Abstract
Systemic arterial hypertension is accompanied by autonomic impairments that, if not contained, promotes cardiac functional and morphological damages. Pyridostigmine bromide (PYR) treatment results in positive effects on autonomic control and beneficial cardiac remodeling. These findings were also observed after aerobic physical training (APT). However, little is known about PYR effects on left ventricular contractility, mainly when it is combined with APT. We aimed to investigate the effects of chronic acetylcholinesterase inhibition on cardiac autonomic tone balance, coronary bed reactivity, and left ventricular contractility in spontaneously hypertensive rats (SHR) submitted to APT. Male SHR (18 weeks) were divided into two groups (N = 16): untrained and submitted to APT for 14 weeks (18th to 32nd week). Half of each group was treated with PYR (15 mg/kg/day) for two weeks (31st to 32nd week). The experimental protocol consisted of recording hemodynamic parameters, double autonomic blockade with atropine and propranolol, and assessment of coronary bed reactivity and ventricular contractility in isolated hearts using the Langendorff technique. PYR and APT reduced blood pressure, heart rate, and sympathetic influence on the heart. The Langendorff technique showed that APT increased coronary perfusion pressure and left ventricle contractility in response to coronary flow and β-agonist administration. However, treatment with PYR annulled the effects of APT. In conclusion, although chronic treatment with PYR reduces cardiac sympathetic tonic influence, it does not favor coronary bed reactivity and cardiac contractility gains. PYR treatment in the trained SHR group nullified the coronary vascular reactivity and cardiac contractility gains.
Keywords: Pyridostigmine bromide, Aerobic physical training, Hypertension, Autonomic control, Coronary reactivity, Contractility, Cardiac contractility
Subject terms: Hypertension, Cardiovascular biology
Introduction
Systemic arterial hypertension (SAH) is a multifactorial disease that represents a significant public health problem due to its clinical consequences and high prevalence1. This condition is followed by critical autonomic impairments that may favor heart failure and acute myocardial infarction development, among other comorbidities2,3. In this case, pharmacological and non-pharmacological therapies that improve the cardiovascular autonomic balance, and consequently, reduce the impairment in cardiac morphology and functionality are necessary.
Regular aerobic physical training (APT) has been an essential therapeutic strategy to prevent and treat SAH with robust benefits on cardiovascular autonomic control, cardiac morphology, and functionality4–6, thus the guidelines for the control and treatment of hypertension recommend the practice of regular physical exercises, mainly aerobic exercises, as the first therapeutic tool or as an adjuvant treatment to pharmacological treatment7–9. Additionally, previous studies have shown that cholinergic stimulation through pyridostigmine bromide (PYR) also has positive effects on cardiac autonomic control and adverse cardiac remodeling, which resemble the effects of APT10,11. In this case, an experimental study with spontaneously hypertensive rats (SHR) demonstrated that the administration of PYR (1.5 mg/kg/day) for 16 weeks reduced vascular reactivity dysfunction by decreasing the generation of reactive oxygen species and increasing the bioavailability of nitric oxide12. Other studies also in SHR demonstrated that the administration of PYR (25 mg/kg/day) for two weeks promoted a decrease in heart rate (HR) and blood pressure (BP) and favored an increase in cardiac vagal tone13,14.
Therefore, the combination of APT with acetylcholinesterase blockade through PYR treatment potentiates the hemodynamic effects observed in isolated treatments, in addition to promoting less cardiac dependence on the sympathetic autonomic component in SHR15. However, despite the various autonomic benefits observed, the effects of chronic acetylcholinesterase blockade on cardiac functionality, more specifically on vascular reactivity and left ventricular contractility, are still inconclusive, as well as the effects of the association of acetylcholinesterase blockade with APT.
Therefore, this study aimed to investigate whether the effects of chronic inhibition of acetylcholinesterase associated with APT on cardiac autonomic balance are also accompanied by positive effects on coronary bed reactivity and cardiac contractility.
Results
Pyridostigmine bromide and aerobic physical training improved hemodynamic and autonomic parameters in SHR, but their association did not potentialize the individual effects
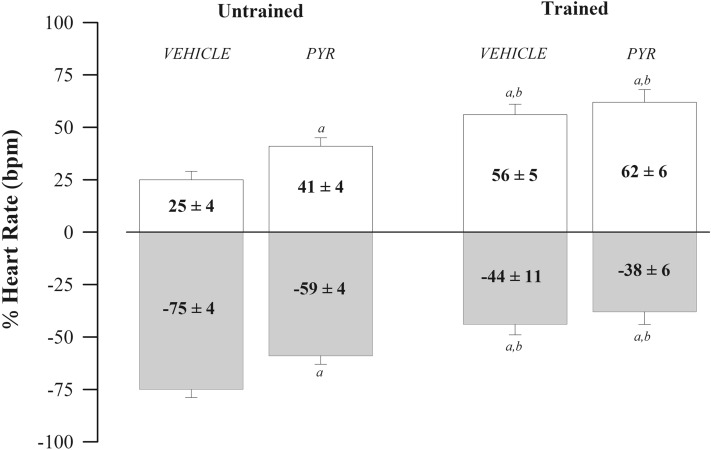
Table 1 presents the hemodynamic parameters and the HR values obtained before and after administering atropine and propranolol. The results show that both PYR (Drug Factor) and the APT (Training Factor) reduced the baseline parameters of SBP (p < 0.041 and p < 0.001), DBP (p < 0.040 and p < 0.032), MBP (p < 0.028 and p < 0.005) and HR (p < 0.001 and p < 0.001). However, HR reduction was more prominent in trained groups. Drug and APT factors resulted in lower HR values after atropine administration, while only the drug factor promoted in lower HR values after propranolol administration. Drug and training factors also promoted a reduction in the Δ HR after atropine administration, but only the training factor resulted in a reduction in the Δ HR after propranolol. In turn, both factors resulted in lower IHR values. Figure 1 shows cardiac autonomic balance in percentage values after administration of atropine (positive values) and propranolol (negative values). Results show that PYR and APT reduced sympathetic and/or increased vagal cardiac influence. However, trained groups had even more significant effects showing vagal predominance in heart rate modulation.
Table 1.
