Abstract
A rapid, anhydrous Suzuki–Miyaura cross-coupling of alkylboronic esters with aryl halides is described. Parallel experimentation revealed that the combination of AntPhos, an oxaphosphole ligand, neopentyldiol alkylboronic esters, and potassium trimethylsilanolate (TMSOK) enables successful cross-coupling. In general, reactions proceed in under 1 h with good yields and high linear/branched (l/b) selectivities. Crucially, two literature examples which previously took >20 h to reach completion were accomplished in a fraction of the time with the method described herein. Mechanistic studies revealed that the reaction proceeds through a stereoretentive pathway and identified the boronic ester skeleton as a predominant pathway for deleterious protodehalogenation.
Graphical Abstract

INTRODUCTION
The venerable Suzuki–Miyaura cross-coupling (SMCC) has revolutionized C–C bond formation for synthetic chemistry.1 Whereas it has been predominantly used for Csp2–Csp2 cross-coupling, within the past two decades, significant advances have been made for the analogous Csp2–Csp3 variant using aryl halides and alkylboronates as the coupling partners.2,3 This type of reaction is of particular interest for the pharmaceutical industry, as potential drug candidates with greater sp3 character are considered to be more drug-like.4–6 To accomplish these challenging cross-couplings, many early reports disclose the use of alkylboranes or boronic acids.2a,7 More recently, the use of potassium alkyltrifluoroborate salts,2c,3b–e,8,9c,10a,11a–d alkylboronic esters,9a,b,10b,11e–g,12–15 and MIDA alkylboronates3f,16 has arisen as a popular strategy to circumvent the oxidative liabilities associated with alkylboronic acids or boranes. Despite these developments, a recent benchmarking study of methods for Csp2–Csp3 cross-coupling employing aryl halides and alkyl nucleophiles as coupling partners revealed that modern B-alkyl SMCC conditions succeeded in obtaining the cross-coupled product 61% of the time for 1° alkylboronates and 20% of the time for 2° alkylboronates, with an overall success rate of only 37%.6
The challenges with this important cross-coupling stem from the slow transmetalation of alkylboronates, and the ability of alkylboronates to undergo β-hydride elimination prior to transmetalation or before reductive elimination. To address these problems, many reports leverage the use of bulky, electron-rich ligands3,7f,17 as well as the use of directing groups at proximal and distal positions, such as benzyl groups,9 ethers,10 carbonyl groups,11 geminal 1,1- and 1,2-diboronates,14 and even hydroxides,15 to affect a variety of B-alkyl cross-couplings. Often, aqueous conditions are employed by necessity, as both potassium trifluoroborates and MIDA boronates require hydrolysis prior to transmetalation,16a,18,19 or for solubilization of an inorganic base. Alternatively, with alkylboronic acids and esters, anhydrous conditions can be used, though these conditions are less common.2b,3a,7c,d,f,12,17a Regardless of the scenario, the often biphasic nature of the reaction mixtures introduces problems related to mass transfer phenomena, resulting in irreproducible reaction kinetics and yields.
Recently, our laboratory disclosed a mechanistic study on the anhydrous, stoichiometric transmetalation of arylboronic esters with an arylpalladium(II) hydroxy dimer, demonstrating that transmetalation of boronic esters can occur without prior hydrolysis to a boronic acid.20 Notably, the structure of the boronic ester greatly affected the rate of transmetalation, with glycol and dimethyl boronic esters providing significant rate increases. To translate these stoichiometric mechanistic insights to a catalytic system, anhydrous conditions must be used, as hydrolysis of the boronic ester to the boronic acid would preclude any beneficial rate enhancements from occurring. Thus, our group discovered that a rapid, homogeneous, and anhydrous Csp2–Csp2 SMCC could be realized by using neopentyldiol boronic esters in tandem with potassium trimethylsilanolate (TMSOK), a soluble base.21 Notably, in the original report, both methyl and cyclopropylboronic esters were successful coupling partners, but they required modified conditions to afford the cross-coupled products. Given the beneficial rate increases observed under anhydrous conditions with TMSOK, we sought to identify a method that could enable a rapid, homogeneous B-alkyl SMCC. Such a method could be of importance for drug discovery, as synthesis tends to be the bottleneck for the design-build-test-learn (DBTL) cycle.6,22
RESULTS
Initial Cross-Coupling Experiments.
Given that protodehalogenation was previously observed with neopentyldiol cyclopropylboronic ester,21a initial experiments began with THF-3,4-diol n-butylboronic ester (2a) (Scheme 1). Whereas the room temperature cross-coupling of 2a with 4-bromotoluene (1a) and t-Bu3P–Pd-G3 failed to produce any cross-coupled product 4aa, heating the reaction mixture to reflux afforded 55% yield of 4aa. Unfortunately, reaction stalling was observed, and modulation of the aryl halide, solvent, or ester structure resulted in lower yields or significant protodehalogenation. To obtain satisfactory cross-coupling, we hypothesized that a different catalyst may be able to enable successful transmetalation of the slower alkylboronic ester while outcompeting deleterious protodehalogenation.
Scheme 1.

Initial Investigation using n-Butylboronic Ester 2aa
aReaction run with 0.20 mmol of aryl halide and 1.20 equiv of boronic ester. Yields determined by GC-FID analysis using biphenyl as internal standard.
Optimization of the Reaction by High-Throughput Experimentation.
Thus, a survey of 24, third-generation Buchwald precatalysts and two boronic ester scaffolds was commenced by running two 24 well plates using Radleys Greenhouse Plus Parallel synthesizer (see Supporting Information for details). The ligand set represents a broad selection of structural diversity for B-alkyl SMCC, including Buchwald-type ligands,8,23 electron-rich phosphines, such as (Ad)3P,24 QPhos,25 AntPhos,17a,26 and BI-DIME,17h,27 and bisphosphines.7d,e The well plates were heated for 16 h at 100 °C combining 1a as the aryl halide and either 2a (Plate 1) or neopentyldiol n-butylboronic ester 3a (Plate 2) as the coupling partner with TMSOK in 1,4-dioxane (Figure 1A). Notably, the use of TMSOK as a base enabled preparation of stock solutions for liquid handling, decreasing the time for plate setup when compared to the laborious manual weighing of solid inorganic bases into each well.28 Aliquots of the reaction mixtures were analyzed by gas chromatography and formation of 4aa, remaining 1a, and protodehalogenation of 1a were quantitated using biphenyl as an internal standard (see Supporting Information).
