Abstract
Bacterial resistance has led to the spread of bacterial infections such as chronic wound infections. Finding solutions for combating resistant bacteria in chronic wounds such as Staphylococcus aureus and Pseudomonas aeruginosa became an attractive theme among researchers. P. aeruginosa is a gram negative opportunistic human pathogenic bacterium that is difficult to treat due to its high resistance to antibiotics. S. aureus (gram negative bacterium) also has a high antibiotic resistance, so that it is resistant to vancomycin (VRSA), tetracycline, fluoroquinolones and beta-lactam antibiotics including penicillin and methicillin (MRSA). In particular, S. aureus and P. aeruginosa have intrinsic and acquired antibiotic resistance, making the clinical management of infection a real challenge, especially in patients with comorbidities. aPDT can be proposed as a new method in the treatment of multi-drug resistant bacteria in chronic wound infection conditions. In this study, the effect of saponin (100 μg/mL) on photodynamic inactivation on planktonic and biofilm forms of P. aeruginosa (ATCC 27853) and S. aureus (ATCC 25923) strains and on Human Dermal Fibroblast (HDF) cells was investigated. Methylene blue (MB) was used as photosensitizer (0, 10, 50, 100 μg/mL). The light source was a red LED source (660 nm; power density: 20 mW/cm2) which is related to the maximum absorption of MB. The results showed that the use of saponin in combination with MB-aPDT (Methylene Blue-antibacterial photodynamic therapy) reduces the phototoxic activity of MB due to decreasing the monomer form of MB. This result was obtained by spectrophotometric study. Also, the result of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide) assay showed that 8 min of irradiation (660 nm) at 10 μg/mL concentration of alone MB had the lowest phototoxic effect on HDF cells. Due to reduced phototoxic properties of MB in this method, detergents containing saponins not recommended to applied at the same time with MB-aPDT in wound infection area.
Keywords: Saponin, Photodynamic inactivation, Biofilm, Chronic wound, Bacteria resistance
Introduction
Epidemiological studies have shown a direct relationship between antibiotic use and the emergence and spread of resistant bacterial strains [27]. Antibiotic resistance can be transmitted through horizontal gene transfer (HGT) between different types of bacteria. Resistance can also be generated spontaneously by mutation. Antibiotics kill drug-sensitive bacteria and leave resistant bacteria to reproduce as a result of natural selection [33]. Despite warnings about overuse of antibiotics, but overuse is evident worldwide [27]. Misdiagnosis of antibiotics also helps to promote resistant bacteria [7]. Microbial resistance to antibiotics has been increasing for the past two decades, both in hospitals and in communities around the world, and may continue to increase soon [34].
Pseudomonas aeruginosa is a Gram-negative opportunistic human pathogenic bacterium that is difficult to treat due to its high resistance to antibiotics [32]. P. aeruginosa is a pathogen that causes a large number of infections in humans [14]. P. aeruginosa leads to greater resistance to antibiotics by producing biofilms [40]. Infections caused by S. aureus (Gram-positive) are also common. Treatment remains challenging due to the emergence of multidrug-resistant strains such as MRSA (methicillin-resistant Staphylococcus) [5]. S. aureus does not usually cause infection on healthy skin, however, if it can enter the bloodstream or internal tissues, it can cause a variety of serious infections [19]. This bacteria also has a high antibiotic resistance, so that it is resistant to vancomycin (VRSA), tetracycline, fluoroquinolones and beta-lactam antibiotics including penicillin and methicillin [29]. One of the most common infections is wound infection that occurs in chronic wounds. Diabetic foot ulcers, pressure ulcers, and leg ulcers are three of the most common types of chronic ulcers with similar pathogenesis [23].
One of the main causes of delayed or unhealed wounds is the presence of infection [4]. wound bacterial infections depend on the host resistance, the number of present microorganisms, and their severity. Since bacterial resistance to antibiotics is increasing and this has led to the spread of bacterial infections such as wound infections, therefore finding better antibacterial compounds that having good and acceptable antibacterial properties and do not cause resistance in bacteria is necessary. Among these compounds, we can name plant compounds such as berberine, curcumin, flavonoids, saponins, etc. Saponins have the same function as detergents and also have anticancer properties in addition to antimicrobial properties [2]. The amphipathic nature of saponins indicates their activity as a surfactant that can be used to increase the penetration of macromolecules such as proteins through cell membranes [36]. As significant commercial products, saponins are widely used in the health, pharmaceutical, cosmetic and food industries [21]. Saponins are derived from plants such as soy, Yucca and Quillaja, which have several beneficial effects on animal health. Saponins are glycosidic compounds composed of a fat-soluble nucleus (aglycone) that are either alkaloid steroids such as tomatoes, yucca and oats, or triterpenoids such as guar, glia, alfalfa and soy. Saponins have several biological effects, such as hemolytic and antibacterial activity. Not all saponins have the same biological activity. Some are beneficial and some are harmful to animal function [12]. In addition, saponins have several properties. Among them, antifungal, antiviral, antioxidant properties and their lethal effect on protozoa can be mentioned [1, 13, 17, 26]. In contrast, Quillaja saponaria saponins have a cytotoxic effect against CHO-K1 cells, whereas against E. coli cells this effect has not been observed [2].
