Abstract
Streptococcus pneumoniae, a medically important opportunistic bacterial pathogen of the upper respiratory tract, is a major public health concern, causing a wide range of pneumococcal illnesses, both invasive and noninvasive. It is associated with significant global morbidity and mortality, including pneumonia, meningitis, sepsis, and acute otitis media. The major purpose of this study was to determine the molecular epidemiology of Streptococcus pneumoniae strains that cause invasive and noninvasive infections in Ethiopia. A prospective study was undertaken in two regional hospitals between January 2018 and December 2019. Whole-genome sequencing was used to analyze all isolates. Serotypes and multilocus sequence types (MLST) were derived from genomic data. The E-test was used for antimicrobial susceptibility testing. Patient samples obtained 54 Streptococcus pneumoniae isolates, 33 from invasive and 21 from noninvasive specimens. Our findings identified 32 serotypes expressed by 25 Global Pneumococcal Sequence Clusters (GPSCs) and 42 sequence types (STs), including 21 new STs. The most common sequence types among the invasive isolates were ST3500, ST5368, ST11162, ST15425, ST15555, ST15559, and ST15561 (2/33, 6% each). These sequence types were linked to serotypes 8, 7 C, 15B/C, 16 F, 10 A, 15B, and 6 A, respectively. Among the noninvasive isolates, only ST15432, associated with serotype 23 A, had numerous isolates (4/21, 19%). Serotype 14 was revealed as the most resistant strain to penicillin G, whereas isolates from serotypes 3, 8, 7 C, and 10 A were resistant to erythromycin. Notably, all serotype 6 A isolates were resistant to both erythromycin and penicillin G. Our findings revealed an abnormally significant number of novel STs, as well as extremely diversified serotypes and sequence types, implying that Ethiopia may serve as a breeding ground for novel STs. Recombination can produce novel STs that cause capsular switching. This has the potential to influence how immunization campaigns affect the burden of invasive pneumococcal illness. The findings highlight the importance of continuous genetic surveillance of the pneumococcal population as a vital step toward enhancing future vaccine design.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-72762-9.
Keywords: Streptococcus pneumoniae, Invasive and noninvasive isolates, Molecular epidemiology
Subject terms: Microbiology, Molecular biology
Introduction
Streptococcus pneumoniae (S. pneumoniae or pneumococcus) is an alarming bacterial pathogen that causes infectious illnesses globally. Pneumococcus infections range from non-invasive to invasive pneumococcal illnesses1. Invasive pneumococcal disease (IPD) occurs when S. pneumoniae is isolated from normally sterile bodily locations, such as blood, cerebrospinal fluid, or pleural fluid. While IPD is relatively uncommon, it is associated with a high case fatality rate2,3. Noninvasive pneumococcal disease (NIPD) includes non-bacteraemic pneumonia cases and the recovery of pneumococcal isolates from nonsterile locations, such as middle ear fluid and ocular discharge1.
The capsular polysaccharide of S. pneumoniae constitutes its most crucial virulence factor, with serotyping servicing as the primary criterion for the classification of pneumococci for decades4,5. Presently, 100 serotypes of S. pneumoniae have been identified based on the differing antigenic properties of the capsule6.
Pneumococcal conjugate vaccine (PCV) is a pneumococcal vaccine prepared using the conjugate vaccination method and used to protect newborns, young children, and adults from sickness caused by the bacterium S. pneumoniae. It contains pure capsular polysaccharides from pneumococcal serotypes linked to a carrier protein, which improves antibody response as compared to the pneumococcal polysaccharide vaccine. The World Health Organization (WHO) recommends using the conjugate vaccine in routine immunizations for children7,8.
Since 2000, three PCVs (PCV7, PCV10, and PCV13) targeting common serotypes of pneumococci have been launched to reduce IPD cases worldwide. As a result, the incidence of pneumococcal illnesses, both invasive and noninvasive, has decreased significantly9–11. However, serotype shifts, characterized by increased detection of serotypes not covered by PCVs, have been found in locations where these vaccinations were introduced12–15. Thus, S. pneumoniae continues to be a leading source of bacterial illnesses such as meningitis, pneumonia, and otitis media.
Over the last two decades, drug-resistant S. pneumoniae has grown globally, with the majority of strains resistant to numerous drugs16,17. Although the distribution of serotypes and the incidence of resistant pneumococci vary significantly by geography, antibiotic resistance is high in particular serotypes, including 6B, 6 A, 9 V, 14, 15 A, 19 F, 19 A, and 23 F, on a worldwide scale18,19.
Ethiopia has licensed PCV10, added to the pediatric immunization program in 2011, to protect against serotypes 4, 6B, 9 V, 14, 18 C, 19 F, 23 F, 1, 5, and 7 F, conjugated to DPT-HepB-Hib (Synflorix, GlaxoSmithKline, Rixensart, Belgium), for routine immunization of infants beginning in November 2011. It is provided beginning at 6 weeks, followed by doses at 10 and 14 weeks (3 + 0), with no catch-up vaccination at a later age20.