Figure 1.
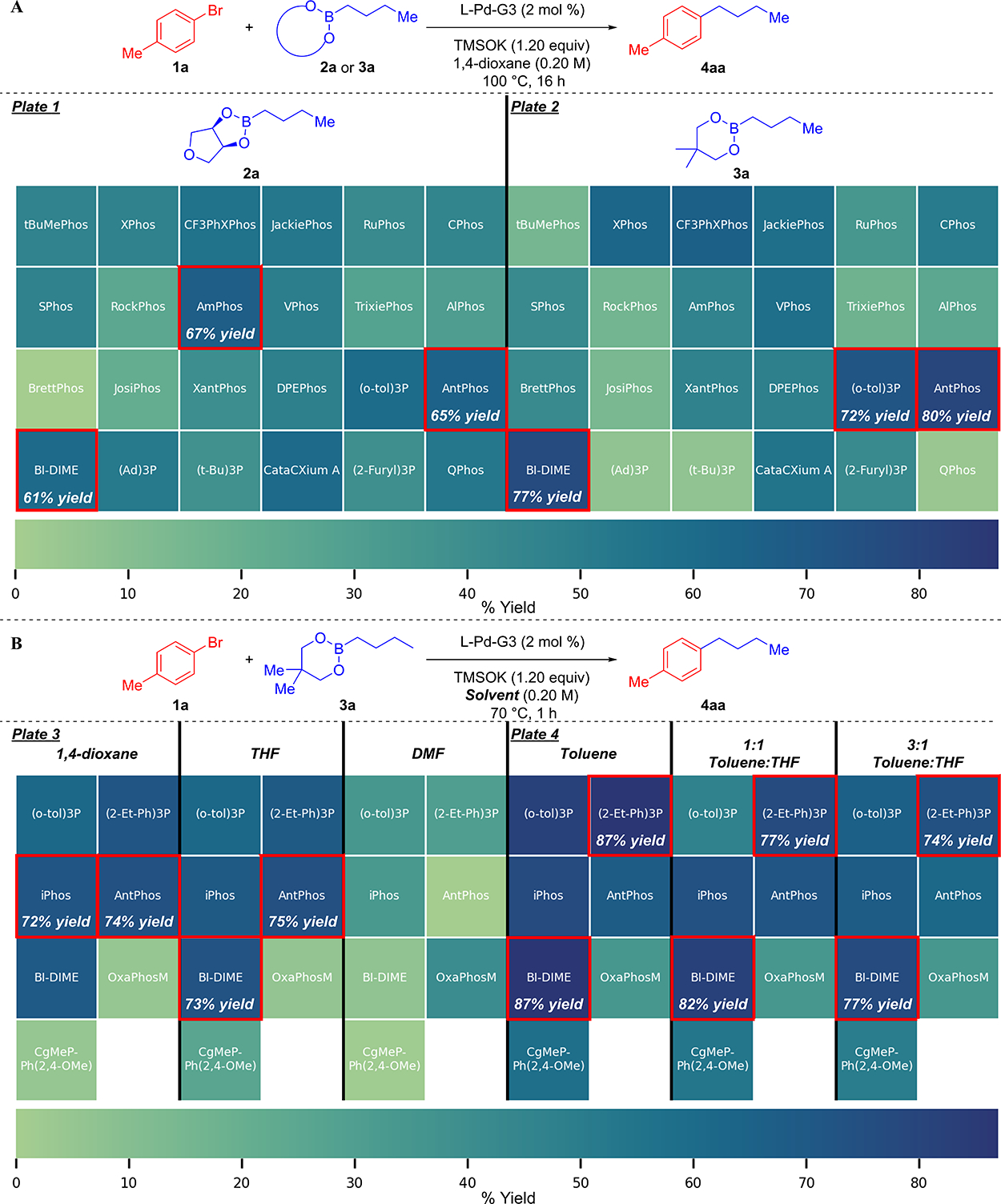
HTE survey using four, 24 well plates. (A) First-generation screen focusing on ligand space. (B) Second-generation screen focusing on solvents and refined ligand space. Reactions run with 0.20 mmol of aryl halide and 1.20 equiv of boronic ester. Yields were determined by GC-FID analysis using biphenyl as an internal standard.
Four trends became apparent from the first-generation survey. First, whereas up to 22% of remaining 1a was found in all wells containing 2a, 1a was completely consumed in nearly every well containing 3a. Presumably, the lack of complete conversion of starting material that was observed with 2a as a coupling partner is due to the sequestration of TMSOK by a more Lewis acidic species that forms as the cross-coupling reaction proceeds. Second, as previously observed, reactions that used 3a had significantly higher amounts of protodehalogenation. Third, good l/b ratios (~20–25/1) were observed in nearly all cases with both 2a and 3a, with 2a providing significantly higher l/b ratios. Fourth, AntPhos-Pd-G3, BI-DIME-Pd-G3, and (o-Tol)3P–Pd-G3 were among the top performing precatalysts in both plates, but, surprisingly, they gave higher yields with 3a. Additionally, Buchwald and bidentate ligands afforded similar yields between the two boronic esters; however, electron-rich ligands were uniquely effective for reactions that employed 2a.
In light of these observations, a second-generation survey with 3a, six solvents, and a refined set of seven, third-generation Buchwald precatalysts was performed using two 24 well plates (Figure 1B). In addition to the best performing ligands in the previous generation, new structural motifs that have been successful in B-alkyl SMCC were selected, such as Burke’s iPhos,3f Tang’s bis-phosphine mono-oxide oxaphosphole (referred here as OxaPhosM),17b and Capretta’s oxaphosphaadamantanes (CgMe-PPh(2,4-OMe)).29 Whereas reactions employing DMF failed to provide 4aa in useful yields with any precatalyst, wells containing THF or 1,4-dioxane afforded 4aa (Plate 3) in comparable yields and selectivities to the first two plates, with AntPhos-Pd-G3, BI-DIME-Pd-G3, and iPhos-Pd-G3 representing the top performing systems. Curiously, the use of toluene or toluene/THF mixtures as the reaction solvent resulted in the formation of 4aa with higher yields than ethereal solvents for all precatalysts, including OxaPhosM-Pd-G3 and CgMePPh(2,4-OMe)-Pd-G3, two precatalysts that failed to significantly react in either THF or 1,4-dioxane (Plate 4). However, the heterogeneity of the reaction, induced by the poor solubility of TMSOK in toluene, precluded any further investigation of its use in the reaction. Nonetheless, both THF and 1,4-dioxane provided satisfactory results for their use in the cross-coupling.