Antimicrobial photodynamic therapy (aPDT) is the use of a photoactive dye followed by radiation, which results in the death of microbial cells by producing reactive oxygen species (ROS) in the presence of oxygen molecules [24]. One of the advantages of aPDT for bacterial infections over antibiotics is the ability to kill microorganisms independently of their antimicrobial resistance pattern. In addition, a wide range of activities, low probability of side effects, very fast response time and average cost of treatment are also among the advantages of this method [28]. They can also damage almost all biological molecules and cause the destruction of cells and any other microorganisms (gram-positive and gram-negative bacteria, viruses, fungi, and parasites) [6].
One promising way is combination of aPDT with conventional antimicrobial drugs to enhance their effects or even overcome microbial resistance. This method points to potential new applications for the treatment of superficial skin infections. This option could help expand the use of aPDT and reduce antibiotic use [32].
Due to the advantages of this combination method, in this study we investigated the effect of aPDT in combination with saponin on two strains of antibiotic-resistant bacteria including Pseudomonas aeruginosa and Staphylococcus aureus. The present research was conducted with the following hypothesis that the combination of saponin and aPDT is effective on the planktonic and biofilm states of S. aureus and P. aeruginosa and the combined method has no harmful effect on normal eukaryotic cells.
Materials and Methods
Bacterial Strain and Growth Conditions
Staphylococcus aureus (ATCC 25923) and P. aeruginosa (ATCC 27853) were used in this study collected from the Institute of Pasture, Tehran, Iran. Strains previously were stored on glycerol, cultured and stored on nutrient agar plates (NA, Merck, Germany) with regular transfer onto fresh medium. For inoculation, the microorganism was grown aerobically in NA plates at 37 °C for 18–24 h and then the strain suspension was prepared in sterile Phosphate Buffered Saline (PBS) (pH 7.4) to reach the turbidity of 0.5 McFarland (a concentration of 1.5 × 108 CFU/mL). Tryptic Soy Broth (TSB, Merck, Germany) was used as the liquid medium for biofilm culture.
Photosensitizer and Light Source
Methylene blue (MB) was used as photosensitizer. The light source used in this study was a red LED source (660 nm; power density: 20 mW/cm2) which is related to the maximum absorption of MB. All experiments were repeated three times. Each experiment was performed with four groups. The first group: only methylene blue at dark, the second group: only MB in presence of LED light irradiation, the third group: MB with saponin at dark, the fourth group: MB with saponin exposed to LED light irradiation.
Controls: Dark control group: bacteria only without treatment at dark, LED control group: bacteria only without treatment exposed to laser light irradiation.
Effect of Pretreatment with Saponin on MB-aPDT Inactivation of Planktonic Form of Staphylococcus aureus and Pseudomonas aeruginosa
To analyze the effect of pretreatment with saponin, the bacteria were first treated with saponin (100 μg/mL, 1h), then different MB concentrations were added (0, 10, 50, 100 μg/mL, 10 min), and finally laser illumination (660 nm, 8 min) was performed. After centrifugation and adding 1 mL PBS (pH 7.4) to each sample, a 1:1000 dilution was prepared. Then 50 μL of cell suspension was cultured on NA and incubated for 24 h at 37 °C. After exact time colonies were counted [11].
Assessment of Biofilm Formation Ability After Pretreatment With Saponin and MB-aPDT Inactivation
Overnight culture of different groups of treated strains was diluted 1:50 in TSB supplemented with 0.2% glucose. Amounts (200 μL) of the bacterial suspension were inoculated in 96-well flat-bottomed sterile polystyrene microplate and incubated at 37 °C for 24 h [8]. Then the wells were washed twice with PBS (pH 7.4) to remove planktonic bacteria. In order to fix the biofilms, 200 μL of ethanol was added to each well and incubated for 10 min at room temperature and then stained with 0.5% crystal violet for 30 min. After the staining, the plates were washed with water to remove excess dye. Then 200 μL of acetic acid was added as a solvent to each well to prepare them for the optical density reading. The absorbance of the solubilized dye was read at 490 nm using a microplate reader (Hyperion, Inc., FL, USA) [25].