Hemodynamic parameters and heart rate values after administration of atropine and propranolol in spontaneously hypertensive rats (SHR) Untrained (N = 16) and Trained (N = 16) treated with vehicle (N = 08) or pyridostigmine bromide (PYR; N = 08).
| Untrained | Trained | Drug factor | Training factor | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vehicle | PYR | Vehicle | PYR | F(d.f) | p | F(d.f) | p | F(d.f) | p | |
| Hemodynamic parameters | ||||||||||
| Systolic Blood Pressure, mmHg | 188 ± 6 | 167 ± 3a | 159 ± 6a | 158 ± 5a,b | F(1,31):4.59 | 0.041 | F(1,31):14.97 | < 0.001 | F(1,31):4.08 | 0.053 |
| Diastolic Blood Pressure, mmHg | 146 ± 8 | 127 ± 3a | 127 ± 7a | 119 ± 4a | F(1,31):4.64 | 0.040 | F(1,31):5.08 | 0.032 | F(1,31):0.86 | 0.36 |
| Mean Blood Pressure, mmHg | 163 ± 7 | 143 ± 3a | 140 ± 6a | 135 ± 4a | F(1,31):5.34 | 0.028 | F(1,31):9.22 | 0.005 | F(1,31):1.99 | 0.168 |
| Tonic autonomic control | ||||||||||
| Heart Rate, bpm | 382 ± 5 | 351 ± 5a | 333 ± 4a,b | 315 ± 6a,b,c | F(1,31):24.81 | < 0.001 | F(1,31):73.03 | < 0.001 | F(1,31):1.24 | 0.208 |
| HR atropine, bpm | 408 ± 5 | 390 ± 3a | 372 ± 3a,b | 366 ± 5a,b | F(1,31):8.74 | 0.006 | F(1,31):53.95 | < 0.001 | F(1,31):2.42 | 0.131 |
| Δ HR atropine, bpm | 26 ± 5 | 39 ± 4a | 39 ± 3a | 52 ± 6a,b,c | F(1,31):7.60 | 0.010 | F(1,31):7.61 | 0.010 | F(1,31):0.07 | 0.979 |
| HR propranolol, bpm | 306 ± 5 | 295 ± 6 | 300 ± 6 | 283 ± 4a,c | F(1,31):7.02 | 0.013 | F(1,31):2.65 | 0.115 | F(1,31):0.42 | 0.52 |
| Δ HR propranolol, bpm | 76 ± 5 | 56 ± 5a | 33 ± 5a,b | 32 ± 6a,b | F(1,31):4.03 | 0.054 | F(1,31):42.06 | < 0.001 | F(1,31):3.66 | 0.066 |
| IHR, bpm | 328 ± 4 | 306 ± 4a | 319 ± 3b | 294 ± 3a,b,c | F(1,31):39.14 | < 0.001 | F(1,31):7.19 | 0.012 | F(1,31):0.19 | 0.67 |
Significant values are in italics.
Values are expressed as mean ± SEM. Two-way analysis of variance associated with the Student–Newman–Keuls test evaluated the effects of pyridostigmine bromide and aerobic physical training. PYR pyridostigmine bromide, HR heart rate, IHR intrinsic heart rate, F factor, d.f. degrees of freedom, mmHg millimeters of mercury. ap < 0.05 vs. untrained vehicle group; bp < 0.05 vs. untrained PYR group; cp < 0.05 vs. trained vehicle group.
Figure 1.
Evaluation of cardiac autonomic control through double pharmacological blockade with methylatropine and propranolol. The bars show the percentage fluctuation of heart rate (HR) after administration of methylatropine (white box) and propranolol (grey box) in spontaneously hypertensive rats (SHR) untrained (N = 16) and trained (N = 16) treated with pyridostigmine bromide (N = 08) or vehicle (N = 08). at ap < 0.05 vs. untrained vehicle group; bp < 0.05 vs. untrained PYR group; cp < 0.05 vs. trained vehicle group.
Pyridostigmine bromide and aerobic physical training enhanced cardiac morphological parameters, but only aerobic physical training was capable of improving cardiac functionality parameters
The results of cardiac morphology and functionality through two-dimensional echocardiography are shown in Table 2. Regarding morphology, the results of the two-way ANOVA analysis showed that drug factor reduced posterior wall thickness (PWT) and intraventricular septum thickness (IVST) (p < 0.001; p = 0.003), respectively, while training factor reduced relative wall thickness (RWT) and increase left ventricular end-diastolic diameter (LVEDD) and left ventricular end-systolic diameter LVESD (p < 0.001; p = 0.007), respectively. However, when associated, it resulted in significant increases in PWT (p < 0.001) and IVST (p < 0.001). The intra-group comparison it was observed that the untrained PYR group shows decrease in PWT and RWT parameters compared to the untrained Vehicle group. In turn, the trained Vehicle group showed lower PWT, RWT, and IVST, and higher LVEDD and LVESD values than the untrained Vehicle group. The trained PYR group showed increases in PWT and IVST when compared to the Vehicle and untrained PYR groups and the Vehicle trained group. Moreover, the same group demonstrated an increase in RWT only in comparison to the trained Vehicle group. Additionally, there was an increase in both LVEDD and LVESD when compared to the Vehicle and PYR untrained groups.
Table 2.
Cardiac morphology and function values obtained through two-dimensional echocardiography in spontaneously hypertensive rats (SHR) untrained (N = 16) and trained (N = 16) treated with vehicle (N = 08) or pyridostigmine bromide (PYR; N = 08).
