.12 |
| Intraoperative complication, n (%) | 31/292 (10.6) | 21/306 (6.8) | OR | 1.61 (0.90 to 2.87) | 0.10 |
| Perioperative stroke, n (%) | 24/292 (8.2) | 7/306 (2.2) | OR | 3.82 (1.62 to 9.02) | 0.002 |
| Perioperative minor symptomatic stroke, n (%) | 13/292 (4.4) | 2/306 (0.6) | OR | 7.08 (1.58 to 31.66) | 0.01 |
| Perioperative major symptomatic stroke, n (%) | 11/292 (3.7) | 3/306 (0.9) | OR | 3.95 (1.09 to 14.31) | 0.036 |
| Good functional outcome at discharge, n (%) | 264/290 (91.0) | 281/303 (92.4) | OR | 0.83 (0.47 to1.50) | 0.55 |
| NIHSS at discharge, median (IQR) | 0 (0-1) | 0 (0-1) | Beta | -0.005 (-0.04 to 0.03) | 0.76 |
| Length of hospital stay, median (IQR) | 4 (2-6) | 3 (2-5) | Beta | 0.02 (-0.00 to 0.06) | 0.06 |
| Follow-up stroke, n (%) | 26/271 (9.5) | 20/294 (6.8) | OR | 1.45 (0.79 to 2.67) | 0.22 |
| Follow-up ischemic stroke, n (%) | 23/292 (7.8) | 18/306 (5.8) | OR | 1.36 (0.72 to 2.59) | 0.33 |
| Follow-up hemorrhagic stroke, n (%) | 3/292 (1.0) | 2/306 (0.6) | OR | 1.57 (0.26 to 9.51) | 0.50 |
Propensity score matching
PSM resulted in 143 matched pairs (Table 3) (Fig. 1). Although symptomatic stroke occurred more in the hypertensive group, it didn’t reach statistical significance (11.1% vs. 7.7%; OR: 1.5; CI: 0.64-3.47, p = 0.34) (Fig. 2). Similarly, perioperative strokes were more common in the hypertension group but were not statistically significant (6.2% vs. 2.1%; OR 3.13; 95% CI: 0.83-11.82, p = 0.09) (Fig. 3). Good functional outcome at discharge was similar between the groups (93% in hypertension vs. 92.3% in non-hypertension; OR 1.1; 95% CI: 0.45-2.69, p = 0.82). NIHSS scores at discharge were comparable (median 0 in both groups, p = 0.86). Length of hospital stay was not significantly different (median 4 days vs. 3 days, p = 0.91). Follow-up stroke rates were similar between the groups (6.2% in hypertension vs. 5.5% in non-hypertension; OR 1.13; 95% CI: 0.42-3.02, p = 0.8) (Table 4).
Table 3.
Comparison of baseline characteristics between matched patients with and without hypertension
| Total = 286 | With hypertension = 143 | Without hypertension = 143 | P-value | |
|---|---|---|---|---|
| Age, mean years (SD) | 42.5 (14.4) | 42.5 (12.2) | 42.5 (12.7) | 0.96 |
| Male, n (%) | 73/286 (25.5) | 38/143 (26.5) | 35/143 (24.4) | 0.68 |
| Race, n (%) | ||||