Effect of Pretreatment with Saponin on MB-aPDT Inactivation of Biofilm form of Staphylococcus aureus and Pseudomonas aeruginosa
Suspensions of S. aureus and P. aeruginosa strain with a turbidity equivalent to a 0.5 McFarland standard was diluted at 1:100 in TSB contaning 0.2% glucose. Aliquots (500 μL) of the diluted suspension were inoculated into 24-well flat-bottomed sterile polystyrene plate and then incubated at 37 °C for 24 h. The TSB medium was removed from the biofilms formed in each well first biofilms were treated with saponin (100 μg/mL, 1h), then treated with MB (0, 10, 50, 100 μg/mL, 10 min) and irradiated with a red laser light irradiation (660 nm, 8 min). To homogenize the samples, biofilms were pipetted with 1 ml of fresh PBS 1: 1000 dilution was prepared from all samples and cultured on NA plates at 37 °C for 24 h. After that the colonies were counted [10, 11].
Phototoxicity Effect of MB and Saponin on Human Dermal Fibroblast
HDF cells ( cells) were cultured in DMEM cell culture medium in 96 well plates and incubated for 24 h at 5% CO2 at 37 °C. Then the culture medium was removed, and the cells treated with saponin (1 h). Then saponin was removed from the wells and the cells in all groups (alone MB without saponin and MB with saponin) were treated with different concentrations of MB for 10 min. All wells were washed with PBS. The aPDT group was irradiated for 8 min. Following this period, the cells were resupplied with medium and incubated overnight at 37 °C. MTT assay was used to evaluate cell viability [25].
Spectrophotometric Study of MB Interactions with saponin
Methylene blue (10 µg⁄mL) and saponin concentration range (0–700 µg/mL) stocks were prepared by using distilled water. Alternations in MB absorbance were recorded by increasing the concentration of saponin at 800–500 nm using water as the blank solution. The obtained data were analyzed, and the molecular binding constants were determined using a suitable theoretical method [15].
Basis for Selection of Saponin and Methylene Blue Concentrations
The concentrations were selected based on the MIC (Minimum Inhibitory Concentration) and MBC (Minimum Bactericidal Concentration) of the material.
In this study, 100 μg/ml saponin concentration was used, which was selected according to MIC (62.5 μg/ml) and MBC (125 μg/ml) of saponin[9].
Methylene blue concentration (μg/mL) required for 6 log10 reduction in CFU, in MB-aPDT method, for s. aureus and p. aeruginosa in planktonic state is equal to 0.62 μg/mL and 10–20 μg/mL, respectively. As a result, 0 and 10 μg/mL and higher amounts 50 and 100 μg/mL of methylene blue concentrations were used in this study [30].
Results
The Effect of Saponin Pretreatment and then Inactivation of MB-aPDT on the Planktonic Form of S. aureus and P. aeruginosa
As shown in the Fig. 1a, In P. aeruginosa, the mean log CFU of the irradiated group without saponin at 100 μg/mL concentration of MB was 5.3 whereas the mean log CFU of the irradiated group with saponin at the same concentration of MB was 6.2.
Fig. 1.
Calculated CFU of P. aeruginosa and S.aureus treated with saponin (100 μg/mL) and different concentrations of MB (0, 10, 50, 100 μg/mL) and also alone MB at dark and red LED irradiation at 660 nm wavelength for 8 min (energy 10 J/cm2). Data are shown as mean ± SD (n = 3). *P < 0.05 was considered significant compared to the dark group
According to Fig. 1b, it can be seen that in S. aureus the mean log CFU of MB with saponin groups were increased in all concentrations of MB compared to the mean log CFU of alone MB.
These results indicate that only MB without saponin groups had a greater cytotoxic effect on both P. aeruginosa and S. aureus planktonic form.
The Effect of Saponin Pretreatment and then MB-aPDT on the Biofilm Formation Ability of S. aureus and P. aeruginosa
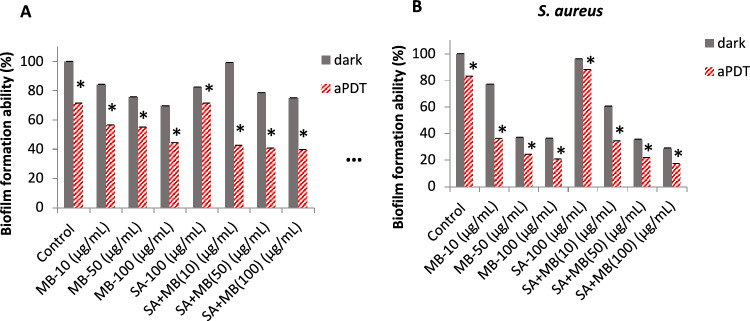
According to Fig. 2a, the MB with saponin groups exposed to red light irradiation at 10, 50 and 100 μg/mL concentrations of MB showed 42.4, 40.5 and 39.5% biofilm formation ability, respectively. Alone MB groups exposed to irradiation at 100 μg/mL concentration of MB showed 44.2% biofilm formation ability which implies a few reductions in formation ability of P. aeruginosa.