| Untrained | Trained | Drug factor | Training factor | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vehicle | PYR | Vehicle | PYR | F(d.f) | p | F(d.f) | p | F(d.f) | p | |
| Morphology | ||||||||||
| Final weight, g | 356 ± 8 | 350 ± 6 | 365 ± 8 | 367 ± 6 | F(1,31): 0.05 | 0.82 | F(1,31):3.09 | 0.09 | F(1,31):0.25 | 0.62 |
| Heart weight, g | 1.94 ± 0.10 | 1.86 ± 0.05 | 1.97 ± 0.10 | 1.88 ± 0.11 | F(1,31):0.78 | 0.39 | F(1,31):0.08 | 0.78 | F(1,31):0.01 | 0.91 |
| Heart relative weight, mg/g | 5.41 ± 0.19 | 5.33 ± 0.20 | 5.42 ± 0.29 | 5.11 ± 0.24 | F(1,31):0.73 | 0.40 | F(1,31):0.22 | 0.65 | F(1,31):0.24 | 0.63 |
| PWT, mm/kg | 9.3 ± 0.12 | 8.8 ± 0.10a | 8.3 ± 0.16a | 9.9 ± 0.22a,b,c | F(1;47):13.65 | < 0.001 | F(1;47):0.103 | 0.750 | F(1;47):47.72 | < 0.001 |
| IVST, mm/kg | 8.8 ± 0.14 | 8.5 ± 0.19 | 8.2 ± 0.11a | 9.3 ± 0.14a,b,c | F(1;47):10.27 | 0.003 | F(1;47):0.348 | 0.560 | F(1;47):18.49 | < 0.001 |
| RWT | 0.51 ± 0.01 | 0.47 ± 0.01a | 0.43 ± 0.01a,b | 0.50 ± 0.01c | F(1;47):2.06 | 0.163 | F(1;47):7.32 | 0.011 | F(1;47):24.48 | < 0.001 |
| LVEDD, mm/kg | 36.3 ± 0.53 | 37.3 ± 0.84 | 38.8 ± 0.56a | 39.8 ± 0.45a,b | F(1;47):2.67 | 0.114 | F(1;47):16.67 | < 0.001 | F(1;47):0.01 | 0.98 |
| LVESD, mm/kg | 22.6 ± 0.46 | 23.7 ± 0.60 | 24.2 ± 0.41a | 24.9 ± 0.44a,b | F(1;47):3.03 | 0.093 | F(1;47):8.40 | 0.007 | F(1;47):0.20 | 0.656 |
| Functionality | ||||||||||
| HR, bpm | 356 ± 6 | 323 ± 5a | 326 ± 5a | 312 ± 6a | F(2,47):18.32 | < 0.001 | F(1,47):13.78 | < 0.001 | F(2,47):2.86 | 0.102 |
| LVEDV, μL/g | 1.39 ± 0.04 | 1.44 ± 0.05 | 1.51 ± 0.05 | 1.63 ± 0.06a,b | F(1;47):2.71 | 0.111 | F(1;47):8.48 | 0.007 | F(1;47):0.621 | 0.437 |
| LVESV, μL/g | 0.35 ± 0.03 | 0.38 ± 0.04 | 0.36 ± 0.03 | 0.40 ± 0.02 | F(1;47):1.39 | 0.248 | F(1;47):0.166 | 0.687 | F(1;47):0.012 | 0.915 |
| Stroke volume, μL/g | 1.04 ± 0.05 | 1.06 ± 0.06 | 1.12 ± 0.06 | 1.23 ± 0.04a,b | F(1;47):0.888 | 0.354 | F(1;47):7.06 | 0.013 | F(1;47):0.458 | 0.504 |
| Ejection fraction, % | 75 ± 2 | 74 ± 3 | 76 ± 3 | 76 ± 2 | F(2,47):0.114 | 0,738 | F(1,47):0.404 | 0.530 | F(2,47):0.025 | 0.875 |
| Shortening fraction, % | 35 ± 1 | 34 ± 2 | 37 ± 2 | 37 ± 1 | F(2,47):0.001 | 0,979 | F(1,47):2.93 | 0.098 | F(2,47):0.072 | 0.790 |
| Cardiac output, mL/min | 115 ± 5 | 129 ± 6 | 137 ± 5 | 141 ± 4 | F(2,47):3.69 | 0.065 | F(1,47):12.05 | 0.002 | F(2,47):1.05 | 0.314 |
| Cardiac index, mL/g | 0.37 ± 0.02 | 0.37 ± 0.03 | 0.38 ± 0.02 | 0.39 ± 0.01 | F(2,47):0.014 | 0.906 | F(1,47):0.492 | 0.489 | F(2,47):0.141 | 0.711 |
Significant values are in italics.
Values are expressed as mean ± SEM. Two-way analysis of variance associated with the Student–Newman–Keuls test evaluated the effects of pyridostigmine bromide and aerobic physical training. PYR pyridostigmine bromide, F factor, d.f. degrees of freedom, F factor, d.f. degrees of freedom, g gram, mg/g milligram per gram, PWT posterior wall thickness, IVST interventricular septum thickness, RWT relative wall thickness, LVEDD left ventricle end-diastolic diameter, LVESD left ventricle end-systolic diameter, mm/kg millimeters per kilo, HR heart rate, LVEDV left ventricular end-diastolic volume, LVESV left ventricular end-systolic volume, μL/g microliters per gram, % percentage, bpm beats per minute, mL/min milliliters per minute, mL/g milliliters per gram. ap < 0.05 vs. untrained vehicle group; bp < 0.05 vs. untrained PYR group; cp < 0.05 vs. trained vehicle group.
Regarding functionality, the TwoWay ANOVA results showed that drug (p < 0.001), and training (p < 0.001) factors decreased HR (p < 0.001). In addition, only training factor increased LVEDV, stroke volume, and cardiac output (p = 0.007; p = 0.013; p = 0.002, respectively). When the treatments were associated, no significant results were found. In the intra-group comparison, the untrained PYR and trained Vehicle groups presented lower HR when compared to the untrained Vehicle group, while the trained PYR group presented, in addition to a decrease in HR, higher values of LVEDV and stroke volume when compared with the untrained Vehicle and PYR groups.
Pyridostigmine bromide counteracted aerobic physical training effects on coronary bed reactivity and on cardiac contractile parameters in response to flow and β-agonist administration in SHR
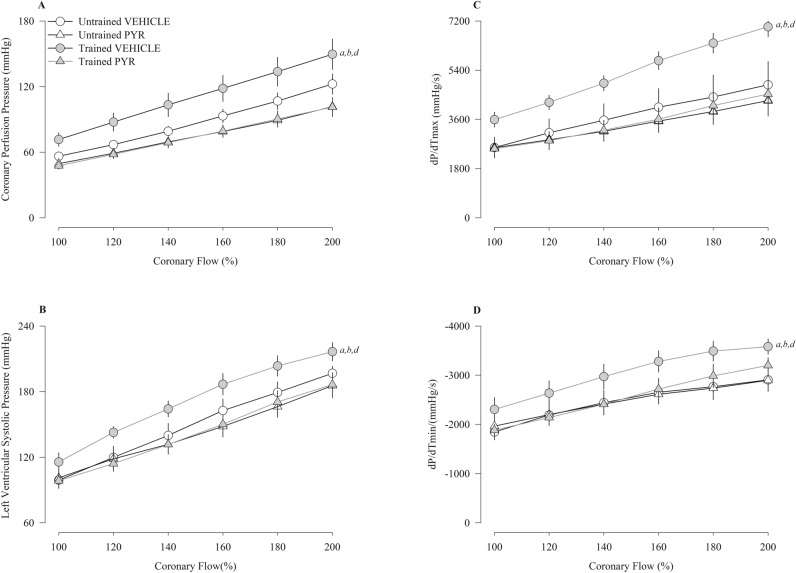
Figure 2 presents the results of coronary reactivity (Fig. 2A), left ventricular pressure (Fig. 2B), maximum dP/dT (Fig. 2C), and minimum dP/dT (Fig. 2D) in response to the progressive increase in flow (100–200%). The trained Vehicle group had higher values for coronary perfusion pressure, left ventricular systolic pressure, and maximum and minimum dP/dT compared to the other groups.
Figure 2.