| Caucasian | 166/286 (58.0) | 48/143 (58.7) | 82/143 (57.3) | 0.90 |
| African-American | 82/286 (28.6) | 41/143 (28.6) | 41/143 (28.6) | 1.00 |
| Asian | 21/286 (7.3) | 10/143 (6.9) | 11/143 (7.6) | 1.00 |
| Hispanic | 10/286 (3.5) | 5/143 (3.5) | 5/143 (3.5) | 1.00 |
| other | 7/286 (2.4) | 3/143 (2.1) | 4/143 (2.8) | 1.00 |
| Diabetes mellitus, n (%) | 45/286 (15.7) | 18/143 (12.5) | 27/143 (18.8) | 0.14 |
| Smoker, n (%) | 112/286 (391) | 54/143 (37.7) | 58/143 (40.5) | 0.62 |
| Family history of moyamoya, n (%) | 7/286 (2.4) | 3/143 (2.1) | 4/143 (2.8) | 1.00 |
| Underlying disease, n (% | ||||
| Sickle cell disease | 14/286 (4.9) | 8/148 (3.4) | 6/143 (7.5) | 0.78 |
| Sickle cell trait | 3/286 (1.0) | 1/143 (0.7) | 2/143 (1.4) | 1.00 |
| Neurofibromatosis | 2/286 (0.7) | 2/143 (1.4) | 0/143 (0) | 0.49 |
| Procedure type, n (%) | ||||
| Indirect Revascularization | 161/286 (56.2) | 80/143 (55.9) | 81/143 (56.6) | 1.00 |
| Direct Revascularization | 147/286 (51.4) | 71/143 (49.6) | 76/143 (53.1) | 0.55 |
| Combined | 22/286 (7.6) | 8/143 (5.5) | 14/143 (9.7) | 0.26 |
| Suzuki grade, n (%) | ||||
| I | 12/286 (4.2) | 6/143 (4.2) | 6/143 (4.2) | 1.00 |
| II | 28/286 (9.7) | 16/143 (11.1) | 12/143 (8.3) | 0.55 |
| III | 90/286 (31.4) | 42/143 (29.3) | 48/143 (33.5) | 0.44 |
| IV | 98/286 (34.2) | 49/143 (34.2) | 49/143 (34.2) | 1.00 |
| V | 42/286 (14.6) | 22/143 (15.3) | 20/143 (13.9) | 0.86 |
| VI | 17/286 (5.9) | 8/143 (5.5) | 9/143 (6.2) | 1.00 |
| Surgery side, n (%) | ||||
| Right hemisphere | 153/286 (53.5) | 67/143 (53.1) | 66/143 (53.8) | 0.90 |
| Left hemisphere | 133/286 (46.5) | 67/143 (46.8) | 66/143 (46.1) | 0.90 |
| Follow-up (months), median months (IQR) | 16 (6-56) | 21 (7-58) | 15 (4-51) | 0.27 |
| mRS (0-2) on admission, n (%) | 255/286 (89.1) | 128/143 (89.5) | 127/143 (88.8) | 1.00 |
| Incidental, n (%) | 61/286 (21.3) | 33/143 (23.0) | 28/143 (19.5) | 0.56 |
| Stroke, n% | ||||
| Ischemic stroke | 161286 (56.2) | 79/143 (55.2) | 82/143 (57.3) | 0.72 |
| TIA | 77/286 (21.3) | 38/143 (23.0) | 39/143 (19.5) | 1.00 |
| Intraventricular hemorrhage | 6/286 (2.1) | 2/143 (1.4) | 4/143 (2.8) | 0.68 |
| Intracerebral hemorrhage | 10/286 (3.5) | 6/143 (4.2) | 4/143 (2.8) | 0.74 |
| Subarachnoid hemorrhage | 20/286 (6.9) | 10/143 (6.9) | 10/143 (6.9) | 1.00 |
Table 4.