Fig. 2.
Biofilm formation ability (%) of P. aeruginosa and S. aureus after treatment with saponin (100 μg/mL) and different concentrations of MB (0, 10, 50, 100 μg/mL) and alone MB at dark and red laser irradiation at 660 nm for 8 min (energy 10 J/cm2). Data are shown as mean ± SD (n = 3). *P < 0.05 was considered significant compared to the dark group
As shown in the Fig. 2b, MB with saponin groups exposed to red irradiation at 50 and 100 μg/mL concentrations of MB showed 21.6 and 17.1% biofilm formation ability, respectively, compared to alone MB groups exposed to radiation at 50 and 100 μg/mL concentrations of MB showed 24.1 and 20.5% biofilm formation ability, respectively. The slow reduction in biofilm formation ability of S. aureus was observed, too. As a result, the effect of MB-aPDT in combination with saponin on preventing biofilm formation had a little difference compared to alone MB-aPDT.
Photodynamic Inactivation of Biofilms with Saponin then MB-aPDT
According to Fig. 3a, it can be seen that the log CFU of alone MB, then irradiation groups at 10, 50 and 100 μg/mL concentrations of MB was 86.8, 85.5 and 85.1%, respectively, and the log CFU of groups treated with saponin, MB then irradiated at the same concentrations of MB were 90.6, 84.3 and 85.1%, respectively.
Fig. 3.
Remaining biofilm (%) of P. aeruginosa and S.aureus treated with saponin (100 μg/mL) and different concentrations of MB (0, 10, 50, 100 μg/mL) and also alone MB at dark and red laser irradiation at 660 nm for 8 min (energy 10 J/cm2). Data are shown as mean ± SD (n = 3). *P < 0.05 was considered significant compared to the dark group
In Fig. 3b, the log CFU of alone MB groups exposed to irradiation at 100 μg/mL of MB was 82.98% and the log CFU of MB with saponin groups exposed to irradiation at the same concentration was 82.98%.
According to the obtained data, the composition of saponin had no significant effect on the destruction of biofilms in both bacteria compared to the groups without saponin.
Effect of Saponin then MB-aPDT on Human Dermal Fibroblast Cells
As shown Fig. 4, alone MB at 50 and 100 μg/mL concentrations had toxic effect on HDF cells. It can also be observed that the lowest toxicity among MB with saponin groups exposed to irradiation was 10 μg/mL concentration of MB which cell viability was about 70%. In the alone MB group, exposed to irradiation at 10 μg/mL, the cell viability was observed about 80%. Therefore, saponin in combination with aPDT can reduced the viability of HDF cells.
Fig. 4.

Effect of MB (0, 10, 50, 100 μg/mL) with and without saponin (100 μg/mL) on the cell viability of human dermal fibroblast cells (HDF) at dark and red laser irradiation at 660 nm for 8 min (energy 10 J/cm2). Data are shown as mean ± SD (n = 3). *P < 0.05 was considered significant compared to the dark group
Interaction of MB with Saponin
Figure 5a shows the spectral change of MB by enhancing concentration of saponin. In Fig. 5b, the changes of absorbance versus saponin concentrations were recorded based on the monomer and dimer MB maximum absorption peaks.
Fig. 5.
Schematic structure of saponin (SA) and methylene blue (MB) (a) MB UV/vis spectrum alternations in the presence of different concentrations of saponin (b). Variation in absorbance at 625 nm (dimeric form of MB) (orange) and 665 nm (monomeric form of MB) (blue) by enhancing saponin (c). Benesi-Hildebrand graph for interaction of MB (10 μg/mL) at 665 nm (monomeric form of MB) (blue line) with saponin at pH = 7 and 25 °C (d). Sap: saponin, MB: Methylene blue
As can be seen in Fig. 5c, the alternation of 1/ΔAbs versus 1/[C] was created based on the Benesi Hildebrand equation [39]. As shown in the diagram, the changes are linear and molecular interaction is 1:1 equilibrated in both monomeric and dimeric forms of MB. By using the Benesi Hildebrand equation, the related binding constant can be estimated. Due to the binding constants, the Gibbs free energy of interactions can be reached. In UV/Vis spectrum interaction MB with saponin, the bathochromic change in the maximum absorption spectrum of MB (at both dimer and monomer absorption wavelengths) showed a decrease in adsorption by increase in the saponin concentration. The results show that saponin can interact with MB in monomeric and dimeric forms. Because saponin has surfactant properties and MB is a hydrophobic molecule, the interaction of these two substances created a micelle structure, which reduced the monomer form of MB and caused to aggregate this form. Dimerization (or aggregation) of MB molecules reduces its phototoxic properties.
