Abstract
Introduction
Medical androgen deprivation therapy (ADT) options have expanded for patients with advanced prostate cancer (PC). Historically, ADT was primarily available in long-acting injectable formulations. In 2020, the first oral formulation was US Food and Drug Administration-approved for adults with advanced PC. This study’s aim was to assess patient preferences for attributes of medical ADT, including mode of administration, side effects, impact on sexual interest, and out-of-pocket (OOP) costs, and to segment respondents into distinct groups based on their treatment choice patterns.
Methods
A cross-sectional survey was conducted among US residents aged > 40 years with PC, employing a discrete choice experiment to assess preferences for ADT attributes. For each choice task, respondents were asked to select the hypothetical treatment profile that they preferred out of two presented. Latent class analysis (LCA) was conducted to estimate attribute-level preference weights and calculate attribute relative importance for groups of respondents with similar treatment preferences.
Results
A total of 304 respondents completed the survey (mean age 64.4 years). LCA identified four preference groups, named according to the attribute each group considered most important: Sexual interest, Cost-sensitive, Favors daily pill, and Favors injection. Most respondents in the Sexual interest group were < 65 years, while the Cost-sensitive group was mostly ≥ 65 years. Favors daily pill had the highest proportion of ADT-naïve individuals. On average, respondents in these groups preferred an oral medication. Favors injection, which had the highest proportion of ADT-experienced individuals, preferred infrequent intramuscular injections, lower chance of post-ADT testosterone recovery, and lower OOP cost.
Conclusion
Respondents differed in their preferences regarding ADT attributes, highlighting the need for patient involvement in their treatment decisions. Effective communication between healthcare providers and patients about the benefits and risks of available therapies should be encouraged to ensure that patients receive the PC treatment that best meets their needs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-024-02955-1.
Keywords: Advanced prostate cancer, Androgen deprivation therapy, Discrete choice experiment, Latent class analysis, Patient preference, Prostatic neoplasms
Plain Language Summary
Prostate cancers often depend on the male sex hormone, testosterone, to grow. Androgen deprivation therapy (ADT) is used to lower testosterone levels in patients with advanced prostate cancer. ADT options available to patients have different characteristics, including how they are taken (injection or pill), side effects, impact on sexual interest, and costs. Researchers wanted to understand which ADT characteristics were most important to groups of patients with similar preferences. To do this, they gave 304 patients a series of two hypothetical (meaning not real) examples of ADT options with different characteristics and asked them to choose the option that they preferred most. Researchers found that patients could be separated into four different groups based on their preferences for ADT characteristics. One group preferred an ADT that had the least impact on their interest in sex. These patients were mainly younger than 65 years old. A second group preferred a lower cost ADT. These patients were mainly 65 years or older. A third group preferred a pill that could be taken once a day by mouth. Most of these patients did not take ADT in the past. A fourth group preferred an ADT that was given in a physician’s office as an injection every 6 months. These patients mainly had taken ADT in the past. This study shows that patients have different preferences for ADT treatment characteristics. It is important for doctors to discuss the different ADT options with patients to find the treatment that best meets their needs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-024-02955-1.
Key Summary Points
| Why carry out this study? |
| Medical androgen deprivation therapy (ADT) for patients with advanced prostate cancer includes several gonadotropin-releasing hormone receptor agonist or antagonist options; these options differ with respect to important attributes (e.g., mode of administration, side effects, impact on sexual interest, out-of-pocket cost). |
| Employing a discrete choice experiment, this study assessed patient preferences for different attributes of medical ADT and segmented respondents into distinct groups based on similar treatment preferences. |
| What was learned from this study? |
| Four preference groups, which differed in their demographic and clinical characteristics, were identified and named according to the attribute each group considered most important: Sexual interest, Cost-sensitive, Favors daily pill, and Favors injection. |
| The results of this study bring into focus the heterogeneity in the priorities and preferences of patients with advanced prostate cancer, and suggest that different groups of patients will generally prioritize factors related to administration, side effects, potential impact on sexual activity, or cost when choosing ADT treatment. |
| Shared decision-making between healthcare providers and patients that includes discussing their individual preferences and expectations from treatment, as well as the benefits and risks associated with different ADT options, should be encouraged to ensure that patients receive the prostate cancer treatment that is best suited for their care and needs. |
Introduction
Nearly 300,000 new prostate cancer (PC) diagnoses and over 35,000 deaths are estimated for the USA in 2024 [1]. Advanced PC encompasses several disease states spanning from biochemical recurrence after primary definitive therapy without evidence of metastases to disease that has spread outside the prostate, to the pelvic lymph nodes, or beyond the pelvis and vertebral column [2, 3]. Increased testosterone levels drive the growth of PC resulting from dysregulation of the androgen receptor signalling pathway [4]. Thus, androgen deprivation therapy (ADT) remains a critical component of treatment for patients with advanced PC, and treatment guidelines support its use in metastatic disease and in conjunction with radiation in specific settings [2, 5, 6]. Approved medical ADTs for advanced PC have historically included injectable gonadotropin-releasing hormone (GnRH) receptor agonists (e.g., goserelin, histrelin, leuprolide, and triptorelin) and an injectable GnRH receptor antagonist (i.e., degarelix) [7, 8]. Relugolix, an oral, daily, GnRH receptor antagonist, became available in 2020 in the USA as an alternative to current injectable formulations [9]. While the different ADT options reach the threshold of achieving sustained testosterone suppression below castrate levels (< 50 ng/dL), they differ in mode and frequency of administration, adverse event profiles, testosterone surge occurrence, and speed of testosterone recovery after treatment discontinuation, as well as out-of-pocket (OOP) costs [8, 10–13].
GnRH receptor agonists are associated with delayed castration due to an initial testosterone surge. In the first 4 weeks of treatment, this testosterone surge sometimes necessitates the use of concomitant first-generation nonsteroidal antiandrogens, such as bicalutamide [2, 8, 14]. In addition, the time to recover to normal testosterone levels (> 300 ng/dL) after treatment cessation can be prolonged with GnRH receptor agonists, particularly in older patients, those with low baseline testosterone levels, or those on long-term GnRH receptor agonist treatment [15, 16]. This can result in persistence of the side effects of low testosterone levels including reduced sexual interest. GnRH receptor agonists may also be associated with an increased risk of cardiovascular events and diabetes [17–20].
GnRH receptor antagonists are not associated with an initial testosterone surge and corresponding clinical sequalae [11, 21]. In addition, some clinical trials and meta-analyses have shown a lower risk of cardiovascular disease with GnRH receptor antagonists compared with agonists [22–24]. However, other studies show no difference or a decreased likelihood of heart failure but increased likelihood of arrythmia with GnRH receptor antagonists versus agonists [25, 26]. The GnRH receptor antagonist degarelix is associated with increased risk of injection site reactions versus agonists [27]. Patients receiving relugolix orally avoid the burden associated with injections and may experience more rapid testosterone recovery to normal levels following treatment cessation compared with GnRH receptor agonists [24].