Coronary perfusion pressure (A), left ventricular systolic pressure (B), dP/dT (max) (C) and dP/dT (min) (D) induced flow in spontaneously hypertensive rats (SHR) untrained (N = 16) and trained (N = 16) treated with pyridostigmine bromide (N = 08) or vehicle (N = 08). mmHg millimeters of mercury, s second, max maximum, min minimum. Values are expressed as means ± standard error of the mean. ap < 0.05 vs. untrained vehicle group; bp < 0.05 vs. untrained PYR group; cp < 0.05 vs. trained vehicle group.
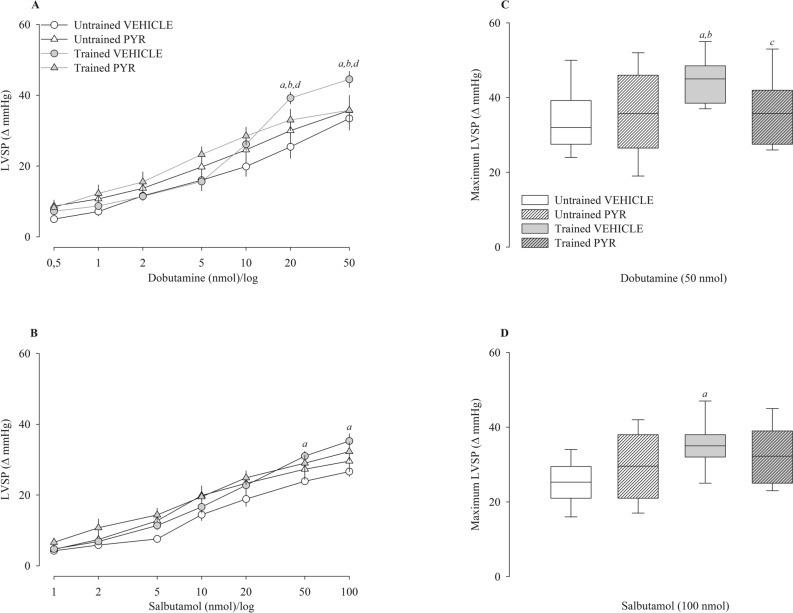
Figure 3 shows the left ventricular systolic pressure variation (Δ) after administration of dobutamine (Fig. 3A) and salbutamol (Fig. 3B) and their respective maximum responses (Fig. 3C,D). The trained Vehicle group displayed significantly greater differences in left ventricular systolic pressure compared to the other groups, but only at the highest dobutamine doses (20 and 50 nmol). Regarding salbutamol, the trained vehicle group similarly displayed significantly higher left ventricular systolic pressure at the highest doses (50 and 100 nmol), but only when compared to the untrained vehicle group.
Figure 3.
Left ventricular systolic pressure (LVSP) after administration of dobutamine (A) and salbutamol (B) in spontaneously hypertensive rats (SHR) untrained (N = 16) and trained (N = 16) treated with pyridostigmine bromide (N = 08) or vehicle (N = 08). The graphs on the right represent the maximum response obtained for dobutamine (50 nmol) (C) and salbutamol (100 nmol) (D). Values are expressed in delta (Δ). mmHg millimeters of mercury. ap < 0.05 vs. untrained vehicle group; bp < 0.05 vs. untrained PYR group; cp < 0.05 vs. trained vehicle group.
Discussion
The effects of chronic acetylcholinesterase blockade on cardiovascular autonomic control in SHR have already been investigated13,15. Our findings showed similar effects of PYR and APT on cardiac autonomic tonic balance, characterized by a reduction in sympathetic autonomic drive and/or an increase in cardiac parasympathetic drive. However, as previously shown in the literature, the association of both did not enhance the observed effects.
In turn, to the best of our knowledge, the literature lacks a study addressing the effects of PYR, associated or not with aerobic physical training, on left ventricular contractility and coronary bed reactivity. Therefore, we proposed to investigate whether chronic blockade of acetylcholinesterase with PYR in SHR previously subjected to APT enhances the responses observed on the reactivity of the coronary bed and the contractility of the left ventricle. Our main results show that acetylcholinesterase blockade with pyridostigmine bromide does not affect left ventricular contractility and coronary bed reactivity. In addition, PYR treatment in previously trained SHR reduced the contractile parameters observed.
Hemodynamic parameters
Concerning isolated treatments, the results showed that both treatment with PYR and APT significantly reduced BP and basal HR, in addition to reducing the participation of the sympathetic component and/or increasing the parasympathetic autonomic component in cardiac autonomic balance13,15. The precise cause of the decrease in BP induced by acetylcholinesterase blockade is still uncertain, but some mechanisms have been identified. Among them, the effect of greater acetylcholine bioavailability altering cardiac functionality, the displacement of the cardiac autonomic tonic balance resulting in less sympathetic influence, and/or more significant vagal influence, associated with high release of nitric oxide via lymphocytes and endothelium13,15–17. In turn, the decrease in BP induced by APT is primarily linked to adaptations that reduce cardiovascular sympathetic drive and improve the vascular endothelial function's vasodilatory response, accompanied by other systemic effects such as decreased oxidative stress10,12. Nevertheless, combining APT and PYR does not enhance the individual outcomes regardless of the mechanisms responsible for the reduction in blood pressure caused by APT and PYR15.
Cardiac morphological and functional evaluation by echocardiography
In the morphological and functional analysis using two-dimensional echocardiography, we observed well-defined differences between the groups studied. While PYR reduced PWT, IVST, and RWT, physical training increased LVESD, LVEDD, LVEDV, stroke volume, cardiac output, and promoted a more prominent reduction in RWT. The reduction of morphological parameters associated with PYR may be related to the increased release of acetylcholine at the neuromuscular junction, consequently resulting in a decrease in cardiac muscle activity and the morphological alterations related to the pressure overload18. In fact, the increase in cardiac morphological parameters associated with hypertension development is attributed to the chronically elevated workload on cardiomyocytes19. In turn, regarding cardiac functionality evaluated by echocardiography, PYR did not promote any effects. The same results were observed in previous research from our laboratory, in which were evaluated the effects of PYR in two different doses, 5 and 15 mg/mL15.
In contrast, adaptations in cardiac morphology and functionality induced by APT are associated with exercise-induced increase in venous return and are commonly expressed by an eccentric remodeling of the left ventricular wall20. These adaptations are necessary for better cardiac performance during physical exercise21. Our results demonstrated that the APT increased LVEDD and LVEDV and reduced RWT as a consequence, in addition to promoting an improvement in stroke volume and cardiac output. Although these effects on cardiac morphology are in accordance with the results seen on normotensive hearts6, they differ from previous research on hypertensive animals, in which the common result observed is concentric left ventricular remodelling21–23. In fact, in a recent publication from our laboratory, it was observed that 14 weeks of aerobic physical training promoted a significant reduction in LVEDD and LVESD in normalized values and an increase in RWT24. Regarding the LVEDD and LVESD values, the difference in the results can be attributed to the reduced body weight values observed in the control group from the Aguilar et al. study. In turn, the difference in physical training effects on RWT may be, at least in part, attributed to differences in the volume of the aerobic physical training protocols.