To validate the results obtained from the screens, the top-yielding precatalysts, AntPhos-Pd-G3, iPhos-Pd-G3, and BI-DIME-Pd-G3, were subjected to further investigation in THF (Scheme 2A). Whereas the reaction mixtures containing iPhos and BI-DIME had significantly unconsumed 1a at 30 min, the reaction containing AntPhos had completely consumed 1a, affording 4aa in 91% yield. With conditions in hand, a control experiment was performed using n-butylboronic acid (5a) as the coupling partner (Scheme 2B). Critically, only a trace amount of product was observed, demonstrating the rate enhancements obtained with the boronic ester scaffold under anhydrous conditions. Finally, brief optimization of the base loading (1.40 equiv) and concentration (0.30 M) further increased the rate of the reaction, providing 92% yield of 4aa in 15 min (Scheme 2C).
Scheme 2.

Post-HTE Experimentsa
aReaction run with 0.20 mmol of aryl halide and 1.20 equiv of boronic ester. Yields determined by GC-FID analysis using biphenyl as internal standard.
Evaluation of Reaction Scope.
Using these optimized conditions, the scope of the aryl halide structure was explored with boronate 3a (Table 1A). Most reactions were complete within 15–20 min and the desired products were obtained with good to excellent yields and high l/b selectivities (38 to 96%, l/b, 13:1 to >99:1). Whereas products formed from electron-neutral and electron-poor arenes (4aa–4ga) were obtained rapidly (<25 min) in generally good yields, products from electron-rich substrates were formed slowly, often requiring extended reaction times or elevated temperatures (4ha–4ja). Notably, products containing an acidic N–H, such as aniline 4ka, could be successfully cross-coupled in good yield using an additional equivalent of TMSOK. Products 4la and 4ma derived from (E)-2-bromostyrene and mesityl bromide, respectively, were formed in good yields and excellent l/b selectivities. The preparation of 1-pentylbenzene (4na), arising from the corresponding benzyl chloride, required elevated temperature and catalyst loading, but nevertheless proceeded in 38% and 13:1 l/b selectivity. Finally, products 4oa and 4ap containing simple heterocycles could be obtained in moderate yields, though higher catalyst loadings for 4oa and temperatures were required.
Table 1.
Reaction Scope of Aryl Halides and Neopentyldiol Boronic Estersa

|
Reactions run with 1.00 mmol of aryl halide and 1.20 equiv of boronic ester. The products are labeled accordingly with the numbering 4xy, where x = the letter of the corresponding halide, and y = the letter of the corresponding boronic ester. l/b = linear/branched ratio. Yields of isolated product after column chromatography.
1,4-Dioxane used at 100 °C.
2.4 equiv of TMSOK used.
4 mol % catalyst used.
Benzyl chloride used as the coupling partner.
Product was further purified to analytical purity; see the Supporting Information.
Next, the scope of alkylboronic esters that could undergo cross-coupling with 2-bromonaphthalene (1c) was explored (Table 1B). In all examples, the coupling products were obtained rapidly and in good yields (50–94%) with perfect l/b selectivities. Products derived from methylboronic- and cyclopropylboronic esters (4cb and 4cc) were formed smoothly. Notably, the use of THF-3,4-diol boronic ester was not required for successful formation of 4cc.21a Interestingly, 4cd was produced in good yield despite the increased steric hindrance introduced from the β-branched methylcyclohexylboronic ester. Products obtained from activated substrates such as benzylboronic ester 4ce could be successfully formed at elevated temperatures. In addition, products containing functional groups such as alkyl chlorides, silyl ethers and acetals were obtained in good yields (4cg, 4ch, 4cj). Notably, attempts to form 4ci containing a tert-butyl ester failed at 70 °C, presumably owing to formation of a palladium enolate that coordinates to the neopentyldiol boronic ester. However, running the reaction at 100 °C in 1,4-dioxane successfully enabled the production of 4ci. Finally, to demonstrate the method can produce more nitrogen-containing targets, 4qd and 4re6 were obtained in good yields from coupling with 3e and 3d, respectively.
To demonstrate the advantages of using anhydrous conditions with boronic esters, two comparisons to literature procedures were executed. First, using anhydrous conditions with TMSOK, 4re was formed from neopentyldiol boronic ester 3e in 53% yield after 5 min. In contrast, 4re had been previously prepared in 53% yield after 72 h using a SMCC with potassium benzyltrifluoroborate and aqueous cesium carbonate.6 Similarly, using potassium phenethyltrifluoroborate and aqueous potassium carbonate, 4sk had been previously synthesized using an SMCC in 74% yield after 16–20 h.30 Using the method described herein, 4sk was obtained in 70% yield after only 20 min. Thus, preparation of both 4re and 4sk highlights the rate enhancements obtained with a soluble base and a boronic ester under anhydrous conditions.
DISCUSSION
Stereospecificity of the Cross-Coupling.
Finally, two sets of mechanistic experiments were performed to investigate the stereospecificity of the cross-coupling with a 2° boronic ester and the origin of protodehalogenation. First, two stereodefined cyclopropylboronic esters, trans-3l31 and cis-3l,32 were prepared as a single diastereomer by cyclopropanation of the corresponding (Z)- and (E)-styrenes. The diastereospecificity (ds)33 of the reaction was determined by using the optimized coupling conditions with 1c. Respectively, products trans-4cl and cis-4cl were obtained in 90% and 94% ds, suggesting that the reaction proceeds through a highly stereoretentive process (Scheme 3).34
Scheme 3.

Stereospecificity of the Coupling Reaction
aReactions run with 1.00 mmol of aryl halide and 1.20 equiv of boronic ester. Yields of isolated product after column chromatography. bProduct was further purified to analytical purity; see the Supporting Information.
Origin of Protodehalogenation.