Comparison of outcomes between matched patients with and without hypertension
| With hypertension = 143 | Without hypertension = 143 | Effect variable | Value (95% CI) | P- value | |
|---|---|---|---|---|---|
| Symptomatic stroke, n (%) | 15/134 (11.1) | 10/129 (7.7) | OR | 1.5 (0.64 to 3.47) | 0.34 |
| Symptomatic ischemic stroke, n (%) | 14/134 (10.4) | 9/129 (6.9) | OR | 1.55 (0.64 to 3.73) | 0.32 |
| Symptomatic hemorrhagic stroke, n (%) | 1/134 (0.75) | 1/129 (0.78) | OR | 0.96 (0.05 to 15.55) | 0.97 |
| Intraoperative complication, n (%) | 12/143 (8.3) | 9/143 (6.2) | OR | 1.36 (0.55 to 3.34) | 0.49 |
| Perioperative stroke, n (%) | 9/143 (6.2) | 3/143 (2.1) | OR | 3.13 (0.83 to 11.82) | 0.09 |
| Perioperative minor symptomatic stroke, n (%) | 6/143 (4.2) | 1/143 (0.7) | OR | 6.21 (0.73 to 52.33) | 0.09 |
| Perioperative major symptomatic stroke, n (%) | 3/143 (2.1) | 2/143 (1.4) | OR | 1.51 (0.24 to 9.17) | 0.65 |
| Good functional outcome at discharge, n (%) | 133/143 (93.0) | 132/143 (92.3) | OR | 1.10 (0.45 to 2.69) | 0.82 |
| NIHSS at discharge, median (IQR) | 0 (0-0) | 0 (0-0) | Beta | -0.004 (-0.05 to 0.04) | 0.86 |
| Length of hospital stay, median (IQR) | 4 (2-4) | 3 (2-5) | Beta | 0.02 (-0.04 to 0.05) | 0.91 |
| Follow-up stroke, n (%) | 9/143 (6.2) | 8/143 (5.5) | OR | 1.13 (0.42 to 3.02) | 0.80 |
| Follow-up ischemic stroke, n (%) | 9/143 (6.2) | 8/143 (5.5) | OR | 1.13 (0.42 to 3.02) | 0.80 |
| Follow-up hemorrhagic stroke, n (%) | 0/143 (0) | 1/143 (0.7) | - | - | 1.00 |
Cox proportional hazard model
After adjusting the model to age, smoking, Suzuki grade, procedure type, diabetes mellitus, underlying disease, surgery side, and incidental MMD, there was no significant difference between the hypertension group and non-hypertension group in terms of symptomatic stroke (HR 1.33; 95% CI: 0.69-2.56, p = 0.38), and follow-up stroke (HR 0.90; 95% CI: 0.43-1.87, p = 0.78) (Supplementary Table 1).
Discussion
In this study, hypertensive patients had a higher rate of symptomatic stroke, both ischemic and perioperative, compared to non-hypertensive patients. However, after propensity score matching, these differences did not reach statistical significance, indicating that confounding factors such as age, diabetes mellitus, and smoking status—more prevalent in the hypertensive group—may have contributed to the increased stroke risk observed in the unmatched analysis. Additionally, our adjusted cox proportional analysis showed no significant difference between the two groups in symptomatic and follow-up stroke rates.
Hypertension is a well-established major risk factor for numerous cardiovascular disorders, including stroke, due to its profound adverse effects on cerebral vascular structure and function [22]. Previous studies have emphasized that hypertension accelerates atherogenesis and is associated with increased cardiovascular morbidity [3, 7, 8, 18].
A meta-analysis was done by Wei et al. [25] to investigate the risk factors for postoperative stroke in MMD patients. Hypertension was found not to be associated with increased risk of postoperative stroke. Our study aligns with these findings, where hypertension was found not to be associated with symptomatic stroke, perioperative stroke, follow-up stroke or functional outcomes.
In a study by Ma et al. which investigated the effect of hypertension on moyamoya patients, they reported a higher rate of unfavorable outcomes and postoperative complications in hypertensive moyamoya patients [16]. In contrast, our study showed no significant differences between hypertensive moyamoya patients and non-hypertensive moyamoya patients in postoperative complications or functional outcomes.
Another study by Wang et al. which analyzed the associations between clinical risk factors and long-term outcomes in moyamoya patients found that hypertension was positively associated with follow-up stroke [24]. However, our study still showed no significant differences between hypertensive moyamoya patients and non-hypertensive moyamoya patients regarding follow-up stroke (whether ischemic or hemorrhagic), both before and after PSM.
The differences between our study and those proposed by Ma et al. [16] and Wang et al. [24] can be explained by the fact that their studies were conducted exclusively on Chinese populations. Although Chinese populations may have lower rates of hypertension compared to the American population, this lower prevalence can lead to lower awareness and control, resulting in higher complication rates [9, 10, 28]. Our study included populations from different ethnicities, with Caucasian populations being the most common. This suggests that treatment protocols and patient demographics can significantly impact outcomes in hypertensive Moyamoya patients.