In turn, the association of PYR and APT resulted in significant increases in cardiac morphological parameters, which can be a compensation for the individual intervention effects observed mainly in heart rate. That is, the prominent heart rate reduction in response to both interventions association was compensated by an increase in the stroke volume, which is directly dependent on gains in cardiac muscle contractility and strength. In this case, a previous study in rats subjected to acute myocardial infarction demonstrated that the association of PYR and APT for three months resulted in positive adaptations in cardiac morphological and functional parameters, as well as favourable effects on autonomic HR modulation and in the inflammatory profile25. The same authors also presented similar results in rats subjected to the resistance physical training model26.
Additionally, a recent study in our laboratory with SHR showed that continuous aerobic training for 12 weeks increased LVEDD, LVESD, and stroke volume15. In turn, unlike our results, Bandoni et al. showed a decrease in LVESD and LVEDD in SHR treated with PYR (40 mg/kg/day) after acute myocardial infarction. However, in this same study, it was also observed that PYR did not alter the ejection fraction, in addition to a significant increase in the “fractional area change”, which is an index of global ventricular function associated with a decrease in the transmitral flow ratio. Per the authors, these changes suggested improvement in systolic and diastolic functions27. Morphological and functional studies with PYR present significantly different results and the cause is associated with constant differences in experimental protocols.
Cardiac functionality evaluated in isolated hearts
Regarding the reactivity of the coronary bed and the contractility of the left ventricle in isolated heart, we are unaware of any study using this approach. Therefore, our study is the first to investigate these parameters after chronic treatment with PYR. In this case, treatment with PYR does not promote changes in the evaluated parameters involving the reactivity of the coronary bed and the contractility of the left ventricle either through the increase in coronary flow or the administration of β-agonists. Unlike PYR, APT increases left ventricular contractility, coronary bed reactivity, dP/dTmax, and dP/dTmin. These results replicate those observed in a previous study with Wistar Kyoto and SHR rats6.
Additionally, administering salbutamol and dobutamine even more promoted significantly more expressive responses in SHR trained at the highest doses. In contrast, treatment with PYR in SHR previously submitted to APT significantly reduced responses in left ventricular contractility and coronary bed reactivity; that is, PYR treatment appears to nullify the effects of APT on coronary bed reactivity and left ventricular contractility in SHR. The cause of this finding is possibly associated with the chronic effect of increased acetylcholine on vagal cardiac nerve endings. The increase in vagal stimulation due to chronic acetylcholinesterase blockade appears to act negatively on left ventricular contractility through two mechanisms. The first principle is that the increased activation of muscarinic M2 receptors in the cardiac cell membrane promotes more significant inhibition of adenyl cyclase and reduces cAMP production, resulting in a lower contractile response in the left ventricle28. In turn, the second mechanism is associated with the inhibitory effect of vagal stimulation on noradrenergic endings in the heart. In this case, elevated vagal activity may reduce, at least in part, ventricular contractility by antagonizing any stimulatory effects that, concomitantly with sympathetic activity, may be exerted on ventricular contractility29. Nonetheless, we do not know whether these mechanisms still influence ventricular contractility in isolated heart preparation. Additionally, a recent study with patients affected by myasthenia gravis treated with acetylcholinesterase inhibitors showed a reduction in cardiac contractility, which the authors characterized as an effect of the disease30. However, we cannot highlight the action of acetylcholinesterase inhibitors on cardiac contractility. In this case, new approaches must be taken to elucidate the findings of Zawadka-Kunikowska et al. and other issues relating to our study.
Limitations and potential impact on clinical practice
Our study presents some limitations that should be taken into consideration when interpreting the results. Although we used a placebo treatment to control the trained groups, we did not use a placebo to control the pharmacological treatment. In addition, the heart rate was used to assess the heart’s sympathetic and parasympathetic autonomic tone, but this parameter can also be influenced by other factors, such as catecholamine levels, that were not controlled in our study in either group.
Regarding the potential impact on clinical practice, it’s important to note that there are no clinical trials that addressed the chronic PYR treatment effects on hypertensive patients, although its low dose safety for this population was confirmed in a study by Arad et al.31. In this case, PYR use in clinical practice for hypertension treatment needs more robust research, mainly clinical trials, with the objective of evaluating its chronic effects in this population. On the other hand, the majority of studies addressed pharmacological treatments that inhibit the sympathetic branch of the autonomic nervous system, although it is well documented that hypertension is also accompanied by a parasympathetic withdrawal32. In this sense, our study contributes with new findings about a cholinergic mimetic and its association with physical training on autonomic modulation of the heart rate and on cardiac functionality, both, in vivo and in isolated hearts.
Conclusions
Although treatment with PYR (15 mg/kg/day for 14 days) and APT has demonstrated its therapeutic potential for the treatment of hypertension, through the improvement of cardiac autonomic control associated with a significant reduction in BP and HR, our findings suggest that chronic acetylcholinesterase blockade does not interfere with the parameters of left ventricular contractility and coronary bed reactivity. Additionally, chronic acetylcholinesterase blockade in previously trained SHR does not potentiate the values observed in left ventricular contractility parameters and coronary bed reactivity. What was observed was a reduction in the values of the parameters obtained in trained SHR. Finally, the reason for this finding needs to be investigated.
Methods
Animals and ethical aspects
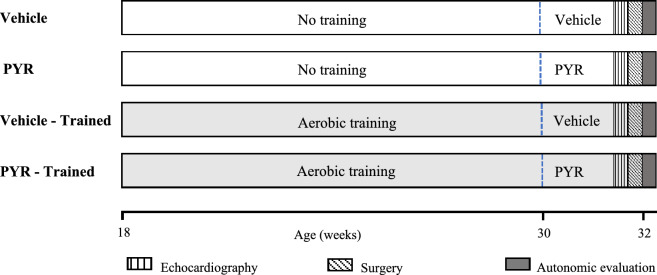
All the experimental protocols of this study are in accordance with the ARRIVE guidelines and were approved by the Committee on Animal Research and Ethics of Ribeirão Preto Medical School, University of São Paulo (protocol nº 044/2020). The sample size was calculated using the ANOVA calculator available on the Sigma Plot 11.0 statistics tool (Systat Software Inc., San Jose, CA, USA, https://grafiti.com/sigmaplot-detail/). The desired power was 80%, and the alpha value was 0.05. Minimum detectable differences in means and expected deviation of residuals were established in accordance with results from previous research6,24. We investigated SHR (N = 32) males aged 18 weeks (University of Sao Paulo—Institute of Biomedical Sciences, São Paulo, SP, Brazil) divided into two groups (N = 16): an untrained group and a group trained through aerobic exercise for 14 weeks. Half of each group (N = 08) underwent pharmacological treatment with PYR (PYR Group, 15 mg/kg/day) or Vehicle (VEHICLE Group) during the last 2 weeks of the experimental protocol (31st and 32nd weeks) (Fig. 4). All animals were kept in the laboratory of the Graduate Program in Rehabilitation and Functional Performance at the Faculty of Medicine of Ribeirão Preto/University of São Paulo, under conditions of light–dark cycle of 12 h, temperature of 22 ± 1 °C and relative humidity of 55%. In addition, the animals had free access to water and food (Nuvilab CR-1, Nuvital, Colombo, PR, Brazil).