Second, the origin of protodehalogenation was investigated by deuterium labeling studies. In analogy to other palladium-catalyzed cross-couplings,35 this side reaction was hypothesized to occur by reductive elimination of an arylpalladium(II) hydride (8) (Figure 2A). Consequently, three potential pathways were identified in which 8 could be generated from an arylpalladium(II) halide (7) (Figure 2B). In path A, β-hydride elimination could occur from the backbone of the boronic ester skeleton, such as coordination of 7 to an alkoxide, such as 9, resulting from fragmentation of the neopentyldiol boronic ester skeleton upon binding of TMSOK. In path B, β-hydride elimination could proceed from the solvent, as in THF adduct 10 or 1,4-dioxane adduct 11.36 In path C, β-hydride elimination could occur from the alkyl chain, such as alkylpalladium(II) complex 12. Initially, investigation of path A and B was chosen (Figure 2C). Thus, neopentyldiol n-butylboronic ester 3a-d437 was subjected to the standard reaction conditions with 2,4,6-triisopropylbromobenzene (1s), a substrate for which protodehalogenation greatly outcompeted cross-coupling. After heating in 1.4-dioxane-h8 at 100 °C for 25 min, 13-h1/d1 was obtained with 77% deuterium incorporation. Executing the same experiment with 3a-h4 in 1,4-dioxane-d8 resulted in formation of 13-h1/d1 with only 2% deuterium incorporation. Together, these results suggest that reduction of the arene predominantly occurs by Path A.
Figure 2.
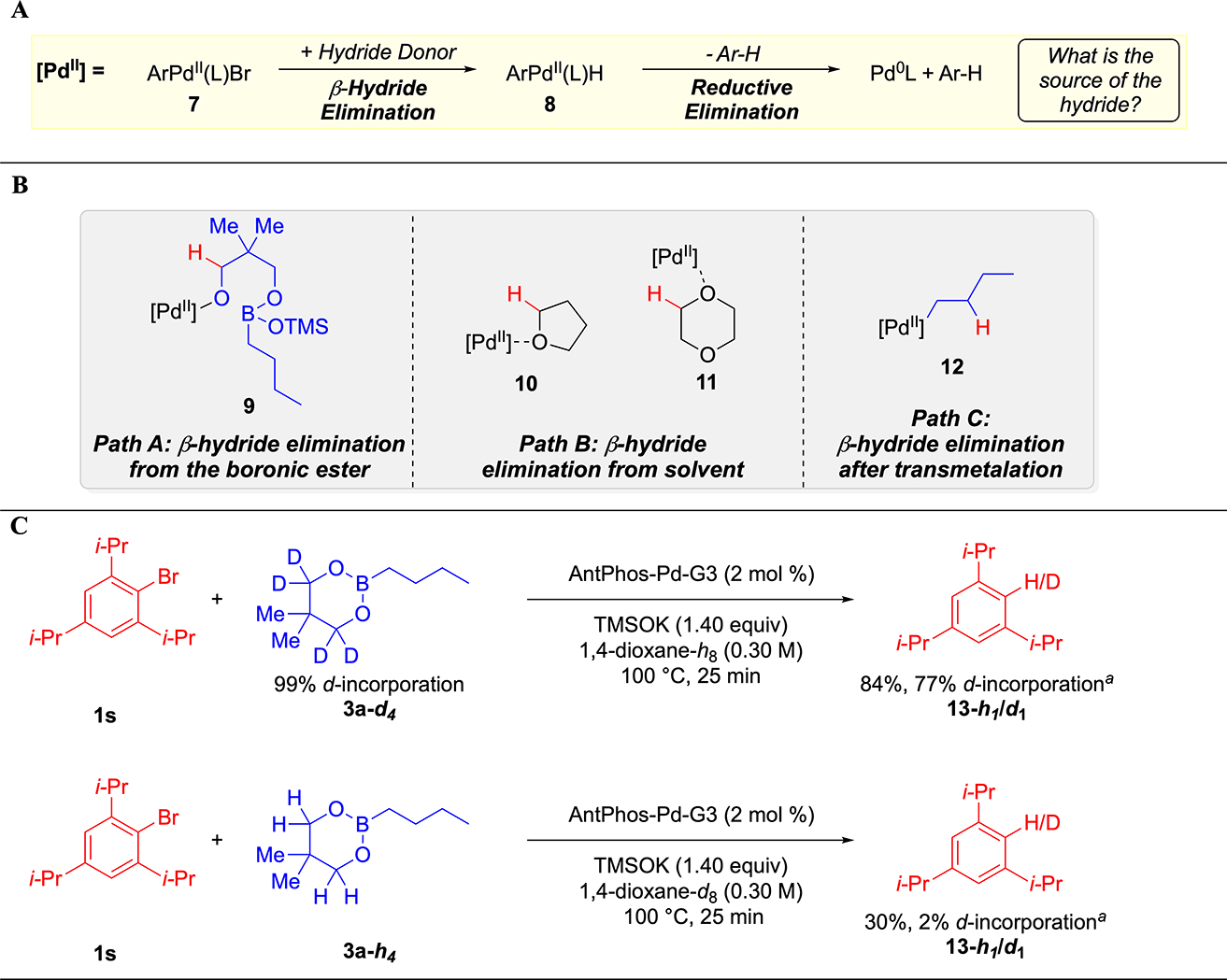
(A). Plausible mechanism for the origin of protodehalogenation. (B). Hypothetical pathways for the source of the hydride. (C). Deuterium labeling experiments. Reactions run with 0.25 mmol of aryl halide and 1.20 equiv of boronic ester. Yields of isolated product after column chromatography. Deuterium incorporation was determined from mass spectrometry; see the Supporting Information for details.
CONCLUSION
In conclusion, a rapid, homogeneous, B-alkyl SMCC has been described, providing a variety of products in 1 h or less. Notably, parallel experimentation involving the use of a soluble base, TMSOK, enabled discovery of a precatalyst and boronic ester that could successfully perform cross-coupling while preventing reaction stalling and outcompeting deleterious protodehalogenation. Mechanistic studies revealed that transmetalation proceeds through a stereoretentive pathway, and the boronic ester skeleton was revealed to be predominantly responsible for the origin of protodehalogenation. Owing to the homogeneity of the reaction, this method could be exceptionally useful for applications in library synthesis and high-throughput experimentation. Additionally, we expect that the rate enhancements for transmetalation provided by alkylboronic esters under anhydrous conditions may enable new reactivity for other metal-catalyzed transformations.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the National Institutes of Health (Grant GM R35 127010) for generous financial support. We thank the UIUC SCS support facilities (microanalysis, mass spectrometry, and NMR spectroscopy) for their assistance. Dr. Gerald Larson (Gelest) is thanked for generous gifts of TMSOK to support these studies. We also thank Wesley Wang (Prof. M. D. Burke) for providing samples of iPhos and CgMe-PPh(2,4-OMe). Vincent Kassel is acknowledged for conducting preliminary studies on the origin of protodehalogenation.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.4c00089.