Discussion
Infection is a common problem in chronic wounds. This is one of the key reasons that stops wound healing and increases the risk of patient mortality [18]. Staphylococcus aureus and Pseudomonas aeruginosa are the most common bacteria isolated from chronic wounds. S. aureus and P. aeruginosa have inherent and acquired antibiotic resistance and this makes clinical management of the infection a real challenge, especially in patients with comorbidities [35]. aPDT could be suggested as a new treatment for multidrug-resistant bacteria in chronic wound infections [22]. So far no resistance has been observed in bacteria against this method [20]. In the present study, MB was used as a photosensitizer at three 10, 50 and 100 μg/mL concentrations. There are few studies on the effect of aPDT with MB on drug-resistant bacteria.
In one study by Perez-Laguna et al. Staphylococcus aureus and Pseudomonas aeruginosa were incubated with MB at 0.03–80 μg/mL concentrations and then irradiated with LED light (625 nm) in combination with gentamicin. The planktonic state of the two bacterial species was reduced to zero at 0.62 and 10 μg/mL concentrations of MB, respectively [30]. But in our study, we observed slightly reduction in amount of CFU in both S. aureus and P. aeruginosa in the presence of mb-aPDT in combination with saponin and only MB without saponin groups had a greater cytotoxic effect on both bacteria species.
In 2019, Yang et al. investigated the effect of MB-based PDT (660 nm) on the inhibition of Pseudomonas aeruginosa biofilm formation by using the violet crystal method. The biofilm formation in the 250 μM to 1 mM concentration range of methylene blue decreased after irradiation with 20 J/cm2 of energy. [41]. In our study, an acceptable effect was observed in preventing the formation of S. aureus and P. aeruginosa biofilms. Also, In the study by Perez-Laguna et al. in 2020, they investigated the degradation of the biofilm forms of Staphylococcus aureus and Pseudomonas aeruginosa, at 64 and 7000 μg/mL concentrations of MB with LED irradiation (625 nm) in combination with gentamicin reduced the biofilm form of two bacterial species to zero, respectively [30]. Our results were similar to the previous results and decreasing in biofilm formation was observed in both species in presence of MB-aPDT and the combination of saponin with MB-APDT had a little effect on preventing biofilm formation compared to alone MB-aPDT.
In another study by Motallebi et al., MTT assay showed that laser irradiation for 5 min (660 nm) had the least phototoxic effect on human skin fibroblast cells [24]. In the present study, the results showed that 8 min of red irradiation at 10 μg/mL concentration of alone MB had the least phototoxic effect on human skin fibroblast cells and the phototoxic effect increased by enhancing MB concentration.
Some plant derivatives such as berberine, curcumin, flavonoids, saponins have antibacterial effect.
According to Arabski et al. investigation, Quillaja Saponaria saponins increased growth of six strains of E. coli [3]. In our study we saw that 100 μg⁄mL concentration of saponin against the planktonic state of S. aureus and P. aeruginosa, led to a slight increase in the CFU⁄mL compared to the control group. Arabski et al. also reported in 2012 that the saponin Quillaja saponaria had a cytotoxic effect against CHO-K1 cells at above 25 μg/mL concentrations [3]. While in our study, saponin with 100 μg/mL concentration compared to the control group showed 13.4% reduction in the survival of human fibroblast cells (HDF).
In a study by Shang et al., when 0.0002 or 0.002 mg/mL concentrations of tea saponin were added to Streptococcus agalactiae, no significant change in its biofilm formation was observed, but in 2 mg⁄mL had a significant inhibitory effect on the formation of Streptococcus agalactiae biofilm [38]. In our study, considering that 100 μg/mL concentration of saponin was used, the result was the same as the low concentrations of saponin in the study of Shang et al.
Given the possibility of regrowth of those microorganisms that have not been inactivated during irradiation, the triple combination of aPDT with conventional antimicrobial drugs may be an appropriate method to achieve an additive or synergistic therapeutic effect or even to overcome microbial resistance [31](Pérez-Laguna et al. 2019c). Therefore, in the present study, we investigated MB-aPDT effect in combination with the antibacterial substance, saponin, on Staphylococcus aureus and Pseudomonas aeruginosa.
In the study by Motallebi et al. in 2020, rutin pretreatment (400 μg/mL, 4 h), then MB (8 μg/mL, 10 min)-aPDT (660 nm, 5 min), led to a significant reduction in planktonic form of P. aeruginosa and S. aureus in comparison with alone MB-aPDT group [24]. However, in our study, in pretreatment with saponin and then MB-aPDT (in all concentrations of MB 10, 50 and 100 μg/mL), caused no reduction compared to alone MB in both P. aeruginosa and S. aureus bacteria.