According to WHO Child Health Epidemiology Reference Group WHO/CHERG estimates from 2014, acute respiratory tract infections (ARI) cause 18% of all under-five deaths worldwide. In 2019, the incidence of lower respiratory infections was 8313.7 (95% UI 7757.6–8918), the death rate was 59.4 (95% UI 49.8–71.4), and the years of life lost were 2404.5 (95% UI 2059.4-2833.3) per 100,000 individuals21. The Ethiopian Ministry of Health’s vaccination policy requires all children to be immunized from birth (oral polio vaccine) to the 14th year for girls (human papillomavirus)22. Ethiopia has 39.09% immunization coverage for children aged 12 to 23 months, with 55.16% receiving their third dose of the PCV23. While few studies have shown that the nasopharyngeal carriage rate of S. pneumoniae ranges from 41 to 65%24–27 and can reach 38.3% in cerebrospinal fluid (CSF)28,29, there is a knowledge gap regarding epidemiological factors linking carriage and transmission rates to serotypes and genetic relatedness.
Epidemiological data on S. pneumoniae isolates are critical for informing treatment and preventative strategies. Therefore, this work investigated the genetic epidemiology and drug resistance profile of S. pneumoniae isolates causing invasive and noninvasive infections in Ethiopia using whole-genome sequencing (WGS).
Materials and methods
Study area, design, and setting
The study was carried out in Ethiopia’s Addis Ababa and Amhara National Region State Hospitals (Fig. 1). From January 2018 to December 2019, we conducted a laboratory-based prospective, cross-sectional investigation. S. pneumoniae was isolated from clinical specimens of all ages including CSF, pleural, and peritoneal fluids and blood classified as IPD, and from eye and middle ear discharges and sputum as NIPD. All individuals culture positive for S. pneumoniae from clinical specimens and volunteers to participate in the study were included in the study.
Fig. 1.
Map of the study area (The study area map was generated using ArcGIS 10.4.1 version (https://www.esri.com/) that encompassed Amhara and Addis Ababa in Ethiopia. This was done by opening a new map document and setting the coordinate system to match the region, typically using a projection suitable for Ethiopia (Universal Transverse Mercator (UTM) system, specifically UTM Zone 37 N, as it covers most of the country).
Microbiological methods
Samples from all clinical specimens were first grown on 5% sheep blood agar (Oxoid, Basingstoke, UK) plates and incubated for 18–24 h at 37 °C and 5% CO2. Morphological and alpha-hemolysis properties were used to make a more specific identification. Blood samples were taken by highly skilled laboratory technicians or physicians. Blood culture bottles (TM media, India) were incubated for a further seven days, with daily visual inspections of turbidity. Finally, subcultivation was done on the seventh day. Every morning, gram staining was conducted to determine whether pneumococcus features were present or absent. All gram-positive diplococci isolated from all specimens were evaluated for additional biochemical confirmation using optochin (Oxoid, Basingstoke, UK) and bile solubility testing30. Positive isolates were kept at − 80 °C in a Skimmed Milk-Trypticase Soy-Glucose-Glycerol (STGG) medium until further characterization and transported to the Norwegian Institute of Public Health Microbiology Laboratory, Oslo, Norway using dry ice for WGS.
Antimicrobial resistance tests
The antimicrobial agents were selected based on the Ethiopian standard treatment guideline for the treatment of S. pneumoniae infection31. In this regard, resistance to the antibiotics penicillin G (PEN), erythromycin (ERY), clindamycin (CD), tetracycline (TET), chloramphenicol (CHL), and trimethoprim-sulfamethoxazole (SXT) was assessed using the strip gradient diffusion method (E-test, Biomerieux®), which followed the 2020 Clinical Laboratory Standards Institute (CLSI) guidelines. Penicillin breakpoints for meningitis are susceptible at values ≤ 0.06 µg/ml and resistant at ≥ 0.12 µg/ml32. Quality control was performed with S. pneumoniae ATCC 49,619 as the control strain.
DNA extraction and whole-genome sequencing
Isolates were cultivated overnight on sheep blood agar at 37 °C with 5% CO2. DNA was extracted from each isolate’s entire growth plate using the QIAamp DNA Mini Kit, QIAGEN, and QIAcube TM BioRobot machinery. The DNA concentrations were quantified with a Qubit 4 fluorometer. DNA libraries for WGS were created using the KAPA HyperPlus DNA library preparation kit and sequenced with NextSeq500 (Illumina). The nucleotide sequence data from this work were submitted to the European Nucleotide Archive’s DNA Data Bank with the accession number PRJEB42770 and uploaded to PathogenWatch on January 1, 2018, under Ethiopia.
Molecular characterization
MLST was used for genotyping. The sequence types of S. pneumoniae strains were identified using seven housekeeping genes (aroE, gdh, gki, recP, spi, xpt, and ddl) obtained from WGS data33. Allelic counts were determined by comparing nucleotide sequence data from a certain locus to all known alleles at that location. The sequencing types (STs) were determined using the seven allele combinations in the S. pneumoniae MLST database (https://pubmlst.org/spneumoniae/). New alleles and STs were sent to the database curator for assignment. The clustering of similar STs was evaluated using eBURST Version 3.034. The isolates’ associations were investigated using eBURST v.3 software (https://eburst.mlst.net//), which assigned a clonal complex (CC) based on the rigorous group definition of five or six out of seven common alleles. STs that shared six identical alleles across seven MLST loci with another ST were categorized as CC. Single-locus variations (SLVs) and double-locus variants (DLVs) were characterized as STs that differ by one or two alleles, respectively. Singletons were characterized as unrelated sequence types that did not fit into any CC. Isolates from the same CC or ST but with distinct serotypes were thought to represent capsular switch variations.