This study has several limitations that should be considered. First, as a retrospective analysis, it is inherently subject to biases related to data collection and interpretation. Second, our focus was primarily on preoperative blood pressure values, without comprehensive monitoring of intraoperative and postoperative blood pressure variations, which could influence outcomes. Also, data on the severity of hypertension and the degree of its medical management were not uniformly available across all centers, limiting our ability to stratify these variables in our analysis. Additionally, the data were collected from multiple centers, leading to potential variability in clinical practices and patient management protocols. Third, the median follow-up period of 17 months is relatively short, which may limit the ability to capture long-term outcomes. Lastly, while propensity score matching was employed to balance baseline characteristics which provides a more accurate comparison between the groups, unmeasured confounders and reduction in statistical power may still affect the results.
Conclusion
In conclusion, hypertensive, and non-hypertensive patients with MMD showed no significant differences in symptomatic stroke rates, perioperative strokes, or functional outcomes. Proper management can lead to comparable recovery in both groups. Further research is needed to optimize treatment strategies for hypertensive Moyamoya patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
None.
Author contributions
B.M, J.M.R, H.A, E.A, K.E.N, C.J.C, R.J, H.S, J.A.G, A.A.D, A.B., M.K., C.S.O, A.J.T., A.M., A.S, G.M.C, R.A.H, G.P., A.M.S, A.J.P, D.M.H, M.G., J.W, S.M.N, K.B, M.M.S, J.B, A.Z, C.M, B.M.H, R.L, R.D, R.A, G.S.S, A.A, A.M., N.A.H, S.I.T, M.R.G, R.H.R, P.J. contributed to the conception and design of the work.
B.M, J.M.R, H.A, E.A, K.E.N, C.J.C, R.J, H.S, J.A.G, A.A.D, A.B., M.K., C.S.O, A.J.T., A.M., A.S, G.M.C, R.A.H, G.P., A.M.S, A.J.P, D.M.H, M.G., J.W, S.M.N, K.B, M.M.S, J.B, A.Z, C.M, B.M.H, R.L, R.D, R.A, G.S.S, A.A, A.M., N.A.H, S.I.T, M.R.G, R.H.R, P.J. were involved in the acquisition of data, and data analysis and interpretation.
B.M, J.M.R, H.A, E.A, K.E.N, C.J.C, R.J, H.S, J.A.G, A.A.D, A.B., M.K., C.S.O, A.J.T., A.M., A.S, G.M.C, R.A.H, G.P., A.M.S, A.J.P, D.M.H, M.G., J.W, S.M.N, K.B, M.M.S, J.B, A.Z, C.M, B.M.H, R.L, R.D, R.A, G.S.S, A.A, A.M., N.A.H, S.I.T, M.R.G, R.H.R, P.J. drafted the work and revised it critically for important intellectual content.
All authors gave final approval of the version to be published and agree to be accountable for all aspects of the manuscript.
Funding
This research received no grant from any funding agency in public, commercial, or not-for-profit sectors.
Data availability
Data can be provided on reasonable request from authors.
Declarations
Ethical approval
All procedures performed in the studies involving human participants were per the Institutional Review Board (IRB) ethical standards and national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
The study protocol was reviewed and approved by the Institutional Review Board. Following institutional guidelines, all protected health information was removed, and individual patient consent was not required in the analysis of the case series.