Experimental procedures and characterization data for all new compounds along with copies of 1H, 11B, 13C, 19F, 31P NMR spectra and HTE experiments (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.joc.4c00089
Contributor Information
Matthew J. Bock, Department of Chemistry, University of Illinois at Urbana–Champaign, Urbana, Illinois 61801, United States
Scott. E. Denmark, Department of Chemistry, University of Illinois at Urbana–Champaign, Urbana, Illinois 61801, United States
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
REFERENCES
- (1).(a) Colacot TJ The 2010 Nobel Prize in Chemistry: Palladium-Catalysed Cross-Coupling. Platin. Met. Rev. 2011, 55, 84–90. [Google Scholar]; (b) Johansson Seechurn CCC; Kitching MO; Colacot TJ; Snieckus V Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem., Int. Ed. 2012, 51, 5062–5085. [DOI] [PubMed] [Google Scholar]; (c) Suzuki A Cross-Coupling Reactions of Organoboranes: An Easy Way To Construct C-C Bonds (Nobel Lecture). Angew. Chem., Int. Ed. 2011, 50, 6722–6737. [DOI] [PubMed] [Google Scholar]
- (2).(a) Chemler SR; Trauner D; Danishefsky SJ The B-Alkyl Suzuki–Miyaura Cross-Coupling Reaction: Development, Mechanistic Study, and Applications in Natural Product Synthesis. Angew. Chem., Int. Ed. 2001, 40, 4544–4568. [DOI] [PubMed] [Google Scholar]; (b) Doucet H Suzuki–Miyaura Cross-Coupling Reactions of Alkylboronic Acid Derivatives or Alkyltrifluoroborates with Aryl, Alkenyl or Alkyl Halides and Triflates. Eur. J. Org. Chem. 2008, 2008, 2013–2030. [Google Scholar]; (c) Molander GA; Sandrock DL Potassium Trifluoroborate Salts as Convenient, Stable Reagents for Difficult Alkyl Transfers. Curr. Opin. Drug Discovery Devel. 2009, 12, 811–823. [PMC free article] [PubMed] [Google Scholar]; (d) Rygus JPG; Crudden CM Enantiospecific and Iterative Suzuki–Miyaura Cross-Couplings. J. Am. Chem. Soc. 2017, 139, 18124–18137. [DOI] [PubMed] [Google Scholar]; (e) El-Maiss J; Mohy El Dine T; Lu C-S; Karamé I; Kanj A; Polychronopoulou K; Shaya J Recent Advances in Metal-Catalyzed Alkyl–Boron (C(sp3)–C(sp2)) Suzuki-Miyaura Cross-Couplings. Catalysts 2020, 10, 296. [Google Scholar]; (f) Ma X; Murray B; Biscoe MR Stereoselectivity in Pd-Catalysed Cross-Coupling Reactions of Enantioenriched Nucleophiles. Nat. Rev. Chem. 2020, 4, 584–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).(a) Littke AF; Dai C; Fu GC Versatile Catalysts for the Suzuki Cross-Coupling of Arylboronic Acids with Aryl and Vinyl Halides and Triflates under Mild Conditions. J. Am. Chem. Soc. 2000, 122, 4020–4028. [Google Scholar]; (b) Molander GA; Yun C-S; Ribagorda M; Biolatto B B-Alkyl Suzuki–Miyaura Cross-Coupling Reactions with Air-Stable Potassium Alkyltrifluoroborates. J. Org. Chem. 2003, 68, 5534–5539. [DOI] [PubMed] [Google Scholar]; (c) Dreher SD; Dormer PG; Sandrock DL; Molander GA Efficient Cross-Coupling of Secondary Alkyltrifluoroborates with Aryl Chlorides–Reaction Discovery Using Parallel Microscale Experimentation. J. Am. Chem. Soc. 2008, 130, 9257–9259. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Li L; Zhao S; Joshi-Pangu A; Diane M; Biscoe MR Stereospecific Pd-Catalyzed Cross-Coupling Reactions of Secondary Alkylboron Nucleophiles and Aryl Chlorides. J. Am. Chem. Soc. 2014, 136, 14027–14030. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zhao S; Gensch T; Murray B; Niemeyer ZL; Sigman MS; Biscoe MR Enantiodivergent Pd-Catalyzed C–C Bond Formation Enabled through Ligand Parameterization. Science 2018, 362, 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Lehmann JW; Crouch IT; Blair DJ; Trobe M; Wang P; Li J; Burke MD Axial Shielding of Pd(II) Complexes Enables Perfect Stereoretention in Suzuki-Miyaura Cross-Coupling of Csp3 Boronic Acids. Nat. Commun. 2019, 10, 1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. [DOI] [PubMed] [Google Scholar]
- (5).Wei W; Cherukupalli S; Jing L; Liu X; Zhan P Fsp3: A New Parameter for Drug-Likeness. Drug Discovery Today 2020, 25, 1839–1845. [DOI] [PubMed] [Google Scholar]
- (6).Dombrowski AW; Gesmundo NJ; Aguirre AL; Sarris KA; Young JM; Bogdan AR; Martin MC; Gedeon S; Wang Y Expanding the Medicinal Chemist Toolbox: Comparing Seven C(sp2)–C(sp3) Cross-Coupling Methods by Library Synthesis. ACS Med. Chem. Lett. 2020, 11, 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) Miyaura N; Ishiyama T; Ishikawa M; Suzuki A Palladium-Catalyzed Cross-Coupling Reactions of B-Alkyl-9-BBN or Trialkylboranes with Aryl and 1-Alkenyl Halides. Tetrahedron Lett. 1986, 27, 6369–6372. [Google Scholar]; (b) Miyaura N; Ishiyama T; Sasaki H; Ishikawa M; Sato M; Suzuki A Palladium-Catalyzed Inter- and Intramolecular Cross-Coupling Reactions of B-Alkyl-9-Borabicyclo[3.3.1]Nonane Derivatives with 1-Halo-1-Alkenes or Haloarenes. Syntheses of Functionalized Alkenes, Arenes, and Cycloalkenes via a Hydroboration-Coupling Sequence. J. Am. Chem. Soc. 1989, 111, 314–321. [Google Scholar]; (c) Wright SW; Hageman DL; McClure LD Fluoride-Mediated Boronic Acid Coupling Reactions. J. Org. Chem. 1994, 59, 6095–6097. [Google Scholar]; (d) Zou G; Reddy YK; Falck JR Ag(I)-Promoted Suzuki–Miyaura Cross-Couplings of n-Alkylboronic Acids. Tetrahedron Lett. 2001, 42, 7213–7215. [Google Scholar]; (e) Molander GA; Yun C-S Cross-Coupling Reactions of Primary Alkylboronic Acids with Aryl Triflates and Aryl Halides. Tetrahedron 2002, 58, 1465–1470. [Google Scholar]; (f) Guo B; Fu C; Ma S Application of LB-Phos·HBF4 in the Suzuki Coupling Reaction of 2-Bromoalken-3-Ols with Alkylboronic Acids. Eur. J. Org. Chem. 2012, 2012, 4034–4041. [Google Scholar]