All isolates were serotyped using SeroBA on raw WGS readouts. SeroBA is implemented in Python3 and is freely available under an open-source GPLv3 license from https://github.com/phe-bioinformatics/PneumoCaT35. If the serotype could not be determined using SeroBA, the isolates were classed as nontypable (NT).
Statistical analysis
Finally, the data were coded, entered, cleaned and analyzed using Statistical Package for the Social Sciences version 20.0 (SPSS v.20) (IBM Corp., Chicago, IL, USA).
Results
Clinical data
From January 2018 to December 2019, fifty-four isolates were obtained, 26 of which were from Addis Ababa and 28 were from the Amhara region. Of the 54 isolates, 32 (59.3%) came from male patients and 22 (40.7%) from females. The patients’ ages ranged from 38 days to 71 years old; 34 (63%) were youngsters under the age of 18, while 20 (37%) were adults. Twenty of the isolates came from CSF, ten from blood, ten from eye discharge, nine from sputum, two from ear discharge, two from pleural fluids, and one from peritoneal fluid. Thus, 33 (61.1%) of the 54 isolates were obtained from aseptic specimens classified as invasion, while 21 (38.9%) were from noninvasive isolates.
Serotypes and antimicrobial resistance
Both invasive and noninvasive isolates had a wide range of serotypes. There were 22 serotypes found among the 33 invasive isolates, one of which was NT, and 13 serotypes identified in the 21 noninvasive isolates, one of which was NT. Only four serotypes were identified among both invasive and noninvasive isolates: 7 C, 10 A, 35B, and 38 (Tables 1 and 2). The PCV-10 vaccination covered six invasive isolates with serotypes (1, 7 F), 14 (2 isolates), 19 F, and 23 F, for an 11.1% serotype coverage.
Table 1.
Antimicrobial resistance patterns of invasive and noninvasive isolates of different serotypes.
| Serotype | No. of isolates | PEN (N) | ERY (N) | CD (N) | TET (N) | CHL (N) | SXT (N) |
|---|---|---|---|---|---|---|---|
| 6 A | 4 | 3 | 4 | 3 | 4 | 0 | 0 |
| 23 A | 4 | 0 | 2 | 0 | 0 | 1 | 0 |
| 7 C | 3 | 0 | 2 | 0 | 2 | 0 | 0 |
| 10 A | 3 | 0 | 2 | 2 | 3 | 0 | 2 |
| 15B | 3 | 1 | 0 | 0 | 0 | 0 | 1 |
| 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 3 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
| 8 | 2 | 0 | 2 | 0 | 0 | 2 | 0 |
| 11B | 2 | 0 | 2 | 1 | 2 | 0 | 0 |
| 14 | 2 | 2 | 1 | 1 | 2 | 0 | 1 |
| 16 F | 2 | 0 | 2 | 0 | 0 | 1 | 0 |
| 17 F | 2 | 0 | 1 | 0 | 1 | 1 | 0 |
| 19 A | 2 | 0 | 1 | 1 | 2 | 0 | 1 |
| 35B | 2 | 0 | 1 | 0 | 0 | 1 | 0 |
| 38 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Others | 17 | 2 | 10 | 2 | 6 | 3 | 6 |
| NT | 2 | 1 | 1 | 0 | 0 | 0 | 2 |
| Total, N (%) | 54 (100) | 10 (18.5) | 33 (61.1) | 10 (18.5) | 21 (38.9) | 10 (18.5) | 13 (24.1) |
PEN = Penicillin, ERY = Erythromycin, CD = Clindamycin, TET = Tetracycline, CHL = Chloramphenicol, SXT = Trimethoprim-sulfamethoxazole, NT = Nontypable, N = Number.
Table 2.
Association of serotypes with STs among invasive and noninvasive pneumococcal isolates.
| Serotype | Sequence type(s) (number of isolates) | |
|---|---|---|
| Invasive pneumococcal isolates (33) | Noninvasive pneumococcal isolates (21) | |
| 1 | 217 (1) | 0 |
| 3 | 6783 (1) | 0 |
| 6 A | 15,420 (1), 15,561 (2), 16,045 (1) | 0 |
| 7 C | 5368 (2) | 5368 (1) |
| 7 F | 218 (1) | 0 |
| 8 | 3500 (2) | 0 |
| 10 A | 15,555 (2) | 3135 (1) |
| 10 F | 0 | 15,601 (1) |
| 11 A | 15,552 (1) | 0 |
| 11B | 0 | 15,598 (1), 15,554 (1) |
| 13 | 0 | 8930 (1) |
| 14 | 15,551 (1), 15,560 (1) | 0 |
| 15 A | 2318 (1) | 0 |
| 15B | 11,162 (1), 15,559 (2) | 0 |
| 15 C | 11,162 (1) | 0 |
| 16 F | 15,425 (2) | 0 |
| 17 F | 0 | 11,922 (1), 15,556 (1) |
| 18 A | 5063 (1) | 0 |
| 19 A | 0 | 2013 (1), 320 (1) |
| 19 F | 15,558 (1) | 0 |
| 20 | 0 | 13,704 (1) |
| 21 | 0 | 15,553 (1) |
| 23 A | 0 | 15,432 (4) |
| 23 F | 6514 (1) | 0 |
| 24 | 6055 (1) | 0 |
| 29 | 0 | 15,550 (1) |
| 35 A | 3214 (1) | 0 |
| 35B | 6455 (1) | 5902 (1) |
| 38 | 15,431 (1) | 15,431 (1) |
| 41 F | 0 | 6449 (1) |
| 45 | 15,549 (1) | 0 |
| 46 | 11,144 (1) | 0 |
| NT | 15,588 (1) | 15,557 (1) |
STs bold refer to new STs identified in this study.