Figure 4.
Schematic representation of the experimental timeline of all studied groups.
Aerobic physical training (APT)
The APT protocol consisted of swimming sessions in a glass tank (80 cm wide, 100 cm long, and 80 cm deep) with a capacity for six animals simultaneously. The tank was filled with warm water (30 ± 2 °C), and the APT program was divided into two stages over 14 weeks (18th to 32nd week of life). The first stage consisted of adapting to the APT, lasting 2 weeks. The training volume was started at 5 min, followed by increments of 5 min per day until reaching 40 min per day. The second stage consisted of 12 weeks of 40-min APT sessions, also performed five times a week.
The intensity of physical training was assessed through blood samples collected from the tail vein of the animals at the end of the 2nd, 5th, 8th, and 11th weeks of APT, immediately before and after the exercise sessions. This procedure aimed to measure the concentration of blood lactate (Accutrend®, Roche Diagnostics, Mannheim, Germany). The expected lactate level was 5.5–6 mmol/L. If the APT did not reach the expected lactate concentration, the physical training intensity was increased by strapping Velcro weight bags to the animal’s torso as previously demonstrated33.
Acetylcholinesterase inhibitor treatment
Pharmacological treatment was carried out in the last 2 weeks (31st and 32nd weeks) of the experimental protocol by administering PYR (Sigma-Aldrich®) diluted in drinking water at a dose of 15 ± 1 mg/kg/day. Intake was measured and corrected daily using graduated drinkers. This dosage was determined based on results from previous studies13,15.
Experimental protocols
Blood pressure and heart rate recording: tail plethysmography
All groups had systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), and HR recorded by tail plethysmography using the CODA® system (CODA Data Acquisition Software, version 4.2.2.0, Kent Scientific Corporation, https://www.kentscientific.com/products/coda-data-acquisition-software/). The evaluation was carried out in the 18th week of the animals' life, before the experimental protocol began, and the objective was to ensure that all SHRs presented similar hemodynamic parameters. The rats were placed in their container, and an inflatable rubber cuff was attached to the proximal region of their tail. The cuff was automatically inflated and deflated at fixed intervals of 15 s. Next to the cuff, a pulse transducer (sensor) was attached that captured the signals sent and were then recorded in a computer system. All animals underwent a period of adaptation to the recording through 3 measurements that were not considered. Subsequently, 12 new measurements were performed; thus, BP and HR were considered the mean of at least ten measurements.
Morphofunctional evaluation of the left ventricle using conventional two-dimensional echocardiography
For the in vivo cardiac morphofunctional analysis, the Vevo 2100® High-Resolution Imaging System (VisualSonics, Toronto, ON, Canada) with a 30 MHz probe was used. The animals were anesthetized with a mixture of 1.5% vaporized isoflurane in 1% oxygen and then placed on a heated plate (37–40 °C) with the paws connected to electrodes for electrocardiographic (ECG) recording. With the two-dimensional visualization of the left ventricle in the long axis, images were obtained in M-mode for analysis of the following cardiac parameters: left ventricle internal diameter, and anterior and posterior walls thickness during systole and diastole. The shortening fraction (%) and ejection fraction (%) values were calculated using the Teichholz method34. The analyses were performed on the fifth day of the 14th week of the experimental protocol (32nd week of life), 24 h before the BP and HR recording cannulation surgery.
The femoral artery and vein cannulation surgery
On the seventh day of the 14th week of the experimental protocol (32nd week of life of the animal) and 48 h after the echocardiographic examination, the animals were anesthetized with ketamine (80 mg/kg) and xylazine (10 mg/kg, i.p.) (Syntec do Brasil LTDA) for cannulation (PE 10) of the left femoral artery and vein for direct recording of BP and drug infusion, respectively. The cannulas were externalized to the dorsal region of the rats. After the surgical procedure, the animals received an intramuscular injection of an antibiotic (40,000 U/kg IM procaine Penicillin G) to prevent infection and subcutaneous non-steroidal anti-inflammatory flunixine meglumine (Banamine, Merck Sharp & Dohme Pharmaceutical LTDA, Brazil) (2.5 mg/kg) for postoperative analgesia. The animals were housed in individual boxes for 24 h for post-surgical recovery.
Assessment of cardiac sympathovagal balance and determination of pacemaker intrinsic heart rate (IHR)
The purpose of this protocol was to evaluate the vagal and sympathetic influence on the determination of HR in different groups. Twenty-four hours after implantation of the cannulas, the following drugs were administered in bolus: atropine (4 mg/kg) and propranolol (5 mg/kg) (Sigma-Aldrich®) to block cardiac autonomic receptors referring to the vagal and sympathetic components, respectively. Before the experiments, baseline HR was recorded for 30 min, and then the drugs were administered. The different experimental groups were studied using two different procedures:
We recorded the baseline HR for 15 min, and soon after, atropine (4 mg/kg) was injected. HR was recorded for 15 min to obtain an index of vagal influence in determining baseline HR. Next, propranolol (5 mg/kg) was administered, and the HR was recorded for another 12 min, in which case the IHR was obtained.
24 h later, in the same animal, we recorded the baseline HR for 15 min, and soon after, propranolol (5 mg/kg) was administered. The HR was recorded for 12 min to obtain an index of the sympathetic influence in determining the baseline HR. Next, atropine (4 mg/kg) was administered, and the HR was recorded for another 15 min to obtain the IHR. The results of the atropine/propranolol and propranolol/atropine protocols were considered separately, except for baseline HR (before any blockade), and for IHR (after the double blockade), which were presented as the mean of both protocols.