Shahmoradi et al. found that the combined use of MB and SeNPs (selenium nanoparticles) with LED emitting diodes significantly reduced the colony forming units (CFU) of 1-day E. faecalis biofilms compared to the control group [37]. In the present study, the combination of saponin (100 mg/mL) in all three concentrations of MB (10, 50 and 100 μg/mL) the LED irradiation (660 nm) did not show significant effect on the degradation of S. aureus and P. aeruginosa biofilms. There was no significant decrease in the CFU/mL content of the biofilms of strains compared to the control group, too.
In a study conducted by Khorsandi et al., spectrophotometric results showed that by increasing the concentration of rutoside (1–60 μg/mL), the adsorption of MB (10 μg/mL) decreased at MB maximum absorption spectra [16]. In the present study, according to the spectrophotometric results, by increasing the concentration of saponin (1–700 μg/mL), the absorption of MB (10 μg/mL) had a decreasing trend.
Conclusion
The result of this study not confirming our hypothesis for this study which was the combination of saponin and aPDT is effective on the planktonic and biofilm states of S. aureus and P. aeruginosa even according to our results, the effect of saponin on photodynamic inactivation on planktonic and biofilm form of Pseudomonas aeruginosa and Staphylococcus aureus, reduced the photoactivity of MB due to reduction of the monomer form of MB, which is the active and photoactive form of MB. Therefore, it can be recommended not to use detergents such as saponins at the same time with MB-aPDT method.
According to the obtained results, it is suggested to investigate the antimicrobial effect of saponin in combination with photodynamic inactivation on other strains of Gram-positive and Gram-negative bacteria in future research. Plus, this method can be test on animal model to confirm the result of in vitro study and make it as a new approach for using in clinics.
Declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Apers S, Baronikova S, Sindambiwe JB, Witvrouw M, De Clercq E, Vanden Berghe D, Van Marck E, Vlietinck A, Pieters L (2001) Antiviral, haemolytic and molluscicidal activities of triterpenoid saponins from Maesa lanceolata: establishment of structure-activity relationships. Planta Med 67:528–532. 10.1055/s-2001-16489 10.1055/s-2001-16489 [DOI] [PubMed] [Google Scholar]
- 2.Arabski M, Węgierek-Ciuk A, Czerwonka G, Lankoff A, Kaca W (2012a) Effects of saponins against clinical E. coli strains and eukaryotic cell line. J Biomed Biotechnol. 10.1155/2012/286216 [DOI] [PMC free article] [PubMed]
- 3.Arabski M, Węgierek-Ciuk A, Czerwonka G, Lankoff A, Kaca W (2012b) Effects of saponins against clinical E. coli strains and eukaryotic cell line. J Biomed Biotechnol. 10.1155/2012/286216 [DOI] [PMC free article] [PubMed]
- 4.Bonham PA (2009) Identifying and treating wound infection: topical and systemic antibiotic therapy. J Gerontol Nurs 35:12–16. 10.3928/00989134-20090903-03 10.3928/00989134-20090903-03 [DOI] [PubMed] [Google Scholar]
- 5.Boucher HW, Corey GR (2008) Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 46. 10.1086/533590 [DOI] [PubMed]
- 6.Brohem CA, Da LB, Cardeal S, Tiago M, Soengas MS, De Moraes Barros SB, Maria-Engler SS (2011) Artificial skin in perspective: concepts and applications. Wiley Online Library 24:35–50. 10.1111/j.1755-148X.2010.00786.x 10.1111/j.1755-148X.2010.00786.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and prevension. 2013. ANTIBIOTIC RESISTANCE THREATS in the United States. [place unknown].