Antimicrobial resistance by serotype has revealed that serotypes 14 and 6 A were the most common among penicillin G-resistant pneumococcal isolates, followed by serotypes 3, 15B, and NT. The most common serotypes found in erythromycin-resistant S. pneumoniae (ERSP) were 6 A, 3, 8, 7 C, 10 A, 23 A, 19 A, and 1 (Table 1).
Multilocus sequence typing
The genotypic study revealed a high level of genetic variability in our community. MLST analysis detected 42 STs from 54 isolates. Table 2 shows the STs of both invasive and noninvasive isolates. 26 STs were found among the invasive isolates, with ST3500, ST5368, ST11162, ST15425, ST15555, ST15559, and ST15561 accounting for 2 out of 33 (6% each). These STs were linked to serotypes 8, 7 C, 15B/C, 16 F, 10 A, 15B, and 6 A, respectively. Twenty-one STs were novel to our study and had been registered in MLST data from Ethiopia. There were 24 STs among the invasive isolates and 16 STs among the noninvasive isolates, with ST5368 (related to serotype 7 C) and ST15431 (associated with serotype 38) detected in both groups. Among the noninvasive isolates, only ST15432 linked with serotype 23 A had numerous isolates (4/21, 19%).
Pneumococcal lineages
Among the 25 isolates, the most prevalent GPSC/serotype combinations were GPSC10 (serotypes 10 A, 17 F, and 19 A), GPSC877;88 (serotypes 15B and 15 C), GPSC92 (serotype 7 C), GPSC224 (serotype 8), GPSC30 (serotype 10 A), GPSC117 (serotype 38) and GPSC880;22;857;882 (serotypes 15 A and 35B). Table S1 summarizes data on the distribution of serotypes, GPSC types, sequence types, and MLST among the pneumococcal serotypes from invasive and non-invasive isolates.
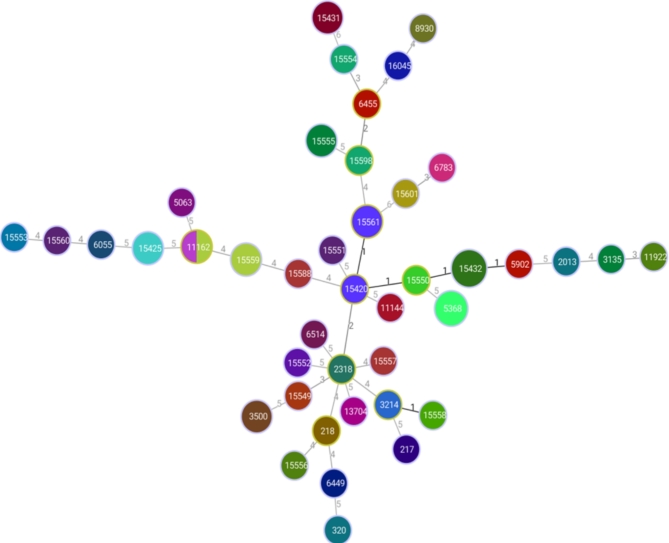
Figure 2 depicts a population snapshot of S. pneumoniae isolates obtained using eBURST analysis. The eBURST analysis identified two CCs in both invasive and noninvasive isolates. The two CCs were the principal founders, ST15420 and ST15432, each with two SLVs. ST3214 and ST15558 may be termed SLVs. ST15420, ST2318, ST15598, and ST6455 may be termed DLVs.
Fig. 2.
A population snapshot of the invasive and noninvasive S. pneumoniae isolates via eBURST analysis. Each number represents one ST, and the color indicates the serotypes of the isolates. All 42 STs are shown here in only one group, and the numbers between the circles indicate the difference between the alleles of each ST.
Discussion
Recent improvements in genome sequencing technologies have provided significant tools for studying microbial genomic diversity. In this study, we detected 32 distinct serotypes and 42 STs in a sample of 54 pneumococcal isolates collected from two regions in Ethiopia between 2018 and 2019. Using MLST, a proven and internationally established approach for monitoring the spread of clones through the pneumococcal population36, the genotyping data revealed significant variability among Ethiopian isolates. Only two CCs received 42 STs. 21 of the 42 STs had not previously been discovered elsewhere in the world. Interestingly, five of these were represented by several isolates in our limited collection, with some detected in both Addis Ababa and Amhara, indicating that they may be widespread in Ethiopia.
In this study, we looked at the antimicrobial resistance profile of S. pneumoniae in regularly administered medications that are recommended by the Ethiopian standard treatment guideline for S. pneumoniae infection in 202131. In Ethiopia, empirical therapy has a significant impact on antibiotic choices, as antibiotics can be purchased without a prescription, which likely leads to overuse and misuse of antibiotics, which can contribute to the emergence of antibiotic resistance in the region37. The resistance drugs in this study can be replaced by medicines with extremely good activity against S. pneumoniae, such as cefotaxime and ceftriaxone, which may be related to their scarcity and high cost in comparison to routinely administered antibiotics38.