- (8).Molander GA; Jean-Gérard L Cross-Coupling Reactions of Organotrifluoroborate Salts. Org. React. 2012, 79, 1–316. [Google Scholar]
- (9).(a) Imao D; Glasspoole BW; Laberge VS; Crudden CM Cross Coupling Reactions of Chiral Secondary Organoboronic Esters With Retention of Configuration. J. Am. Chem. Soc. 2009, 131, 5024–5025. [DOI] [PubMed] [Google Scholar]; (b) Matthew SC; Glasspoole BW; Eisenberger P; Crudden CM Synthesis of Enantiomerically Enriched Triarylmethanes by Enantiospecific Suzuki–Miyaura Cross-Coupling Reactions. J. Am. Chem. Soc. 2014, 136, 5828–5831. [DOI] [PubMed] [Google Scholar]; (c) Roh B; Farah AO; Kim B; Feoktistova T; Moeller F; Kim KD; Cheong PH-Y; Lee HG Stereospecific Acylative Suzuki–Miyaura Cross-Coupling: General Access to Optically Active α-Aryl Carbonyl Compounds. J. Am. Chem. Soc. 2023, 145, 7075–7083. [DOI] [PubMed] [Google Scholar]
- (10).(a) Molander GA; Wisniewski SR Stereospecific Cross-Coupling of Secondary Organotrifluoroborates: Potassium 1-(Benzyloxy)Alkyltrifluoroborates. J. Am. Chem. Soc. 2012, 134, 16856–16868. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) LaPorte AJ; Shi Y; Hein JE; Burke MD Stereospecific Csp3 Suzuki–Miyaura Cross-Coupling That Evades β-Oxygen Elimination. ACS Catal. 2022, 12, 10905–10912. [Google Scholar]
- (11).(a) Molander GA; Petrillo DE Suzuki–Miyaura Cross-Coupling of Potassium Trifluoroboratohomoenolates. Org. Lett. 2008, 10, 1795–1798. [DOI] [PubMed] [Google Scholar]; (b) Molander GA; Jean-Gérard L Scope of the Suzuki–Miyaura Cross-Coupling Reaction of Potassium Trifluoroboratoketohomoenolates. J. Org. Chem. 2009, 74, 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Molander GA; Jean-Gérard L Use of Potassium β-Trifluoroborato Amides in Suzuki–Miyaura Cross-Coupling Reactions. J. Org. Chem. 2009, 74, 5446–5450. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Sandrock DL; Jean-Gérard L; Chen C; Dreher SD; Molander GA Stereospecific Cross-Coupling of Secondary Alkyl β-Trifluoroboratoamides. J. Am. Chem. Soc. 2010, 132, 17108–17110. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ohmura T; Awano T; Suginome M Stereospecific Suzuki–Miyaura Coupling of Chiral α-(Acylamino)Benzylboronic Esters with Inversion of Configuration. J. Am. Chem. Soc. 2010, 132, 13191–13193. [DOI] [PubMed] [Google Scholar]; (f) Awano T; Ohmura T; Suginome M Inversion or Retention? Effects of Acidic Additives on the Stereochemical Course in Enantiospecific Suzuki–Miyaura Coupling of α-(Acetylamino)Benzylboronic Esters. J. Am. Chem. Soc. 2011, 133, 20738–20741. [DOI] [PubMed] [Google Scholar]; (g) Hoang GL; Takacs JM Enantioselective γ-Borylation of Unsaturated Amides and Stereo-retentive Suzuki–Miyaura Cross-Coupling. Chem. Sci. 2017, 8, 4511–4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Sato M; Miyaura N; Suzuki A Cross-Coupling Reaction of Alkyl- or Arylboronic Acid Esters with Organic Halides Induced by Thallium(I) Salts and Palladium-Catalyst. Chem. Lett. 1989, 18, 1405–1408. [Google Scholar]
- (13).Yang C-T; Zhang Z-Q; Tajuddin H; Wu C-C; Liang J; Liu J-H; Fu Y; Czyzewska M; Steel PG; Marder TB; Liu L Alkylboronic Esters from Copper-Catalyzed Borylation of Primary and Secondary Alkyl Halides and Pseudohalides. Angew. Chem., Int. Ed. 2012, 51, 528–532. [DOI] [PubMed] [Google Scholar]
- (14).(a) Endo K; Ohkubo T; Hirokami M; Shibata T Chemoselective and Regiospecific Suzuki Coupling on a Multi-substituted Sp3-Carbon in 1,1-Diborylalkanes at Room Temperature. J. Am. Chem. Soc. 2010, 132, 11033–11035. [DOI] [PubMed] [Google Scholar]; (b) Lee JCH; McDonald R; Hall DG Enantioselective Preparation and Chemoselective Cross-Coupling of 1,1-Diboron Compounds. Nat. Chem. 2011, 3, 894–899. [DOI] [PubMed] [Google Scholar]; (c) Mlynarski SN; Schuster CH; Morken JP Asymmetric Synthesis from Terminal Alkenes by Cascades of Diboration and Cross-Coupling. Nature 2014, 505, 386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Sun C; Potter B; Morken JP A Catalytic Enantiotopic-Group-Selective Suzuki Reaction for the Construction of Chiral Organoboronates. J. Am. Chem. Soc. 2014, 136, 6534–6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Blaisdell TP; Morken JP Hydroxyl-Directed Cross-Coupling: A Scalable Synthesis of Debromohamigeran E and Other Targets of Interest. J. Am. Chem. Soc. 2015, 137, 8712–8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).