- 8.Coenye T, Nelis HJ (2010) In vitro and in vivo model systems to study microbial biofilm formation. J Microbiol Methods 83:89–105. 10.1016/j.mimet.2010.08.018 10.1016/j.mimet.2010.08.018 [DOI] [PubMed] [Google Scholar]
- 9.Dong S, Yang X, Zhao L, Zhang F, Hou Z, Xue P (2020) Antibacterial activity and mechanism of action saponins from Chenopodium quinoa Willd. husks against foodborne pathogenic bacteria. Ind Crops Prod 149:112350. 10.1016/j.indcrop.2020.112350
- 10.Fontana CR, Abernethy AD, Som S, Ruggiero K, Doucette S, Marcantonio RC, Boussios CI, Kent R, Goodson JM, Tanner ACR, Soukos NS (2009) The antibacterial effect of photodynamic therapy in dental plaque-derived biofilms. J Periodontal Res 44:751–759. 10.1111/j.1600-0765.2008.01187.x 10.1111/j.1600-0765.2008.01187.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghasemi M, Khorsandi K, Kianmehr Z (2021) Photodynamic inactivation with curcumin and silver nanoparticles hinders Pseudomonas aeruginosa planktonic and biofilm formation: evaluation of glutathione peroxidase activity and ROS production. World J Microbiol Biotechnol 37:149. 10.1007/s11274-021-03104-4 10.1007/s11274-021-03104-4 [DOI] [PubMed] [Google Scholar]
- 12.Hassan SM, Byrd JA, Cartwright AL, Bailey CA (2010) Hemolytic and antimicrobial activities differ among saponin-rich extracts from guar, quillaja, yucca, and soybean. Appl Biochem Biotechnol 162:1008–1017. 10.1007/s12010-009-8838-y 10.1007/s12010-009-8838-y [DOI] [PubMed] [Google Scholar]
- 13.Hu WL, Liu JX, Ye JA, Wu YM, Guo YQ (2005) Effect of tea saponin on rumen fermentation in vitro. Anim Feed Sci Technol 120:333–339. 10.1016/J.ANIFEEDSCI.2005.02.029 10.1016/J.ANIFEEDSCI.2005.02.029 [DOI] [Google Scholar]
- 14.Kerr KG, Snelling AM (2009) Pseudomonas aeruginosa: a formidable and ever-present adversary. J Hosp Infect 73:338–344. 10.1016/J.JHIN.2009.04.020 10.1016/J.JHIN.2009.04.020 [DOI] [PubMed] [Google Scholar]
- 15.Khorsandi K, Hosseinzadeh R, Chamani E (2020) Molecular interaction and cellular studies on combination photodynamic therapy with rutoside for melanoma A375 cancer cells: an in vitro study. Cancer Cell Int 20:525. 10.1186/s12935-020-01616-x 10.1186/s12935-020-01616-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khorsandi K, Hosseinzadeh R, Chamani E (2020) Molecular interaction and cellular studies on combination photodynamic therapy with rutoside for melanoma A375 cancer cells: an in vitro study. Cancer Cell Int 20:1–15. 10.1186/S12935-020-01616-X/FIGURES/12 10.1186/S12935-020-01616-X/FIGURES/12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YA, Kong CS, Lee JI, Kim H, Park HY, Lee HS, Lee C, Seo Y (2012) Evaluation of novel antioxidant triterpenoid saponins from the halophyte Salicornia herbacea. Bioorg Med Chem Lett 22:4318–4322. 10.1016/J.BMCL.2012.05.017 10.1016/J.BMCL.2012.05.017 [DOI] [PubMed] [Google Scholar]
- 18.Landis SJ (2008) Chronic wound infection and antimicrobial use. Adv Skin Wound Care 21. 10.1097/01.ASW.0000323578.87700.A5 [DOI] [PubMed]
- 19.Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339:520–532. 10.1056/NEJM199808203390806 10.1056/NEJM199808203390806 [DOI] [PubMed] [Google Scholar]
- 20.Maisch T (2015) Resistance in antimicrobial photodynamic inactivation of bacteria. Photochem Photobiol Sci 14:1518–1526. 10.1039/C5PP00037H 10.1039/C5PP00037H [DOI] [PubMed] [Google Scholar]
- 21.Meng ZY, Zhang JY, Xu SX, Sugahara K (1999) Steroidal saponins from Anemarrhena asphodelaides and their effects on superoxide generation. Planta Med 65:661–663. 10.1055/s-2006-960842 10.1055/s-2006-960842 [DOI] [PubMed] [Google Scholar]
- 22.Mirzahosseinipour M, Khorsandi K, Hosseinzadeh R, Ghazaeian M, Shahidi FK (2020) Antimicrobial photodynamic and wound healing activity of curcumin encapsulated in silica nanoparticles. Photodiagnosis Photodyn Ther 29:101639. 10.1016/J.PDPDT.2019.101639 10.1016/J.PDPDT.2019.101639 [DOI] [PubMed] [Google Scholar]
- 23.Mishra SC, Chhatbar KC, Kashikar A, Mehndiratta A (2017) Diabetic foot. BMJ 359:j5064. 10.1136/BMJ.J5064 10.1136/BMJ.J5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motallebi M, Khorsandi K, Sepahy AA, Chamani E, Hosseinzadeh R (2020a) Effect of rutin as flavonoid compound on photodynamic inactivation against P. aeruginosa and S. aureus. Photodiagnosis Photodyn Ther 32:102074. 10.1016/j.pdpdt.2020.102074 [DOI] [PubMed]