Nonetheless, enough antimicrobial medicine is essential for the treatment of IPD, and drug resistance is a significant hurdle to effective treatment. In this study, the most prevalent penicillin-resistant S. pneumoniae serotypes were 6 A, 15B, 3, 14, and NT. This finding is consistent with previous studies conducted in the global population (Africa region for serotypes 6 A, 14 and 15B; Latin America, Asia, the Middle East, North America, and Europe for serotypes 3, 6, and 14)39, Malaysia40 for serotypes 6 A/B and NT, and Brazil41 for serotypes 6 A, 14, and NT. Penicillin is the first-line antibiotic for treating pneumococcal infections; thus, widespread resistance is a public health concern.
The major drawback of PCV is its inability to protect against pneumococcal illness caused by nonvaccine serotypes, increasing the risk of replacement infections. In our study, 15B was the most prevalent serotype among non-PCV invasive isolates resistant to penicillin. Following widespread PCV vaccination, the global population (South Africa and Mauritius)39, Taiwan42, and the United States43 have seen an increase in serogroup 15 infections.
Furthermore, we identified that serotypes 1, 3, 6 A, 19 A, 8, 7 C, 10 A, and 23 A were resistant to erythromycin. Erythromycin-resistant serotype 19 A was discovered in the global population except for the Middle East39, Bulgaria44, Tunisia45, Finland46, and China47, whereas serotype 23 A was found in North America39, Bulgaria44, and Colombia48, and serotype 3 was found in Asia, North America, and Europe39.
In general, vaccine-targeted serotypes were more resistant than unprotected serotypes. In the current investigation, the antibiotic resistance rate was higher in the most common serotypes (3, 6 A, 14, and 19 A) than in the other serotypes. Pneumococcal vaccine 13-valent (PCV13) is indicated for active immunization for the prevention of pneumonia and invasive disease caused by S. pneumoniae serotypes 1, 3, 4, 5, 6 A, 6B, 7 F, 9 V, 14, 18 C, 19 A, 19 F and 23 F. The PCV13 vaccine includes serotypes related to antibiotic resistance that were not covered by PCV10 (serotypes 3, 6 A, and 19 A). Serotype 1, in comparison to other vaccination serotypes, is more sensitive to the drugs being tested. In a comparable investigation from the global population (Asia and North America) and Morocco, this serotype was identified as a rarely associated serotype with antibiotic resistance39,49. This could be explained by the short period of asymptomatic colonization of this serotype in the human nasopharynx, where genetic elements are exchanged by recombination with other streptococci50,51.
This study describes the genetic structure of the pneumococcal population following routine PCV10 usage in Ethiopia. The prevalence of genotypes such as ST15561 (serotype 6 A), ST5368 (serotype 7 C), ST3500 (serotype 8), ST15555 (serotype 10 A), ST15559 (serotype 15B), ST15425 (serotype 16 F), and ST15432 (serotype 23 A), which appear to be uncommon in other parts of the world, emphasizes the importance of molecular characterization of pneumococci from Ethiopia, but ST217 (serotype 1) is common in sub-Saharan Africa52, Global population39 and Kenya53. ST320 (common in Spain54, Asia55, and Trinidad and Tobago56) was found among our serotype 19 A isolates.
Furthermore, significant STs circulating among invasive and noninvasive isolates from several African nations differ from those found internationally (Morocco49, Kenya53, and Gambia57). Such variances could be explained by differences in the original bacterial population, host features, or antibiotic selective pressures across nations. These findings emphasize the importance of molecular data from the African continent.
Isolates with the same sequence type are typically of the same serotype, but a novel or atypical serotype-sequence type combination is frequently indicative of capsular switching. Capsular switching is a typical occurrence in S. pneumoniae isolates, which requires horizontal recombination of capsular DNAs via transformation; for example, a single clone’s serotype can be changed by altering or exchanging its cps locus50. In our study, one capsular transition occurred between serotypes 15B and 15 C using ST11162. Because genotypic changes might occur temporally, either in the absence of known pressure or as a result of other selection pressures such as antibiotic usage, we were unable to detect such changes using only one year of MLST data.
The disease-causing pneumococcal lineages in Ethiopia are a combination of globally distributed and locally distinct strains. In the context of GPSCs, three isolates from GPSC10 expressing serotypes 10 A, 17 F, and 19 A are high-risk lineages that can persist and spread globally58. Our study’s biggest limitation is that we only evaluated a small number of isolates, there is no prevaccination data, and this is not vaccine effectiveness research. Another constraint was that we did not have detailed demographic and clinical information on the patients to conduct a thorough analysis of the data.
Conclusions
IPD is a burden for both children and adults in developing nations, particularly Ethiopia, where monitoring data is scarce. This study provided baseline epidemiological data on both invasive and noninvasive pneumococcal illnesses, as well as better resolution for extremely virulent serotype analysis. Longitudinal and multicenter surveillance with more background information on pneumococcal isolates is required to confirm the current findings and assess the impact of PCV universal immunization in the future. Long-term, large-scale surveillance should be carried out to analyze the impact and effectiveness of pneumococcal vaccines to develop alternative or complementary preventive and control methods and policies in the country.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the study participants. We are indebted to the microbiology laboratory staff of the hospitals and the University of Gondar for collecting and rechecking the isolates. We want to thank the Norwegian Institute of Public Health Microbiology Laboratory staff for performing antimicrobial resistance, serotyping, and whole-genome sequencing.