Competing interests
Dr. Jabbour is a consultant for Medtronic, MicroVention, Balt and Cerus Endovascular. Dr. Tjoumakaris is a consultant for MicroVention. Dr. Gooch is a consultant for Stryker. Dr. Spiotta is a consultant for Terumo, Stryker, Penumbra, RapidAI, Cerenovus. Dr. Patel is a consultant for MicroVention and Medtronic. Dr. Du is a consultant for grand rounds. Dr. Burkhardt is a consultant for Longeviti Neuro solutions, Q-Apel Medical, Stryker. Dr. Hanel is a consultant for Medtronic, Balt, Stryker, Q’Apel Medical, Inc, Codman Neuro (J&J), Cerenovus, Microvention, Imperative Care, Inc, Phenox, Inc, Rapid Medical. Dr. Siddiqui is a consultant for Amnis Therapeutics, Apellis Pharmaceuticals, Inc., Boston Scientific, Canon Medical Systems USA, Inc., Cardinal Health 200, LLC, Cerebrotech Medical Systems, Inc., Cerenovus, Cerevatech Medical, Inc., Cordis, Corindus, Inc., Endostream Medical, Ltd, Imperative Care, InspireMD, Ltd., Integra, IRRAS AB, Medtronic, MicroVention, Minnetronix Neuro, Inc., Peijia Medical, Penumbra, Q’Apel Medical, Inc., Rapid Medical, Serenity Medical, Inc., Silk Road Medical, StimMed, LLC, Stryker Neurovascular, Three Rivers Medical, Inc., VasSol, Viz.ai, Inc. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Amlie-Lefond C, Ellenbogen RG (2015) Factors associated with the presentation of moyamoya in childhood. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc 24(6):1204–1210. 10.1016/j.jstrokecerebrovasdis.2015.01.018 10.1016/j.jstrokecerebrovasdis.2015.01.018 [DOI] [PubMed] [Google Scholar]
- 2.Amoah J, Stuart EA, Cosgrove SE et al (2020) Comparing propensity score methods versus traditional regression analysis for the evaluation of observational data: a case study evaluating the treatment of gram-negative bloodstream infections. Clin Infect Dis Off Publ Infect Dis Soc Am 71(9):e497–e505. 10.1093/cid/ciaa169 10.1093/cid/ciaa169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anim JI, Kofi AD (1989) Hypertension, cerebral vascular changes and stroke in Ghana: cerebral atherosclerosis and stroke. East Afr Med J 66(7):468–475 [PubMed] [Google Scholar]
- 4.Chiu D, Shedden P, Bratina P, Grotta JC (1998) Clinical features of moyamoya disease in the United States. Stroke 29(7):1347–1351. 10.1161/01.str.29.7.1347 10.1161/01.str.29.7.1347 [DOI] [PubMed] [Google Scholar]
- 5.Cuschieri S (2019) The STROBE guidelines. Saudi J Anaesth 13(Suppl 1):S31–S34. 10.4103/sja.SJA_543_18 10.4103/sja.SJA_543_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Naamani K, Chen CJ, Jabre R et al (2024) Direct versus indirect revascularization for moyamoya: a large multicenter study. J Neurol Neurosurg Psychiatry 95(3):256–263. 10.1136/jnnp-2022-329176 10.1136/jnnp-2022-329176 [DOI] [PubMed] [Google Scholar]
- 7.Ellenga Mbolla BF, Gombet TR, Atipo-Ibara BI, Etitiele F, Kimbally-Kaky G (2012) Impact of severe hypertension in acute heart failure in Brazzaville (Congo). Med Sante Trop 22(1):98–99. 10.1684/mst.2012.0017 10.1684/mst.2012.0017 [DOI] [PubMed] [Google Scholar]
- 8.Ewen E, Zhang Z, Kolm P et al (2009) The risk of cardiovascular events in primary care patients following an episode of severe hypertension. J Clin Hypertens Greenwich Conn 11(4):175–182. 10.1111/j.1751-7176.2009.00097.x 10.1111/j.1751-7176.2009.00097.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FastStats. April 29, 2024. https://www.cdc.gov/nchs/fastats/hypertension.htm. Accessed 22 June 2024
- 10.Hypertension China 2023 country profile. https://www.who.int/publications/m/item/hypertension-chn-2023-country-profile. Accessed 22 June 2024
- 11.Imputing Missing Data with R; MICE package | DataScience+. https://datascienceplus.com/imputing-missing-data-with-r-mice-package/. Accessed 10 Aug 2024