- 25.Motallebi M, Khorsandi K, Sepahy AA, Chamani E, Hosseinzadeh R (2020b) Effect of rutin as flavonoid compound on photodynamic inactivation against P. aeruginosa and S. aureus. Photodiagnosis Photodyn Ther [Internet]. 32:102074. 10.1016/j.pdpdt.2020.102074 [DOI] [PubMed]
- 26.Mshvildadze V, Favel A, Delmas F, Pharmazie RE, 2000 U. (2000) Antifungal and antiprotozoal activities of saponins from Hedera colchica. pascal-francis.inist.fr. [PubMed]
- 27.Nature E (2013) The antibiotic alarm. Nature 2013 495:141–141. 10.1038/495141a [DOI] [PubMed]
- 28.O’Riordan K, Akilov OE, Hasan T (2005) The potential for photodynamic therapy in the treatment of localized infections. Photodiagn Photodyn Ther 2:247–262. 10.1016/S1572-1000(05)00099-2 10.1016/S1572-1000(05)00099-2 [DOI] [PubMed] [Google Scholar]
- 29.Pantosti A, Sanchini A, Monaco M (2007) Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiol 2:323–334. 10.2217/17460913.2.3.323 10.2217/17460913.2.3.323 [DOI] [PubMed] [Google Scholar]
- 30.Pérez-Laguna V, García-Luque I, Ballesta S, Pérez-Artiaga L, Lampaya-Pérez V, Rezusta A, Gilaberte Y (2020) Photodynamic therapy using methylene blue, combined or not with gentamicin, against Staphylococcus aureus and Pseudomonas aeruginosa. Photodiagnosis Photodyn Ther 31:101810. 10.1016/j.pdpdt.2020.101810 10.1016/j.pdpdt.2020.101810 [DOI] [PubMed] [Google Scholar]
- 31.Pérez-Laguna V, García-Malinis AJ, Aspiroz C, Rezustata A, Gilaberteerte Y (2018) Antimicrobial effects of photodynamic therapy. G Ital Dermatol Venereol 153:833–846. 10.23736/S0392-0488.18.06007-8 [DOI] [PubMed]
- 32.Pérez-Laguna V, Gilaberte Y, Millán-Lou MI, Agut M, Nonell S, Rezusta A, Hamblin MR (2019) A combination of photodynamic therapy and antimicrobial compounds to treat skin and mucosal infections: a systematic review. Photochem Photobiol Sci 18:1020–1029. 10.1039/c8pp00534f 10.1039/c8pp00534f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Read AF, Woods RJ (2014) Management antibiotic resistance. Evol Med Public Health, 147. 10.1093/emph/eou024 [DOI] [PMC free article] [PubMed]
- 34.Santajit S, Indrawattana N (2016) Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed Res Int. 10.1155/2016/2475067 [DOI] [PMC free article] [PubMed]
- 35.Serra R, Grande R, Butrico L, Rossi A, Settimio UF, Caroleo B, Amato B, Gallelli L, De Franciscis S (2015) Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev Anti Infect Ther 13:605–613. 10.1586/14787210.2015.1023291 10.1586/14787210.2015.1023291 [DOI] [PubMed] [Google Scholar]
- 36.Shafiei M, Ali AA, Shahcheraghi F, Saboora A, Noghabi KA (2014) Eradication of Pseudomonas aeruginosa biofilms using the combination of n-butanolic Cyclamen coum extract and Ciprofloxacin. Jundishapur J Microbiol 7:14358. 10.5812/JJM.14358 10.5812/JJM.14358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shahmoradi S, Shariati A, Zargar N, Yadegari Z, Asnaashari M, Amini SM, Darban-Sarokhalil D (2021) Antimicrobial effects of selenium nanoparticles in combination with photodynamic therapy against Enterococcus faecalis biofilm. Photodiagn Photodyn Ther 35:102398. 10.1016/J.PDPDT.2021.102398 10.1016/J.PDPDT.2021.102398 [DOI] [PubMed] [Google Scholar]
- 38.Shang F, Wang H, Xue T (2020) Anti-biofilm effect of tea saponin on a Streptococcus agalactiae strain isolated from bovine mastitis. Animals 10:1713. 10.3390/ANI10091713 [DOI] [PMC free article] [PubMed]
- 39.Wang R, Yu Z (2007) Validity and reliability of Benesi-Hildebrand method. Acta Phys Chim Sin 23:1353–1359. 10.1016/S1872-1508(07)60071-0 10.1016/S1872-1508(07)60071-0 [DOI] [Google Scholar]
- 40.Wu W, Jin Y, Bai F, Jin S (2015) Pseudomonas aeruginosa. Molecular Med Microbiol Second Edition 2–3:753–767. 10.1016/B978-0-12-397169-2.00041-X 10.1016/B978-0-12-397169-2.00041-X [DOI] [Google Scholar]
- 41.Yang SM, Lee DW, Park HJ, Kwak MH, Park JM, Choi MG (2019) Hydrogen peroxide enhances the antibacterial effect of methylene blue-based photodynamic therapy on biofilm-forming bacteria. Photochem Photobiol 95:833–838. 10.1111/PHP.13056 10.1111/PHP.13056 [DOI] [PubMed] [Google Scholar]