Abbreviations
- ST
Sequence type
- IPD
Invasive pneumococcal disease
- CSF
Cerebrospinal fluid
- NIPD
Noninvasive pneumococcal disease
- PCV
Pneumococcal conjugate vaccine
- WHO
World Health Organization
- CHERG
Child Health Epidemiology reference group
- ARI
Acute respiratory infection
- WGS
Whole genome sequence
- CLSI
Clinical Laboratory Standard Institute
- MLST
Multilocus sequence typing
- SLV
Single locus variant
- DLV
Double locus variant
- CC
Clonal complex
Author contributions
B.S. conceived and designed the experiment, collected data and experimental samples, analyzed the sequenced and overall data, and wrote the original manuscript; F.M., G.Y., A.M., W.A., T.A.L., D.A.C., B.T., and S.U.F. designed, supervised, analyzed the data, and revised the manuscript; T.A.L., S.T.F., D.V., and D.A.C. analyzed the sequenced data and revised the manuscript. All authors reviewed and approved the manuscript.
Funding
This research was funded by the University of Gondar (Ethiopia) and the Norwegian Institute of Public Health. The funders had no role in the design, data collection, analysis and interpretation, or decision to publish or writing of the manuscript.
Data availability
All data generated or analyzed during this study are included in this manuscript.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was reviewed and approved by the Ethical Review Board of the University of Gondar (No. O/V/P/RCS/05/377/2017) under the 1964 Helsinki Declaration. Permission was obtained from each hospital laboratory for collecting the isolates. Written informed consent was obtained from all patients and a parent or legal guardian of patients under the age of 18 years after explaining the purpose and objective of the study.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Centers for Disease Control and Prevention, Epidemiology & Prevention of Vaccine-Preventable Diseases. and. Washington D.C: Public Health Foundation; (2015). https://www.cdc.gov/vaccines/pubs/pinkbook/front-matter.html
- 2.Pletz, M. W. & Welte, T. Pneumococcal and Influenza Vaccination. Community-Acquired Pneumonia. European Respiratory Monographs Vol. 63, 266–285 (European Respiratory Society, 2014). [Google Scholar]
- 3.Andresen, D. N. & Collignon, P. J. Invasive pneumococcal disease in the Australian Capital Territory and Queanbeyan region: do high infant rates reflect more disease or better detection? J. Paediatr. Child. Health. 40, 184–188 (2004). 10.1111/j.1440-1754.2004.00334.x [DOI] [PubMed] [Google Scholar]
- 4.Kadioglu, A., Weiser, J. N., Paton, J. C. & Andrew, P. W. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol.6, 288–301 (2008). 10.1038/nrmicro1871 [DOI] [PubMed] [Google Scholar]
- 5.Sorensen, U. B. Typing of pneumococci by using 12 pooled antisera. J. Clin. Microbiol.31, 2097–2100 (1993). 10.1128/jcm.31.8.2097-2100.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganaie, F. et al. A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral streptococcus. mBio. 11 (3), e00937–e1020 (2020). 10.1128/mBio.00937-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper –February 2019. Wkly. Epidemiol. Rec.94(8), 85–104 (2019) (hdl:10665/310970.). [Google Scholar]
- 8.World Health Organization (WHO). Summary of WHO Position Paper on Pneumococcal Conjugate Vaccines in Infants and Children Under 5 Years of Age, February 2019 (PDF). World Health Organization (WHO). 21 April 2019. Archived from the original (PDF) on 8 March 2022 (2019).
- 9.Navarro Torné, A. et al. ECDC country experts for pneumococcal disease. European enhanced surveillance of invasive pneumococcal disease in 2010: data from 26 European countries in the post heptavalent conjugate vaccine era. Vaccine. 32 (29), 3644–3650 (2014). 10.1016/j.vaccine.2014.04.066 [DOI] [PubMed] [Google Scholar]
- 10.Chiba, N. et al. Changes in capsule and drug resistance of pneumococci after introduction of PCV7, Japan, 2010–2013. Emerg. Infect. Dis.20 (7), 1132–1139 (2014). 10.3201/eid2007.131485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilishvili, T. et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J. Infect. Dis.201 (1), 32–41 (2010). & Active Bacterial Core Surveillance/Emerging Infections Program Network 10.1086/648593 [DOI] [PubMed] [Google Scholar]
- 12.Waight, P. A. et al. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: An observational cohort study. Lancet Infect. Dis.15, 629 (2015). 10.1016/S1473-3099(15)70044-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gladstone, R. A. et al. Five winters of pneumococcal serotype replacement in UK carriage following PCV introduction. Vaccine. 33 (17), 2015–2021 (2015). 10.1016/j.vaccine.2015.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinberger, D. M., Malley, R. & Lipsitch, M. Serotype replacement in disease following pneumococcal vaccination: A discussion of the evidence. Lancet378, 1962–1973 (2011). 10.1016/S0140-6736(10)62225-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slotved, H-C., Dalby, T. & Hoffmann, S. The effect of pneumococcal conjugate vaccines on the incidence of invasive pneumococcal disease caused by ten nonvaccine serotypes in Denmark. Vaccine. 34 (6), 769–774 (2016). 10.1016/j.vaccine.2015.12.056 [DOI] [PubMed] [Google Scholar]
- 16.Mera, R. M., Miller, L. A., Daniels, J. J., Weil, J. G. & White, A. R. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States over a 10-year period: Alexander project. Diagn. Microbiol. Infect. Dis.51, 195–200 (2005). 10.1016/j.diagmicrobio.2004.10.009 [DOI] [PubMed] [Google Scholar]
- 17.Kim, S. H. et al. ANSORP Study Group. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: An Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob. Agents Chemother.56, 1418–1426 (2012). 10.1128/AAC.05658-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linares, J., Ardanuy, C., Pallares, R. & Fenoll, A. Changes in antimicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period. Clin. Microbiol. Infect.16, 402–410 (2010). 10.1111/j.1469-0691.2010.03182.x [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). Drug resistance. (2017). www.cdc.gov/pneumococcal/drug-resistance.html. Accessed December 16, 2020.