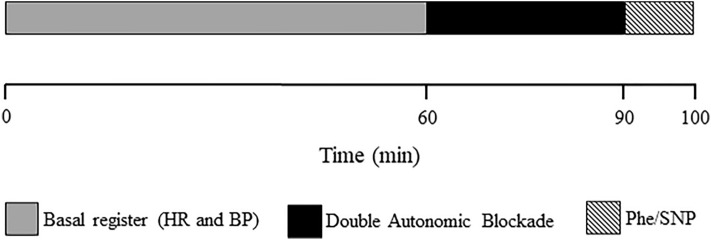
Figure 5 presents a schematic of the experimental procedures sequence performed, except for the isolated heart experiments.
Figure 5.
Double autonomic blockade. HR Heart rate, BP blood pressure, Phe phenylephrine, SNP sodium nitroprusside.
Evaluation of coronary perfusion pressure and left ventricular contractility in isolated hearts: Langendorff technique
For this procedure, the animals were heparinized (5000 IU/kg) and anesthetized by intraperitoneal injections with ketamine (80 mg/kg) and xylazine (10 mg/kg).
After being anesthetized, the animals were decapitated and their hearts were removed and perfused (8 mL/g/min) with a Krebs–Henseleit solution containing (mM): NaCl 118.4; KCl 4.7; CaCl2 2.5; MgSO47H2O 1.2, NaHCO3 25, KH2PO4 1.2; glucose 11.2; and pyruvate 2.0. The Krebs solution was aerated with 95% O2 and 5% CO2 at 37 °C35. Coronary perfusion pressure and intraventricular pressure were obtained by cannulating the aorta and implanting a latex balloon inside the left ventricle, respectively, which were connected to two pressure transducers (MLT844, ADInstruments, Bella Vista, Australia). The latex balloon was inflated with water to achieve a left-ventricular end-diastolic pressure (preload) of 8–10 mm of mercury36. Coronary perfusion and intraventricular pressure were recorded and assessed by computerized graphic software (PowerLab, Chart 7; ADInstruments, Bella Vista, Australia, https://www.adinstruments.com/support/downloads/windows/labchart-0).
The experimental protocol, previously carried out in our laboratory6, lasted approximately one hour. In this case, after 10 min of baseline stabilization, the coronary bed reactivity was evaluated by progressively increasing the baseline flow by 20% every 2 min until a 100% increase was reached. For each flow value, the coronary perfusion pressure and the left intraventricular pressure were recorded, in addition to the maximum velocity of contraction (dP/dTmax) and the maximum velocity of relaxation (dP/dTmin) of the left ventricle.
Subsequently, after a 10-min stabilization, β-adrenergic receptor sensitivity was evaluated using a dose–response curve plotting the maximum intraventricular pressure response obtained by the administration of dobutamine (β1-adrenergic agonist) and salbutamol (β2-adrenergic agonist) (Sigma-Aldrich®). The protocol consisted of the alternate administration of seven increasing bolus doses of dobutamine (0.5, 1, 2, 5, 10, 20, and 50 nmol) and salbutamol (1, 2, 5, 10, 20, 50, and 100 nmol), with a 2–3-min interval between them, or until the evaluated parameters returned to baseline values.
Statistical analysis
Results are presented as mean ± standard error. The effects of PYR (Drug Factor) and APT (Training Factor) treatment were evaluated using a two-way analysis of variance. Post-hoc comparisons were performed when appropriate using the Student–Newman–Keuls test. Coronary vascular bed reactivity and dose–response curves of cardiac contractility after administration of dobutamine and salbutamol were analyzed using a multivariate repeated measures model, and post-hoc comparisons were performed when appropriate. Differences were considered statistically significant at p < 0.05. All statistical tests were performed using SigmaPlot 11.0 software (Systat Software Inc., San Jose, CA, USA, https://grafiti.com/sigmaplot-detail/).
Institutional review and board statement
This study was performed in accordance with relevant guidelines and regulations. Animal experiments were carried out in accordance with the ARRIVE guidelines and were approved by the Committee on Animal Research and Ethics of Ribeirão Preto Medical School, University of São Paulo (protocol nº 044/2020).
Author contributions
K.P.R. and H.C.D.S. conceived and designed research; K.P.R., B.A.A. and J.C.S.D. performed experiments and analyzed data; A.C.V., T.E.V., M.E.D., and N.T.C. applied aerobic physical training and drug treatment to the rats. K.P.R. and B.A.A. drafted the manuscript. H.C.D.S. edited and revised the manuscript. All authors approved the final version of the manuscript.
Funding
This study was founded in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Financial Code 001. Process nº 2021/14938-7 and 2022/02553-6, Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Data availability
The data that supports the findings of this study are available on the request of the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Virani, S. S. et al. Heart disease and stroke statistics—2021 update: A report from the American Heart Association. Circulation143, E254–E743 (2021). 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 2.Campbell, R. C. et al. Diretrizes de 2021 da Organização Mundial Da Saúde sobre o tratamento medicamentoso da hipertensão arterial: Repercussões para as políticas na região das américas. Rev. Panam. Salud Publica46, 1–10 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kjeldsen, S. E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res.129, 95–99 (2018). 10.1016/j.phrs.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 4.Cattadori, G., Segurini, C., Picozzi, A., Padeletti, L. & Anzà, C. Exercise and heart failure: An update. ESC Heart Fail.5, 222–232 (2018). 10.1002/ehf2.12225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muthiah, V. et al. Baseline blood pressure, the 2017 ACC/AHA high blood pressure guidelines, and long-term cardiovascular risk in SPRINT. Am. J. Med.131, 956–960 (2018). 10.1016/j.amjmed.2017.12.049 [DOI] [PubMed] [Google Scholar]
- 6.Vieira, S. et al. Integrative physiological study of adaptations induced by aerobic physical training in hypertensive hearts. Front. Physiol.13, 1–10 (2022). 10.3389/fphys.2022.920196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barroso, W. S. et al. Brazilian guidelines of hypertension—2021. Arq. Bras. Cardiol.116, 516–658 (2021). 10.36660/abc.20201238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams, B. et al. ESC/ESH guidelines for the management of arterial hypertension. Kardiologia Polska77, 71–159 (2018). 10.5603/KP.2019.0018 [DOI] [PubMed] [Google Scholar]