Patients’ involvement in their own treatment decision-making is understood to improve treatment outcomes [28–30]; however, there are few studies evaluating the overall preferences of patients for the different ADT options for advanced PC [29, 31]. Thus, the present study aimed to quantify the drivers of treatment preferences and explore variations in preferences among patients with PC for key attributes that differentiate currently available medical ADT options.
Methods
Study Design and Population
In this quantitative study, a cross-sectional, online survey (Supplementary Appendix S1), including a discrete choice experiment (DCE) to elicit patient preferences for hypothetical medical ADT options, was administered to enrolled participants between February 17, 2022 and July 25, 2022. Eligible individuals were English-speaking US residents aged > 40 years with a self-reported physician diagnosis of PC (and no other cancer diagnosis) and enrolled in healthcare coverage for the past 3 years. The study was designed to include a roughly equal number of ADT-experienced and ADT-naïve participants to allow for varying ADT treatment history. Therefore, participants were excluded if they were unable to recall if they had been treated with medical ADT (Supplementary Fig. S1).
Participants were recruited using the actively managed Kantar Profiles online consumer panel, which uses several recruitment methodologies, including opt-in email, co-registration, e-newsletter campaigns, traditional banner placements, and internal and external affiliate networks [32]. Participants were required to provide electronic informed consent.
The final protocol and informed consent documentation were reviewed by Sterling International Review Board (Atlanta, GA); an exemption determination was granted on October 28, 2021 (Protocol ID # 9398-MMaculaitis). The study complied with all legal and regulatory requirements, as well as with scientific purpose, value, and rigor, and followed generally accepted research practices described in Good Practices for Outcomes Research issued by the International Society for Pharmacoeconomics and Outcomes Research [33–35].
Survey Development
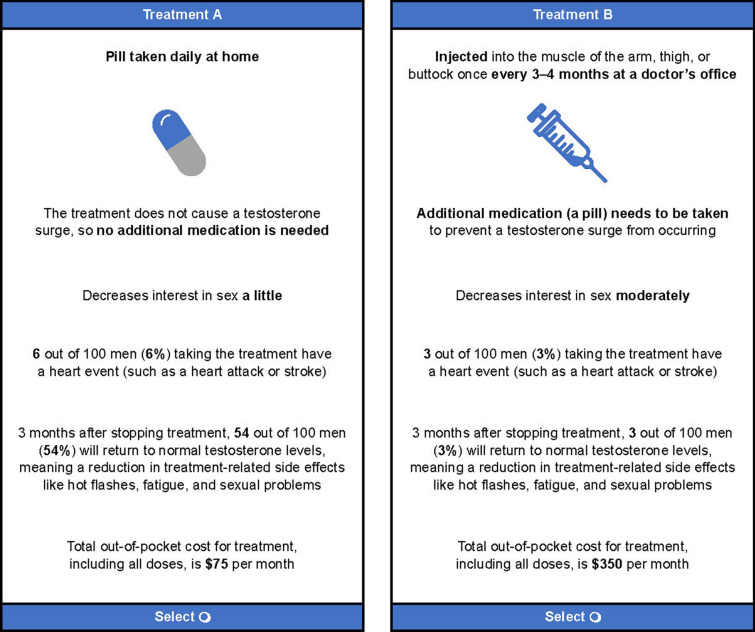
The survey included a DCE to elicit patient preferences for hypothetical medical ADT options. DCE attributes and attribute levels were identified on the basis of consultations with clinical experts and published literature [9, 11, 24, 36–39], and focused on attributes that differ among currently approved ADTs. The following ADT attributes were included in the DCE: mode and frequency of administration, needing to take a pill to prevent testosterone surge at initiation of ADT, impact on sexual interest, risk of cardiovascular events, chance of achieving normal testosterone levels 3 months after discontinuation, and monthly OOP cost. Although the attributes “impact on sexual interest” and “chance of achieving normal testosterone levels 3 months after discontinuation” are clinically related [40], in the pretest interviews participants considered these to be separate issues. Therefore, these were presented as separate attributes in the DCE. A description of all attribute levels is provided in Table 1. As sustained testosterone suppression below castrate levels at rates ranging from approximately 90% to 100% [7, 24, 27, 41, 42] have been reported for approved medical ADTs, efficacy in sustaining castration levels of testosterone was not included. The survey instrument also collected data on sociodemographic characteristics (e.g., age, race/ethnicity, insurance type, and marital status), clinical characteristics (cancer location, time since diagnosis, and comorbidities), and treatment history (procedures and treatments ever received for PC).
Table 1.
Discrete choice experiment attributes and levels
| Attribute (not shown) | Label | Levels |
|---|---|---|
| Mode, frequency, and location of administration | How the medication is taken | Injected under the skin of the abdomen once a month at a doctor's office [10, 39] |
| Injected into the muscle of the arm, thigh, or buttock once every 3–4 months at a doctor's office [10, 38] | ||
| Injected into the muscle of the arm, thigh, or buttock once every 6 months at a doctor's office [10, 38] | ||
| Pill taken daily at home [9, 10] | ||
| Testosterone surge within the first few days of administration, which can be treated with additional medication | Additional medication is needed to prevent a testosterone surge from occurring | Additional medication (a pill) is needed to prevent a testosterone surge; taken daily for approximately 3 weeks to prevent a testosterone surge from occurring [2] |
| The treatment does not cause a testosterone surge, so no additional medication is needed [24] | ||
| Impact on sexual interest | Decreases interest in sex | Decreases interest in sex very much [11] |
| Decreases interest in sex moderately [36] | ||
| Decreases interest in sex a little [37] | ||
| Risk of cardiovascular events | X out of 100 men (X%) taking the treatment have a heart event (such as a heart attack or stroke) | 3% [24] |
| 6% [24] | ||
| Percentage of patients achieving normal testosterone after 3 months | 3 months after stopping treatment, X out of 100 men (X%) will return to normal testosterone levels, meaning a reduction in treatment-related side effects, like hot flashes, fatigue, and sexual problems | 3% [24] |
| 16% [11] | ||
| 54% [24] | ||
| Out-of-pocket cost | Total out-of-pocket cost for treatment, including all doses, is $X/montha | $5 |
| $75 | ||
| $200 | ||
| $350 |
aThe levels of this attribute were not obtained from the literature, although they were evaluated in the cognitive interviews
Prior to administration, the survey was pretested using 45-min structured cognitive interviews with a convenience sample of 12 participants who met the inclusion criteria for the study. Moderators conducted the interviews via telephone using a secure desktop-sharing platform to allow the participants to view the survey instrument. The purpose of the pretest interviews was to assess the relevance of the DCE attributes to participants’ treatment choices, as well as the understandability of the survey instrument and DCE exercise. Participants’ feedback during the interviews was used to refine the final DCE survey instrument.