(a) Knapp DM; Gillis EP; Burke MD A General Solution for Unstable Boronic Acids: Slow-Release Cross-Coupling from Air-Stable MIDA Boronates. J. Am. Chem. Soc. 2009, 131, 6961–6963. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Duncton MAJ; Singh R Synthesis of Trans-2-(Trifluoromethyl)Cyclopropanes via Suzuki Reactions with an N-Methyliminodiacetic Acid Boronate. Org. Lett. 2013, 15, 4284–4287. [DOI] [PubMed] [Google Scholar]; (c) St. Denis JD; Scully CCG; Lee CF; Yudin AK Development of the Direct Suzuki–Miyaura Cross-Coupling of Primary B-Alkyl MIDA-Boronates and Aryl Bromides. Org. Lett. 2014, 16, 1338–1341. [DOI] [PubMed] [Google Scholar]; (d) Li J; Ballmer SG; Gillis EP; Fujii S; Schmidt MJ; Palazzolo AME; Lehmann JW; Morehouse GF; Burke MD Synthesis of Many Different Types of Organic Small Molecules Using One Automated Process. Science 2015, 347, 1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).(a) Li C; Xiao G; Zhao Q; Liu H; Wang T; Tang W Sterically Demanding Aryl–Alkyl Suzuki–Miyaura Coupling. Org. Chem. Front. 2014, 1, 225–229. [Google Scholar]; (b) Li C; Chen T; Li B; Xiao G; Tang W Efficient Synthesis of Sterically Hindered Arenes Bearing Acyclic Secondary Alkyl Groups by Suzuki–Miyaura Cross-Couplings. Angew. Chem., Int. Ed. 2015, 54, 3792–3796. [DOI] [PubMed] [Google Scholar]; (c) Si T; Li B; Xiong W; Xu B; Tang W Efficient Cross-Coupling of Aryl/Alkenyl Triflates with Acyclic Secondary Alkylboronic Acids. Org. Biomol. Chem. 2017, 15, 9903–9909. [DOI] [PubMed] [Google Scholar]; (d) Sivendran N; Pirkl N; Hu Z; Doppiu A; Gooßen LJ Halogen-Bridged Methylnaphthyl Palladium Dimers as Versatile Catalyst Precursors in Coupling Reactions. Angew. Chem., Int. Ed. 2021, 60, 25151–25160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Molander GA; Biolatto B Palladium-Catalyzed Suzuki–Miyaura Cross-Coupling Reactions of Potassium Aryl- and Heteroaryltrifluoroborates. J. Org. Chem. 2003, 68, 4302–4314. [DOI] [PubMed] [Google Scholar]
- (19).(a) Butters M; Harvey JN; Jover J; Lennox AJJ; Lloyd-Jones GC; Murray PM Aryl Trifluoroborates in Suzuki–Miyaura Coupling: The Roles of Endogenous Aryl Boronic Acid and Fluoride. Angew. Chem., Int. Ed. 2010, 49, 5156–5160. [DOI] [PubMed] [Google Scholar]; (b) Lennox AJJ; Lloyd-Jones GC Organotrifluoroborate Hydrolysis: Boronic Acid Release Mechanism and an Acid–Base Paradox in Cross-Coupling. J. Am. Chem. Soc. 2012, 134, 7431–7441. [DOI] [PubMed] [Google Scholar]
- (20).Thomas AA; Zahrt AF; Delaney CP; Denmark SE Elucidating the Role of the Boronic Esters in the Suzuki–Miyaura Reaction: Structural, Kinetic, and Computational Investigations. J. Am. Chem. Soc. 2018, 140, 4401–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).(a) Delaney CP; Kassel VM; Denmark SE Potassium Trimethylsilanolate Enables Rapid, Homogeneous Suzuki–Miyaura Cross-Coupling of Boronic Esters. ACS Catal. 2020, 10, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kassel VM; Hanneman CM; Delaney CP; Denmark SE Heteroaryl–Heteroaryl, Suzuki–Miyaura, Anhydrous Cross-Coupling Reactions Enabled by Trimethyl Borate. J. Am. Chem. Soc. 2021, 143, 13845–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Blakemore DC; Castro L; Churcher I; Rees DC; Thomas AW; Wilson DM; Wood A Organic Synthesis Provides Opportunities to Transform Drug Discovery. Nat. Chem. 2018, 10, 383–394. [DOI] [PubMed] [Google Scholar]
- (23).Martin R; Buchwald SL Palladium-Catalyzed Suzuki–Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands. Acc. Chem. Res. 2008, 41, 1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Chen L; Ren P; Carrow BP Tri(1-Adamantyl)Phosphine: Expanding the Boundary of Electron-Releasing Character Available to Organophosphorus Compounds. J. Am. Chem. Soc. 2016, 138, 6392–6395. [DOI] [PubMed] [Google Scholar]
- (25).Kataoka N; Shelby Q; Stambuli JP; Hartwig JF Air Stable, Sterically Hindered Ferrocenyl Dialkylphosphines for Palladium-Catalyzed C–C, C–N, and C–O Bond-Forming Cross-Couplings. J. Org. Chem. 2002, 67, 5553–5566. [DOI] [PubMed] [Google Scholar]
- (26).Tang W; Keshipeddy S; Zhang Y; Wei X; Savoie J; Patel ND; Yee NK; Senanayake CH Efficient Monophosphorus Ligands for Palladium-Catalyzed Miyaura Borylation. Org. Lett. 2011, 13, 1366–1369. [DOI] [PubMed] [Google Scholar]
- (27).Tang W; Capacci AG; Wei X; Li W; White A; Patel ND; Savoie J; Gao JJ; Rodriguez S; Qu B; Haddad N; Lu BZ; Krishnamurthy D; Yee NK; Senanayake CH A General and Special Catalyst for Suzuki–Miyaura Coupling Processes. Angew. Chem., Int. Ed. 2010, 49, 5879–5883. [DOI] [PubMed] [Google Scholar]
- (28).Mennen SM; Alhambra C; Allen CL; Barberis M; Berritt S; Brandt TA; Campbell AD; Castañón J; Cherney AH; Christensen M; Damon DB; Eugenio de Diego J; García-Cerrada S; García-Losada P; Haro R; Janey J; Leitch DC; Li L; Liu F; Lobben PC; MacMillan DWC; Magano J; McInturff E; Monfette S; Post RJ; Schultz D; Sitter BJ; Stevens JM; Strambeanu II; Twilton J; Wang K; Zajac MA The Evolution of High-Throughput Experimentation in Pharmaceutical Development and Perspectives on the Future. Org. Process Res. Dev. 2019, 23, 1213–1242. [Google Scholar]