- 9.Whelton, P. K. et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: A report of the American College of Cardiology/American Heart Association Task F. Hypertension2018, 71 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Bezerra, O. C. et al. Cholinergic stimulation improves oxidative stress and inflammation in experimental myocardial infarction. Sci. Rep.7, 1–12 (2017). 10.1038/s41598-017-14021-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu, Y. et al. Long-term administration of pyridostigmine attenuates pressure overload-induced cardiac hypertrophy by inhibiting calcineurin signalling. J. Cell Mol. Med.21, 2106–2116 (2017). 10.1111/jcmm.13133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lataro, R. M. et al. Chronic treatment with acetylcholinesterase inhibitors attenuates vascular dysfunction in spontaneously hypertensive rats. Am. J. Hypertens.32, 579–587 (2019). 10.1093/ajh/hpz036 [DOI] [PubMed] [Google Scholar]
- 13.Blanco, J. H. D. et al. Chronic cholinergic stimulation promotes changes in cardiovascular autonomic control in spontaneously hypertensive rats. Auton. Neurosci.193, 97–103 (2015). 10.1016/j.autneu.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 14.Cavalcante, G. L. et al. Benefits of pharmacological and electrical cholinergic stimulation in hypertension and heart failure. Acta Physiol.232, e13663 (2011). 10.1111/apha.13663 [DOI] [PubMed] [Google Scholar]
- 15.Gardim, C. B., Veiga, A. C., Aguilar, B. A., Philbois, S. V. & Souza, H. C. D. Effects of chronic cholinergic stimulation associated with aerobic physical training on cardiac morphofunctional and autonomic parameters in spontaneously hypertensive rats. Sci. Rep.11, 17141 (2021). 10.1038/s41598-021-96505-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernatova, I. & Csizmadiova, Z. Effect of chronic social stress on nitric oxide synthesis and vascular function in rats with family history of hypertension. Life Sci.78, 1726–1732 (2006). 10.1016/j.lfs.2005.08.005 [DOI] [PubMed] [Google Scholar]
- 17.Eglen, R. M. Muscarinic receptor subtypes in neuronal and non-neuronal cholinergic function. Auton. Autacoid Pharmacol.26, 219–233 (2006). 10.1111/j.1474-8673.2006.00368.x [DOI] [PubMed] [Google Scholar]
- 18.Verma-Ahuja, S., Husain, K., Verhulst, S., Espinosa, J. A. & Somani, S. M. Delayed effects of pyridostigmine and exercise training on acetylcholinesterase and muscle tension in mouse lower extremity. Arch. Toxicol.74, 539–546 (2000). 10.1007/s002040000169 [DOI] [PubMed] [Google Scholar]
- 19.Nadruz, W. Myocardial remodeling in hypertension. J. Hum. Hypertens.29, 1–6 (2015). 10.1038/jhh.2014.36 [DOI] [PubMed] [Google Scholar]
- 20.Weiner, R. B. & Baggish, A. L. Exercise-induced cardiac remodeling. Prog. Cardiovasc. Dis.54, 380–386 (2012). 10.1016/j.pcad.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 21.Engel, L. E. et al. The high-intensity interval training mitigates the cardiac remodeling in spontaneously hypertensive rats. Life Sci.1, 1209–1259 (2022). [DOI] [PubMed] [Google Scholar]
- 22.da Costa Rebelo, R. M., Schreckenberg, R. & Schlüter, K. D. Adverse cardiac remodelling in spontaneously hypertensive rats: Acceleration by high aerobic exercise intensity. J. Physiol.590, 5389–5400 (2012). 10.1113/jphysiol.2012.241141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schultz, R. L. et al. Effects of excessive long-term exercise on cardiac function and myocyte remodeling in hypertensive heart failure rats. Hypertension50, 410–416 (2007). 10.1161/HYPERTENSIONAHA.106.086371 [DOI] [PubMed] [Google Scholar]
- 24.Aguilar, B. A. et al. Physical exercise is essential for increasing ventricular contractility in hypertensive rats treated with losartan. Hypertens. Res.47, 1350–1361 (2024). 10.1038/s41440-024-01611-z [DOI] [PubMed] [Google Scholar]
- 25.Feriani, D. J. et al. Pyridostigmine improves the effects of resistance exercise training after myocardial infarction in rats. Front. Physiol.9, 1–11 (2018). 10.3389/fphys.2018.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feriani, D. J. et al. Impact of exercise training associated to pyridostigmine treatment on autonomic function and inflammatory profile after myocardial infarction in rats. Int. J. Cardiol.15, 757–765 (2017). 10.1016/j.ijcard.2016.10.061 [DOI] [PubMed] [Google Scholar]
- 27.Bandoni, R. L. et al. Cholinergic stimulation with pyridostigmine modulates a heart-spleen axis after acute myocardial infarction in spontaneous hypertensive rats. Sci. Rep.11, 9563 (2021). 10.1038/s41598-021-89104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harvey, R. D. & Belevych, A. E. Muscarinic regulation of cardiac ion channels. Br. J. Pharmacol.139, 1074–1084 (2003). 10.1038/sj.bjp.0705338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuder, H., Rink, D. & Muscholl, E. Sympathetic nerve stimulation on the perfused rat heart affinities of N-Methylatropine and pirenzepine at pre- and postsynaptic muscarine Receptors. Naunyn Schmiedebergs Arch Pharmacol318, 210–219 (1982). 10.1007/BF00500482 [DOI] [PubMed] [Google Scholar]
- 30.Zawadka-Kunikowska, M. et al. Association of cardiac autonomic responses with clinical outcomes of myasthenia gravis: Short-term analysis of the heart rate and blood pressure variability. J. Clin. Med.11, 1–15 (2022). 10.3390/jcm11133697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arad, M. et al. Safety of pyridostigmine in hypertensive patients receiving beta blockers. Am. J. Cardiol.69, 518–522 (1992). 10.1016/0002-9149(92)90997-D [DOI] [PubMed] [Google Scholar]
- 32.Mancia, G. & Grassi, G. The autonomic nervous system and hypertension. Circ. Res.114, 1804–1814 (2014). 10.1161/CIRCRESAHA.114.302524 [DOI] [PubMed] [Google Scholar]
- 33.Maida, K. D. et al. Amlodipine and enalapril promote distinct effects on cardiovascular autonomic control in spontaneously hypertensive rats: The role of aerobic physical training. J. Hypertens.34, 2383–2392 (2016). 10.1097/HJH.0000000000001112 [DOI] [PubMed] [Google Scholar]
- 34.Teichholz, L. E., Kreulen, T., Herman, M. & Gorlin, R. Problems in echocardiographic-angiographic correlations in the presence or absence of asynergy. Am. J. Cardiol.37, 7–11 (1976). 10.1016/0002-9149(76)90491-4 [DOI] [PubMed] [Google Scholar]
- 35.Skrzypiec-Spring, M., Grotthus, B., Szeląg, A. & Schulz, R. Isolated heart perfusion according to Langendorff—Still viable in the new millennium. J. Pharmacol. Toxicol. Methods55, 113–126 (2007). 10.1016/j.vascn.2006.05.006 [DOI] [PubMed] [Google Scholar]
- 36.Dhein, S., Mohr, F. W., Delmar, M. Practical Methods in Cardiovascular Research 155–172 (Springer, 2005).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings of this study are available on the request of the corresponding author.