- 12.Kim JS (2016) Moyamoya disease: epidemiology, clinical features, and diagnosis. J Stroke 18(1):2–11. 10.5853/jos.2015.01627 10.5853/jos.2015.01627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuwayama F, Hamasaki Y, Shinagawa T et al (2001) Moyamoya disease complicated with renal artery stenosis and nephrotic syndrome: reversal of nephrotic syndrome after nephrectomy. J Pediatr 138(3):418–420. 10.1067/mpd.2001.111330 10.1067/mpd.2001.111330 [DOI] [PubMed] [Google Scholar]
- 14.Leuven E, Sianesi B PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Stat Softw Compon. Published online February 1, 2018. https://ideas.repec.org//c/boc/bocode/s432001.html. Accessed 21 June 2024
- 15.Liu X, Zhang D, Shuo W, Zhao Y, Wang R, Zhao J (2013) Long term outcome after conservative and surgical treatment of haemorrhagic moyamoya disease. J Neurol Neurosurg Psychiatry 84(3):258–265. 10.1136/jnnp-2012-302236 10.1136/jnnp-2012-302236 [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Zhao M, Deng X et al (2020) Comparison of clinical outcomes and characteristics between patients with and without hypertension in moyamoya disease. J Clin Neurosci Off J Neurosurg Soc Australas 75:163–167. 10.1016/j.jocn.2019.12.016 10.1016/j.jocn.2019.12.016 [DOI] [PubMed] [Google Scholar]
- 17.Macyszyn L, Attiah M, Ma TS et al (2017) Direct versus indirect revascularization procedures for moyamoya disease: a comparative effectiveness study. J Neurosurg 126(5):1523–1529. 10.3171/2015.8.JNS15504 10.3171/2015.8.JNS15504 [DOI] [PubMed] [Google Scholar]
- 18.Mourad JJ (2013) Severe hypertension: definition and patients profiles. Rev Prat 63(5):672–676 [PubMed] [Google Scholar]
- 19.van der Vliet JA, Zeilstra DJ, Van Roye SF, Merx JL, Assmann KJ (1994) Renal artery stenosis in moyamoya syndrome. J Cardiovasc Surg (Torino) 35(5):441–443 [PubMed] [Google Scholar]
- 20.Scott RM, Smith ER (2009) Moyamoya disease and moyamoya syndrome. N Engl J Med 360(12):1226–1237. 10.1056/NEJMra0804622 10.1056/NEJMra0804622 [DOI] [PubMed] [Google Scholar]
- 21.Shang S, Zhou D, Ya J et al (2020) Progress in moyamoya disease. Neurosurg Rev 43(2):371–382. 10.1007/s10143-018-0994-5 10.1007/s10143-018-0994-5 [DOI] [PubMed] [Google Scholar]
- 22.Sobey CG, Faraci FM (2001) Novel mechanisms contributing to cerebral vascular dysfunction during chronic hypertension. Curr Hypertens Rep 3(6):517–523. 10.1007/s11906-001-0015-9 10.1007/s11906-001-0015-9 [DOI] [PubMed] [Google Scholar]
- 23.Suzuki J, Takaku A (1969) Cerebrovascular, “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 20(3):288–299. 10.1001/archneur.1969.00480090076012 10.1001/archneur.1969.00480090076012 [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Zhang Z, Wang Y et al (2021) Clinical and genetic risk factors of long-term outcomes after encephaloduroarteriosynangiosis in moyamoya disease in China. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc 30(7):105847. 10.1016/j.jstrokecerebrovasdis.2021.105847 10.1016/j.jstrokecerebrovasdis.2021.105847 [DOI] [PubMed] [Google Scholar]
- 25.Wei W, Chen X, Yu J, Li XQ (2019) Risk factors for postoperative stroke in adults patients with moyamoya disease: a systematic review with meta-analysis. BMC Neurol 19(1):98. 10.1186/s12883-019-1327-1 10.1186/s12883-019-1327-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whelton PK, Carey RM, Aronow WS et al (2018) ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertens Dallas Tex 71(6):1269–1324. 10.1161/HYP.0000000000000066 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 27.Yamada I, Himeno Y, Matsushima Y, Shibuya H (2000) Renal artery lesions in patients with moyamoya disease: angiographic findings. Stroke 31(3):733–737. 10.1161/01.str.31.3.733 10.1161/01.str.31.3.733 [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Shi Y, Zhou B et al (2023) Prevalence, awareness, treatment, and control of hypertension in China, 2004–18: findings from six rounds of a national survey. BMJ 380:e071952. 10.1136/bmj-2022-071952 10.1136/bmj-2022-071952 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be provided on reasonable request from authors.