- 20.Ministry of Health Federal Republic of Ethiopia In Introducing pneumococcal conjugate vaccine in Ethiopia: A Training Manual for Health Workers (Addis Ababa, Ethiopia, 2011). [Google Scholar]
- 21.Yigezu, A. et al. Burden of lower respiratory infections and associated risk factors across regions in Ethiopia: A subnational analysis of the Global Burden of diseases 2019 study. BMJ Open.13(9), e068498 (2023). 10.1136/bmjopen-2022-068498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.FMOH, Expanded program on immunization. 1–4. (2022).
- 23.Sako, S., Gilano, G. & Hailegebreal, S. Determinants of childhood vaccination among children aged 12–23 months in Ethiopia: A community-based cross-sectional study. BMJ Open.13, e069278 (2023). 10.1136/bmjopen-2022-069278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keenan, J. D. et al. Nasopharyngeal pneumococcal serotypes before and after mass azithromycin distributions for trachoma. J. Pediatr. Infect. Dis. Soc.5, 222–226 (2016). 10.1093/jpids/piu143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assefa, A., Gelaw, B., Shiferaw, Y. & Tigabu, Z. Nasopharyngeal carriage and antimicrobial susceptibility pattern of Streptococcus pneumoniae among pediatric outpatients at Gondar University Hospital, North West Ethiopia. Pediatr. Neonatol. 54, 315–321 (2013). 10.1016/j.pedneo.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 26.Gebre, T. et al. Nasopharyngeal carriage and antimicrobial susceptibility patterns of Streptococcus pneumoniae among children under five in Southwest Ethiopia. Children. 4, 1–11 (2017). 10.3390/children4040027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sime, W. T. et al. Serotype and molecular diversity of nasopharyngeal Streptococcus pneumoniae isolates from children before and after vaccination with the ten-valent pneumococcal conjugate vaccine (PCV10) in Ethiopia. BMC Infect. Dis.19, 409 (2019). 10.1186/s12879-019-4024-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhe, L. & Klugman, K. P. Pneumococcal and Haemophilus influenzae meningitis in a children’s hospital in Ethiopia: Serotypes and susceptibility patterns. Trop. Med. Int. Health.4, 421–427 (1999). 10.1046/j.1365-3156.1999.00417.x [DOI] [PubMed] [Google Scholar]
- 29.Tegene, B., Denekew, K. & Mesele, G. Phenotypic characterization and serotypes identification of CSF isolates in Acute bacterial meningitis. Am. J. Infect. Dis. Microbiol.5 (3), 100–105 (2017). [Google Scholar]
- 30.Slotved, H. C. et al. Vestrheim DF. External Quality Assurance for Laboratory Identification and capsular typing of Streptococcus pneumoniae. Sci. Rep.7, 13280 (2017). 10.1038/s41598-017-13605-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ministry of Health (MoH). Standard Treatment Guidelines for General Hospitals, 4th edition (2021).
- 32.Wayne, P. A. Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI supplements M100. CLSI; (2020).
- 33.Enright, M. C. & Spratt, B. G. A multilocus sequence typing scheme for Streptococcus pneumoniae: Identification of clones associated with serious invasive disease. Microbiology144, 3049–3060 (1998). 10.1099/00221287-144-11-3049 [DOI] [PubMed] [Google Scholar]
- 34.Francisco, A. P., Bugalho, M., Ramirez, M. & Carriço, J. A. Global optimal eBURST analysis of multilocus typing data using a graphic matroid approach. BMC Bioinform.10, 152 (2009). 10.1186/1471-2105-10-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epping, L. et al. Keane JA. SeroBA: rapid high-throughput serotyping of Streptococcus pneumoniae from whole-genome sequence data. Microb. Genomics10.1099/mgen.0.000186 (2018). 10.1099/mgen.0.000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aanensen, D. M. & Spratt, B. G. The multilocus sequence typing network: mlst.net. Nucleic Acids Res.33, W728–W733 (2005). 10.1093/nar/gki415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abera, B., Kibret, M. & Mulu, W. Knowledge and beliefs on antimicrobial resistance among physicians and nurses in hospitals in Amhara Region, Ethiopia. BMC Pharmacol. Toxicol.15, 26 (2014). 10.1186/2050-6511-15-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iliyasu, G., Habib, A. G. & Aminu, M. B. Antimicrobial susceptibility pattern of invasive pneumococcal isolates in North West Nigeria. J. Glob Infect. Dis.7, 70–74 (2015). 10.4103/0974-777X.154440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hackel, M. et al. Serotype prevalence and antibiotic resistance in Streptococcus pneumoniae clinical isolates among global populations. Vaccine. 31 (42), 4881–4887 (2013). 10.1016/j.vaccine.2013.07.054 [DOI] [PubMed] [Google Scholar]
- 40.Goh, S. L. et al. & Ju Teh CS. Molecular detection and genotypic characterization of Streptococcus pneumoniae isolated from children in Malaysia. Pathogens Global Health; 2047–7732. (2020). [DOI] [PMC free article] [PubMed]