DCE Design
A DCE presents respondents with a series of choice tasks in which they must select between hypothetical treatment options. Individuals’ responses on the DCE tasks subsequently allow for trade-offs between different treatment attributes to be evaluated. A fundamental assumption of the DCE design is that individuals’ preferences can be attributed to the collection of attributes (e.g., benefits, risks, etc.) associated with the specific treatment options under consideration. In the current study, each respondent completed a series of 11 DCE tasks, in which they were asked to choose between two hypothetical ADT treatment profiles consisting of the same attributes, but with varying attribute levels. The hypothetical treatment profiles were not designed to reflect currently available ADT options, but rather each treatment profile comprised a combination of attribute levels. The treatment profiles, profile pairings, and series of choice tasks in the DCE were generated using a balanced experimental design [34]. The DCE questions were designed to ensure that the series of questions provided sufficient choice information to statistically infer the trade-offs that respondents were willing to make among the attributes. An example choice task is shown in Fig. 1.
Fig. 1.
Example discrete choice experiment choice task. The prompt shown to participants with each choice task was as follows: “If the following were your only options for a prostate cancer androgen deprivation therapy/hormonal treatment, please indicate which option you would choose: Treatment A or Treatment B. Assume there are no other differences between Treatment A versus B besides what is shown.” Treatment A and B are not actual products; rather, they are a mix of attributes from currently available androgen deprivation therapies
Data Analysis
Empirical evidence suggests that a sample size of 300 respondents would be sufficient to estimate a two-alternative, forced-choice DCE with the number of attributes and levels included in this study [43]. Respondent characteristics were summarized using means and standard deviations (SDs) for continuous or discrete/count data, with categorical data presented as frequencies and percentages. Hierarchical Bayesian modeling was used to analyze the DCE data and estimate preference weights for all attribute levels in each latent class [33]. A Bayesian latent class analysis (LCA) was conducted to identify distinct groups within which preferences were homogeneous and between which preference differed [44, 45]. For the LCA analysis, multinomial logistic regression was used to identify segments of respondents with similar preferences. Identification of the optimal number of latent class groups was based on Bayesian information criteria. Each respondent was assigned to a class based on their class-assignment probability.
The change in utility (i.e., preference) associated with a change in the levels of each attribute is represented by the difference between the preference weights for any two levels of an attribute, with larger differences reflecting greater contribution to the overall utility for a given treatment choice. The model assumes that treatment choice reflects the utility of the choice alternatives and that utility is a function of the attributes of the treatment. The modeling yielded a distribution of preference weights reflecting the relative contribution of each attribute level to overall utility and, thus, treatment choice. A mean and SD were estimated for each preference weight in each latent class. Attribute relative importance was computed by latent class by dividing the range of preference weights for each attribute (preference weight of the most preferred level minus that of the least preferred level) by the sum of the ranges of all attributes and then standardizing to sum to 100% across the attributes. Attributes with higher relative importance are more influential to respondents’ treatment choice. Attribute relative importance and selected respondent characteristics were then compared across the class groups using one-way analysis of variance (continuous or discrete/count data) or chi-square tests (categorical data). P values < 0.05 (two-tailed) were considered statistically significant.
Data from the DCE tasks were analyzed using Sawtooth’s Lighthouse Studio (v9.13.1; Provo, UT); LCA was conducted using Latent Gold (v6.0; Arlington, MA); all other analyses were performed using IBM SPSS (v28.0; Armonk, NY).
Results
Respondent Characteristics
The majority of the 304 respondents were aged ≥ 65 years, White, married, and had been diagnosed with PC for a mean of 5.4 years (Table 2). Approximately half of respondents reported each of the following characteristics: ADT-naïve, college educated, diagnosed with PC confined to the prostate, and planning to be sexually active. In addition, cardiovascular comorbidities (congestive heart failure, heart disease, myocardial infarction, and/or stroke) were present in 15.1% of the full sample.
Table 2.
Patient characteristics by latent class group and for the total sample
| Variables | Total sample (N = 304) |
Sexual interest (n = 89) |
Favors injection (n = 59) |
Favors daily pill (n = 56) |
Cost sensitive (n = 100) |
P valuea |
|---|---|---|---|---|---|---|
| Race/ethnicityb, n (%) | ||||||
| Whitec | 198 (65.1) | 68 (76.4) | 35 (59.3) | 24 (42.9) | 71 (71.0) | < 0.001 |
| Black/African American | 51 (16.8) | 15 (16.9) | 9 (15.3) | 13 (23.2) | 14 (14.0) | 0.510 |
| Hispanic/Latino | 21 (6.9) | 3 (3.4) | 5 (8.5) | 7 (12.5) | 6 (6.0) | 0.186 |
| Otherd | 34 (11.2) | 3 (3.4) | 10 (16.9) | 12 (21.4) | 9 (9.0) | |
| Age, mean (SD), years | 64.4 (7.2) | 62.4 (8.1) | 65.1 (5.8) | 63.7 (6.1) | 66.2 (7.2) | 0.002 |
| Age categories, n (%) | 0.026e | |||||
| < 65 years | 143 (47.0) | 51 (57.3) | 25 (42.4) | 30 (53.6) | 37 (37.0) | |
| ≥ 65 years | 161 (53.0) | 38 (42.7) | 34 (57.6) | 26 (46.4) | 63 (63.0) | |
| Insurance typef, n (%) | ||||||
| Individual/family insurance plans | 160 (52.6) | 55 (61.8) | 35 (59.3) | 30 (53.6) | 40 (40.0) | 0.015 |
| Medicaid/MediCal | 20 (6.6) | 6 (6.7) | 2 (3.4) | 3 (5.4) | 9 (9.0) | 0.558 |
| Medicare | 151 (49.7) | 34 (38.2) | 30 (50.8) | 25 (44.6) | 62 (62.0) | 0.010 |
| Marital status, n (%) | ||||||
| Committed relationship/married | 220 (72.4) | 69 (77.5) | 34 (57.6) | 37 (66.1) | 80 (80.0) | 0.001 |
| Education, n (%) | ||||||
| College degree or higher | 144 (47.4) | 56 (62.9) | 22 (37.3) | 17 (30.4) | 49 (49.0) | 0.003 |
| Employment status, n (%) | < 0.001e | |||||
| Retired | 178 (58.6) | 31 (34.8) | 41 (69.5) | 38 (67.9) | 68 (68.0) | |
| Employed (full-time or part-time) | 91 (29.9) | 49 (55.1) | 12 (20.3) | 11 (19.6) | 19 (19.0) | |
| Unemployed or disabled | 31 (10.2) | 9 (10.1) | 4 (6.8) | 6 (10.7) | 12 (12.0) | |
| Cancer locationg, n (%) | ||||||
| Cancer is in prostate only | 168 (55.3) | 56 (62.9) | 34 (57.6) | 18 (32.1) | 60 (60.0) | 0.002 |
| Cancer has spread to lymph nodes | 57 (18.8) | 12 (13.5) | 15 (25.4) | 17 (30.4) | 13 (13.0) | 0.015 |
| Cancer has spread to bones and/or other organs | 61 (20.1) | 11 (12.4) | 10 (16.9) | 18 (32.1) | 22 (22.0) | 0.030 |
| Do not know/recall | 21 (6.9) | 11 (12.4) | 0 | 4 (7.1) | 6 (6.0) | 0.035 |
| Comorbiditiesf, n (%) | ||||||
| High blood pressure | 136 (44.7) | 36 (40.4) | 22 (37.3) | 31 (55.4) | 47 (47.0) | 0.191 |
| High cholesterol | 119 (39.1) | 38 (42.7) | 16 (27.1) | 19 (33.9) | 46 (46.0) | 0.083 |
| Lower back pain | 77 (25.3) | 26 (29.2) | 13 (22.0) | 9 (16.1) | 29 (29.0) | 0.231 |
| Anxiety or depression | 57 (18.8) | 18 (20.2) | 5 (8.5) | 8 (14.3) | 26 (26.0) | 0.038 |
| Diabetes | 49 (16.1) | 10 (11.2) | 7 (11.9) | 11 (19.6) | 21 (21.0) | 0.200 |
| CV comorbiditiesg | 46 (15.1) | 8 (9.0) | 14 (23.7) | 11 (19.6) | 13 (13.0) | 0.064 |
| Sexually active, n (%) | ||||||
| Plan to be sexually active | 152 (50.0) | 69 (77.5) | 25 (42.4) | 16 (28.6) | 42 (42.0) | < 0.001 |
| Time since diagnosish, mean (SD) years | 5.4 (4.8) | 5.1 (3.9) | 5.7 (5.1) | 4.6 (3.5) | 6.0 (5.9) | 0.346 |
| ADT experience, n (%) | 0.622 | |||||
| ADT-naïve | 155 (51.0) | 47 (52.8) | 27 (45.8) | 32 (57.1) | 49 (49.0) | |
| ADT-experienced | 149 (49.0) | 42 (47.2) | 32 (54.2) | 24 (42.9) | 51 (51.0) | |
| Procedures ever receivedf, n (%) | ||||||
| Prostatectomy | 132 (43.4) | 35 (39.3) | 27 (45.8) | 27 (48.2) | 43 (43.0) | 0.736 |
| Radiation | 180 (59.2) | 54 (60.7) | 32 (54.2) | 39 (69.6) | 55 (55.0) | 0.268 |
| Treatments ever receivedf, n (%) | ||||||
| Chemotherapyi | 114 (37.5) | 21 (23.6) | 31 (52.5) | 35 (62.5) | 27 (27.0) | < 0.001 |
| First-generation antiandrogensj | 133 (43.8) | 34 (38.2) | 30 (50.8) | 27 (48.2) | 42 (42.0) | 0.408 |
| Next-generation hormonal therapiesk | 75 (24.7) | 18 (20.2) | 12 (20.3) | 18 (32.1) | 27 (27.0) | 0.319 |
| Targeted therapiesl | 30 (9.9) | 7 (7.9) | 6 (10.2) | 10 (17.9) | 7 (7.0) | 0.148 |
| Immunotherapiesm | 45 (14.8) | 13 (14.6) | 9 (15.3) | 13 (23.2) | 10 (10.0) | 0.173 |
ADT androgen deprivation therapy; BMI body mass index; CV cardiovascular; SD standard deviation
aP values reflect tests (one-way analysis of variance for continuous/discreet data and chi-square tests for categorical data) of the overall effect across the 4 latent class groups on each variable shown
bPercentages may not add to 100 because of rounding
cDefined as all respondents who did not self-report any race other than White
dDefined as all respondents who self-reported as Asian, Native Hawaiian or other Pacific Islander, mixed race, other ethnicity, or prefer to not answer
eThe P value shown represents the test for differences in the distribution across all response categories for the characteristic
fParticipants could select > 1 response option
gIncludes self-reported diagnosis of ≥ 1 of the following: congestive heart failure, heart disease, myocardial infarction, and/or stroke
hFive patients were excluded from disease duration analysis; three patients did not recall year of diagnosis and two patients provided illogical response for year of diagnosis
iChemotherapies such as cabazitaxel, mitoxantrone hydrochloride, or docetaxel
jFirst-generation antiandrogens such as nilutamide, flutamide, or bicalutamide
kNext-generation hormonal therapies such as darolutamide, abiraterone acetate, enzalutamide, or apalutamide
lTargeted therapies such as radium-223 dichloride, rucaparib, or olaparib
mImmunotherapies such as sipuleucel-T or pembrolizumab
Latent Class Analysis
LCA identified four groups, named according to the attribute each group found most important: Sexual interest, Favors Injection, Favors daily pill, and Cost-sensitive (Fig. 2, Table 2). The groups identified by LCA differed significantly on certain sociodemographic and clinical variables (Table 2). Respondents in the Sexual interest group were predominantly aged < 65 years and highly educated (college degree or higher); they were also most likely to report that their cancer was confined to the prostate only and that they planned to be sexually active. The Favors injection group had the highest proportion of ADT-experienced individuals and the highest rate of cardiovascular comorbidities. The Favors daily pill group had the highest proportion of ADT-naïve individuals, were most likely to report their PC had spread to lymph nodes or other organs, and were least often planning to be sexually active. Respondents in the Cost-sensitive group were more likely to be aged ≥ 65 years and had the highest rates of anxiety or depression, high cholesterol, and diabetes among all four groups.
Fig. 2.
Attribute-level preference weights for latent class groups. Attributes are shown in the order in which they were presented to respondents in the discrete choice experiment tasks. The change in relative importance associated with a change in the levels of each attribute is represented by the vertical distance between the preference weights for any two levels of that attribute. Larger differences between preference weights indicate that the change between those two levels is perceived by respondents as relatively more influential to overall utility
ADT Treatment Preferences by Group
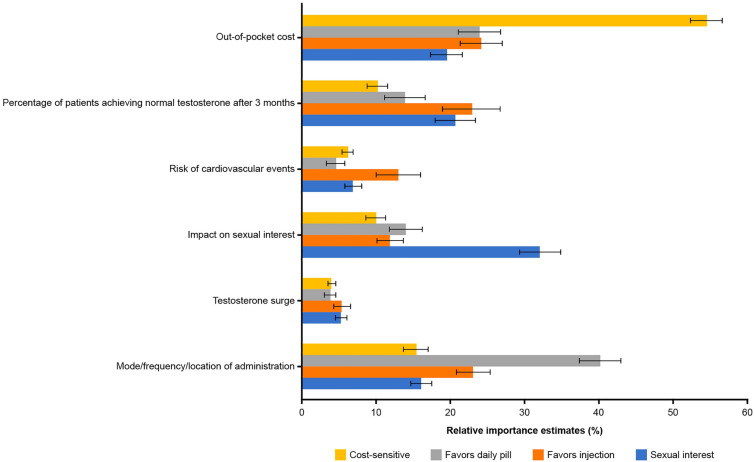
Respondents in the Sexual interest, Favors daily pill, and Cost-sensitive groups placed higher relative importance on a single attribute, at a level that was higher than for any other group for that attribute (Fig. 3). The Cost-sensitive group placed the highest relative importance on OOP costs (relative importance = 54.5%) versus 19.4% to 24.1% for the other LCA groups. The Sexual interest group placed highest relative importance on impact on sexual interest (relative importance = 32.0%) versus 10.0% to 14.0% for the other LCA groups, and the Favors daily pill group placed the highest relative importance on mode of administration (relative importance = 40.1%) versus 15.3% to 23.0% for the other LCA groups. On average, respondents in these groups preferred an oral medication (Fig. 2). In contrast, respondents in the Favors injection group (19.4% of the sample) preferred intramuscular injections every 6 months at a doctor’s office to daily oral administration (Fig. 2). The Favors injection group placed a higher relative importance for risk of cardiovascular events than any of the other LCA groups (relative importance = 12.9% vs. 4.5% to 6.9%); however, other attributes ranked higher in relative importance for respondents within this group (Fig. 3). The Favors injection and Sexual interest groups placed higher relative importance on achieving normal testosterone 3 months after treatment discontinuation than did respondents in the other two groups. The need to take an additional medication to prevent testosterone surge had the lowest relative importance for all groups.
Fig. 3.
Attribute relative importance estimates for latent class groups. For all one-way analysis of variance tests of the overall effect of the latent class group on attribute relative-importance estimates, P < 0.01. Error bars represent 95% confidence intervals. Relative importance values sum to 100% across attributes for each group
The Sexual interest group favored less impact on sexual interest (change from “very much” to “moderately”, 0.13 − [− 2.28] = 2.41) and a greater chance to achieve normal testosterone within 3 months of treatment discontinuation (change from “16% chance” to “54% chance”, 1.41 − [− 0.26] = 1.67; Fig. 2). Improvement in impact on sexual interest was the most important attribute to respondents in this group (relative importance = 32.0%) and was 1.6 to 6.2 times more important than each of the other attributes (Fig. 3).
The Favors injection group preferred infrequent ADT injections (change from “a once-daily pill” to “an intramuscular injection once every 6 months at a doctor’s office”, 0.81 − [− 0.30] = 1.11) and lower OOP cost (change from “$200 OOP monthly” to “$75 OOP monthly”, 0.57 − [− 0.37] = 0.94; Fig. 2). The data also indicate that this group preferred a lower chance, rather than a higher chance, of achieving normal testosterone within 3 months after treatment discontinuation (change from “54% chance” to “16% chance”, 0.47 − [− 1.23] = 1.70). With respect to relative importance, reducing OOP costs (relative importance = 24.1%), changes in mode and frequency of administration (relative importance = 23.0%), and reduced chance of achieving normal testosterone 3 months after discontinuation (relative importance = 22.8%) were perceived as being similarly high in importance for the Favors injection group (Fig. 3).
The Favors daily pill group preferred a once-daily ADT pill (change from “a subcutaneous injection once every 6 months at a doctor’s office” to “a once-daily pill”, 3.38 − [− 0.35] = 3.73; Fig. 2). Changes in mode of administration was 1.7 to 10.8 times more important to members of this group than each of the other attributes (relative importance = 40.1% vs. 3.7% to 23.8%; Fig. 3).
The Cost-sensitive group preferred a lower OOP cost (change from “$200 OOP monthly” to “$75 OOP monthly”, 2.10 − [− 1.34] = 3.44; Fig. 2). Reducing OOP costs was of highest importance (relative importance = 54.5%) to respondents in this group and was 3.6 to 14.0 times more important than each of the other attributes (Fig. 3).
Discussion
To our knowledge, this is the first study to evaluate patient preferences for attributes that differ among currently available injectable and oral ADTs. This study focused on medical options only. The findings from this study indicate that patients with varying underlying clinical and demographic characteristics prioritize different factors when choosing PC treatment. Four groups were identified with distinct ADT preferences; for three of the groups (Cost-sensitive, Favors daily pill, and Sexual interest), ADT treatment choice was principally driven by a single attribute. Specifically, the Cost-sensitive group, which included the highest proportion of respondents who were aged ≥ 65 years, was most influenced by affordability. The Favors daily pill group, which included the highest proportion of ADT-naïve individuals, was most interested in having the option of an oral once-daily treatment. The Sexual interest group, which included the highest proportion of respondents who were aged < 65 years, favored an option with minimal impact on sexual activity and greater chance of normal testosterone recovery after discontinuation of treatment.
In contrast, a combination of attributes including preferring infrequent intramuscular injections, a lower OOP cost, and a lower chance of normal testosterone recovery after discontinuation drove ADT treatment choice for the Favors injection group. This group included the highest proportion of ADT-experienced individuals and second highest proportion of respondents aged ≥ 65 years. Thus, for some patients, testosterone recovery is of high importance, while for others it is not, which may be related to the age and level of sexual activity of these patients. Alternatively, it is possible that those who preferred a lower chance of testosterone recovery believed that continued testosterone suppression would reduce the chance of tumor recurrence or progression. This highlights the importance of clinician–patient shared decision-making and education on ADT cessation, benefits of testosterone recovery, and potential improvement in quality of life. The results of the current study add context to prior literature which identified the impact on libido or sexual functioning as an important attribute in PC treatment choice [31, 46]. These results may be particularly relevant for patients with nonmetastatic disease. These patients are eligible for ADT with radiotherapy or intermittent therapy with a limited treatment duration, a regimen that has been shown to minimize some of the adverse effects of ADT, including sexual dysfunction [47]. However, additional studies are required to determine the underlying reason for the observed differences in preferences, as this study did not explore this question. For example, it is not known why ADT-experienced individuals and those with cardiovascular comorbidities preferred injections to an oral medication.
While effectiveness is an important consideration in the treatment preferences of patients with PC [31], the present study did not include this attribute as sustained testosterone suppression below castrate levels at rates from approximately 90% to 100% have been reported for currently available oral and injectable ADTs [7, 24, 27, 41, 42]. Additionally, although risk of cardiovascular events was an attribute in the DCE, the risk of cardiovascular events with GnRH receptor agonists or antagonists remains uncertain [23, 25, 26, 48, 49]. A 2023 systematic review of 11 randomized trials including 4248 patients suggests that GnRH receptor antagonists are potentially associated with fewer major adverse cardiovascular events compared with agonists, but cautions that findings may not be definitive because of limitations in the size and quality of the data set [49]. High-quality studies comparing current ADT options are needed to help answer this question. Our findings indicate that different groups of patients will generally prioritize factors related to administration, side effects, potential impact on sexual activity, or cost when choosing ADT treatment. These findings highlight the need for different ADT treatment options, as well as open dialogue between patients and their physicians to determine the best option for the individual patient.
Limitations
An online self-reported survey likely underrepresents individuals without adequate internet access or with discomfort with online administration, including residents of rural areas, institutionalized adults, and those with the most severe comorbidities and disabilities. Additionally, 55% of respondents reported that they had cancer confined to the prostate only (Table 2); these respondents may have been more likely to be ADT-naïve or to have received a limited treatment course rather than life-long treatment, compared with respondents with metastatic disease [2]. Further, OOP costs for cancer treatments in the USA are not generalizable, as they vary substantially depending on type of insurance, treatment plan, geographic location, and other factors [50].
The accuracy of self-reported data (e.g., PC diagnosis, comorbidities, treatments, etc.) could not be confirmed. Prior research has shown that DCEs can predict real-world treatment choices, supporting the external validity of this approach [51]; nonetheless, stated preferences do not perfectly correspond with actual treatment choices. While the DCE was designed to closely mirror available clinical evidence and was informed by qualitative research with the target population of interest, it may not capture all aspects involved in real-world treatment decisions. A DCE cannot accommodate all factors that could potentially influence treatment preferences; unmeasured variables may lead to error in the estimation of preferences. Additionally, the study did not capture other important factors in treatment selection, such as the rates of adherence associated with the different modes of administration, nor were patients asked to provide explanations for their stated preferences for different attributes. To help guide treatment decision-making, research assessing the impact of treatment preferences on adherence and identifying the many factors underlying patients’ preferences for ADT attributes is warranted.
Conclusions
Four preference groups were identified according to the ADT treatment attribute each group considered most important: Sexual interest, Favors injection, Favors daily pill, and Cost-sensitive. The results of this study bring into focus the heterogeneity in priorities and preferences of patients with PC, which may be influenced by the needs of their disease state and a variety of factors personal to the individual, including lifestyle, age, and income. An effective dialogue between healthcare providers and their patients is a critical first step towards understanding what is most important to patients, with the goal of identifying the therapy that can provide patients with an optimal treatment journey.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the participants for their involvement in the study. The authors acknowledge Robert Dufour (former employee of Myovant Sciences, Inc. at the time of the study), Kathleen M. Beusterien (employee of Oracle Life Sciences), Lewis Kopenhafer (employee of Oracle Life Sciences), and Esmond Nwokeji (former employee of Pfizer Inc. at the time of the study) for their contributions to the study concept and design, as well as Oliver Will (employee of Oracle Life Sciences) for support with statistical analysis.
Medical Writing, Editorial, and Other Assistance
Medical writing and editorial support were provided by Michelle Mancher, MPH, and Rosie Henderson, MSc, of Onyx (a division of Prime, London, UK); funded by Pfizer Inc., in collaboration with Sumitomo Pharma Switzerland GmbH.
Author Contributions
Conception and design: Brett Hauber, Elke Hunsche, Martine C. Maculaitis, Sean P. Collins. Financial support: Agnes Hong. Administrative support: Agnes Hong. Collection and assembly of data: Martine C. Maculaitis. Data analysis and interpretation: Brett Hauber, Agnes Hong, Elke Hunsche, Martine C. Maculaitis, Sean P. Collins. Manuscript writing: Brett Hauber, Agnes Hong, Elke Hunsche, Martine C. Maculaitis, Sean P. Collins. Final approval of manuscript: Brett Hauber, Agnes Hong, Elke Hunsche, Martine C. Maculaitis, Sean P. Collins. Accountable for all aspects of the work: Brett Hauber, Agnes Hong, Elke Hunsche, Martine C. Maculaitis, Sean P. Collins.
Funding
This work, including the journal’s Rapid Service and Open Access fees, was funded by Pfizer Inc., in collaboration with Sumitomo Pharma Switzerland GmbH.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due the data collection only being granted exemption determination from an IRB for this specific protocol but are available from the corresponding author on reasonable request for non-commercial use.
Declarations
Conflict of Interest
Brett Hauber and Agnes Hong are employees of Pfizer Inc., with stock ownership in Pfizer Inc. Elke Hunsche is an employee of Sumitomo Pharma Switzerland GmbH (formerly Myovant Sciences GmbH). Martine C. Maculaitis is an employee of Oracle Life Sciences, which received funding from Pfizer Inc. to conduct and report on the study. Sean P. Collins is currently Vice Chair of Faculty Affairs in the Department of Radiation Oncology at the University of South Florida; he is a consultant for Boston Scientific Corporation, Sumitomo Pharma America, Inc. (formerly Myovant Sciences Inc.), and Pfizer Inc.; and has received honoraria and research funding from Accuray Inc.
Ethical Approval
Participants were required to provide electronic informed consent. The final protocol and informed consent documentation were reviewed by Sterling International Review Board (Atlanta, GA); an exemption determination was granted on October 28, 2021 (Protocol ID # 9398-MMaculaitis). The study complied with all legal and regulatory requirements, as well as with scientific purpose, value, and rigor, and followed generally accepted research practices described in Good Practices for Outcomes Research issued by the International Society for Pharmacoeconomics and Outcomes Research.
Footnotes
Prior Presentation: The data were presented at The American Society of Clinical Oncology Genitourinary Symposium in San Francisco, CA February 16–19, 2023 and at the annual congress of The American Urological Association in Chicago, IL April 28–May 1, 2023.
References
- 1.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. 10.3322/caac.21820. 10.3322/caac.21820 [DOI] [PubMed] [Google Scholar]
- 2.Lowrance W, Dreicer R, Jarrard DF, et al. Updates to advanced prostate cancer: AUA/SUO guideline (2023). J Urol. 2023;209(6):1082–90. 10.1097/JU.0000000000003452. 10.1097/JU.0000000000003452 [DOI] [PubMed] [Google Scholar]
- 3.Moul JW. The evolving definition of advanced prostate cancer. Rev Urol. 2004;6(Suppl 8):S10–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Militaru FC, Militaru V, Crisan N, et al. Molecular basis and therapeutic targets in prostate cancer: a comprehensive review. Biomol Biomed. 2023;23(5):760–71. 10.17305/bb.2023.8782. 10.17305/bb.2023.8782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6(2):76–85. 10.1038/ncpuro1296. 10.1038/ncpuro1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker C, Castro E, Fizazi K, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(9):1119–34. 10.1016/j.annonc.2020.06.011. 10.1016/j.annonc.2020.06.011 [DOI] [PubMed] [Google Scholar]
- 7.Raja T, Sud R, Addla S, et al. Gonadotropin-releasing hormone agonists in prostate cancer: a comparative review of efficacy and safety. Indian J Cancer. 2022;59(Suppl 1):S142–59. 10.4103/ijc.IJC_65_21. 10.4103/ijc.IJC_65_21 [DOI] [PubMed] [Google Scholar]
- 8.Van Poppel H, Abrahamsson P-A. Considerations for the use of gonadotropin-releasing hormone agonists and antagonists in patients with prostate cancer. Int J Urol. 2020;27(10):830–7. 10.1111/iju.14303. 10.1111/iju.14303 [DOI] [PubMed] [Google Scholar]
- 9.U.S. Food & Drug Administration. Prescribing information: ORGOVYX (relugolix) tablets, for oral use. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214621s000lbl.pdf. Accessed May 16, 2024.
- 10.Crawford ED, Heidenreich A, Lawrentschuk N, et al. Androgen-targeted therapy in men with prostate cancer: evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019;22(1):24–38. 10.1038/s41391-018-0079-0. 10.1038/s41391-018-0079-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dearnaley DP, Saltzstein DR, Sylvester JE, et al. The oral gonadotropin-releasing hormone receptor antagonist relugolix as neoadjuvant/adjuvant androgen deprivation therapy to external beam radiotherapy in patients with localised intermediate-risk prostate cancer: a randomised, open-label, parallel-group phase 2 trial. Eur Urol. 2020;78(2):184–92. 10.1016/j.eururo.2020.03.001. 10.1016/j.eururo.2020.03.001 [DOI] [PubMed] [Google Scholar]
- 12.Henry MA, Leung A, Filson CP. Cost considerations for systemic therapy for patients with advanced genitourinary malignancies. Cancer. 2018;124(14):2897–905. 10.1002/cncr.31355. 10.1002/cncr.31355 [DOI] [PubMed] [Google Scholar]
- 13.Borrelli E, McGladrigan CG. PCN80 estimating the difference in annual out-of-pocket costs for relugolix and leuprolide for medicare patients with metastatic prostate cancer. Value Health. 2021;24(Suppl 1):S34. 10.1016/j.jval.2021.04.172. 10.1016/j.jval.2021.04.172 [DOI] [Google Scholar]
- 14.Thompson IM. Flare associated with LHRH-agonist therapy. Rev Urol. 2001;3(Suppl 3):S10–4. [PMC free article] [PubMed] [Google Scholar]
- 15.Kaku H, Saika T, Tsushima T, et al. Time course of serum testosterone and luteinizing hormone levels after cessation of long-term luteinizing hormone-releasing hormone agonist treatment in patients with prostate cancer. Prostate. 2006;66(4):439–44. 10.1002/pros.20341. 10.1002/pros.20341 [DOI] [PubMed] [Google Scholar]
- 16.Nascimento B, Miranda EP, Jenkins LC, Benfante N, Schofield EA, Mulhall JP. Testosterone recovery profiles after cessation of androgen deprivation therapy for prostate cancer. J Sex Med. 2019;16(6):872–9. 10.1016/j.jsxm.2019.03.273. 10.1016/j.jsxm.2019.03.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fradin J, Kim FJ, Lu-Yao GL, Storozynsky E, Kelly WK. Review of cardiovascular risk of androgen deprivation therapy and the influence of race in men with prostate cancer. Cancers (Basel). 2023;15(8):2316. 10.3390/cancers15082316. 10.3390/cancers15082316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klimis H, Pinthus JH, Aghel N, et al. The burden of uncontrolled cardiovascular risk factors in men with prostate cancer. JACC: CardioOncol. 2023;5(1):70–81. 10.1016/j.jaccao.2022.09.008. 10.1016/j.jaccao.2022.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448–56. 10.1200/JCO.2006.06.2497. 10.1200/JCO.2006.06.2497 [DOI] [PubMed] [Google Scholar]
- 20.O’Farrell S, Garmo H, Holmberg L, Adolfsson J, Stattin P, Van Hemelrijck M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2015;33(11):1243–51. 10.1200/JCO.2014.59.1792. 10.1200/JCO.2014.59.1792 [DOI] [PubMed] [Google Scholar]
- 21.Shore ND, Abrahamsson P-A, Anderson J, Crawford ED, Lange P. New considerations for ADT in advanced prostate cancer and the emerging role of GnRH antagonists. Prostate Cancer Prostatic Dis. 2013;16(1):7–15. 10.1038/pcan.2012.25. 10.1038/pcan.2012.25 [DOI] [PubMed] [Google Scholar]
- 22.Margel D, Peer A, Ber Y, et al. Cardiovascular morbidity in a randomized trial comparing GnRH agonist and GnRH antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. J Urol. 2019;202(6):1199–208. 10.1097/JU.0000000000000384. 10.1097/JU.0000000000000384 [DOI] [PubMed] [Google Scholar]
- 23.Albertsen PC, Klotz L, Tombal B, Grady J, Olesen TK, Nilsson J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65(3):565–73. 10.1016/j.eururo.2013.10.032. 10.1016/j.eururo.2013.10.032 [DOI] [PubMed] [Google Scholar]
- 24.Shore ND, Saad F, Cookson MS, et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382(23):2187–96. 10.1056/NEJMoa2004325. 10.1056/NEJMoa2004325 [DOI] [PubMed] [Google Scholar]
- 25.Lopes RD, Higano CS, Slovin SF, et al. Cardiovascular safety of degarelix versus leuprolide in patients with prostate cancer: the primary results of the PRONOUNCE randomized trial. Circulation. 2021;144(16):1295–307. 10.1161/CIRCULATIONAHA.121.056810. 10.1161/CIRCULATIONAHA.121.056810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dragomir A, Touma N, Hu J, Perreault S, Aprikian AG. Androgen deprivation therapy and risk of cardiovascular disease in patients with prostate cancer based on existence of cardiovascular risk. J Natl Compr Canc Netw. 2023;21(2):163–71. 10.6004/jnccn.2022.7083. 10.6004/jnccn.2022.7083 [DOI] [PubMed] [Google Scholar]
- 27.Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102(11):1531–8. 10.1111/j.1464-410X.2008.08183.x. 10.1111/j.1464-410X.2008.08183.x [DOI] [PubMed] [Google Scholar]
- 28.Noteboom EA, May AM, van der Wall E, de Wit NJ, Helsper CW. Patients’ preferred and perceived level of involvement in decision making for cancer treatment: a systematic review. Psychooncology. 2021;30(10):1663–79. 10.1002/pon.5750. 10.1002/pon.5750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menges D, Piatti MC, Cerny T, Puhan MA. Patient preference studies for advanced prostate cancer treatment along the medical product life cycle: systematic literature review. Patient Prefer Adherence. 2022;16:1539–57. 10.2147/PPA.S362802. 10.2147/PPA.S362802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kehl KL, Landrum MB, Arora NK, et al. Association of actual and preferred decision roles with patient-reported quality of care: shared decision making in cancer care. JAMA Oncol. 2015;1(1):50–8. 10.1001/jamaoncol.2014.112. 10.1001/jamaoncol.2014.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connor MJ, Genie MG, Burns D, et al. A systematic review of patients’ values, preferences, and expectations for the treatment of metastatic prostate cancer. Eur Urol Open Sci. 2022;36:9–18. 10.1016/j.euros.2021.10.003. 10.1016/j.euros.2021.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kantar Health. Panels & Audiences. 2020. https://www.kantar.com/expertise/research-services/panels-and-audiences. Accessed 20 Nov 2023.
- 33.Hauber AB, González JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016;19(4):300–15. 10.1016/j.jval.2016.04.004. 10.1016/j.jval.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 34.Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13. 10.1016/j.jval.2012.08.2223. 10.1016/j.jval.2012.08.2223 [DOI] [PubMed] [Google Scholar]
- 35.Bridges JFP, Hauber AB, Marshall D, et al. Conjoint analysis applications in health–a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–13. 10.1016/j.jval.2010.11.013. 10.1016/j.jval.2010.11.013 [DOI] [PubMed] [Google Scholar]
- 36.Spry NA, Kristjanson L, Hooton B, et al. Adverse effects to quality of life arising from treatment can recover with intermittent androgen suppression in men with prostate cancer. Eur J Cancer. 2006;42(8):1083–92. 10.1016/j.ejca.2006.01.029 [DOI] [PubMed] [Google Scholar]
- 37.Kim MS, Jung SI, Chung HS, Chang Hwang E, Kwon D. Effects of leuprolide acetate on the quality of life of patients with prostate cancer: a prospective longitudinal cohort study. Prostate Int. 2021;9(3):132–9. 10.1016/j.prnil.2020.11.001. 10.1016/j.prnil.2020.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.U.S. Food & Drug Administration. Prescribing information: LUPRON DEPOT (leuprolide acetate dor depot suspension). 1989. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/019732s045,020517s043lbl.pdf. Accessed Feb 5, 2024.
- 39.U.S. Food & Drug Administration. Prescribing information: FIRMAGON® (degarelix for injection). 2008. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022201s016lbl.pdf. Accessed Feb 5, 2024.
- 40.Schover LR. Sexual healing in patients with prostate cancer on hormone therapy. Am Soc Clin Oncol Educ Book. 2015;35(1):e562–6. 10.14694/EdBook_AM.2015.35.e562. 10.14694/EdBook_AM.2015.35.e562 [DOI] [PubMed] [Google Scholar]
- 41.Shim M, Bang WJ, Oh CY, Lee YS, Cho JS. Effectiveness of three different luteinizing hormone-releasing hormone agonists in the chemical castration of patients with prostate cancer: goserelin versus triptorelin versus leuprolide. Investig Clin Urol. 2019;60(4):244–50. 10.4111/icu.2019.60.4.244. 10.4111/icu.2019.60.4.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlegel PN, Histrelin Study Group. Efficacy and safety of histrelin subdermal implant in patients with advanced prostate cancer. J Urol. 2006;175(4):1353–8. 10.1016/S0022-5347(05)00649-X. 10.1016/S0022-5347(05)00649-X [DOI] [PubMed] [Google Scholar]
- 43.Yang J-C, Johnson FR, Kilambi V, Mohamed AF. Sample size and utility-difference precision in discrete-choice experiments: a meta-simulation approach. J Choice Model. 2015;16:50–7. 10.1016/j.jocm.2015.09.001. 10.1016/j.jocm.2015.09.001 [DOI] [Google Scholar]
- 44.Louviere JJ. What you don’t know might hurt you: some unresolved issues in the design and analysis of discrete choice experiments. Environ Resource Econ. 2006;34(1):173–88. 10.1007/s10640-005-4817-0. 10.1007/s10640-005-4817-0 [DOI] [Google Scholar]
- 45.Boeri M, Saure D, Schacht A, Riedl E, Hauber B. Modeling heterogeneity in patients’ preferences for psoriasis treatments in a multicountry study: a comparison between random-parameters logit and latent class approaches. Pharmacoeconomics. 2020;38(6):593–606. 10.1007/s40273-020-00894-7. 10.1007/s40273-020-00894-7 [DOI] [PubMed] [Google Scholar]
- 46.Sculpher M, Bryan S, Fry P, de Winter P, Payne H, Emberton M. Patients’ preferences for the management of non-metastatic prostate cancer: discrete choice experiment. BMJ. 2004;328(7436):382. 10.1136/bmj.37972.497234.44. 10.1136/bmj.37972.497234.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai H-T, Penson DF, Makambi KH, Lynch JH, Van Den Eeden SK, Potosky AL. Efficacy of intermittent androgen deprivation therapy vs conventional continuous androgen deprivation therapy for advanced prostate cancer: a meta-analysis. Urology. 2013;82(2):327–33. 10.1016/j.urology.2013.01.078. 10.1016/j.urology.2013.01.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abufaraj M, Iwata T, Kimura S, et al. Differential impact of gonadotropin-releasing hormone antagonist versus agonist on clinical safety and oncologic outcomes on patients with metastatic prostate cancer: a meta-analysis of randomized controlled trials. Eur Urol. 2021;79(1):44–53. 10.1016/j.eururo.2020.06.002. 10.1016/j.eururo.2020.06.002 [DOI] [PubMed] [Google Scholar]
- 49.Nelson AJ, Lopes RD, Hong H, et al. Cardiovascular effects of GnRH antagonists compared with agonists in prostate cancer: a systematic review. JACC CardioOncol. 2023;5(5):613–24. 10.1016/j.jaccao.2023.05.011. 10.1016/j.jaccao.2023.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.American Cancer Society. The costs of cancer. 2020. https://www.fightcancer.org/sites/default/files/National%20Documents/Costs-of-Cancer-2020-10222020.pdf. Accessed Feb 05, 2024.
- 51.de Bekker-Grob EW, Swait JD, Kassahun HT, et al. Are healthcare choices predictable? The impact of discrete choice experiment designs and models. Value Health. 2019;22(9):1050–62. 10.1016/j.jval.2019.04.1924. 10.1016/j.jval.2019.04.1924 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due the data collection only being granted exemption determination from an IRB for this specific protocol but are available from the corresponding author on reasonable request for non-commercial use.