- (29).(a) Adjabeng G; Brenstrum T; Wilson J; Frampton C; Robertson A; Hillhouse J; McNulty J; Capretta A Novel Class of Tertiary Phosphine Ligands Based on a Phospha-Adamantane Framework and Use in the Suzuki Cross-Coupling Reactions of Aryl Halides under Mild Conditions. Org. Lett. 2003, 5, 953–955. [DOI] [PubMed] [Google Scholar]; (b) Brenstrum T; Gerristma DA; Adjabeng GM; Frampton CS; Britten J; Robertson AJ; McNulty J; Capretta A Phosphaadamantanes as Ligands for Palladium Catalyzed Cross-Coupling Chemistry: Library Synthesis, Characterization, and Screening in the Suzuki Coupling of Alkyl Halides and Tosylates Containing β-Hydrogens with Boronic Acids and Alkylboranes. J. Org. Chem. 2004, 69, 7635–7639. [DOI] [PubMed] [Google Scholar]
- (30).Petrillo DE; Molander GA 1-(4-Acetylphenyl)-2-Phenylethane from Potassium 2-Phenethyltrifluoroborate and 4-Bromoacetophenone. Org. Synth. 2007, 84, 317. [Google Scholar]
- (31).Takechi R; Nishimura T Enantioselective 1,4-Addition of Cyclopropylboronic Acid Catalyzed by Rhodium/Chiral Diene Complexes. Chem. Commun. 2015, 51, 8528–8531. [DOI] [PubMed] [Google Scholar]
- (32).Prepared in two steps from phenylacetylene. See the Supporting Information for details
- (33).In analogy to enantiospecificity, diastereospecificity was calculated by the following formula: % ds = (deproduct/destarting material) * 100. See also: Seebach, D.; Naef, R. Helv. Chim. Acta 1981, 64, 2704–2708. [Google Scholar]
- (34).Because all of the couplings in this work employ primary boronic esters we chose to test cyclopropylboronic esters for simplicity. These are biased secondary boronates; a more rigorous set of esters will be tested in future studies of secondary esters. For studies pertaining to the stereospecificity of primary alkylboron SMCC, see: Ridgway BH; Woerpel KA Transmetalation of Alkylboranes to Palladium in the Suzuki Coupling Reaction Proceeds with Retention of Stereochemistry. J. Org. Chem. 1998, 63, 458–460. Matos K; Soderquist JA Alkylboranes in the Suzuki-Miyaura Coupling: Stereochemical and Mechanistic Studies. J. Org. Chem. 1998, 63, 461–470. Murray B; Zhao S; Aramini JM; Wang H; Biscoe MR The Stereochemical Course of Pd-Catalyzed Suzuki Reactions Using Primary Alkyltrifluoroborate Nucleophiles. ACS Catal. 2021, 11, 2504–2510.
- (35).(a) Surry DS; Buchwald SL Dialkylbiaryl Phosphines in Pd-Catalyzed Amination: A User’s Guide. Chem. Sci. 2011, 2, 27–50. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mann G; Hartwig JF Palladium Alkoxides: Potential Intermediacy in Catalytic Amination, Reductive Elimination of Ethers, and Catalytic Etheration. Comments on Alcohol Elimination from Ir(III). J. Am. Chem. Soc. 1996, 118, 13109–13110. [Google Scholar]; (c) Widenhoefer RA; Buchwald SL Electronic Dependence of C–O Reductive Elimination from Palladium (Aryl)Neopentoxide Complexes. J. Am. Chem. Soc. 1998, 120, 6504–6511. [Google Scholar]; (d) Viciu MS; Grasa GA; Nolan SP Catalytic Dehalogenation of Aryl Halides Mediated by a Palladium/Imidazolium Salt System. Organometallics 2001, 20, 3607–3612. [Google Scholar]; (e) Navarro O; Kaur H; Mahjoor P; Nolan SP Cross-Coupling and Dehalogenation Reactions Catalyzed by (N-Heterocyclic Carbene)Pd(Allyl)Cl Complexes. J. Org. Chem. 2004, 69, 3173–3180. [DOI] [PubMed] [Google Scholar]; (f) Navarro O; Marion N; Oonishi Y; Kelly RA; Nolan SP Suzuki–Miyaura, α-Ketone Arylation and Dehalogenation Reactions Catalyzed by a Versatile N-Heterocyclic Carbene–Palladacycle Complex. J. Org. Chem. 2006, 71, 685–692. [DOI] [PubMed] [Google Scholar]; (g) Yuen OY; So CM; Man HW; Kwong FY A General Palladium-Catalyzed Hiyama Cross-Coupling Reaction of Aryl and Heteroaryl Chlorides. Chem. – Eur. J. 2016, 22, 6471–6476. [DOI] [PubMed] [Google Scholar]
- (36).Although rare, reduction by ethereal solvents has been reported in palladium(II)-catalyzed systems before. See: Lau SYW; Andersen NG; Keay BA Optimization of Palladium-Catalyzed Polyene Cyclizations: Suppression of Competing Hydride Transfer from Tertiary Amines with Dabco and an Unexpected Hydride Transfer from 1,4-Dioxane. Org. Lett. 2001, 3, 181–184. Martins A; Candito DA; Lautens M Palladium-Catalyzed Reductive Ortho-Arylation: Evidence for the Decomposition of 1,2-Dimethoxyethane and Subsequent Arylpalladium(II) Reduction. Org. Lett. 2010, 12, 5186–5188. Molina de la Torre JA; Espinet P; Albéniz AC Solvent-Induced Reduction of Palladium-Aryls, a Potential Interference in Pd Catalysis. Organometallics 2013, 32, 5428–5434.
- (37).3a-d4 was prepared by esterification of 5a with neopentyl-1,3-diol-d4. See the Supporting Information for more details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.