- 41.Pinto, T. C. A. et al. Evolution of penicillin nonsusceptibility among Streptococcus pneumoniae isolates recovered from asymptomatic carriage and Invasive Disease over 25 years in Brazil, 1990–2014. Front. Microbiol.10, 486 (2019). 10.3389/fmicb.2019.00486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiang, C. Y. et al. Successful control of Streptococcus pneumoniae 19A replacement with a catch-up primary vaccination program in Taiwan. Clin. Infect. Dis.69(9), 1581–1587 (2019). 10.1093/cid/ciy1127 [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez, B. E., Hulten, K. G., Lamberth, L., Kaplan, S. L. & Mason, E. O. Jr. Streptococcus pneumoniae serogroups 15 and 33: An increasing cause of pneumococcal infections in children in the United States after the introduction of the pneumococcal 7-valent conjugate vaccine. Pediatr. Infect. Dis.25, 301–305 (2006). 10.1097/01.inf.0000207484.52850.38 [DOI] [PubMed] [Google Scholar]
- 44.Setchanova, L. et al. Serotype changes and antimicrobial nonsusceptibility rates of invasive and noninvasive Streptococcus pneumoniae isolates after implementation of 10-valent pneumococcal nontypable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Bulgaria. Braz. J. Infcet Dis.21(4), 433–440 (2017). 10.1016/j.bjid.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raddaoui, A. et al. Serotype distribution Antibiotic Resistance and Clonality of Streptococcus pneumoniae isolated from immunocompromised patients in Tunisia. PLoS ONE10(10), e0140390 (2015). 10.1371/journal.pone.0140390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siira, L. et al. Clonality behind the increase of multidrug-resistance among noninvasive pneumococci in Southern Finland. Eur. J. Clin. Microbiol. Infect. Dis.31, 867–871 (2012). 10.1007/s10096-011-1386-8 [DOI] [PubMed] [Google Scholar]
- 47.Yi-jie, Z. et al. Serological and molecular capsular typing, antibiotic susceptibility and multilocus sequence typing of Streptococcus pneumoniae isolates from invasive and noninvasive infections. Chin. Med. J.126(12), 2296–2303 (2013). 10.3760/cma.j.issn.0366-6999.20122925 [DOI] [PubMed] [Google Scholar]
- 48.Palacios, P. A., Duarte, C., Sanabria, O. & Moreno, J. Molecular characterization of nonvaccine Streptococcus pneumoniae serotypes 11A, 15 B/C and 23A recovered from invasive isolates in Colombia. Biomédica. 37, 390–396 (2017). 10.7705/biomedica.v37i3.3223 [DOI] [PubMed] [Google Scholar]
- 49.Nzoyikorera, N. et al. Whole genomic comparative analysis of Streptococcus pneumoniae serotype 1 isolate causing invasive and noninvasive infections among children under 5 years in Casablanca, Morocco. BMC Genom.22, 39 (2021). 10.1186/s12864-020-07316-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams, T. M. et al. Genome analysis of a highly virulent serotype 1 strain of Streptococcus pneumoniae from West Africa. PLoS ONE. 7 (10), e26742 (2012). 10.1371/journal.pone.0026742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lessa, F. C. et al. Streptococcus mitis expressing pneumococcal serotype 1 Capsule. Sci. Rep.8, 17959 (2018). 10.1038/s41598-018-35921-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donkor, E. S. Molecular typing of the pneumococcus and its application in epidemiology in sub-saharan Africa. Front. Cell. Infect. Microbiol.3, 12. 10.3389/fcimb.2013.00012 (2013). 10.3389/fcimb.2013.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brueggemann, A. B. et al. Population genetic structure of Streptococcus pneumoniae in Kilifi, Kenya, prior to the introduction of pneumococcal conjugate vaccine. PLoS ONE8(11), e81539 (2013). 10.1371/journal.pone.0081539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Picazo, J. et al. Clonal and clinical profile of Streptococcus pneumoniae serotype 19A causing pediatrics invasive infections: A 2-year (2007–2009) laboratory-based surveillance in Madrid. Vaccine29, 1770–1776 (2011). 10.1016/j.vaccine.2010.12.114 [DOI] [PubMed] [Google Scholar]
- 55.Shin, J., Baek, J. Y., Kim, S. H., Song, J-H. & Ko, K. S. Predominance of ST320 among Streptococcus pneumoniae serotype 19A isolates from 10 Asian countries. J. Antimicrob. Chemother.66, 1001–1004 (2011). 10.1093/jac/dkr048 [DOI] [PubMed] [Google Scholar]
- 56.Nures-Lucas, M., McGee, L., Hawkins, P. A., Swanston, W. H. & Akpaka, P. E. Serotypes and genotypes of Streptococcus pneumoniae isolates from Trinidad and Tobago. Intern. J. Infect. Dis.46, 100–106 (2016). 10.1016/j.ijid.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Antonio, M. et al. Molecular epidemiology of pneumococci obtained from Gambian children aged 2–29 months with invasive pneumococcal disease during a trial of a 9-valent pneumococcal conjugate vaccine. BMC Infect. Dis.8, 81 (2008). 10.1186/1471-2334-8-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lo, S. W. et al. The global pneumococcal sequencing Consortium. Emergence of a multidrug-resistant and virulent Streptococcus pneumoniae lineage mediates serotype replacement after PCV13: An international whole-genome sequencing study. Lancet Microbe3, e735–e743 (2022). 10.1016/S2666-5247(22)00158-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript.