Abstract
Guangdong Province, China’s largest economy, has a high incidence of tuberculosis (TB). At present, there are few reports on the distribution, transmission and drug resistance of Mycobacterium tuberculosis (Mtb) strains in this region. In this study, we performed minimum inhibitory concentration testing for 14 anti-TB drugs and whole-genome sequencing of 713 clinical Mtb isolates from 20,662 sputum culture-positive tuberculosis patients registered at 31 tuberculosis drug resistance surveillance sites covering 20 cities in Guangdong Province from 2016 to 2018. Moreover, we evaluated genome-wide associations between mutations and drug resistance, and further investigated the differences in the MICs of mutations. The epidemiology, drug-resistant phenotypes and whole genome sequencing data of 713 clinical Mtb isolates were analyzed, revealing the lineage distribution and drug-resistant gene profiles in Guangdong Province. WGS combined with quantitative MIC measurements identified several novel loci associated with resistance, of which 16 loci were found to be related to resistance to more than one drug. This study analyzed the lineage distribution, prevalence characteristics and resistance-corresponding gene profiles of Mtb isolates in Guangdong province, and provided a theoretical basis for the formulation of tuberculosis prevention and control policy in the province.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12088-024-01236-3.
Keywords: Mycobacterium tuberculosis, Whole-genome sequencing, Drug resistance
Introduction
According to the “Global Tuberculosis Report 2022”, there were nearly 10.6 million new tuberculosis (TB) cases in the world in 2021, including 1.6 million TB-associated deaths, which are recorded as the deadliest infectious disease other than COVID-19 [1]. Owing to improper treatment and poor management, Mycobacterium tuberculosis (Mtb) gradually becomes resistant to existing drugs and spreads, causing drug-resistant TB, especially resistant to at least isoniazid and rifampicin TB or rifampicin-resistant TB (MDR/RR–TB) [2]. China ranks third among countries with a high burden of MDR/RR-TB globally, and the MDR/RR-TB rates in newly treated patients is more than double the global average [1].
In 2017, Guangdong had one of the highest TB burden in China. Approximately 80,000 new TB cases were reported each year for three consecutive years, with a total drug resistance rate of 16.4%. It is worth mentioning that the economic aggregate of Guangdong Province ranks first in China. As the province with the largest permanent resident population in mainland China, Guangdong is also the province with the earliest and largest number of Chinese immigrants, accounting for approximately two-thirds of China’s total, coming from more than 160 countries and regions in the world. Guangdong, with its diverse geographical features and extremely unbalanced regional economic development, has become the epitome of China’s economy. Some studies have shown that the regional TB incidence is closely related to poverty, poor living conditions and population concentration. In addition, unbalanced regional development and its consequences on socio-economic conditions play an important role in the incidence of TB [3–5]. Therefore, a comprehensive investigation of the distribution, transmission, and drug resistance spectrum of TB strains are of great significance for the prevention and control of TB in the Guangdong Province.
In recent years, whole-genome sequencing (WGS) assays, which has played an important role in clinical and public health fields [6], can not only identify Mtb lineages or subspecies, distinguish TB incidence patterns, identify sources of infection and specific transmission directions, but it can also be applied to the prediction of drug resistance and microevolution of Mtb [2, 7–10]. Although WGS is regarded as an effective tool for the comprehensive diagnosis of drug resistance in developed countries, studies have shown that popular data analysis tools (TB Profiler, PhyResSE, Mykrobe Predictor, TGS-TB, KvarQ and CASTB) vary greatly in predicting the sensitivity and specificity of different anti-TB drugs. The prediction results for first-line drugs (isoniazid and rifampicin) are high, but the prediction accuracy for second-line injectable drugs and fluoroquinolones is lower, so the functionality and practicability of the software need to be improved [8, 10, 11]. Moreover, it should be noted that owing to differences in regional economic levels, clinical drug preferences, patient compliance, and different strain pedigrees, there will be differences in drug resistance profiles in various regions, which may also be one of the reasons for the low prediction accuracy. Current TB drug resistance studies focus mostly on comparative analyses of clinically isolated sensitive and drug-resistant strains. This makes it difficult to completely eliminate the background differences between strains, or to determine the evolutionary trajectory of Mtb drug resistance, changes in the origin of Mtb drug resistance, and key factors causing multidrug resistance. Therefore, before implementing genomic prediction, it is necessary to fully understand the local Mtb lineage, drug resistance characteristics, and resistance-conferring mutation profile.
It has been suggested that increased levels of resistance in many pathogens are due to the spread of a few relatively highly successful antibiotic resistant lineages. This phenomenon may be caused by these successful lineages accidentally acquiring rare antibiotic resistance genes through mutation [12, 13] or horizontal gene transfer [14]. Another possibility is that some strains are more likely to evolve resistance than others because of their higher mutation rate, or that they carry “enhancer” genes that open up new genetic pathways for the evolution of resistance [15]. If so, it will be possible to identify strains that are at high risk for antibiotic resistance and prevent this outcome and associated treatment failure by changing antibiotic regimens.
To remedy these deficiencies, WGS and drug sensitivity tests were performed on Mtb strains from pulmonary tuberculosis patients enrolled in 31 TB drug resistance surveillance sites in Guangdong province from 2016 to 2018 to comprehensively characterize the lineage distribution, prevalence characteristics and resistance-corresponding gene profiles, and further determine the correlation between minimum inhibitory concentration (MIC) and drug resistance mutations. This study provides a theoretical basis for the formulation of TB prevention and control policies in Guangdong Province, and will help to guide clinical rational drug use.
Materials and methods
Clinical sample information
This study is a retrospective study. The clinical isolates and epidemiological data of 20,662 sputum culture-positive tuberculosis patients registered at 32 tuberculosis drug resistance surveillance sites covering 21 cities in Guangdong Province from 2016 to 2018 were collected. All isolates were preserved in the ultra-low temperature freezers in the Bio-Bank of Clinical Resources on Tuberculosis (Centre for Tuberculosis Control of Guangdong Province, China). Cluster random sampling was used to obtain 1901 representative samples of sputum culture-positive Mtb isolates (1553 naïve and 348 previously treated), of which 916 could not be used due to missing strains or incomplete epidemiological data. 985 samples were re-cultured in Löwenstein–Jensen (LJ) medium, of which 155 failed and 62 were non-tuberculosis mycobacteria (NTM). Finally, a total of 768 samples were obtained for drug susceptibility testing (DST) and WGS. Detailed sampling methods are shown in the Supplementary methods.
Drug Susceptibility Testing
The drug susceptibility tests of 713 clinical isolates to 14 anti-TB drugs were carried out using the Sensititre MYCOTB MIC plate (Baso, Zhuhai, Guangdong Province, China). The critical concentrations were defined as 1.0 mg/L for rifampicin (RIF), 0.2 mg/L for isoniazid (INH), 5.0 mg/L for ethambutol (EMB), 100 mg/L for pyrazinamide (PZA), 2.0 mg/L for ofloxacin (OFX), 2.0 mg/L for levofloxacin (LFX), 2.0 mg/L for streptomycin (SM), 0.5 mg/L for moxifloxacin (MFX), 5.0 mg/L for kanamycin (KM), 1.0 mg/L for amikacin (AKM), 2.0 mg/L for para-aminosalicylic acid (PAS), 2.5 mg/L for prothionamide (PTO), 2.0 mg/L for capreomycin (CAP), and 0.5 mg/L for rifabutin (RBU), respectively. The results were determined after 7–14 days incubation at 37 ℃.
Association Analysis of Co-resistance of Anti-TB Drugs
This assay was performed according the previously published literature [16]. Fisher’s exact test was used to investigate the co-resistance frequency for anti-TB drugs. The statistical significance (p-value) of two drugs was determined based on the numbers of drug-resistant and drug-susceptible strains. If the p-value did not exceed 0.05, it meant a high frequency of co-resistance of two drugs. If the p-value exceeded 0.05, it meant a low frequency of co-resistance of two drugs.
Whole Genome Sequencing and Phylogenetic Analyses
Whole genome sequencing was carried out according to conventional methods [17]. For detailed parameters and experimental procedures, please refer to the Supplementary methods.
Whole-Genome Sequencing for Prediction of Drug Resistance and Determination of Resistance Level
WGS predictions of phenotypic susceptibility were based on the mutations in or upstream of drug-resistance-related genes using TB profiler (v3.0.8) (https://tbdr.lshtm.ac.uk/) according to previously published procedures [18]. Detailed resistance-related mutations of drugs and analytical method [17] are shown in the Supplementary material (Supplementary methods and Table S8).
Genome-Wide Association Study
We adopted a GWAS approach to identify nucleotide variation and loci underlying drug resistance as successfully applied in Mtb [19, 20]. The detailed parameters were shown in supplementary methods.
Statistical Analysis
Chi-square test or Fisher exact test was used for categorical data. All statistical analysis was performed in the SPSS version 26.0 software (SPSS Inc., Chicago, Illinois.). P < 0.05 was considered statistically significant.
Results
Epidemiological and Drug-Resistance Characteristics of Clinical Mtb Isolates
We screened 713 clinical Mtb isolates from 985 re-cultured samples originating from 21 prefecture-level cities in Guangdong Province from to 2016–2018 (Fig. 1 and Supplementary Table S1). Detailed epidemiological information on the corresponding patients with pulmonary TB is shown in Supplementary Table S2.
Fig. 1.
Distribution of the 713 clinical Mtb isolates from from the 32 tuberculosis surveillance sites in Guangdong Province. A Province-wide distribution of available sequencing Mtb isolates in this study. The vector diagram was purchased from Chengdu Xuegao Jihuan Network Technology Co., LTD., and some modifications were made based on it, including the color and the number of available sequencing Mtb isolates for each region. B Screening process of the Mtb isolates. Drug susceptible tests were performed using 14 anti-tuberculosis drugs. The figure in brackets indicates the number of Mtb strains. Abbreviations: DS, drug susceptible; MDR, multidrug-resistant
The antimicrobial susceptibility profile of each clinical strain was established by evaluating the MICs of 14 anti-TB drugs using the micro-broth dilution method. The results showed that 175 were drug-susceptible strains, 216 were RR-TB, including 196 MDR-TB, and 322 were DR-TB resistant to one or more drugs other than rifampicin (Supplementary Tables S2 and S3). The resistance rate of drug-resistant strains to first-line anti-TB drugs ranged from high to low: isoniazid (46.1%) > rifampicin (30.3%) > streptomycin (25.5%) > pyrazinamide (19.2%) > ethambutol (18.7%). The rate of resistance to second-line anti-TB drugs ranged from high to low: ofloxacin (22.7%) > rifabutin (22.2%) > moxifloxacin (19.8%) > para-aminosalicylic acid (19.5%) > levofloxacin (18.7%) > capreomycin (13.2%) > amikacin (12.6%) > kanamycin (8.0%) > prothionamide (6.7%) (Supplementary Table S2).
We further investigated the distribution of MDR-TB strains with different drug-resistance profiles among the four TB surveillance areas divided by economic level and geographic location (Supplementary Table S4). The results showed that 68.37% of MDR strains were resistant to at least five anti-TB drugs and more than 39% of MDR strains resistant to at least eight drugs, reflecting the serious situation of drug resistance in Guangdong. By further investigating the multidrug co-resistance patterns among the four monitoring regions, we found that the co-resistance frequency of first-line anti-TB drugs was higher than that of second-line anti-TB drugs, except fluoroquinolones. The MDR strains in each region possessed their own characteristic co-resistance patterns. For example, the co-resistance frequency of MDR strains to aminoglycoside drugs (AKM and KM) in the Pearl River Delta and eastern Guangdong was higher than that in western and northern Guangdong, while the co-resistance frequency of MDR strains to PTO and RBU was lowest in northern Guangdong (Supplementary Figure S1; Supplementary Table S4). Different patterns of multidrug co-resistance may be associated with preferred drug combinations in different regions and other unmeasured variables.
Whole Genome Sequencing for Genotyping and Canonical Resistance Regions of Clinical Mtb Isolates in Guangdong
Whole genome resequencing analysis showed that Mtb strains in Guangdong Province were mainly divided into three main lineages, namely lineage 1 (L1, Indo-Oceanic, 3.1% [22/713]); lineage 2 (L2, East Asian, 74.1% [528/713]); and lineage 4 (L4, Euro-American, 22.9% [163/713]). Further analysis revealed that two sub-lineages within L2 strains were ancient Beijing genotype L2.2 (98.40%) and proto-Beijing L2.1 (1.60%), while L4 strains mainly included L4.2 (12.74%), L4.4.2 (40.76%) and L4.5 (38.85%) (Fig. 2 and Supplementary Table S7). The distribution of lineages in each geographic region of Guangdong also varied, especially in the L1 strains, which were mainly distributed in eastern Guangdong, accounting for approximately 12.20% of all Mtb strains in this area (Supplementary Figure S2 and Supplementary Table S7). Interestingly, compared to lineage 4, lineage 2 was significantly more likely to be resistant to first-line anti-TB drugs (except EMB), RBU and PAS (P < 0.005), followed by MFX (P = 0.0616) and AKM (P = 0.0781). MDR-TB strains were significantly more likely to be present in lineage 2 (P < 0.0001) (Supplementary Table S6).
Fig. 2.
The phylogenetic tree of the 713 clinical Mtb isolates. A The maximum likelihood phylogenetic tree of 713 Mtb isolates was constructed with the software IQ-Tree and visualized with the iTol software (http://itol.embl.de). Geographic information, lineages and drug resistance information were subsequently labeled on the phylogenetic tree. Abbreviations: Pre-MDR, pre‒multidrug-resistant TB (resistant to 1 of 2 first-line drugs: isoniazid or rifampin); MDR, multidrug-resistant, (resistant to 2 first-line drugs: isoniazid and rifampin); Pre-XDR, Pre-extensively drug resistant tuberculosis (resistant to either fluoroquinolones or injectable drugs in addition to MDR); XDR, extensively drug-resistant (resistant to fluoroquinolones and injectable drugs in addition to MDR) (https://www.who.int/tb/publications/global_report/en); RIF, rifampin; INH, isoniazid; EMB, ethambutol; PZA, pyrazinamide; FQs, fluoroquinolones including OFX (ofloxacin), MFX (moxifloxacin) and LFX (levofloxacin); SM, streptomycin; KM, kanamycin; AMK, amikacin; CAP, capreomycin; PTO, prothionamide; PAS, para-aminosalicylic acid. B The number of strains with different drug-resistant phenotypes tested by the Sensititre MYCOTB MIC plate (BASO, China)
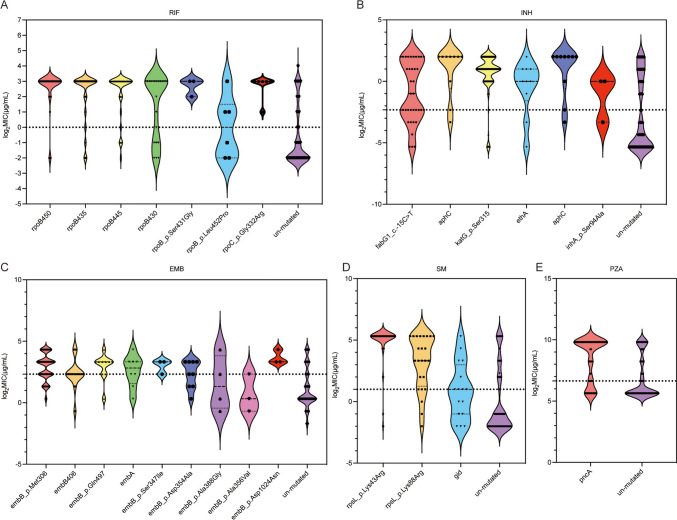
Next, we investigated the canonical resistance regions collected using the TB profiler (https://github.com/jodyphelan/tbdb). Regarding first-line anti-TB drugs, the three most frequently occurring mutations associated with the high-level rifampicin resistance were rpoB Ser450 (43.98%, 95/216), rpoB His445 (11.11%, 24/216), rpoB Asp435 (9.26%, 20/216). Although less frequently, rpoB Ser431Gly and rpoC Gly332Arg were also associated with higher levels of rifampicin resistance. For isoniazid, high-level resistance was caused by the katG Ser315 mutation (59.57%, 196/329) and aphC (2.74%, 9/329). The fabG1 c.-15C > T (5.78%, 19/329) could elevate the MICs, but almost half were at or below the resistance threshold. Ethambutol resistance mutations occurred mainly in embB (56.39%,75/133), with embB Met306 being the most frequently mutated point (31.58%, 42/133), which elevated the MIC but mostly at sub-threshold levels similar to embA mutations. Additionally, embB Gln497 and embB Asp1024Asn, were relative to high-level resistance. Streptomycin resistance mutations occurred majorly in rpsL Lys43Arg (44.51%, 81/182) and rpsL Lys88Arg (9.89%, 18/182), causing high-level resistance (Fig. 3 and Supplementary Table S7). Most mutations associated with PZA resistance were scattered throughout the pncA gene (19.12%, 26/136) and its promoter (19.12%, 26/136), and elevated the MICs to a higher level.
Fig. 3.
Distribution and log2MIC of different mutations of first-line drugs. Mutations that were noted fewer than three time are not shown. Epidemiological cut-off is denoted by dotted line. Detailed numbers are shown in the Supplementary Table S7. Abbreviations: RIF, rifampin; INH, isoniazid; EMB, ethambutol; SM, streptomycin; PZA, pyrazinamide
For second-line drugs, except rpoB Ser450Leu and rpoC Gly332Arg, the variants in rpoB were related to lower elevations in rifabutin MIC, compared to rifampicin. High-level resistance-conferring mutations in amikacin, kanamycin, and capreomycin were mainly detected in rrs A1401G. We also detected mutations in the eis promoter, which significantly increased AKM’s MIC, but had no significant effect on KAN and CAP’s MICs. PTO, a structural analog of isoniazid and ethionamide (ETH), shows a high degree of cross-resistance to these two drugs; therefore, ETH and INH drug-resistance related gene mutations in the TB profiler became the main basis for studying PTO resistance. Surprisingly, in addition to fabG1 c.-15C > T, which could elevate the MICs mostly at or below the resistance threshold, other mutations, such as KatG Ser315, ethA, and inhA Ser94Ala, had less effect on MIC (Fig. 4 and Supplementary Table S7). The mechanism of resistance to fluoroquinolones (OFX, MFX and LFX) was mutation in either the subunit of DNA gyrase (gyrA or gyrB), where the two most frequently occurring variants were gyrA Asp94 (38.64–42.69%) and Ala90 (12.88–14.18%). Para-aminosalicylic acid high-level resistance-conferring mutations were detected in thyX_c.-16C > T, and we also detected folC and thyA mutations, which had less effect on MIC elevation than thyX (Fig. 4 and Supplementary Table S7).
Fig. 4.
Distribution and log2MIC of different mutations of second-line drugs. Mutations that were noted fewer than three time are not shown. Epidemiological cut-off is denoted by dotted line. Detailed numbers are shown in the Supplementary Table S7. Abbreviations: AKM, amikacin; KM, kanamycin; CAP, capreomycin; LFX, levofloxacin; PTO, prothionamide; PAS, para-aminosalicylic acid; RBU, rifabutin
We attempted to predict Mtb susceptibility using WGS based on canonical loci in a TB profiler. Isolates occurred resistance-conferring mutations were considered phenotypically resistant, whereas isolates containing only wild-type genes were predicted to be phenotypically susceptible. However, the results were not as good as expected. For the first-line drugs, INH, RIF, EMB, PZA and SM, the sensitivities were 85.81%, 89.95%, 66.12%, 73.61%, and 84.0%, while the specificities were 80.90%, 92.80%, 91.05%, 86.90%, and 86.91%, respectively. For the second-line drugs, except PTO, the sensitivity varied from 34.88 to 77.34% (Supplementary Table S8).
Genome-Wide Association for Drug Resistance
It should be noted that for each drug, there was a large number of strains that did not contain the corresponding classical drug resistance mutations (listed in the TB profile) (Supplementary Table S7), but were still resistant to the drug, suggesting the possibility of other mutations or regulatory mechanisms. Using Pyseer (v1.3.9) software, genome-wide association study (GWAS) was performed for each drug separately using a gene/non-coding region binary burden score with a false discovery rate of < 9.44e−06 or the single locus test with a false discovery rate of < 4.49e−05 (Supplementary Tables S10 and S11). The resulting quantile–quantile (QQ) plots of the P-value distribution has shown that the observed P-value distribution (except for the short tail) is consistent with the expected line, suggesting that the correction for population structure was adequate. The significant loci in the short tails were indicated in Figs. 5 and 6.
Fig. 5.
QQ-plots of GWAS results based on the gene level to 14 drugs for each drug
Fig. 6.
QQ-plots of GWAS results based on the locus level to 14 drugs for each drug
For all drug-recognized loci, most were identified by GWAS with the highest frequency and lowest P value of all the significant hits (Supplementary Tables S9, S10 and S11). The compensatory gene rpoC was found to be strongly associated with resistance to both rifabutin (likelihood ratio test (lrt)-p value = 2.23E−27) and RIF (lrt-p value = 1.07E−20). Among the variants of rpoC, the most significant mutation locus was rpoC 483 V. Futhermore, gyrA S95T was the most significant hit among the fluoroquinolones, followed by DS94GT and DS94NT. In addition to OFX, gyrA A90V was not significantly associated with MFX and LFX (Supplementary Table S11). However, we did not find significant associations between the compensatory gene rpoA and RIF resistance, embA/embC and EMB resistance, or gyrB and fluoroquinolone resistance.
We also identified several novel loci that are related to resistance to one or more antibiotics (Supplementary Table S9). Sixteen loci were found to be related to resistance to more than one drug. Eight loci within genes were related to resistance to RIF or RBU, including the gene encoding the transcriptional regulator WhiB6, esxO, Rv3798, purE, and four PE/PPE family proteins. Six intergenic regions were found to be related to RIF/RBU resistance, including the intergenic regions whiB6-Rv3863 and PPE39-glyS, as well as regions near to ESX/type VII secretion system-related genes, such as esxO-esxP. cfp2 (Rv2376c, a major and early secreted component of Mtb) and 542,959 c > t in the intergenic regions Rv0452-PPE11 were related to resistance to SM. The most significantly associated non-canonical locus was the gene Rv1841c with INH (lrt-pvalue = 2.81E−06). The locus Rv0046c which encodes ino1, inositol-3-phosphate synthase, guaB2 (Rv3411c, inosine-5’-monophosphate dehydrogenase) and Rv1248c (2-hydroxy-3-oxoadipate synthase), was associated with resistance to PTO.
Discussion
We analyzed the epidemiological and drug-resistant phenotypes and whole genome sequencing data of 713 clinical Mtb isolates from the drug resistance surveillance program in Guangdong Province from 2016 to 2018 to characterize the distribution of Mtb lineages and drug-resistant gene profiles in Guangdong Province. Using WGS combined with quantitative MIC measurements, we further assessed the correlation of various mutations with phenotypic resistance levels of commonly used first- and second-line anti-TB agents. These findings provide a reference for TB diagnosis, treatment and formulation of prevention and control strategies in the future.
The results of this study showed that more than 90% (196/216) of RR-TB strains were MDR-TB, which was consistent with previous meta-analysis [21]. Worryingly, more than 30% of MDR-TB were found additional resistance to fluoroquinolones, known as pre-XDR-TB, which is only one step away from XDR-TB [22]. In western Guangdong and eastern Guangdong, the drug resistance rate of fluoroquinolones or AKM in MDR exceeded 40%. For pyrazinamide, one of the common drug used to treat MDR/RR-TB in China, we found 47.96% of MDR-TB strains were identified as phenotypically resistant, which was higher in Heilongjiang (28.6%), Guangxi (30.8%), Sichuan (33.3%) and Shanghai (38.5%) [23, 24]. For other drugs used in short-term treatment of RR-TB in China, 52.04%, 34.18% and 14.29% of MDR-TB strains were identified as phenotypically resistant to EMB, MFX and PTO, respectively. Overall, the severe situation of drug resistance in Guangdong highlights the urgent need for comprehensive anti-TB drug resistance detection before designing treatment regimens.
Phenotypic antibiotic susceptibility testing for Mtb is slow and cumbersome. Rapid molecular diagnostics promise to help guide therapy, but such tests rely on a complete understanding of the molecular determinants that altered antibiotic susceptibility. In recent years, several studies have used WGS to predict TB drug resistance [7, 25, 26]. We also assessed the efficiency of WGS based on canonical resistant loci collected in the TB profiler to confirm resistance to the most commonly used anti-TB agents compared to phenotypic susceptibility testing. Except for INH, RIF and SM, the sensitivity for the remaining drugs was all below 80%. The results were not satisfactory, as had previously been reported, which might be related to a limited sample size, preferred drug combinations in different regions, a limited number of mutations included and other unmeasured variables. The feasibility of this method in predicting drug resistance in TB in Guangdong needs to be tested on a large number of samples.
A GWAS was performed to further capture the significant genetic regions associated with antibiotic resistance and several novel mutations were enriched. Although these loci have not been used to predict or diagnose antibiotic resistance in patients with TB, several have recently been associated with resistance either in vitro or in other genome-wide association studies [27]. gyrA S95T was the most significant hit among the fluoroquinolones, followed by DS94GT and DS94NT.
Several mutations in the intergenic regions, esxO-esxP and Rv3862c (whiB6)-Rv3863, were found to be related to RIF/RBU resistance, which had also been observed in other clinical strains. esxO and esxP are type VII secretion system related genes [28]. Li et al. developed a CRISPR interference-based functional genomics method to systematically titrate gene expression in Mtb, revealed that downregulated esxO and Rv3863 could cause drug resistance to RIF [29]. Mutations in ino1, guaB2 and Rv1248c were found associated with resistance to PTO. It was found that the downregulated ino 1 could cause Mtb co-resistance to EMB and LFX and sensitization to clarithromycin and vancomycin; Downregulated guaB2 could sensitize Mtb to INH and RIF, and cause resistance to EMB and LFX; Downregulated Rv1248c could sensitize Mtb to INH and RIF, and cause resistance to EMB. Another study reported that Ino1, a secretion system-related protein, and PE/PPE family proteins were significantly under-expressed in Mtb over 48 h of RIF exposure by liquid chromatography coupled to mass spectrometry (LC–MS) [30]. These results indicated that in addition to regulating transcriptional activity, Mtb can antagonize the effects of RIF by modulating processes involved in bacterial cell wall synthesis and lipid metabolism.
Conclusion
This study comprehensively analyzed and described the lineage distribution and drug resistance prevalence of Mtb strains in the Guangdong Province. The relationship between classical drug resistance mutations and drug resistance levels was analyzed by integrating WGS and the drug sensitivity phenotypes of 14 anti-TB drugs. This study found that except for a few drugs, such as rifampicin, isoniazid and streptomycin, the effect of predicting drug resistance of clinical tuberculosis isolates based on classical gene mutations was not ideal. Therefore, the effectiveness of WGS in predicting bacterial resistance in this province needs more evaluation. The newly discovered drug-resistant mutations need further experimental verification in the future. This study provides important guidance and suggestions for TB prevention and control in Guangdong province, especially for MDR-TB. At the same time, it also has a guiding significance for clinical medication.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the partners of TB drug resistance monitoring stations in Guangdong province for their hard work in sorting out patient information and collecting Mtb strains. The authors thank Tao Chen, Zhenyan Li and Li Yu, formerly working in Center for Tuberculosis Control of Guangdong Province, for their hard work in the establishment of biobank. The authors express appreciation of Zhichao Chen from Shanghai Gene-Optimal Science &Technology Co., Ltd for help with WGS analysis.
Abbreviations
- TB
Tuberculosis
- Mtb
Mycobacterium tuberculosis
- MDR/RR–TB
Isoniazid and rifampicin TB or rifampicin-resistant TB
- WGS
Whole-genome sequencing
- MIC
Minimum inhibitory concentration
- INH
Isoniazid
- RIF
Rifampicin
- SM
Streptomycin
- PZA
Pyrazinamide
- EMB
Ethambutol
- OFX
Ofloxacin
- RBU
Rifabutin
- MFX
Moxifloxacin
- PAS
Para-aminosalicylic acid
- LFX
Levofloxacin
- CAP
Capreomycin
- AKM
Amikacin
- KM
Kanamycin
- PTO
Prothionamide
- ETH
Ethionamide
- GWAS
Genome-wide association study
Quantile-quantile
- LJ
Löwenstein–Jensen
- NTM
Non-tuberculosis mycobacteria
- DST
Drug susceptibility testing
Author Contributions
Wenjing Wei, Yuhui Chen and Meiling Yu conceived the study. Wenjing Wei and Yuhui Chen acquired funding, supervised and administered the project. Chenchen Zhang, Zhuhua Wu, Xinchun Huang, Yuchuan Zhao, Meiling Yu and Qi Sun processed the samples and performed the experiments. Chenchen Zhang, Meiling Yu, Yanmei Chen, Qi Sun, Huixin Guo and Wenya Dong were responsible for collecting clinical strains and conducting drug susceptibility tests. Chenchen Zhang, Yuchuan Zhao and Wenjing Wei analyzed the data. Qinghua Liao, Huizhong Wu and Xunxun Chen were responsible for providing guidance on clinical TB drug use. Anqi Liang and Wenya Dong were responsible for clinical strain information collection and collation. Chenchen Zhang, Wenjing Wei and Yuhui Chen prepared the manuscript. All the authors have read the final version of the manuscript and have approved it.
Funding
This work was supported by a Major Infectious Disease Prevention and Control of the National Science and Technique Major Project [2018ZX10715004], the Science and Technology Planning Project of Guangzhou [202201010785] and Medical Science Foundation of Guangdong Province [B2021012].
Availability of Data and Materials
The raw data of WGS has already been submitted to the Sequence Read Archive (SRA) database. The BioProject accession numbers are PRJNA866200.
Declarations
Coonflict of interests
The authors declare no competing interests.
Ethics Approval and Consent to Participate
The clinical Mtb isolates used in this study were collected from the Major Infectious Disease Prevention and Control of the National Science and Technique Major Project and preserved by the Biobank of Center for Tuberculosis Control of Guangdong Province. Written informed consent was also obtained from each patient in face-to-face interviews by trained clinicians. The study was performed in accordance with the Declaration of Helsinki and approved by the Medical Ethics Committee of Center for Tuberculosis Control of Guangdong Province, China.
Consent for Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chenchen Zhang, Zhuhua Wu, Xinchun Huang, and Yuchuan Zhao have contributed equally to this work.
Contributor Information
Meiling Yu, Email: gdyumeiling@163.com.
Yuhui Chen, Email: pistachia@163.com.
Wenjing Wei, Email: wenjingwei2014@163.com.
References
- 1.World Health Organization. Global tuberculosis report 2022. Available at: https://www.who.int/publications/i/item/9789240061729. Accessed 27 Oct 2022
- 2.Satta G, Lipman M, Smith GP, Arnold C, Kon OM, McHugh TD (2018) Mycobacterium tuberculosis and whole-genome sequencing: how close are we to unleashing its full potential? Clin Microbiol Infect 24:604–609 10.1016/j.cmi.2017.10.030 [DOI] [PubMed] [Google Scholar]
- 3.de Paiva J, Magalhaes M, Leal TC, Da SL, Da SL, Do CR, de Souza C (2022) Time trend, social vulnerability, and identification of risk areas for tuberculosis in Brazil: an ecological study. PLoS ONE 17:e247894 10.1371/journal.pone.0247894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, Xu C, Hu M, Qiao J, Chen W, Li T, Qian S, Yan M (2021) Spatio-temporal variation in tuberculosis incidence and risk factors for the disease in a region of unbalanced socio-economic development. BMC Public Health 21:1–1817 10.1186/s12889-021-11833-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Najafizada M, Rahman A, Taufique Q, Sarkar A (2021) Social determinants of multidrug-resistant tuberculosis: a scoping review and research gaps. Indian J Tuberc 68:99–105 10.1016/j.ijtb.2020.09.016 [DOI] [PubMed] [Google Scholar]
- 6.Hota SR, Padhi SK, Pahari A, Behera BK, Panda B, Mor SK, Singh VK, Goyal SM, Sahoo N (2022) Characterization and whole genome sequencing of chromobacterium violaceum OUAT_2017: a zoonotic pathogen found fatal to a wild asiatic elephant. Indian J Microbiol 62:627–633 10.1007/s12088-022-01047-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu D, Huang F, Zhang G, He W, Ou X, He P, Zhao B, Zhu B, Liu F, Li Z et al (2022) Whole-genome sequencing for surveillance of tuberculosis drug resistance and determination of resistance level in China. Clin Microbiol Infect 28:731–739 10.1016/j.cmi.2021.09.014 [DOI] [PubMed] [Google Scholar]
- 8.van Beek J, Haanperä M, Smit PW, Mentula S, Soini H (2019) Evaluation of whole genome sequencing and software tools for drug susceptibility testing of Mycobacterium tuberculosis. Clin Microbiol Infec 25:82–86 10.1016/j.cmi.2018.03.041 [DOI] [PubMed] [Google Scholar]
- 9.Chen X, He G, Wang S, Lin S, Chen J, Zhang W (2019) Evaluation of whole-genome sequence method to diagnose resistance of 13 anti-tuberculosis drugs and characterize resistance genes in clinical multi-drug resistance mycobacterium tuberculosis isolates from China. Front Microbiol 2019, 10 [DOI] [PMC free article] [PubMed]
- 10.Schleusener V, Koser CU, Beckert P, Niemann S, Feuerriegel S (2017) Mycobacterium tuberculosis resistance prediction and lineage classification from genome sequencing: comparison of automated analysis tools. Sci Rep 7:46327 10.1038/srep46327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngo TM, Teo YY (2019) Genomic prediction of tuberculosis drug-resistance: benchmarking existing databases and prediction algorithms. BMC Bioinform 20:68 10.1186/s12859-019-2658-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersson DI (2003) Persistence of antibiotic resistant bacteria. Curr Opin Microbiol 6:452–456 10.1016/j.mib.2003.09.001 [DOI] [PubMed] [Google Scholar]
- 13.Chopra I, O Neill AJ, Miller K (2003) The role of mutators in the emergence of antibiotic-resistant bacteria. Drug Resist Update 6:137–145 [DOI] [PubMed]
- 14.Davies J (1994) Inactivation of antibiotics and the dissemination of resistance genes. Science (American Association for the Advancement of Science) 264:375–382 10.1126/science.8153624 [DOI] [PubMed] [Google Scholar]
- 15.Smith T, Wolff KA, Nguyen L (2012) molecular biology of drug resistance in mycobacterium tuberculosis. In vol. 374. Springer, Berlin, 53–80 [DOI] [PMC free article] [PubMed]
- 16.Huang H, Ding N, Yang T, Li C, Jia X, Wang G, Zhong J, Zhang J, Jiang G, Wang S et al (2019) Cross-sectional whole-genome sequencing and epidemiological study of multidrug-resistant mycobacterium tuberculosis in China. Clin Infect Dis 69:405–413 10.1093/cid/ciy883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Q, Liu Q, Ji L, Li J, Zeng Y, Meng L, Luo G, Yang C, Takiff HE, Yang Z et al (2020) Citywide transmission of multidrug-resistant tuberculosis under China’s rapid urbanization: a retrospective population-based genomic spatial epidemiological study. Clin Infect Dis 71:142–151 10.1093/cid/ciz790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coll F, McNerney R, Preston MD, Guerra-Assuncao JA, Warry A, Hill-Cawthorne G, Mallard K, Nair M, Miranda A, Alves A et al (2015) Rapid determination of anti-tuberculosis drug resistance from whole-genome sequences. Genome Med 7:51 10.1186/s13073-015-0164-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei W, Zhao Y, Zhang C, Yu M, Wu Z, Xu L, Peng K, Wu Z, Li Y, Wang X (2023) Whole-genome sequencing and transcriptome-characterized in vitro evolution of aminoglycoside resistance in Mycobacterium tuberculosis. Microb Genom 9(5) [DOI] [PMC free article] [PubMed]
- 20.Lees JA, Galardini M, Bentley SD, Weiser JN, Corander J (2018) Pyseer: a comprehensive tool for microbial pangenome-wide association studies. Bioinformatics 34:4310–4312 10.1093/bioinformatics/bty539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z, Dong H, Wu B, Zhang M, Zhu Y, Pang Y, Wang X (2019) Is rifampin resistance a reliable predictive marker of multidrug-resistant tuberculosis in China: a meta-analysis of findings. J Infection 79:349–356 10.1016/j.jinf.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 22.Riccardi N, Saderi L, Borroni E, Tagliani E, Cirillo DM, Marchese V, Matteelli A, Piana A, Castellotti P, Ferrarese M et al (2021) Therapeutic strategies and outcomes of MDR and pre-XDR-TB in Italy: a nationwide study. Int J Tuberc Lung Dis 25:395–399 10.5588/ijtld.21.0036 [DOI] [PubMed] [Google Scholar]
- 23.Kurbatova EV, Cavanaugh JS, Dalton T, Click ES, Cegielski JP (2013) Epidemiology of pyrazinamide-resistant tuberculosis in the United States, 1999–2009. Clin Infect Dis 57:1081–1093 10.1093/cid/cit452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu P, Wu J, Yang C, Luo T, Shen X, Zhang Y, Nsofor CA, Zhu G, Gicquel B, Gao Q (2016) Prevalence and transmission of pyrazinamide resistant Mycobacterium tuberculosis in China. Tuberculosis (Edinb) 98:56–61 10.1016/j.tube.2016.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker TM, Miotto P, Koser CU, Fowler PW, Knaggs J, Iqbal Z, Hunt M, Chindelevitch L, Farhat M, Cirillo DM et al (2022) The 2021 WHO catalogue of Mycobacterium tuberculosis complex mutations associated with drug resistance: a genotypic analysis. Lancet Microbe 3:e265–e273 10.1016/S2666-5247(21)00301-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Yang T, Hong C, Yang Z, Wu L, Gao Q, Yang H, Tan W, Carvalho-Assef APDA (2022) Whole-genome sequencing for resistance level prediction in multidrug-resistant tuberculosis. Microbiol Spectrum 10:e271421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crook DW, Rodrigues C, Ismail NA, Mistry N, Iqbal Z, Merker M, Moore D, Walker AS, Thwaites G, Niemann S et al (2022) Genome-wide association studies of global Mycobacterium tuberculosis resistance to 13 antimicrobials in 10,228 genomes identify new resistance mechanisms. PLOS Biol 20:e3001755 10.1371/journal.pbio.3001755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mustafa AS (2022) Early secreted antigenic target of 6 kda-like proteins of mycobacterium tuberculosis: diagnostic and vaccine relevance. Int J Mycobacteriol 11:10–15 10.4103/ijmy.ijmy_232_20 [DOI] [PubMed] [Google Scholar]
- 29.Li S, Poulton NC, Chang JS, Azadian ZA, DeJesus MA, Ruecker N, Zimmerman MD, Eckartt KA, Bosch B, Engelhart CA et al (2022) CRISPRi chemical genetics and comparative genomics identify genes mediating drug potency in Mycobacterium tuberculosis. Nat Microbiol 7:766–779 10.1038/s41564-022-01130-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meneguello JE, Arita GS, Silva JVDO, Ghiraldi-Lopes LD, Caleffi-Ferracioli KR, Siqueira VLD, Scodro RBDL, Pilau EJ, Campanerut-Sá PAZ, Cardoso RF (2020) Insight about cell wall remodulation triggered by rifampicin in Mycobacterium tuberculosis. Tuberculosis (Edinb) 120:101903 10.1016/j.tube.2020.101903 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data of WGS has already been submitted to the Sequence Read Archive (SRA) database. The BioProject accession numbers are PRJNA866200.